Abstract
Emerging economy countries in epidemiological transition have been especially challenged in the fight against cancer. This was an ecological study that aimed to describe the temporal trend of cancer mortality in a Brazilian northeastern state with a medium Human Development Index using official Brazilian mortality data from 1980 to 2018. We calculated the mortality crude rate (CR) and age-standardized rate (ASR) based on official population counts and estimates. The Joinpoint Regression Program, National Cancer Institute, USA, was used to calculate time trends of cancer mortality. There were 34,214 deaths from cancer, excluding nonmelanoma skin cancer, in Sergipe. The overall cancer mortality ASR was 70.1 and 57.9 per 100,000 men and women, respectively. For the last five years, the leading causes of cancer deaths were prostate (21.3), trachea, bronchus and lung (11.7), stomach (6.5), oral cavity (5.4) and liver and intrahepatic bile ducts (5.1) in males and breast (13.8), trachea, bronchus and lung (6.6), cervix (6.4), colon/rectum (5.8) and central nervous system (3.6) in females. In addition, there was a significant reduction in deaths from ill-defined causes in the series. Our results show that although there has been an increase in cancer mortality rates associated with Western lifestyles, such as prostate, breast and colon/rectum, high rates of cancer related to poverty and infections, such as stomach and cervix, still persist in Sergipe.
Subject terms: Cancer, Medical research, Oncology
Introduction
Cancer is a major health problem worldwide and has a considerable impact on the population and health systems, especially in countries with emerging economies facing epidemiological transition1,2, such as Brazil.
According to GLOBOCAN 2018 estimates, there were 18.1 million new cases of cancer (17.0 million excluding nonmelanoma skin cancer—NMSC) and 9.6 million cancer deaths (9.5 million excluding NMSC) in 20181. There has been a global decrease in cancer mortality rates in recent years, although it is less pronounced in underdeveloped regions, such as some Latin American countries. However, the total number of deaths is still rising, especially in emerging economy countries1,3–5.
The Brazilian National Cancer Institute (INCA) periodically publishes cancer incidence estimates of the nineteen main cancer sites in Brazil. For the 2020–2022 triennium, 625 000 new cases of cancer are expected each year (450 000 excluding NMSC). In 2018, there were 224,727 cancer-related deaths in Brazil, accounting for approximately 17% of the total deaths6–8.
There are some disparities in the incidence and mortality rates for specific types of cancer between the five geographic regions of Brazil, depending on the degree of social and economic development, exposure to risk factors and lifestyle. For example, in the Southeast Region, the epidemiological profile is similar to that in developed countries, while in the North and Northeast Regions, high rates of cancer incidence and mortality related to underdevelopment still persist6.
There are few studies on cancer incidence and mortality in Sergipe; existing studies are focused on a particular group or type of cancer9–15. This study aimed to describe the temporal trends of cancer mortality in Sergipe in a comprehensive way to compare the results with regions with similar HDI and signal priorities for actions against cancer. We also intended to assess the quality of official mortality data in our state.
Methods
This was an exploratory ecological study of time trends. Data from 1980 to 2018 were obtained from the Mortality Information System (SIM database).
Sergipe is located on the coast of the Northeast Region of Brazil in the 24 south zone. It has a tropical climate, a population of 2,068,017 inhabitants (census 2010), an estimated population of 2,278,308 for 2018 and a Human Development Index (HDI) of 0.665 (census 2010). Only the capital, Aracaju, has a high HDI (0.77) and is home to approximately 28% of the state’s population, while the other 74 municipalities have medium or low HDI16–18.
We included individuals who died from cancer of any site or ill-defined site residing in the state of Sergipe. We calculated crude rates (CRs) and age-standardized rates (ASRs), adjusted by the world population19,20, for all ages and age groups by stage of life (0–19, 20–44, 45–64 and 65+) for both sexes and by the main sites of cancer, based on official population counts and estimates from the Brazilian Institute of Geography and Statistics (IBGE).
According to the primary site, the cases were classified and sometimes grouped based on the International Classification of Diseases, 10th edition (ICD-10), as follows: oral cavity—C00 to C10; esophagus—C15; stomach—C16; colon and rectum—C18 to C21; liver and intrahepatic bile ducts—C22; larynx—C32; trachea, bronchus and lung—C33 and C34; melanoma skin cancer—C43; nonmelanoma skin cancer—C44; female breast—C50; cervix uteri—C53; corpus uteri—C54; ovary—C56; prostate—C61; bladder—C67; central nervous system—C70 to C72; thyroid gland—C73; Hodgkin’s lymphoma—C81; non-Hodgkin’s lymphoma—C82 to C85 and C96; leukemias—C91 to C95. The other sites were grouped as ‘other’ and added to cancers of unspecified topography. For the temporal analysis of ill-defined causes of death, we selected cases with codes R69, R98 and R99 based on ICD-10.
The Joinpoint Regression Program, version 4.7.0.0, from the National Cancer Institute, USA21, was used to calculate time trends for age-standardized rates of cancer mortality using a model based on the assumption of a minimal number of join points where statistically significant changes in the curves occur. Additionally, the annual percent change (APC) and the average annual percent change (AAPC), which are the summary measures of the trends over the analyzed period, with their respective 95% confidence intervals (CI 95%) and p values, were calculated by the program. A significant change in a trend was defined as p < 0.05.
The present project was submitted to the Research and Ethics Committee of Federal University of Sergipe (Universidade Federal de Sergipe—UFS), and it was approved and registered under CAAE number 57995416.9.0000.5546. We declare that all methods were in agreement with the relevant guidelines and regulations. For the sake of confidentiality, we used deidentified databases. Therefore, it was not possible to obtain informed consent. The Research and Ethics Committee of the Federal University of Sergipe exempted informed consent, in agreement with Resolution 466 of December 2012 of the Ministry of Health, Brazil.
Results
During the time series, there were 34,672 deaths from cancer in Sergipe (34,214 deaths excluding NMSC), accounting for a total of 9.27% of all deaths. There were 17,322 deaths in males and 17,350 in females. Excluding NMSC, the overall age-standardized mortality rate was 70.1/100,000 men and 57.9/100,000 women for the whole period. Table 1 shows the distribution of the cancer deaths, with the respective mortality CRs and ASRs, by the main sites for both sexes.
Table 1.
Distribution of the number of cancer deaths (N) by the main sites, with respective age-standardized mortality rates (ASR) per 100,000 men and women and 95% confidence intervals (95% CI) in Sergipe, 1980–2018.
| Sites | Male | Female | ||||
|---|---|---|---|---|---|---|
| N | ASR | 95% CI | N | ASR | 95% CI | |
| Oral cavity | 878 | 3.8 | 3.51; 4.01 | 299 | 1.0 | 0.89; 1.11 |
| Esophagus | 709 | 3.0 | 2.82; 3.26 | 210 | 0.7 | 0.61; 0.81 |
| Stomach | 1339 | 5.7 | 5.37; 5.98 | 786 | 2.6 | 2.45; 2.82 |
| Colon and rectum | 698 | 2.9 | 2.71; 3.14 | 992 | 3.3 | 3.12; 3.54 |
| Liver and intrahepatic bile ducts | 965 | 4.1 | 3.82; 4.33 | 881 | 3.0 | 2.81; 3.21 |
| Larynx | 613 | 2.7 | 2.45; 2.88 | 93 | 0.3 | 0.26; 0.40 |
| Trachea, bronchus and lung | 2232 | 9.7 | 9.28; 10.08 | 1442 | 5.0 | 4.75; 5.26 |
| Melanoma skin cancer | 115 | 0.5 | 0.38; 0.55 | 94 | 0.3 | 0.24; 0.37 |
| Prostate | 3354 | 13.5 | 13.07; 13.98 | – | – | – |
| Breast | – | – | – | 2773 | 9.5 | 9.13; 9.84 |
| Cervix | – | – | – | 1925 | 6.6 | 6.26; 6.84 |
| Corpus uteri | – | – | – | 163 | 0.6 | 0.49; 0.67 |
| Ovary | – | – | – | 643 | 2.2 | 2.04; 2.38 |
| Bladder | 406 | 1.7 | 1.54; 1.87 | 158 | 0.5 | 0.44; 0.60 |
| Central nervous system | 723 | 2.7 | 2.49; 2.89 | 749 | 2.5 | 2.29; 2.65 |
| Thyroid | 47 | 0.2 | 0.14; 0.25 | 103 | 0.4 | 0.29; 0.43 |
| Hodgkin’s lymphoma | 90 | 0.3 | 0.24; 0.37 | 49 | 0.2 | 0.11; 0.20 |
| Non-Hodgkin lymphoma | 453 | 1.7 | 1.54; 1.85 | 329 | 1.1 | 0.95; 1.18 |
| Leukemias | 748 | 2.5 | 2.35; 2.71 | 655 | 2.0 | 1.86; 2.17 |
| Other sites | 3715 | 15.2 | 14.69; 15.67 | 4785 | 16.2 | 15.70; 16.62 |
| Subtotal | 17,085 | 70.1 | 69.06; 71.17 | 17,129 | 57.9 | 57.01; 58.75 |
| Nonmelanoma skin cancer | 237 | 1.0 | 0.83; 1.07 | 221 | 0.7 | 0.57; 0.74 |
| Total | 17,322 | 71.1 | 70.01; 72.13 | 17,350 | 58.5 | 57.66; 59.41 |
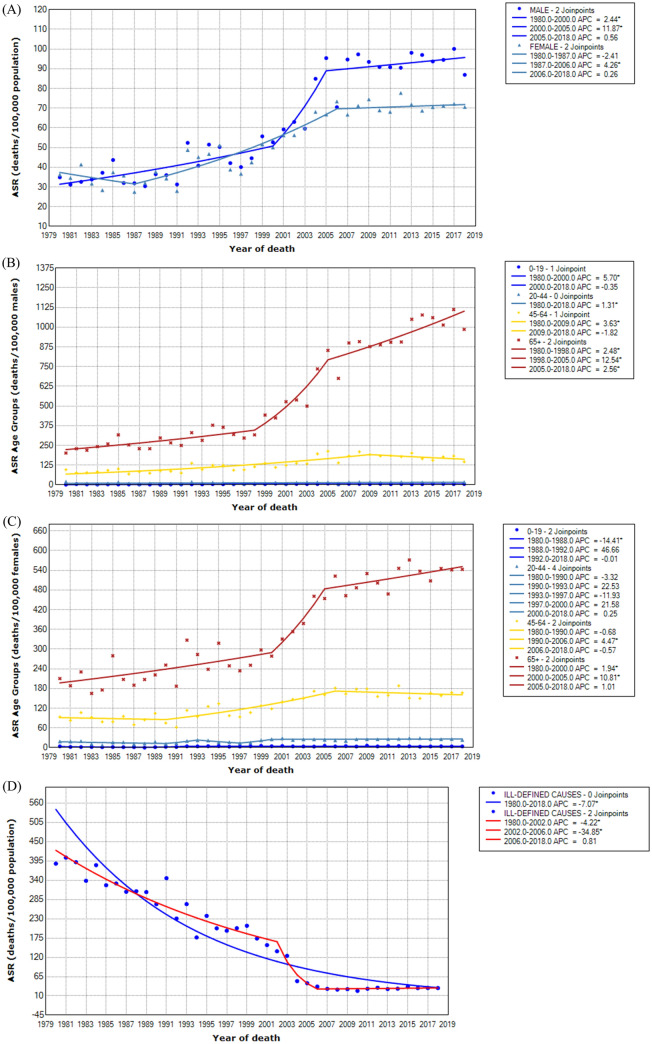
Table 2 depicts the joinpoint analysis of cancer mortality by all ages and age groups for both sexes and for the ill-defined causes of death (male and female). For males, there was a statistically significant upward trend from 1980 to 2000 (APC 2.44) and from 2000 to 2005 (APC 11.87) and subsequent stabilization of the curve. For females, the curve was stable until 1987, and there was a statistically significant upward trend from 1987 to 2006 (APC 4.26) and new stabilization thereafter. The trend curve of the ill-defined causes of ASR showed a statistically significant decrease from the beginning of the series until 2006 and stabilized later. The curves modeled by Joinpoint are represented in Fig. 1.
Table 2.
Joinpoint analysis of cancer mortality for age-standardized rates for all ages and specific for age groups for both sexes. Sergipe, 1980–2018.
| Age groups | JP | APC | 95% CI | AAPC | 95% CI |
|---|---|---|---|---|---|
| Male | |||||
| All | 2 | (1980–2000) 2.44a | 1.15; 3.74 | 2.98a | 1.55; 4.42 |
| – | (2000–2005) 11.87a | 1.91; 22.80 | |||
| – | (2005–2018) 0.56 | − 0.60; 1.74 | |||
| 0–19 | 1 | (1980–2000) 5.70a | 3.11; 8.35 | 2.79a | 1.17; 4.43 |
| – | (2000–2018) − 0.35 | − 2.44; 1.78 | |||
| 20–44 | 0 | (1980–2018) 1.31a | 0.53; 2.10 | 1.31a | 0.53; 2.10 |
| 45–64 | 1 | (1980–2009) 3.63a | 2.78; 4.49 | 2.31a | 1.43; 3.21 |
| – | (2009–2018) − 1.82 | − 4.48; 0.91 | |||
| 65+ | 2 | (1980–1998) 2.48a | 0.91; 4.06 | 4.29a | 3.08; 5.51 |
| – | (1998–2005) 12.54a | 7.13; 18.21 | |||
| – | (2005–2018) 2.56a | 1.51; 3.62 | |||
| Female | |||||
| All | 2 | (1980–1987) − 2.41 | − 7.82; 3.31 | 1.73a | 0.55; 2.93 |
| – | (1987–2006) 4.26a | 3.24; 5.30 | |||
| – | (2006–2018) 0.26 | − 0.89; 1.42 | |||
| 0–19 | 2 | (1980–1988) − 14.41a | − 21.48; − 6.70 | 1.28 | − 3.31; 6.09 |
| – | (1988–1992) 46.66 | − 2.72; 121.09 | |||
| – | (1992–2018) − 0.01 | − 1.02; 1.01 | |||
| 20–44 | 4 | (1980–1990) − 3.32 | − 7.58; 1.13 | 2.24 | − 3.91; 8.79 |
| – | (1990–1993) 22.53 | − 27.45; 106.93 | |||
| – | (1993–1997) − 11.93 | − 29.67; 10.28 | |||
| – | (1997–2000) 21.58 | − 21.34; 87.90 | |||
| – | (2000–2018) 0.25 | − 0.76; 1.28 | |||
| 45–64 | 2 | (1980–1990) − 0.68 | − 4.39; 3.18 | 2.13a | 0.57; 3.71 |
| – | (1990–2006) 4.47a | 2.92; 6.06 | |||
| – | (2006–2018) 0.57 | − 1.91; 0.78 | |||
| 65+ | 2 | (1980–2000) 1.94a | 0.76; 3.12 | 2.56a | 1.65; 3.48 |
| – | (2000–2005) 10.81a | 1.81; 20.61 | |||
| – | (2005–2018) 1.01 | − 0.04; 2.08 | |||
| Ill-defined causes | |||||
| Total | 2 | (1980–2002) − 4.22a | − 4.85; − 3.59 | − 7.07a | − 8.04; − 6.09 |
| – | (2002–2006) − 34.85a | − 47.65; − 18.93 | |||
| – | (2006–2018) 0.81 | − 2.69; 4.44 | |||
JP number of join points, APC annual percent change, AAPC average annual percent change, CI Confidence interval.
aAPC/AAPC statistically significant.
Figure 1.
Time trends of cancer mortality for age-standardized rates (ASR) for all ages and sites in both sexes (A), for specific age groups in males (B) and in females (C) and for ill-defined causes of death (D).
Considering only the last five years of the series, which are the ones that best reflect the current scenario and projections, the mortality ASR was 97.2 and 72.0 per 100,000 men and women, respectively. For this period, the leading causes of cancer death were prostate, lung/trachea/bronchus, stomach, oral cavity and liver/intrahepatic bile ducts in males and breast, lung/trachea/bronchus, cervix, colon/rectum and central nervous system (CNS) in females (Table 3).
Table 3.
Number of cases of the leading causes of cancer deaths (N), with respective age-standardized mortality rates (ASR) per 100,000 men and women and 95% confidence intervals (95% CI) in Sergipe, 2014–2018.
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| Sites | N | ASR | 95% CI | Sites | N | ASR | 95% CI |
| Prostate | 971 | 21.3 | 19.97; 22.65 | Breast | 853 | 13.8 | 12.90; 14.76 |
| Lung/Tr/Br | 529 | 11.7 | 10.67; 12.66 | Lung/Tr/Br | 396 | 6.6 | 5.92; 7.21 |
| Stomach | 304 | 6.5 | 5.80; 7.27 | Cervix | 391 | 6.4 | 5.73; 6.99 |
| Oral cavity | 261 | 5.4 | 4.78; 6.10 | Colon/rectum | 356 | 5.8 | 5.17; 6.37 |
| Liver/IHBD | 236 | 5.1 | 4.49; 5.80 | CNS | 211 | 3.6 | 3.15; 4.13 |
| Totala | 4552 | 97.2 | 94.42; 100.07 | Totala | 4408 | 72.0 | 69.91; 74.17 |
ASR age-standardized rate (world population), CI Confidence interval, Br bronchus, Tr trachea, CNS Central nervous system, IHBD Intrahepatic bile ducts.
aAll cancer deaths, except NMSC.
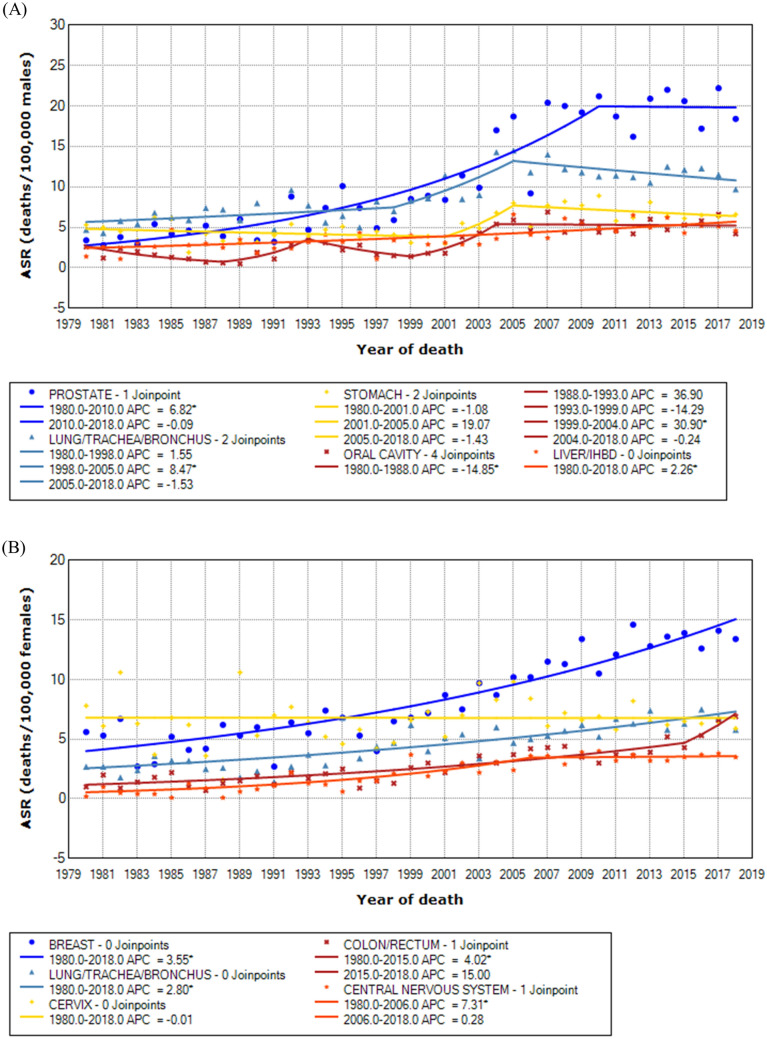
Table 4 shows the joinpoint analysis of cancer mortality by the main sites for both sexes. The curves modeled by Joinpoint are shown in Fig. 2.
Table 4.
Joinpoint analysis of cancer mortality by the main sites for age-standardized rates for both sexes. Sergipe, 1980–2018.
| Sites | JP | APC | 95% CI | AAPC | 95% CI |
|---|---|---|---|---|---|
| Male | |||||
| Prostate | 1 | (1980–2010) 6.82a | 5.40; 8.25 | 5.32a | 3.87; 6.80 |
| – | (2010–2018) − 0.09 | − 4.66; 4.69 | |||
| Lung/trachea/bronchus | 2 | (1980–1998) 1.55 | − 0.42; 3.57 | 1.72a | 0.11; 3.35 |
| – | (1998–2005) 8.47a | 1.37; 16.08 | |||
| – | (2005–2018) − 1.53 | − 3.16; 0.13 | |||
| Stomach | 2 | (1980–2001) − 1.08 | − 2.89; 0.76 | 0.74 | − 2.25; 3.83 |
| – | (2001–2005) 19.07 | − 9.37; 56.42 | |||
| – | (2005–2018) − 1.43 | − 3.57; 0.75 | |||
| Oral cavity | 4 | (1980–1988) − 14.85a | − 26.35; − 1.55 | 1.79 | − 5.17; 9.25 |
| – | (1988–1993) 36.90 | − 4.90; 97.07 | |||
| – | (1993–1999) − 14.29 | − 31.03; 6.52 | |||
| – | (1999–2004) 30.90a | 2.20; 67.66 | |||
| – | (2004–2018) − 0.24 | − 2.33; 1,90 | |||
| Liver/IHBD | 0 | (1980–2018) 2.26a | 1.46; 3.07 | 2.26a | 1.46; 3.07 |
| Female | |||||
| Breast | 0 | (1980–2018) 3.55a | 3.01; 4.09 | 3.55a | 3.01; 4.09 |
| Lung/trachea/bronchus | 0 | (1980–2018) 2.80a | 2.14; 3.48 | 2.80a | 2.14; 3.48 |
| Cervix | 0 | (1980–2018) − 0.01 | − 0.76; 0.74 | − 0.01 | − 0.76; 0.74 |
| Colon/rectum | 1 | (1980–2015) 4.02a | 3.08; 4.98 | 4.85a | 3.25; 6.48 |
| – | (2015–2018) 15.00 | − 3.22; 36.65 | |||
| CNS | 1 | (1980–2006) 7.31a | 5.02; 9.65 | 5.04a | 3.31; 6.80 |
| – | (2006–2018) 0.28 | − 2.51; 3.14 | |||
JP number of join points, APC annual percent change, AAPC average annual percent change, CI Confidence interval, CNS central nervous system, IHBD intrahepatic bile ducts.
aAPC/AAPC statistically significant.
Figure 2.
Time trends of cancer mortality for age-standardized rates (ASR) for the main sites in males (A) and females (B).
Discussion
The mortality curves for all cancers showed a significant increase until 2005 for men and 2006 for women and then they stabilized. At the same time, there was a significant drop in the mortality ASR from ill-defined causes. We believe that this is due to several factors: the implementation of the Unified Health System (SUS) in 1988 and family health programs, which made it possible for poor people to access health services, diagnostic resources and screening programs; government encouragement for health professionals to work in smaller cities and remote areas; greater coverage of SIM in the national territory with consequent reduction of underregistration deaths also in Sergipe22,23; and the improvement of the SIM database based on employee training in data coding.
According to GLOBOCAN 2018, mortality rates for all cancers combined worldwide are nearly 50% higher in males than in females1. We also verified a higher mortality ASR among males both in the entire series (70.1 versus 57.9) and in the last five years (97.2 versus 72.0). The average mortality ASRs from 2014 to 2018 were similar to those observed for the group of medium HDI countries in GLOBOCAN 20181.
As expected for chronic diseases, the 65+ age group showed the greatest increase in the mortality trend, most pronouncedly among men. Interestingly, the male age group 20–44 years old presented a slight increasing trend during the entire series (APC/AAPC of 1.31), which likely reflects improved information on death certificates and decreased deaths from ill-defined causes. The curves for the other age groups remained stable for the past decade in both sexes.
In our study, lung cancer was the leading cause of cancer-related death for both sexes combined. It also remains the leading cause of cancer death worldwide, accounting for 18.4% of all cancer-related deaths, followed by colorectal (9.2%), stomach (8.2%), liver (8.2%) and breast (6.6%)1.
Among men only, prostate cancer was the main cause of death in our population, followed by lung/trachea/bronchus, stomach, oral cavity and liver/intrahepatic bile ducts. Among women only, breast cancer was the main cause of death in our population, followed by lung/trachea/bronchus, cervix, colorectal and CNS. This profile is similar to more developed countries where higher mortality rates from lung, breast and colorectal cancers are observed and it is also similar to less developed countries where high rates of cervix, stomach and oral cavity cancers are observed1–5,24.
In our population, there was a statistically significant increase in lung cancer mortality in the period from 1998 to 2005 among men (APC 8.47) with stabilization of the curve afterwards. Among women, there was a steady, but less dramatic, increase across the series (APC/AAPC 2.8), possibly reflecting a lower degree of exposure to risk factors. The average mortality ASRs in the last 5 years of analysis—11.7/100,000 men and 6.6/100,000 women—were similar to those observed in low/medium HDI countries1. Lung cancer incidence and mortality have changed substantially over time, expressing different trends by sex, age group and region throughout the world, according to historic patterns of smoking prevalence, air pollution and other risk factors1,25. Most developed countries have experienced periods of increased incidence and mortality, first among men and then among women. After a certain period, their rates started to stabilize and then decline, first among men and then among women, mainly due to the decrease in smoking prevalence across generations. More recently, medium HDI countries seem to follow this same pattern3,4,24,25.
Mortality from prostate cancer showed a statistically significant increase until 2010 (APC 6.82) and then it stabilized, reaching an ASR of 18.4 per 100,000 men in 2018 in our study. Lima et al., in a study on mortality from prostate cancer in Aracaju, also found an upward trend with a statistically significant APC of 2.1 and an average ASR of 23.2/100,000 men from 1996 to 200610. Mortality rates from prostate cancer have been increasing in many countries in Central and South America (including Brazil) and in less developed countries in Asia and Europe. In contrast, mortality trends have stabilized or have been decreasing in recent years in the USA and other developed countries from Europe, Oceania and Asia, where early diagnosis appears to have played an important role1,4,24.
Breast cancer had the highest mortality ASR among our female population (13.8 in the last five years of the series), which is similar to the world media (13.0) and to trends observed in the Pan-American region1,26. In our study, the temporal trend continues rising throughout the series. We also observed that three age groups—20–44, 45–64 and 65+ years old—showed statistically significant upward trends throughout the series (data not shown). Breast cancer is the leading cause of cancer death among women in over 100 countries1. Martínez-Mesa et al. analyzed whether the HDI could explain differences in the incidence and mortality rates from breast cancer and gynecological cancers in the Pan-American region. They found that the HDI showed a positive correlation with breast cancer incidence and mortality rates and a negative correlation with cervical cancer incidence and mortality rates26.
In our study, the cervical cancer mortality ASR (6.4) was similar to that observed for the world (6.8); it was higher in relation to North America (2.6), but it was lower than that observed in South America (8.6), Central America (8.9) and the Caribbean (8.6)26. Although the temporal trend in mortality from cervical cancer was stable across the series in our state, we expect rates to decrease in the future after the addition of the tetravalent HPV vaccine to the national immunization calendar. The vaccination campaign started in 2014 for girls and in 2017 for boys. Currently, the target age group for vaccination is 9–14 years old27.
Colorectal cancer was the fourth leading cause of cancer death among women and the seventh among men in the period from 2014 to 2018. It presented a statistically significant upward trend curve with an AAPC of 4.85 and 3.96 across the series for females and males, respectively (data not shown). This means that this site could soon be among the top five for males in our state. The mortality rate for colorectal cancer has been increasing in Latin America, the Caribbean and South Africa, while it has been decreasing in most developed countries from Northern and Western Europe, North America and Oceania1,3,4,28. It is the third leading cause of death among men and women, separately, in the United States and the second and third leading causes of death in men and women, respectively, in Europe4,24. We believe that screening strategies, such as fecal occult blood tests or even colonoscopy, should be implemented for specific age groups to decrease mortality.
Stomach cancer was the third cause of cancer mortality in our male population, and it showed a stationary curve across the series. Since Helicobacter pylori causes approximately 75% of noncardia carcinomas of the stomach3,29, we assume that the prevalence of H. pylori among men is still high in Sergipe. There was a decrease in the mortality of stomach cancer in some countries in Asia (from 2000 to 2010)3 and in most countries in Latin America mainly thanks to improvements in basic sanitation, food preservation, health surveillance actions and the control of H. pylori infection. Carioli et al. pointed out that although there was a decrease in stomach cancer mortality from 1980 to 2014, rates still remained high in Latin America, particularly in Chile5.
According to the INCA protocol, the following sites were grouped as oral cavity: lips, oral cavity, salivary glands and oropharynx (C00–C10)6. The most well-known risk factors for these cancers are smoking, heavy alcohol intake, unprotected sun exposure (for lip cancer), HPV infection (especially for oropharynx cancer) and obesity6,30. Oral cavity cancer was the fourth leading cause of cancer death among men in our study (ASR of 5.4 in the last 5 years), but it showed a steady trend over the past decade. This mortality ASR was similar to the average observed among countries with low/medium HDI for men (5.0) and higher than the average observed for countries with high/very high HDI (1.5), according to GLOBOCAN 2018. Moreover, this cancer group was not among the main causes of cancer death among women, as observed for other regions of the world, regardless of the HDI1. Miranda-Filho and Bray pointed out that both incidence and mortality rates from oral cavity cancer were consistently higher among males than females globally, mainly reflecting the level of exposure to risk factors30.
For liver/intrahepatic bile duct mortality, we observed an upward trend with a statistically significant APC of 2.26 among men in Sergipe. According to GLOBOCAN 2018, mortality rates were 2 to 3 times higher among men in most world regions1. Hashim et al. also observed an upward trend in liver cancer mortality in North America, most Latin America and some Asian countries3. We emphasize that some of our cases correspond to metastatic carcinomas of undetermined primary sites. This limited our analysis on the role of hepatitis B and C virus infections in the onset of primary liver cancer.
In our study, the CNS was the fifth main site of cancer mortality among women and showed a stationary trend in the past decade. Like the liver, the CNS is a frequent site of metastasis. As we did not have access to histopathological data on patients, our analysis of primary CNS cancer mortality was limited.
As a strength of our study, we highlight that it presents cancer mortality over a long time period, and it was based on official data. Furthermore, there has been a progressive improvement in quality data of death certificates and in SIM coverage in our state, which can be verified by the progressive decrease in the mortality rates from ill-defined/undetermined causes since the beginning of the series in Sergipe.
Our study has some limitations. We did not include race in the analysis because we think this information is not reliable in our population. There is a high degree of miscegenation in Brazil, especially in the northeastern region. Furthermore, the race classification is based on how the individual self-reports, usually reflecting some social prejudices. We also did not have access to patients’ medical records, including the date of diagnosis, histopathological reports, treatments performed or hospitalizations. Further studies may go deeper into those topics.
Conclusion
Our results show that although there has been a statistically significant increase in cancer mortality rates associated with Western lifestyles, such as prostate, breast and colon/rectum (especially in women), high rates of cancer related to poverty and infections, such as stomach, cervix and oral cavity, still persist. This profile is similar to that observed in regions that have low and medium HDI and that are usually facing epidemiological transition.
In addition, due to population aging, we expect cancer mortality to rise in our state. Therefore, the government should implement or emphasize some measures and control strategies for the prevention of the most common types of cancer, which are evidence-based and well-established in developed countries, such as tobacco-control policies, vaccination programs (e.g., HPV and hepatitis B viruses), treatment for H. pylori, screening programs such as Papanicolau tests for cervical cancer and mammography and encouraging a healthier lifestyle.
We hope that this brief review of the epidemiology of mortality for the main sites of cancer in a low/medium HDI region will contribute to global findings. We also emphasize the importance of having high quality national databases with wide coverage so that they reflect real-world situations in the best possible way.
Acknowledgements
The authors wish to thank the personnel of the Cancer Registry for their great work in collecting data and preparing the database for this research work: José Erinaldo Lobo de Oliveira, Elma Santana de Oliveira, Maria das Graças Prata França, Sueli Pina Vieira, Marina Kobilsek, Maria Cristina Conceição Coelho Santos, Maria das Graças Rodrigues de Melo, and Cecília Ferreira.
Author contributions
Conception and design: M.S.L. and C.A.L.; analysis and interpretation of data: M.S.L. and C.A.L.; drafting of the article: M.S.L., H.F.F.S., A.R.M., E.C.H., A.D.M. and E.A.C.B.; critical revision of the article: C.A.L., H.L.F.B.; R.C.; final approval of the article: all authors approved the manuscript.
Competing interests
This research was partially supported by the Research Development Grant from the Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe—FAPITEC/SE (CALL MS/CNPq/FAPITEC/SE/SES-No 06/2018) to C.A.L. Protocol: 019.203. 00961/2018-2. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sierra MS, et al. Cancer patterns and trends in Central and South America. Cancer Epidemiol. 2016;44(Suppl 1):S23–S42. doi: 10.1016/j.canep.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Hashim D, et al. The global decrease in cancer mortality: Trends and disparities. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27:926–933. doi: 10.1093/annonc/mdw027. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Carioli G, et al. Cancer mortality predictions for 2017 in Latin America. Ann. Oncol. 2017;28:2286–2297. doi: 10.1093/annonc/mdx301. [DOI] [PubMed] [Google Scholar]
- 6.Ministério da Saúde/INCA. Estimativa 2020: Incidência de câncer no Brasil. Rio de Janeiro: Instituto Nacional de Câncer José Alencar Gomes da Silva (2019).
- 7.Atlas On-line de Mortalidade. https://www.inca.gov.br/MortalidadeWeb/pages/Modelo03/consultar.xhtml#panelResultado.
- 8.TabNet Win32 3.0: Mortalidade-Brasil. http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sim/cnv/obt10uf.def.
- 9.Andrade, L. A. et al. Spatial analysis and trends of mortality due to penile cancer in sergipe, 2000–2015. Cogitare Enferm.25, e64676 (2020).
- 10.Lima CA, da Silva AM, Kuwano AY, Rangel MRU, Macedo-Lima M. Trends in prostate cancer incidence and mortality in a mid-sized Northeastern Brazilian city. Rev. Assoc. Med. Bras. 2013;59:15–20. doi: 10.1590/S0104-42302013000100006. [DOI] [PubMed] [Google Scholar]
- 11.Lima CA, et al. Do cancer registries play a role in determining the incidence of non-melanoma skin cancers? Eur. J. Dermatol. 2018;28:169–176. doi: 10.1684/ejd.2018.3248. [DOI] [PubMed] [Google Scholar]
- 12.de Brito ÉAC, et al. Assessing trends of breast cancer and carcinoma in situ to monitor screening policies in developing settings. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Góes, J. A. P. et al. Trend and spatial analysis of prostate cancer mortality in the state of Sergipe, Brazil. Geospat. Health13, 732 (2018). [DOI] [PubMed]
- 14.Lima CA, Rangel MRU, Macedo-Lima M, Da Silva AM. Time trends in breast cancer incidence and mortality in a mid-sized northeastern Brazilian city. BMC Public Health. 2012;12:1. doi: 10.1186/1471-2458-12-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Melo, A. U. C. et al. Análise das estimativas de incidência de câncer de boca no Brasil e em Sergipe (2000–2010) Estimates’ evaluation of mouth cancer incidence in Brazil and Sergipe (2000–2010). Odontol. Clín.-Cient.11(1), 65–70 (2012).
- 16.Instituto Brasileiro de Geografia e Estatística - IBGE. Cidades/Sergipe/Panorama. Available at: https://cidades.ibge.gov.br/brasil/se/panorama. Accessed 19 Oct 2020.
- 17.Instituto Brasileiro de Geografia e Estatística - IBGE. Cidades/Sergipe/Aracaju/Panorama. Available at: https://cidades.ibge.gov.br/brasil/se/aracaju/panorama. Accessed 19 Oct 2020.
- 18.Instituto Brasileiro de Geografia e Estatística - IBGE. Projeção da população. Available at: https://www.ibge.gov.br/apps/populacao/projecao/. Accessed 19 Oct 2020.
- 19.Doll, R. et al.Cancer Incidence in Five Continents, Vol. I: International Union Against Cancer. International Agency for Research on Cancer (1966).
- 20.Segi, M. & Fujisaku, S. Cancer Mortality for Selected Sites in 24 Countries (1950–1957). Sendai, Japan: Department of Public Health, Tohoku University, School of Medicine (1960).
- 21.Joinpoint Regression Program, Version 4.7.0.0 - February 2019; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- 22.de Frias PG, Pereira PMH, de Andrade CLT, Szwarcwald CL. Sistema de Informações sobre Mortalidade: estudo de caso em municípios com precariedade dos dados. Cad. Saude Publica. 2008;24:2257–2266. doi: 10.1590/S0102-311X2008001000007. [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen L, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–1406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 24.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Cheng T-YD, et al. The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J. Thorac. Oncol. 2016;11:1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Mesa J, et al. Exploring disparities in incidence and mortality rates of breast and gynecologic cancers according to the Human Development Index in the Pan-American region. Public Health. 2017;149:81–88. doi: 10.1016/j.puhe.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Instituto Nacional do Câncer - INCA. Prevenção do câncer do colo do útero. Available at: https://www.inca.gov.br/controle-do-cancer-do-colo-do-utero/acoes-de-controle/prevencao. Accessed 15 Oct 2020.
- 28.Araghi M, et al. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer. 2019;144:2992–3000. doi: 10.1002/ijc.32055. [DOI] [PubMed] [Google Scholar]
- 29.Peleteiro B, La Vecchia C, Lunet N. The role of Helicobacter pylori infection in the web of gastric cancer causation. Eur. J. Cancer Prev. 2012;21:118–125. doi: 10.1097/CEJ.0b013e32834a7f66. [DOI] [PubMed] [Google Scholar]
- 30.Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551. doi: 10.1016/j.oraloncology.2019.104551. [DOI] [PubMed] [Google Scholar]