Summary
Host-microbiota interactions create a unique metabolic milieu that modulates intestinal environments. Integration of 16S ribosomal RNA (rRNA) sequences and mass spectrometry (MS)-based lipidomics has a great potential to reveal the relationship between bacterial composition and the complex metabolic network in the gut. In this study, we conducted untargeted lipidomics followed by a feature-based molecular MS/MS spectral networking to characterize gut bacteria-dependent lipid subclasses in mice. An estimated 24.8% of lipid molecules in feces were microbiota-dependent, as judged by > 10-fold decrease in antibiotic-treated mice. Among these, there was a series of unique and microbiota-related lipid structures, including acyl alpha-hydroxyl fatty acid (AAHFA) that was newly identified in this study. Based on the integrated analysis of 985 lipid profiles and 16S rRNA sequence data providing 2,494 operational taxonomic units, we could successfully predict the bacterial species responsible for the biosynthesis of these unique lipids, including AAHFA.
Subject Areas: Microbiome, Omics, Lipidomics
Graphical Abstract
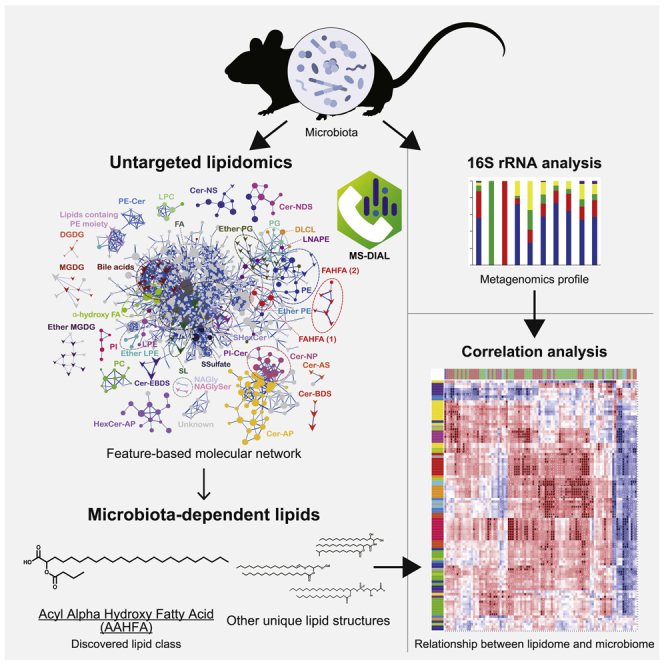
Highlights
-
•
Feature-based molecular networking was explored in untargeted lipidomics analysis
-
•
Of all lipids, 24.8% of fecal lipids were decreased >10-fold in antibiotics-treated mice
-
•
Acyl alpha-hydroxy fatty acid (AAHFA) was identified as microbiota-specific lipid
-
•
With 16S rRNA data, we revealed the relevance of microbiome and lipidome in mice
Microbiome; Omics; Lipidomics
Introduction
The symbiotic bacteria in the gut affect host health and disease (Cho and Blaser, 2012; Honda and Littman, 2016). Genetic and environmental factors induce microbial imbalance called dysbiosis, which causes various diseases, including inflammatory bowel disease (IBD), diabetes, rheumatoid arthritis, and autism (Wang et al., 2011, 2015; Hsiao et al., 2013; Yoshimoto et al., 2013; Kim et al., 2014; Becattini et al., 2016; Pedersen et al., 2016; Rabot et al., 2016). Bacteria produce various bioactive metabolites, including short-chain fatty acids (SCFAs) and secondary bile acids (Chávez-Talavera et al., 2017; Levy et al., 2017; Sheng et al., 2017). These molecules are distributed to various host tissues via blood circulation and control host tissue homeostasis, disease phenotypes, and drug sensitivities (Kaddurah-Daouk et al., 2011; Fujisaka et al., 2018). In addition to hydrophilic metabolites, recent studies have revealed that bacteria also produce a series of hydrophobic lipids involved in host immune regulation, such as α-galactosylceramide (An et al., 2014), monoglucosyldiacylglycerol (Imai et al., 2018), and ceramide conjugated with phosphoinositol (PI-Cer) and phosphoethanolamine (PE-Cer) (Brown et al., 2019). Further, the structural specificity of these lipids has been indicated, as their different acyl chains are important determinants of their agonist activity on corresponding host receptors, as shown for SCFAs (Koh et al., 2016) and α-galactosylceramide (An et al., 2014). However, the comprehensive profiling of complex lipid structures in the gut environment and their biological relevance have not been fully addressed.
For a comprehensive understanding of the metabolic network in host-microbiota interactions, a deep metabolic profiling method is essential. Mass spectrometry (MS)-based untargeted analysis has been employed to detect unique bacterial metabolites (Wikoff et al., 2009; Dodd et al., 2017). Recent studies have provided the global metabolomic landscape of the gut microbiome in large cohort studies, revealing the dysregulated metabolism of bile acids and fatty acids in patients with IBD (Lloyd-Price et al., 2019; Franzosa et al., 2019). On the other hand, there are still many unknowns in MS data, and only 20% of mass ion signals are currently annotated, even with method blank subtraction and consideration of molecular adducts (Seitzer and Searle, 2019). Moreover, existing software programs and MS databases mostly cover host-derived metabolites but not much of the bacteria-derived ones. Thus, global metabolic profiling covering the molecular diversity of both host- and bacteria-derived metabolites is an emerging need for understanding metabolism in host-microbiota interactions.
Recently, we developed an advanced untargeted lipidomics platform that unbiasedly covers more than 8000 molecular species of 117 lipid subclasses (Tsugawa et al., 2020), although the relationship of lipids with the microbiome still remains a challenging issue. In this study, comprehensive profiling of lipids and the microbiome was achieved by liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based untargeted lipidomics and 16S ribosomal RNA (rRNA) gene analyses. We employed feature-based molecular networking (FBMN, Nothias et al., 2020), which utilizes MS-DIAL feature detections followed by the MS/MS spectral networking algorithm (Tsugawa et al. 2019, 2020). The MS-DIAL-FBMN approach was used to (1) obtain the fecal lipidome landscape, including known and unknown lipids, (2) reveal structural features of known microbiota-derived lipids, and (3) elucidate unknown lipid structures associated with the gut microbiota. Furthermore, a correlation analysis of lipid profiles and 16S rRNA sequence data was performed to determine the bacterial species responsible for producing unique lipids present in the gut.
Results
Characterization of Fecal Microbiome and Lipidome in Antibiotic-Treated Mice
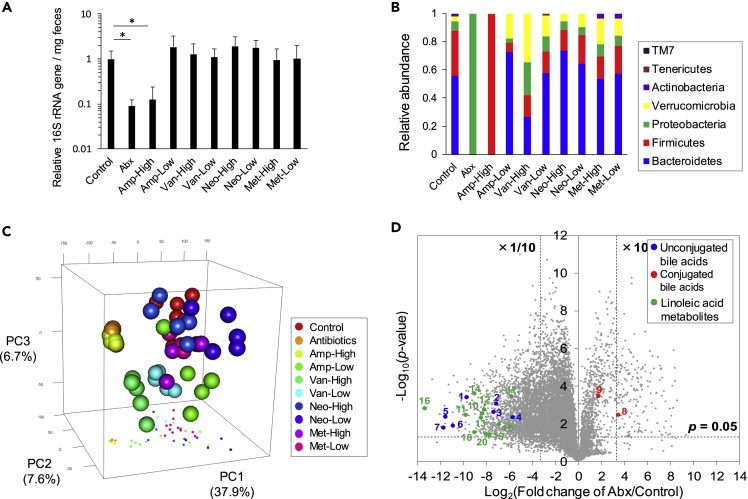
In this study, the fecal microbiome and lipidome in antibiotic-treated mice were investigated using 16S rRNA sequencing and LC-MS/MS analyses. The administration of antibiotic-cocktails (Abx) containing ampicillin, vancomycin, neomycin, and metronidazole for two weeks substantially decreased the amount of bacterial 16S rRNA genes in feces (Figure 1A). Although 16S rRNA amplicon sequence analysis demonstrated the presence of Proteobacteria in the Abx-treated group (Figure 1B), most commensal bacteria were eliminated. The remaining Proteobacteria were confirmed as Escherichia coli (Data S1), suggesting an accidental outbreak of antibiotic-resistant E. coli, as reported in a previous study (Ayres et al., 2012). Single antibiotic treatment of either high or low dose showed differential effects on the fecal microbiome composition, while the total bacterial amount was kept within two-folds of control levels, except for the condition with high-dose of ampicillin (Figures 1A and 1B). At the phylum level, the relative abundances of Firmicutes and Bacteroidetes were decreased in the low dose ampicillin- or the high-dose vancomycin-treated groups, respectively (Figure 1B, Data S1). Principal coordinate analysis of 16S rRNA gene sequencing data also showed unique features of differential bacterial populations among the different antibiotic-treated groups (Figure S1). Score plots of Abx- and high-dose ampicillin- and vancomycin-treated groups were clearly separated from those of the other groups, with relatively mild perturbation of phylum composition in the PCo1 axis (Figure S1).
Figure 1.
Microbiome and Lipidome Profiles in Antibiotic-Treated Mouse Feces
(A) The relative amount of 16S rRNA gene in the feces of mice treated with either low or high dose of ampicillin (Amp, 0.1 or 1 mg/mL), vancomycin (Van, 0.05 or 0.5 mg/mL), neomycin (Neo, 1 or 4 mg/mL), metronidazole (Met, 0.1 or 1 mg/mL), or their high dose mixture (Abx). The asterisk indicates the significance of the t-test (p < 0.001). Data are mean ± SEM (n = 5, except for the high dose Amp condition (n = 3)).
(B) The microbial composition in the feces of antibiotic-treated mice. Data are mean (n = 5).
(C) Principle component analysis of lipid profiles measured by targeted and untargeted lipidomics (n = 5 mice/condition).
(D) Volcano plot of lipids between Abx-treated and control mouse feces. Blue dots: unconjugated bile acids (1: MCA, 2: CA, 3: hyodeoxycholic acid (HDCA), 4: deoxycholic acid (DCA), 5: lithocholic acid (LCA), 6: isoLCA, 7: isoalloLCA). Red dots: conjugated bile acids (8: tauroMCA, 9: tauroCA). Green dots: linoleic acid metabolites (10: 10-hydroxy-cis-12-octadecenoic acid (HYA), 11: 10-hydroxyoctadecanoic acid (HYB), 12: 10-hydroxy-trans-11-octadecenoic acid (HYC), 13: 10-oxo-cis-12-octadecenoic acid (KetoA), 14: 10-oxo-octadecanoic acid (KetoB), 15: 10-oxo-trans-11-octadecenoic acid (KetoC), 16: 13-hydroxy-9-octadecenoic acid, 17: 13-oxo-9-octadecenoic acid, 18: 10,13-hydroxyoctadecanoic acid, 19: 10-hydroxy-cis-12-cis-15-octadecadienoic acid (αHYA), 20: 10-hydroxy-trans-11-cis-15-octadecadienoic acid (αHYC)). See also Figure S1 and Data S1.
LC coupled to triple quadrupole (QqQ)- and quadrupole time-of-flight (QTOF)/MS platforms were used for targeted and untargeted LC-MS/MS analyses, respectively, to measure mouse fecal lipidome. The targeted analysis profiled a total of 136 molecules of free fatty acids and oxylipins, including HYA, a linoleic acid-derived bioactive metabolite produced by gut microbiota (Kishino et al., 2013). Of these, 23 fatty acid metabolites were >10-fold decreased in Abx treatment, with no increase observed in any metabolite (Table 1). Untargeted lipidomics using data-dependent acquisition provided a total of 10,010 chromatographic peak features after background subtraction, and a quarter of these was decreased in Abx treatment by > 10-fold (Table 1). Score plots of principal component analysis using the integrated lipid profiles from targeted and untargeted analyses showed that Abx and high-dose ampicillin and vancomycin groups were clearly distinguished from the other groups in the first principal component (PC1) axis. In addition, we found unique antibiotic treatment-dependent clusters in the three-dimensional score plots, suggesting that such treatments have substantial effects on metabolic profiles in addition to the perturbation of bacterial compositions (Figure 1C). Volcano plot between the control and Abx groups showed that 2,513 and 169 lipid ions were significantly decreased and increased over 10-folds, respectively (Figures 1D and Table 1). Thus, 26.7% of lipids in feces were estimated to be microbiota-dependent, as judged by > 10-fold increase or decrease in antibiotic-treated mice.
Table 1.
Summary of Lipidomics in this Study
| The Number of Chromatographic Peak Features (Targeted + Untargeted Analyses) | ||
|---|---|---|
| Detected | Total | 10,146 (136 + 10,010) |
| MS2-acquired | 6096 (136 + 5960) | |
| Identified | 985 (136 + 849) | |
| >10-folds decrease in Abx treatment (p < 0.05) | Total | 2513 (23 + 2493) |
| MS2-acquired | 1692 (23 +1671) | |
| Identified | 244 (23 + 225) | |
| > 10-folds increase in Abx treatment (p < 0.05) | Total | 169 (0 + 169) |
| MS2-acquired | 152 (0 + 152) | |
| Identified | 1 (0 + 1) | |
Bile acids, which are known to be metabolized by the microbiome, were identified based on retention times and MS/MS spectra matched with authentic standards. The peak abundances of unconjugated primary and secondary bile acids were significantly decreased in the Abx-treated group (ID 1–7 of Figure 1D). On the other hand, the amount of taurine-conjugated muricholic acid (MCA) was increased in the Abx-treated mouse feces (ID 8 of Figure 1D). The amount of taurine-conjugated cholic acid (CA) was also increased, although the fold change was less than 10-folds (ID 9 of Figure 1D). These results indicated a harmonized bioreaction of the intestinal microbiome, where the deconjugation of primary bile acids followed by the conversion to secondary bile acids was coordinately catalyzed (Wahlström et al., 2016). Similarly, a series of unique linoleic acid metabolites, including HYA, were significantly reduced by Abx treatment (ID 10–20 of Figure 1D). These results demonstrated that a significant portion of fecal metabolites was dramatically reduced in Abx-treated mice.
Characterization of Microbiome-Derived Lipids via Molecular Spectrum Networking
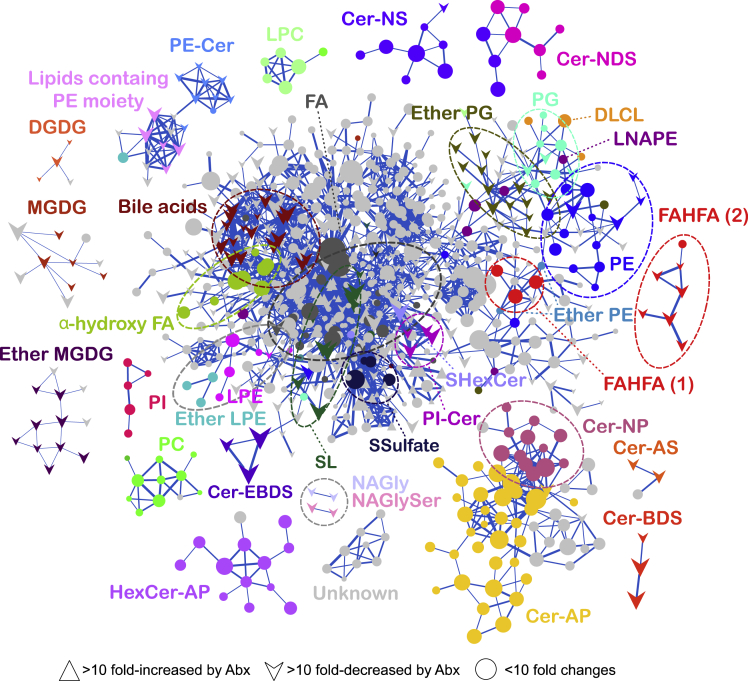
The LC-QTOF/MS-based untargeted lipidomics data were processed by MS-DIAL (Tsugawa et al., 2020), which provided 5960 peak features with the MS/MS spectra information. Of these, we focused on 1671 and 152 peak features that were significantly decreased and increased by >10 fold after Abx treatment, respectively (Table 1). To determine the metabolic signatures of these lipids, including annotated and unknown molecules, we employed the FBMN technique (Figure 2). In FBMN, a node denotes a metabolic ion feature, and the nodes are linked if the MS/MS spectra have a high spectral similarity, indicating the existence of the same or similar substructure moieties.
Figure 2.
Feature-Based Molecular Spectrum Networking of Fecal Lipidome
Nodes corresponding to molecular species were linked based on the similarity of MS/MS spectra (Bonanza score > 0.85). The nodes of circle and up- and down-arrows represent lipid ions with less than 10-fold changes and more than 10-fold increase and decrease, respectively, in the Abx treatment group when compared to the control group. Node size and thickness of links denote the magnitude of measured ion intensity and Bonanza score, respectively. Nomenclatures of identified lipids are listed in Data S3. Mean intensity was used (n = 5). See also Figure S3 and Data S2.
By FBMN analysis, the MS/MS spectra of similar lipid subclasses were clustered by their fragmentation patterns, and unique metabolic profiles of each lipid subclass were revealed. Node shapes of triangle, reverse-triangle, and circle denote more than 10-fold increase, 10-fold decrease, and less than 10-fold change in the Abx-treated group, respectively, when compared to those in the control group. Lipid structures were characterized by curating the MS/MS spectra in the MS-DIAL software program (Tsugawa et al., 2020). The lipid nomenclature used in this study is available in Data S3 and at http://prime.psc.riken.jp/compms/msdial/lipidnomenclature.html. These results demonstrated that the abundances of bile acids, monoglucosyl/galactosyl diacylglycerol (MGDG), diglucosyl/galactosyl DG (DGDG), alkylacyl MGDG (Ether MGDG), alkylacyl phosphatidylglycerol (ether PG), sulfonolipid (SL, also known as sulfobacin), N-acyl glycine (NAGly) and N-acyl glycine serine (NAGlySer), PI-Cer, PE-Cer, acylated ceramide (AcylCer), ceramide alpha-hydroxy fatty acid-sphingosine (Cer-AS), ceramide beta-hydroxy fatty acid-sphinganine (Cer-BDS), and fatty acid esters of hydroxy fatty acid (FAHFA) were decreased by >10-fold in Abx-treated mouse feces (Figure 2). The structures of most lipid molecules were annotated by curating ESI(−)-MS/MS fragment ions in MS-DIAL (Tsugawa et al., 2020) to determine the lipid subclass and the O- and N-acyl chain properties of carbon number and ring/double bond equivalents (Data S2). As a result, we revealed a total of 226 lipid structures significantly perturbed by Abx treatment (Table 1). Although the structures have not been fully resolved, the lipid ion features containing the m/z 196.038 product ion in ESI(−)-MS/MS, indicating the existence of a PE-polar head moiety, were also decreased in the Abx-treated group.
Some of the drastically decreased lipid subclasses have been previously reported as bacterial lipids. For example, plasmalogen PG and MGDG are widely distributed in anaerobic bacteria, including intestinal Clostridium species (Řezanka et al., 2012). SL is known as a marker of Alistipes (Walker et al., 2017). The production of Cer-BDS, PE-Cer, and PI-Cer by Bacteroides fragilis, B. thetaiotaomicron, and B. ovatus has also been reported (Wieland Brown et al., 2013; An et al., 2014; Brown et al., 2019). In the MS/MS spectrum of AcylCer annotated by MS-DIAL, we found the characteristic fragment ion, revealing that the hydroxy moiety linked to the esterified acyl chain is located in the beta carbon position of the N-acyl chain; thus, the ceramide backbone can be termed Cer-BDS. Therefore, these AcylCer lipid subclasses in mouse feces were annotated as Cer-EBDS, where the character ‘E’ denotes the esterified moiety of fatty acid in Cer-BDS (Tsugawa et al., 2017, 2020). Ceramide clusters containing nonhydroxy-fatty acid-phytosphingosine (Cer-NP), ceramide containing nonhydroxy-fatty acid-sphinganine (Cer-NDS), ceramide containing nonhydroxy-fatty acid-sphingosine (Cer-NS), ceramide alpha-hydroxy fatty acid-phytosphingosine (Cer-AP), phosphatidylinositol (PI), dilysocardiolipin (DLCL), N-acyl lysophosphatidylethanolamine (LNAPE), and alpha-hydroxy fatty acids showed relatively mild changes under Abx treatment (>2-fold changes, Figure S3). Phosphatidylcholine (PC), lyso PC, lyso PE, and hexosyl Cer-AP did not change drastically (changes were less than 2-fold). Of interest, FAHFA molecules in two different clusters were observed in FBMN: one designated as “FAHFA (1)” displayed a mild change in abundance, while the other, annotated as “FAHFA (2)” showed a drastic decrease under Abx treatment (see the next section for further structure elucidation). These results demonstrated that the molecular networking approach based on untargeted lipidomics data is a powerful method not only for the characterization of lipid subclasses but also for the determination of bacteria dependency.
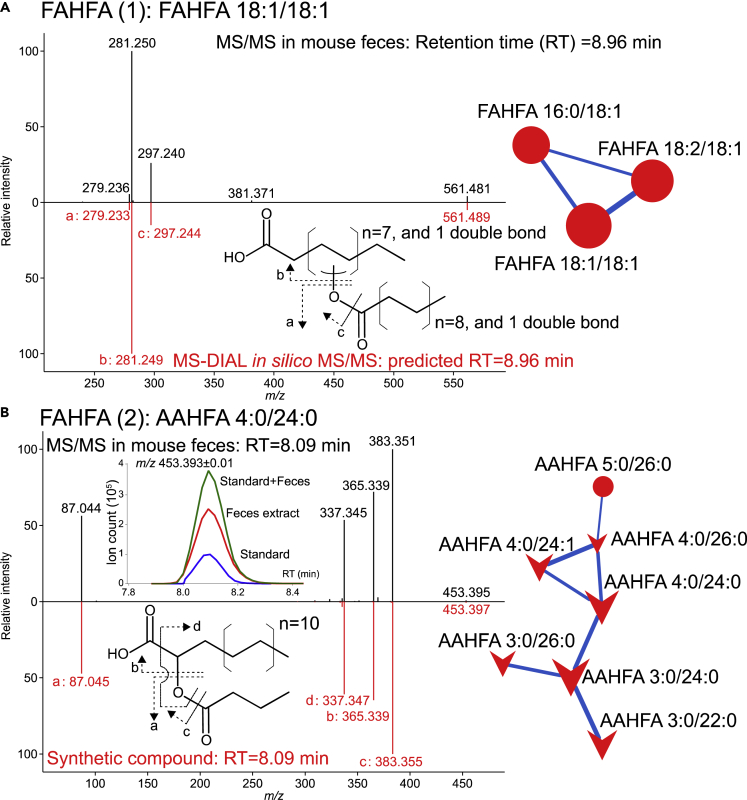
Identification of a Discovered Lipid Subclass Acyl Alpha-Hydroxy Fatty Acid
The FBMN analysis revealed two distinct groups of FAHFA (Figure 2). However, only FAHFA (2) showed a higher microbiome-dependency, suggesting that the producers and structures of the two FAHFA groups were different. We observed the same product ion, indicating an esterified acyl chain moiety in both FAHFA (1) and FAHFA (2) MS/MS spectra (cleavage indicated in Figures 3A and 3B), while the chain length of the esterified acyl chain moiety was estimated as four (possibly butyrate) in FAHFA (2). Furthermore, we found a unique fragment ion (cleavage indicated in Figure 3B) in the FAHFA (2) MS/MS spectrum, which was estimated as the neutral loss of carboxylic acid moiety (46.008 Da). This fragmentation behavior enabled us to hypothesize that the hydroxy moiety of the fatty acid backbone of FAHFA (2) is located at the alpha carbon—the carboxylic acid neutral loss is not observed in well-known endogenous FAHFA molecules, such as palmitic-acid-9-hydroxy-stearic acid (9-PAHSA) and 5-PAHSA (Yore et al., 2014). To confirm the hypothesis, a compound with the proposed structure was chemically synthesized by the condensation of α-hydroxy fatty acid and butyric chloride (see Transparent Methods). Since the LC retention time and MS fragmentation pattern of the synthetic compound matched those of the endogenous signal annotated as FAHFA 4:0/24:0 in feces (Figure 3B), the hydroxy position of FAHFA (2) was proposed as the alpha carbon position. We also analyzed the synthetic butyric acid esters of β-hydroxy 24:0 and found that the retention time and fragmentation pattern were totally different from those of butyric acid esters of α-hydroxy 24:0 in the fecal samples (Figure S2). The lipid species grouped in FAHFA (2) included SCFA (C3-C5) in the esterified acyl chain and long carbon chain (C22-C26) in the α-hydroxy fatty acid backbone (Figure 3B). We also confirmed the existence of acetate esterified to α-hydroxy fatty acid (C24) in the lipidomics data, although it was not located in the same cluster of FAHFA (2) because the fragment ion of acetate (m/z = 59, FA 2:0) was not measured due to our mass range condition (m/z = 70‒1750). Since the structure is not recorded in any metabolite database, including the human metabolome database, KEGG, and LIPIDMAPS, we referred to this microbiome-dependent new lipid subclass composed of SCFA and α-hydroxy fatty acids as acyl alpha-hydroxy fatty acids, abbreviated as AAHFAs. The levels of α-hydroxy fatty acids and their precursor, very long-chain fatty acids, were partially reduced in the feces of Abx-treated mice (15–40% of control, Figure S4), while the reduction rate was lower than that of AAHFA (<10% of control). The levels of SCFAs such as acetate, propionate, and butyrate were significantly decreased by 25.1-, 225- and 1790-folds, respectively (Figure S5), suggesting that the dramatic reduction in AAHFA levels in Abx-treated mice feces can be regarded as an additive effect of decreases in both α-hydroxy fatty acids and SCFAs.
Figure 3.
Identification of FAHFA and AAHFA Molecules in Feature-Based Molecular Network
(A) Measured (black) and in silico (red) MS/MS spectra of conventional FAHFA.
(B) Measured MS/MS spectra in the feces (black) and synthetic standard (red) of AAHFA. The chromatograms of the synthetic standard of AAHFA (blue), the fecal extract (red), and their mixture (green) are also described. The chemical structures represent the estimated fragmentation patterns corresponding to the measured mass spectra. The feature-based molecular networks of FAHFA and AAHFA presented on the right side were extracted from Figure 2.
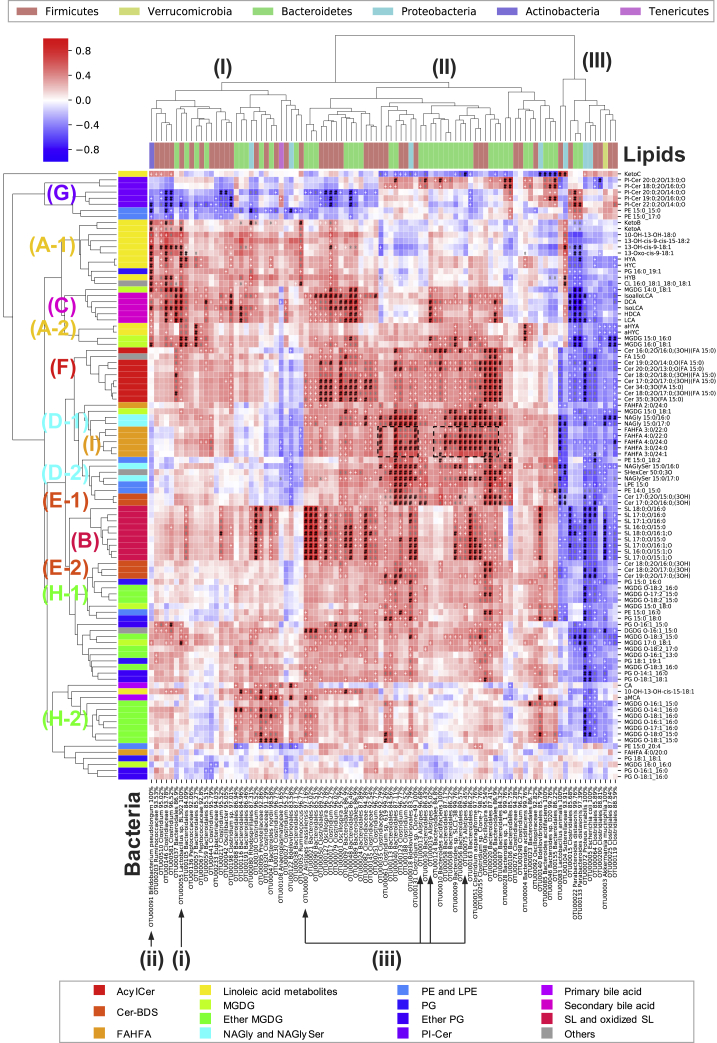
Integrated Analysis of 16S rRNA Amplicon Sequences and Lipidomics Data
To investigate the relationship between lipidome and microbiome, the integrated analysis involving lipidomics and 16S rRNA amplicon sequencing was performed. The Spearman’s rank correlation between microbial profiles and lipid profiles among the control and single antibiotic treatment groups was analyzed, where bacterial operational taxonomic units (OTUs) of over 30 reads and lipids with a >50-fold decrease in the Abx-treated group were mapped (Figure 4). The hierarchical clustering separated the bacterial profiles into three groups: (I) Firmicutes and Bacteroidetes, (II) Bacteroidetes-enriched, and (III) others. The molecular species of lipids were roughly classified according to the lipid categories (A-I of Figure 4). The amount of hydroxy- (HYA, HYB, and HYC) and oxo-forms (KetoA, KetoB, and KetoC) of linoleic acids (lipid class A-1 of Figure 4) positively correlates with the abundance of Lactobacillus sp. BL302 (bacteria i of Figure 4). Positive correlations were also found between α-linolenic acid metabolites (αHYA etc., lipid class A-2) and Lactobacillus sp. BL302. The profile of Bifidobacterium pseudolongum (bacteria ii), which is a closely related species of B. breve, a known producer of HYB (O'Connell et al., 2013), also showed a positive correlation with linoleic acid metabolites (lipid class A-1). Similarly, a clear positive correlation was found between the abundance of SL (lipid class B) and its known producer Alistipes (bacteria iii) (Walker et al., 2017). The metabolic profiles of secondary bile acids containing DCA, HDCA, LCA, and LCA isomers (lipid class C) were positively correlated with the bacterial spectra of both the orders Bacteroidales and Clostridiales (bacteria group I and II). These results are consistent with those of a previous study that reported the involvement of Bacteroides and Clostridium bacteria in the deconjugation of conjugated bile acids (Masuda, 1981), which are subsequently converted to secondary bile acids. N-acyl amides, NAGly, and NAGlySer (lipid class D-1 and D-2), which are ligands for Toll-like receptor 2 present in the oral bacteria Porphyromonas gingivalis (Olsen and Nichols, 2018), were also enriched in Bacteroidales (bacteria group II), which highlights the ubiquitous presence of N-acyl amides in gut Bacteroidetes (Cohen et al., 2017). Correlation analysis also demonstrated a positive correlation between the abundance of various Bacteroidetes and sphingolipids containing odd-chain sphingoid base and/or fatty acids. For example, beta-hydroxy ceramide (Cer-BDS) and the esterified ceramide (Cer-EBDS) (lipid class E−1, and E−2, and F, respectively) showed positive correlations with Bacteroidales bacteria (bacteria group II). On the other hand, the bacterial correlation of PI-Cer (lipid class G) was different from that of Cer-BDS and Cer-EBDS, suggesting that the bacteria in different genera of the phylum Bacteroidetes produce different sphingolipid subclasses. The pattern of positive correlation was also different based on the chain length of PI-Cer molecules. Cer-BDS molecules (lipid classes E−1 and E−2) were classified into the different clusters based on the chain length of the sphingoid base- and N-acyl chain moieties. The total chain length of Cer-BDS in lipid class E−1, composed of C17 sphingoid base and C15–C16 N-acyl chain, was smaller than that in E−2, composed of C18–C19 sphingoid base and C16-C17 N-acyl chain. A similar feature was observed in ether MGDG with di- and mono-unsaturated fatty alcohols (lipid classes H-1 and H-2). This implies that the preferences of acyl chain length and degree of unsaturation of the metabolite precursor for the biosynthesis of even a single lipid subclass and the specificity would depend on the bacterial species. Finally, the abundance of the newly identified lipid AAHFA (lipid class I) was positively correlated with the bacteria in the Bacteroidales and Clostridiales orders (dashed square in Figure 4), indicating the relationship between these bacteria in AAHFA biosynthesis.
Figure 4.
Correlation Analysis of Microbial Composition and Lipid Profiles
A heatmap representation of Spearman's rank correlation between the abundance of lipids and the read numbers of 16S rRNA gene sequence (n = 40). The bacteria operational taxonomic units (OTUs) of over 30 reads and the lipids with a >50-fold decrease in the Abx-treated group were used. The symbols indicate the significance (+: p < 0.01, ∗: p < 0.001, #: p < 0.0001). The annotation of bacteria was performed at the phylum, order, family, genus, or species level based on a sequence similarity threshold of 70%, 80%, 90%, 95%, or 97%, respectively. Vertical and horizontal color labels denote lipid subclasses and bacterial phyla, as described in the color legends.
Discussion
In this study, we revealed a global gut lipidome by LC-MS/MS-based untargeted lipidomics with molecular spectrum networking and characterized the relationship between bacterial composition and fecal lipid molecules by integrating 16S rRNA gene sequence data. Our untargeted lipidomics with the FBMN technique revealed 225 molecules with different lipid subclasses as microbiota-related lipids. The integrated analysis of lipidomics and 16S rRNA amplicon sequencing enabled us to predict the bacteria species responsible for the biosynthesis of the unique lipids. Moreover, our study identified a novel lipid subclass, namely AAHFA, derived from gut microbiota.
Molecular spectrum networking, where spectrum propagation is achieved to characterize unknowns, has increasingly been applied to plant metabolomics (Wang et al., 2016). Besides, we believe that the FBMN approach has a strong potential in untargeted lipidomics because the mass fragmentation pattern in the same lipid subclasses is similar among the various acyl chain properties (Tsugawa et al., 2020). Notably, the molecular spectrum networking revealed not only the microbiota-dependent lipid clusters but also discovered lipid structures, such as AAHFA, by their unique MS/MS fragmentation patterns.
The integrated analysis of lipidomics and 16S rRNA amplicon sequencing highlighted potential microbiota-derived lipid biosynthesis (Figure 4). Of note, many of our results, including the relevant HYA and Lactobacillus, SL and Alistipes, and ceramides and Bacteroides, were consistent with previous reports. Additionally, our study showed that the metabolic profile of Cer-BDS and Cer-EBDS in mouse feces was highly correlated with Bacteroidales bacteria (bacteria group II in Figure 4), while some Cer-EBDS molecules also had positive correlations with Clostridium bacteria (Figure 4). Based on these results, it could be speculated that the biosynthesis of Cer-EBDS using Cer-BDS as the substrate would take place in both Bacteroidales and Clostridium bacteria. Interestingly, there were lipid molecules of a single lipid subclass classified into two independent clusters based on acyl chain length and/or degree of unsaturation in the sphingoid base, O-acyl chain, and N-acyl chain moieties (Figure 4). Notably, the acyl chain-specific activity in bacterial lipids has also been reported, as described for SCFAs (Koh et al., 2016) and α-galactosylceramides (An et al., 2014). Therefore, it is important to determine precise lipid structures by MS-based lipidomics to understand host-microbiome interactions at the molecular level.
Our correlation analysis revealed a positive correlation of the newly identified lipid subclass AAHFA with the bacterial profiles of Bacteroidales and Clostridiaceae. This positive correlation was reasonable since the substrates of AAHFA, which are SCFAs such as butyrate and propionate, were reportedly produced by Clostridium and Bacteroides (Koh et al., 2016). The other part of AAHFA, namely α-hydroxy fatty acid, is potentially synthesized by bacterial CYP enzyme, which plays a role in oxygen detoxification in obligate anaerobic bacteria (Girhard et al., 2007). Moreover, α-hydroxy fatty acids can originate from the host-derived enzyme, fatty acid 2-hydroxylase (FA2H), which is highly expressed in the colon (Alderson et al., 2004). Our data indicated that the levels of α-hydroxy fatty acids were only partially reduced in Abx-treated mouse feces (Figure S4), suggesting that α-hydroxy fatty acids for AAHFA biosynthesis were derived from both bacteria and host. Furthermore, our results showed that the bacteria correlating with AAHFA metabolites were highly correlated with NAGly and NAGlySer molecules, which also have the structural backbone of fatty acid esters of hydroxy fatty acyls (lipid classes D-1 and D-2 in Figure 4). Importantly, NAGly molecules are biosynthesized in Bacteroides by N-acyltransferase (glsB), followed by the O-acyltransferase (glsA) enzymatic reaction (Lynch et al., 2019). Thus, it indicates that these bacteria would have an esterifying enzyme for α-hydroxy fatty acids to form AAHFAs. Although the activity of AAHFA was not determined in this study, structurally similar FAHFA is reported to be bioactive to stimulate insulin and GLP-1 secretion via the GPR120 receptor (Yore et al., 2014).
Consequently, we revealed a global fecal lipidome with the molecular spectrum networking and characterized the relationship between different gut microbiota and lipid profiles. The lipid molecular networking captured unique metabolic changes unbiasedly in the untargeted analysis and thus would be a powerful tool to open up a new avenue to discover potential links between microbial lipid metabolism and host biological phenotypes.
Limitations of the Study
The complete chemical assignment of lipid structures should be confirmed by chemically synthesized standards. The structures of N-acyl amides, ether MGDG, Cer-EBDS, and SL were characterized based on the exact mass and mass fragmentation patterns described in a previous study (Tsugawa et al., 2020). In addition, precise determination of acyl chain structures (straight, iso, or anteiso), unsaturation properties (unsaturated bond or cyclopropane), and sugar isomers (glucose or galactose, etc.) were not addressed in this study. Further, the precise determination of gut bacterial composition in mice was a challenge due to the lack of murine microbiome 16S rRNA reference (Xiao et al., 2015).
Resource Availability
Lead Contact
Further information should be directed to and will be fulfilled by the Lead Contact, Makoto Arita (makoto.arita@riken.jp).
Materials Availability
This study did not generate new unique materials.
Data Availability
MS data are available at the DropMet section of RIKEN PRIMe (http://prime.psc.riken.jp/) via the index of DM0032. 16S rRNA gene sequence data is available at DDBJ of the National Institute of Genetics (https://www.ddbj.nig.ac.jp/index-e.html) via the index of DRA010247.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was mainly supported by AMED LEAP under grant number JP18gm0010003 (for M.A.). The work was also supported partially by the JSPS Grant-in-Aid for Scientific Research on Innovative Areas “LipoQuality” (15H05897, 15H05898 for M.A.) and JSPS KAKENHI (18H02432, 18K19155 for H.T.). We thank our lab members, especially Akira Sen, Mie Honda, Aya Hori, Kanako Igarashi, Kaori Itaya, Yosuke Isobe, Masahiro Ueda, Kakou Ko, and Satoshi Morozumi, for their skillful technical supports and suggestions. We acknowledge Editage (www.editage.jp) for English language editing.
Authors Contributions
S.Y. and M.A. designed the study. S.Y. performed biological experiments. S.Y. and K.I. performed LC-MS/MS analyses, and Y.O., W.S., and M.H. performed 16S rRNA gene analyses. S.Y., N.O., and H.T. performed data analyses. S.Y., N.O., H.T., and M.A. wrote the manuscript. S.Y., N.O., H.T., H.A., and M.A. thoroughly discussed this project, and all authors helped improve the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101841.
Supplemental Information
References
- Alderson N.L., Rembiesa B.M., Walla M.D., Bielawska A., Bielawski J., Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- An D., Oh S.F., Olszak T., Neves J.F., Avci F.Y., Erturk-Hasdemir D., Lu X., Zeissig S., Blumberg R.S., Kasper D.L. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres J.S., Trinidad N.J., Vance R.E. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat. Med. 2012;18:799–806. doi: 10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becattini S., Taur Y., Pamer E.G. Antibiotic-Induced changes in the intestinal microbiota and disease. Trends Mol. Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.M., Ke X., Hitchcock D., Jeanfavre S., Avila-Pacheco J., Nakata T., Arthur T.D., Fornelos N., Heim C., Franzosa E.A. Bacteroides-derived sphingolipids are critical for Maintaining intestinal homeostasis and symbiosis. Cell Host Microbe. 2019;25:668–680.e7. doi: 10.1016/j.chom.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694.e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L.J., Esterhazy D., Kim S.H., Lemetre C., Aguilar R.R., Gordon E.A., Pickard A.J., Cross J.R., Emiliano A.B., Han S.M. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D., Spitzer M.H., Van Treuren W., Merrill B.D., Hryckowian A.J., Higginbottom S.K., Le A., Cowan T.M., Nolan G.P., Fischbach M.A. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa E.A., Sirota-Madi A., Avila-Pacheco J., Fornelos N., Haiser H.J., Reinker S., Vatanen T., Hall A.B., Mallick H., McIver L.J. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S., Avila-Pacheco J., Soto M., Kostic A., Dreyfuss J.M., Pan H., Ussar S., Altindis E., Li N., Bry L. Diet, Genetics, and the gut microbiome drive dynamic changes in plasma metabolites. Cell Rep. 2018;22:3072–3086. doi: 10.1016/j.celrep.2018.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girhard M., Schuster S., Dietrich M., Dürre P., Urlacher V.B. Cytochrome P450 Monooxygenase from Clostridium acetobutylicum: a new α-fatty acid hydroxylase. Biochem. Biophys. Res. Commun. 2007;362:114–119. doi: 10.1016/j.bbrc.2007.07.155. [DOI] [PubMed] [Google Scholar]
- Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Matsumura T., Mayer-Lambertz S., Wells C.A., Ishikawa E., Butcher S.K., Barnett T.C., Walker M.J., Imamura A., Ishida H. Lipoteichoic acid anchor triggers Mincle to drive protective immunity against invasive group A Streptococcus infection. Proc. Natl. Acad. Sci. U S A. 2018;115:E10662–E10671. doi: 10.1073/pnas.1809100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddurah-Daouk R., Baillie R.A., Zhu H., Zeng Z.B., Wiest M.M., Nguyen U.T., Wojnoonski K., Watkins S.M., Trupp M., Krauss R.M. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS One. 2011;6:e25482. doi: 10.1371/journal.pone.0025482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.G., Udayanga K.G.S., Totsuka N., Weinberg J.B., Núñez G., Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe. 2014;15:95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino S., Takeuchi M., Park S.B., Hirata A., Kitamura N., Kunisawa J., Kiyono H., Iwamoto R., Isobe Y., Arita M. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. U S A. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Levy M., Blacher E., Elinav E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017;35:8–15. doi: 10.1016/j.mib.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., Andrews E., Ajami N.J., Bonham K.S., Brislawn C.J. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A., Tammireddy S.R., Doherty M.K., Whitfield P.D., Clarke D.J. The Glycine lipids of Bacteroides thetaiotaomicron are important for fitness during growth in vivo and in vitro. Appl. Environ. Microbiol. 2019;85:1–14. doi: 10.1128/AEM.02157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N. Deconjugation of bile salts by Bacteroides and Clostridium. Microbiol. Immunol. 1981;25:1–11. doi: 10.1111/j.1348-0421.1981.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Olsen I., Nichols F.C. Are sphingolipids and serine dipeptide lipids underestimated virulence factors of Porphyromonas gingivalis? Infect. Immun. 2018;86:1–10. doi: 10.1128/IAI.00035-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothias L.F., Petras D., Schmid R., Dührkop K., Rainer J., Sarvepalli A., Protsyuk I., Ernst M., Tsugawa H., Fleischauer M. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods. 2020;17:905–908. doi: 10.1038/s41592-020-0933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’connell K.J., Motherway M.O.C., Hennessey A.A., Brodhun F., Ross R.P., Feussner I., Stanton C., Fitzgerald G.F., Van Sinderen D. Identification and characterization of an oleate hydratase-encoding gene from Bifidobacterium breve. Bioengineered. 2013;4:313–321. doi: 10.4161/bioe.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen H.K., Gudmundsdottir V., Nielsen H.B., Hyotylainen T., Nielsen T., Jensen B.A.H., Forslund K., Hildebrand F., Prifti E., Falony G. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- Rabot S., Membrez M., Blancher F., Berger B., Moine D., Krause L., Bibiloni R., Bruneau A., Gérard P., Siddharth J. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep32484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzer P.M., Searle B.C. Incorporating in-source fragment information improves metabolite identification accuracy in untargeted LC-MS data sets. J. Proteome Res. 2019;18:791–796. doi: 10.1021/acs.jproteome.8b00601. [DOI] [PubMed] [Google Scholar]
- Sheng L., Jena P.K., Liu H.X., Kalanetra K.M., Gonzalez F.J., French S.W., Krishnan V.V., Mills D.A., Wan Y.J.Y. Gender differences in bile acids and microbiota in relationship with gender dissimilarity in steatosis induced by diet and FXR inactivation. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-01576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H., Ikeda K., Tanaka W., Senoo Y., Arita M., Arita M. Comprehensive identification of sphingolipid species by in silico retention time and tandem mass spectral library. J. Cheminform. 2017;9:1–12. doi: 10.1186/s13321-017-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H., Nakabayashi R., Mori T., Yamada Y., Takahashi M., Rai A., Sugiyama R., Yamamoto H., Nakaya T., Yamazaki M. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods. 2019;16:295–298. doi: 10.1038/s41592-019-0358-2. [DOI] [PubMed] [Google Scholar]
- Tsugawa H., Ikeda K., Takahashi M., Satoh A., Mori Y., Uchino H., Okahashi N., Yamada Y., Tada I., Bonini P. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020;38:1159–1163. doi: 10.1038/s41587-020-0531-2. [DOI] [PubMed] [Google Scholar]
- Wahlström A., Sayin S.I., Marschall H.U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Walker A., Pfitzner B., Harir M., Schaubeck M., Calasan J., Heinzmann S.S., Turaev D., Rattei T., Endesfelder D., Castell W.Z. Sulfonolipids as novel metabolite markers of Alistipes and Odoribacter affected by high-fat diets. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-10369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–65. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., Gu X., Huang Y., Zamanian-Daryoush M., Culley M.K. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Carver J.J., Phelan V.V., Sanchez L.M., Garg N., Peng Y., Nguyen D.D., Watrous J., Kapono C.A., Luzzatto-Knaan T. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland Brown L.C., Penaranda C., Kashyap P.C., Williams B.B., Clardy J., Kronenberg M., Sonnenburg J.L., Comstock L.E., Bluestone J.A., Fischbach M.A. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013;11:e1001610. doi: 10.1371/journal.pbio.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Feng Q., Liang S., Sonne S.B., Xia Z., Qiu X., Li X., Long H., Zhang J., Zhang D. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015;33:1103–1108. doi: 10.1038/nbt.3353. [DOI] [PubMed] [Google Scholar]
- Yore M.M., Syed I., Moraes-Vieira P.M., Zhang T., Herman M.A., Homan E.A., Patel R.T., Lee J., Chen S., Peroni O.D. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S., Iwakura Y., Oshima K., Morita H., Hattori M. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Řezanka T., Křesinová Z., Kolouchová I., Sigler K. Lipidomic analysis of bacterial plasmalogens. Folia Microbiol. 2012;57:463–472. doi: 10.1007/s12223-012-0178-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MS data are available at the DropMet section of RIKEN PRIMe (http://prime.psc.riken.jp/) via the index of DM0032. 16S rRNA gene sequence data is available at DDBJ of the National Institute of Genetics (https://www.ddbj.nig.ac.jp/index-e.html) via the index of DRA010247.