Abstract
Prostate cancer (PCa) commonly metastasizes to the bone where the cells frequently undergo dormancy. The escape of disseminated tumor cells from cellular dormancy is a major cause of recurrence in marrow. Abscisic acid (ABA), a phytohormone, is known to regulate dormancy of plant seeds and to regulate other stress responses in plants. Recently, ABA was found to be synthesized by mammals cells and has been linked to human disease. Yet the role of ABA in regulating tumor dormancy or reactivation is unknown. We found that ABA is produced by human marrow cells, and exogenous ABA inhibits PCa cell proliferation while increasing the expression of p27, p21, and p16 and decreasing the expression of the proliferation marker, Ki67. Further, ABA significantly increased the percentage of PCa cells in the G0 phase of the cell cycle as well as the duration the cells were arrested in G0. We found that ABA regulates an increase of PPARγ receptor expression and suppressed phosphorylation of mTOR/p70S6K signaling and resulting in the induction of the cellular dormancy. We then confirmed that ABA regulates G0 cell cycle arrest through PPARγ receptor signaling in vitro and under co-culture conditions with osteoblasts. Finally, we demonstrate that ABA regulates PCa dormancy in vivo following intratibial injection in an animal model. Together these data suggest that the ABA and PPARγ signaling pathways contribute to the establishment of PCa cellular dormancy in the bone marrow microenvironment. These findings may suggest critical pathways for targeting metastatic disease.
Keywords: Prostate cancer, Disseminated tumor cells, Dormancy, Abscisic acid, PPARγ, Bone marrow microenvironment
Abbreviations: ABA, Abscisic acid; DTCs, Disseminated tumor cells; GAS6, Growth arrest specific 6; PCa, Prostate cancer
Introduction
Prostate cancer (PCa) commonly metastasizes to the bone [1]. PCa frequently takes more than 5 y to progress to biochemical recurrence and lethal metastatic disease after curative surgery or radiation therapy. These observations suggest that disseminated tumor cells (DTCs) may enter into a state of cellular dormancy for long periods within the bone marrow and possibly other sites [2], [3], [4], [5]. Once in the marrow, DTC dormancy and subsequent reactivation are likely to be governed by systemic and local signals derived from the metastatic microenvironment [6,7].
Previously, we established that osteoblast-secreted growth arrest specific 6 (GAS6) signaling through TAM receptors (Tyro3, AXL, and MER) expressed on PCa cells regulates dormancy. Specifically, we demonstrated that AXL signaling directly induces DTC quiescence [8,9], and that PCa cells which express low AXL/TYRO3 ratios escape from dormancy [10]. We also demonstrated that PCa cells binding to osteoblasts in the bone marrow induces a higher expression of TANK binding kinase 1 (TBK1), which induces dormancy (Ki67-negative cells) [11] and GAS6 significantly increases G1 arrested cells by altering the signaling networks associated with G1 arrest and S phase delay [12]. Further, GAS6-AXL signaling induces transforming growth factor beta (TGFβ) autocrine signaling to induce dormancy [13]. Conversely, MER signaling stimulates PCa dormancy escape through a MAP kinase dependent mechanism and loss of MER delays the formation of bone metastasis [14].
Other groups have also identified important regulators of dormancy [15], [16], [17]. Bone marrow-derived TGFβ2 activates p38, resulting in a high p38/ERK ratio (activation of p38 and inactivation of ERK signaling), which induces dormancy of head and neck squamous cell carcinoma cells [16]. TGFβ2-induced dormancy of malignant DTCs required TGFβ receptor-I (TGFβ-RI), TGFβ-RIII, and SMAD1/5 activation through induction of DEC2/SHARP1 and p27 and downregulation of CDK4 [16]. A recent report showed that downregulation of TGFβ2 is associated with escape from dormancy of PCa xenografts [17]. Moreover, the transcription factors NR2F1, NANOG, and retinoic acid receptor β were identified as transcriptional regulators of dormancy in head and neck, prostate, and breast cancers [15]. In addition it has been demonstrated that indolent cancer cells secrete a high level of secreted protein acidic and rich in cysteine (SPARC), which significantly stimulates expression of bone morphogenetic protein 7 (BMP7) in bone marrow stromal cells [18]. The secreted BMP7 regulates PCa dormancy by inducing senescence, reducing stemness, and activating dormancy-associated p38 MAPK signaling and p21 expression [19].
In this work, we sought to expand our understanding of the regulators of dormancy by exploring clues from other systems in which regulators of growth are known. Abscisic acid (ABA) was identified in the early 1960s as a phytohormone that plays a significant role in dormancy of plant seeds and other stress responses in plants [20], [21], [22], [23], [24], [25]. More recently, ABA has received considerable attention in mammalian cells and linked to human disease [26,27]. Growing evidence suggests that ABA is a new and crucial signaling regulator targeting mesenchymal stem cells (MSC) and hematopoietic stem cells (HSC) in the bone marrow microenvironment [26,28,29]. In addition, a few recent studies have demonstrated that ABA effectively inhibits the proliferation of tumor cells [26,27,30]. Yet the role of ABA in regulating metastatic tumor cell dormancy or reactivation in the bone marrow remains unknown.
In the present study, we explored the role of ABA on the induction of dormancy of PCa cells in the marrow. We demonstrate that ABA inhibits PCa cell proliferation and significantly increased G0 cell cycle arrest of PCa cells. Moreover, ABA regulates an increase in expression of the ABA receptor PPARγ, through suppression of phosphorylation of mTOR/p70S6K signaling during cellular dormancy [31,32]. We further demonstrate that ABA signaling through PPARγ contributes to PCa cellular dormancy in vitro when co-cultured with osteoblasts and in an in vivo intratibial animal model. Our results suggest that ABA and PPARγ signaling pathway contributes to the establishment of PCa cellular dormancy in bone marrow microenvironment.
Materials and methods
Cell cultures
Human PCa cell lines (LNCaP, PC3, and DU145) were obtained from the American Type Culture Collection (Rockville, MD). The metastatic subclone of LNCaP, C42B, was originally isolated from a lymph node of a PCa patient with disseminated bony and lymph node involvement. Murine osteoblast precursor cells (MC3T3-E1) were obtained from the American Type Culture Collection (Rockville, MD). All PCa cell lines were routinely grown in RPMI 1640 (Life Technologies, Carlsbad, CA), and MC3T3-E1 cells were grown in α-MEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS, GEMINI Bio-Products, Sacramento, CA), 1% penicillin-streptomycin (P/S, Life Technologies) and maintained at 37 °C, 5% CO2, and 100% humidity. Normal human prostate epithelial PNT2 cells (cat no. 95012613, Sigma, St. Louis, MO) were cultured in RPMI 1640, 2 mM glutamine (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, GEMINI Bio-Products, Sacramento, CA), 1% penicillin-streptomycin (P/S, Thermo Fisher Scientific, Waltham, MA). Human MSCs were obtained from Lonza (cat. PT-2501 Lonza, Walkersville, MD). Human osteoblasts (HOBs) were obtained using a modification of methods described by Taichman and Emerson [33]. Human bone marrow endothelial cells (HBMECs) were obtained using a modification of methods described by Masek and Sweetenham [34]. The human cells were cultured in DMEM (Life Technologies) supplemented with 10% FBS and 1% P/S.
Proliferation assays
PCa cell proliferation assays were performed in 1% FBS culture conditions with ABA treatment using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assays (cat no. G5421, Promega, Madison WI).
Cytotoxicity assays
PCa cell cytotoxicity assays were performed in 10% FBS culture condition with various doses of ABA treatment for 4 h by CytoTox96 Non-Radioactive Cytotoxicity Assays (cat no. G1780, Promega).
Cell cycle assays
We used gene delivery via lentivirus to stably integrate two different combinations of cell cycle reporters in the PC3 cell line (PC3-Venus-Cherry (PC3VC) cells [35]. Together these reporters permit us to monitor cell cycle dynamics, including active cycling and quiescence. In the first combination, we used the reporters generated by Oki et al [36] that distinguish quiescence (G0) from G1. The G0 reporter is a modified inactive form of p27 cyclin-dependent kinase inhibitor protein fused to “Venus” fluorescent protein (G0-Venus). G0-Venus is upregulated upon entry into quiescence and is tagged for degradation by Kip1 ubiquitination-promoting complex in late G1 and the Skp2 ubiquitin ligase in the G1-S transition [36]. Therefore, this reporter is high during G0, but low upon G1 entry and G1-S transition. The G1 reporter is based upon a portion of human Cdt1, a replication licensing gene [37], fused to “Cherry” fluorescent protein (G1-Cherry) [38]. Like endogenous Cdt1, this reporter is high during G0 and G1, but degraded during S-phase by Skp2-dependent degradation [37]. Together, these two reporters can be used to quantify proliferation vs quiescence. Cells that are in G0 exhibit both reporters with relatively stronger G0-Venus, while cells in G1 exhibit low G0-Venus with stronger G1-Cherry expression. This is visible by live imaging and quantifiable by flow cytometry, because the degradation of G0-Venus precedes the degradation of G1-Cherry upon cell cycle entry. Cell cycle monitoring was performed in PC3VC cell culture with direct ABA (50 µM) (cat no. ab120860, Abcam, Cambridge, MA) treatment.
In coculture experiments, PC3VC cells were cocultured with MC3T3-E1 cells in culture conditions of α-MEM and RPMI media (1:1 ratio) with 5% FBS and 1% penicillin-streptomycin following direct ABA (50 µM) treatment. For evaluating of cell cycle phase, live cells were selected by DAPI-negative cells (cat. NBP2-31156, DAPI, NOVUS) first, and then the live cells were negatively gated for anti-mouse H-2kd (cat no. 116622, PE/Cy7, BioLegend, San Diego, CA), which were then positively gated for HLA-A,B,C (cat no. 311426, APC/Cy7, BioLegend). After these gates were applied, cells were plotted as Venus (FITC) vs Cherry (PE-TxRed) for cell cycle analysis. All analyses were performed using a FACS Aria IIu three-laser flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and data were analyzed with FACS-DIVA software (Becton Dickinson).
Additionally, PC3VC cell imaging was captured by video. PC3VC cells were cultured for 24 h in RPMI with 10% FBS, 1% P/S and then, treated with vehicle or ABA (50 µM) for 24 h. Five spots of cells in each group were set for tracking. Video images were taken for 24 h at 15 min intervals using a Deltavision Elite Microscope (GE Healthcare Life Science, Pittsburgh, PA). The duration of G0 phase in single cells was measured (n = 20/group).
Ki67 staining and FACS analyses
Cells were fixed with cold 70% ethanol, and stained with an APC conjugated anti-human Ki67 antibody (cat no. 350513, BioLegend) in PBS containing 2% FBS for 30 min at room temperature. Ki67 negative PCa cells were examined using a FACS Aria IIu three-laser flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and data were analyzed with FACS-DIVA software (Becton Dickinson).
Quantitative RT-PCR
Total RNA was extracted from cells using the RNeasy mini or micro kit (Qiagen, Valencia, CA) and converted into cDNA using a First-Strand Synthesis Kit (Invitrogen). Quantitative PCR was performed on an ABI 7700 sequence detector (Applied Biosystems) using TaqMan Universal PCR Master Mix Kit (Applied Biosystems) according to the directions of manufacturer. TaqMan MGB probes, p27 (CDKN1B, Hs01597588_m1), p21 (CDKN1A, Hs99999142_m1), p16 (CDKN2A, Hs00923894_m1), Ki67 (Hs01032440_m1), and PPARγ (peroxisome proliferator-activated receptor γ, Hs01115513_m1) (Applied Biosystems) were used. β-actin (Hs01060665-g1) was used as an internal control for normalization of target gene expression.
Stable knockdown of PPARγ
Lentiviral particles with PPARγ human shRNA or negative control (cat no. TL320459V, Origene, Rockville, MD) were infected into PC3VC cells in the presence of 5 μg/mL polybrene for 48 h. Infected cells were selected for 7 d in media containing 1 μg/mL Puromycin and analyzed by real-time PCR or ELISA. Human PPARγ silencing was verified by real-time PCR and Western blot.
ELISA
An antibody sandwich ELISA was used to evaluate ABA production in conditioned culture media of MSC, HOB, HBMEC, and MC3T3-E1 cells at 72 h and serum and bone marrow fluids from 3-wk-old C57/BL6 mice (n = 8) by following the directions of the manufacturer (cat. LS- F4483-1, Life Span Biosciences, Seattle WA). ABA levels were normalized to total protein.
Western blot
PCa cells were cultured in RPMI 1640 with 10% FBS and 1% P/S. Whole cell lysates were prepared from cells, separated on 4% to 20% Tris-Glycine gels and transferred to PVDF membranes. The membranes were incubated with 5% milk for 1 h and incubated with primary antibodies overnight at 4 °C. Primary antibodies used were as follows: p27Kipl (1:1000 dilution, cat. 3686, Cell Signaling), p21 Waf1/Cipl (1:1000 dilution, cat. 2947, Cell Signaling), p16 (1:1000 dilution, cat. 80772, Cell Signaling), PPARγ (1:1000 dilution, cat. 2443, Cell Signaling), p70S6K (1:1000 dilution, cat. 2708, Cell Signaling), phospho-p70S6K (1:1000 dilution, cat. 9234, Cell Signaling). Blots were incubated with peroxidase-coupled anti-rabbit IgG secondary antibody (cat. 7074, 1:2000 dilution, Cell Signaling) for 1 h, and protein expression was detected with SuperSignal West Dura Chemiluminescent Substrate (cat. Prod 34075, Thermo Scientific, Rockford, IL). Membranes were reprobed with monoclonal anti-β-actin antibody (1:1000 dilution, cat. 4970, Cell Signaling) to control for equal loading.
Animals
Five- to seven-wk-old male SCID mice (CB.17 SCID; Taconic, Germantown, NY) were used as transplant recipients. All animal procedures were performed in compliance with the institutional ethical requirements and approved by the University of Michigan Institutional Committee for the Use and Care of Animals.
In vivo experiments
Control cells (PC3VC-Control) or PPARγ silenced PC3VC (PC3VC- shPPARγ) cells (2 × 105 cells) were suspended in 30 μL of PBS and injected into 5- to 7-wk-old male CB.17 SCID mice by intratibial injection [13]. After PCa cell injection, ABA (20 mg/kg) treatment for 8 times (twice daily) [39] was followed by intraperitoneal injection. At 96 h, mice were sacrificed, and the tibiae which PCa cells were injected were collected for analyzing cell cycle phase of PC3VC cells by FACS analyses. For evalation of cell cycle phase, DAPI negative live cells (cat no. NBP2-31156, DAPI, NOVUS) were negatively gated for anti-mouse H-2kd (cat no. 116622, PE/Cy7, BioLegend), which were then positively gated for HLA-A,B,C (cat no. 311426, APC/Cy7, BioLegend). After these gates were applied, cells were plotted as Venus vs Cherry (PE-TxRed) using a FACS Aria IIu three-laser flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and data were analyzed with FACS-DIVA software (Becton Dickinson).
Statistical analyses
Results are presented as mean ± standard deviation (SD). Significance of the difference between two measurements was determined by unpaired Student's t test, and multiple comparisons were evaluated by the Newman-Keuls multiple comparison test. Values of P< 0.05 were considered significant.
Results
Human marrow cells produce ABA
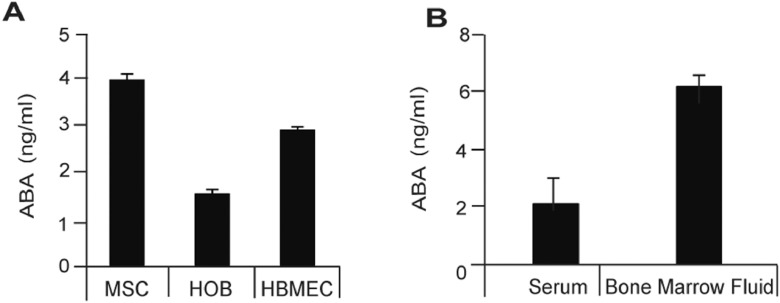
Signals from bone marrow microenvironment play a critical role in establishing dormancy of PCa cells [7,8]. To determine which marrow cells produce ABA, human mesenchymal stem cells (MSC), human osteoblasts (HOB), and HBMECs were cultured for 72 h and examined for ABA production. Significant levels of ABA production were detected in the culture media from all of three cell types (Figure 1A). To ensure that ABA production from cell lines is not an artifact of cell culture, we examined ABA levels in the serum and bone marrow fluids from 3-wk-old C57/BL6 mice. Significant levels of ABA production were detected from both serum and bone marrow fluids of the mice. (Figure 1B). Together these data suggest that ABA is present in the bone marrow and serum at significant levels, and that normal resident cells of the bone marrow secrete ABA.
Figure 1.
Human marrow cells produce ABA. (A) ABA production was identified in culture media of MSC, HOB, and HBMEC cells at 72 h as quantified by ELISA (cat. LS- F4483-1, Life Span Biosciences). ABA production was normalized to total protein. (B) Serum and bone marrow fluids were collected from 3-wk-old C57/BL6 mice (n = 8). ABA production was evaluated in serum and bone marrow fluids by ELISA. ABA production was normalized to total protein. Data in A, B are representative of mean with SD (Student's t test).
ABA induces G0 cell cycle arrest in PCa cells
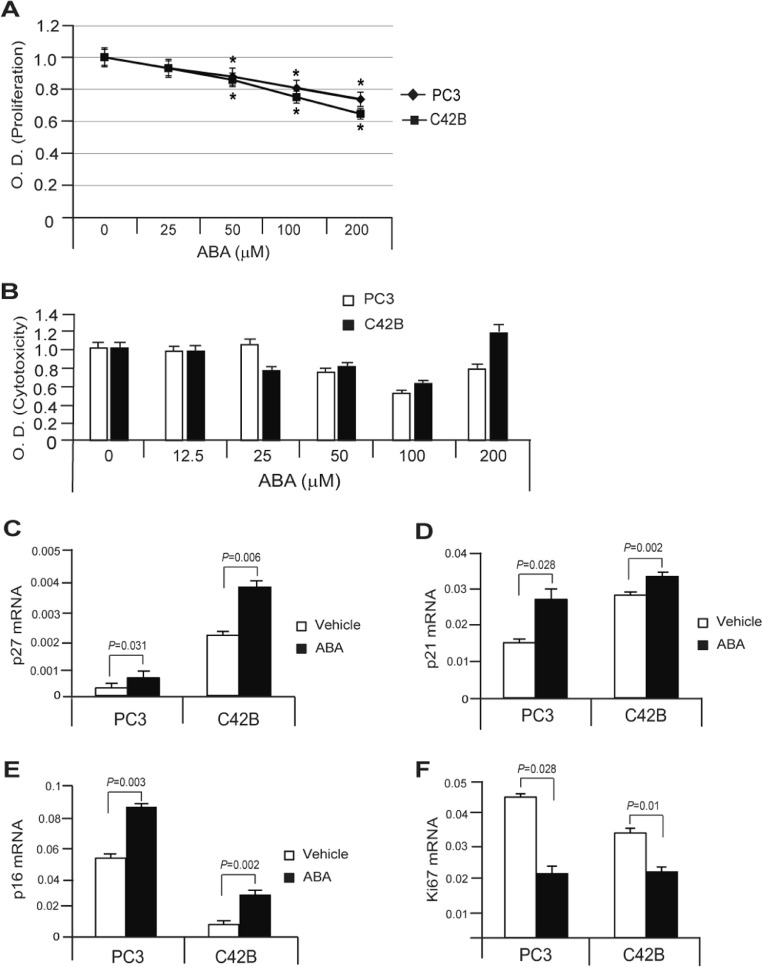
ABA has been reported to alter cellular proliferation in many different cell types. To evaluate the impact of ABA on PCa proliferation, proliferation assays were performed along with gene expression assays for cell cycle markers. ABA inhibited proliferation of PCa cells in a dose dependent manner (Figure 2A), and importantly did not induce cell death (Figure 2B). Levels of mRNA expression for p27, p21, and p16 all indicative of cell dormancy were dramatically increased by several PCa cells in response to ABA, whereas mRNA expression level for Ki67, indicative of cell proliferation was significantly decreased (Figure 2C–F).
Figure 2.
ABA inhibits cell proliferation and regulates cell cycle markers in PCa cells. (A) PCa cell proliferation assays were performed in 1% FBS culture condition with ABA treatment. (B) PCa cell cytotoxicity assays were performed in 10% FBS culture condition with 4 h of the ABA treatment. Data in Figure 1A, B are representative of mean with SD (Student's t test). *, P < 0.001 to 0.01. mRNA expression levels of (C) p27, (D) p21, (E) p16, and (F) Ki67 were measured in PCa cells with vehicle or ABA (50 µM) treatment by real-time PCR. Data in C-F are representative of mean with SD (Student's t test).
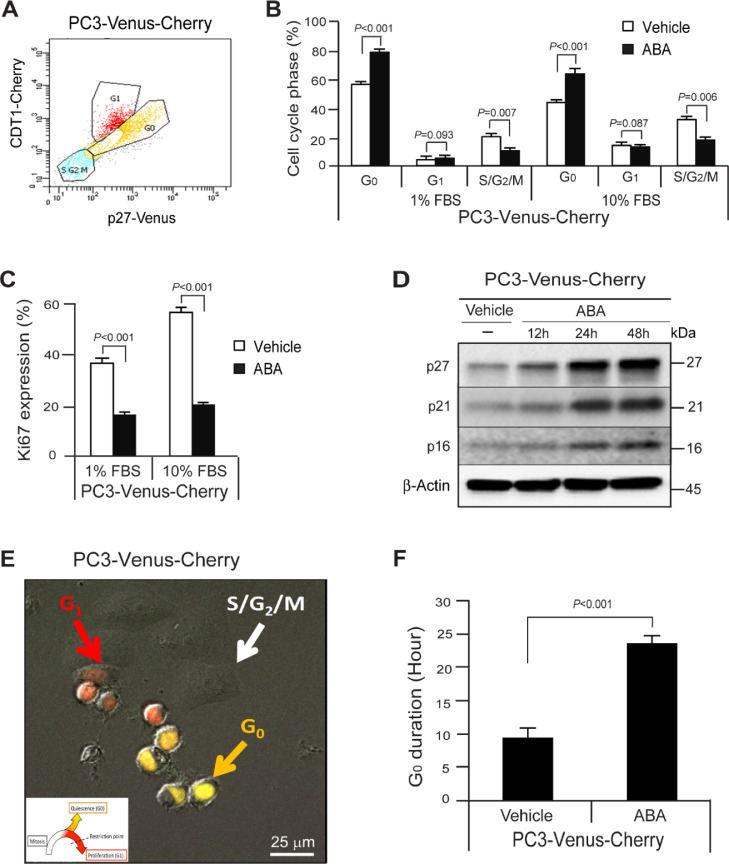
We further explored how ABA regulates the cell cycle in PCa cells. For these investigations, cell-cycle specific p27-G0-Venus and Cdt1-G1-Cherry reporters were employed, which facilitates the monitoring cell cycle dynamics, including active cycling and quiescence [13] (Figure 3A). We treated PC3VC cells with ABA and examined alternations of the cell cycle. We found that ABA induces quiescence of PCa cells, as defined by an increase of G0 cell cycle arrest phase and reduction of S,G2 and M phases of the cell cycle (Figure 3B) and down-regulation of Ki67 expression (Figure 3C) in 1% or 10% FBS culture conditions at 72 h. We also found that ABA increased p27, p21, and p16 protein expression (Figure 3D). We also examined the duration that the cells remained in G0 phase of the cell cycle by live cell imaging. PC3VC cells were cultured for 24 h in RPMI with 10% FBS and 1% P/S and then treated with vehicle or ABA (50 μΜ) for 24 h. We found that ABA treated PC3VC remained in the G0 phase for 23.6 h vs 9.4 h for vehicle treated cells (Figure 3E,F). Together, the data demonstrate that ABA regulates cellular dormancy of PCa cells.
Figure 3.
ABA induces G0 cell cycle arrest in PCa cells. (A) A flow profile of the cell cycle phase; G0, G1, and S/G2/M in PC3VC cells. (B) % of G0, G1, or S/G2/M cell cycle phase and (C) Ki67 expression in PC3VC cells following the treatment of vehicle or ABA (50 µM) in 1% FBS or 10% FBS culture conditions at 72 h as quantified by FACS analyses. Data in B and C are representative of mean with s.d. (Student's t test). (D) Expression of p27, p21, and p16 by vehicle or ABA treatment as quantified by Western blot. (E) Live cell imaging of PC3VC (G0: yellowish-orange arrow, G1: red arrow, and S/G2/M: white arrow) following vehicle or ABA (50 µM) treatment by video for 24 h with 15 min interval by Deltavision Elite Microscope. Bar=25 µm. Insert: The color scheme indicates the cell cycle phase, either quiescenct /G0 (orange),G1 (red) and the combination of S, G2 and M phases (none). (F) Quantification of G0 duration in single cells from E (n = 10/group). Data are representative of mean with SD (Student's t test).
ABA increases dormancy through PPARγ receptor signaling in PCa cells
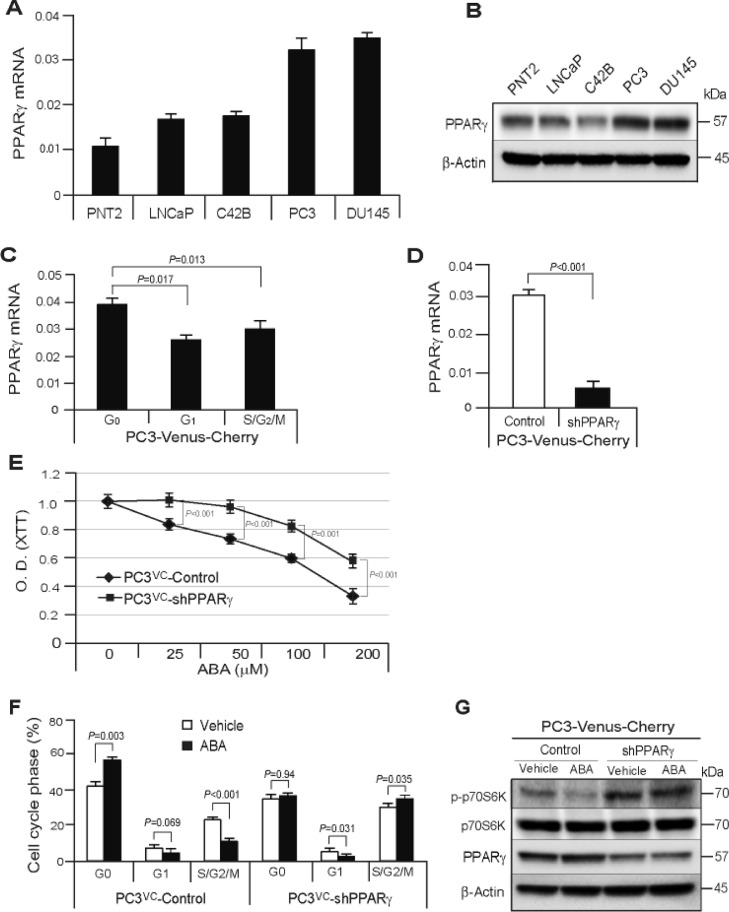
Under normal culture conditions, significant levels of expression of the ABA receptor mRNA, PPARγ were detected in normal prostate epithelial cells and PCa cell lines (Figure 4A,B). To examine whether PPARγ receptor signaling is associated with ABA induced dormancy, we first examined the PPARγ receptor expression in different cell cycle phases. We isolated different cell cycle phase of PC3VC cells by FAC sorting and then, examined PPARγ mRNA expression. We found that PPARγ mRNA expression was highly elevated in G0 phase compared with G1 or S/G2/M phase of PC3VC cells, suggesting the association of PPARγ receptor expression during cellular dormancy (Figure 4C).
Figure 4.
ABA induces cellular dormancy through PPARγ receptor signaling in PCa cells. (A) Basal levels of mRNA expression of the receptor of ABA, PPARγ in PCa cell lines as quantified by real-time PCR. (B) Basal levels of protein expression of the receptor of ABA, PPARγ in PCa cell lines as quantified by Western blot. (C) mRNA expression of PPARγ in G0, G1, and S/G2/M cell cycle phase of PC3VC cells. (D) Verification of PPARγ mRNA expression in PPARγ silencing PC3VC as quantified by real-time PCR. (E) Proliferation assays in PC3VC-Control or PC3VC-shPPARγ cells were performed in 1% FBS culture condition with ABA treatment. (F)% of G0, G1, or S/G2/M cell cycle phase in PC3VC-Control or PC3VC-shPPARγ cells following vehicle or ABA (50 µM) treatment in 1% FBS culture conditions at 72 h as quantified by FACS analyses. Data in C–F are representative of mean with SD (Student's t test). (G) Activation of down-stream effector, p70S6K of mTOR signaling in PC3VC-Control or PC3VC-shPPARγ cells following vehicle or ABA (50 µM) treatment as quantified by Western blot.
To further explore the impact of PPARγ on dormancy, PPARγ receptor expression was stably silenced in PC3VC cells (Figure 4D). Although ABA inhibited the proliferation in both PC3VC-Control and PC3VC-shPPARγ cells in a dose dependent manner, PPARγ silenced PCa cells were less affected by ABA treatment compared to control PCa cells, suggesting that ABA signals through the PPARγ receptor, although other signaling cascades are possible (Figure 4E). We also examined cell cycle phase with ABA treatment in PC3VC-Control cells or PC3VC-shPPARγ cells. We found a decrease of G0 cell cycle arrest and an increase of S/G2/M phase in shPPARγ PCa cells compared with control cells (Figure 4F). We further examined how ABA regulates PPARγ receptor expression and how ABA-PPARγ signaling is linked to PCa cellular dormancy. Here, we found that control PCa cells respond to ABA with a significant increase of PPARγ receptor signaling through suppression of down-stream effector of mTOR signaling, phosphorylation of p70S6K (p-p70S6K), whereas PPARγ silencing dramatically reversed the activation of p-p70S6K and did not respond to ABA (Figure 4G). These data suggest that engagement of PPARγ by ABA suppresses the activity of mTOR signaling, which is essential for anti-proliferation or cellular dormancy [40], [41], [42].
ABA and PPARγ signaling pathway induces PCa dormancy intheBone marrow microenvironment
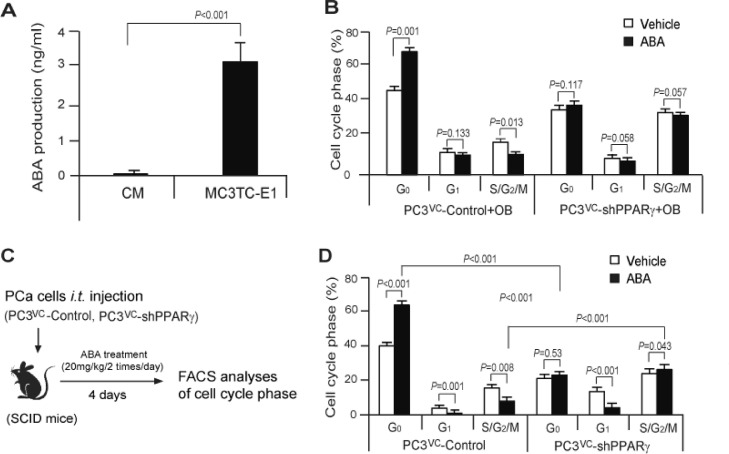
PCa cells are known to develop cellular dormancy, particularly within the marrow. To explore the role that ABA plays in the marrow we first performed co-culture experiments with PCa cells and osteoblasts (MC3TC-E1). First, we examined ABA production from MC3TC-E1 cells and significant levels of ABA production were detected in the culture media from MC3TC-E1 cells (Figure 5A). Interestingly, more G0 arrested cells and fewer cells in S/G2/M were found in PC3VC- cells in the cocultured with osteoblasts compared with PC3VC cell alone (Figure 5B). Importantly, we found that the numbers of G0 arrested cells were not changed when PPARγ was silenced from PC3 cells when cocultured with osteoblasts (Figure 5B). These data suggest that ABA may regulate PCa growth arrest through PPARγ signaling in the bone marrow microenvironment.
Figure 5.
ABA and PPARγ signaling pathway induces PCa cellular dormancy in the bone marrow microenvironment. (A) ABA production was identified in culture media of MC3TC-E1 cells at 72 h as quantified by ELISA (cat. LS- F4483-1, Life Span Biosciences). ABA production was normalized to total protein. (B) % of G0, G1, or S/G2/M cell cycle phase when PC3VC-Control or PC3VC-shPPARγ cells are co-cultured with murine osteoblasts (MC3T3-E1 cells) with or without ABA (50 µM) treatment. Live cells (cat. NBP2-31156, DAPI, NOVUS) were negatively gated for anti-mouse H-2kd (cat no. 116622, PE/Cy7, BioLegend), which were then positively gated for (human) HLA-A,B,C (cat no. 311426, APC/Cy7, BioLegend). After these gates were applied, the cells were plotted on the Venus-Cherry spectrum. Cell cycle phase was determined using FACS analyses. (C) Diagram of the experimental procedures for in vivo animal model. PC3VC-Control or PC3VC-shPPARγ cells (2 × 105 cells) were suspended in 30μl of PBS and injected into 5- to 7-wk-old male CB.17. SCID mice by i.t. injection. After PCa cell injection, vehicle or ABA (20 mg/kg) treatment for 8 times (twice daily) was followed by i.p. injection. At 4 d, mice were sacrificed, and the tibiae which PCa cells were injected were collected for analyzing cell cycle phase of PC3VC cells by FACS analyses. Antibody staining were applied as same as in coculture study in A. (D) Quantification of % of G0, G1, or S/G2/M cell cycle phase from 5C (n = 4/group). Data in A, B and D are representative of mean with SD (Student's t test).
To further explore the impact of ABA on PCa DTCs, animal experiments were performed (Figure 5C). PC3VC-Control or PC3VC-shPPARγ cells (2 × 105 cells) were first injected intratibially. Thereafter, the animals were treated vehicle or ABA (20 mg/kg) twice daily by intraperitoneal injection over 4 d for a total of 8 treatments. Subsequently, the cell cycle phase of PC3VC cells in the marrow was evaluated by FACS analyses (Figure 5C). More G0 arrested PCa cells were identified and fewer cells in S/G2/M were identified in animals treated with ABA than the vehicle treated animals. Further, animals which were inoculated with the PC3VC-shPPARγ cells had fewer of the recovered cells in G0 following ABA treatment compared to the PC3VC-Control cells (Figure 5D). The data suggest ABA may regulate the PCa growth arrest through PPARγ signaling in bone marrow microenvironment.
Discussion
Significant progress has been made towards understanding the regulators of DTC fate and dormancy in marrow [7,[14], [15], [16], [17], [18], [19]]. Here, we investigated a possible new regulator of dormancy, which is conserved throughout evolution and known to regulate growth and dormancy. We demonstrated that marrow niche cells produce ABA and PPARγ receptor signaling in PCa cells plays a pivotal role in regulating the metastatic PCa tumor cell dormancy just as it regulates seed dormancy of plants. Understanding how plants regulate cellular growth and applying this knowledge to tumors may open multiple new targetable mechanisms controlling dormancy regulation. Other mechanisms for dormancy regulation in plants will provide rational and hypotheses for cancer dormancy investigations.
Growing evidence suggests that ABA is a new and crucial signaling regulator targeting MSC and HSC in the bone marrow microenvironment [26,28,29]. Stress signals (e.g., TNF-α, RANTES, or IL-8) secreted by peripheral blood mononuclear cells have been shown to stimulate MSC to release ABA. MSC-derived ABA in turn enhances hematopoietic progenitors numbers through the cyclic adenosine diphosphate ribose (cADPR)/[Ca2+]i-mediated signaling pathways [28,29]. BMP7 and lymphocyte conditioned medium also stimulates ABA production by MSCs [29], which promotes growth-stimulatory effects on early hematopoietic cells expressing CD34 [26,43]. Further, ABA stimulates granulocyte production in the bone marrow [26,28] and ABA-treated CD34 hematopoietic progenitor cells are known to release VEGF and IL-8, which regulates the growth of hematopoietic cells, stromal cells, and endothelial cells [28]. Collectively, these data illustrate the connectedness of hematopoietic and marrow stromal populations with regards to ABA signaling networks. Given that DTCs parasitize the hematopoietic stem cell niche during metastasis [44], it is not surprising that ABA would regulate DTC dormancy. We have also discussed other parallels between DTC and HSC regulation [6].
Recently several studies have demonstrated that ABA inhibits the proliferation of tumor cells [26,27] and ABA negatively regulates Ki67 expression in glioma cell lines, U87MG and A172 cells [30]. Another report shows that ABA inhibits proliferation and induces cell arrested in G0/G1 phase in human hepatocellular carcinoma, SMMC-7221cells [26]. ABA also induces expression of the p27CIP/KIP ortholog ICK1 (an inhibitor of CDK action at the G1-S phase transition) [21,22,45,46], and a single application of ABA reduced the percentages of cells in mitosis and cells replicating DNA in the meristem of Sinapis plants [23,47]. Together, these studies support our findings that ABA inhibits the proliferation with an increase of G0 cell cycle arrest in PCa cells suggesting that ABA may have wider ranging impacts of tumor proliferation than heretofore appreciated.
Mammalian ABA signaling is known to be mediated by at least two receptors, lanthionine synthetase C-like 2 (LANCL2) and PPARγ [26,27,48]. LANCL2, a peripheral membrane protein, regulates cell survival by Akt activation through its interaction with mTOR signaling [49]. Interestingly, recent reports also demonstrate that LANCL2 expression is expressed by DTCs in bone marrow of breast cancer patients [3,48]. We found that PPARγ gene expression was associated in the cell cycle phase. However, LANCL2 gene expression may not be associated in the G0 cell cycle phase (data not shown). Based on these findings, we pursued studies with PPARγ and demonstrated that enhancement of PPARγ receptor signaling by ABA treatment promotes PCa cellular dormancy with G0 cell cycle arrest in vitro culture conditions and in vivo animal model. Moreover, PPARγ has a role as a tumor suppressor in several types of cancer cells including PCa [42,[50], [51], [52], [53], [54], [55]]. As such, what role LANCL2 ultimately plays in DTC biology of DTCs will require further studies, but clearly PPARγ appears to play the predominant role in response to ABA in PCa cells.
Interestingly, PPARγ appears to play a significant role in regulating PCa growth in response to a number of ligands in addition to ABA. For example, recent studies demonstrate that troglitazone (TZD), an agonist of PPARγ, has an anti-proliferative action on the PCa cells, in part through inhibition of the AR pathway [55]. Further, TZD also reduced the growth of PC3 and LNCaP tumors in nude mouse xenograft models [56], [57], [58]. Additionally, TZD upregulate CDK inhibitors, which promote cell cycle arrest and apoptotic effects to PCa cells [57,59,60]. The study showed that TZD increases G0/G1 cell cycle arrest of PC3 cells in dose dependent manner and further demonstrated that PPARγ-mediated growth inhibition was linked to the upregulation of the cyclin dependent kinase inhibitors p21 and p27 and/or repression of cyclin D1 expression [60]. Similarly, TZD increased p21 expression in PC3 and C42B cells [57]. Moreover, TZD has been shown to induce apoptosis of PC3 cells by reducing the activity of the anti-apoptotic proteins Bcl-2 and Bcl-xL [59].
One mechanism whereby ABA induces dormancy of PCa cells through the activation of PPARγ receptor is by suppression of mTOR signaling. Several reports now show that PPARγ agonists and/or ligands inhibit key pathways of the insulin/IGF axis, such as PI3K/mTOR, MAPK, and GSK3β/Wnt/β-catenin cascades, which regulate cancer cell proliferation and tumor growth [40], [41], [42]. Our findings are intriguing in that ABA increases PPARγ receptor expression while suppressing mTOR/p70S6K signaling; however, the mTOR/p70S6K signaling was reversed in PPARγ silenced PCa cells. In fact, suppression of PPARγ expression leads to G0 cell cycle arrest under in vitro culture conditions and in vivo animal models. These results suggest that the suppression of mTOR signaling is essential for ABA-PPARγ signaling-mediated cellular dormancy in PCa cells.
In summary, this work provides evidence as to the role of ABA in the marrow in the establishment of PCa DTC cellular dormancy. We further demonstrate that ABA-PPARγ receptor signaling strongly suppress mTOR activity, thus inducing PCa cellular dormancy. Importantly, this work contributes to the identification of new regulator of metastatic PCa dormancy and may have important implications for targeting metastatic disease.
Author contributions
Y.J. and R.S.T. designed the experiments. Y.J., F.C.C., K.Y., A.M.D., Y.W., M.H., E.L. performed experiments and analyzed the data. L.B. provided the PC3VC cells and discussed the results and gave valuable critique on the paper. Y.J. and R.S.T. wrote the manuscript.
Acknowledgments
The authors wish to thank Dr. Taocong Jin for technical and logistical support in Molecular Core at University of Michigan and Dr. Jin Koo Kim at University of California Los Angeles gave valuable critique on the signaling pathway of PPARγ and mTOR.
Footnotes
Funding: This work is directly supported by the National Cancer Institute (CA093900 and CA163124), the Department of Defense (W81XW-15–1-0413 and W81XWH-14–1-0403), and the Prostate Cancer Foundation.
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Guise TA, Mundy GR. Cancer and bone. Endocrine reviews. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 2.Roberts WB, Han M. Clinical significance and treatment of biochemical recurrence after definitive therapy for localized prostate cancer. Surgical oncology. 2009;18:268–274. doi: 10.1016/j.suronc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naume B, Zhao X, Synnestvedt M, Borgen E, Russnes HG, Lingjærde OC, Strømberg M, Wiedswang G, Kvalheim G, Kåresen R. Presence of bone marrow micrometastasis is associated with different recurrence risk within molecular subtypes of breast cancer. Molecular oncology. 2007;1:160–171. doi: 10.1016/j.molonc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clinical Cancer Research. 2009;15:677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. The Journal of urology. 2000;164:101–105. [PubMed] [Google Scholar]
- 6.Cackowski FC, Taichman RS. Parallels between hematopoietic stem cell and prostate cancer disseminated tumor cell regulation. Bone. 2019;119:82–86. doi: 10.1016/j.bone.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yumoto K, Eber MR, Berry JE, Taichman RS, Shiozawa Y. Molecular pathways: niches in metastatic dormancy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:3384–3389. doi: 10.1158/1078-0432.CCR-13-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12 doi: 10.1593/neo.91384. IN4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra A, Wang J, Shiozawa Y, McGee S, Kim J, Jung Y, Joseph J, Berry JE, Havens A, Pienta KJ. Hypoxia stabilizes GAS6/Axl signaling in metastatic prostate cancer. Mol Cancer Res. 2012;10:703–712. doi: 10.1158/1541-7786.MCR-11-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, Yumoto K, Berry JE, Shiozawa Y, Pienta KJ. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PloS one. 2013;8:e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JK, Jung Y, Wang J, Joseph J, Mishra A, Hill EE, Krebsbach PH, Pienta KJ, Shiozawa Y, Taichman RS. TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia. 2013;15:1064–1074. doi: 10.1593/neo.13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee E, Decker AM, Cackowski FC, Kana LA, Yumoto K, Jung Y, Wang J, Buttitta L, Morgan TM, Taichman RS. Growth arrest‐specific 6 (GAS6) promotes prostate cancer survival by G1 arrest/S phase delay and inhibition of apoptosis during chemotherapy in bone marrow. Journal of cellular biochemistry. 2016;117:2815–2824. doi: 10.1002/jcb.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yumoto K, Eber MR, Wang J, Cackowski FC, Decker AM, Lee E, Nobre AR, Aguirre-Ghiso JA, Jung Y, Taichman RS. Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Scientific reports. 2016;6:36520. doi: 10.1038/srep36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cackowski FC, Eber MR, Rhee J, Decker AM, Yumoto K, Berry JE, Lee E, Shiozawa Y, Jung Y, Aguirre‐Ghiso JA. Mer tyrosine kinase regulates disseminated prostate cancer cellular dormancy. Journal of cellular biochemistry. 2017;118:891–902. doi: 10.1002/jcb.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, Ekpin E, George A, Zheng Y, Lam H-M. NR2F1 controls tumour cell dormancy via SOX9-and RARβ-driven quiescence programmes. Nature communications. 2015;6:6170. doi: 10.1038/ncomms7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM, Aguirre-Ghiso JA. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nature cell biology. 2013;15:1351. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruppender N, Larson S, Lakely B, Kollath L, Brown L, Coleman I, Coleman R, Nguyen H, Nelson PS, Corey E. Cellular Adhesion Promotes Prostate Cancer Cells Escape from Dormancy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Xing F, Liu Y, Wu K, Said N, Pochampally R, Shiozawa Y, Lin H-K, Balaji K, Watabe K. Secreted protein acidic and rich in cysteine (SPARC) mediates metastatic dormancy of prostate cancer in the bone. Journal of Biological Chemistry. 2016;291(37):19351–19363. doi: 10.1074/jbc.M116.737379. jbc. J Biol Chem. 2016 Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S-Y, Chien C-T, Baskin JM, Baskin CC. Storage behavior and changes in concentrations of abscisic acid and gibberellins during dormancy break and germination in seeds of Phellodendron amurense var. wilsonii (Rutaceae. Tree physiology. 2009;30:275–284. doi: 10.1093/treephys/tpp111. [DOI] [PubMed] [Google Scholar]
- 21.Himmelbach A. Signalling of abscisic acid to regulate plant growth. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1998;353:1439–1444. doi: 10.1098/rstb.1998.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvath DP, Anderson JV, Chao WS, Foley ME. Knowing when to grow: signals regulating bud dormancy. Trends in plant science. 2003;8:534–540. doi: 10.1016/j.tplants.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Jacqmard A, Houssa C, Bernier G. Abscisic acid antagonizes the effect of cytokinin on DNA-replication origins. Journal of Experimental Botany. 1995;46:663–666. [Google Scholar]
- 24.Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A. A molecular timetable for apical bud formation and dormancy induction in poplar. The Plant Cell. 2007;19:2370–2390. doi: 10.1105/tpc.107.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meguro A, Sato Y. Salicylic acid antagonizes abscisic acid inhibition of shoot growth and cell cycle progression in rice. Scientific reports. 2014;4:4555. doi: 10.1038/srep04555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H-H, Hao R-L, Wu S-S, Guo P-C, Chen C-J, Pan L-P, Ni H. Occurrence, function and potential medicinal applications of the phytohormone abscisic acid in animals and humans. Biochemical pharmacology. 2011;82:701–712. doi: 10.1016/j.bcp.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Sakthivel P, Sharma N, Klahn P, Gereke M, Bruder D. Abscisic acid: a phytohormone and mammalian cytokine as novel pharmacon with potential for future development into clinical applications. Current medicinal chemistry. 2016;23:1549–1570. doi: 10.2174/0929867323666160405113129. [DOI] [PubMed] [Google Scholar]
- 28.Scarfì S, Fresia C, Ferraris C, Bruzzone S, Fruscione F, Usai C, Benvenuto F, Magnone M, Podestà M, Sturla L. The plant hormone abscisic acid stimulates the proliferation of human hemopoietic progenitors through the second messenger cyclic ADP‐ribose. Stem Cells. 2009;27:2469–2477. doi: 10.1002/stem.173. [DOI] [PubMed] [Google Scholar]
- 29.Scarfì S, Ferraris C, Fruscione F, Fresia C, Guida L, Bruzzone S, Usai C, Parodi A, Millo E, Salis A. Cyclic ADP‐ribose‐mediated expansion and stimulation of human mesenchymal stem cells by the plant hormone abscisic acid. Stem Cells. 2008;26:2855–2864. doi: 10.1634/stemcells.2008-0488. [DOI] [PubMed] [Google Scholar]
- 30.Zhou N, Yao Y, Ye H, Zhu W, Chen L, Mao Y. Abscisic‐acid‐induced cellular apoptosis and differentiation in glioma via the retinoid acid signaling pathway. International journal of cancer. 2016;138:1947–1958. doi: 10.1002/ijc.29935. [DOI] [PubMed] [Google Scholar]
- 31.Guri AJ, Hontecillas R, Bassaganya-Riera J. Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: possible action of the cAMP/PKA/PPAR gamma axis. Clin Nutr. 2010;29:646–653. doi: 10.1016/j.clnu.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hontecillas R, Bassaganya-Riera J. Expression of PPAR gamma in intestinal epithelial cells is dispensable for the prevention of colitis by dietary abscisic acid. ESPEN J. 2012;7:e189–e195. doi: 10.1016/j.clnme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masek LC, Sweetenham JW. Isolation and culture of endothelial cells from human bone marrow. Br J Haematol. 1994;88:855–865. doi: 10.1111/j.1365-2141.1994.tb05128.x. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Yumoto K, Yasuhara K, Nadres ET, Kikuchi Y, Buttitta L, Taichman RS, Kuroda K. Anticancer polymers designed for killing dormant prostate cancer cells. Sci Rep. 2019;9:1096. doi: 10.1038/s41598-018-36608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oki T, Nishimura K, Kitaura J, Togami K, Maehara A, Izawa K, Sakaue-Sawano A, Niida A, Miyano S, Aburatani H. A novel cell-cycle-indicator, mVenus-p27K−, identifies quiescent cells and visualizes G0–G1 transition. Scientific reports. 2014;4:4012. doi: 10.1038/srep04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura-Ishizu A, Takizawa H, Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141:4656–4666. doi: 10.1242/dev.106575. [DOI] [PubMed] [Google Scholar]
- 39.Qi C-C, Zhang Z, Fang H, Liu J, Zhou N, Ge J-F, Chen F-H, Xiang C-B, Zhou J-N. Antidepressant effects of abscisic acid mediated by the downregulation of corticotrophin-releasing hormone gene expression in rats. International Journal of Neuropsychopharmacology. 2015;18:1–9. doi: 10.1093/ijnp/pyu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He G, Sung YM, Digiovanni J, Fischer SM. Thiazolidinediones inhibit insulin-like growth factor-i-induced activation of p70S6 kinase and suppress insulin-like growth factor-I tumor-promoting activity. Cancer Res. 2006;66:1873–1878. doi: 10.1158/0008-5472.CAN-05-3111. [DOI] [PubMed] [Google Scholar]
- 41.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Molecular cancer therapeutics. 2006;5:430–437. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 42.Vella V, Nicolosi ML, Giuliano S, Bellomo M, Belfiore A, Malaguarnera R. PPAR-gamma Agonists As Antineoplastic Agents in Cancers with Dysregulated IGF Axis. Frontiers in endocrinology. 2017;8:31. doi: 10.3389/fendo.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruzzone S, Moreschi I, Usai C, Guida L, Damonte G, Salis A, Scarfi S, Millo E, De Flora A, Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proceedings of the National Academy of Sciences. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francis D, Sorrell DA. The interface between the cell cycle and plant growth regulators: a mini review. Plant growth regulation. 2001;33:1–12. [Google Scholar]
- 46.Kang S-G, Choi J-H, Suh S-G. A leaf-specific 27 kDa protein of potato Kunitz-type proteinase inhibitor is induced in response to abscisic acid, ethylene, methyl jasmonate, and water deficit. Molecules and cells. 2002;13:144–147. [PubMed] [Google Scholar]
- 47.Kinet J, Bodson M, Jacqmard A, Bernier G. The Inhibition of Flowering by Abscisic Acid in Sinapis alba L. Zeitschrift für Pflanzenphysiologie. 1975;77:70–74. [Google Scholar]
- 48.Fresia C, Vigliarolo T, Guida L, Booz V, Bruzzone S, Sturla L, Di Bona M, Pesce M, Usai C, De Flora A. G-protein coupling and nuclear translocation of the human abscisic acid receptor LANCL2. Scientific reports. 2016;6:26658. doi: 10.1038/srep26658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng M, Van Der Donk WA, Chen J. Lanthionine synthetase C–like protein 2 (LanCL2) is a novel regulator of Akt. Molecular biology of the cell. 2014;25:3954–3961. doi: 10.1091/mbc.E14-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elix C, Pal SK, Jones JO. The role of peroxisome proliferator-activated receptor gamma in prostate cancer. Asian J Androl. 2018;20:238–243. doi: 10.4103/aja.aja_15_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 52.Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikka S, Chen L, Sethi G, Kumar AP. Targeting PPARgamma Signaling Cascade for the Prevention and Treatment of Prostate Cancer. PPAR Res. 2012;2012 doi: 10.1155/2012/968040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koeffler HP. Peroxisome proliferator-activated receptor γ and cancers. Clinical Cancer Research. 2003;9:1–9. [PubMed] [Google Scholar]
- 55.Hisatake J-i, Ikezoe T, Carey M, Holden S, Tomoyasu S, Koeffler HP. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor γ in human prostate cancer. Cancer research. 2000;60:5494–5498. [PubMed] [Google Scholar]
- 56.Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 57.Lyles BE, Akinyeke TO, Moss PE, Stewart LV. Thiazolidinediones regulate expression of cell cycle proteins in human prostate cancer cells via PPARgamma-dependent and PPARgamma-independent pathways. Cell Cycle. 2009;8:268–277. doi: 10.4161/cc.8.2.7584. [DOI] [PubMed] [Google Scholar]
- 58.Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002;110:923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiau CW, Yang CC, Kulp SK, Chen KF, Chen CS, Huang JW, Chen CS. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res. 2005;65:1561–1569. doi: 10.1158/0008-5472.CAN-04-1677. [DOI] [PubMed] [Google Scholar]
- 60.Zhu H, Pan X, Qi H, Wang X, Hou S, Han B, Liu Z, Xu L. Troglitazone attenuates epidermal growth factor receptor signaling independently of peroxisome proliferator-activated receptor in PC-3 cells. Oncol Rep. 2011;25:81–90. [PubMed] [Google Scholar]