Abstract
Leishmaniasis is a major neglected tropical disease which contributes a huge economic burden on already meager economic resources. The World Health Organization (WHO) has estimated an annual incidence of 700,000--1,000,000 patients and about 20,000–30,000 deaths per year. Approximately 66,941 patients of cutaneous leishmaniasis are reported annually in the Americas. In recent years, Nicaragua has presented alarmingly high numbers of patients and elevated incidence rates. Unfortunately, there are no detailed spatial descriptions on the epidemiological situation of leishmaniasis in this country. The objective of this study is to present descriptive data about the epidemiology of leishmaniasis in Nicaragua in the context of the distribution of this neglected tropical disease (NTD) in the Americas. This paper also provides an epidemiological update on different forms of leishmaniasis found in the three administrative regions of Nicaragua and its municipalities. Health authorities from the Ministry of Health of Nicaragua (MINSA) provided the entomological and epidemiological information for the different forms of the disease from 2001 to 2018. Prevalence, incidence rates, clinical classification of disease, age groups, sex, and geographic distribution by municipality and department are described in this study. Approximately 90%–95% of the national patients corresponded to CL and 5–10% correspond to MCL. The disease is distributed in the three regions of the country, with a higher burden in the Departments of Jinotega, Matagalpa and Atlántico Norte. The municipalities with the highest proportion of patients were El Cuá (23.92%), Waslala (14.16), Santa Maria de Pantasma (9.62%), Rancho Grande (9.03%) and Siuna (7.67%). There is an expansion of spatial distribution of CL and MCL in the North Central and South Atlantic regions of the country. These results could inform interventional strategies to address the burden of leishmaniasis in Nicaragua, which would improve the likelihood of meeting the goals for the Leishmaniasis Plan of Action for the Americas.
Keywords: Leishmaniasis, Epidemiology, The Americas, Nicaragua
1. Introduction
Leishmaniasis are a group of multiorgan diseases caused by more than twenty species of the parasite Leishmania spp. This microorganism is transmitted through the bite of female sand-flies of the genus Lutzomyia in the New World and Phlebotomus in the Old World. The disease comprises a variety of clinical syndromes that include cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL) and visceral leishmaniasis (VL) (Torres-Guerrero et al., 2017). All persons exposed to the vector are susceptible to infection, especially men because of occupational exposure (farmers, military, and loggers) and children because of their immunological susceptibility (World Health Organization, 2020a). CL and MCL usually involve uncovered areas of the body, such as face and extremities (Savoia, 2015). Even after healing, the scars of these lesions can disfigure a person's face, leading to social stigma and psychological repercussions (Handler et al., 2015). On the other hand, VL mainly affects internal organs, such as liver and spleen. It has a high case–fatality rate (10–20%), making it the second most dangerous parasitic tropical disease after malaria (Turan et al., 2015).
As a major neglected tropical disease (NTD), a zoonosis and a vector transmitted infection, leishmaniasis is an extremely difficult burden to control in developing nations (Kamhawi, 2017). The WHO calculates an incidence of 700,000-1,000,000 patients per year and 20,000–30,000 deaths (Ministerio de Salud, 2019). Different from the reported incidence of other NTDs, the incidence of leishmaniasis is increasing, most likely due to improvements in the access to diagnosis and treatment (Georgiadou et al., 2015). More than 90% of the total number of leishmaniasis patients in the world are reported in six countries: Brazil, Ethiopia, India, Somalia, South Sudan, and Sudan (World Health Organization, 2020b).
Approximately 66,941 patients of CL are reported annually in the Americas which accounts for approximately 30% of the total patients of CL worldwide (Pan American Health Organization, 2019). The countries with the highest number of patients are Brazil, Peru, and Colombia (Cruz-Chan et al., 2015) (WHO, 2016). In Central America, 39,000 patients are reported annually, which corresponds to 12% of Latin America's annual incidence. Most of the patients are reported in Nicaragua, Panama, and Costa RicSa (Hotez et al., 2014).
The main vectors in the Central American region are Lutzomyia spp., which are known as “chirizo,” “rodador” or “papalomoyo” in Nicaragua. More than 30 species of Lutzomyia and one species of Brumptomyia have been reported in the Nicaraguan territory (Cruz-Chan et al., 2015) (Raymond et al., 2010). The anthropophilic species responsible for the transmission of CL and MCL in the country are Lu. trapidoi, Lu. ylephyletor, Lu. cruciata, Lu. shannoni, Lu. gomezi and Lu. panamensis (Maroli et al., 2013). VL is transmitted by Lu. longipalpis and Lu. evansi. Lu. longipalpis and Lu. evansi is mainly found in the pacific plains of the country where atypical cutaneous leishmaniasis is endemic. The vectors described in the central highlands and the Atlantic plains are Lu. barrettoi majuscule, Lu. shannoni and Lu. panamensis. Additionally, Lu. panamensis was isolated from El Cua, El Balsamo, and Rosa Grande and is considered a suspected vector of CL in the central highlands and northern region (Pan American Health Organization, 2018).
The clinical form of the disease depends greatly on the subspecies of the parasite and the genetic and immunologic factors of the human host (Torres-Guerrero et al., 2017). The localized cutaneous leishmaniasis (CL) patients in the country are mainly caused by Leishmania mexicana while MCL is mainly caused by L. braziliensis and L. panamensis. The patients of atypical cutaneous leishmaniasis are caused by Leishmania infantum (syn. L. chagasi) (Belli et al., 1999). This last species was also the agent of VL before its elimination from Nicaragua. L. mexicana, L. braziliensis, and L. panamensis are transmitted mainly in the northern central highlands (Jinotega and Matagalpa) and Atlantic region. Conversely, atypical cutaneous leishmaniasis caused by L. infantum (syn. L. chagasi) has been frequently diagnosed in the pacific plain and southern central region (Maroli et al., 2013). The etiologic agents for cutaneous leishmaniasis may coexist with other species present in the pacific and southern regions. Patients of atypical cutaneous leishmaniasis are mainly distributed in the municipalities of Leon, Madriz and Chontales (Belli et al., 1999). The transmission cycle of leishmaniasis in Nicaragua is mainly peri domiciliary and intradomiciliary in peri/urban regions. The reservoirs identified in these areas include opossums (Didelphis spp.), sloths (Choloepus spp., Bradypus spp.), rodents (Rattus spp., Proechimys spp., Nectomys spp.), and dogs (Cruz-Chan et al., 2015).
Four forms of the disease have been described in Nicaragua since year 2001: CL, MCL, atypical cutaneous leishmaniasis, and VL. Most of the time, CL clinical signs appear after 2–8 weeks of incubation, as localized erythematous lesions at the sand-fly biting site. Subsequently, a macule develops in the same location followed by a papule and a nodule that will eventually ulcerate to form a typical localized cutaneous ulcerative lesion (Reithinger et al., 2007). These ulcers usually present hardened and elevated borders with a central erosion of the skin covered by a scab (Convit et al., 2006). Sometimes the lesions disappear spontaneously after 3–18 months, leaving behind atrophic/cribriform hypopigmented scars (Savoia, 2015). When the lesion affects the ear and its cartilage, it is called chiclero ulcer. When the lesions are not localized, they can appear as multiple diffuse nodular lesions in the face or trunk. Atypical cutaneous leishmaniasis is also known as the nodular or tuberculoid form of the disease and it is characterized by presenting diffused papules and nodules in uncovered areas of the body (Handler et al., 2015). The nodules can vary in size (3–30 mm), they are soft to the touch and will not ulcerate. Nicaragua presents one of the highest incidence rates for atypical cutaneous leishmaniasis, possibly due to the high content of inorganic volcanic particles in the skin of inhabitants. Convit et al. detected a high concentration of silica and aluminum in biopsies from patients with atypical nodular lesions of CL from Nicaragua, Honduras, and Guatemala (Convit et al., 2006). The presence of these inorganic particles in lesions from areas of Central American countries characterized by the presence of large amounts of volcanic ash suggests that environmental factors may contribute significantly to the frequency and clinical manifestations of this type of leishmaniasis (Convit et al., 2006). Other studies reported that leprosy caused by Mycobacterium leprae was found in many children and adults with atypical cutaneous leishmaniasis lesions (Soto et al., 2017). Additionally, Soto et al. detected individuals with a coinfection of Leishmania spp. and Mycobacterium leprae in blood and nasal swabs from Nicaragua and Honduras. The relationship between these coinfections still needs to be elucidated (Soto et al., 2017). MCL is a metastatic form of CL in the New World. The incubation period can vary from 1 to 3 months or even years after the scaring of the initial ulcer (Handler et al., 2015). These lesions can invade nasal mucosa and disseminate to oral, pharyngeal, laryngeal, or even lip mucosa. Complications of this clinical form are hoarseness, loss of voice, odynophagia, dysphagia, cachexia, over infection and death (Handler et al., 2015).
General reports have described the distribution of leishmaniasis in the Americas and the world in previous years (Pan American Health Organization, 2019) (Alvar et al., 2012). Alvar et al. documented an in-depth global estimate of the burden of leishmaniasis from 2007 to 2011 that revealed a high incidence and mild underreporting of leishmaniasis in Nicaragua (Alvar et al., 2012). Unfortunately, there are no detailed spatial descriptions on the epidemiological situation of leishmaniasis in Nicaragua. The objective of this study is to present descriptive data about the epidemiology of leishmaniasis in Nicaragua in the context of the distribution of this NTD in the Americas. This study also provides an epidemiological update on different forms of leishmaniasis found in the three administrative regions of Nicaragua and its municipalities. The descriptive information provided in this report could be of interest for local and international public health professionals and institutions as a background for the design of more advanced studies and interventions to control leishmaniasis in the most affected country of Central America.
2. Materials and methods
This clinical-epidemiological review was made through a non-systematic revision of Pubmed publications related to the keywords leishmaniasis, epidemiology, clinical manifestations, and prevention, from 2006 to 2018. Epidemiological information was collected from the World Health Organization (WHO) and the Pan-American Health Organization (PAHO) to draw a general overview of the disease in Nicaragua. Regional and local reports in English and Spanish were considered to report statistical information. Health authorities from the Ministry of Health of Nicaragua (MINSA) provided the entomological and epidemiological information for the different forms of the disease from 2001 to 2018. Prevalence, incidence rates, clinical classification of disease, age groups, sex, and geographic distribution by municipality and department were described in this study. The geospatial data was represented through ArcGIS version 10.6.1 (http://www.esri.com/arcgis).
2.1. Study area
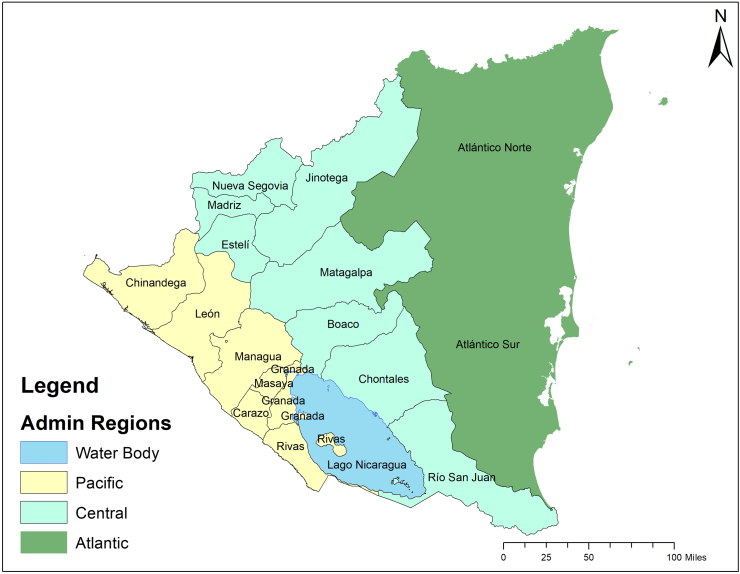
The study site is the Republic of Nicaragua, which is the largest country in the Central American isthmus, bordered by Honduras to the northwest, the Caribbean to the east, Costa Rica to the south, and the Pacific Ocean to the southwest. It is located between latitude 10° and 15° 45′ North and longitude 79° 30′ and 88° West. The nation occupies a landmass of 130,967 km2 (50,567 sq. mi) and contains a population of 6460, 411 inhabitants. Nicaragua has three distinct geographical regions: The Pacific lowlands, the Amerrisque Mountains (North-central highlands), and the Mosquito Coast (Atlantic lowlands/Caribbean lowlands) (Fig. 1). The country is divided into 17 administrative departments and 139 municipalities (Supplementary materials).
Fig. 1.
Administrative Regions of Nicaragua. Source: Adapted from Instituto Nicaragüense de Estudios Territoriales. INETER. (https://www.ineter.gob.ni/)
3. Results
3.1. Case detection and diagnosis
Leishmaniasis patients are actively detected through epidemiological and clinical diagnosis by a community network of health workers called ESAFC (Equipo de Salud Familiar y Comunitario) at the community level or the daily consultation of patients (Organización Panamericana de la Salud/Organización Mundial de la Salud, 2019). The ESAFC consists of doctors, nurses and social workers that are distributed in each SILAIS, by municipality and department. The Leishmania spp. Parasite is usually present in the cutaneous borders of the ulcer and mucosa. Therefore, the smear sample is obtained by scrape, punch, or aspiration biopsy from the borders of the most recent lesion. The smear samples are studied at the municipal or departmental level using Giemsa stain to detect the protozoan by microscopy. If the smear results are negative, new samples are sent to the Departmental or Central level to do culture or molecular studies of the tissue (Ministerio de Salud, 2014a). The parasite culture is done with the tissue sampled from the patient using the special media (Novy-McNeal-Nicole) for Leishmania spp. The culture can reveal flagellated forms of the parasite or promastigotes. In the case of epidemiological and clinical suspicion of VL, it is recommended to test the patient's serum using the Ag-rK39 immunochromatographic test. Another test that is frequently used is the Intradermal Leishmanin Test or Montenegro Test, which is an injection of killed Leishmania spp. promastigotes that will induce a Delayed-Type Hypersensitivity response in persons with previous exposure to the Leishmania parasite. This test usually appears negative in persons with no previous exposure to the parasite or anergic patients, and it is positive in persons with a history of localized cutaneous lesions. A positive result appears as a subcutaneous induration (>5 mm after 72 h) at the injection site (Torres-Guerrero et al., 2017). A person can be positive after three months of the initial infection and it can remain this way indefinitely. Indirect Immunofluorescence and PCR (Polymerase Chain Reaction) are done at the central level in Managua at the CNDR (Centro Nacional de Diagnóstico y Referencia) (Ministerio de Salud, 2014a). Biopsy specimens and/or scrapings of cutaneous or mucosal ulcerations are taken from the border of the most recent lesion and used as PCR samples (Ministerio de Salud, 2014a). Ninety-five percent of the patients included in this study were diagnosed by clinical examination in endemic areas, followed by microscopic examination of the tissue sample and PCR at the CNDR. Approximately 5% of the patients were diagnosed only by clinical and microscopic examination of the tissue sample due to geographic and logistical difficulties of shipping samples to Managua for specialized diagnosis at CNDR (Ministerio de Salud, 2014a).
3.2. Epidemiology
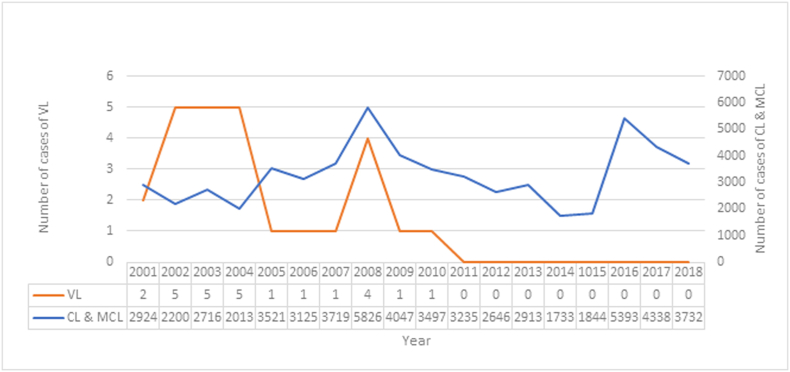
During 2001–2018, the Republic of Nicaragua reported 59,448 patients (Fig. 2). Approximately 90%–95% of the national patients corresponded to CL and 5–10% correspond to MCL. The disease is distributed in the three regions of the country, with a higher burden in the Central region, followed by the Atlantic and the Pacific (Ministerio de Salud, 2019). Twenty-six patients of VL were reported from 2001 to 2010. No more patients of VL have been reported after this period (Fig. 2, Fig. 3, Fig. 4). The increase in the amount of cases of CL reported in 2008 and 2016 (Fig. 3) was responsible for the peaks observed in the overall leishmaniasis case count in Fig. 2. The burden that MCL and atypical cutaneous leishmaniasis produce the country (Fig. 4) is not enough to cause drastic changes in the overall case count for all forms of leishmaniasis (Fig. 2).
Fig. 2.
Number of patients with visceral leishmaniasis (VL) and cutaneous & mucocutanoeus leishmaniasis (CL & MCL) in Nicaragua. 2001–2018.
(Source: Pan-American Health Organization. Ministry of Health. Managua, Nicaragua.)
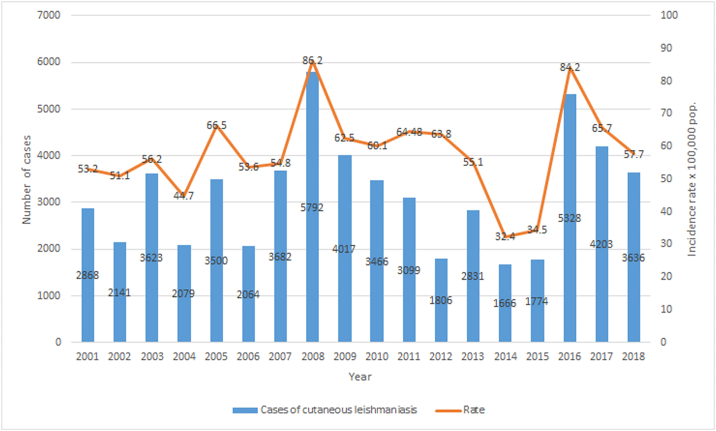
Fig. 3.
Number of Patients and Incidence Rates of localized cutaneous leishmaniasis in Nicaragua. 2013–2018.
(Source: Ministry of Health. Managua, Nicaragua.)
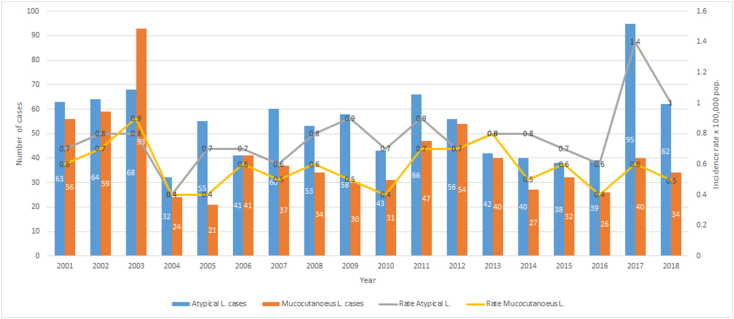
Fig. 4.
Number of Patients and Incidence Rates of localized atypical and mucocutaneous leishmaniasis in Nicaragua. 2013–2018.
(Source: Ministry of Health. Managua, Nicaragua.)
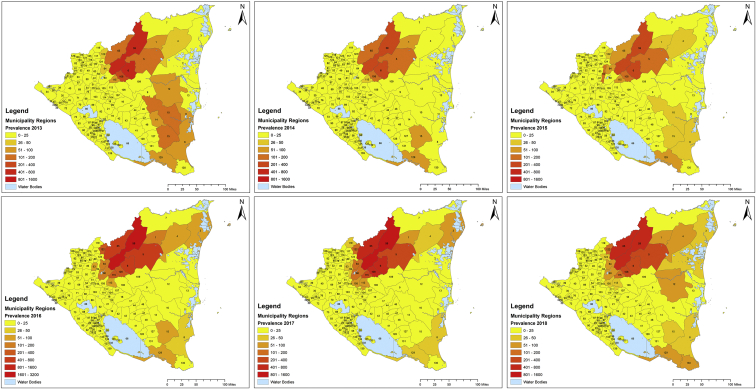
In 2011 and 2012 Nicaragua presented the second highest incidence rate in the Americas with 65.38 and 65.9 patients per 100,000 population, respectively. Almost one-half of the patients reported in 2011 corresponded to children under 10 years old (49.1%). In 2013, Nicaragua reported 59 patients per 100,000 population and total case count of 2913 (Fig. 5). Apart from detecting patients in the North-Central Region of the country, the MINSA detected a wide distribution of the disease the Atlántico Norte Department (Fig. 5). In 2014, Nicaragua registered the highest incidence rate out of 16 Latin American countries studied by PAHO for that year (62.97 patients per 100,000 habitants) with a predominance in Jinotega and Matagalpa, as show in Fig. 5 (Pan American Health Organization, 2014). The leishmaniasis composite indicator (which considers the average number of cases and incidence from the past three years for CL and VL) remained intense for the year 2015 and the disease showed similar distribution as in previous years (Fig. 5). The composite indicator measures the mortality and morbidity effects of leishmaniasis into a single indicator that can serve as a tool to compare the burden of the disease across populations. In 2016, the incidence rate dramatically increased to 84.30/100,000 inhabitants. 5393 patients of leishmaniasis were reported in the ¨Sistemas Locales de Atención Integral en Salud¨ (SILAIS) of Jinotega (2378), Matagalpa (1544), Las Minas (651) and Nueva Segovia (415). The municipalities that presented the highest prevalence were El Cuá (965), San José de Bocay (867), Rancho Grande (384) and Wiwilí (383) (Ministerio de Salud, 2019). This increase in the number of patients was recorded as the highest rate in the Americas due to an outbreak in El Cuá, a town located in the Jinotega Department (Fig. 5). This phenomenon was probably due to environmental factors that facilitated the reproduction of Lutzomyia spp. in endemic areas, such as the increase in moisture caused by the presence of hurricanes and tropical storms and to the increase in the screening by social service physicians during that year (La Prensa, 2017) (Ministerio de Salud, 2016). The high altitude, sylvatic landscape and abundance of vectors and reservoirs present in the Central and Atlantic Regions of the country may serve as a risk factor for the population at risk. In 2017, the incidence rate was 65.90/100,000 inhabitants with a total prevalence of 4338 patients. Most of the patients were reported in San José de Bocay (657), Rancho Grande (530), El Cuá (527), and Waslala (452), as presented in Fig. 5 2018 had a slight decline in new patients with an incidence rate of 57.67 per 100,000 inhabitants. Most of the patients were reported in the North Central (76.54%) and Atlantic (21.63%) regions of the country (Fig. 8). The least number of patients were registered in the Central and South Pacific regions (1.83%). Fifteen administrative departments presented patients of leishmaniasis. Most of the patients were reported in the municipalities of San Jose de Bocay (510), El Cua (441) and Rancho Grande (379) in the central region; Siuna (341), Waslala (314) and Bonanza (72) in the Atlantic region; Telica (Hotez et al., 2014) and Managua (World Health Organization, 2020b) in the pacific region. CL and MCL were reported in 90 municipalities of Nicaragua in 2018 (Fig. 5).
Fig. 5.
Prevalence of cutaneous leishmaniasis by Municipalities in Nicaragua, 2013–2018.*.
*This image presents the spatial distribution of case counts from 2013 to 2018. The number assigned to each municipality can be found in Supplementary materials.
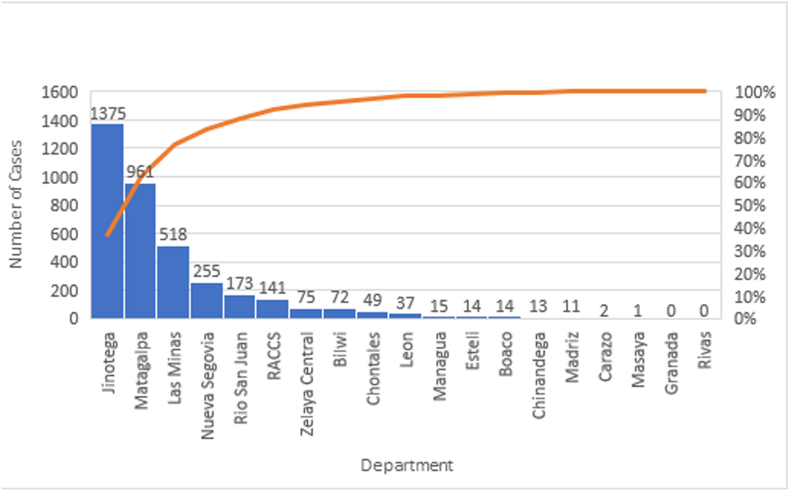
Fig. 8.
Prevalence of cutaneous leishmaniasis by Department in Nicaragua, 2018.
Source: Adapted from Mapa de Padecimientos. MINSA. (http://mapasalud.minsa.gob.ni/mapa-de-padecimientos-de-salud-de-nicaragua/)
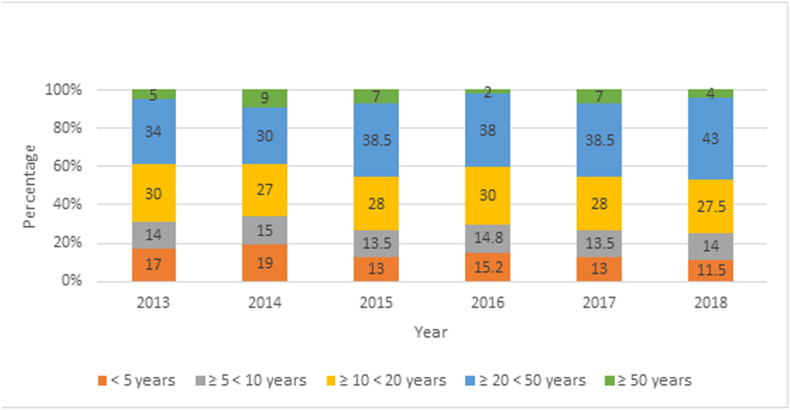
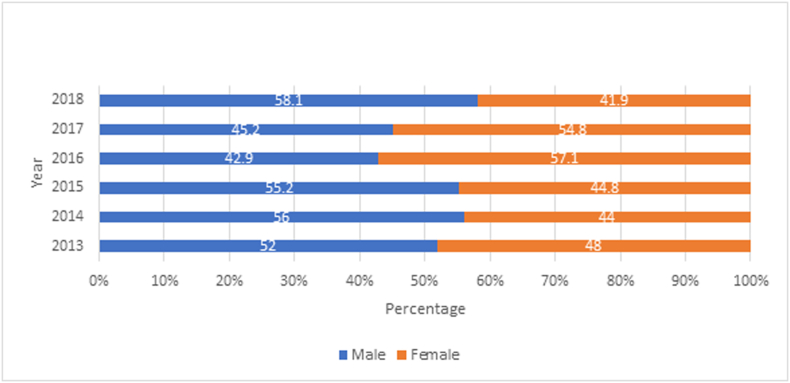
From 2013 to 2018, the disease was detected in the three regions of the country, with a higher burden in the Departments of Jinotega, Matagalpa and Atlántico Norte. The municipalities with the highest proportion of patients were El Cuá (23.92%), Waslala (14.16), Santa Maria de Pantasma (9.62%), Rancho Grande (9.03%) and Siuna (7.67%). 14.78% of the patients corresponded to children under 5 years old and 14.13% to children ≥5 <10 years of age. 71.8% of the patients reported during this period were 10 or more years old (Fig. 6). The disease is almost evenly distributed among sexes, with a slight predominance in males over females (Fig. 7). Females aged 1–9 were more likely to present leishmaniasis lesions than males in the same age range for the year 2018. The similar proportion in this age range in both sexes is probably due to intra domiciliary exposure during childhood. After the first decade of life, males represented the majority of leishmaniasis patients in comparison with females (Fig. 6, Fig. 7). Laboral exposure to the vector is a major risk factor of infection during adulthood in Nicaragua.
Fig. 6.
Proportion of cutaneous and mucocutaneous leishmaniasis by age group in Nicaragua, 2013–2018.
(Source: Ministry of Health. Managua, Nicaragua.)
Fig. 7.
Proportion of cutaneous and mucocutaneous leishmaniasis by sex in Nicaragua, 2013–2018.
(Source: Ministry of Health. Managua, Nicaragua.)
3.3. Treatment
The pharmacological treatment available in Nicaragua for uncomplicated CL and MCL is meglumine antimoniate (Glucantime). The recommended dosage for CL and MCL is 20 mg/kg IM or IV for 20 days and 20 mg/kg IV or IM for 28 days, respectively (Ministerio de Salud, 2014a). The intramuscular injection should be applied in the gluteus maximus. Intralesional treatment with glucantime is recommended in patients with contraindications for systemic treatment or when the cutaneous lesion presents the following characteristics: single ulcer over 900 mm2 (in any location, except the head and joint regions), absence of immunosuppression and a high tolerance for its application (Ministerio de Salud, 2014a). CL responds better and faster to glucantime than the MCL form. The most common adverse effects of the systemic medication are anorexia, nausea, vomiting, abdominal pain, myalgia, arthralgia, and lethargy (Ministerio de Salud, 2014b). Electrocardiographic alterations such as wave T inversion, QT interval prolongation, and arrhythmias, can depend on the dosage of the drug and duration of treatment. Therefore, blood tests and electrocardiograms are indicated during treatment (Ministerio de Salud, 2016). Glucantime is contraindicated in pregnant women and persons with cardiac, hepatic, or severe renal diseases. Amphotericin B (AmBisome) is reserved for therapeutic failure to glucantime and special patients, such as immunodeficiencies, the elderly, or patients of visceral leishmaniasis. It is common to present chills, fever, and thrombophlebitis after the infusion of AmBisome. Discontinuation of treatment is recommended when nephrotoxicity is detected (Ministerio de Salud, 2014a).
3.4. Prevention
Leishmaniasis was included in the list of Mandatory Notifiable Disease in 1980 by the MINSA. Ever since, the disease is reported regularly through the ENO (Enfermedad de Notificatión Obligatoria) form (Ministerio de Salud, 2014b). The document is then inputted into an electronic database by epidemiologists at the municipal and departmental level. The MINSA uses a community model called MOSAFC (Modelo de Salud Familiar y Comunitario) which focuses the prevention at the community level (Ministerio de Salud, 2014b). Prevention of the disease starts with the education of the population at risk by the ESAFC. This team is also in charge of conducting field investigations to detect the focus of peri-domiciliary/intra-domiciliary infection in the community. The ESAFC team needs to wear protective clothes, repellent and impregnated bed nets with deltamethrin/lambda-cyhalothrin when studying an outbreak in the communities. When the peri-domiciliary transmission is detected, the epidemiologist of the municipality must identify infected dogs and other animals that can serve as a reservoir for the infection (Ministerio de Salud, 2014b). It is recommended to spray with deltamethrin or lambda-cyhalothrin all pig pens, barns corrals, etc. When the transmission is intra-domiciliary the social workers must spray the interior walls with residual insecticides and provide the family with impregnated bed nets with deltamethrin/lambda-cyhalothrin (Ministerio de Salud, 2014b).
4. Discussion
This study portrays epidemiological data for the Republic of Nicaragua from 2001 to 2018. Prevalence, incidence rates, clinical classification of disease, age groups, sex and geographic distribution by municipality and department were described. Approximately 90%–95% of the national patients corresponded to CL and 5–10% correspond to MCL. The disease is distributed in the three regions of the country, with a higher burden in the Departments of Jinotega, Matagalpa, and Atlántico Norte. The municipalities with the highest proportion of patients were El Cuá, Waslala, Santa Maria de Pantasma, Rancho Grande, and Siuna.
In Nicaragua, the MINSA and PAHO are currently implementing strategies to maintain zero patients of VL and reduce the incidence of atypical cutaneous leishmaniasis for future years by decreasing the number of sylvatic and domiciliary reservoirs (Organización Panamericana de la Salud/Organización Mundial de la Salud, 2019). However, even with several control measures being employed, the PAHO still considers Nicaragua a high-intensity country for CL due to an extremely high yearly incidence rate, a rating Nicaragua has failed to dissociate from for over a decade (Maia-Elkhoury et al., 2016). In fact, the country has presented more than two-thousand patients per year for more than 15 years with peaks of leishmaniasis prevalence detected in 2008 and 2016 (Fig. 2, Fig. 3, Fig. 4). This phenomenon may be due to an increase in diagnosed patients by social service nurses and physicians during those years. It is important to consider that most of the patients were reported in the poorest municipalities of the country, where underreporting issues still remain due to difficulties in accessing population, low number of health care personnel, and low number of health care infrastructure. Alvar et al. estimated mild levels of underreporting for cutaneous leishmaniasis (2.8–4.6-fold) and visceral leishmaniasis (1.2–1.8-fold) through epidemiological questionnaires to national/international experts and literature searches (Alvar et al., 2012). These results suggest that leishmaniasis is a bigger burden for Nicaragua and that it is necessary to measure the magnitude of underestimation within the country to fully understand the true picture of the disease.
About 40–45% of the Nicaraguan population lives in high transmission zones, such as sylvatic or mountainous areas that are potential transmission sites for leishmaniasis. Furthermore, intense deforestation has been reported in the Central and Atlantic regions of the country (Liscow ZD, 2013) (Gourdji et al., 2015), which may increase the prevalence of the disease due to the alteration of the ecosystems and human invasion of territories with high vector density (Purse et al., 2017). Most of the persons living in Central or Atlantic regions of the country acquire the disease after the first ten years of age, doing agricultural or military work. After the first decade of life, the disease becomes predominantly a male infection due to occupational exposure to the bites of sand-flies in coffee or cacao plantations in endemic regions of the country, such as Jinotega and Matagalpa (Fig. 8). The clinical manifestation in females is mainly detected when they are less than ten years of age (13.0%). The percentage of affected women in Nicaragua is 41.9% as compared to 58.1% for men in 2018. This data suggests a high domicile or peri-domicile infection rate for women and a high infection rate for economically active men working in agricultural settings (Fig. 5, Fig. 6). The relationship between poverty and leishmaniasis is very complex: if the poverty of a region increases, the risk of acquiring leishmaniasis and presenting severe forms of the disease, also increases. Additionally, the disease by itself can exacerbate the poverty in affected families due to high expenditure in healthcare, the inability of family providers to work in the field of even high mobility/mortality for family members (Okwor and Uzonna, 2016). In Nicaragua there is a steady internal rural-rural migration stream due to seasonal labour for the agricultural industry from the Atlantic coast to the Central region and vice versa (World Health Organization, 2010). There is also a stable migration from rural areas of the Atlántico Norte and Atlántico Sur to urban cities in the central and pacific regions This migratory flow of persons from endemic regions to non-endemic municipalities can change the distribution of the disease as seen in Fig. 5. In addition to the socioeconomic and migratory factors that play a role in the high burden of leishmaniasis in Nicaragua, it is also important to consider the environmental variables that facilitate the transmission of the infection in endemic areas. Different studies have shown that environmental factors, such as temperature, elevation, topography, and vegetation may create a perfect habitat for the vector and intermediate hosts of the parasite and therefore enhance the transmission of the disease (Macours and Vakis, 2010). It is possible that the high elevation, vegetation, and humidity of the endemic areas are directly linked to a high number of Lutzomyia spp. habitats, a high number of non-human hosts (especially dogs), and human infection.
The price of the medication for leishmaniasis is relatively expensive and it causes a high economic burden for endemic countries with very high incidence rates (World Health Organization, 2010). As mentioned above, Nicaragua's treatment regime focusses on meglumine antimoniate (Glucantime) and Amphotericin B (AmBisome) only, without considering other PAHO recommended drugs such as miltefosine which usually is not used due to its unavailability in the region (Macours and Vakis, 2010). Control strategies should always consider the socio-economic context of the country and the affected communities. Since CL and MCL are not lethal diseases and prevention strategies for leishmaniasis are very costly and complex, the control strategies designed for these forms of leishmaniasis have been focused on the medical treatment and not on the control of non-human reservoirs and Lutzomyia species. Even though the prevention strategies used in Nicaragua are relatively effective, they could be enhanced to reduce the transmission of the disease in hotspots by using satellite images and remote sensing techniques. Health promotion, communication, education, and social mobilization should be a priority for the Regions of Jinotega, Matagalpa, and Atlántico Norte (Las Minas), which are the most affected departments (Fig. 8). Additionally, the supplies and medication for diagnosis and treatment should always be present in endemic regions, and the investigation for outbreaks must be performed promptly for successful control. PAHO/WHO recommendations are currently being followed to promote actions to reduce sources of infection for the vector, through entomological surveillance and integrated vector/canine surveillance (World Health Organization, 2019).
As a major NTD, a zoonosis, and a vector transmitted infection, leishmaniasis is an extremely difficult burden to control in developing nations. The disease presents complex characteristics in the Americas since it uses different intermediate hosts, parasite species, vector species and even clinical manifestations, which can complicate the diagnosis and clinical management. It is necessary to work towards analytical studies of disease outbreaks to create scientifically based strategies to control leishmaniasis at the local, regional, and national level. Determining the risks factors and environmental conditions that play a role in the transmission of leishmaniasis is necessary to create effective control measures in Nicaragua. It is imperative to continue building strong collaborations between government, non-profit organizations, pharmaceutical companies, and philanthropists to control and eradicate the different clinical forms of leishmaniasis worldwide.
5. Conclusions
National and international control efforts have been effective in the elimination of visceral leishmaniasis in Nicaragua since 2011. Unfortunately, there has been an expansion of the distribution of CL and MCL in the North Central and South Atlantic regions of the country. The historical data provided in this study suggests that there has been little or no improvement in the control of CL, MCL, and atypical cutaneous leishmaniasis from 2001 to 2018. These results could inform interventional strategies to address the burden of leishmaniasis in Nicaragua, which would improve the likelihood of meeting the goals described in the PAHO's Leishmaniasis Plan of Action for the Americas. A limitation of this study is that we could only retrieve the case counts of leishmaniasis for 2001–2012, without the data for sex distribution, age-range, and municipality. Health education initiatives for both healthcare personnel and the general population are extremely important to support the national leishmaniasis elimination plan. Optimized diagnostic and vector control measures are imperative for the control of leishmaniasis in Nicaragua.
Key messages
There has been an expansion of the distribution of CL and MCL in the North Central and South Atlantic regions of Nicaragua. Epidemiological and geospatial data could inform interventional strategies to address the burden of leishmaniasis in Nicaragua.
Acknowledgments
Acknowledgement
We thank Dr. Clara Isabel Gonzalez Moncada, Dr. Ana Gabriela Duarte, and MSc. Josefa Moran from UNAN-Managua for reviewing previous versions of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parepi.2020.e00192.
Contributor Information
Santiago E. Hernández-Bojorge, Email: santiagohern@usf.edu.
Ricardo Izurieta, Email: ricardoi@usf.edu.
Appendix A. Supplementary data
Supplementary material
References
- Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035671. [Internet]. e35671. Available from. May 31 [cited 2018 Oct 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli A., García D., Palacios X., Rodriguez B., Valle S., Videa E. Widespread atypical cutaneous Leishmaniasis caused by Leishmania (L.) Chagasi in Nicaragua. Am. J. Trop. Med. Hyg. 1999;61(3):380–385. doi: 10.4269/ajtmh.1999.61.380. https://www.ncbi.nlm.nih.gov/pubmed/10497975 [Internet]. Sep. Available from. [DOI] [PubMed] [Google Scholar]
- Convit J., Ulrich M., Castillo J., De Lima H., Pérez M., Caballero N. Inorganic particles in the skin of inhabitants of volcanic areas of Central America: their possible immunomodulatory influence in leishmaniasis and leprosy. Trans. R. Soc. Trop. Med. Hyg. 2006;100(8):734–739. doi: 10.1016/j.trstmh.2005.09.012. https://www.ncbi.nlm.nih.gov/pubmed/16406036 [Internet]. Aug. Available from. [DOI] [PubMed] [Google Scholar]
- Cruz-Chan J.V., Valenzuela J., Dumonteil E. Leishmaniasis in the Americas. In: Franco-Paredes C., Santos-Preciado J.I., editors. Neglected Tropical Diseases - Latin America and the Caribbean [Internet] Springer; Vienna: 2015. pp. 113–128.https://link.springer.com/chapter/10.1007%2F978-3-7091-1422-3_6 [cited 2020 Jan 20]. (Neglected Tropical Diseases). Available from. [DOI] [Google Scholar]
- Georgiadou S.P., Makaritsis K.P., Dalekos G.N. Leishmaniasis revisited: current aspects on epidemiology, diagnosis and treatment. J. Transl. Intern. Med. 2015;3(2):43–50. doi: 10.1515/jtim-2015-0002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936444/ [Internet]. [cited 2018 Nov 4]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdji S., Läderach P., Valle A.M., Martinez C.Z., Lobell D.B. Historical climate trends, deforestation, and maize and bean yields in Nicaragua. Agric. For. Meteorol. 2015;200:270–281. doi: 10.1016/j.agrformet.2014.10.002. http://www.sciencedirect.com/science/article/pii/S0168192314002536 [Internet]. Jan 15 [cited 2019 Nov 19]. Available from. [DOI] [Google Scholar]
- Handler M.Z., Patel P.A., Kapila R., Al-Qubati Y., Schwartz R.A. Cutaneous and mucocutaneous leishmaniasis: clinical perspectives. J. Am. Acad. Dermatol. 2015;73(6):897–908. doi: 10.1016/j.jaad.2014.08.051. https://www.ncbi.nlm.nih.gov/pubmed/26568335 [Internet]. Dec. quiz 909–10. Available from. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Woc-Colburn L., Bottazzi M.E. Neglected tropical diseases in Central America and Panama: review of their prevalence, populations at risk and impact on regional development. Int. J. Parasitol. 2014;44(9):597–603. doi: 10.1016/j.ijpara.2014.04.001. https://www.ncbi.nlm.nih.gov/pubmed/24846528 [Internet]. Aug. Available from. [DOI] [PubMed] [Google Scholar]
- Kamhawi S. The yin and yang of leishmaniasis control. PLoS Negl. Trop. Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005529. [Internet]. Available from. Apr 20 [cited 2018 Nov 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Prensa . La Prensa; 2017. 13 Muertos y más de 14 mil Personas Afectadas Tras el Paso de la Tormenta Nate en Nicaragua [Internet]https://www.laprensa.com.ni/2017/10/06/nacionales/2309258-en-vivo-estragos-en-nicaragua-tormenta-nate [cited 2020 Jan 22]. Available from. [Google Scholar]
- Liscow ZD Do property rights promote investment but cause deforestation? Quasi-experimental evidence from Nicaragua. J. Environ. Econ. Manag. 2013;65(2):241–261. doi: 10.1016/j.jeem.2012.07.001. http://www.sciencedirect.com/science/article/pii/S0095069612000642 [Internet]. Mar 1 [cited 2019 Nov 19]. Available from: [DOI] [Google Scholar]
- Macours K., Vakis R. Seasonal migration and early childhood development. World Dev. 2010;38(6):857–869. doi: 10.1016/j.worlddev.2010.02.012. Jun 1. Available from. [DOI] [Google Scholar]
- Maia-Elkhoury A.N.S., Yadón Z.E., Díaz M.I.S., Lucena De A.F., Castellanos L.G., Sanchez-Vazquez M.J. Exploring spatial and temporal distribution of cutaneous leishmaniasis in the Americas, 2001–2011. PLoS Negl. Trop. Dis. 2016;10(11) doi: 10.1371/journal.pntd.0005086. [Internet]. e0005086. Available from. Nov 8 [cited 2019 Nov 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroli M., Feliciangeli M.D., Bichaud L., Charrel R.N., Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013;27(2):123–147. doi: 10.1111/j.1365-2915.2012.01034. https://www.ncbi.nlm.nih.gov/pubmed/22924419 [Internet]. Jun. Available from: [DOI] [PubMed] [Google Scholar]
- Ministerio de Salud . Formulario Nacional de Medicamentos FNM. 7ma Edición [Internet] Ministerio de Salud; Managua: 2014. Antileishmaniásicos; pp. 100–103.http://www.minsa.gob.ni/index.php/repository/Descargas-MINSA/Divisi%C3%B3n-General-de-Insumos-M%C3%A9dicos/Formulario-Nacional-de-Medicamentos/Formulario-Nacional-de-Medicamentos-2014-7ma-Edeci%C3%B3n/ [cited 2019 Nov 20]. Available from. [Google Scholar]
- Ministerio de Salud . Ministerio de Salud; Managua: 2014. Manual de Procedimientos Para la Prevención, Control y Atención de las Leishmaniasis; pp. 23–24. Report No.: 132. [Google Scholar]
- Ministerio de Salud Dirección General de Docencia e Investigación. Ubicación de ingreso de médicos al servicio social. [Internet] 2016. http://www.minsa.gob.ni/index.php/repository/Descargas-MINSA/Direcci%C3%B3n-General-de-Docencia-e-Investigaci%C3%B3n/orderby,3/ [cited 2020 Jan 22]. Available from.
- Ministerio de Salud Mapa de Padecimientos de Salud de Nicaragua [Internet] 2019. http://mapasalud.minsa.gob.ni/mapa-de-padecimientos-de-salud-de-nicaragua/ [cited 2018 Jan 4]. Available from.
- Okwor I., Uzonna J. Social and economic burden of human leishmaniasis. Am. J. Trop. Med. Hyg. 2016;94(3):489–493. doi: 10.4269/ajtmh.15-0408. [Internet]. [cited 2018 Jan 23]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organización Panamericana de la Salud/Organización Mundial de la Salud . Pan American Health Organization/World Health Organization; 2019. Personal de Salud Consolida sus Competencias en Vigilancia e Investigación de Leishmaniasis Visceral y Cutánea Atípica [Internet]https://www.paho.org/nic/index.php?option=com_content&view=article&id=973:personal-de-salud-consolida-sus-competencias-en-vigilancia-e-investigacion-de-leishmaniasis-visceral-y-cutanea-atipica&Itemid=244 [cited 2019 Nov 19]. Available from. [Google Scholar]
- Pan American Health Organization . Pan American Health Organization/World Health Organization; Washington D.C: 2014. Leishmaniasis: Epidemiological Report of The Americas [Internet] pp. 1–4.http://iris.paho.org/xmlui/handle/123456789/51679 Report No.: 2. Available from. [Google Scholar]
- Pan American Health Organization . Pan American Health Organization/World Health Organization; Washington D.C: 2018. Leishmaniasis. Epidemiological Report of the Americas [Internet] pp. 1–6.http://iris.paho.org/xmlui/handle/123456789/34856 Report No.: 6. Available from. [Google Scholar]
- Pan American Health Organization . Pan American Health Organization/World Health Organization; Washington D.C: 2019. Leishmaniasis: Epidemiological Report in the Americas [Internet]http://iris.paho.org/xmlui/bitstream/handle/123456789/50505/Leishreport2019_eng.pdf?ua=1 Report No.: 7. Available from. [Google Scholar]
- Purse B.V., Masante D., Golding N., Pigott D., Day J.C., Ibañez-Bernal S. How will climate change pathways and mitigation options alter incidence of vector-borne diseases? A framework for leishmaniasis in South and Meso-America. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0183583. [Internet]. e0183583. Available from. Oct 11 [cited 2019 Nov 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond R.W., McHugh C.P., Kerr S.F. Sand flies of Nicaragua: a checklist and reports of new collections. Mem. Inst. Oswaldo Cruz. 2010;105(7):889–894. doi: 10.1590/s0074-02762010000700008. https://www.ncbi.nlm.nih.gov/pubmed/21120358 [Internet]. Nov. Available from. [DOI] [PubMed] [Google Scholar]
- Reithinger R., Dujardin J.-C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7(9):581–596. doi: 10.1016/S1473-3099(07)70209-8. https://www.ncbi.nlm.nih.gov/pubmed/17714672 [Internet]. Sep. Available from. [DOI] [PubMed] [Google Scholar]
- Savoia D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Ctries. 2015;9(6):588–596. doi: 10.3855/jidc.6833. https://www.ncbi.nlm.nih.gov/pubmed/26142667 [Internet]. Jul 4. Available from. [DOI] [PubMed] [Google Scholar]
- Soto L.A., Caballero N., Fuentes L.R., Muñoz P.T., Gómez Echevarría J.R., López M.P. Leprosy associated with atypical cutaneous leishmaniasis in Nicaragua and Honduras. Am. J. Trop. Med. Hyg. 2017;97(4):1103–1110. doi: 10.4269/ajtmh.16-0622. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5637581/ [Internet]. Oct 11 [cited 2019 Nov 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Guerrero E., Quintanilla-Cedillo M.R., Ruiz-Esmenjaud J., Arenas R. Leishmaniasis: a review. F1000Research. 2017;6 doi: 10.12688/f1000research.11120.1. [Internet]. Available from. May 26 [cited 2019 Nov 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan E., Kandemir H., Yeşilova Y., Ekinci S., Tanrıkulu O., Kandemir S.B. Assessment of psychiatric morbidity and quality of life in children and adolescents with cutaneous leishmaniasis and their parents. Adv. Dermatol. Allergol. Dermatol. Alergol. 2015;32(5):344–348. doi: 10.1371/journal.pone.0223313. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4692819/ [Internet]. Oct [cited 2018 Oct 16]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO [Internet] World Health Organization; 2016. WHO to implement online epidemiological surveillance for leishmaniasis.http://www.who.int/neglected_diseases/news/WHO_implement_epidemiological_surveillance_le [cited 3 Jun 2017]. Available. [Google Scholar]
- World Health Organization Control of the leishmaniases. World Health Organ. Tech. Rep. Ser. 2010;949:1–186. https://www.ncbi.nlm.nih.gov/pubmed/21485694 xii–xiii. back cover. Available from. [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2019. Access to Essential Antileishmanial Medicines and Treatment [Internet]http://www.who.int/leishmaniasis/research/en/ [cited 2019 Dec 4]. Available from: [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2020. Neglected Tropical Diseases [Internet]http://www.who.int/neglected_diseases/diseases/en/ [cited 2018 Oct 16]. Available from. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2020. Epidemiological Situation [Internet]http://www.who.int/leishmaniasis/burden/en/ [cited 2019 Dec 16]. Available from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material