Abstract
Objective
To investigate the relationship between blood levels of cadmium (Cd) and lead (Pb) and the expression of miRNA-21 among bladder cancer (BC) patients.
Material and methods
The blood concentrations of Cd and Pb in 268 BC patients and 132 controls were determined by inductively coupled plasma optical emission spectrometry (ICP-OES). The blood concentrations of Cd and Pb were interpreted according to the type and stage of the carcinoma. The expression of miRNA-21 was assessed by quantitative reverse transcription polymerase chain reaction in cancerous and adjacent non-cancerous bladder tissues among the patient groups.
Results
The blood concentrations of Cd and Pb were statistically elevated in BC patients compared to those of the controls. This elevation is more prevalent in groups with muscle-invasive bladder cancer (MIBC) than those with non-muscle invasive bladder cancer (NMIBC). Among the BC group, miRNA-21 was upregulated in cancerous tissues relative to adjacent non-cancerous tissues. Moreover, the expression was significantly higher in patients with MIBC compared to those with NMIBC. The expression of miRNA-21 in cancerous tissues was significantly associated with blood concentration of Cd and Pb among BC patients.
Conclusion
There is a relationship between Cd and Pb body burden and the tissue expression of miRNA-21 among BC patients. This indicates the role of miRNA-21 in Cd and Pb induced BC.
Keywords: Environmental pollution, Gene expression, Cancer research, Oncology, Toxicology, Clinical research, Cadmium, Lead, miRNA-21, Bladder cancer
Environmental pollution; Gene expression; Cancer research; Oncology; Toxicology; Clinical research; Cadmium; Lead; miRNA-21; Bladder cancer.
1. Introduction
Cadmium (Cd) and lead (Pb) are biohazard heavy metals that threaten human health. Although they are distributed naturally, their concentrations are increased in the environment due smelting, batteries manufacture, ceramics and pigments industries (Andjelkovic et al., 2019). Cd and Pb are present in air, food and water, and their toxic effects depend upon the route and the dose of exposure (Tchounwou et al., 2012). The World Health Organization (WHO) has reported that Cd and Pb are two of the most ten toxic chemicals to human health (WHO 2010). Cd and Pb are in the seventh and second orders on the priority list of serious matters (ATSDR 2019). Both metals exert toxic effects on liver, kidneys, cardiovascular, reproductive and hematopoietic systems by different mechanisms (Andjelkovic et al., 2019). Oxidative stress and binding to body proteins and enzymes are the most important mechanisms of Cd and Pb toxicity (Matović et al., 2015; Vujotić et al., 2020). Heavy metals, including Cd and Pb, may also cause damage of DNA (Flora et al., 2012).
Bladder cancer is a common neoplasm of the urinary system. Some environmental factors including heavy metals participate in the etiology of BC (Chang et al., 2016). Epidemiological studies showed possible associations between Cd or Pb and BC (Feki-Tounsi et al. 2013, 2014; Golabek et al., 2009). United States Environmental Protection Agency (US-EPA) has defined Cd as possible human carcinogen (Huff et al., 2007). The mechanism of Cd carcinogenesis is complex however gene expression deregulation plays a critical part in this matter (Feki-Tounsi and Hamza-Chaffai 2014). Elevated levels of Pb were detected in blood and cancerous tissues of BC patients, suggesting its contribution to development of BC (Golabek et al., 2009).
MicroRNAs (miRNAs) are short non-coding RNA sequences. It can control gene expression and regulate vital cellular pathways. They are involved in initiation and development of cancers (Hou et al., 2011). Evidences demonstrates that exposure toxic metals affect miRNA expression (Wallace et al., 2020). miRNA-21 has been reported to be one of the most commonly up-regulated miRNAs in various human cancers functioning as a key regulator of carcinogenic process, and has been considered as a novel target in cancer monitoring (Bautista-Sánchez et al., 2020). It is interesting that recent studies also show that miRNA-21 is critically involved in heavy metals-induced cell malignant transformation and tumorigenesis (Wallace et al., 2020). Alteration of miRNA expression is associated with Cd carcinogenesis in prostatic cancer (Moustafa et al., 2018), pancreatic cancer (Wallace et al., 2019), thyroid cancer (Buha et al., 2018) and ovarian cancer (Wang et al., 2018). Association between Pb toxicity and changes in expression of different types of miRNA has also be observed in many diseases such as neurotoxicity (Masoud et al., 2016), nephropathy (Siddeek et al., 2014) and cancers (Feng and Tsao 2016). Few studies were conducted to investigate changes in the expression of miRNAs in response to Cd and Pb exposure (Lei et al., 2019). It would be very interesting to determine whether miRNA-21 is involved in the correlation between BC progression and Cd and Pb exposure.
To the best of our knowledge, the present study is the first to explore the relation between Cd and Pb concentrations in blood and expression of miRNA-21 in cancerous tissues among BC patients. The study aims to add additional information about BC pathogenesis that may be helpful for reducing incidence and recurrence of the disease.
2. Materials and methods
2.1. Study population
The pre-operative study was conducted at the Urology and Nephrology Center, Mansoura University that serve Dakahlia Governorate in Egypt during 3 years period from 2015-2018. The A total of 268 patients with different stages of BC were included in the study. The patients recruited for the study were first diagnosed with BC. The diagnosis of BC was confirmed by cystoscopy, biopsy and histopathological examinations. Patients were grouped according to their histopathological reports as transitional cell carcinoma (TCC, n = 148) or squamous cell carcinoma (SCC, n = 120). Each group was subdivided into two sub-groups, non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) based on the stage of cancer. Exclusion criteria were the presence of known occupational exposure to heavy metals, chemotherapy, radiotherapy and other malignant diseases. The study included a control group of 132 matched individuals with neither tumor nor heavy metal exposure history. Informed consent was obtained from each person. All participants had normal kidney functions (estimated by plasma creatinine analysis) and normal hepatic functions (determined by analysis of plasma transaminases and bilirubin). The study was approved by our local Institutional Review Board (IRB) (IRB #RP-17.12.301). The characteristics of all groups were summarized in Table 1.
Table 1.
Characteristics of the study groups.
| Control group | SCC group | TCC group | p value∗ | |
|---|---|---|---|---|
| Number of subjects | 132 | 120 | 148 | |
| NMIBC:MIBC |
- |
21:99 |
31:117 |
|
|
Categorical variables, n (%) | ||||
| Gender | ||||
| Male | 100 (75.7) | 91 (75.8) | 115 (77.7) | 0.44 |
| Female | 32 (24.3) | 29 (24.2) | 33 (22.3) | 0.51 |
| Smokers | 34 (25.7) | 34 (28.3) | 40 (27.0) | 0.32 |
| Hypertension | 29 (22.0) | 30 (25.0) | 36 (24.3) | 0.18 |
| Diabetes |
23 (17.4) |
21 (17.5) |
28 (18.9) |
0.28 |
|
Continuous variables, Mean ± SD | ||||
| Age (years) | 54.0 ± 7.7 | 61.4 ± 6.4 | 58.5 ± 8.9 | 0.46 |
| BMI (kg/m2) | 25.9 ± 4.0 | 27.4 ± 3.7 | 26.1 ± 4.9 | 0.52 |
| Serum creatinine (mg dL−1) | 0.81 ± 0.07 | 0.89 ± 0.06 | 0.84 ± 0.08 | 0.71 |
| ALT (IU L−1) | 26.8 ± 4.1 | 31.7 ± 4.9 | 29.4 ± 5.8 | 0.44 |
| AST (IU L−1) | 16.5 ± 3.9 | 18.4 ± 3.7 | 21.9 ± 4.3 | 0.36 |
| Bilirubin (mg dL−1) | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.66 |
One way ANOVA for continuous variables and Chi-square test for categorical variables.
2.2. Analysis of Cd and Pb
Venous blood samples for analysis of Cd and Pb were collected from all individuals in metal free tubes (Royal blue vacutainer tubes) containing K2EDTA and were preserved at -20 °C. Samples were digested within a month of collection as previously prescribed (Mortada et al., 2017). Briefly, 1 mL of blood sample was mixed with 4 mL of HNO3 and 2 mL of H2O2 in Teflon digested tube and then kept for 20 min at room temperature. The closed tubes were processed in the microwave digestion system (Speed wave four, Berghof Products, Germany) according to the following program: power, 1600 W (100%); ramp time, 15 min; temperature, 200 °C; hold time, 15 min; and cooling time, 15 min. After cooling, the solution was diluted to 10 mL by distilled water and filtered (if necessary), then subjected for analysis of Cd and Pb by inductively coupled plasma optical emission spectrometry (Agilent technologies 700 ICP-OES Series, Santa Clara, CA, USA). The instrumental parameters were adjusted according to the manufacturer specifications (Table 2). Accuracy was tested at the start of each run by analysis of certified reference material (Seronorm™ Trace Elements Whole Blood L-2, Sero, Norway).
Table 2.
ICP-OES instrument parameters for determination of Cd and Pb.
| Metal ion | Power (kW) | Plasma gas flow (L min−1) | Auxiliary gas flow (L min−1) | Nebulizer gas flow rate (L min−1) | Delay time (sec) | Wavelength (nm) | Detection limit (μg L−1) |
|---|---|---|---|---|---|---|---|
| Cd | 1.4 | 15 | 1.5 | 0.7 | 12 | 226.502 | 0.2 |
| Pb | 220.353 | 1.8 |
2.3. miRNA-21 quantification by real-time (RT)-qPCR
Two tissue samples were obtained from each BC patient: the first was collected from central part of the tumor and the second which served as a control was taken from non-lesion part of the tissue. Tissue samples for miRNA analysis were preserved in RNA later at -80 °C. Total RNA including miRNAs was extracted from bladder tissues using miRNeasy Mini Kit (cat.# 217004, Qiagen, Hilden, Germany), then it reverse transcribed to cDNA in a final volume of 20 μL using the miScript Reverse Transcription kit (cat.# 218161,Qiagen, Hilden, Germany). The qPCR measurements were done in triplicate using miScript SYBR-Green PCR kit (SYBR® Green PCR Kit) (cat.# 218073,Qiagen, Hilden, Germany) and miScript primer assay for miRNA-21 on the Rotor-Gene Q 5-Plex (Qiagen) according to the manufacturer's instructions. The results were normalized to SNORD68_11 (sno68) (Qiagen). The amplification profile was denatured at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min and 70 for 30 °C s. The expression level of miRNA gene was represented by fold change, which was calculated using the equation 2−ΔΔCT (Livak and Schmittgen 2001).
2.4. Statistical analyses
Statistical computations were performed by SPSS-PC software (MAS Medical & Scientific Eq. Co, IL, USA). Kolmogorov-Smirnov test indicated normal distribution of all variables. Accordingly, continuous data were expressed as mean ± standard deviation (SD) while categorical variables were presented as number and percentage. Comparisons between groups were made using ANOVA and Chi-square test as appropriate. The partial correlation coefficient (r) was estimated to determine the relation between blood levels of Cd and Pb with cancerous tissue expression of mi-RNA21, after controlling for age and BMI. p-value ≤ 0.05 was defined as significant.
3. Results
Table 1 displays the descriptive features of study groups. The mean age of the study groups were comparable. Most of BC cases were MIBC (n = 99, 85.5% for SCC; n = 117, 79.1% for TCC). No significant differences were observed between the groups concerning gender, BMI, smoking habit, clinical and biochemical data.
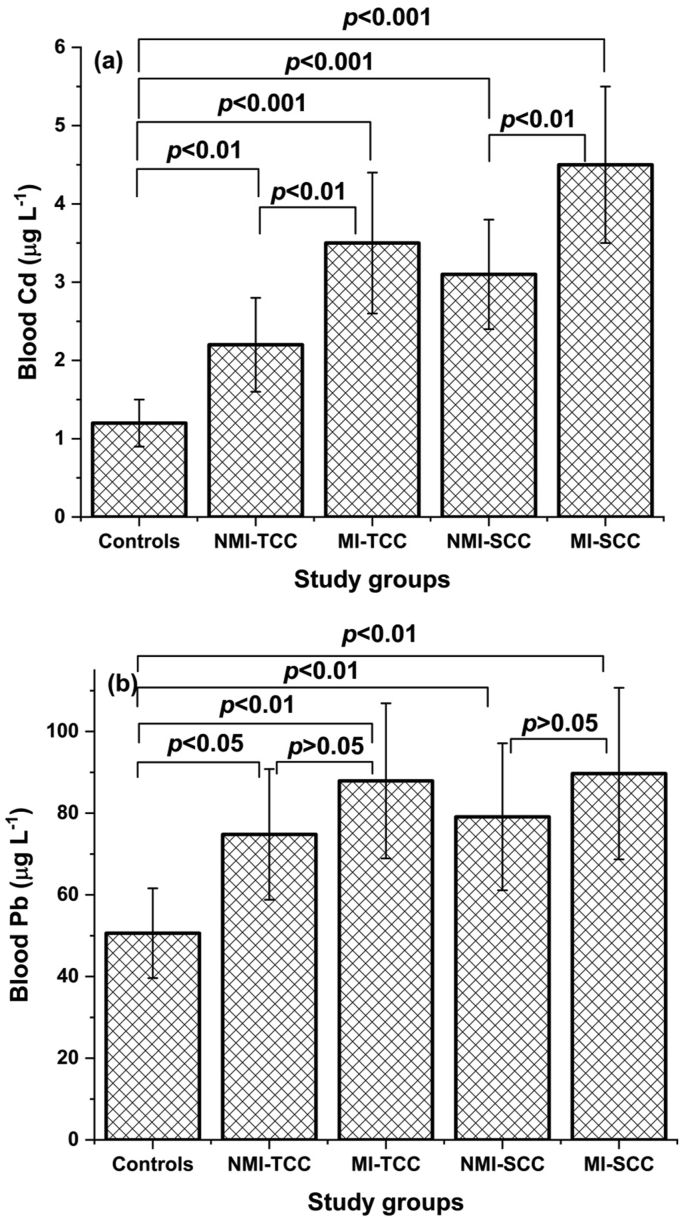
Figure 1 presents the levels of blood Cd and Pb from cancer and control groups. Significantly elevated concentrations of blood Cd and Pb were detected in TCC and SCC groups when compared to the controls (p < 0.001). It is also evident that Cd and Pb levels in blood among both TCC and SCC groups were significantly higher in MIBC patients than those with NMIBC (p < 0.01 in case of Cd and p < 0.05 regarding Pb). The elevated levels in the study groups didn't reach the level of toxicity stated by the international communities. In adults, Pb blood levels up to 100 μg L−1 are considered normal (CDC 2013), while the normal blood Cd concentration is less than 5 μg L−1 (Moyer 2015). The elevated levels in our patients didn't reach these levels.
Figure 1.
(a) Cd and (b) Pb levels in NMIBC and MIBC of TCC and SCC groups versus controls (NMI-TCC = non-muscle invasive transition cell carcinoma, MI-TCC = muscle invasive transition cell carcinoma, NMI-SCC = non-muscle invasive squamous cell carcinoma, MI-SCC = muscle invasive squamous cell carcinoma).
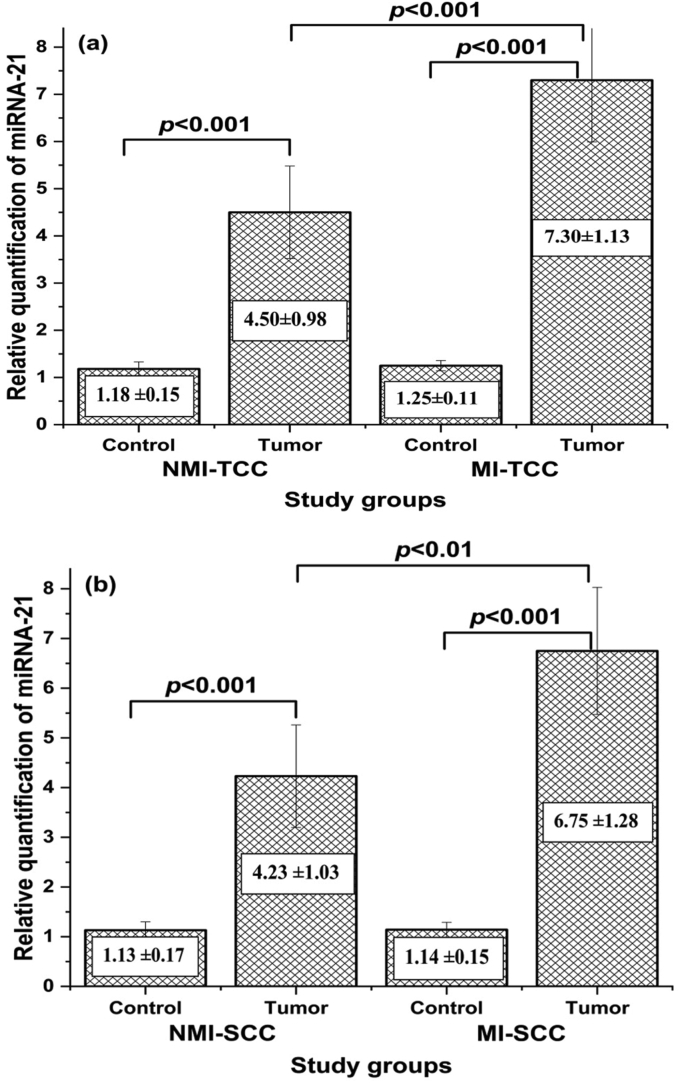
The results in Figure 2 display comparison between expression of mi-RNA21 in cancerous bladder tissues and adjacent non-cancerous bladder tissues in SCC and TCC groups. The expressions of mi-RNA21 in bladder cancerous tissues in both SCC and TCC groups were significantly higher (p < 0.001) when compared to those in the adjacent non-cancerous tissues. Moreover, miRNA-21 was upregulated in MIBC patients when compared to those of NMIBC patients within the same group (p < 0.001 in TCC group and p < 0.01 in SCC).
Figure 2.
The expression of miRNA-21 in cancerous and adjacent non-cancerous tissues in (a) TCC group and (b) SCC group (NMI-TCC = non-muscle invasive transition cell carcinoma, MI-TCC = muscle invasive transition cell carcinoma, NMI-SCC = non-muscle invasive squamous cell carcinoma, MI-SCC = muscle invasive squamous cell carcinoma).
Table 3 presents the partial correlation coefficient values (r) between levels of blood Cd or Pb with expression of miRNA-21 in cancerous bladder tissue among TCC and SCC groups. As shown, expression of miRNA-21 in cancerous tissue is positively correlated with blood levels of Cd or Pb (p < 0.001 except between blood Cd and miRNA-21 in SCC group).
Table 3.
Correlation coefficients (r) between blood Cd and Pb with miRNA-21 expression in cancerous tissues.
| TCC |
SCC |
|||
|---|---|---|---|---|
| Cd blood | Pb blood | Cd blood | Pb blood | |
| miRNA-21 | 0.584 (<0.001) | 0.659 (<0.001) | 0.363 (<0.01) | 0.531 (<0.001) |
All values represent r (p values).
4. Discussion
The recurrence of BC continues to impede its treatment. Multiple factors are involved in the pathogenesis of BC, however genetics and environmental factors are the most contributed sources (Volanis et al., 2010). Heavy metals such as Cd and Pb increase the risk of urothelial carcinogenesis (Chang et al., 2016). The exact mechanism of carcinogenesis of Cd and Pb is still undefined. Alteration in oxidative stress (Abdeen et al., 2019) and failure in DNA repair may cause genetic mutation accompanied by abnormal gene expression that involve in the cancer prognosis (Al Bakheet et al., 2013). Exposure to Cd+2 led to a cancerous transformation in human urothelial cell lines (Sens et al., 2004). Both Cd and Pb also induced genes linked to cell signaling and metabolism (Koedrith et al., 2013; Koizumi and Yamada 2003). Generally, heavy metals initiate or promote cancer by different cellular mechanisms including cellular redox imbalance and changes in genetic and epigenetic factors (Koedrith et al., 2013). The role of miRNA in carcinogenic effects of heavy metals is still unclear due to limited studies (Humphries et al., 2016). Therefore, our study is aimed to study the correlation between Cd and Pb levels in blood and expression of miRNA-21 in patients with different stages of TCC and SCC.
Blood Cd and Pb are considered good indicators of body burden as they are strongly correlated with urinary and tissue levels (Järup and Åkesson 2009; Mendy et al., 2012). In the present work, BC patients (TTC and SCC) exhibited higher levels of Cd and Pb compared to controls. These findings agree with other previous studies. Many investigators have observed a relationship between Cd and Pb exposure to elevated risk of BC. Golabek et al., (2009) reported higher blood levels of Pb in BC patients compared to controls. In a Belgian case-control study, exposure to Cd elevated the risk of BC development (Kellen et al., 2007). The statistically significant elevation of Cd and Pb blood levels among MIBC patients compared to those with NMIBC suggest a correlation between exposure to Cd and Pb and the progression of BC.
miRNAs are short non-coding RNAs that have a significant function in controlling differentiation, proliferation, apoptosis and autophagy (Liu et al., 2017). Dysregulation of miRNAs was observed in different types of cancer and their expressions were associated with pathogenesis and features of the disease (Ambros 2004). In our study, high expression of miRNA-21 was observed in cancerous tissues of BC patients in comparison with the non-cancerous tissues and the expressions were more prevalence in the MIBC groups. These findings corroborated the previously reported data (Pignot et al., 2013; Zhang et al., 2015) indicating a significant role of miRNA-21 in bladder carcinogenesis.
The presence of a significant positive correlation between blood levels of Cd and Pb with the expression of miRNA-21 in TCC and SCC groups suggests that there is a role of these metals in genetic alteration accompanied BC. These findings agree with other reports which suggest the involvement of miRNA-21 in metal-induced carcinogenesis (Ling et al., 2012). Liu et al. reported expression of a number of miRNAs in Cd transformed human bronchial epithelial cells (Liu et al., 2015). These results come together to refer that miRNA-21 has a role in metal induced carcinogenesis. No data are available in the literature about the relation between Cd and Pb exposure and expression of miRNAs among BC patients. However, Cd and Pb may alter miRNAs expression in other diseases. In an experimental study, Fay et al. reported that alteration in expression of 44 miRNAs may play a role in the pathophysiology of Cd nephrotoxicity (Fay et al., 2018). In another study, the exposure to Pb during early life affected the expression of miRNAs that related to Alzheimer disease (Masoud et al., 2016). Among occupationally exposed workers, miRNA-21 was associated with Pb toxicity (Xu et al., 2017).
5. Conclusion
The present work proposed the positive correlation between blood concentrations Cd and Pb with the expression of miRNA-21 in BC tissues. These finding provide additional information about the role of miRNA-21 in metal induced bladder carcinogenesis. Descriptive and functional studies are strongly recommended to speak about the role of miR-21 in metal-induced bladder carcinogenesis.
Declarations
Author contribution statement
A. Awadalla: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
W. Mortada: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
H. Abol-Enein and A. Shokeir: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Science & Technology development Fund (STDF), Ministry of Scientific Research, Egypt (5236).
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdeen A. Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ. Sci. Pollut. Control Ser. 2019;26:25167–25177. doi: 10.1007/s11356-019-05783-x. [DOI] [PubMed] [Google Scholar]
- Al Bakheet S.A., Attafi I.M., Maayah Z.H., Abd-Allah A.R., Asiri Y.A., Korashy H.M. Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environ. Pollut. 2013;181:226–232. doi: 10.1016/j.envpol.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Publ. Health. 2019;16:274. doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR ATSDR’s substance priority list. 2019. https://www.atsdr.cdc.gov/spl/index.html#2019spl
- Bautista-Sánchez D. The promising role of miR-21 as a cancer biomarker and its importance in RNA-based therapeutics. Mol. Ther. Nucleic Acids. 2020 doi: 10.1016/j.omtn.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buha A. Overview of cadmium thyroid disrupting effects and mechanisms. Int. J. Mol. Sci. 2018;19:1501. doi: 10.3390/ijms19051501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . US Department of Health and Human Services, CDC, National Institute for Occupational Safety and Health; 2013. Adult Blood Lead Epidemiology and Surveillance (ABLES)https://www.cdc.gov/niosh/topics/ables/description.html [Google Scholar]
- Chang C.H. Association between levels of urinary heavy metals and increased risk of urothelial carcinoma. Int. J. Urol. 2016;23:233–239. doi: 10.1111/iju.13024. [DOI] [PubMed] [Google Scholar]
- Fay M.J. Cadmium nephrotoxicity is associated with altered microRNA expression in the rat renal cortex. Toxics. 2018;6:16. doi: 10.3390/toxics6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feki-Tounsi M., Hamza-Chaffai A. Cadmium as a possible cause of bladder cancer: a review of accumulated evidence. Environ. Sci. Pollut. Control Ser. 2014;21:10561–10573. doi: 10.1007/s11356-014-2970-0. [DOI] [PubMed] [Google Scholar]
- Feki-Tounsi M., Olmedo P., Gil F., Khlifi R., Mhiri M.-N., Rebai A., Hamza-Chaffai A. Cadmium in blood of Tunisian men and risk of bladder cancer: interactions with arsenic exposure and smoking. Environ. Sci. Pollut. Control Ser. 2013;20:7204–7213. doi: 10.1007/s11356-013-1716-8. [DOI] [PubMed] [Google Scholar]
- Feki-Tounsi M., Olmedo P., Gil F., Mhiri M.-N., Rebai A., Hamza-Chaffai A. Trace metal quantification in bladder biopsies from tumoral lesions of Tunisian cancer and controls subjects. Environ. Sci. Pollut. Control Ser. 2014;21:11433–11438. doi: 10.1007/s11356-014-3099-x. [DOI] [PubMed] [Google Scholar]
- Feng Y.-H., Tsao C.-J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016;5:395–402. doi: 10.3892/br.2016.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G., Gupta D., Tiwari A. Toxicity of lead: a review with recent updates. Interdiscipl. Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabek T., Darewicz B., Borawska M., Markiewicz R., Socha K., Kudelski J. Lead concentration in the bladder tissue and blood of patients with bladder cancer Scandinavian. J. Urol. Nephrol. 2009;43:467–470. doi: 10.3109/00365590903198991. [DOI] [PubMed] [Google Scholar]
- Hou L., Wang D., Baccarelli A. Environmental chemicals and microRNAs. Mutat. Res. Fund Mol. Mech. Mutagen. 2011;714:105–112. doi: 10.1016/j.mrfmmm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J., Lunn R.M., Waalkes M.P., Tomatis L., Infante P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health. 2007;13:202–212. doi: 10.1179/oeh.2007.13.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries B., Wang Z., Yang C. The role of microRNAs in metal carcinogen-induced cell malignant transformation and tumorigenesis. Food Chem. Toxicol. 2016;98:58–65. doi: 10.1016/j.fct.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järup L., Åkesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Kellen E., Zeegers M.P., Hond E.D., Buntinx F. Blood cadmium may be associated with bladder carcinogenesis: the Belgian case–control study on bladder cancer. Canc. Detect. Prevent. 2007;31:77–82. doi: 10.1016/j.cdp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Koedrith P., Kim H., Weon J.-I., Seo Y.R. Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int. J. Hyg Environ. Health. 2013;216:587–598. doi: 10.1016/j.ijheh.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Yamada H. DNA microarray analysis of altered gene expression in cadmium-exposed human cells. J. Occup. Health. 2003;45:331–334. doi: 10.1539/joh.45.331. [DOI] [PubMed] [Google Scholar]
- Lei L.-J. MiR-21 as a potential biomarker for renal dysfunction induced by cadmium exposure. Int. J. Clin. Exp. Med. 2019;12:1631–1639. [Google Scholar]
- Ling M. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radic. Biol. Med. 2012;52:1508–1518. doi: 10.1016/j.freeradbiomed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zheng C., Shen H., Zhou Z., Lei Y. MicroRNAs-mRNAs expression profile and their potential role in malignant transformation of human bronchial epithelial cells induced by cadmium. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/902025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu X., Wu Y., Wu Q., Wang Q., Yang Z., Li L. MicroRNAs in biofluids are novel tools for bladder cancer screening. Oncotarget. 2017;8:32370. doi: 10.18632/oncotarget.16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Masoud A.M., Bihaqi S.W., Machan J.T., Zawia N.H., Renehan W.E. Early-life exposure to lead (Pb) alters the expression of microRNA that target proteins associated with Alzheimer’s disease. J. Alzheim. Dis. 2016;51:1257–1264. doi: 10.3233/JAD-151018. [DOI] [PubMed] [Google Scholar]
- Matović V., Buha A., Ðukić-Ćosić D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Mendy A., Gasana J., Vieira E.R. Urinary heavy metals and associated medical conditions in the US adult population. Int. J. Environ. Health Res. 2012;22:105–118. doi: 10.1080/09603123.2011.605877. [DOI] [PubMed] [Google Scholar]
- Mortada W., Kenawy I., Abdel-Rhman M., El-Gamal G., Moalla S. A new thiourea derivative [2-(3-ethylthioureido) benzoic acid] for cloud point extraction of some trace metals in water, biological and food samples. J. Trace Elem. Med. Biol. 2017;44:266–273. doi: 10.1016/j.jtemb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Moustafa A.A., Kim H., Albeltagy R.S., El-Habit O.H., Abdel-Mageed A.B. MicroRNAs in prostate cancer: from function to biomarker discovery. Exp. Biol. Med. 2018;243:817–825. doi: 10.1177/1535370218775657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer T. Toxic metals. In: CA Burtis D.B., editor. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics. seventh ed. edn. Elsevier; Amsterdam: 2015. p. 598. [Google Scholar]
- Pignot G. microRNA expression profile in a large series of bladder tumors: identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. Int. J. Canc. 2013;132:2479–2491. doi: 10.1002/ijc.27949. [DOI] [PubMed] [Google Scholar]
- Sens D.A., Park S., Gurel V., Sens M.A., Garrett S.H., Somji S. Inorganic cadmium-and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol. Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- Siddeek B. MicroRNAs as potential biomarkers in diseases and toxicology. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;764:46–57. doi: 10.1016/j.mrgentox.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Tchounwou P., Yedjou C., Patlolla A., Sutton D. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. Exp. Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanis D., Kadiyska T., Galanis A., Delakas D., Logotheti S., Zoumpourlis V. Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicol. Lett. 2010;193:131–137. doi: 10.1016/j.toxlet.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Vujotić L. Association between oxidative stress biomarkers and concentrations of some metal ions in the blood of patients with brain tumors and hydrocephalus Archives of Medical Science. AMS. 2020;16:811. doi: 10.5114/aoms.2019.87409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.R., Spandidos D.A., Tsatsakis A., Schweitzer A., Djordjevic V., Djordjevic A.B. Potential interaction of cadmium chloride with pancreatic mitochondria: implications for pancreatic cancer. Int. J. Mol. Med. 2019;44:145–156. doi: 10.3892/ijmm.2019.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.R. Toxic-metal-induced alteration in miRNA expression profile as a proposed mechanism for disease development. Cells. 2020;9:901. doi: 10.3390/cells9040901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Chen J., Luo L., Li Y., Liu J., Zhang W. Effect of cadmium on kitl pre-mRNA alternative splicing in murine ovarian granulosa cells and its associated regulation by miRNAs. J. Appl. Toxicol. 2018;38:227–239. doi: 10.1002/jat.3516. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2010. Ten Chemicals of Major Public Health Concern.https://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/ [Google Scholar]
- Xu M. Identification of differential plasma miRNA profiles in Chinese workers with occupational lead exposure. Biosci. Rep. 2017;37 doi: 10.1042/BSR20171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.H., Qi F., Cao Y.H., Zu X.B., Chen M.F. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol. Lett. 2015;10:2610–2616. doi: 10.3892/ol.2015.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]