Abstract
Low education is considered an important modifiable risk factor for dementia worldwide, despite the lack of a formal consensus definition of low education. The primary aim of this systematic review was to document and address the inconsistency in measuring and operationalising education in dementia studies. A secondary aim was to consider the dose of education required to reduce dementia risk. The protocol was registered at PROSPERO with registration ID CRD42018096168. CINAHL, Cochrane, PsycInfo, and Pubmed databases were searched using terms related to education, dementia and/or MCI, and incidence. Studies were eligible for inclusion if a risk ratio for education and any dementia, Alzheimer's Disease (AD), Vascular Dementia (VaD) or Mild Cognitive Impairment (MCI) was reported in a population cognitively healthy at baseline. Sample sizes for 65 studies meeting selection criteria ranged from 152 to 12,881, representing populations from 24 countries. Risk of bias, assessed using a tool designed specifically for dementia risk studies, was found to be medium or low for all studies. There were 23 continuous, 29 dichotomous, and 31 categorical operationalisations of education reported. Random effects meta-analyses from continuous operationalisations suggested each year of education reduced risk by eight percent for AD (95% CI:5–12%) and seven percent for any dementia (95% CI:6–9%). Dichotomous operationalisations indicated an increased risk for low education of 45% (95% CI:29–63%) for any dementia and 85% (95% CI:56–118%) for AD, however definitions of low education were heterogeneous, ranging from zero to 12 years. There were too few studies to produce summary ratios for VaD or MCI. We conclude that, while the evidence of an association between low education and dementia incidence is robust, inconsistency in the definition, measurement and operationalisation of education hinders the translation of this evidence into practical policy recommendations to reduce dementia risk.
Keywords: Education, Dementia risk, Systematic review, Modifiable risk factors for dementia, Dementia risk methodology, Social determinants of dementia risk
1. Introduction
Among potentially modifiable risk factors for dementia, low education has perhaps the greatest impact on population risk worldwide (Livingston et al., 2017; Norton et al., 2014). To date there have been seven major systematic reviews summarising the literature on an association between education and dementia (Valenzuela & Sachdev, 2005; Caamaño-Isorna et al., 2006; Sharp & Gatz, 2011; Fratiglioni & Wang, 2007; Xu et al., 2016; Meng & D'Arcy, 2012; Prince et al., 2014). Summary odds ratios for the increased risk of low education on any dementia were calculated in five of the seven systematic reviews, ranging from 1.59 (95% CI: 1.26–2.01) to 1.89 (95% CI: 1.61–2.22) (Valenzuela & Sachdev, 2005; Caamaño-Isorna et al., 2006; Xu et al., 2016; Meng & D'Arcy, 2012; Prince et al., 2014). The consistency in the reported effect sizes make the evidence for low education as a dementia risk factor compelling. Such a robust effect is remarkable given the heterogeneity of measurements of education, variation in types of dementia and diagnostic methods, diversity of study populations, and differences in statistical indicators used in the individual studies.
Given such strong evidence for the effect of low education on dementia risk, the merit of performing yet another systematic review on the topic would be dubious, if not for an outstanding issue that remains unaddressed – it is unclear what is meant by (low) education and, consequently, how much education is required to reduce dementia risk (Then et al., 2016). In the absence of a consensus definition of what is meant by education in general, and low education in particular, since it is considered a primary risk factor, it is challenging to translate findings regarding education and dementia risk into policy recommendations. Five of the seven systematic reviews of an association between education and dementia mentioned the heterogeneity in education across studies as a limitation (Caamaño-Isorna et al., 2006; Fratiglioni & Wang, 2007; Prince et al., 2014; Sharp & Gatz, 2011; Xu et al., 2016). The objectives of this review then, were threefold: 1. To provide an updated systematic review and meta-analysis of studies reporting on education as a risk factor for dementia, incorporating recent and previously unconsidered studies, and attempting to minimise the effect of heterogeneity between studies by grouping studies according to how education was operationalised; 2. To document the inconsistency in measuring and operationalising education when used in studies that examine it as a risk factor for dementia; and 3. To examine existing evidence of the dose of education required to reduce dementia risk. For the first time to our knowledge, tables, forest plots and summary ratios are presented separately for continuous, dichotomous and categorical operationalisations of education. Sensitivity analysis is also conducted to consider the impact of a specific cut-off for definitions of low education on dementia risk.
2. Methodology
2.1. Protocol registration
The protocol for this review was registered at PROSPERO with registration ID CRD42018096168, including the review question, search strategy, inclusion and exclusion criteria, plans for risk of bias assessment and data analysis and synthesis. Changes to the protocol were registered at PROSPERO with justification.
2.2. Search strategy
The following databases were searched for relevant literature: CINAHL, Cochrane, PsycInfo, and Pubmed; using search terms related to dementia and MCI along with search terms related to education and search terms related to incidence. The following demonstrates the terms and Boolean operators used for PubMed as an example: ((((dementia [Title] OR Alzheimer* [Title] OR “mild cognitive impairment" [Title] OR “MCI" [Title]))) AND ((education* OR “cognitive reserve” OR “brain reserve"))) AND ((incidence OR ratio)). Searches for the other databases varied on this, subject to search conventions specific to each database, and are documented in Appendix A. A research librarian was consulted for advice on the appropriateness of search terms and strategy to answer the research question. All searches were limited to the dates January 1, 1990 to May 15, 2019. No other restrictions were imposed on the search strategy.
Studies from previously published systematic reviews and meta-analyses were also identified and included if they met our study criteria; as were studies identified from reference lists of included studies.
2.3. Outcome of interest
Our outcomes of interest were any dementia, Alzheimer's disease (AD), vascular dementia (VaD), and mild cognitive impairment (MCI). Any documented diagnosis of these outcomes was included.
2.4. Exposure of interest
Education was the main exposure of interest. As the focus of the review was the inconsistency in how education was measured and operationalised, the operationalisation of education was also of interest e.g. continuous, dichotomous or categorical variable and variations within these operationalisations.
2.5. Population
Studies were included if they involved 100 participants or more; if participants were recruited from a population-based sample; and if participants were cognitively healthy at baseline.
2.6. Inclusion criteria
Studies with a longitudinal component that measured incident dementia or MCI outcomes were included, whether they were retrospective or prospective, so long as they reported a risk, hazard or odds ratio for education (or a ratio could be extrapolated from incidence rates). As education is a risk factor that is typically fixed long before dementia outcome, case control studies were also included if controls had been screened for cognition and excluded in the case of possible dementia. Randomised controlled trials were included in the protocol, although it was not anticipated that many results would be returned, due to the typical long delay between exposure and outcome.
2.7. Exclusion criteria
Cross-sectional studies were excluded, as a longitudinal component is required to study incidence of cognitive outcomes. Also excluded were studies of prevalent dementia or MCI, as incident cases are required to investigate etiology. Studies not reporting a risk, hazard or odds ratio (or at least providing enough information to allow a ratio to be extrapolated) were excluded, as these ratios were required for comparison. Longitudinal studies of populations that were not screened and excluded for cognitive impairment at baseline, using MMSE or similar, were also excluded to avoid confusion of prevalent and incident cases. Finally, papers not in the English language were excluded due to lack of resources available in the study team.
2.8. Screening and extraction process
Two reviewers (JM and RP) screened all studies for inclusion and exclusion criteria independently, consulting in the case of disagreement and reaching a consensus decision for each study. The process was repeated by both reviewers (JM and RP) at title and abstract and full text screening stages. The screening process was managed with the aid of Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Both reviewers extracted data for ten per cent of the studies. As good agreement was obtained for data extraction (defined as eighty per cent agreement in the protocol), one reviewer (JM) proceeded with extraction for the remaining studies.
2.9. Risk of bias
Following Hoy et al. (Hoy et al., 2012a, 2012b), our aim was to assess whether included studies had attempted to minimise bias in the study design and implementation, rather than to judge whether bias was present or not in a particular study. To achieve this aim, we modified existing tools (Hosking et al., 2018; Hoy et al., 2012a, 2012b; Pedditizi, Peters, & Beckett, 2016), and expanded upon existing criteria (Fratiglioni & Wang, 2007) to develop our own risk of bias tool specific to dementia incidence studies (Appendix B). For example, to reduce the risk of selection bias, studies could have randomly selected participants from both the general community and institutions, and ideally reported a participation rate to reflect representativity. To prevent bias in the measurement of the exposure variable, studies could have obtained objective measures of education justifying operationalisation of the measure with reference to the literature. To decrease the likelihood of ascertainment bias, studies could have assessed all participants for dementia/MCI in the same way, using standard diagnostic criteria. Including relevant and justified confounding variables could minimise the risk of confounders biasing any reported associations. To avoid the risk of study-length bias, studies could have had several follow-ups over a long time period, with not too long in-between follow-ups. To prevent bias due to attrition, studies could have clearly reported losses to follow-up and differences between those who dropped out of the study and those who remained. Further, following up the medical records and death certificates of those who had died and including them as cases if appropriate could help alleviate attrition bias.
2.10. Reporting of results
Data were extracted from all relevant papers, however in the case of more than one paper reporting on the same study using the same population, timeframe, operationalisation of education and outcome, the most recent publication was reported. This decision was based on the expectation that, compared to earlier publications, the most recent publications were most likely to have i) the longest follow-up periods, ii) the largest sample sizes, iii) updated case numbers, and iv) use the latest methodological and statistical approaches; all of which reduce the risk of bias influencing study results. As the focus of this review is how the operationalisation of education influences results, a study may be represented in the tables and forest plots more than once if more than one operationalisation of education was used (but never more than once for the same operationalisation and dementia outcome).
The most adjusted risk ratio was extracted for each study. Random effects meta-analyses were performed and forest plots were produced for each of the three operationalisations of education – continuous, dichotomous and categorical, for outcomes that had sufficient studies to conduct a meta-analysis (pre-determined as a minimum of five studies), however no summary ratio was produced for categorical operationalisations due to heterogeneity in categories among studies. Meta-analyses were conducted using Stata version 15.1 (StataCorp LLC, Texas, US).
For dichotomous operationalisations that compared high education to a low education reference, the inverse of the risk ratio and confidence intervals was included in the meta-analysis so that the summary ratio would represent the combined risk of low education. Ratios for categorical operationalisations were separated by whether they presented the risk of low education versus high or high education versus low, then ordered by their reference category cut-offs in the forest plot, from reference categories with the lowest amount of education to reference categories with the highest.
Extracted data, risk of bias ratings and results of meta-analyses including weights and forest plots were incorporated into Graphical Overview for Evidence Review (GOfER) charts, following recent publication of this method of collating review evidence (Sievert et al., 2019). Incidence rate ratios were extrapolated from incident rates when they were not provided. Confidence intervals for extrapolated incident rate ratios were calculated using Stata version 15.1.
2.11. Reporting of subgroup results
Findings from studies that stratified results by gender and/or ethnicity were reported descriptively.
2.12. Sensitivity analysis
A sensitivity analysis was undertaken to examine the effect of definitions of low education for dichotomous operationalisations, provided there were at least five studies remaining after exclusions. This was achieved by excluding studies that used a definition of low education that was the equivalent of more than eight years of education. Eight years was an arbitrary cut-off designed to test sensitivity, chosen because it serves as a midpoint for definitions of low education used in high and low income country contexts and reflects a level of education that is more than primary school but less than high school. Comparisons of the effect measure and the I2 statistic are presented, with the I2 statistic representing “the percentage of total variation across studies that is due to heterogeneity rather than chance” (Higgins et al., 2003).
2.13. Measure of publication bias
Funnel plots and Egger's regression test statistics were produced to evaluate the risk of publication bias for continuous and dichotomous operationalisations of education and the risk of AD and any dementia.
3. Results
Objective 1: providing an updated systematic review and meta-analysis, grouping studies according to operationalisation of education.
3.1. Number of studies found
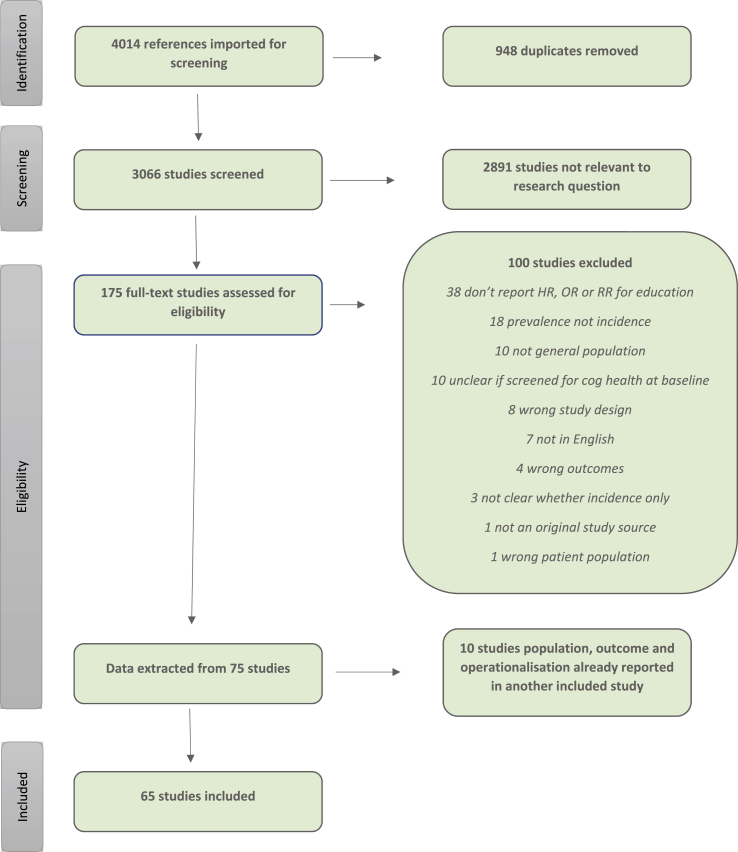
Data were extracted from 65 original research articles identified as matching the study selection criteria [ (Then et al., 2016), (Beard et al., 1992; Bermejo-Pareja et al., 2008; Bickel & Cooper, 1994; Borenstein et al., 2014; Borenstein et al., 2005; Brayne et al., 2010; Cadar et al., 2018; Chen et al., 2011; Contador et al., 2015; de Bruijn et al., 2015; De Deyn et al., 2011; Dekhtyar et al., 2015; Dekhtyar et al., 2016; Di Carlo et al., 2002; Evans et al., 1997; Fischer et al., 2008; Fitzpatrick et al., 2004; Geerlings et al., 1999; Harmanci et al., 2003; He, Zhang, & Zhang, 2000; Hendrie et al., 2018; Karp et al., 2009; Katz et al., 2012; Kaup et al., 2014; Kerola et al., 2010; Kotaki et al., 2019; Kukull et al., 2002; Lee et al., 2008; Letenneur et al., 1999; Letenneur et al., 2000; Lindsay, 2002; Lobo et al., 2011; Lopez et al., 2003; Luukinen et al., 2005; Marengoni et al., 2011; McDowell et al., 2007; Moceri et al., 2000; Nakahori et al., 2018; Nitrini et al., 2004; Noale et al., 2013; Ojagbemi, Bello, & Gureje, 2016; Prince et al., 2012; Scarmeas et al., 2001)], 16 of which had not been considered in previous reviews due to recency of publication or not meeting specific review selection criteria (Borenstein et al., 2014; Cadar et al., 2018; Contador et al., 2015; de Bruijn et al., 2015; Dekhtyar et al., 2015, 2016; Hendrie et al., 2018; Kotaki et al., 2019; Lobo et al., 2011; Nakahori et al., 2018; Ojagbemi et al., 2016; Sullivan et al., 2019; Then et al., 2016; Yu et al., 2017; Yuan et al., 2016; Zahodne et al., 2016). This was the result of abstract screening of the initial search results of 3066 articles, and full-text screening of 175 articles considered relevant, as outlined in the PRISMA diagram in Fig. 1. Supplementary Table A lists the excluded articles along with their reasons for exclusion. 100 articles were excluded during full-text screening due to ineligibility in terms of meeting selection criteria (e.g. not reporting HR, OR or RR for education, reporting prevalence rather than incidence), and ten studies were excluded during data extraction due to the fact that the population, outcome and operationalisation reported were already included in another, more recent article.
Fig. 1.
PRISMA flow diagram of process identifying studies eligible for inclusion.
Of 65 articles meeting selection criteria, there were 58 cohort studies, 6 case control studies and one randomised controlled trial. Dates of publication ranged from 1992 to 2019. The majority of articles (57) included study populations from High Income Country (HIC) settings. Of the remaining articles, six were from Lower-Middle Income Country (LMIC) settings, one was from an Upper-Middle Income Country (UMIC) setting and one was from a Low Income Country (LIC) setting. There was representation from 24 countries in the 65 included articles, however over a third of articles (25) reported studies that were conducted in the USA or Canada and the majority of the remaining articles reported results from studies in Europe (28). Study periods ranged from 1982 to 2017, with numbers of follow-ups ranging from one to approximately 16 and number of follow-up years ranging from one to 28. Sample sizes in terms of participants included in the analysis ranged from 152 to 12,881.
3.2. Summary ratios
3.2.1. Any dementia
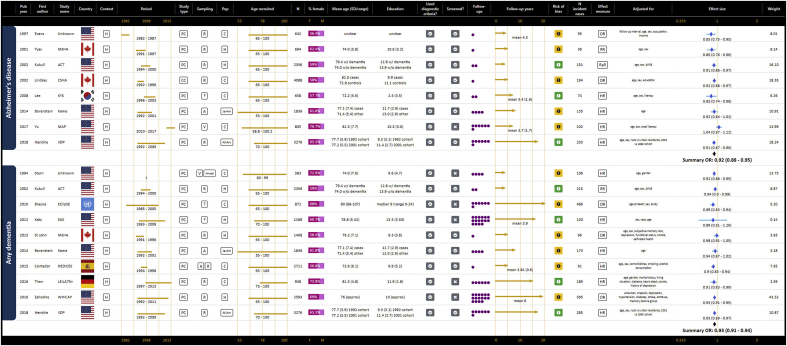
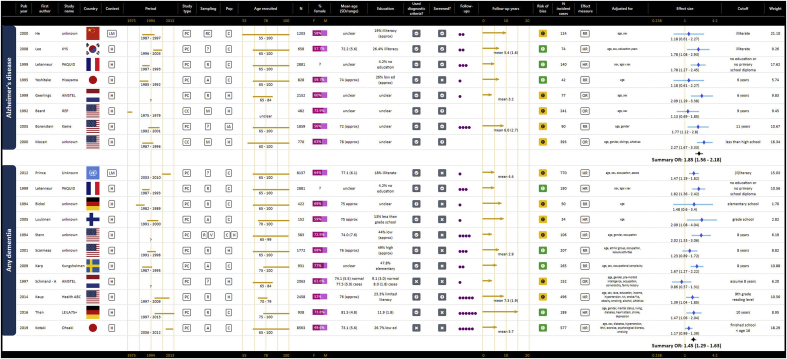
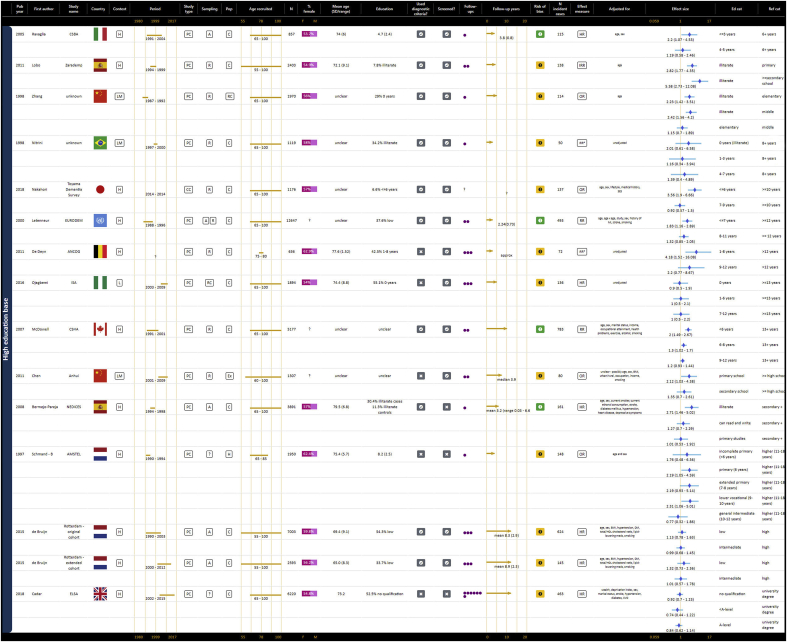
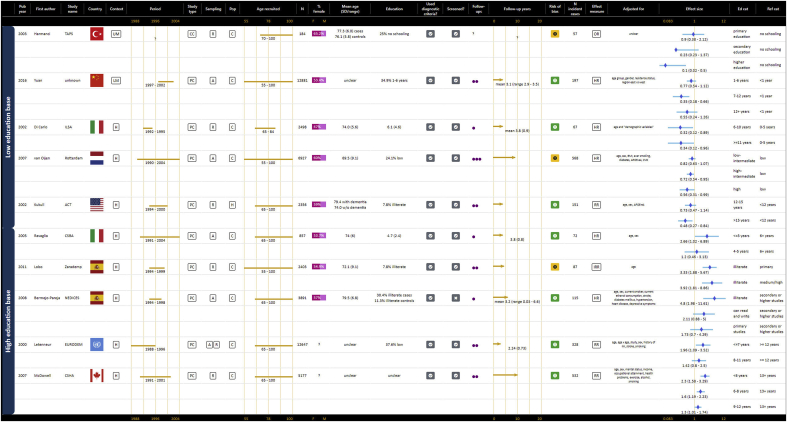
As represented in GOfER 1, 10 prospective cohort studies contributed to the summary odds ratio for a continuous association between years of education and risk of any dementia of 0.93 (95% CI: 0.91–0.94) per year (Borenstein et al., 2014; Brayne et al., 2010; Contador et al., 2015; Hendrie et al., 2018; Katz et al., 2012; Kukull et al., 2002; St John & Montgomery, 2013; Stern et al., 1994; Then et al., 2016; Zahodne et al., 2016). The I2 statistic for this meta-analysis was 0.0%, p = 0.719. For the dichotomous risk of low education versus all other education, the summary odds ratio for any dementia from 11 contributing prospective cohort studies was 1.45 (95% CI: 1.29–1.63), shown in GOfER 2 (Bickel & Cooper, 1994; Karp et al., 2009; Kaup et al., 2014; Kotaki et al., 2019; Letenneur et al., 1999; Luukinen et al., 2005; Prince et al., 2012; Scarmeas et al., 2001; Schmand et al., 1997a; Stern et al., 1994; Then et al., 2016). The I2 statistic of 33.0% suggested low heterogeneity, p = 0.135.
GOfER 1.
Graphical Overview for Evidence Review presenting extracted data, forest plots and summary odds ratios for continuous operationalisations of education and the risk of AD or any dementia.
GOfER 2.
Graphical Overview for Evidence Review presenting extracted data, forest plots and summary odds ratios for dichotomous operationalisations of education and the risk of AD or any dementia.
The forest plot of categorical operationalisations in GOfER 3a, GOfER 3b demonstrates that nine of 22 studies (40.9%) did not report any association between education levels and any dementia (Cadar et al., 2018; de Bruijn et al., 2015; Dekhtyar et al., 2015, 2016; Nitrini et al., 2004; Ojagbemi et al., 2016; Sullivan et al., 2019; Then et al., 2016; Valenzuela et al., 2011). Of 13 studies that did report an association, while there was mostly a significant association between the most extreme level of education and the reference education category, this association was often not significant for middle categories (Bermejo-Pareja et al., 2008; Chen et al., 2011; De Deyn et al., 2011; Di Carlo et al., 2002; Kukull et al., 2002; Letenneur et al., 2000; Lobo et al., 2011; McDowell et al., 2007; Nakahori et al., 2018; Ravaglia et al., 2005; Schmand et al., 1997b; Yuan et al., 2016; Zhang et al., 1998). Only three studies (13.6%) reported an association for all categories versus the reference category, and all of these studies used very low levels of education for the low education category: illiteracy (Lobo et al., 2011), less than one year (Yuan et al., 2016), or zero to five years (Di Carlo et al., 2002).
GOfER 3a.
Graphical Overview for Evidence Review presenting extracted data and forest plots for categorical operationalisations of education and the risk of any dementia, including only studies that used low education as the reference category.
GOfER 3b.
Graphical Overview for Evidence Review presenting extracted data and forest plots for categorical operationalisations of education and the risk of any dementia, including only studies that used high education as the reference category.
3.2.2. Alzheimer's disease
For the risk of AD, seven prospective cohort studies and one case control study contributed to the summary odds ratio for a continuous association for years of education of 0.92 (95% CI: 0.88–0.96), depicted in GOfER 1 (Borenstein et al., 2014; Evans et al., 1997; Hendrie et al., 2018; Kukull et al., 2002; Lee et al., 2008; Lindsay, 2002; Tyas et al., 2001; Yu et al., 2017). The I2 statistic of 55.4% suggested medium heterogeneity was present, p = 0.028. The dichotomous association for low education from six prospective cohort and two case control studies was represented in a summary odds ratio of 1.85 (95% CI: 1.56–2.18), as shown in GOfER 2 (Beard et al., 1992; Borenstein et al., 2005; Geerlings et al., 1999; He et al., 2000; Lee et al., 2008; Letenneur et al., 1999; Moceri et al., 2000; Yoshitake et al., 1995). The I2 statistic of 22.4% suggested low heterogeneity, p = 0.252. The continuous summary ratio did not change when the case control study (Lindsay, 2002) was removed, however the dichotomous summary ratio was increased to 1.91 (95% CI: 1.62–2.25) when the two case control studies (Beard et al., 1992; Moceri et al., 2000) were excluded.
For categorical operationalisations, all ten contributing studies showed an association between at least one level of education and the reference category, with three studies (30.0%) demonstrating an association for all levels of education (Di Carlo et al., 2002; Lobo et al., 2011; McDowell et al., 2007). As for any dementia, categorical associations appeared stronger for the most extreme category versus the reference, but weakened for the middle categories, as represented in GOfER 4 (Bermejo-Pareja et al., 2008; Di Carlo et al., 2002; Harmanci et al., 2003; Kukull et al., 2002; Letenneur et al., 2000; Lobo et al., 2011; McDowell et al., 2007; Ravaglia et al., 2005; van Oijen et al., 2007; Yuan et al., 2016).
GOfER 4.
Graphical Overview for Evidence Review presenting extracted data and forest plots for categorical operationalisations of education and the risk of AD, separating studies according to whether they used low or high education as the reference category.
3.2.3. Vascular dementia
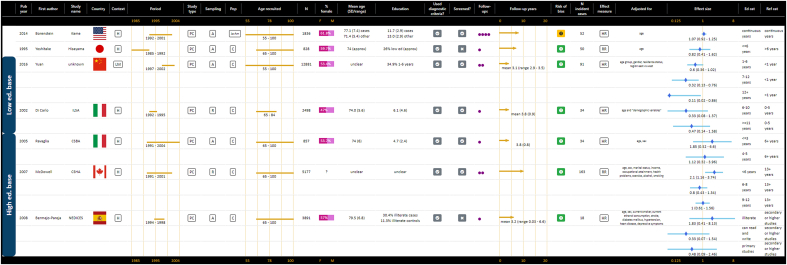
The evidence on the association between education and VaD was mixed and no meta-analyses were possible, as only seven studies reported risks relating to education and VaD (Bermejo-Pareja et al., 2008; Borenstein et al., 2014; Di Carlo et al., 2002; McDowell et al., 2007; Ravaglia et al., 2005; Yoshitake et al., 1995; Yuan et al., 2016), and five of these used categorical operationalisations that could not be summarised (Bermejo-Pareja et al., 2008; Di Carlo et al., 2002; McDowell et al., 2007; Ravaglia et al., 2005; Yuan et al., 2016). Of these seven studies, five (71.4%) reported no association between education and VaD at all (Bermejo-Pareja et al., 2008; Borenstein et al., 2014; Di Carlo et al., 2002; Ravaglia et al., 2005; Yoshitake et al., 1995) and two reported associations for some but not all education levels versus the reference category (McDowell et al., 2007; Yuan et al., 2016). Details of the individual studies and forest plots of hazards and odds ratios are presented in GOfER 5.
GOfER 5.
Graphical Overview for Evidence Review presenting extracted data and forest plots for all operationalisations of education and the risk of VaD.
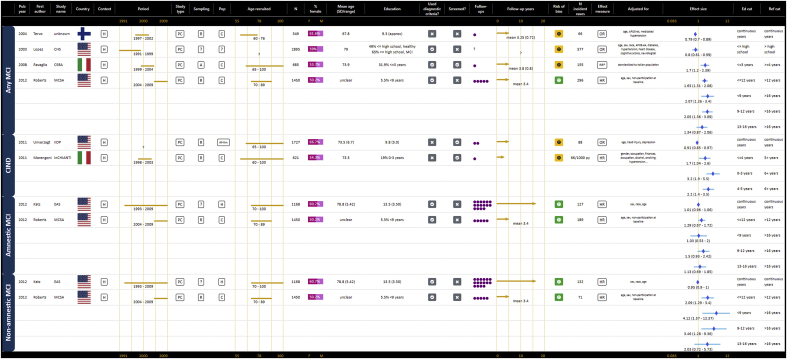
3.2.4. Mild cognitive impairment
Seven publications provided estimates for the risk of MCI related to education, however MCI types and definitions varied and data synthesis was not possible (Katz et al., 2012; Lopez et al., 2003; Marengoni et al., 2011; Ravaglia et al., 2008; Roberts et al., 2012; Tervo et al., 2004; Unverzagt et al., 2011). Details of studies and hazard and odds ratios are presented in GOfER 6.
GOfER 6.
Graphical Overview for Evidence Review presenting extracted data and forest plots for all operationalisations of education and the risk of MCI.
3.2.5. Other results not included in meta-analyses
Too few studies stratified results by gender or racial and/or ethnic differences to allow synthesis of findings, however a summary of these studies is included in Appendix C.
Also reported in Appendix C are findings from studies that used alternative measures of education, such as literacy. These are provided for descriptive purposes only as literacy was not a focus of this review.
Objective 2: documentation of inconsistency in measuring and operationalising education.
3.3. Operationalisation of education exposure
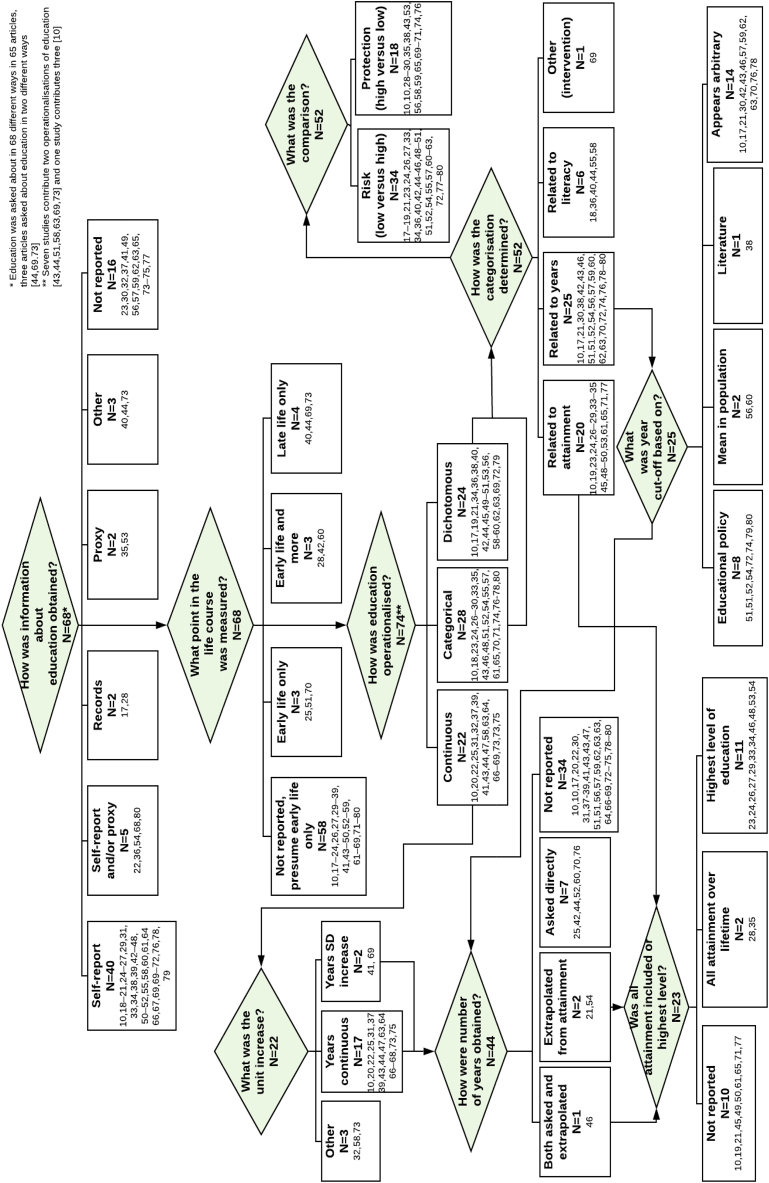
The flow chart in Fig. 2 demonstrates the inconsistency in the measurement and operationalisation of education between publications. Overall, there were 23 continuous, 29 dichotomous and 31 categorical operationalisations represented in the 65 publications, however not all are included in the flow chart. Seven studies reported two different operationalisations of education (Kukull et al., 2002; Lee et al., 2008; Marengoni et al., 2011; Prince et al., 2012; Stern et al., 1994; Unverzagt et al., 2012; Yu et al., 2017) and one study investigating the impact of operationalisations of education reported effects for twelve different operationalisations (Then et al., 2016). Only one each of dichotomous, continuous and categorical operationalisations were used for the flow chat from this latter study, so as not to bias the chart with results from one study, hence 22 continuous, 28 categorical and 24 dichotomous operationalisations are included. Overall, there was a lack of consistency in: 1. how education information was obtained, with many studies not reporting this; 2. whether life course education was considered; 3. how education was operationalised; 4. how categorisations were determined; 5. how number of years was obtained when used; 6. how cut-offs were decided, with very few studies providing justification for this; 7. whether all educational attainment was included or just the highest level; and 8. The use of low or high education as a reference category.
Fig. 2.
Categorisation of included studies by measurement and operationalisation of education. (Reference numbers for studies can be found in Supplementary Table B).
Objective 3: identifying the dose of education required to reduce dementia risk.
3.4. Definitions of low education
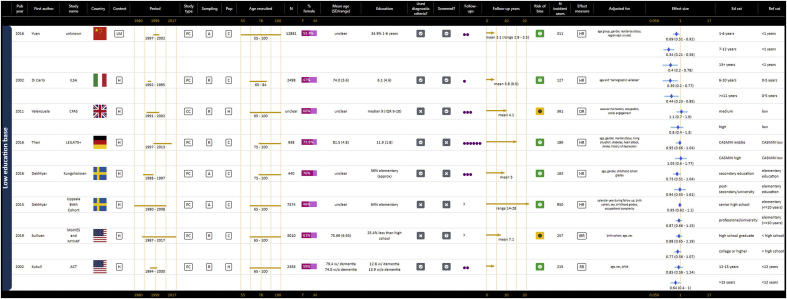
The definition of low education varied widely between studies, as represented in Table 1. Many studies defined low education in terms of years but there was wide variation in the cut-offs used, with the number of years defined as “low education” ranging from zero to less than one, three, six, seven, eight, nine, ten, 11 or 12 years of study. Other studies used levels of attainment to classify education as low, but here again this ranged from not completing primary, grade or elementary school to not completing high school, to having no qualification. Definitions of low education that used illiteracy or a very low number of years were more often used in LMIC (e.g. Turkey, Nigeria, China, Brazil) and Southern European (Spain and Italy) settings, and definitions with less than 12 years or high school originated more often from the United States of America or the United Kingdom. This was not consistent, however, with some studies from the same country using very different definitions of low education (e.g. different studies from Japan defined low education as less than six years (Yoshitake et al., 1995) or finishing high school before age 16 (Kotaki et al., 2019)).
Table 1.
Definitions of low education used in studies with categorical and dichotomous operationalisations of education.
| Author | Year | Country | Definition of low education |
|---|---|---|---|
| Categorical operationalisations | |||
| Bermejo-Pareja | 2008 | Spain | illiterate |
| Lobo | 2011 | Spain | illiterate |
| Harmanci | 2003 | Turkey | no schooling |
| Ojagbemi | 2016 | Nigeria | 0 years |
| Zhang | 1998 | China | 0 years |
| Nitrini | 2004 | Brazil | 0 years illiterate |
| DiCarlo | 2002 | Italy | 0–5 years |
| Yuan | 2016 | China | <1 year |
| Ravaglia | 2005 | Italy | <3 years |
| Chen | 2011 | China | primary school or less |
| deBruijn | 2015 | Netherlands | < primary or lower vocational |
| vanOijen | 2007 | Netherlands | primary |
| Dekhtyar | 2015 | Sweden | elementary or less |
| AlHazzouri Zeki | 2013 | USA | <6 |
| McDowell | 2007 | Canada | <6 years |
| Schmand | 1997 | Netherlands | <6 years |
| Nakahori | 2018 | Japan | ≤6 years |
| Then | 2016 | Germany | < elementary or basic vocational |
| Letenneur | 2000 | Denmark, France, Netherlands, UK | ≤7 years |
| Dekhtyar | 2016 | Sweden | <8 years |
| DeDeyn | 2011 | Belgium | <9 years |
| Roberts | 2012 | USA | <9 years |
| Kukull | 2002 | USA | <12 years |
| Fitzpatrick | 2004 | USA | <high school |
| Sullivan | 2018 | USA | <high school |
| Cadar | 2018 | England | no qualification |
| Valenzuela | 2011 | United Kingdom | Unclear |
| Dichotomous operationalisations | |||
| Prince | 2012 | Cuba, Dominican Republic, Venezuela, Peru, Mexico, China | illiterate |
| Lee | 2008 | Korea | illiterate |
| He | 2000 | China | illiterate |
| Noale | 2013 | Italy | <3 years |
| Marengoni | 2011 | Italy | ≤3 |
| Geerlings | 1999 | Netherlands | <6 years |
| Yoshitake | 1995 | Japan | <6 years |
| Letenneur | 1999 | France | <primary school |
| Bickel | 1994 | Germany | <=elementary or less |
| Luukinen | 2005 | Finland | <grade school |
| Scarmeas | 2001 | USA | <8 years |
| Stern | 1994 | USA | <8 years |
| Karp | 2009 | Sweden | <8 years |
| Schmand | 1997 | Netherlands | Assume <8 years |
| Beard | 1992 | USA | <9 years |
| Kaup | 2014 | USA | <9th grade reading level literacy |
| Shadlen | 2006 | USA | <10 years |
| Then | 2016 | Germany | <10 years |
| Borenstein | 2005 | USA | <11 years |
| Kotaki | 2019 | Japan | finished school before age 16 |
| Lopez | 2003 | USA | <=high school |
| Moceri | 2000 | USA | <=high school |
3.5. Dose of education required for a statistically significant effect
Studies are ranked in GOfER 2 from lowest to highest in terms of the cut-off used to dichotomise education. This means that studies using illiteracy or zero years to define low education are represented at the top in the GOfER and corresponding forest plot, and studies using a definition of low education that includes 12 years or less are represented at the bottom. A visual trend in effect size towards the null value may be perceptible as the definition of low education includes more years or a higher level of attainment, particularly for any dementia. There was, however, no apparent trend in terms of statistical significance and no obvious dose or threshold required for an association between low education and dementia.
3.6. Sensitivity analyses
Restriction of analysis to studies that defined low education with a cut-off that was less than or equal to eight years resulted in slightly higher summary odds ratios of 1.93 (95%CI: 1.61–2.30) for AD and 1.54 (95%CI: 1.35–1.75) for any dementia (compared with 1.85 (95% CI: 1.56–2.18) for AD and 1.45 (95% CI: 1.29–1.63) for any dementia when all studies were included). The I2 statistic indicated less variability between studies when this restricted definition of low education was used, with an I2 of 0.0% for AD and 13.0% for any dementia, compared to 22.4% and 33.0% respectively when all definitions of low education were included.
3.7. Risk of bias
3.7.1. Publication bias
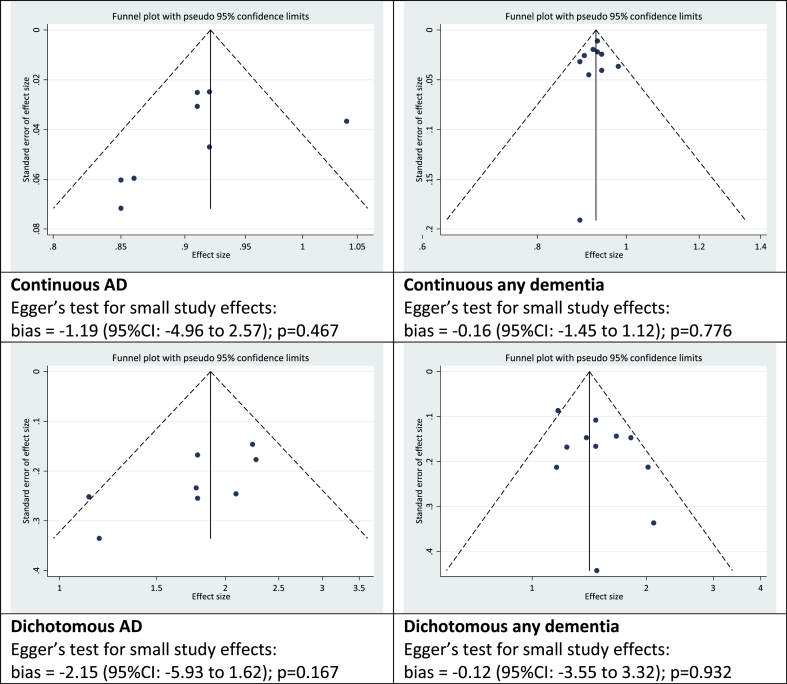
Funnel plots representing the risk of publication bias, along with the results of Egger's test for small study effects, are reproduced in Fig. 3. Visually there is a suggestion of asymmetry with under-representation of smaller studies with non-significant results, however this does not serve as proof of bias and could reflect other sources of heterogeneity between studies (Sterne & Harbord, 2004). Egger's regression test statistics did not support evidence of publication bias.
Fig. 3.
Funnel plots.
3.7.2. Study risk of bias
Using a customised risk of bias tool for dementia studies, 20 of 65 studies were assessed to be at low risk of bias (Bermejo-Pareja et al., 2008; Dekhtyar et al., 2015, 2016; Di Carlo et al., 2002; Hendrie et al., 2018; Karp et al., 2009; Katz et al., 2012; Kotaki et al., 2019; Kukull et al., 2002; Lee et al., 2008; Letenneur et al., 1999, 2000; McDowell et al., 2007; Noale et al., 2013; Ravaglia et al., 2005; Roberts et al., 2012; Scarmeas et al., 2001; Then et al., 2016; Yoshitake et al., 1995; Yuan et al., 2016). All of the remaining 45 studies were categorised as medium risk [ (Beard et al., 1992), (Bickel & Cooper, 1994; Borenstein et al., 2005, 2014; Brayne et al., 2010; Cadar et al., 2018; Chen et al., 2011; Contador et al., 2015; de Bruijn et al., 2015; De Deyn et al., 2011), (Evans et al., 1997; Fischer et al., 2008; Fitzpatrick et al., 2004; Geerlings et al., 1999; Harmanci et al., 2003; He et al., 2000), (Evans et al., 1997; Fischer et al., 2008; Fitzpatrick et al., 2004; Geerlings et al., 1999; Harmanci et al., 2003; He et al., 2000), (Evans et al., 1997; Fischer et al., 2008; Fitzpatrick et al., 2004; Geerlings et al., 1999; Harmanci et al., 2003; He et al., 2000), (Lindsay, 2002; Lobo et al., 2011; Lopez et al., 2003; Luukinen et al., 2005; Marengoni et al., 2011), (Lindsay, 2002; Lobo et al., 2011; Lopez et al., 2003; Luukinen et al., 2005; Marengoni et al., 2011), (Lindsay, 2002; Lobo et al., 2011; Lopez et al., 2003; Luukinen et al., 2005; Marengoni et al., 2011), (Lindsay, 2002; Lobo et al., 2011; Lopez et al., 2003; Luukinen et al., 2005; Marengoni et al., 2011), (Schmand et al., 1997a, 1997b; Shadlen et al., 2006; St John & Montgomery, 2013; Stern et al., 1994; Sullivan et al., 2019; Tervo et al., 2004; Tyas et al., 2001; Unverzagt et al., 2011, 2012; Valenzuela et al., 2011; van Oijen et al., 2007), (Schmand et al., 1997a, 1997b; Shadlen et al., 2006; St John & Montgomery, 2013; Stern et al., 1994; Sullivan et al., 2019; Tervo et al., 2004; Tyas et al., 2001; Unverzagt et al., 2011, 2012; Valenzuela et al., 2011; van Oijen et al., 2007), (Zahodne et al., 2016; Zeki Al Hazzouri et al., 2013; Zhang et al., 1998), (Zahodne et al., 2016; Zeki Al Hazzouri et al., 2013; Zhang et al., 1998)], with no studies found to be at a high risk of bias influencing results. The risk of bias tool exposed the following aspects as being the most common in terms of increasing the vulnerability of studies to bias: having a short follow-up period or only one follow-up; not describing the differences between included and excluded study participants; not including institutionalised participants; and not defining the exposure measurement and operationalisation. The overall rating for each study is available in GOfER 1, GOfER 2, GOfER 3a, GOfER 3b, GOfER 4, GOfER 5, GOfER 6 and Supplementary Table B. The risk of bias tool is presented in Appendix B.
4. Discussion
Finding 1: A revised, attenuated and more precise summary odds ratio for low education and any dementia.
This systematic review has updated and reiterated the evidence for an association between education in early life and reduced risk of AD and any dementia incidence, with the addition of 16 previously unconsidered studies. Results of meta-analyses suggest reduced risks of eight per cent for AD and seven per cent for any dementia for each year of education from continuous operationalisations (95% CI for AD: 5–12% reduced risk per year of education; 95% CI for any dementia: 6–9% reduced risk per year of education); and an 85% increased risk of AD (95% CI: 56–118%) and 45% (95% CI: 29–63%) increased risk of any dementia for those with low education from dichotomous operationalisations.
Evidence was less conclusive for studies using categorical operationalisations of education: of 22 studies of the incidence of any dementia, 40.9% showed no association between any of the education levels and the reference category, 45.5% showed an association for some but not all levels, and only 13.6% demonstrated an association for all levels. Of ten studies of AD incidence, 70.0% showed an association between some but not all levels and the reference category, with the remaining 30.0% demonstrating an association for all levels. The majority of seven studies investigating education and VaD did not provide evidence of an association (71.4%), however there were too few studies for both VaD and the multiple MCI outcomes to attempt formal syntheses of effect sizes.
The finding of an association between low education and any dementia reported here confirms findings from previous systematic reviews, although the summary odds ratio of 1.45 (95% CI: 1.29–1.63) has higher precision and is somewhat attenuated compared to other meta-analyses that reported ratios of 1.89 (95% CI: 1.61–2.22) (Valenzuela & Sachdev, 2005), 1.59 (95% CI: 1.26–2.01) (Caamaño-Isorna et al., 2006), (Livingston et al., 2017), 1.88 (95% CI: 1.51–2.34) (Meng & D'Arcy, 2012), 1.72 (95% CI: 1.52–1.96) (Prince et al., 2014), and 1.81 (95% CI: 1.59–2.06) (Xu et al., 2016). One other meta-analysis, restricted to only four studies due to the requirement that all studies use the same cut-off of eight years of education, reported a higher summary odds ratio of 1.99 (95% CI: 1.30–3.04) (Beydoun et al., 2014). This higher ratio is comparable to the outcome of our sensitivity analysis using the same eight year cut-off, resulting in a ratio of 1.54 (95%CI: 1.35–1.75). To the best of our knowledge, ours was the first review with the objective of minimising heterogeneity by separating operationalisations of education into continuous, dichotomous and categorical groupings before conducting meta-analyses, and this may be one factor underlying the lower odds ratios and narrower confidence intervals for our estimates.
Finding 2: Inconsistency in definitions of low education calling any summary odds ratio into question.
The wide variation of definitions of low education demonstrated in Table 1, combined with the multiple and inconsistent approaches to measuring and operationalising education demonstrated in Fig. 2, provide evidence that (low) education is not necessarily comparable across studies of dementia incidence published to date. In this context, it is important to question how meaningful any summary odds ratio truly is, in terms of representing an overall risk of dementia for people with low education. Risk ratios determined from an individual study will always depend on the reference group risk in that population. This was illustrated in one of the included studies by Then et al., where several cut-offs for low education were tested. Of adjusted hazard ratios produced cutting education years at nine, ten and 12 years respectively, only the adjusted ratio for ten years showed an association with incident dementia (adjusted OR for risk of ≥ 9 years education: 0.72, 95% CI: 0.51–1.00; adjusted OR for risk of ≥ 10 years education: 0.68, 95% CI: 0.49–0.95; adjusted OR for risk of ≥ 12 years education: 0.86, 95% CI: 0.61–1.22) (Then et al., 2016). This finding of statistically significant and non-significant ratios depending on what year cut-off was used has two implications: the first is that we must be wary of potential publication bias in representations of a dichotomous association between low versus high education and dementia. As observational studies do not typically publish their protocols prior to analysis and publication, it is possible that a definition of low education is decided based on a cut-off that provides a statistically significant association, thus increasing chances of study publication. The second implication is that we must question the appropriateness of combining ratios that have not used comparable reference values because we cannot be sure that they represent comparable risks in the respective populations. This is problematic not only because we do not know whether low education defined as less than three years in one study is comparable with low education defined as less than twelve years in another study; but also because we do not know whether low education defined as ten years is comparable in two different populations, given the wide global variation in education systems and socioeconomic structures underlying access to them, both now and historically.
Finding 3: Lack of evidence as to the dose required for dementia prevention.
In the context of the discussion point above, it is unsurprising that no clear dose of education emerged from the literature as sufficient to reduce the risk of dementia. Visual examination of the dichotomous forest plots and results of sensitivity analysis hint that definitions of low education that include fewer years of education may result in higher summary odds ratios in terms of dementia risk, but this requires further testing in a study designed to test this hypothesis. Theoretically, the finding from the continuous forest plots of a reduced risk per year of education should have a mathematical limit that could provide insight as to a dose, but to our knowledge this has not yet been described in the literature and was beyond the scope of our study. Although a dose itself remains elusive, Xu et al. have previously reported a dose-response trend for both low and high education for risk of AD and any dementia using studies with categorical operationalisations (Xu et al., 2016).
Interestingly, the results from our categorical operationalisation of education demonstrate the difficulty of choosing cut-offs to determine risks associated with education. Visual examination of the forest plots show a trend: as the reference education level grows larger in terms of years of education included, effect sizes grow smaller and are more likely to cross the null value of OR = 1.0, regardless of whether a low or high education level has been used as the reference. In most categorical investigations, low education does not appear to be a risk compared to all education groups, but only compared to high education. This implies that the effect of low education in studies using dichotomous operationalisations that arbitrarily divide a population into two parts may be diluted by the lack of association between low education and “education somewhere in the middle”. In fact, some of the summary odds ratios produced in prior systematic reviews and reproduced widely in the literature were based on individual ratios for the lowest education category compared to the highest, thus inflating the risk of low education in terms of the general population (Caamaño-Isorna et al., 2006; Livingston et al., 2017; Valenzuela & Sachdev, 2005; Xu et al., 2016). Clarity regarding whether the risk of low education is in reference to a select group of people with high education or to anyone else in the population without low education is vital to making policy recommendations to reduce population risk of dementia.
4.1. Limitations
Wide variation in adjustment strategies for the effect of confounding, ranging from no adjustment, to adjustment for age and sex only, to adjustment for multiple confounders, including confounders related to cognition, is a factor for consideration when pooling results of studies. Although we have attempted consistency in pooling the most adjusted ratios from each study, the summary odds ratios may be influenced by under- or over-adjustment for confounders in individual studies. There were not enough studies reporting gender, racial, ethnic or cultural differences to provide information about whether there is an interaction between these factors and the association between education and dementia. Such differences should be explored in future studies as it is possible that the associations reported here do not apply for all genders, races, ethnicities and cultures, due to structural inequalities in access to education.
This review has attempted to draw attention to inconsistency in measurements of education in studies associating it with AD, other dementias and MCI. While this is an achievable task in terms of the empirical measurement and operationalisation of education, it would be less feasible if we wanted to compare the content and quality of education across studies. A limitation of this study, therefore, is that the effect of the heterogeneity in operationalisation of education may only be a partial explanation for the heterogeneity of results, as these may largely be explained by contextual differences in what is offered as education and how it is received. Rehkopf et al. discuss this problem in the context of the consistency assumption that underlies the translation of findings from observational studies to interventions aimed at improving health outcomes. Specifically addressing the consistency of measurements of education, these authors acknowledge that it is unknown how findings related to education should be translated into interventions, with areas to target including ages to begin or end compulsory schooling, class sizes and student/teacher ratios, teacher skill levels, classroom time and specific curriculae. According to Rehkopf et al., “the link between what we measure in most observational studies of education, and what matters for health, is not necessarily close” (Rehkopf, Glymour, & Osypuk, 2016). In this sense it is disappointing that even when a systematic review of the evidence from epidemiological studies of the association between education and dementia incidence to date is attempted, as we have here, inconsistency in measurements prevents translations of these findings into recommendations for intervention, even at the most basic level of a dose of the number of years required to reduce dementia risk.
4.2. The case for standardising the measurement and operationalisation of education
The main focus of this analysis was the considerable inconsistency in measurements of education in general, and cut-offs used to operationalise low education in particular, in epidemiological studies of dementia risk. The fact that an association between low education and dementia persists despite inconsistency in educational measurements, operationalisations and study contexts serves as testament to the robustness of the association. However, it is clear that evidence in the field would be strengthened and could provide further opportunities for practical translation into policy if there was consistency in these measurements and definitions cross-nationally and cross-culturally (Glymour & Whitmer, 2019). The issue of measurement is one for the future, that needs to be taken into consideration in the design of studies from now on. The measurements we have today are the legacy of studies that were designed and implemented many years ago. Given the context-specific nature of the impact of education on different populations worldwide, and the consistency consideration discussed above, this will take some consideration and collaboration among researchers. In the meantime, there is a pressing need to develop a standardised method for operationalising the measurements of education that we already have. It is possible that standardisation may need to be context-specific, however this need not prevent a consensus definition of low education that could be applied on a country-by-country basis and then compared more broadly. One possible example might be a cut-off related to the normal distribution, defining low education as values falling in the lowest quartile. A consistent definition such as this would provide information about whether the risk factor of low education is related to an actual number of years, or rather from being at the lowest end of what would be described as a gradient effect (Marmot, 2015).
5. Conclusion
It is possible that underlying the lack of consensus in the handling of the education variable in epidemiological investigations is a lack of consensus among disciplines regarding what we are trying to measure and the mechanisms by which it might reduce dementia risk. From a neuroscientific standpoint, the aim may be to measure the sum total of an exposure that is directly neuroprotective, as might be implied by using number of years of education. From a psychological perspective, it may be desirable to measure an overall level of attainment and achievement, with accompanying psychosocial characteristics of persistence and diligence, as could be inferred by measuring a highest level of attainment. From a sociological viewpoint, it may be more appropriate to develop a measure of education that represents its status as a socioeconomic milestone that opens doors to a lifetime of better opportunities, including better health in general and enhanced cognitive health in particular. According to Sharp and Gatz, “education is best described as a proxy for a trajectory of life events, beginning prior to and extending beyond the years of formal education, that either increase or decrease an individual's risk for dementia” (Sharp & Gatz, 2011). Given this status of education as a multidisciplinary proxy variable, it is no wonder that epidemiologists struggle to measure it. Our conclusion from this review is that, while the evidence for an effect of education on dementia risk is robust and appears to withstand heterogeneity in study contexts, it could be strengthened to provide practical policy recommendations for dementia prevention if consensus were achieved on ways to define, measure and operationalise (low) education.

Ethical statement
This research was not subject to ethical review as it involved secondary analysis of data from previously published studies. All data was obtained from publications of original studies that were subject to individual ethical review processes.
The review protocol for this research was registered at PROSPERO with registration ID CRD42018096168.
Funding sources
JM is supported by an Australian Government Research Training Program Scholarship and Supplementary PhD Scholarship Awards from the ARC Centre of Excellence in Population Ageing Research (CEPAR) and Neuroscience Research Australia (NeuRA). KJA is funded by NHMRC Research Fellowship number 1102694, RP is funded by the NHMRC Dementia Centre for Research Collaboration. We acknowledge funding from the NHMRC Dementia Collaborative Research Centre and the ARC Centre of Excellence in Population Ageing Research.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank Steven Bagshaw from Shore Informatics for writing code to display the Graphical Overview for Evidence Reviews (GOfERs).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2020.100654.
Appendices A, B and C. Supplementary data
The following is the Supplementary data to this article:
References
- Beard C.M., Kokmen E., Offord K.P. Lack of association between Alzheimer's disease and education, occupation, marital status, or living arrangement. Neurology. 1992;42:2063–2068. doi: 10.1212/wnl.42.11.2063. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F., Benito-León J., Vega S. Incidence and subtypes of dementia in three elderly populations of central Spain. Journal of the Neurological Sciences. 2008;264:63–72. doi: 10.1016/j.jns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Beydoun M.A., Beydoun H.A., Gamaldo A.A. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel H., Cooper B. Incidence and relative risk of dementia in an urban elderly population: Findings of a prospective field study. Psychological Medicine. 1994;24:179–192. doi: 10.1017/S0033291700026945. [DOI] [PubMed] [Google Scholar]
- Borenstein A.R., Wu Y., Bowen J.D. Incidence rates of dementia, alzheimer disease, and vascular dementia in the Japanese American population in Seattle, WA: The kame project. Alzheimer Disease and Associated Disorders. 2014;28:23–29. doi: 10.1097/WAD.0b013e3182a2e32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein A.R., Wu Y., Mortimer J.A. Developmental and vascular risk factors for Alzheimer's disease. Neurobiology of Aging. 2005;26:325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Brayne C., Ince P.G., Keage H.A.D. Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- de Bruijn R.F., Bos M.J., Portegies M.L. The potential for prevention of dementia across two decades: The prospective, population-based rotterdam study. BMC Medicine. 2015;13 doi: 10.1186/s12916-015-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamaño-Isorna F., Corral M., Montes-Martínez A. Education and dementia: A meta-analytic study. Neuroepidemiology; Basel. 2006;26:226–232. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- Cadar D., Lassale C., Davies H. Individual and area-based socioeconomic factors associated with dementia incidence in england: Evidence from a 12-year follow-up in the English longitudinal study of ageing. JAMA Psychiatry. 2018;75:723–732. doi: 10.1001/jamapsychiatry.2018.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Hu Z., Wei L. Incident dementia in a defined older Chinese population. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contador I., Bermejo-Pareja F., Puertas-Martin V. Childhood and adulthood rural residence increases the risk of dementia: NEDICES study. Current Alzheimer Research. 2015;12:350–357. doi: 10.2174/1567205012666150324181327. [DOI] [PubMed] [Google Scholar]
- De Deyn P.P., Goeman J., Vervaet A. Prevalence and incidence of dementia among 75–80-year-old community-dwelling elderly in different districts of Antwerp, Belgium: The Antwerp Cognition (ANCOG) Study. Clinical Neurology and Neurosurgery. 2011;113:736–745. doi: 10.1016/j.clineuro.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Dekhtyar S., Wang H.-X., Fratiglioni L. Childhood school performance, education and occupational complexity: A life-course study of dementia in the kungsholmen project. International Journal of Epidemiology. 2016:dyw008. doi: 10.1093/ije/dyw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekhtyar S., Wang H.-X., Scott K. A life-course study of cognitive reserve in dementia—from childhood to old age. American Journal of Geriatric Psychiatry. 2015;23:885–896. doi: 10.1016/j.jagp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Di Carlo A., Baldereschi M., Amaducci L. Incidence of dementia, Alzheimer's disease, and vascular dementia in Italy. The ILSA study. Journal of the American Geriatrics Society. 2002;50:41–48. doi: 10.1046/j.1532-5415.2002.50006.x. [DOI] [PubMed] [Google Scholar]
- Evans D.A., Hebert L.E., Beckett L.A. Education and other measures of socioeconomic status and risk of incident alzheimer disease in a defined population of older persons. Archives of Neurology. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- Fischer P., Zehetmayer S., Jungwirth S. Risk factors for alzheimer dementia in a community-based birth cohort at the age of 75 years. Dementia and Geriatric Cognitive Disorders. 2008;25:501–507. doi: 10.1159/000128577. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A.L., Kuller L.H., Ives D.G. Incidence and prevalence of dementia in the cardiovascular health study: Incidence OF dementia IN CHS. Journal of the American Geriatrics Society. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L., Wang H.-X. Brain reserve hypothesis in dementia. Journal of Alzheimer's Disease. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- Geerlings M.I., Schmand B., Jonker C. Education and incident Alzheimer's disease: A biased association due to selective attrition and use of a two-step diagnostic procedure? International Journal of Epidemiology. 1999;28:492–497. doi: 10.1093/ije/28.3.492. [DOI] [PubMed] [Google Scholar]
- Glymour M.M., Whitmer R.A. Using cross-cultural studies to improve evidence on dementia prevention: Lessons from the special issue Sponsored by the international research network on dementia prevention (IRNDP) Journal of Alzheimer's Disease. 2019;70 doi: 10.3233/JAD-190304. S5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmanci H., Emre M., Gurvit H. Risk factors for alzheimer disease: A population-based case-control study in istanbul, Turkey. Alzheimer Disease and Associated Disorders. 2003;17:139–145. doi: 10.1097/00002093-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Hendrie H.C., Smith-Gamble V., Lane K.A. The association of early life factors and declining incidence rates of dementia in an elderly population of african Americans. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2018;73:S82–S89. doi: 10.1093/geronb/gbx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.L., Zhang X.K., Zhang M.Y. Psychosocial risk factors for Alzheimer s disease. Hong Kong Journal of Psychiatry. 2000;10(2):2–7. [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking D.E., Ayton S., Beckett N. More evidence is needed. Iron, incident cognitive decline and dementia: A systematic review. Ther Adv Chronic Dis. 2018;9:241–256. doi: 10.1177/2040622318788485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D., Bain C., Williams G. A systematic review of the global prevalence of low back pain. Arthritis & Rheumatism. 2012;64:2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Woolf A. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. Journal of Clinical Epidemiology. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Karp A., Andel R., Parker M.G. Mentally Stimulating activities at work during midlife and dementia risk after age 75: Follow-up study from the kungsholmen project. American Journal of Geriatric Psychiatry. 2009;17:227–236. doi: 10.1097/JGP.0b013e318190b691. [DOI] [PubMed] [Google Scholar]
- Katz M.J., Lipton R.B., Hall C.B. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and alzheimer dementia in blacks and whites: A report from the einstein aging study. Alzheimer Disease and Associated Disorders. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup A.R., Simonsick E.M., Harris T.B. Older adults with limited literacy are at increased risk for likely dementia. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014;69:900–906. doi: 10.1093/gerona/glt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerola T., Nieminen T., Hartikainen S. B-type natriuretic peptide as a predictor of declining cognitive function and dementia—a cohort study of an elderly general population with a 5-year follow-up. Annals of Medicine. 2010;42:207–215. doi: 10.3109/07853891003652542. [DOI] [PubMed] [Google Scholar]
- Kotaki Y., Tomata Y., Tanji F. Joint impact of seven risk factors on incident dementia in elderly Japanese: The ohsaki cohort 2006 study. Journal of Neurology. 2019;266:1222–1229. doi: 10.1007/s00415-019-09252-w. [DOI] [PubMed] [Google Scholar]
- Kukull W.A., Higdon R., Bowen J.D. Dementia and alzheimer disease incidence: A prospective cohort study. Archives of Neurology. 2002;59:1737. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- Lee J.-Y., Chang S.M., Jang H.-S. Illiteracy and the incidence of Alzheimer's disease in the yonchon county survey, korea. International Psychogeriatrics. 2008;20 doi: 10.1017/S1041610208007333. [DOI] [PubMed] [Google Scholar]
- Letenneur L., Gilleron V., Commenges D. Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. Journal of Neurology, Neurosurgery & Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letenneur L., Launer L.J., Andersen K. Education and the risk for Alzheimer's disease: Sex makes a difference. EURODEM pooled analyses. EURODEM incidence research group. American Journal of Epidemiology. 2000;151:1064–1071. doi: 10.1093/oxfordjournals.aje.a010149. [DOI] [PubMed] [Google Scholar]
- Lindsay J. Risk factors for Alzheimer's disease: A prospective analysis from the Canadian study of health and aging. American Journal of Epidemiology. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V. Dementia prevention, intervention, and care. The Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Lobo A., Lopez-Anton R., Santabárbara J. Incidence and lifetime risk of dementia and Alzheimer's disease in a Southern European population: Incidence and LTR of dementia and AD. Acta Psychiatrica Scandinavica. 2011;124:372–383. doi: 10.1111/j.1600-0447.2011.01754.x. [DOI] [PubMed] [Google Scholar]
- Lopez O.L., Jagust W.J., Dulberg C. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: Part 2. Archives of Neurology. 2003;60:1394. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- Luukinen H., Viramo P., Herala M. Fall-related brain injuries and the risk of dementia in elderly people: A population-based study. European Journal of Neurology. 2005;12:86–92. doi: 10.1111/j.1468-1331.2004.00953.x. [DOI] [PubMed] [Google Scholar]
- Marengoni A., Fratiglioni L., Bandinelli S. Socioeconomic status during lifetime and cognitive impairment No-dementia in late life: The population-based aging in the chianti area (InCHIANTI) study. Journal of Alzheimer's Disease. 2011;24:559–568. doi: 10.3233/JAD-2011-101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M. Bloomsbury; London: 2015. The health gap: The challenge of an unequal world. [DOI] [PubMed] [Google Scholar]
- McDowell I., Xi G., Lindsay J. Mapping the connections between education and dementia. Journals of Clinical and Experimental Neuropsychology. 2007;29:127–141. doi: 10.1080/13803390600582420. [DOI] [PubMed] [Google Scholar]
- Meng X., D'Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moceri V.M., Kukull W.A., Emanuel I. Early-life risk factors and the development of Alzheimer's disease. Neurology. 2000;54:415–420. doi: 10.1212/wnl.54.2.415. [DOI] [PubMed] [Google Scholar]
- Nakahori N., Sekine M., Yamada M. A pathway from low socioeconomic status to dementia in Japan: Results from the toyama dementia survey. BMC Geriatrics. 2018;18 doi: 10.1186/s12877-018-0791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitrini R., Caramelli P., Jr E.H. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Disease and Associated Disorders. 2004;18:6. [PubMed] [Google Scholar]
- Noale M., Limongi F., Zambon S. Incidence of dementia: Evidence for an effect modification by gender. The ILSA study. International Psychogeriatrics. 2013;25:1867–1876. doi: 10.1017/S1041610213001300. [DOI] [PubMed] [Google Scholar]
- Norton S., Matthews F.E., Barnes D.E. Potential for primary prevention of Alzheimer's disease: An analysis of population-based data. The Lancet Neurology. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- van Oijen M., de Jong F.J., Hofman A. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimer's and Dementia. 2007;3:92–97. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Ojagbemi A., Bello T., Gureje O. Cognitive reserve, incident dementia, and associated mortality in the ibadan study of ageing. Journal of the American Geriatrics Society. 2016;64:590–595. doi: 10.1111/jgs.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedditizi E., Peters R., Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age and Ageing. 2016;45:14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- Prince M., Acosta D., Ferri C.P. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: A 10/66 dementia research group population-based cohort study. The Lancet. 2012;380:50–58. doi: 10.1016/S0140-6736(12)60399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Albanese E., Guerchet M. 2014. World alzheimer report 2014: Dementia and risk reduction. An analysis of protective and modifiable factors. [Google Scholar]
- Ravaglia G., Forti P., Maioli F. Incidence and etiology of dementia in a large elderly Italian population. Neurology. 2005;64:1525–1530. doi: 10.1212/01.WNL.0000160107.02316. [DOI] [PubMed] [Google Scholar]
- Ravaglia G., Forti P., Montesi F. Mild cognitive impairment: Epidemiology and dementia risk in an elderly Italian population: Mild cognitive impairment IN elderly ITALIANS. Journal of the American Geriatrics Society. 2008;56:51–58. doi: 10.1111/j.1532-5415.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- Rehkopf D.H., Glymour M.M., Osypuk T.L. The consistency assumption for causal inference in social epidemiology: When a rose is not a rose. Curr Epidemiol Rep. 2016;3:63–71. doi: 10.1007/s40471-016-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.O., Geda Y.E., Knopman D.S. The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Levy G., Tang M.X. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B., Smit J.H., Geerlings M.I. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychological Medicine. 1997;27:1337–1344. doi: 10.1017/S0033291797005461. [DOI] [PubMed] [Google Scholar]
- Schmand B., Smit J., Lindeboom J. Low education is a genuine risk factor for accelerated memory decline and dementia. Journal of Clinical Epidemiology. 1997;50:1025–1033. doi: 10.1016/S0895-4356(97)00121-2. [DOI] [PubMed] [Google Scholar]
- Shadlen M.-F., Siscovick D., Fitzpatrick A.L. Education, cognitive test Scores, and black-white differences in dementia risk: EDUCATION, race, and dementia. Journal of the American Geriatrics Society. 2006;54:898–905. doi: 10.1111/j.1532-5415.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Sharp E.S., Gatz M. The relationship between education and dementia an updated systematic review. Alzheimer Disease and Associated Disorders. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert K., Hussain S.M., Page M.J. Effect of breakfast on weight and energy intake: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2019:l42. doi: 10.1136/bmj.l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John P., Montgomery P. Does self-rated health predict dementia? Journal of Geriatric Psychiatry and Neurology. 2013;26:41–50. doi: 10.1177/0891988713476369. [DOI] [PubMed] [Google Scholar]
- Sterne J.A.C., Harbord R.M. Funnel plots in meta-analysis. STATA Journal: Promoting communications on statistics and Stata. 2004;4:127–141. doi: 10.1177/1536867X0400400204. [DOI] [Google Scholar]
- Stern Y., Gurland B., Tatemichi T.K. Influence of education and occupation on the incidence of Alzheimer's disease. Journal of the American Medical Association. 1994;271:1004–1010. [PubMed] [Google Scholar]
- Sullivan K.J., Dodge H.H., Hughes T.F. Declining incident dementia rates across four population-based birth cohorts. Journal of Gerontology: Series A. 2019;74:1439–1445. doi: 10.1093/gerona/gly236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo S., Kivipelto M., Hänninen T. Incidence and risk factors for mild cognitive impairment: A population-based three-year follow-up study of cognitively healthy elderly subjects. Dementia and Geriatric Cognitive Disorders. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- Then F.S., Luck T., Angermeyer M.C. Education as protector against dementia, but what exactly do we mean by education? Age and Ageing. 2016;45:523–528. doi: 10.1093/ageing/afw049. [DOI] [PubMed] [Google Scholar]
- Tyas S.L., Manfreda J., Strain L.A. Risk factors for Alzheimer's disease: A population-based, longitudinal study in manitoba, Canada. International Journal of Epidemiology. 2001;30:590–597. doi: 10.1093/ije/30.3.590. [DOI] [PubMed] [Google Scholar]
- Unverzagt F.W., Guey L.T., Jones R.N. ACTIVE cognitive training and rates of incident dementia. Journal of the International Neuropsychological Society. 2012;18:669–677. doi: 10.1017/S1355617711001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt F.W., Ogunniyi A., Taler V. Incidence and risk factors for cognitive impairment no dementia and mild cognitive impairment in african Americans. Alzheimer Disease and Associated Disorders. 2011;25:4–10. doi: 10.1097/WAD.0b013e3181f1c8b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M., Brayne C., Sachdev P. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. American Journal of Epidemiology. 2011;173:1004–1012. doi: 10.1093/aje/kwq476. [DOI] [PubMed] [Google Scholar]
- Valenzuela M.J., Sachdev P. Brain reserve and dementia: A systematic review. Psychological Medicine. 2005;36:441. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Xu W., Tan L., Wang H.-F. Education and risk of dementia: Dose-response meta-analysis of prospective cohort studies. Molecular Neurobiology. 2016;53:3113–3123. doi: 10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]
- Yoshitake T., Kiyohara Y., Kato I. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: The hisayama study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- Yuan J., Zhang Z., Wen H. Incidence of dementia and subtypes: A cohort study in four regions in China. Alzheimer's and Dementia. 2016;12:262–271. doi: 10.1016/j.jalz.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Yu L., Wilson R.S., Schneider J.A. Financial and health literacy predict incident Alzheimer's disease dementia and pathology. Journal of Alzheimer's Disease. 2017;56:1485–1493. doi: 10.3233/JAD-161132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne L.B., Schupf N., Brickman A.M. Dementia risk and protective factors differ in the context of memory trajectory groups. Journal of Alzheimer's Disease. 2016;52:1013–1020. doi: 10.3233/JAD-151114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A., Haan M.N., Neuhaus J.M. Cardiovascular risk score, cognitive decline, and dementia in older Mexican Americans: The role of sex and education. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Katzman R., Yu E. A preliminary analysis of incidence of dementia in Shanghai, China. Psychiatry and Clinical Neurosciences. 1998;52:S291–S294. doi: 10.1111/j.1440-1819.1998.tb03248.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.