Summary
Complications after vaccination, lack of vaccines against certain infections, and the emergence of antibiotic-resistant microorganisms point to the need for alternative ways of protection and treatment of infectious diseases. Here, we proposed a therapeutic approach to control salmonellosis based on adoptive cell therapy. We showed that the T cell receptor (TCR) repertoire of salmonella-specific memory cells contains 20% of TCR variants with the dominant-active α-chain. Transduction of intact T lymphocytes with the dominant salmonella-specific TCRα led to their enhanced in vitro proliferation in response to salmonella. Adoptive transfer of transduced T cells resulted in a significant decrease in bacterial loads in mice infected with salmonella before or after the adoptive transfer. We demonstrated that adoptive immunotherapy based on T cells, transduced with dominant-specific TCRα could be successfully applied for treatment and prevention of infectious diseases and represent a useful addition to vaccination and existing therapeutic strategies.
Subject Areas: Immunology, Microbiology
Graphical Abstract
Highlights
-
•
A regular TCR repertoire of memory T cells contains alpha-chain-centric TCRs
-
•
Dominant-active TCRα, paired with random TCRβ, recognizes specific microbial antigens
-
•
Adoptive immunotherapy could be applied for treatment of infections
Immunology; Microbiology
Introduction
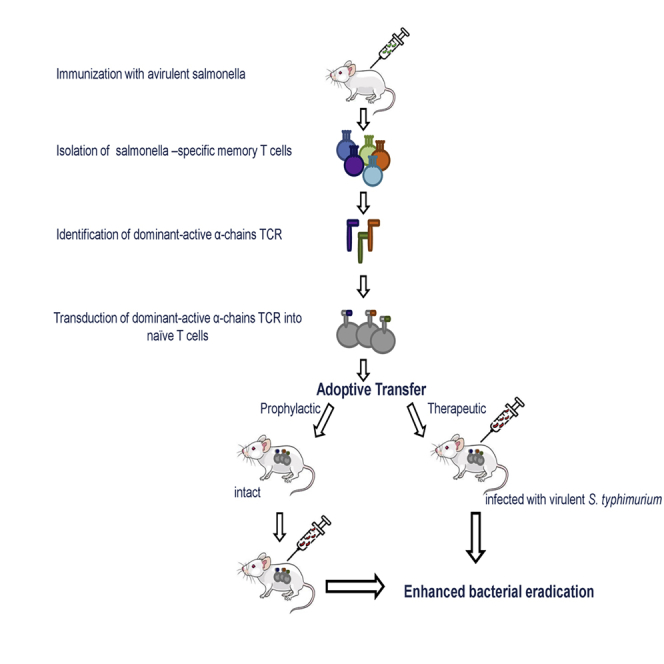
Vaccination and antibiotics remain the most effective approaches for prophylaxis and control of infectious diseases. However, the emergence of antibiotic-resistant microorganisms, lack of vaccines against certain infections, safety concerns arising with the application of live attenuated strains or plasmid vaccines point out the need for the development of alternative prophylactic and therapeutic strategies. Here, we described an approach representing “vaccination without vaccines” based on adoptive immunotherapy using T cells, transduced with pathogen-specific T cell receptors (TCRs) that allows quickly forming a pool of effector T cells ready to fight infection. Moreover, here, we proposed an experimental technology for identification of therapeutic dominant-active α-chains, originated from chain-centric antigen-specific TCRs.
Adoptive immunotherapy has been discovered and successfully applied to treat patients with cancer (Zhang and Chen, 2018; Met et al., 2019). Gene modification of patient's lymphocytes with tumor-specific TCRs or chimeric antigen receptors (CARs) could significantly improve the efficiency of the adoptive cell therapy as it was demonstrated for treatment of melanoma, synovial sarcoma, colorectal cancer, etc. using TCR-modified lymphocytes or lymphomas using CAR-transduced T cells (Met et al., 2019). However, adoptive immunotherapy based on TCR-modified T cells remains expensive so far (Rosati et al., 2017) because of time- and labor-consuming processes of T cell cloning, sequencing of both TCR α- and β-chains, and controlling of their pairing. In this respect, focusing on chain-centric TCRs, i.e. identification of a dominant-specific chain (either TCRα or TCRβ) (Yokosuka et al., 2002; Nakatsugawa et al., 2015; Ochi et al., 2015; Zhao et al., 2016) could significantly lower costs and benefit the overall efficiency of adoptive cell therapy.
Previously, we demonstrated the dominant role of TCRα in recognition of the tumor alloantigen (Zamkova et al., 2019) that was consistent with other findings (Yokosuka et al., 2002; Nakatsugawa et al., 2015). Although several studies pointed out β-chain-centric TCRs (Ochi et al., 2015; Zhao et al., 2016), we assume that the leading role of TCRα in antigen recognition could be a regular phenomenon. It is known that during the thymic development, TCRα genes undergo editing to produce an α-chain that can not only successfully pair with a rearranged β-chain but also form a TCR with which a thymocyte will survive the positive selection (Huang et al., 2005; Hale and Fink, 2010). Furthermore, due to the lack of α-chain allelic exclusion, some mature T cells can express two TCR α-chains (Padovan et al., 1993) and, hence, two TCRs, allowing to avoid skewing the TCR repertoire specificity and immunodeficiency that arise in β-chain transgenic animals (Silaeva et al., 2014). Structural analyses of TCR - peptide/major histocompatibility complex (pMHC) complexes demonstrated that TCRα could dictate the orientation of the whole trimolecular complex and have more contacts with a pMHC (Stadinski et al., 2011), influencing TCRβ interaction with a pMHC and changing the TCR specificity (Yokosuka et al., 2002).
In studies here, we aimed to prove that dominant antigen-specific α-chain-centric TCRs frequently generate during the immune response. Moreover, we interested if adoptive immunotherapy could be applied for prevention and treatment of infectious diseases. For this, induction of salmonellosis in mice was used as an experimental infectious model.
Salmonella infections remain a serious health problem worldwide for both human and animals (Ahmad, 2002). Salmonella infections can cause in human local intestinal diseases (by nontyphoid serovars) or typhoid fever (by S. enterica serovars Typhi and Paratyphi) and pose the significant mortality threat (Wick, 2011; Griffin and McSorley, 2011; Santos, 2014; Behnsen et al., 2015). Although the innate and humoral immune responses are required to control salmonellosis (Cummings et al., 2009; Griffin and McSorley, 2011; Galen et al., 2016; Benoun et al., 2018), the T cell response is a crucial factor of immunity to salmonella, required for both eradication of the primary infection and establishment of protection against re-challenge (Mittrücker et al., 2002; Cummings et al., 2009; Benoun et al., 2018). Vaccination with live attenuated bacteria strains is considered as one of the promising approaches to provide for the host defense to the salmonella infection (Galen et al., 2016; Benoun et al., 2018). However, unlike other enteric pathogens, infection with salmonella does not induce a long-term protective immunity, and the candidate vaccines developed to date have moderate protective efficacy (Levine, 2006; Galen et al., 2016; Benoun et al., 2018). In this respect, a search of alternative preventive and therapeutic strategies to improve recovery from salmonellosis is of particular practical importance.
Here, we described an experimental approach to control salmonellosis based on adoptive transfer therapy. Using a murine model of in vivo establishment of memory T cells, specific to salmonella antigens (Lo et al., 1999), we generated salmonella-specific memory T cells and created cDNA libraries of their TCRs. In this work, we selected therapeutic TCRs based on the chain centricity of TCRs on the part of antigen-specific T lymphocytes (Nakatsugawa et al., 2015; Zamkova et al., 2019). This approach allowed us to identify TCR α-chains capable to form antigen-specific TCRs after pairing with various endogenous β-chains arisen in the T cell repertoire of a secondary host. By practicing this approach, we lost a part of the antigen-specific TCRs but greatly benefited in the time of identification of the desired TCR, avoiding T cell cloning or cloning of TCR genes from individual T cells. We showed that nearly 20% of the memory cell TCR repertoire, formed during the primary in vivo immune response to salmonella comprised of α-chain-centric TCRs. Our data demonstrated that ex vivo transduction of naive murine T lymphocytes with the single dominant-active salmonella-specific TCRα of memory T cells followed by the adoptive transfer to the host, infected with the virulent strain of S. enterica serovar Typhimurium facilitated reduction of bacterial loads. Moreover, the adoptive transfer of transduced T cells to naive mice provided for the protective effect against S. typhimurium.
Here, we demonstrated for the first time the possibility to operatively create efficient protective immunity without either vaccination or induction of the primary immune response in a recipient as adoptively transferred TCRα-transduced T cells could immediately fight the pathogen. This could represent a versatile therapeutic approach to control various infectious diseases. This strategy may be particularly important in challenging with antibiotic-resistant strains and fast-spreading infections or microorganisms for which there are no vaccines.
Results
The general scheme of experiments is represented in Figure S1.
Generation of Memory T Cells Specific to S. typhimurium
Here, we aimed to generate salmonella-specific memory T cells to subsequently create cDNA libraries of their TCRs and search dominant-active salmonella-specific α-chains of these TCRs.
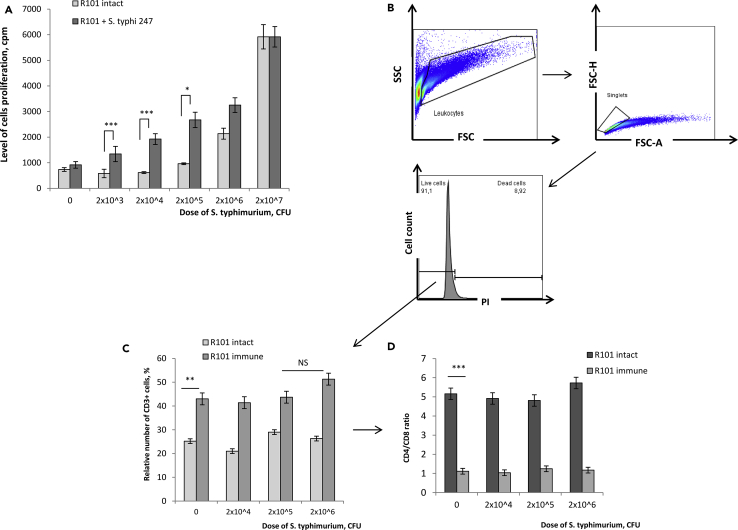
For this, B10.D2(R101) mice were i.p. immunized with S. typhimurium avirulent strain 247, and 3 weeks later, we analyzed functional activity of their splenocytes by in vitro stimulation with 2.0 x 103 - 2.0 × 107 CFU of heat-inactivated S. typhimurium virulent strain IE 147 for 72 h (Figure 1A). We observed 2.3-, 3.1-, and 2.8-fold increased cell proliferation of immunized mice in response to 2.0 x 103, 2.0 x 104, and 2.0 × 105 CFU S. typhimuriun, respectively, as compared to non-immunized (intact) mice (Figure 1A). Further increase of the bacterial dose to 2.0 × 106 CFU resulted only in 1.5-fold enhanced proliferation of splenocytes of immunized mice as compared to the control. At the highest dose of S. typhimuriun (2.0 × 107 CFU), no differences in cell proliferation could be detected in both experimental groups (Figure 1A), suggesting possible involvement of T cell independent salmonella antigens in the proliferative response. Thus, this dose of salmonella was not subsequently used in in vitro studies here.
Figure 1.
Functional and Phenotypic Characteristics of Salmonella-Specific Memory T Cells
B10.D2(R101) mice were i.p. immunized with S. typhimurium avirulent strain 247, and 3 weeks post-immunization total spleen cells of immunized mice were stimulated in vitro with increased doses of heat-inactivated S. typhimurium virulent strain IE 147 for 72 h. Total spleen cells of intact (non-immunized) B10.D2(R101) mice were used as the background control.
(A) Analysis of the salmonella-induced in vitro proliferative response.
(B–D) Flow cytometry analyses of lymphocytes 72 h post in vitro stimulation. (B) Cells were gated as follows: leukocytes were gated based on SSC-A vs. FSC-A, and then singlets were gated based on FSC-H vs. FSC-A, and dead cells were excluded by staining with PI. (C) The relative number (%) of T cells (CD3+) in the population of live singlet leukocytes. (D) CD4/CD8 T cells ratio in the population of CD3+ live singlet leukocytes.
Data are representative of 2 independent experiments and shown as mean ± SD (n = 3). ∗p < 0.03, ∗∗p < 0.01, ∗∗∗p < 0.001 (unpaired Student's t-test). NS, not significant. See also Figures S1 and S4.
Next, we analyzed the relative count of CD3+ lymphocytes and CD4+ and CD8+ T cell subsets in the cultures of splenocytes of immunized mice 72 h post-restimulation in vitro with bacterial cells (Figures 1B–1D). Spleen cells of intact mice, similarly cultured were used as the background control. Flow cytometry analyses showed similar cell viability in all culture conditions that averaged to 90% (Figure 1B). Immunization of B10.D2(R101) mice with avirulent strain 247 resulted in the 2.0-fold increased relative counts of T lymphocytes in their spleen as compared to intact control mice (Figure 1C(0)) and the 5.0-fold decreased CD4/CD8 ratio (Figure 1D(0)), indicating dramatic accumulation of CD8 T cells. However, in vitro restimulation of splenocytes of immunized mice with S. typhimurium did not lead to any further changes in the relative number of CD3+ cells (Figure 1C) and CD4/CD8 ratios (Figure 1D).
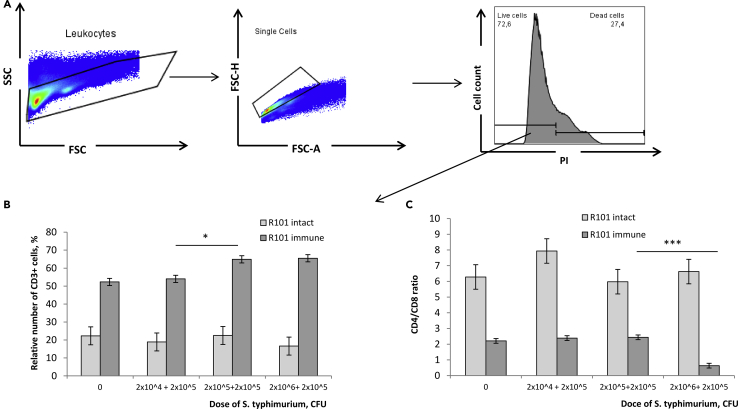
To enrich the pool of salmonella-specific memory cells, next, we performed two rounds of in vitro restimulation. For this, spleen cells of immunized animals were cultured with 2.0 x 104 - 2.0 × 106 CFU of heat-inactivated virulent strain IE 147 for 72 h followed by the second restimulation with 2.0 × 105 CFU for another 72 h (Figure 2). Cell viability on Day 6 of culture approximated to 70% (Figure 2A). We observed 1.2- fold increase in the relative number of CD3+ lymphocytes in cell cultures with 2.0 x 105 - 2.0 x 106 + 2.0 × 105 CFU of salmonella (Figure 2B). Moreover, double stimulation of splenocytes of immunized mice with 2.0 x 106 + 2.0 × 105 CFU S. typhimurium led to the proliferation of CD8 T cells that resulted in 4.0-fold decrease of the CD4/CD8 ratio as compared to lower bacterial doses (Figure 2C). Hence, for the subsequent generation of cDNA libraries of memory cells TCRs, we enriched salmonella-specific memory T cells in vitro as follows: co-culture with 2.0 × 106 CFU of heat-inactivated S. typhimurium virulent strain IE 147 for 72 h followed by the second restimulation with 2.0 × 105 CFU for another 72 h.
Figure 2.
Phenotypic Characteristics of T Cells Post Two in vitro re-Stimulations by Salmonella
B10.D2(R101) mice were immunized with S. typhimurium avirulent strain 247, and 3 weeks later their total spleen cells were stimulated in vitro with heat-inactivated S. typhimurium virulent strain IE 147 for 72 h. Cells were then additionally re-stimulated for another 72 h followed by the flow cytometry analyses. Total spleen cells of intact B10.D2(R101) mice, similarly cultured for 6 days were used as a control.
(A) Leukocytes were gated based on SSC-A vs. FSC-A, and then singlets were gated based on FSC-H vs. FSC-A, and dead cells were excluded by staining with PI.
(B) The relative number (%) of T cells (CD3+) in the population of live singlet leukocytes.
(C) CD4/CD8 T cells ratio in the population of CD3+ live singlet leukocytes.
Data are representative of 2 independent experiments and shown as mean ± SD (n = 3). ∗p < 0.05, ∗∗∗p < 0.001 (unpaired Student's t-test).
Screening of α-Chains TCR In Vitro
Based on the analyses of the generated cDNA libraries (see Transparent Methods), we selected 23 enriched T cell clones with 1.5–3.5-fold increased frequency after the second in vitro restimulation by salmonella as compared to the pool of memory T cells without in vitro restimulation (Table 1).
Table 1.
Clone Frequencies in cDNA Libraries of Salmonella-Specific Memory T Cells
| TCRα | CDR3 Amino Acid Sequence | V Segment | J Segment | T Clone Frequency, % |
|
|---|---|---|---|---|---|
| Without In Vitro Re-stimulation | Post In Vitro Re-Stimulation | ||||
| SM1 | CAARVAGYNKLTF | TRAV14D-1 | TRAJ11 | 0.003 | 0.008 |
| SM3 | CAASAVDTGYQNFYF | TRAV14D-3-DV8 | TRAJ49 | 0.002 | 0.007 |
| SM4 | CVVGKTGFASALTF | TRAV11 | TRAJ35 | 0.002 | 0.007 |
| SM5 | CALSDDSGYNKLTF | TRAV12-1 | TRAJ11 | 0.002 | 0.006 |
| SM6 | CAASSGTYQRF | TRAV14D-3-DV8 | TRAJ13 | 0.003 | 0.006 |
| SM7 | CATVMSNYNVLYF | TRAV8-1 | TRAJ21 | 0.002 | 0.004 |
| SM9 | CAVNNAGAKLTF | TRAV3-1 | TRAJ39 | 0.002 | 0.004 |
| SM10 | CVRSLSDTNAYKVIF | TRAV6-2 | TRAJ30 | 0.002 | 0.003 |
| SM11 | CALSDLPNTNKVVF | TRAV6-7-DV9 | TRAJ34 | 0.002 | 0.003 |
| SM12 | CAVSAWTNTNKVVF | TRAV9-1 | TRAJ34 | 0.002 | 0.003 |
| SM13 | CAVSVMDSNYQLIW | TRAV3-1 | TRAJ33 | 0.002 | 0.003 |
| SM14 | CAVSAWGNTGKLIF | TRAV9-1 | TRAJ37 | 0.001 | 0.002 |
| SM15 | CAVSPVNTGNYKYVF | TRAV7-5 | TRAJ40 | 0.001 | 0.002 |
| SM16 | CAMREINQGGSAKLIF | TRAV16N | TRAJ57 | 0.001 | 0.002 |
| SM17 | CAAEGDDNNNAPRF | TRAV4-3 | TRAJ43 | 0.001 | 0.002 |
| SM18 | CALSGSGGSNAKLTF | TRAV12-3 | TRAJ42 | 0.001 | 0.002 |
| SM19 | CAMREGTSSFSKLVF | TRAV16D-DV11 | TRAJ50 | 0.001 | 0.002 |
| SM20 | CAVITASLGKLQF | TRAV3-1 | TRAJ24 | 0.005 | 0.01 |
| SM21 | CAMREVMDSNYQLIW | TRAV16N | TRAJ33 | 0.003 | 0.007 |
| SM22 | CAVSAWDNNNAPRF | TRAV9-1 | TRAJ43 | 0.001 | 0.002 |
| SM23 | CAASAGSNYNVLYF | TRAV5-4 | TRAJ21 | 0.001 | 0.002 |
| SM24 | CAVSMRSGSFNKLTF | TRAV9N-3 | TRAJ4 | 0.002 | 0.003 |
| SM27 | CAASVNYGNEKITF | TRAV5-4 | TRAJ48 | 0.001 | 0.002 |
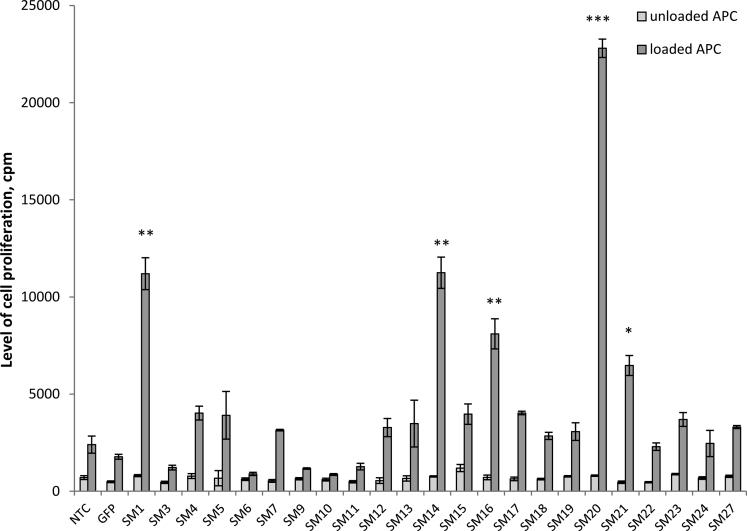
T cells expressing the transgenic variants of TCRα of these 23 memory T cell clones were generated by the retroviral transduction and cultured in vitro with antigen-presenting cells (APCs), loaded with heat-inactivated S. typhimurium virulent strain IE 147 (see Transparent Methods). The level of cell proliferation in cultures with unloaded APC was used as the background control. The level of the antigen-induced proliferative response of T cells, transduced with TCRα was measured in 72 h and compared to the proliferation levels of non-transduced T cells (NTCs) and GFP-transduced lymphocytes (negative controls) (Figure 3). We considered TCRα as a dominant-specific to the S. typhimurium antigens if the level of the antigen-induced proliferation of respective transduced T cells was at least 2.0-fold higher than of the negative controls. Screening in vitro revealed 5 such functionally dominant-active variants of TCRα: SM1, SM14, SM16, SM20, and SM21 (Figure 3).
Figure 3.
In vitro Screening of α-Chains TCR
Lymphocytes of intact B10.D2(R101) mice were transduced with the indicated TCRα or GFP and cultured in vitro for 72 h with unloaded antigen-presenting cells (APCs) (to assess the background proliferation) or with APC, loaded with heat-inactivated S. typhimurium virulent strain IE 147 (to assess the antigen-induced proliferation). The level of antigen-induced proliferation of non-transduced lymphocytes (NTCs) was used as the reference. Data are representative of one of 2 independent experiments and shown as mean ± SD for three technical repeats. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 (unpaired Student's t-test). See also Figures S1, S2, and S5, Tables S1, and S3.
Physicochemical Properties of the CDR3 Region of Dominant-Active Salmonella-Specific TCRα
Analyses of the amino acid (AA) sequences in the CDR3 region of 5 functional dominant-active salmonella-specific TCRα (SM1, SM14, SM16, SM20, and SM21) and evaluation of their physicochemical properties were performed using VDJ tools (Shugay et al., 2015).
CDR3 of TCRα SM16 and SM21 contained 5 hydrophilic AAs (41.7% and 45.4%, respectively), whereas CDR3 of SM1, SM14, and SM20 TCRα had only 2 and 3 hydrophilic AAs, respectively (33.3%, 20.0%, and 22.2%, respectively) (Figure S2A). Furthermore, only SM16 and SM21 contained negatively charged AA (1 and 2, respectively) in their CDR3α region (Figure S2B). Importantly, SM16 and SM21 TCRα belong to the same Va16 family, as determined by the analyses of generated cDNA libraries (Table 1).
The middle part of CDR3 (specifically, 5 central AAs) often contacts with the presented antigen (Egorov et al., 2018), and its physicochemical characteristics could influence the properties of the whole CDR3 region. Thus, next, we analyzed the averaged values of strength, hydropathicity, and polarity for the central 5 AAs of the CDR3α (cCDR3α) of each TCRα (Table S1). The cCDR3α of all dominant-active TCRα, except for SM16, contained one strongly interacting AA (Table S1) that could form hydrophobic interactions, presumably enhancing TCR binding affinity (Egorov et al., 2018; Lu et al., 2019). According to GRAVY, only the cCDR3α of SM1 and SM20 TCRα were hydrophobic (Table S1), with cCDR3α of SM20 having the highest hydrophobic index. Interestingly, naive T cells, transduced with this SM20 TCRα exhibited the highest proliferative potential in vitro in response to salmonella antigens (Figure 3). However, we could not find any correlation between the cCDR3α GRAVY and the proliferative potential of the corresponding TCRα-transduced T cells for other four salmonella-specific dominant-active TCRα (Table S1, Figure 3). The mean polarity of cCDR3α of all five dominant-active TCRα was comparable (Table S1).
Phenotypic Characteristics of T Lymphocytes, Transduced with Salmonella-Specific TCRα
To prove the functional activity of the selected 5 variants of TCRα, we performed several in vivo adoptive transfer experiments. But first, we analyzed the phenotypic characteristic of transduced T cells on Day 3 post-transduction.
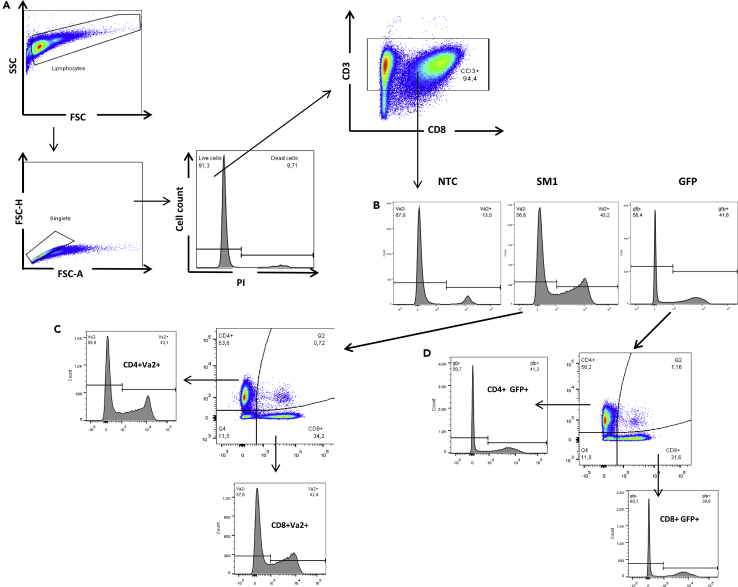
Activation and expansion of spleen cells in vitro in the presence of ConA and IL-2 resulted in accumulation of >90% CD3+ T lymphocytes (Figure 4A). Transduction of T cells with either salmonella-specific TCRα SM1 or GFP did not influence cell viability (over 90% on Day 6 of culture in total according to PI staining; Figure 4A).
Figure 4.
Flow Cytometry Analyses of CD3+ T Cells Prior to Adoptive Transfer
Splenocytes of intact B10.D2(R101) mice were activated with ConA (3 μg/mL) and IL-2 (10 U/mL) for 24 h and transduced with SM1 TCRα or GFP. Flow cytometry analyses were performed on Day 3 post-transduction (6 days in vitro cultivation in total). Non-transduced cells (NTC), similarly cultured for 6 days were used as the control.
(A) Cells were gated as follows: lymphocytes were gated based on SSC-A vs. FSC-A followed by singlets gating based on FSC-H vs. FSC-A. Dead cells were excluded by staining with PI. The relative count of CD3+ cells were determined within the population of live singlets after staining with anti-CD3 monoclonal antibodies.
(B) The level of transduction measured by anti-Va2 staining for TCRα SM1 or by GFP expression (used as the reference) in the population of CD3+ live singlets.
(C) The relative number of CD4+ and CD8+ cells in the subset of SM1 transduced T cells. The relative count of Vα2+ cells were analyzed in CD4+ and CD8+ T cells subsets.
(D) The relative number of CD4+ and CD8+ cells in the subset of GFP transduced T cells. The relative count of GFP+ cells were analyzed in CD4+ and CD8+ T cells subsets. One of two representative experiments is shown (n = 2). Data are presented as mean ± SD.
Based on the AA sequences of the selected TCRα (Table 1), their corresponding Vα families were determined (Table S2). To assess the efficiency of transduction with SM1 TCRα, the relative number of Vα2+ cells was measured in the SM1-transduced T cells culture and compared with the counts of Vα2+ cells in the NTC culture (Figure 4B). We observed at least a 3.0-fold increase of Vα2+ cells in the population of SM1-transduced T lymphocytes as compared to the NTC control. Of particular note, the efficiency of SM1 TCRα transduction corresponded well with the level of transduction with GFP (Figure 4B).
As there are no commercially available antibodies to Vα3, Vα5, and Vα16 families, the efficiency of transduction with the respective TCRα was determined using a level of the reporter expression in the culture of GFP-transduced cells as the reference. Levels of transduction were 40 - 70%.
Analyses of transduced T cells showed that modification with either SM1 TCRα or GFP resulted in the comparable CD4/CD8 ratio, and SM1 TCRα or GFP expressed similarly in both CD4+ and CD8+ T lymphocytes (Figures 4C and 4D). Thus, in adoptive transfer experiments here, we used modified T lymphocytes composed of CD4+ and CD8+ cells at the ratio 3:2, both expressing the transduced TCRα.
Evaluation of the Therapeutic Potential of Salmonella-Specific TCRα In Vivo
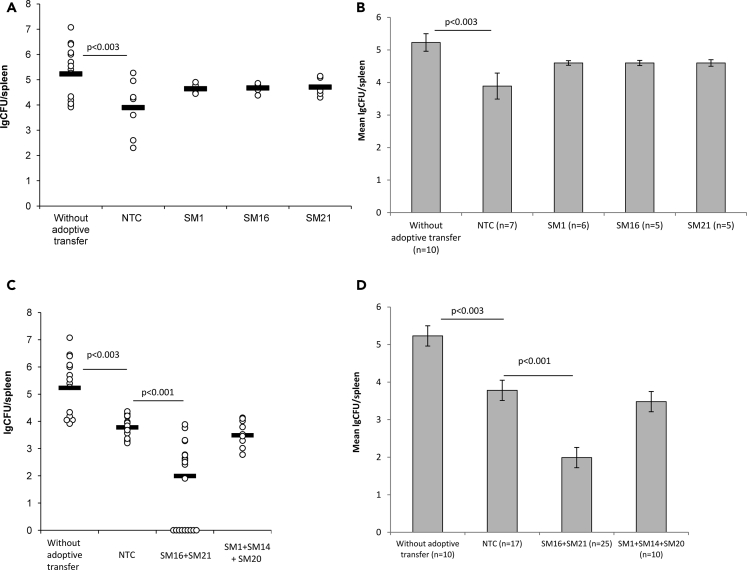
The salmonella infection is naturally spread by the oral route. Thus, in all studies here, we infected experimental animals with 4.0–6.0 x 106 CFU/mouse (LD27) (see Transparent Methods) of S. typhimurium virulent strain IE 147 per os via a gavage needle. To assess the therapeutic potential of salmonella-specific TCRα in vivo, T lymphocytes, transduced with either TCRα SM1, SM16, or SM21 were independently i.v. injected in a dose of 3.0 x105 cells/mouse to mice on Day 3 post-infection. Control NTC was similarly transferred to an independent experimental group as the negative control. Bacterial loads in the spleen of mice were analyzed on Day 7 post-infection, i.e. before any animal mortality could occur (Figure S3B).
The adoptive transfer of NTC to infected animals resulted in a 1.4-log decrease in the bacterial loads as compared to mice without the adoptive transfer (Figure 5). These data clearly indicated that polyclonal activated (by ConA and IL-2) T cells are involved in the immune response to salmonella and could provide the significant therapeutic effect by themselves. Thus, in all experiments here, the NTC group was used as the main control for groups with the adoptive transfer of T cells, transduced with salmonella-specific TCRα.
Figure 5.
Therapeutic Potential of Transduced T Cells, Adoptively Transferred to Infected B10.D2(R101) Mice
B10.D2(R101) mice were enterogastrally infected with LD27 (4.0–6.0 x 106 CFU/mouse) of the S. typhimurium strain IE 147. On Day 3 post-infection, mice were i.v. injected with either non-transduced cells (NTC) or T cells, transduced with the single salmonella-specific TCRα in the dose of 3.0 x 105 cells/mouse. Infected mice without the adoptive transfer were used as the control. The log number of bacterial colony-forming units (lgCFUs) was counted in the spleen on Day 7 post-infection.
(A and B) Adoptive transfer of individual types of T cells, transduced with either single TCRα SM1, SM16, or SM21.
(C and D) Adoptive co-transfer of individual types of T cells, transduced with the single TCRα as specified. (A and C) The lgCFU number per spleen. (B and D) The mean lgCFU number per spleen.
Data are presented as mean ± SD. One-way ANOVA, corrected with Tukey's multiple comparisons. See also Figures S1, S3, and S4.
The adoptive transfer of T cell transduced with SM1, SM16, or SM21 did not lead to a further decrease of bacterial loads in the spleen of mice as compared to the NTC group (Figures 5A and 5B). Next, we decided to inject a mixture of transduced T cells. Lymphocytes, independently transduced with TCRα SM1, SM14, SM16, SM20, or SM21 were randomly divided into 2 groups and equally mixed as follows: Mix 1 (SM16 + SM21) and Mix 2 (SM1 + SM14 + SM20). These two mixtures of transduced T cells were i.v. transferred to infected animals (the total dose of T cells was 3.0 x 105 cells/mouse). We observed the significant 1.8 -log decrease in the bacterial loads in the spleen of mice with adoptively transferred Mix 1 (SM16 + SM20) as compared to the NTC control and 3.24-log decrease as compared to animals without the adoptive transfer (Figures 5C and 5D). Moreover, the adoptive transfer of transduced T cells SM16 + SM21 resulted in complete salmonella eradication in 36% of mice (9 of 25) by Day 7 post-infection (Figure 5C). However, no therapeutic effect was observed with the adoptive transfer of Mix 2 (SM1 + SM14 + SM20) as compared to the NTC group (Figures 5C and 5D).
Our data demonstrated that co-transfer of several types of T lymphocytes, independently transduced with the single salmonella-specific TCRα could improve control of the salmonella infection and resulted in enhanced elimination of the bacteria.
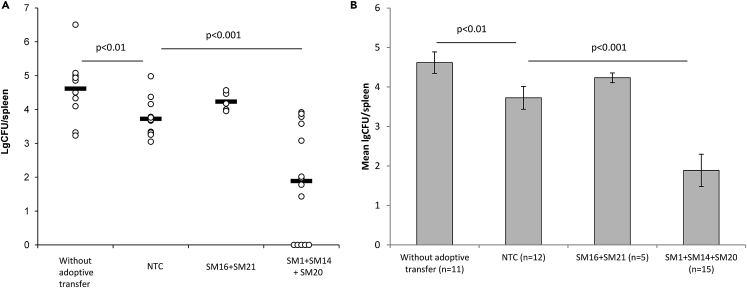
Analysis of the Prophylactic Potential of Salmonella-Specific TCRα In Vivo
Previously, we demonstrated that transduced T cells, adoptively transferred to a naive host could persist in the recipient's organism and exhibit their functional activity within 14 days post-transfer (Zamkova et al., 2019). In this respect, we assumed that the adoptive transfer of T cells, transduced with salmonella-specific TCRα could provide protection from salmonellosis to a naive host.
In order to assess the prophylactic potential of salmonella-specific TCRα in vivo, T cells independently transduced with TCRα SM1, SM14, SM16, SM20, or SM21 were divided into two groups, equally mixed and i.v. injected to intact B10.D2(R101) mice as described above. These mice were infected with 4.0–6.0 x 106 CFU/mouse (LD27) of S. typhimurium virulent strain IE 147 on Day 3 post - adoptive transfer. The analysis of the bacterial load in the spleen of mice was performed on Day 7 post-infection. Again, we observed the significant 0.95-log decrease in the bacterial loads in the spleen of mice, received NTC as compared to mice without the adoptive transfer (Figure 6). Co-transfer of transduced T cells SM1+ SM14 + SM20 (Mix 2) resulted in the significant 1.83-log decrease in the CFU number as compared to the NTC control and 2.72-log decrease as compared to animals without the adoptive transfer (Figure 6). Moreover, in 30% of mice (5 of 15) complete bacteria eradication was achieved (Figure 6A). No prophylactic potential of T cells, modified by α-chains TCR SM16 or SM21 (Mix 1), was demonstrated in this experimental system (Figure 6).
Figure 6.
Prophylactic Potential of Adoptive Transfer of T Cells, Transduced with the Single Salmonella-Specific TCRα
Intact B10.D2(R101) were i.v. injected with either non-transduced T cells (NTCs) or mixture of individual types of T cells, transduced with the single TCRα as specified. On Day 3. post-adoptive, transfer mice were enterogastrally infected with LD27 (4.0–6.0 x 106 CFU/mouse) of S. typhimurium strain IE 147. Mice without the adoptive transfer were used as the control. The log number of bacterial colony-forming units (lgCFUs) was counted in the spleen of mice on Day 7 post-infection. (A) The lgCFU number per spleen.
(B) The mean lgCFU number per spleen. Data are presented as mean ± SD. One-way ANOVA, corrected with Tukey's multiple comparisons.
NS, not significant. See also Figures S1, S3, and S4.
It seems possible that further optimizations of the method by parameters of doses of adoptively transferred cells and/or time frame between the adoptive transfer and infection would increase the protective potential of T cells transduced with salmonella-specific TCRα.
Discussion
In studies here, we proposed an experimental technology for identification and application of individual α-chains TCR, originated from alpha-chain-centric TCRs (Yokosuka et al., 2002; Nakatsugawa et al., 2015; Ochi et al., 2015; Zhao et al., 2016). Such TCRα, paired with various β-chains can dictate the same TCR specificity (Yokosuka et al., 2002; Nakatsugawa et al., 2015; Zamkova et al., 2019). Previously, we showed that TCRα of memory T cells determines the TCR specificity to the tumor antigen both in the TCRα transgenic mice model and when transduced into naive T lymphocytes of wild-type mice (Zamkova et al., 2019). Here, we confirmed that a regular TCR repertoire contains α-chain-centric TCRs and demonstrated that such dominant-active TCRα could be used for adoptive cell therapy and protection from infectious disease.
Adoptive immunotherapy is mainly applied for the treatment of cancer. The possibility of its use for prevention and treatment of infectious diseases has been declared, but no direct data have been obtained. Here, we employed Salmonella typhimurium as a model infection in mice and described an experimental therapeutic strategy to control salmonellosis based on the adoptive transfer of T lymphocytes, transduced with a single dominant-active TCRα of salmonella-specific memory T cells.
The enriched pool of salmonella-specific T lymphocytes was generated by in vitro re-stimulation of memory cells, established during the primary in vivo immune response to the avirulent Salmonella strain 247 (Lo et al., 1999). Analyses of TCRα cDNA libraries revealed 23 memory T cell clones with at least 1.5-fold increased frequency after in vitro re-challenge with salmonella. The single TCRα of all these clones were individually transduced into activated T lymphocytes of intact mice and challenged in vitro with salmonella antigens. T cells modified with 5 variants of TCRα exhibited a profound salmonella-specific in vitro response (Figure 3). Thus, we proved that TCRα can dominate in the antigen recognition by memory T cells and such α-chain-centric TCRs comprise the significant fraction (∼20%) of the memory cells TCR repertoire.
Once established in mice by immunization with an attenuated vaccine strain (Lo et al., 1999; Mittrücker et al., 2002; Kirby et al., 2004), the salmonella-specific immune defense cannot be fully transferred to a naive host (Nauciel, 1990; Mastroeni et al., 1993; Benoun et al., 2018). Several reports demonstrated that the adoptive transfer of salmonella-specific T cell lines confers only partial defense to naive susceptible mice (Mastroeni et al., 1993; Paul et al., 1988). Adoptive transfer experiments here showed that T cells, transduced with a single salmonella-specific TCRα facilitated elimination of salmonella in mice with the established infection and contributed to complete bacteria eradication in over 30% of infected mice (Figure 5). Thus, the therapeutic adoptive transfer of transduced T cells could form the first line of protection in the acute phase of salmonellosis by creating a pool of the pathogen-specific effector T cells, ready to immediately fight the infection. These transferred salmonella-specific T lymphocytes could act before any adaptive immune response arises in the host, alleviating the infectious process and promoting the recipient's immune response.
Moreover, our data indicated that T cells, transduced with the dominant active salmonella-specific TCRα could render the host defense against salmonellosis. The adoptive co-transfer of several types of TCRα-modified T lymphocytes to a naive host improved control of salmonellosis and resulted in the rapid (by Day 7 post-infection) eradication of the bacteria in 30% of infected animals (Figure 6). We assume that such preventive adoptive transfer, aimed at multiple molecular targets of a pathogen, could be an effective addition to existing prophylactic measures for various infectious diseases.
Based on our recent data, obtained in the anti-tumor model system (Zamkova et al., 2019), we expect that transduced salmonella-specific T cells, adoptively transferred to the host either before or during the infection would not impair the formation of the host immunological memory to salmonella. Both adoptively transferred transduced T cells and the recipient's T lymphocytes could contribute to the formation of memory cells, thus, creating the enhanced immune protection against salmonella re-challenge.
Of particular note, both therapeutic and prophylactic effect in studies here was only achieved when several independently modified pools of T lymphocytes, transduced with the single salmonella-specific TCRα were co-transferred to mice (Figures 5 and 6). Although a relatively low number of immunizing antigens of Salmonella spp. have been determined and characterized to date (McSorley et al., 2000; Cummings et al., 2009), it seems likely that TCRs of salmonella-specific memory T cells possess specificity to different bacterial antigens. Interestingly, dominant-active TCRα SM16 and SM21 belong to one Vα16 family (Table 1) and share some common physicochemical features (Table S1, Figure S2). Hence, we proposed that TCRα SM16 and SM21 could recognize very similar salmonella antigens, whereas TCRα SM1, SM14, and SM20 that belong to various Vα families could presumably recognize a broader range of the antigens. Thus, cotransfer of several TCRα could expand the diversity of target salmonella antigens, recognized by transduced lymphocytes, hence, reducing the possibility of the pathogen escaping the immune response and improving the overall therapeutic efficiency.
Strikingly, in studies here, Mix 1 of transduced T cells (TCRα SM16 and SM21) with presumably more narrow antigen specificity provided therapeutic but no prophylactic effect upon adoptive transfer, whereas Mix 2 (TCRα SM1, SM14, and SM20) rendered the opposite effect (Figures 5 and 6). Taking this into account, we assumed that the availability of target antigens in vivo could also contribute to the efficacy of the adoptive transfer therapy here.
The therapeutic adoptive transfer was performed on Day 3 post-infection, i.e., in the acute phase of salmonellosis, when only surface-associated antigens of live salmonella could be available for the host immune system (Barat et al., 2012). These surface-associated antigens are abundant and tend to be low immunogenic (Barat et al., 2012). It seemed possible that TCRα SM16 and SM21 could recognize some of these abundant antigens, and the therapeutic adoptive transfer of Mix 1 could generate a large pool of specific effectors, ready to eliminate the infection. On the contrary, transduced T cells in Mix 2, although having a presumably wider range of the antigen specificity, could not find enough targets likely less presented by host cells to implement any significant therapeutic effect, when adoptively transferred in the acute phase of salmonellosis.
On the other hand, the preliminary adoptive transfer of Mix 2 could significantly broaden the repertoire of peripheral T cells, introducing a large pool of effectors with various specificity to salmonella antigens. It is known that shortly after salmonella infection, some bacterial mortality is observed (Grant et al., 2008), and internal antigens of killed salmonella could be released and presented to host T cells (Barat et al., 2012). Thus, the broader range of antigens, presented by live and killed bacterial cells in the initial phase of infection, could be recognized by ready effectors with the wider TCR repertoire, formed by the prophylactic adoptive transfer of Mix 2. Conversely, lack of sufficient specific targets for Mix 1 could make it ineffective in this experimental system.
It should be noted that in our in vivo experiments both therapeutic and prophylactic effects were observed when activated non-transduced T cells were transferred to infected or naive mice, respectively. In vitro culture of lymphocytes with ConA and IL-2, provided for in our adoptive transfer system, resulted in polyclonal activation of T cells and production of different cytokines (i.e., IL-2, IFNγ, IL-4) (Colle et al., 1993; Ruzek et al., 1996; Azadmehr et al., 2016). Thus, in vivo adoptive transfer of activated T cells could act as an adjuvant of the immune response of host T cells (Paul et al., 1985). It was also shown that cytokines alone could provide some degree of protection against the S. typhimurim infection (Fukazawa et al., 1983; Cummings et al., 2009). Earlier studies demonstrated that the inflammatory microenvironment within salmonella-infected tissues could provide activation stimuli to T cells in addition to a direct cognate TCR stimulation (Fukazawa et al., 1983). It is plausible that in studies here transferred polyclonal activated T cells with a lower activation threshold could be stimulated in a noncognate manner in infected tissues of mice and be involved in the host immune response to S. typhimurium (Pham and McSorley, 2015). However, it should be noted that despite of significant non-specific effects of non-transduced lymphocytes, observed in studies here the adoptive transfer of dominant-specific TCRα-modified T cells could further improve the control of salmonellosis and benefited the overall therapeutic efficiency. Moreover, only immunotherapy with these transduced T cells resulted in complete bacteria eradication in 30% of mice, infected either before or post-adoptive transfer. This effect was not observed when using non-transduced control lymphocytes.
It is well recognized that both CD4 and CD8 T cells are required for the effective adaptive immune response to S. typhimurim (Paul et al., 1988; Mittrücker et al., 2002; Cummings et al., 2009; Behnsen et al., 2015). In studies here, we performed the adoptive transfer of transduced T cells, comprised both CD4 and CD8 cells that equally expressed the salmonella-specific TCRα (Figures 4C and 4D). As salmonella replicates in phagocytic vacuoles of infected host cells, CD4 T cells are essential to control salmonellosis via production of the superoxide radical in infected cells (Hess et al., 1996; McSorley et al., 2000). S. typhimurium induces a strong T-helper 1 (Th1) response in mice, and Th1 cytokines (IFNγ, TNFα) activate bactericidal mechanisms in macrophages (McSorley et al., 2000; Mittrücker and Kaufmann, 2000). CD4 T cells are required for salmonella-specific antibody production, providing help for B cell isotype switching and affinity maturation (Mittrücker et al., 2002; Cummings et al., 2009). CD4 T cells also take part in granuloma formation that controls the bacteria dissemination (Mittrücker and Kaufmann, 2000). Establishment and maintenance of salmonella-specific CD8 response is also Th1-dependent (Shedlock and Shen, 2003; Sun et al., 2004). Salmonella-specific CD8 T cells can lyse infected host cells (Lo et al., 1999; Sun et al., 2004) and produce granulysin that has a direct bactericidal effect on salmonella (Stenger et al., 1998; Luu et al., 2006). It was demonstrated that memory CD8 T cells are more important during re-infection (Lo et al., 1999). In addition, both CD4+ and CD8+ cells produce cytokines, necessary for recruitment and activation of phagocytes (Mittrücker et al., 2002). Thus, in our T cell adoptive transfer system, the therapeutic and prophylactic effect could be achieved by the combined action of both CD4+ and CD8+ cell subsets, expressing the transduced single dominant-active salmonella-specific TCRα.
In conclusion, our studies here demonstrated that chain-centric TCRs are ordinarily contained in the normal TCR repertoire, and dominant-active α-chains represent the significant part (20%) of such chain-centric TCRs. Furthermore, we proved that dominant-active TCRα can recognize microbial antigens, and naive T cells, transduced with these TCRα could respond to the specific bacteria both in vitro and in vivo. These findings are of particular importance as we suggest an approach to identify the therapeutic TCRα, suitable for the adoptive immunotherapy that allows avoiding time - and labor-consuming processes of TCR cloning or amplification of both α- and β-chains TCR from single T cells and controlling of their pairing when transduced into the host T cells. Once identified, therapeutic dominant-active TCRα could be promptly used for transduction of T cells of an MHC-matched recipient in order to generate a large pool of antigen-specific effectors, ready to immediately provide the therapeutic effect.
Furthermore, our experimental approach can open the great perspective for creation of TCRα transgenic animals with innate resistance to certain pathogens without narrowing the diversity of the host TCR repertoire due to the lack of allelic exclusion for α-chain genes in developing T cells.
In studies here, we proved that adoptive immunotherapy based on T cells, transduced with the dominant-specific TCRα could be successfully applied for treatment and prevention of infectious diseases and at the first time represented an example of “vaccination without vaccines” as a perspective alternative to vaccination and existing therapeutic strategies. This approach may be especially effective in the fight against antibiotic-resistant microorganisms, against infections for which there are no vaccines or in a case of a significant risk of side effects after vaccination.
Limitations of the Study
We developed a novel therapeutic approach of adoptive immunotherapy for infectious diseases based on dominant-active antigen-specific α-chains of chain-centric TCRs using salmonellosis in mice as an experimental infectious model. However, considering a vast variety of infections and complex mechanisms of their pathogenesis, further studies using various infectious models are required to prove the efficacy and the universality of the developed therapeutic strategy.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Dmitry B. Kazansky, kazansky1@yandex.ru.
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The published article includes all data sets generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Belyavskii A.V. for providing us with the helper plasmid - pCL-Eco. This study was supported by Russian Foundation for Advanced Research Projects (The Agreement # 6/053/2015-2018).
Author Contributions
Conceptualization, D.B.K., L.M.K., and A.A.K.; Methodology, D.B.K., A.A.K., L.N.N., A.V.B., D.M.C., M.I., and O.V.B.; Validation, A.A.K., L.M.K., L.N.N., and D.V.B.; Formal Analysis, A.A.K., L.N.N., and L.M.K.; Investigation, A.A.K., L.N.N., D.V.B., and A.V.B.; Resources, D.B.K., L.M.K., D.M.C., M.I., and O.V.B.; Data Curation, L.M.K, D.B.K.; Writing- Original Draft - A.A.K, A.V.B., D.B.K.; Writing - Review & Editing - A.A.K., L.M.K., and D.B.K.; Visualization, A.A.K. and A.V.B.; Supervision, L.M.K. and D.B.K.; Project Administration, L.M.K. and D.B.K., Funding Acquisition, L.M.K. and D.B.K.
Declaration of Interests
The authors declare no competing interests. The authors have a patent of the Russian Federation, related to this work (Kazansky, D.B., Khromykh, L.M., Kalinina, A.A., Silaeva, Y.Y., Zamkova, M.A., Bruter, A.V., Persiyantseva, N.A., Chikileva, I.O., Jolokhava, L.Kh., Nesterenko, L.N. et al. (2018). A method of creating anti-infectious immunologic defense against Salmonella typhimurium and Listeria monocytogenes by transgenesis of T lymphocytes. Patent №2706554).
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101854.
Supplemental Information
References
- Ahmad K. Experts call for surveillance of drug-resistant typhoid at a global level. Lancet. 2002;359:592. doi: 10.1016/S0140-6736(02)07777-2. [DOI] [PubMed] [Google Scholar]
- Azadmehr A., Hajiaghaee R., Molla Hassan A.T., Jafari Vesiehsari M., Oladnabidozin M. Splenocyte proliferation, NK cell activation and cytokines production by extract of Scrophularia variegata; an in vitro study on mice spleen cells. Res. J. Pharmacognosy. 2016;3:9–15. [Google Scholar]
- Barat S., Steeb B., Mazé A., Bumann D. Extensive in vivo resilience of persistent Salmonella. PLoS One. 2012;7:e42007. doi: 10.1371/journal.pone.0042007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J., Perez-Lopez A., Nuccio S.P., Raffatellu M. Exploiting host immunity: the Salmonella paradigm. Trends Immunol. 2015;36:112–120. doi: 10.1016/j.it.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoun J.M., Peres N.G., Wang N., Pham O.H., Rudisill V.L., Fogassy Z.N., Whitney P.G., Fernandez-Ruiz D., Gebhardt T., Pham Q.M. Optimal protection against Salmonella infection requires noncirculating memory. Proc. Natl. Acad. Sci. U S A. 2018;115:10416–10421. doi: 10.1073/pnas.1808339115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle J.H., Saron M.F., Truffa-Bachi P. Altered cytokine genes expression by ConA-activated spleen cells from mice infected by lymphocytic choriomeningitis virus. Immunol. Lett. 1993;35:247–253. doi: 10.1016/0165-2478(93)90190-d. [DOI] [PubMed] [Google Scholar]
- Cummings L.A., Deatherage B.L., Cookson B.T. Adaptive immune responses during salmonella infection. EcoSal Plus. 2009;3:3. doi: 10.1128/ecosalplus.8.8.11. [DOI] [PubMed] [Google Scholar]
- Egorov E.S., Kasatskaya S.A., Zubov V.N., Izraelson M., Nakonechnaya T.O., Staroverov D.B., Angius A., Cucca F., Mamedov I.Z., Rosati E. The changing landscape of naive T cell receptor repertoire with human aging. Front. Immunol. 2018;9:1618. doi: 10.3389/fimmu.2018.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y., Kagaya K., Ishibashi Y. Effect of delayed-type hypersensitivity reaction and transferred lymphokine on the resistance of mice to Salmonella typhimurium infection. Infect. Immun. 1983;39:986–989. doi: 10.1128/iai.39.2.986-989.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen J.E., Buskirk A.D., Tennant S.M., Pasetti M.F. Live attenuated human salmonella vaccine candidates: tracking the pathogen in natural infection and stimulation of host immunity. EcoSal Plus. 2016;7:1–17. doi: 10.1128/ecosalplus.ESP-0010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A.J., Restif O., McKinley T.J., Sheppard M., Maskell D.J., Mastroeni P. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol. 2008;6:e74. doi: 10.1371/journal.pbio.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A.J., McSorley S.J. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 2011;4:371–382. doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale J.S., Fink P.J. T-cell receptor revision: friend or foe? Immunology. 2010;129:467–473. doi: 10.1111/j.1365-2567.2010.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Ladel C., Miko D., Kaufmann S.H. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J. Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- Huang C.Y., Sleckman B.P., Kanagawa O. Revision of T cell receptor a chain genes is required for normal T lymphocyte development. Proc. Natl. Acad. Sci. U S A. 2005;102:14356–14361. doi: 10.1073/pnas.0505564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A.C., Sundquist M., Wick M.J. In vivo compartmentalization of functionally distinct, rapidly responsive antigen-specific T-cell populations in DNA-immunized or Salmonella enterica serovar Typhimurium-infected mice. Infect. Immun. 2004;72:6390–6400. doi: 10.1128/IAI.72.11.6390-6400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.M. Enteric infections and the vaccines to counter them: future directions. Vaccine. 2006;24:3865–3873. doi: 10.1016/j.vaccine.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Lo W.F., Ong H., Metcalf E.S., Soloski M.J. T cell responses to gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to salmonella Infection and the involvement of MHC class Ib molecules. J. Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- Lu J., Van Laethem F., Bhattacharya A., Craveiro M., Saba I., Chu J., Love N.C., Tikhonova A., Radaev S., Sun X. Molecular constraints on CDR3 for thymic selection of MHC-restricted TCRs from a random pre-selection repertoire. Nat. Commun. 2019;10:1019. doi: 10.1038/s41467-019-08906-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu R.A., Gurnani K., Dudani R., Kammara R., van Faassen H., Sirard J.C., Krishnan L., Sad S. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J. Immunol. 2006;177:1516–1525. doi: 10.4049/jimmunol.177.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Adoptive transfer of immunity to oral challenge with virulent Salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect. Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley S.J., Cookson B.T., Jenkins M.K. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- Met Ö., Jensen K.M., Chamberlain C.A., Donia M., Svane I.M. Principles of adoptive T cell therapy in cancer. Semin. Immunopathol. 2019;41:49–58. doi: 10.1007/s00281-018-0703-z. [DOI] [PubMed] [Google Scholar]
- Mittrücker H.W., Köhlern A., Kaufmann S.H. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrücker H.W., Kaufmann S.H. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- Nakatsugawa M., Yamashita Y., Ochi T., Tanaka S., Chamoto K., Guo T., Butler M.O., Hirano N. Specific roles of each TCR hemichain in generating functional chain-centric TCR. J. Immunol. 2015;194:3487–3500. doi: 10.4049/jimmunol.1401717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 1990;145:1265–1269. [PubMed] [Google Scholar]
- Ochi T., Nakatsugawa M., Chamoto K., Tanaka S., Yamashita Y., Guo T., Fujiwara H., Yasukawa M., Butler M.O., Hirano N. Optimization of T-cell reactivity by exploiting TCR chain centricity for the purpose of safe and effective antitumor TCR gene therapy. Cancer Immunol. Res. 2015;3:1070–1081. doi: 10.1158/2326-6066.CIR-14-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan E., Casorati G., Dellabona P., Meyer S., Brockhaus M., Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- Paul C., Shalala K., Warren R., Smith R. Adoptive transfer of murine host protection to salmonellosis with T-cell growth factor-dependent, salmonella-specific T-cell lines. Infect. Immun. 1985;48:40–43. doi: 10.1128/iai.48.1.40-43.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C.C., Norris K., Warren R., Smith R.A. Transfer of murine host protection by using interleukin-2- dependent T-lymphocyte lines. Infect. Immun. 1988;56:2189–2192. doi: 10.1128/iai.56.8.2189-2192.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham O.H., McSorley S.J. Protective host immune responses to Salmonella infection. Future Microbiol. 2015;10:101–110. doi: 10.2217/fmb.14.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati E., Dowds C.M., Liaskou E., Henriksen E.K., Karlsen T.H., Franke A. Overview of methodologies for T-cell receptor repertoire analysis. BMC Biotechnol. 2017;17:61. doi: 10.1186/s12896-017-0379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzek M.C., O'Brien D.P., Mathur A. Decreased production of IL-2 and IFN-y by stimulated splenocytes from mice bearing plasma cell tumors is associated with alteration of DNA-binding factors. Int. Immunol. 1996;8:1971–1982. doi: 10.1093/intimm/8.12.1971. [DOI] [PubMed] [Google Scholar]
- Santos R.L. Pathobiology of salmonella, intestinal microbiota, and the host innate immune response. Front. Immunol. 2014;5:252. doi: 10.3389/fimmu.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock D.J., Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Shugay M., Bagaev D.V., Turchaninova M.A., Bolotin D.A., Britanova O.V., Putintseva E.V., Pogorelyy M.V., Nazarov V.I., Zvyagin I.V., Kirgizova V.I. VDJtools: unifying post-analysis of T cell receptor repertoires. PLoS Comput. Biol. 2015;11:e1004503. doi: 10.1371/journal.pcbi.1004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silaeva Y.Y., Grinenko T.S., Vagida M.S., Kalinina A.A., Khromykh L.M., Kazansky D.B. Immune selection of tumor cells in TCR β-chain transgenic mice. J. Immunotoxicol. 2014;1:393–399. doi: 10.3109/1547691X.2013.861548. [DOI] [PubMed] [Google Scholar]
- Stadinski B.D., Trenh P., Smith R.L., Bautista B., Huseby P.G., Li G., Stern L.J., Huseby E.S. A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity. 2011;35:694–704. doi: 10.1016/j.immuni.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger S., Hanson D.A., Teitelbaum R., Dewan P., Niazi K.R., Froelich C.J., Ganz T., Thoma-Uszynski S., Melián A., Bogdan C. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–150. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- Sun J.C., Williams M.A., Bevan M.J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M.J. Innate immune control of Salmonella enterica serovar Typhimurium: mechanisms contributing to combating systemic salmonella Infection. J. Innate Immun. 2011;3:543–549. doi: 10.1159/000330771. [DOI] [PubMed] [Google Scholar]
- Yokosuka T., Takase K., Suzuki M., Nakagawa Y., Taki S., Takahashi H., Fujisawa T., Arase H., Saito T. Predominant role of T cell receptor (TCR)-alpha chain in forming preimmune TCR repertoire revealed by clonal TCR reconstitution system. J. Exp. Med. 2002;195:991–1001. doi: 10.1084/jem.20010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamkova M., Kalinina A., Silaeva Y., Persiyantseva N., Bruter A., Deikin A., Khromykh L., Kazansky D. Dominant role of the α-chain in rejection of tumor cells bearing a specific alloantigen in TCRα transgenic mice and in in vitro experiments. Oncotarget. 2019;10:4808–4821. doi: 10.18632/oncotarget.27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen J. Current status and future directions of cancer immunotherapy. J. Cancer. 2018;9:1773–1781. doi: 10.7150/jca.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Nguyen P., Vogel P., Li B., Jones L.L., Geiger T.L. Autoimmune susceptibility imposed by public TCRβ chains. Sci. Rep. 2016;6:37543. doi: 10.1038/srep37543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data sets generated or analyzed during this study.