Abstract
Objectives
This study aimed to determine the effect of ambient air pollution and temperature on stillbirth in Tehran.
Methods
In this time-series study, the effect of O3 (ppb), CO (ppm), NO2 (ppb), SO2 (ppb), PM2.5 (μg/m3), and minimum, maximum, and mean daily temperature (°C) on stillbirth was evaluated in Tehran, Iran between March 2015 and March 2018. Using a quasi-Poisson regression model in combination with a Distributed Lag Non-linear Models (DLNM), the Relative Risk (RR) was estimated through comparing the high temperature (99th, 95th, and 75th percentiles) and low temperature (1st, 5th, and 25th percentiles) with the median. The effect of air pollution was estimated for each 1-, 5-, or 10-unit increase in the concentration during lags (days) 0–21.
Results
Among air pollutants, only a 5-ppm increase in the SO2 concentration in lag 0 increased the risk of stillbirth significantly (RR = 1.062; 1.002–1.125). The largest effect of heat was observed while comparing the 99th percentile of minimum daily temperature (26.9 °C) with the median temperature (13.2 °C), which was not statistically significant (RR = 1.25; 0.95–1.65). As for cold, a non-significant protective effect was observed while comparing the 1st percentile of maximum daily temperature (3.1 °C) with the median temperature (23.2 °C) (RR = 0.92; 0.72–1.19).
Conclusion
Each 5-ppm increase in the mean daily SO2 in lag 0 increased the risk of stillbirth by 6% while other air pollutants had no significant effects on stillbirth. In lags 0 and 1, the heat increased the risk of stillbirth while the cold had protective effects, which were not statistically significant.
Keywords: Stillbirth, Ambient temperature, Air pollution, Distributed lag non-linear models
Introduction
According to the World Health Organization (WHO) estimates, 2.6 million stillbirths occur in the world annually [1] with rates ranging from 3.1 in 1000 births in high-income countries to 29 in 1000 births in Sub-Saharan Africa and 12.9 in 1000 births in the Middle East [2]. The rate of stillbirth has varied from 8.57 to 11.7 in 1000 births in different parts of Iran in recent years [3, 4]. Although several maternal and neonatal factors have been identified, stillbirth of unknown cause still comprises 25–60% of the total cases [5, 6]. Recent studied have evaluated the effects of environmental risk factors like ambient air pollution and low and high temperature on stillbirth [7–11].
A systematic review of the studies published by November 2016 found only four studies about the effect of ambient temperature on stillbirth, which were all conducted in developed countries and indicated the adverse effects of the heat on stillbirth [7]. As for air pollution, ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and particulate matter (PM), in addition to their effects on increased mortality due to different diseases worldwide [12], based on the evidence from systematic review and meta-analysis studies, it is also possible that they have adverse effects on pregnancy outcomes [9, 13]. However, further studies benefiting from better measurement of air pollutants and controlling confounding variables were recommended to strengthen the evidence due to controversial results [9, 14].
In Iran, however the results of studies have shown that air pollutants especially particulate matter generated by anthropogenic source and dust storm condition has other health effects [15–18], but only one study evaluated the effect of air pollution on stillbirth in Ahvaz [19] and no study has investigated the effect of ambient temperature on stillbirth. On the other hand, the effect of temperature is not linear and also the effects of ambient air pollution and temperature may not be limited to the exposure day. Therefore, this study was conducted to determine the effect of ambient air pollution and temperature on stillbirth in Tehran, Iran using the Distributed lag non-linear models (DLNM) which is capable of evaluating the non-linear and lag effects of exposure on the outcome [20].
Materials and methods
Study population
In this time-series study, the data of 3460 stillbirth cases in all public and private hospitals of Tehran from March 21, 2015 to March 20, 2018 that occurred to mothers permanently residing in Tehran were obtained from the Iranian Maternal and Neonatal (IMaN) Network, belong to Neonatal Health Office, Iranian Ministry of Health and Medical Education. Stillbirth was defined as the birth of a fetus with no signs of life at or after 22 weeks of pregnancy.
Exposure
Meteorological data, including the daily minimum, maximum, and mean temperature and relative humidity were collected from three stations (Mehrabad, Shemiran, and Geophysics stations) of the Iran Meteorological Organization [21] and averaged for each parameter. The missing data were extracted from the National Oceanic and Atmospheric Administration website [22].
The data of air pollution variables were collected from 37 stations including 21 stations of Tehran Air Quality Control Company [23, 24] and 16 stations of the Department of Environment. The data of the above stations were first validated according to the WHO criteria [25] and the data of the stations that lack sufficient validity were excluded from the study. Finally, the daily data of each air pollutant were calculated according to the WHO and US Environmental Protection Agency (EPA) indexes [25, 26], including the maximum 8-h moving average O3 (ppb) and CO (ppm) concentration, maximum 1-h average NO2 concentration (ppb), mean 24-h average SO2 (ppb), and PM2.5 (μg/m3), using the average values of valid stations.
Statistical analysis
The measures of central tendency and dispersion are used to describe quantitative data, and percentage and frequency are used to present qualitative data. A quasi-Poisson regression model in combination with the DLNM was applied for time-series analysis of the effects of ambient air pollution and temperature on stillbirth as follows:
Where Yt represents the number of stillbirths on day t, α is the intercept, and cb is the “cross-basis” function [27, 28] for ambient temperature. Temperature and lag (day) were defined with 4 and 5 degrees of freedom (df), respectively. The effects of relative humidity, seasonal and long-term trends, and weekdays and holidays were controlled in the model. ns represents a natural cubic spline function [27, 28] and β is the regression coefficient. The Akaike Information Criterion (AIC) was used to select the df for temperature and lag. Moreover, for evaluating the effect of temperature, the effect of air pollutants was adjusted using the ns function.
A separate model was designed for each air pollutant to evaluate the effect of ambient air pollution and the cb function was considered for each pollutant.
In the model used for investigating the effect of air pollution, to adjust the effect of temperature, the mean daily temperature was introduced into the model using the ns function with df = 3.
In this study, the effect of exposure was assessed in lags (days) 0–21 prior to the outcome. For minimum, maximum, and mean daily temperature, the 99th, 95th, and 75th percentiles for heat and the 1st, 5th, and 25th percentiles for cold were compared with the median temperature and relative risk (RR) and 95% confidence interval (95% CI) were calculated. To study the effect of air pollution, RR was calculated for each 10-unit increase in PM2.5, NO2, and O3, each 5-unit increase in SO2, and each 1-unit increase in CO. Data were analyzed using DLNM package incorporated in the R software version 3.5.2 [20, 27]. All analyses were two-sided and P value less than 0.05 were considered significant.
Ethical considerations
The study protocol was approved by the Institutional Research Ethics Committee of School of Public Health & Allied Sciences, Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1397.156). The required clearances for data collection from related organizations were obtained from the Research Deputy of the School of Public Health, Tehran University of Medical Sciences. The data were used and reported anonymously.
Results
Descriptive statistics
Of 516,570 births registered in the Iranian Maternal and Neonatal Network during 2015–2018 for Tehran, 3460 were stillbirths (6.69 in 1000 births). The daily distribution of stillbirth as well as the descriptive statistics of exposure variables including temperature and air pollutants is presented in Table 1. Using the average values of the three stations during three years, the lowest temperature according to minimum temperature was −7.22 °C and the highest temperature according to maximum temperature was 42.22 °C. Concentration of maximum 8-h moving average for O3, maximum 1-h average for NO2, mean 24-h average for PM2.5, maximum 8-h moving average for CO, mean 24-h average for SO2 during three years were, 32.6 ppb, 71.1 ppb, 33.0 μg/m3, 3.6 ppm and 12.5 ppb respectively.
Table 1.
Daily distribution of stillbirth, meteorological and pollutant variables in Tehran during March 2015 to March 2018
| Variable | Mean ± SD | Min | 1th percentile | 5th percentile | 25th percentile | Median | 75th percentile | 95th percentile | 99th percentile | Max |
|---|---|---|---|---|---|---|---|---|---|---|
| Stillbirth (cases) | 2.96 ± 1.92 | 0 | 0 | 0 | 2 | 3 | 4 | 5 | 7 | 10 |
| Minimum temperature(°C) | 12.8 ± 8.7 | −7.2 | −4.1 | −0.1 | 5.3 | 13.2 | 20.9 | 24.8 | 26.9 | 28.2 |
| Mean temperature(°C) | 17.7 ± 9.7 | −4.2 | −0.6 | 3.2 | 9.1 | 17.9 | 26.9 | 31.3 | 33.1 | 36.5 |
| Maximum temperature(°C) | 22.9 ± 10.4 | −1.4 | 3.1 | 6.7 | 13.8 | 23.2 | 32.6 | 37.3 | 39.0 | 42.2 |
| Relative humidity (%) | 35.45 ± 17.76 | 9.54 | 11.66 | 14.33 | 21.59 | 31.38 | 45.09 | 70.89 | 86.93 | 96.38 |
| maximum 8-h moving average for O3 (ppb) | 32.60 ± 15.84 | 5.38 | 8.13 | 11.00 | 19.88 | 30.94 | 41.86 | 62.09 | 75.24 | 96.13 |
| maximum 1-h average for NO2 (ppb) | 71.08 ± 18.78 | 30.7 | 37.05 | 44.25 | 58.19 | 68.47 | 82.10 | 54.40 | 133.25 | 149.33 |
| mean 24-h average for PM2.5 (μg/m3) | 33.03 ± 14.12 | 7.54 | 11.54 | 16.00 | 24.42 | 29.31 | 37.95 | 62.27 | 80.07 | 119.29 |
| maximum 8-h moving average for CO (ppm) | 3.64 ± 0.83 | 2.03 | 2.24 | 2.49 | 3.03 | 3.54 | 4.09 | 5.17 | 5.91 | 7.47 |
| mean 24-h average for SO2 (ppb) | 12.50 ± 4.36 | 3.19 | 4.31 | 5.71 | 8.97 | 12.97 | 15.12 | 19.62 | 25.04 | 31.78 |
Effect of ambient temperature on stillbirth
The overall effects of ambient temperature according to RR in different lags and temperatures and according to minimum, maximum, and mean daily temperature are presented in Fig. 1 as a three-dimensional graph. RR was calculated through comparing the risk of stillbirth at different temperatures with the corresponding median temperature. The effects of daily heat and cold according to the comparison of different percentiles with the median temperature for minimum, maximum, and mean temperature for the first seven lags are demonstrated in Table 2. Moreover, the results for all lags (0–21) are presented in Figs. 2, 3, 4. The highest RR for heat was observed in comparison of the 99th percentile with the median temperature according to minimum daily temperature, which was not significant (RR = 1.25; 0.95 to 1.65). The cold had non-significant protective effect in comparison of the 1st percentile with the median value according to maximum daily temperature (RR = 0.92; 0.72–1.19).
Fig. 1.
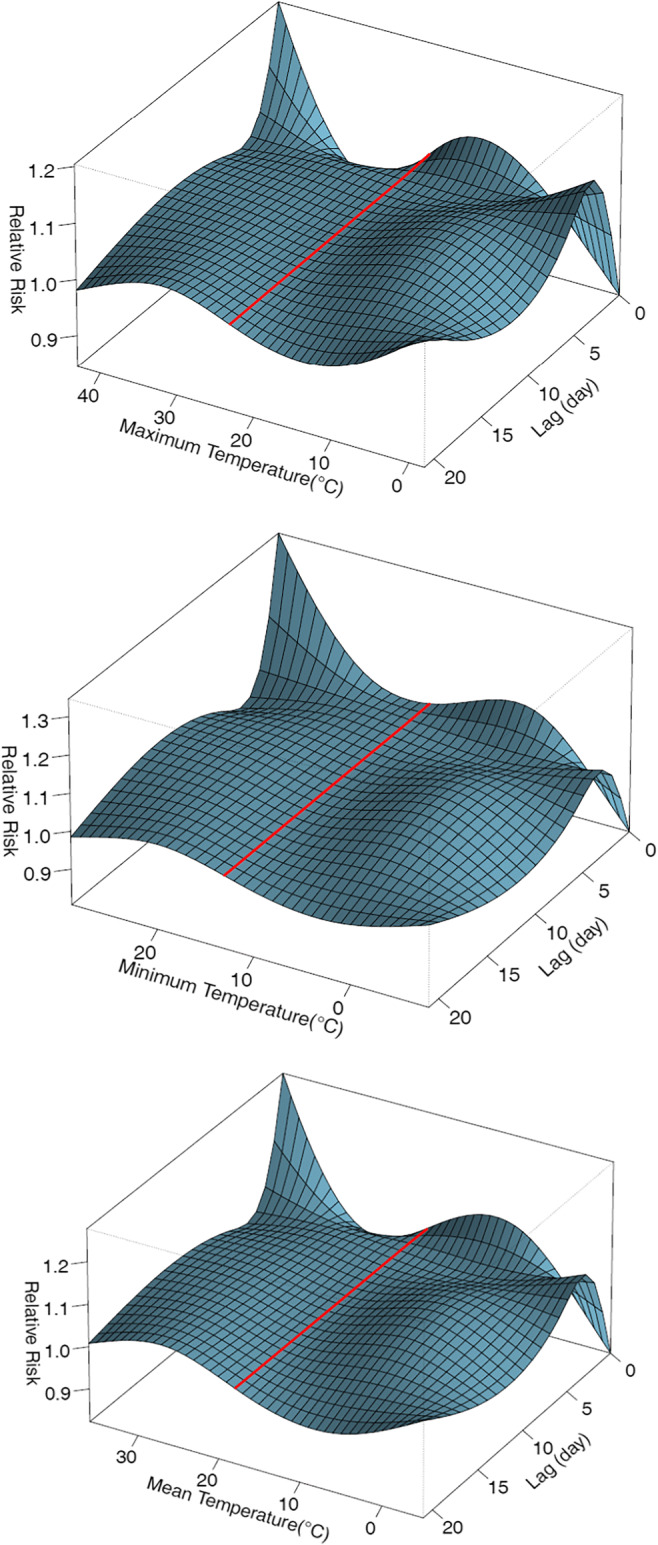
Three-dimensional graph of the effect of maximum, minimum and mean daily temperature on stillbirth in Tehran (reference: median for maximum temperature: 23.2 °C, for minimum temperature: 13.2 °C, for mean temperature: 17.9 °C)
Table 2.
The effect of heat and cold according to maximum, minimum and mean daily temperature on stillbirth in Tehran
| Relative Risk (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Heat Effect | Cold Effect | ||||||
| Variable | Lag (day) | 99th vs 50th percentile | 95th vs 50th percentile | 75th vs 50th percentile | 1th vs 50th percentile | 5th vs 50th percentile | 25th vs 50th percentile |
| Maximum daily temperature | 0 | 1.07 (0.81–1.41) | 1.02 (0.79–1.29) | 0.93 (0.77–1.13) | 0.92 (0.72–1.19) | 0.98 (0.78–1.23) | 1.05 (0.89–1.24) |
| 1 | 1.03 (0.90–1.18) | 1.01 (0.89–1.13) | 0.96 (0.87–1.06) | 0.98 (0.87–1.11) | 0.997 (0.89–1.12) | 1.02 (0.94–1.11) | |
| 2 | 1.01 (0.92–1.11) | 1.00 (0.92–1.09) | 0.99 (0.92–1.06) | 1.03 (0.95–1.11) | 1.01 (0.94–1.09) | 1.01 (0.95–1.07) | |
| 3 | 1.00 (0.90–1.11) | 1.00 (0.91–1.10) | 0.999 (0.93–1.08) | 1.05 (0.96–1.15) | 1.02 (0.94–1.12) | 1.00 (0.94–1.07) | |
| 4 | 0.999 (0.90–1.11) | 1.00 (0.91–1.10) | 1.01 (0.93–1.08) | 1.05 (0.96–1.15) | 1.03 (0.95–1.12) | 1.00 (0.94–1.07) | |
| 5 | 1.00 (0.92–1.09) | 1.01 (0.93–1.09) | 1.01 (0.95–1.07) | 1.05 (0.97–1.12) | 1.03 (0.97–1.11) | 1.01 (0.96–1.06) | |
| 6 | 1.01 (0.94–1.08) | 1.01 (0.95–1.07) | 1.01 (0.96–1.06) | 1.04 (0.98–1.10) | 1.04 (0.98–1.09) | 1.02 (0.98–1.06) | |
| 7 | 1.01 (0.95–1.08) | 1.01 (0.96–1.07) | 1.01 (0.96–1.06) | 1.03 (0.98–1.08) | 1.04 (0.99–1.09) | 1.03 (0.99–1.06) | |
| Minimum daily temperature | 0 | 1.25 (0.95–1.65) | 1.17 (0.94–1.47) | 1.05 (0.89–1.25) | 0.93 (0.74–1.16) | 1.03 (0.85–1.24) | 1.06 (0.93–1.22) |
| 1 | 1.11 (0.98–1.26) | 1.08 (0.97–1.19) | 1.03 (0.95–1.12) | 0.97 (0.87–1.08) | 1.00 (0.91–1.10) | 1.02 (0.95–1.09) | |
| 2 | 1.02 (0.93–1.12) | 1.02 (0.94–1.10) | 1.01 (0.95–1.08) | 0.99 (0.92–1.07) | 0.99 (0.92–1.06) | 0.99 (0.94–1.04) | |
| 3 | 0.98 (0.88–1.09) | 0.99 (0.91–1.09) | 1.01 (0.94–1.08) | 1.01 (0.92–1.10) | 0.99 (0.91–1.07) | 0.98 (0.93–1.04) | |
| 4 | 0.98 (0.88–1.08) | 0.99 (0.91–1.08) | 1.01 (0.94–1.08) | 1.01 (0.93–1.10) | 0.99 (0.92–1.08) | 0.99 (0.93–1.04) | |
| 5 | 0.99 (0.91–1.07) | 0.999 (0.93–1.07) | 1.01 (0.96–1.07) | 1.01 (0.94–1.08) | 1.01 (0.95–1.07) | 0.998 (0.95–1.04) | |
| 6 | 1.00 (0.94–1.07) | 1.01 (0.96–1.07) | 1.02 (0.97–1.07) | 1.01 (0.95–1.07) | 1.02 (0.97–1.07) | 1.01 (0.97–1.05) | |
| 7 | 1.02 (0.96–1.08) | 1.02 (0.97–1.08) | 1.02 (0.98–1.07) | 1.00 (0.95–1.06) | 1.03 (0.98–1.08) | 1.02 (0.98–1.05) | |
| Mean daily temperature | 0 | 1.14 (0.86–1.52) | 1.07 (0.83–1.39) | 0.97 (0.79–1.17) | 0.93 (0.73–1.19) | 1.01 (0.81–1.26) | 1.07 (0.91–1.26) |
| 1 | 1.07 (0.93–1.23) | 1.04 (0.92–1.18) | 0.98 (0.89–1.09) | 0.98 (0.87–1.10) | 1.01 (0.90–1.12) | 1.03 (0.95–1.11) | |
| 2 | 1.02 (0.93–1.13) | 1.01 (0.93–1.11) | 0.998 (0.93–1.08) | 1.01 (0.94–1.09) | 1.00 (0.93–1.08) | 0.998 (0.94–1.06) | |
| 3 | 1.00 (0.89–1.12) | 1.01 (0.91–1.11) | 1.01 (0.93–1.10) | 1.03 (0.94–1.13) | 1.01 (0.92–1.09) | 0.99 (0.93–1.06) | |
| 4 | 0.999 (0.89–1.11) | 1.01 (0.91–1.11) | 1.01 (0.93–1.10) | 1.03 (0.95–1.13) | 1.01 (0.93–1.10) | 0.99 (0.93–1.06) | |
| 5 | 1.01 (0.92–1.10) | 1.01 (0.93–1.10) | 1.02 (0.95–1.09) | 1.03 (0.96–1.11) | 1.02 (0.96–1.09) | 1.01 (0.96–1.06) | |
| 6 | 1.01 (0.94–1.09) | 1.02 (0.95–1.09) | 1.02 (0.97–1.08) | 1.02 (0.97–1.09) | 1.03 (0.98–1.08) | 1.02 (0.98–1.06) | |
| 7 | 1.02 (0.96–1.09) | 1.02 (0.96–1.09) | 1.02 (0.97–1.08) | 1.02 (0.97–1.09) | 1.03 (0.99–1.08) | 1.03 (0.99–1.06) | |
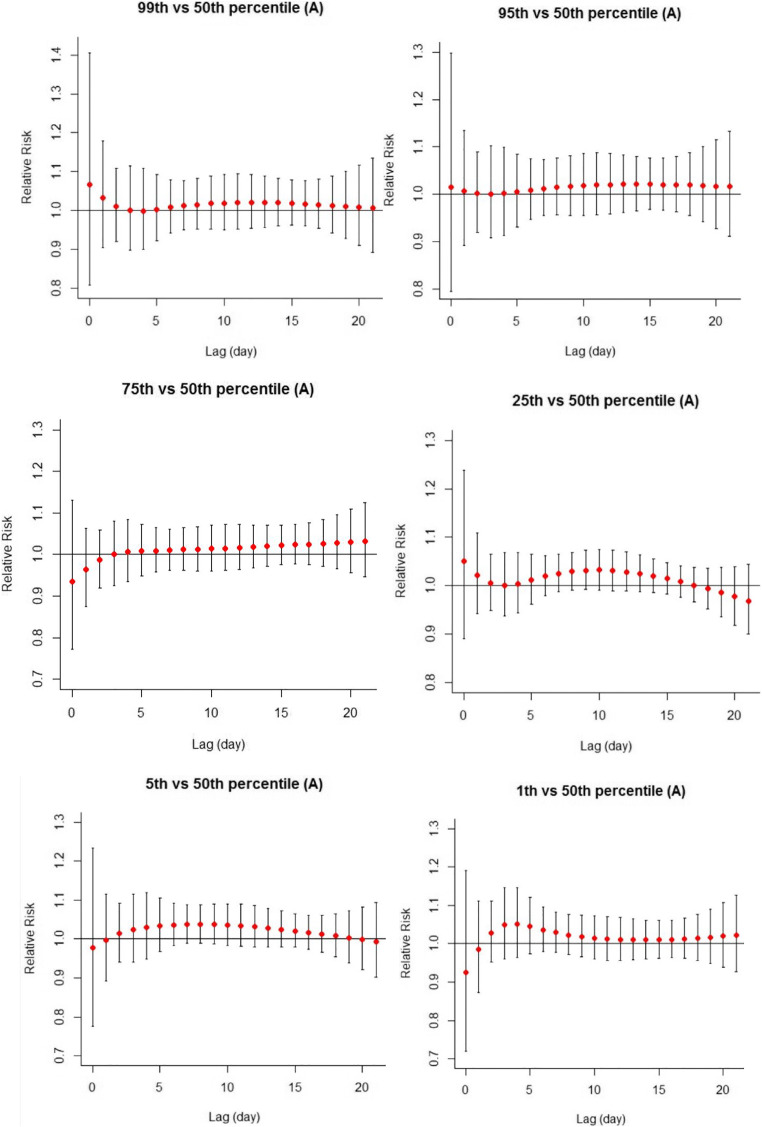
Fig. 2.
The effect of heat (99th, 95th, and 75th percentiles) and cold (1st, 5th, and 25th percentiles) according to maximum daily temperature on stillbirth in Tehran (reference: median for maximum temperature: 23.2 °C)
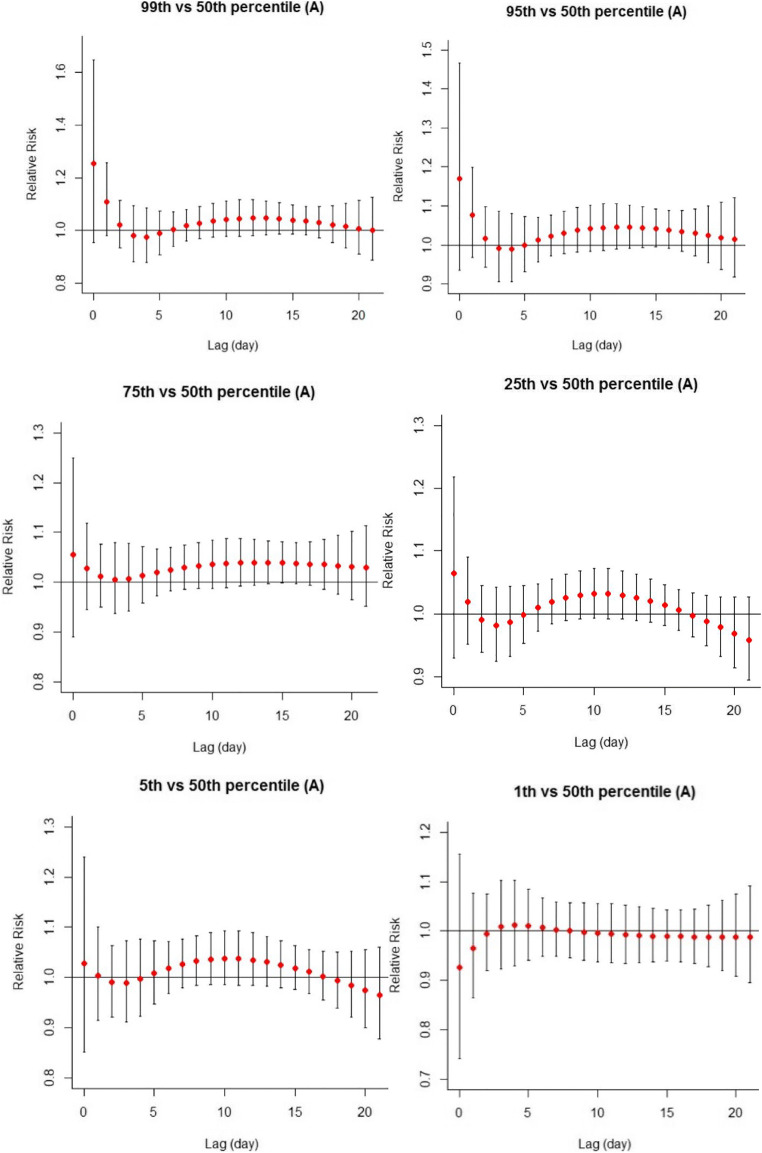
Fig. 3.
The effect of heat (99th, 95th, and 75th percentiles) and cold (1st, 5th, and 25th percentiles) according to minimum daily temperature on stillbirth in Tehran (reference: median for minimum temperature: 13.2 °C)
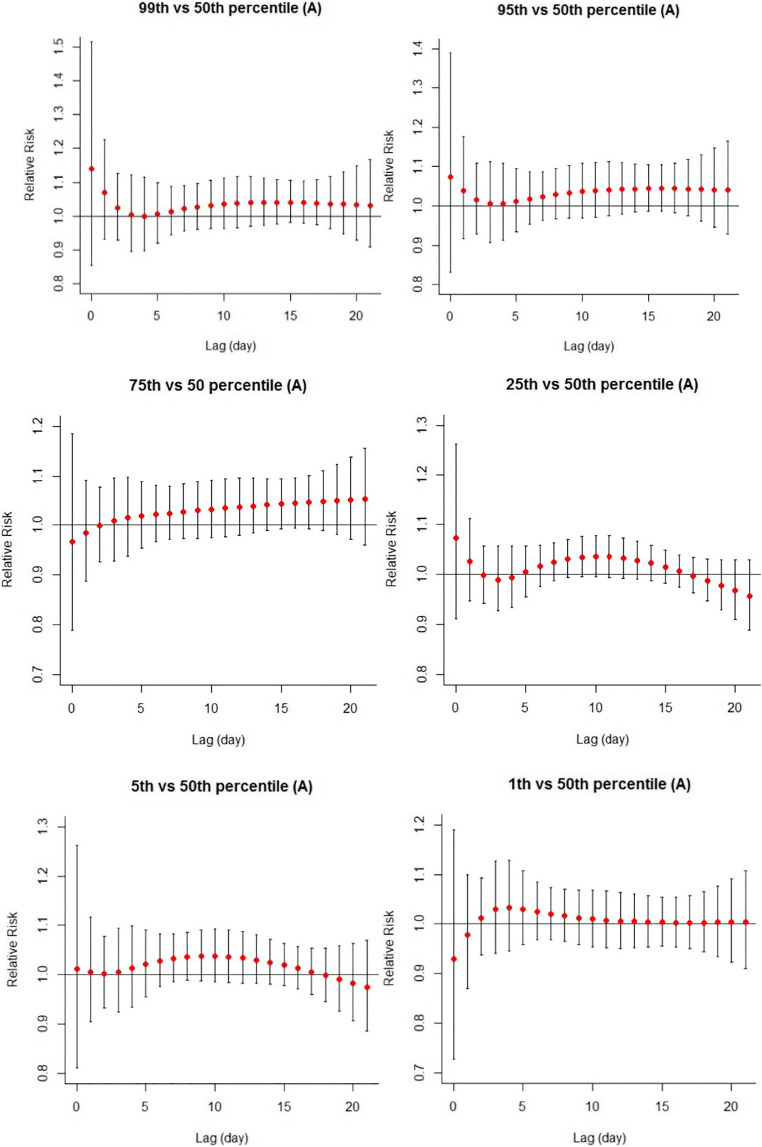
Fig. 4.
The effect of heat (99th, 95th, and 75th percentiles) and cold (1st, 5th, and 25th percentiles) according to mean daily temperature on stillbirth in Tehran (reference: median for mean temperature: 17.9 °C)
Effect of ambient air pollution on stillbirth
Table 3 shows the effects of air pollutants on stillbirth in lags 0–7 post exposure. SO2 had a significant effect on the increased risk of stillbirth in lag 0 (RR = 1.062; 1.002 to 1.125) while the association of other air pollutants with stillbirth was not statistically significant (the results for lags 8–21 are not reported due to lack of significance and similar effects).
Table 3.
The effect of air pollution on stillbirth in Tehran
| Relative Risk (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Lag (day) | 10 μg/m3 increase in PM2.5 | 10 ppb increase in NO2 | 10 ppb increase in O3 | 5 ppb increase in SO2 | 1 ppm increase in CO |
| 0 | 1.009 (0.989–1.029) | 1.008 (0.990–1.026) | 0.994 (0.955–1.034) | 1.062 (1.002–1.125) | 1.007 (0.972–1.044) |
| 1 | 1.004 (0.994–1.015) | 1.004 (0.995–1.014) | 0.993 (0.970–1.017) | 1.028 (0.993–1.064) | 1.003 (0.983–1.024) |
| 2 | 1.001 (0.992–1.009) | 1.002 (0.995–1.009) | 0.994 (0.975–1.012) | 1.005 (0.979–1.032) | 0.999 (0.985–1.015) |
| 3 | 0.999 (0.991–1.007) | 1.000 (0.993–1.007) | 0.994 (0.976–1.012) | 0.991 (0.966–1.018) | 0.997 (0.982–1.012) |
| 4 | 0.998 (0.989–1.006) | 0.999 (0.991–1.006) | 0.994 (0.977–1.013) | 0.984 (0.958–1.010) | 0.995 (0.980–1.010) |
| 5 | 0.998 (0.990–1.005) | 0.998 (0.991–1.005) | 0.995 (0.978–1.012) | 0.980 (0.956–1.006) | 0.993 (0.979–1.007) |
| 6 | 0.998 (0.991–1.005) | 0.998 (0.991–1.004) | 0.995 (0.979–1.011) | 0.980 (0.957–1.004) | 0.992 (0.980–1.005) |
| 7 | 0.998 (0.992–1.005) | 0.997 (0.992–1.003) | 0.996 (0.981–1.010) | 0.982 (0.959–1.006) | 0.992 (0.980–1.004) |
Effects of ambient air pollution and temperature with adjusting the effects of each other
In the ambient temperature model, adjustment for effects of air pollutants did not change the results. Furthermore, adjustment of the air pollution model for the effect of mean temperature did not affect the results.
Discussion
In this study, heat had non-significant increasing and cold had non-significant protective effects on stillbirth in lags 0 and 1. As for air pollutants, SO2 had a significant effect on the risk of stillbirth only in lag 0 as every 5-ppb increase in the 24-h average SO2 concentration increased the risk of stillbirth by 6%.
Few studies have investigated the effects of temperature (heat and cold) on the risk of stillbirth. A recent systematic review of the studies published by November 2016 showed only four studies in this regard [7]. A retrospective cohort study by Strand in Brisbane, Australia according to the data of 2005–2009 showed that increasing temperatures in the last four weeks of pregnancy significantly increased the risk of stillbirths for fetuses of <28 weeks’ gestation. As for the effect of cold, the hazard ratio (HR) for stillbirth was 0.3 at 12 °C relative to 21 °C, which was not statistically significant [29]. According to a time-series study by Arroyo et al. in Madrid, Spain using the 2001–2009 data, every 10 °C increase in the minimum and maximum temperature in the second and third trimester significantly increased the risk of late fetal death by 1.003 and 1.037 times, respectively [30]. Auger et al. conducted a case-crossover study in Quebec, Canada using the 1981–2011 data. In this study, the risk of term stillbirth at maximum daily temperatures of 28 °C, 30 °C, and 32 °C relative to the reference 20°C significantly increased by 1.16, 1.22, and 1.28 times respectively while these effects decreased gradually for lower gestational weeks. Although the effect of cold was protective in some sub groups, it was not statistically significant [31]. Another study by Basu et al. in California using the 1999–2009 data showed a 10.4% increase in the risk of stillbirth for every 10 °F (5.6 °C) increase in the temperature (cumulative effects of lags 2–6) while no associations were found during cold seasons [32].
Except for the above four studies, a study in Sweden evaluated the effect of cold on stillbirth. The births from 1915 to 1929, which—unlike most societies today—experienced fewer indoor-heating amenities from protection from cold, were included in this study. The mean (standard deviation) temperature was 5.0 (2.4) °C during pregnancy. The hazard ratio of stillbirth was 1.08 (95% CI 1.00–1.17) for a 1 °C decrease in temperature during pregnancy [33]. In another study in Brisbane, Australia, exposure to cold (5th percentile) and high (95th percentile) temperature during the second trimester relative to the threshold temperature increased the risk of stillbirth significantly (HR = 1.23 and 1.47, respectively) [34]. In another study of the data of 12 cities in the United States, chronic exposure to high and low temperature during pregnancy significantly increased the risk of stillbirth by 3.71 and 4.75 times, respectively. Moreover, a 1 °C increase in temperature in the week before delivery in hot seasons significantly increased the risk of stillbirth by 1.6 times while this effect was not significant for low temperatures [35].
In general, the results of previous studies suggest the harmful effects of the high ambient temperature on the risk of stillbirth but sufficient evidence is lacking for the effect of low temperature. There is marked heterogeneity among previous studies in terms of duration of exposure (acute or chronic), lag period of exposure and outcome, sample size, the climate of different regions in terms of extreme heat and cold, which could be the reason for slight differences in the results.
Although the exact mechanism of the effect of temperature on stillbirth is unclear, several biological mechanisms have been suggested for the effect of high temperature compared to low temperature on stillbirth. Gestation causes physiological changes in the pregnant mother’s body that may change thermoregulation ability [31, 36] causing dehydration when exposed to high temperatures [37], decreased blood flow to the fetus and uterine contraction [37–39]. Moreover, heat stress may lead to induction of labor through secreting some hormones [37, 38, 40]. Exposure to high temperatures may also cause injury to the cells, placenta, or vascular systems [41]. In terms of behavior, people try to minimize their exposure to low temperature through staying indoor and using warm clothes and indoor heating amenities.
Regarding the effect of ambient air pollution, Siddika et al. [9] conducted a systematic review and meta-analysis of 13 published articles by April 2015, including 11 studies on long-term effects and 2 studies on the effects of short-term exposure. As for the long-term effects, for estimating the pooled effect for each air pollutant, according to the entire pregnancy or each trimester, 2–3 studied were included in the meta-analysis in each sub-group. The pooled effect of exposure to air pollutants, despite indicating elevated risks, were not statistically significant. Furthermore, point estimates were slightly elevated in the third trimester compared to other trimesters. To evaluate the effect of short-term exposure, two time-series studies used a maximum lag time of 6 days before delivery, and both found significant effects for air pollutants. Faiz et al. used the 1980–2004 New Jersey data and found that a one interquartile range (IQR) increase in the mean concentration of CO and SO2 two days before delivery increased the risk of stillbirth by 1.20 and 1.11 times respectively while this effect was not significant for other air pollutants [42]. Pereira et al. also found that stillbirth had a marginal association with SO2 and CO [43]. The results of these two studies regarding SO2 are consistent with our study, which may indicate the stronger acute effects of these pollutants because our study also showed acute effects of SO2 in lag 0. The results of the studies that are published after this meta-analysis are inconsistent. In a recent, study in Harris County, Texas, a 3.6-ppb increase in O3 exposure increased the risk of stillbirth by 1.09 times while acute exposure had no significant effects [44]. A cohort study conducted in China also reported the effects of long-term exposure to O3 and PM2.5 [45]. A study in the United States showed the acute and chronic effects of O3 exposure on stillbirth [46].
In the only similar study conducted in Ahvaz, Iran by Dastoorpoor et al., although the results showed that premature delivery and spontaneous abortion had a significant correlation with increased concentrations of SO2, NO2, CO, and PM2.5, no significant relationship was found between air pollution and stillbirth [19]. The possible mechanisms of the effect of ambient air pollution on pregnancy outcomes are summarized in a systematic review by S. Shah et al. [13]. SO2, which was found to have a significant effect on stillbirth in our study, may induce developmental and functional toxicities through affecting neuromuscular coordination, causing adverse effects on the function and structure of the embryo or fetus [13, 47].
Strengths and limitations
One of the limitations of this study was that exposure was assessed according to the daily temperature reported by Iran Meteorological Organization, which may not indicate the true exposure because people may use heating or cooling amenities or stay indoor during polluted days. Moreover, this study only evaluated the acute effects of exposure to ambient temperature and air pollution and it was not possible to assess their chronic effects.
The stillbirth data were obtained from the Iranian Maternal and Neonatal Network of the Ministry of Health, which covers all hospitals in Tehran and therefore represents the Tehran population. This was the first study of the effect of ambient temperature and air pollution on stillbirth in Tehran (capital of Iran) where the birth rate is the highest compared to other Iranian cities and air pollution is a major problem.
Conclusion
A 5-ppm increase in the 24-h average SO2 concentration in lag 0 increased the risk of stillbirth by 6% while other air pollutants including O3, NO2, CO and PM2.5 had no significant effects on stillbirth in lags 0–21. Extreme heat (the 99th percentile compared with the median temperature) had non-significant increasing and extreme cold (the 1st percentile compared with the median temperature) had non-significant protective effects on stillbirth in lags 0 and 1.
Acknowledgments
The authors wish to thank the personnel of the Neonatasl Health Office, Iranian Ministry of Health and Medical Education, for their assistance in collecting the primary data.
Funding
This study was the result of a PhD thesis and was financially supported by Tehran University of Medical Sciences.
Compliance with ethical standards
Conflict of interests
Authors have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sexual and reproductive health; The neglected tragedy of stillbirths. World Heath Organization. 2017. http://www.who.int/reproductivehealth/topics/maternal_perinatal/stillbirth/en/.
- 2.Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, Gardosi J, Day LT, Stanton C. Stillbirths: where? When? Why? How to make the data count? Lancet. 2011;377(9775):1448–1463. doi: 10.1016/s0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 3.Hajian-Tilaki K, Esmaielzadeh S, Sadeghian G. Trend of stillbirth rates and the associated risk factors in Babol, northern Iran. Oman medical journal. 2014;29(1):18–23. doi: 10.5001/omj.2014.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahani MA, Akbarian Rad Z, Naghavian M, Salmanian T, Haghshenas MM. Factors affecting stillbirth rate in the hospitals affiliated to Babol University of Medical Sciences. Iranian Journal of Neonatology IJN. 2015;6(3):22–27. [Google Scholar]
- 5.Nankali A, Hematti M, Mahdavi Z. Study of the factors associated with stillbirth in pregnant women admitted in Imam Reza Teaching Hospital in Kermanshah (2011–2014) The Iranian Journal of Obstetrics, Gynecology and Infertility. 2017;20(1):1–9. doi: 10.22038/ijogi.2017.8617. [DOI] [Google Scholar]
- 6.Liu L-C, Wang Y-C, Yu M-H, Su H-Y. Major risk factors for stillbirth in different trimesters of pregnancy—A systematic review. Taiwanese Journal of Obstetrics and Gynecology. 2014;53(2):141–145. doi: 10.1016/j.tjog.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Yu C, Wang L. Temperature exposure during pregnancy and birth outcomes: an updated systematic review of epidemiological evidence. Environ Pollut. 2017;225:700–712. doi: 10.1016/j.envpol.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Liu Y, Chen Y, Yao C, Che Z, Cao J. Maternal exposure to fine particulate matter (PM 2.5) and pregnancy outcomes: a meta-analysis. Environ Sci Pollut Res. 2015;22(5):3383–3396. doi: 10.1007/s11356-014-3458-7. [DOI] [PubMed] [Google Scholar]
- 9.Siddika N, Balogun HA, Amegah AK, Jaakkola JJ. Prenatal ambient air pollution exposure and the risk of stillbirth: systematic review and meta-analysis of the empirical evidence. Occup Environ Med. 2016;73(9):573–581. doi: 10.1136/oemed-2015-103086. [DOI] [PubMed] [Google Scholar]
- 10.Bonzini M, Carugno M, Grillo P, Mensi C, Bertazzi P, Pesatori AC. Impact of ambient air pollution on birth outcomes: systematic review of the current evidences. La Medicina del lavoro. 2010;101(5):341–363. [PubMed] [Google Scholar]
- 11.Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Quality, Atmosphere & Health. 2012;5(4):369–381. doi: 10.1007/s11869-010-0106-3. [DOI] [Google Scholar]
- 12.World Health Organization. Ambient air pollution: Health impacts. 2019. https://www.who.int/airpollution/ambient/health-impacts/en/. Accessed 2019.
- 13.Shah PS, Balkhair T. Births KSGoDoPL. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37(2):498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen M. Is it still important to study if ambient air pollution triggers stillbirth? Occup Environ Med. 2016;73(9):571–572. doi: 10.1136/oemed-2016-103625. [DOI] [PubMed] [Google Scholar]
- 15.Hajizadeh Y, Jafari N, Mohammadi A, Momtaz SM, Fanaei F, Abdolahnejad A. (2020) Concentrations and mortality due to short- and long-term exposure to PM(2.5) in a megacity of Iran (2014–2019). Environmental science and pollution research international. doi:10.1007/s11356-020-09695-z. [DOI] [PubMed]
- 16.Jaafari J, Naddafi K, Yunesian M, Nabizadeh R, Hassanvand MS, Ghozikali MG et al. Study of PM10, PM2. 5, and PM1 levels in during dust storms and local air pollution events in urban and rural sites in Tehran. 2018;24(2):482–93.
- 17.Jaafari J, Naddafi K, Yunesian M, Nabizadeh R, Hassanvand MS, Shamsipour M et al. (2020) The acute effects of short term exposure to particulate matter from natural and anthropogenic sources on inflammation and coagulation markers in healthy young adults 139417. [DOI] [PubMed]
- 18.Jaafari J, Naddafi K, Yunesian M, Nabizadeh R, Hassanvand MS, Ghozikali MG et al. (2020) Characterization, risk assessment and potential source identification of PM10 in Tehran. 154:104533.
- 19.Dastoorpoor M, Idani E, Goudarzi G, Khanjani N. Acute effects of air pollution on spontaneous abortion, premature delivery, and stillbirth in Ahvaz, Iran: a time-series study. Environ Sci Pollut Res 2017:1–12. [DOI] [PubMed]
- 20.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29(21):2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meteorological Data Request System. I.R. of Iran Meteorological Organization, Tehran, Iran. 2019. http://www.irimo.ir/far/index.php.
- 22.National Climatic Data Center, Global Summary of the Day (GSOD). National Oceanic and Atmospheric Administration, , U.S. Department of Commerce. https://www7.ncdc.noaa.gov/CDO/cdoselect.cmd?datasetabbv=GSOD. Accessed 2019 2019.
- 23.Goldman-Mellor S, Olfson M, Lidon-Moyano C, Schoenbaum M. Association of Suicide and Other Mortality with Emergency Department Presentation. JAMA Netw Open. 2019;2(12):e1917571. doi: 10.1001/jamanetworkopen.2019.17571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tehran Annual Air Quality Report. period of March 2017- March 2018, QM97/02/01(U)/1″. 2018. http://air.tehran.ir/. 1.
- 25.Breuer D, Bower J. Monitoring ambient air quality for health impact assessment. WHO Regional Office Europe; 1999. [PubMed]
- 26.United States Environmental Protection Agency. NAAQS Table. US EPA. 2019. https://www.epa.gov/criteria-air-pollutants/naaqs-table.
- 27.Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43(8):1–20. doi: 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muggeo VM. Analyzing temperature effects on mortality within the R environment: the constrained segmented distributed lag parameterization. J Stat Softw. 2010;32(12):1–17. doi: 10.18637/jss.v032.i12. [DOI] [Google Scholar]
- 29.Strand LB, Barnett AG, Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol. 2011;175(2):99–107. doi: 10.1093/aje/kwr404. [DOI] [PubMed] [Google Scholar]
- 30.Arroyo V, Díaz J, Carmona R, Ortiz C, Linares C. Impact of air pollution and temperature on adverse birth outcomes: Madrid, 2001–2009. Environ Pollut. 2016;218:1154–1161. doi: 10.1016/j.envpol.2016.08.069. [DOI] [PubMed] [Google Scholar]
- 31.Auger N, Fraser WD, Smargiassi A, Bilodeau-Bertrand M, Kosatsky T. Elevated outdoor temperatures and risk of stillbirth. Int J Epidemiol. 2017;46(1):200–208. doi: 10.1093/ije/dyw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu R, Sarovar V, Malig BJ. Association between high ambient temperature and risk of stillbirth in California. Am J Epidemiol. 2016;183(10):894–901. doi: 10.1093/aje/kwv295. [DOI] [PubMed] [Google Scholar]
- 33.Bruckner TA, Modin B, Vågerö D. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Ann Epidemiol. 2014;24(2):116–121. doi: 10.1016/j.annepidem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Chen G, Jaakkola JJ, Williams G, Guo Y. Temporal change in the impacts of ambient temperature on preterm birth and stillbirth: Brisbane, 1994–2013. Sci Total Environ. 2018;634:579–585. doi: 10.1016/j.scitotenv.2018.03.385. [DOI] [PubMed] [Google Scholar]
- 35.Ha S, Liu D, Zhu Y, Soo Kim S, Sherman S, Grantz KL, Mendola P. Ambient temperature and stillbirth: a multi-center retrospective cohort study. Environ Health Perspect. 2017;125(6):067011. doi: 10.1289/EHP945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vähä-Eskeli K, Erkkola R. The effect of short-term heat stress on uterine contractility, fetal heart rate and fetal movements at late pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1991;38(1):9–14. doi: 10.1016/0028-2243(91)90200-5. [DOI] [PubMed] [Google Scholar]
- 37.Liang Z, Lin Y, Ma Y, Zhang L, Zhang X, Li L, Zhang S, Cheng Y, Zhou X, Lin H, Miao H, Zhao Q. The association between ambient temperature and preterm birth in Shenzhen, China: a distributed lag non-linear time series analysis. Environ Health. 2016;15(1):84. doi: 10.1186/s12940-016-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stan C, Boulvain M, Hirsbrunner-Amagbaly P, Pfister R. Hydration for treatment of preterm labour. The Cochrane database of systematic reviews. 2002(2):Cd003096. doi:10.1002/14651858.cd003096. [DOI] [PubMed]
- 39.Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. Am J Epidemiol. 2010;172(10):1108–1117. doi: 10.1093/aje/kwq170. [DOI] [PubMed] [Google Scholar]
- 40.Yackerson N, Piura B, Sheiner E. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J Perinatol. 2008;28(10):707–711. doi: 10.1038/jp.2008.69. [DOI] [PubMed] [Google Scholar]
- 41.Li D-K, Janevic T, Odouli R, Liu L. Hot tub use during pregnancy and the risk of miscarriage. Am J Epidemiol. 2003;158(10):931–937. doi: 10.1093/aje/kwg243. [DOI] [PubMed] [Google Scholar]
- 42.Faiz AS, Rhoads GG, Demissie K, Lin Y, Kruse L, Rich DQ. Does ambient air pollution trigger stillbirth? Epidemiology. 2013;24:538–544. doi: 10.1097/EDE.0b013e3182949ce5. [DOI] [PubMed] [Google Scholar]
- 43.Pereira LA, Loomis D, Conceicao GM, Braga AL, Arcas RM, Kishi HS, et al. Association between air pollution and intrauterine mortality in Sao Paulo. Brazil Environ Health Perspect. 1998;106(6):325–329. doi: 10.1289/ehp.98106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rammah A, Whitworth KW, Han I, Chan W, Symanski E. Time-varying exposure to ozone and risk of stillbirth in a nonattainment urban region. Am J Epidemiol. 2019;188(7):1288–1295. doi: 10.1093/aje/kwz095. [DOI] [PubMed] [Google Scholar]
- 45.Zang H, Cheng H, Song W, Yang M, Han P, Chen C, Ding R. Ambient air pollution and the risk of stillbirth: a population-based prospective birth cohort study in the coastal area of China. Environ Sci Pollut Res Int. 2019;26(7):6717–6724. doi: 10.1007/s11356-019-04157-7. [DOI] [PubMed] [Google Scholar]
- 46.Mendola P, Ha S, Pollack AZ, Zhu Y, Seeni I, Kim SS, Sherman S, Liu D. Chronic and acute ozone exposure in the week prior to delivery is associated with the risk of stillbirth. Int J Environ Res Public Health. 2017;14(7):731. doi: 10.3390/ijerph14070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh J. Neonatal development altered by maternal sulfur dioxide exposure. Neurotoxicology. 1989;10(3):523–527. [PubMed] [Google Scholar]