Abstract
Background
Since fungi spores have high concentrations in the atmosphere during most of the year, they have an important place in respiratory allergies. In this regard, the preparation of calendars showing fungi spore loads for residential areas has much importance in the treatment of the patients. The first aim of this study was to present the airborne fungal spore research results from Eastern Anatolia in Turkey. Then, the mold spores’ relationships with the meteorological parameters and skin prick test results were also evaluated. The presence of fungal spores was investigated using a volumetric spore trap in 2018 year.
Methods
In this study, fungal spores within the atmosphere of the Elazığ city of Turkey was measured through the volumetric method, using a Lanzoni VPPS 2000 device (VPPS 2000 Lanzoni, Bologna, Italy), in 2018 year. Annual data of temperature, humidity, precipitation and wind speed were used for comparing meteorological data with airborne fungal spore counts. In addition, 637 children who were admitted to a pediatric allergy clinic with allergic complaints were enrolled in the study.
Results
A total of 145,099 spores/m3 and 20 fungal taxa belonging to the molds were recorded. Ustilago was the predominant genus (18.10%), followed by Oidium (18.01%), Drechslera (12.82%), and Fusarium (11.60%), which were the most common fungal spores found in Elazig’s atmosphere. The total mold spores in the atmosphere reached the highest level, with 28,153 spores/m3, in July (mid-summer). Moreover, we found a positive correlation between the mold spores and the temperature, but negative correlations with the humidity and wind speed. In the skin prick tests in the children with allergic complaints, we detected sensitization to Alternaria alternata in 4.4%, Cladosporium herbarum in 3.0%, Penicillium notatum in 1.4%, and Aspergillus fumigatus in 1.1%. Additionally, there was no correlation between fungal spore concentration in the atmosphere with fungal spores sensitization in the skin prick test.
Conclusions
This study was the first aerofungal survey of the Eastern Anatolia region in Turkey; therefore, new information has been introduced in the field of aerobiology in Turkey.
Keywords: Airborne fungal spores, Meteorological parameters, Skin prick test, Volumetric method, Turkey
Background
It has been estimated that approximately 1.5 million fungal species are present all over the world, and approximately 110 of them have been found to cause respiratory allergies. The fungi are separated into two groups, molds and yeasts, based to their morphological structures. Both molds and yeast spores are found in the atmosphere, and both can cause allergic diseases [1, 2]. Inhaled fungal allergens are also separated into two groups: indoor and outdoor fungi. However, when one considers the fact that the atmospheric air is circulating both outdoors and inside the house, fungal spores can be found in both the outer and inner environments. Many fungal species that are usually present outside can be transported to the insides of buildings, including those from the following genera: Penicillium, Cladosporium, Aspergillus, Ulocladium, Aureobasidium, Alternaria, Phoma, Nigrospora, Rhizopus, Mucor, Epicoccum, Stemphylium, Curvularia, Fusarium, Scopulariopsis, Cephalosporium, Trichoderma, Chaetomium, Streptomyces, Candida, Cryptococcus, and Rhodotorula [3–5]. Fungal spore sizes can vary from 1 to 30 μm. Because fungal spores are smaller in diameter than pollens (approximately 1/1000), they can stay in the atmosphere for longer periods of time, and they can be carried farther distances by the wind [1, 2, 6]. All of the fungi that cause allergic diseases are saprophytic, and most of these fungi belong to the classes Ascomycetes and Deuteromycetes [2]. Since fungal spores are small in diameter, they can reach the lower airways and cause both allergic rhinitis and allergic asthma [3–5].
The number and types of fungi in a region can vary depending on the geographic location, climate, and season. Moreover, fungal spores in the air can be affected by many factors, such as the wind, temperature, humidity, season, and rainfall. Although outdoor fungal spores are present in the atmosphere year-round, they increase in the atmosphere during certain periods like pollens [1, 2, 6–9].
In our study, we aimed to investigate the relationships between the numbers and seasonal distributions of the fungal spores seen frequently in the atmosphere around Elazig, as well as the meteorological parameters. In addition, we planned to evaluate the relationships between the fungal spores in the atmosphere and the fungal antigens detected in the skin prick tests of children with allergic complaints.
Materials and methods
Study area, flora, climate, and meteorological data
This study was conducted in the city of Elazig, which is located in the Eastern Anatolia region. The city lies between east longitudes 40° 21′ and 38° 30′ and north latitudes 38° 17′ and 39° 11′ (Fig. 1). Elazig has a surface area of 9.281 km2 and an altitude of 1.067 m. The total surface area of the city is composed of 8.332 km2 of land, 826 km2 of dams and natural lakes, and 123 km2 of forests. This area is located in the transition zone between the Mediterranean, Euro-Siberian, and Irano-Turanian phytogeographical regions. Elazig has a continental climate in which the winters are cold and rainy and the summers are hot and dry. The temperatures range between −15 °C and + 42 °C, and the average annual rainfall is 433 mm. The maximum rainfall is observed during the spring. Recently, lakes that have been created as a result of the construction of new dams have caused partial deviations in the climate. The most marked change is that the winters, which were very cold and snowy in the past, are now relatively mild [10]. Meteorological data from the Elazığ city in 2018 year were obtained from the General Directorate of Meteorology [11].
Fig. 1.
Map is showing the location the city of Elazığ in Turkey
Airborne fungi sampling
In this study, fungal spores within the atmosphere of the Elazığ city of Turkey was measured through the volumetric method, using a Lanzoni VPPS 2000 device (VPPS 2000 Lanzoni, Bologna, Italy), in 2018 year. Lanzoni VPPS 2000 device was placed on the roof of a 15 m height building above ground level. This building was chosen for the following reasons: The build was located at a place representing the features of the city. There is no other building in all directions about 100 m away from the building where the device is placed on the roof. Thereby, air flow is not interrupted by the surrounding buildings.The device was placed on a tripod foot of approximately 1 m on roof. Thus, position of the sampler allowed air movement from all four sides. The tape which is coated with silicone oil was placed in the sampler. The adhesive tape rolled on the drum in the fungal trap which completes its full rotation in a week was regularly changed weekly for one year. The adhesive tape from the device were sent to the Aerobiology Laboratory of the Department of Biology of Kafkas University’s Faculty of Science and Literature for analysis.
Isolation and identification of airborne fungi
VPPS 2000 Lanzoni device operated continuously and was calibrated to aspirate 10 L/m3/min air. There is an aluminum disc inside the device to which the tape used in 7-day sampling is mounted. The disc mounted on the device rotates 2 mm per hour, traveling 48 mm in 24 h and 336 mm in a week. Liquid silicone solution is applied with a brush to adhere fungi with vacuum effect on the tape. The adhesive tape was cut into seven equal pieces of 48 mm in length, representing 1 day. Reference preparations were prepared for daily microscopic examination using the “woodhouse” method [12]. The tape fragments were mounted to slides and covered with glycerin jelly mixed with basic fuchsine. After this step, basic fuchsin glycerin is stained with gelatin dye and made into daily preparations. The slides were examined with an Olympus light microscopy at ×400 magnification. The fungal counts converted into the concentration of airborne fungal spores per cubic meter. Atmospheric sampling and analysis were performed according to the European Aerobiology Network recommendations [13].
Skin prick test analyses
In addition, 637 children who were admitted to a pediatric allergy clinic with allergic complaints were enrolled in the study in 2018 year. The patients, allergic to at least one inhalant allergen as detected by a skin prick test, who were born in the city of Elazığ and who continuous residence there were included in the study. Standard skin prick tests were performed using antigens derived from the following mixtures: a grasses/cereals mixture, a weed mixture, tree mixtures I and II, Dermatophagoides farinae, Dermatophagoides pteronyssinus, mold mixtures I and II, Blattella germanica, cat epithelia, and dog epithelia. Mold mixture I included the following antigens: Alternaria alternata, Cladosporium herbarum, Botrytis cinerea, Curvularia lunata, Fusarium moniliforme, and Helminthosporium halodes. Mold mixture II included the following antigens: Aspergillus fumigatus, Mucor mucedo, Penicillium notatum, Pullularia pullulans, Rhizopus nigricans, and Sepula lacrymans. Additionally, skin prick tests were performed with Alternaria alternata, Cladosporium herbarum, Aspergillus fumigatus, and Penicillium notatum in those patients who had positive results from the skin prick test for mold mixtures I and II. For the skin prick test, standard allergen extracts from the firm Allergopharma (Allergopharma GmbH & Co. KG, Reinbek, Germany) were used. Histamine 10 mg/ml and diluent (0.9% sodium chloride) were used as positive and negative controls, respectively. Allergens and positive-negative controls were applied to volar surface of arm epidermally. Skin prick test were evaluated 15 min after application. A positive test was defined a reaction with as a mean wheal diameter of ≥3 mm greater than the negative control [14]. Our university’s clinical research ethics committee approved this study, and written informed consent was obtained from all of the subjects and/or their parents.
Statistical analyses
All of the statistical analyses were performed using IBM SPSS Statistics for Windows version 21.0 (IBM Corp., Armonk, NY, USA). The descriptive statistics were expressed as the mean ± standard deviation, median, and range. The relationships between the mold spore counts and the selected meteorological parameters (annual data: temperature, humidity, rainfall, and wind speed) were evaluated using Spearman’s rank correlation coefficient. A p value of <0.05 was considered to be statistically significant.
Results
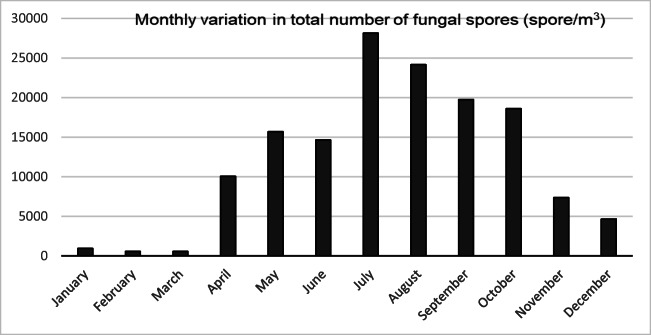
Using the volumetric method, we determined that during the study period there was a total of 145,099 fungal spores/m3 for the 20 taxa in Elazig’s atmosphere. The genera of the most commonly found fungal spores were Ustilago (18.10%), Oidium (18.01%), Drechslera (12.82%), Fusarium (11.60%), Pteridophyta (5.25%), Boletus (5.18%), Chaetomium (3.76%), Puccinia (3.62%), Cladosporium (3.42%), Epicoccum (3.34%), Periconium (2.86%), Melanomma (2.66%), and Alternaria (2.43%) (Table 1). When the monthly distribution of the total fungal spores in the atmosphere was examined, the spores reached the highest concentration in July (mid-summer), with 28,153 spores/m3 (19.40%), followed by a high concentration in August (late-summer), with 24,158 spores/m3 (16.65%), and in September (early autumn), with 19,754 spores/m3 (13.61%). In contrast, the lowest fungal spore concentrations occurred during January and February (winter) and March (early spring) (Fig. 2). When the monthly distribution of the fungal genera was analyzed, we found that Ustilago reached its highest concentration in August, with 8400 spores/m3 (31.98%), followed by Oidium in July, with 9154 spores/m3 (35.01%), Drechslera in May, with 9260 spores/m3 (49.77%), Fusarium in July, with 7421 spores/m3 (44.06%), Pteridophyta in August, with 3136 spores/m3 (41.12%), and Boletus in October, with 3421 spores/m3 (45.44%). We found that Cladosporium and Alternaria, which were the most frequently recorded species in many of the aerofungal studies conducted in Turkey, reached their highest concentrations in June, with 2071 spores/m3 (41.64%), and July, with 1521 spores/m3 (43.10%), respectively. Oidium spores were the only fungal genus found in the atmosphere throughout the year (Table 1).
Table 1.
Monthly distribution of fungal spores in the atmosphere of Elazığ in 2018 year (spore/m3)
| Fungi | January | February | March | April | May | June | July | August | September | October | November | December | Annual total (spor/m3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ustilago | 105 | – | 12 | 133 | 285 | 1154 | 3217 | 8400 | 5353 | 6197 | 952 | 455 | 26,263 |
| Oidium | 7 | 19 | 74 | 157 | 1222 | 4395 | 9156 | 3961 | 5956 | 1012 | 75 | 112 | 26,146 |
| Drechslera | – | 75 | 49 | 7487 | 9260 | 461 | 370 | 289 | 470 | 66 | 76 | – | 18,603 |
| Fusarium | – | – | – | 278 | 1152 | 1343 | 7421 | 2650 | 1964 | 1573 | 408 | 53 | 16,842 |
| Pteridopyhta | – | – | – | 663 | 986 | 293 | 1066 | 3136 | 712 | 593 | 177 | – | 7626 |
| Boletus | 264 | 19 | – | – | 368 | 57 | 63 | 164 | 1105 | 3421 | 1353 | 713 | 7527 |
| Chaetomium | – | – | – | 17 | 36 | 2194 | 2077 | 31 | 23 | 908 | 183 | – | 5469 |
| Puccinia | 222 | 184 | – | – | 331 | 238 | 118 | 96 | 805 | 1785 | 1071 | 405 | 5255 |
| Cladosporium | – | – | 177 | 231 | 546 | 2071 | 916 | 630 | 352 | 9 | 41 | – | 4973 |
| Epicoccum | 321 | 216 | – | – | 95 | 182 | 524 | – | – | 30 | 714 | 2770 | 4852 |
| Periconium | – | – | 9 | 17 | 12 | 942 | 1341 | 1820 | 9 | – | – | – | 4150 |
| Melanomma | – | – | – | 3 | 108 | 5 | 19 | – | – | 1401 | 2233 | 98 | 3867 |
| Alternaria | – | – | 47 | 29 | 65 | 765 | 1521 | 952 | 145 | 5 | – | – | 3529 |
| Curvularia | – | 14 | 103 | 320 | 56 | 13 | 23 | 1540 | 518 | 487 | – | – | 3074 |
| Torula | – | – | 1 | 640 | 960 | 391 | 170 | 32 | 196 | 11 | 23 | – | 2424 |
| Exosporium | – | – | – | – | – | – | – | 244 | 789 | 256 | – | 54 | 1343 |
| Dictyosporium | 10 | 32 | – | – | 112 | – | – | 74 | 889 | 210 | 3 | – | 1330 |
| Pithomyces | 3 | – | 91 | – | 33 | 22 | 4 | 94 | 468 | 423 | 43 | 4 | 1185 |
| Stemphylium | – | – | – | 69 | 52 | 112 | 147 | 45 | – | 204 | 12 | – | 641 |
| Bipolaris | – | – | – | – | – | 23 | 18 | 1 | – | – | – | – | 42 |
| Monthly total (spor/mm3) | 932 | 559 | 563 | 10,044 | 15,679 | 14,638 | 28,153 | 24,158 | 19,754 | 18,591 | 7364 | 4664 | 145,099 |
*The values in bold indicate that the fungal species spores have reached the highest concentration levels throughout one year
Fig. 2.
Monthly variation in total number of fungal spores in the atmosphere of Elazığ in 2018 year
When the relationship between the fungal spores and the meteorological parameters was evaluated, we found a significant positive correlation between the monthly average spore levels of Ustilago, Oidium, Fusarium, Drechslera, Pteridophyta, Cladosporium, Periconium, Alternaria, Torula, Stemphylium, and Bipolaris and the maximum-minimum air temperatures. However, there was a negative correlation between the spore levels of Ustilago, Fusarium, Drechslera, Oidium, Pteridophyta, Cladosporium, Periconium, Alternaria, Torula, Stemphylum, and Bipolaris and the monthly average relative humidity. In addition, we found a negative correlation between the Boletus, Stemphylium, and Puccinia spore levels and the monthly average wind speed. There was also a negative correlation between the Bipolaris spore levels and the monthly total rainfall (Table 2). The meteorological parameter data collected during the aerofungal study is shown in Table 3.
Table 2.
Spearman’s rank correlation coefficients between fungal spores and meteorological parameters
| Fungi | Average monthly temperature (°C) | Monthly average maximum temperature (°C) | Monthly average minimum temperature (°C) | Average monthly relative humidity (%) | Average monthly wind speed (m/s) | Average monthly strong wind speed (m/s) | Total monthly precipitation (mm) |
|---|---|---|---|---|---|---|---|
| Ustilago |
0.648 0.02 |
0.699 0.01 |
0.664 0.01 |
−0.704 0.01 |
−0.142 0.6 |
−0.250 0.4 |
−0.550 0.06 |
| Oidium |
0.893 0.0001 |
0.874 0.0001 |
0.895 0.0001 |
−0.886 0.0001 |
0.177 0.6 |
−0.272 0.4 |
−0.459 0.1 |
| Drechslera |
0.723 0.008 |
0.655 0.02 |
0.732 0.007 |
−0.886 0.0001 |
0.242 0.4 |
−0.452 0.1 |
0.112 0.7 |
| Fusarium |
0.868 0.0001 |
0.873 0.0001 |
0.880 0.0001 |
−0.878 0.0001 |
0.107 0.7 |
0.309 0.3 |
−0.540 0.07 |
| Pteridopyhta |
0.890 0.0001 |
0.879 0.0001 |
0.897 0.0001 |
−0.838 0.002 |
−0.242 0.4 |
−0.391 0.2 |
−0.346 0.3 |
| Boletus |
−0.114 0.4 |
−0.067 0.8 |
−0.081 0.8 |
0.074 0.8 |
−0.600 0.04 |
0.265 0.4 |
−0.175 0.6 |
| Puccinia |
−0.191 0.4 |
−0.158 0.6 |
−0.158 0.6 |
0.123 0.7 |
−0.572 0.05 |
0.318 0.3 |
0.042 0.9 |
| Cladosporium |
0.942 0.0001 |
0.916 0.0001 |
0.937 0.0001 |
−0.836 0.001 |
0.336 0.3 |
−0.593 0.04 |
−0.314 0.3 |
| Periconium |
0.882 0.0001 |
0.857 0.0001 |
0.868 0.0001 |
−0.793 0.002 |
0.442 0.1 |
−0.561 0.6 |
−0.338 0.3 |
| Alternaria |
0.947 0.0001 |
0.947 0.0001 |
0.929 0.0001 |
−0.899 0.0001 |
0.394 0.2 |
−0.451 0.1 |
−0.456 0.1 |
| Torula |
0.755 0.005 |
0.697 0.01 |
0.761 0.004 |
−0.610 0.03 |
0.286 0.3 |
−0.480 0.1 |
−0.095 0.7 |
| Stemphylium |
0.628 0.03 |
0.609 0.03 |
0.638 0.02 |
−0.632 0.03 |
−0.588 0.04 |
−0.179 0.6 |
−0.123 0.7 |
| Bipolaris |
0.736 0.006 |
0.725 0.008 |
0.734 0.007 |
−0.731 0.007 |
0.270 0.4 |
−0.366 0.2 |
−0.667 0.001 |
Significance level: p < 0.05 (values in bold)
Table 3.
Meteorological data of the city of Elazığ in 2018 year
| Months | Average monthly temperature (°C) | Monthly average maximum temperature (°C) | Monthly average minimum temperature (°C) | Average monthly relative humidity (%) | Average monthly wind speed (m/s) | Average monthly strong wind speed (m/s) | Total monthly precipitation (mm) |
|---|---|---|---|---|---|---|---|
| January | 2,3 | 7.4 | −1.9 | 73.8 | 2.1 | 13.8 | 40.7 |
| February | 4.3 | 11.2 | −1.2 | 56.7 | 2.4 | 13.1 | 31.5 |
| March | 9.1 | 15.3 | 4.2 | 58.0 | 2.7 | 12.6 | 36.1 |
| April | 14.7 | 20.1 | 7.9 | 52.4 | 2.8 | 12.6 | 64.8 |
| May | 19.0 | 26.2 | 11.5 | 44.6 | 2.4 | 13.0 | 31.6 |
| June | 24.4 | 31.7 | 15.1 | 30.2 | 2.4 | 12.6 | 5.1 |
| July | 27.7 | 34.7 | 18.8 | 23.4 | 3.4 | 12.9 | 0.0 |
| August | 27.3 | 35.1 | 18.1 | 23.4 | 2.3 | 11.5 | 0.0 |
| September | 20.6 | 29.2 | 13.2 | 33.9 | 2.0 | 12.8 | 7.9 |
| October | 13.2 | 21.6 | 6.4 | 41.4 | 2.5 | 13.6 | 17.9 |
| November | 9.1 | 14.7 | 4.8 | 67.0 | 2.0 | 11.5 | 32.1 |
| December | −1.2 | 2.2 | −4.3 | 72.6 | 2.0 | 15.9 | 12.6 |
Of the 637 children, 58.2% (n = 371) were males, 41.8% (n = 266) were females, and their ages ranged from 2 to 18 years old. The patients were diagnosed as follows: rhinitis in 41% (n = 261), rhinitis and asthma in 26.4% (n = 168), and asthma in 32.6% (n = 208). There was a single allergen sensitivity in 47% (n = 316), with multiple allergens in 53% (n = 358) of the patients. The most common sensitization in the skin prick test was to the grasses-cereals mixture (51.5%, n = 328), followed by Dermatophagoides farina in 22.9% (n = 146), Dermatophagoides pteronyssinus in 21.6% (n = 138), the weed mixture in 19.9% (n = 108), mold mixture I in 11.6% (n = 74), tree mixture I in 10.5% (n = 67), tree mixture II in 7.3% (n = 47), and mold mixture II in 3.3% (n = 21). Additionally, the most common sensitization in the skin prick test with the single commercial fungal extracts was to Alternaria alternata (4.4%, n = 28), followed by Cladosporium herbarum in 3.0% (n = 19), Penicillium notatum in 1.4% (n = 9), and Aspergillus fumigatus in 1.1% (n = 7) (Table 4). In our study, there was no correlation between fungal spore concentration in the atmosphere with fungal spores sensitization in the skin prick test. We determined Cladosporium (2.49%) in ninth lines and Alternaria (1.52%) in thirteenth lines in the atmosphere fungal concentration. However, in our skin prick test results, we found the outdoor fungal mixture, which includes Alternaria and Cladosporium, in the first lines in individuals with fungal sensitivity.
Table 4.
Distribution of the rates of sensitization for each allergen in 637 children with allergic complaints
| Allergens | n (%) |
|---|---|
| Grasses-cereals mixture | 328 (51.5) |
| House dust mite | |
| Dermatophagoides farinae | 146(22.9) |
| Dermatophagoides pteronyssinus | 138 (21.6) |
| Weed mixture | 108 (19.9) |
| Trees mixture I | 67 (10.5) |
| Trees mixture II | 47 (7.3) |
| Mould mixture I | 74 (11.6) |
| Mould mixture II | 21 (3.3) |
| Cladosporium herbarum | 19 (3.0) |
| Alternaria alternata | 28 (4.4) |
| Aspergillus fumigatus | 7 (1,1) |
| Penicillium notatum | 9 (1.4) |
| Animal dander | |
| Cat | 61 (9.5) |
| Dog | 38 (6.0) |
| Cockroach (Blattella germanica) | 25 (3.9) |
Tree mixture I: Alnus glutinosa, Corylus avellana Populus alba, Salix caprea, Ulmus scabra,
Tree mixture II: Betula alba, Quercus robur, Platanus orientalis, Fagus sylvatica,
Grasses-cereals mixture: Holcus lanatus, Dactylis glomerata, Festuca pratensis, Lolium perenne, Poa pratensis, Phleum pratense, Secale cereale, Triticum sativum, Avenaa fatua and Hordeum vulgare
Weed mixture: Artemisia vulgaris, Urtica dioica, Taraxacum vulgare, Plantago lanceolata ve Parietaria officinalis
Discussion
In the present study, a total of 145,099 spores/m3 belonging to 20 fungal taxa were found in the atmosphere around Elazig in 2018 year. The first aerofungal research study in Turkey was conducted by Özkaragöz et al. in the atmosphere around Ankara, in which 13 fungal taxa were detected using the culture medium opening method [15]. Over the next few years, many aerofungal studies were conducted using the same method, and the dominant fungal spores identified were in the following genera: Alternaria, Cladosporium, Aspergillus, Penicillium, Fusarium, and Monilia [7, 16–20]. In the following years, the gravitational method (Durham sampler) was used in the aerofungal investigations, and it was reported that the dominant fungal spores detected in the atmosphere were from the genera Cladosporium, Alternaria, and Ustilago [21, 22]. In Turkey, the first study to use the volumetric method was performed in 1990. After that, Çeter and Pınar investigated the daily, monthly, and annual concentrations of the spores belonging to 35 different fungal taxa in the atmosphere around Ankara in 2003. Then, they prepared an annual spore calendar for the city of Ankara. The major fungal spore genera detected in that study were Cladosporium (75.5%), Alternaria (6.1%), Leptosphaeria (2.2%), Ustilago (2.2%), Exosporium (2%), Pleospora (1.6%), and Drechslera (1.3%) [23]. In a study conducted in Bursa, the most commonly detected fungal spores were from the genera Cladosporium, Alternaria, Aspergillus, Penicillium, Fusarium, and Epicoccum [24]. Ceter et al. reported that the fungal spores found most frequently in the atmosphere in the Mediterranean region of Turkey were from the genera Cladosporium, Alternaria, Epicoccum, Exosporium, Drechslera, and Periconia [25]. Akgül et al. measured a total of 21,1521 spores/m3 from 47 fungal taxa in the atmosphere around the city of Gaziantep, which is in the South Anatolian Region [26]. In that study, Cladosporium spores were detected most frequently (56.48%) in the atmosphere. The other taxa included hyphal particles (14.94%), Ustilago (13.96%), and Alternaria (5.79%). Çeter et al. identified Cladosporium, Alternaria, Leptosphaeria, Periconia, and Ustilago as the major fungal spores in the atmosphere around the black sea region of Turkey [27]. In addition, in a study conducted by using the Merck MAS 100 air sampler in the city of Manisa, Turkey. Cladosporium that was found as the predominant genus followed by Penicillium, Aspergillus, and Alternaria [28].
Mezzari et al. investigated the fungal spores in the atmosphere around the city of Porto Alegre, Brazil. In that study, the most commonly found fungal spore genera were Cladosporium (17.86%), Aspergillus/Penicillium (15.03%), Helminthosporium (2.49%), Botrytis (1.22%), Alternaria (1.19%), Culcuvaria (0.87%), Nigrospora (0.61%), and Fusarium (0.08%) [29]. Hasnain et al. investigated the fungal spores in the atmosphere around the city of Karachi by volumetric spore trap. In that study, Cladosporium spp. (44.8%), Alternaria spp. (15.5%), Periconia spp. (6.1%), Curvularia spp. (2.1%), Stemphylium spp. (1.3%) and Aspergillus/Penicillium type (1%) emerged to be major components constituting more than 70% of the airborne fungal flora [30]. In a study investigating fungal spore levels in Havana/Cuba, a total of 27 fungal spore types were identified using the standard methodology recommended by the Spanish Aerobiology Network. The total spore count was 200,451. Cladosporium was the most abundant, followed by Leptosphaeria, Coprinus and Aspergillus/Penicillium [31]. Kasprzyk et al. studied the fungal spores in the atmosphere around the city of Rzeszow in Poland. In that study, the most frequently detected fungal spores were Alternaria, Botrytis, Cladosporium, Drechslera, Epicoccum, Ganoderma, Pithomyces, Polythrincium, Stemphylium, and Torula. Additionally, although the fungal spores were present in the atmosphere throughout the whole year, they reached peak levels in July and August [32].
Our research was the first study to investigate the 20 fungal taxa in the atmosphere using the volumetric method in the Eastern Anatolia Region of Turkey. The fungal spores most commonly found in the present study were from the following genera: Ustilago (18.10%), Oidium (18.01%), Drechslera (12.82%), Fusarium (11.60%), Pteridophyta (5.25%), Boletus (5.18%), Chaetomium (3.76%), Puccinia (3.62%), Cladosporium (3.42%), Epicoccum (3.34%), Periconium (2.86%), Melanomma (2.66%), and Alternaria (2.43%). Our results differ from the data from the other aerofungal studies performed using the volumetric method in our country. The dominant fungal spores in the other aerofungal studies conducted in Turkey were Cladosporium, Alternaria, Aspergillus, and Penicillium [21–27]. However, in the present study, Cladosporium (3.42%) was in the ninth line and Alternaria (2.43%) was in the thirteenth line. We observed that the fungal spores that often cause diseases in agricultural plants were in the first line in the atmosphere around Elazig. These fungal spores were from the genera Ustilago, Drechslera, Fusarium, and Oidium. Moreover, the previous aerofungal studies conducted in Turkey have reported that the peak concentration of fungal spores occurs from July–September [21–27]. In our study, we found that the fungal spores in the atmosphere increased between July and August, and that they reached the highest level, with 28,153 spores/m3 (19.40%), in July. We believe that this condition was due to the different climate conditions, geographic characteristics, and agricultural activities in Elazig. Because, agriculture is an important source of income for the people of the Elazığ city. Agricultural products grown in our region include the cereals (wheat, barley, oats, rye, maize), vegetables and grapes. However, the pollination season for the pollen and fungal spores in a region may vary from year to year [33]. Therefore, the fact that our research duration was only one year may have been one factor limiting our results.
The amount, distribution, and diversity of the fungal spores in the atmosphere vary depending on the geographic location, climatic conditions, and season. The climate and other meteorological conditions have a significant influence on the amount and diversity of the fungal spores in the atmosphere. Although the spores from the genera Aureobasidium, Didymella, Ganoderma, Fusarium, Leptosphaeria, Phoma, Pleospora, Sporormiella, Trichoderma, Basidiospore, and Ascospora increased in the humid weather, the spores from the genera Cladosporium, Alternaria, Penicillium, Aspergillus, Epicoccum, Drechslera, Pithomyces, Helminthosporium, and Curvularia were found in high amounts during the dry and windy weather. The temperature was reported to have a positive effect on the release of fungal spores, thus increasing the concentration [34]. Akgul et al. reported a positive correlation between the temperature and the atmospheric spore levels of Ascomycota, Basidiomycota, Epicoccum, Cladosporium, Alternaria, and Drechslera [26]. Stepalska and Wolek studied the changes in the fungal spores according to the meteorological parameters, and they found a positive correlation between the spore concentrations and the minimum and maximum temperatures and sunlight [35]. Our results showed a positive correlation between the spore levels of Ustilago, Oidium, Drechslera, Fusarium, Pteridophyta, Cladosporium, Periconium, Alternaria, Torula, Stemphylium, and Bipolaris and the average monthly temperature and maximum and minimum temperatures. Oliveira et al. demonstrated a negative correlation between the fungal spore concentrations and the wind speed, but positive correlations between the fungal spore concentrations and the relative humidity and the temperature [36]. Çelenk et al. reported a positive correlation between the atmospheric fungal spore concentrations and the air temperature, but a negative correlation between the atmospheric fungal spore concentrations and the rainfall, relative humidity, and wind speed [37]. Calderon et al. found positive correlations between the atmospheric fungal spore concentrations and low relative humidity, a high wind speed, and a high air temperature, and negative correlations between the fungal spore concentrations and rainy weather and a high relative humidity [38] . In our study, there were negative correlations between the spore levels of Ustilago, Oidium, Drechslera, Fusarium, Pteridophyt, Cladosporium, Periconium, Alternaria, Torula, Stemphylium, and Bipolaris and the monthly average relative humidity. In addition, we found statistically negative correlations between the spore levels of Boletus, Stemphylium, Puccinia and the monthly wind speed.
When compared to pollen grains, fungal spores are released in greater quantities into the atmosphere. There are approximately 1000 fungal spores per each pollen grain in one m3 of air in the atmosphere [1, 2, 6]. Although fungal spores are very intensive in the atmosphere, it is known that fungal susceptibility in allergic patients is not as common as mite or pollen susceptibility. Previously, it has been reported that this is possibly due to the lower allergenicity of fungal spores or due to a problem in the standardization of the allergen extracts used in skin prick tests [6]. In one multicenter study conducted in Europe, a sensitivity to Alternaria and/or Cladosporium was demonstrated in 9.46% of the patients with rhinitis and asthma symptoms. The highest susceptibility was observed in Spain, with a rate of 20%, and the lowest susceptibility was found in Portugal, with a rate of 3%. The very different results from these two countries with the same climatic and geographic features is believed to be due to the use of two different commercial allergen solutions [6]. This suggests that there is still a problem in the standardization of fungal allergen extracts. According to the skin prick test results of the allergy patients, there is a fungal sensitivity in 3–10% of people worldwide [1, 2]. It has been reported that the most common allergic disease causing fungal allergens are Cladosporium, Alternaria, Penicillium, Aspergillus, Rhizopus, Chaetomium, Curvularia, Fusarium, Helminthosporium, Phoma, Rhodotorula, Aureobasidium, and Trichoderma [1, 2, 6]. In Europe, the fungal spore sensitization frequency in allergy cases has been reported to vary at rates ranging from 5% to 38.5%. In a study conducted in Hungary, the sensitivities to Alternaria and Cladosporium were 18.6% and 12.8%, respectively [39]. In the United States, the fungal spore sensitization frequency in allergic subjects has been reported at rates ranging from 6% to 80% [40]. The fungal sensitization in the skin tests of allergic Turkish children determined rates ranging from 5% to 32.3% [41–44]. In our study, sensitivity rates of 11.6% to a mixture of outdoor fungi and 3.3% to mixture of indoor fungi were found in the skin prick tests of children with allergic complaints. We also found sensitivity rates of 4.4% to Alternaria alternata, 3.0% to Cladosporium herbarum, 1.4% to Penicillium notatum, and 1.1% to Aspergillus fumigatus in the skin prick tests. In the literature, susceptibilities to Alternaria, Cladosporium, and Aspergillus fumigatus have been reported as 10.4%, 12.8%, and 26%, respectively [39, 41, 45]. We used the standard skin prick test that did not contain Ustilago, Pteridophyta, Drechslera, Oidium, and Boletus allergens because these fungal allergens are not commercially present in our country. Therefore, detailed information about the sensitization to each of these fungal spores could not be obtained. However, In our study, there was no correlation between fungal spore concentration in the atmosphere with fungal spores sensitization in the skin prick test. We determined Cladosporium (2.49%) in ninth lines and Alternaria (1.52%) in thirteenth lines in the atmosphere fungal concentration. However, in our skin prick test results, we found the outdoor fungal mixture, which includes Alternaria and Cladosporium, in the first lines in individuals with fungal sensitivity.
Conclusion
This research was the first study that evaluated the fungal spores in the atmosphere in the Eastern Anatolia Region of Turkey using the volumetric method. A total of 145,099 spores/m3 belonging to 20 taxa was demonstrated in the atmosphere around Elazig during a one-year period. Additionally, a one-year fungal spore calendar was prepared. The fungal spores most commonly detected were Ustilago, Pteridophyta, Drechslera, Fusarium, and Oidium. We found that the total fungal spores in the atmosphere reached the highest level in July, with 28,153 spores/m3. Additionally, a positive correlation was found between the fungal spores in the atmosphere and the temperature, while negative correlations were found between the fungal spores in the atmosphere and the humidity and wind speed. Additionally, in our study, there was no correlation between fungal spore concentration in the atmosphere with fungal spores sensitization in the skin prick test. Overall, the results of this study can provide new information in the fields of aerobiology and allergy.
Authors’ contributions
MK conceived the project, wrote the proposal, and wrote the initial draft. MK and ET conceived of the research question, supervised the study, involved in data collection, performed statistical analysis, and wrote the manuscript. MK, ET, and AHE analyzed the data. MKA, GEA, and SA were responsible for laboratory assay. MKA, GEA, and SA conducted the isolation and identification of airborne fungi. All the authors read, commented on and approved the final manuscript.
Funding
This study was supported by the Scientific Research Projects Coordination Unit of Fırat University.
Compliance with ethical standards
Ethics approval and consent to participate
This study was approved by the clinical research ethics committee of Fırat University. All participants signed a consent form before enrolling into the study.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kurup VP, Shen HD, Banerjee B. Respiratory fungal allergy. Microbes Infect. 2000;2:1101–1110. doi: 10.1016/s1286-4579(00)01264-8. [DOI] [PubMed] [Google Scholar]
- 2.Bush RK, Portnoy JM. The role and abatement of fungal allergens in allergic diseases. J Allergy Clin Immunol. 2001;107:430–440. doi: 10.1067/mai.2001.113669. [DOI] [PubMed] [Google Scholar]
- 3.Chao HJ, Schwartz J, Milton DK, Burge HA. Populations and determinants of airborne fungi in large office buildings. Environ Health Perspect. 2002;110:777–782. doi: 10.1289/ehp.02110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgson M. Indoor environmental exposures and symptoms. Environ Health Perspect. 2002;110:663–667. doi: 10.1289/ehp.02110s4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wargocki P, Sundell J, Bischof W, Brundrett G, Fanger PO, Gyntelberg F, Hanssen SO, Harrison P, Pickering A, Seppänen O, Wouters P. Ventilation and health in non-industrial indoor environments: report from a European multidisciplinary scientific consensus meeting (EUROVEN) Indoor Air. 2002;12:113–128. doi: 10.1034/j.1600-0668.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- 6.D'Amato G, Chatzigeorgiou G, Corsico R, Gioulekas D, Jäger L, Jäger S, Kontou-Fili K, Kouridakis S, Liccardi G, Meriggi A, Palma-Carlos A, Palma-Carlos ML, Aleman AP, Parmiani S, Puccinelli P, Russo M, Spieksma FTM, Torricelli R, Wuthrich B. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. Allergy. 1997;52:711–716. doi: 10.1111/j.1398-9995.1997.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 7.Çolakoğlu G. Mould counts in the atmosphere at the Europe quarter of İstanbul. Turkey. J Basic Microbiol. 1996;36(6):389–392. doi: 10.1002/jobm.3620360302. [DOI] [PubMed] [Google Scholar]
- 8.Jones AM, Harrison RM. The effects of meteorological factors on atmospheric bioaerosol concentrations-a review. Sci Total Environ. 2004;326:151–180. doi: 10.1016/j.scitotenv.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Kilic M, Altintas DU, Yilmaz M, Güneşer Kendirli S, Bingöl Karakoc G, Taskin E, et al. The effects of meteorological factors and Alternaria spore concentrations on children sensitised to Alternaria. Allergol Immunopathol (Madr) 2010;38:122–128. doi: 10.1016/j.aller.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Akman Y, Ketenoǧlu O. The climate and vegetation of Turkey. Proceedings of the Royal Society of Edinburgh. Section B. Biological Sciences. 1986;89:123–134. [Google Scholar]
- 11.Turkish State Meteorological Service Official Web Sites-MGM. https://mgm.gov.tr/?il=Elazig
- 12.Wodehouse RP. Pollen Grains. New York: Hofner Publishing co; 1959. [Google Scholar]
- 13.Gala’n C, Smith M, Thibaudon M, Frenguelli G, Oteros J, Gehrig R, et al. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia. 2014;30:385–395. [Google Scholar]
- 14.Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, Canonica GW, Carlsen KH, Cox L, Haahtela T, Lodrup Carlsen KC, Price D, Samolinski B, Simons FER, Wickman M, Annesi-Maesano I, Baena-Cagnani CE, Bergmann KC, Bindslev-Jensen C, Casale TB, Chiriac A, Cruz AA, Dubakiene R, Durham SR, Fokkens WJ, Gerth-van-Wijk R, Kalayci O, Kowalski ML, Mari A, Mullol J, Nazamova-Baranova L, O’Hehir RE, Ohta K, Panzner P, Passalacqua G, Ring J, Rogala B, Romano A, Ryan D, Schmid-Grendelmeier P, Todo-Bom A, Valenta R, Woehrl S, Yusuf OM, Zuberbier T, Demoly P. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 15.Özkaragöz K. A study of airborne fungi in the Ankara area of Turkey in 1966. Allergy. 1969;24:147–156. [PubMed] [Google Scholar]
- 16.Çolakoğlu G. Fungal spore concentrations in the atmosphere at the Anatolia quarter of İstanbul. Turkey J Basic Microbiol. 1996;36:155–162. doi: 10.1002/jobm.3620360302. [DOI] [PubMed] [Google Scholar]
- 17.Asan A, Şen B, Sarıca S. Airborne fungi in urban air of Edirne city. Biologia. 2002;57:59–68. [Google Scholar]
- 18.Asan A, İlhan S, Sen B, Erkara IP, Filik C, Cabuk A, et al. Airborne fungal and Actinomycetes concentrations in urban air of Eskisehir City (Turkey) Indoor and Built Environment. 2004;13:63–74. [Google Scholar]
- 19.Topbaş M, Tosun I, Can G, Keklikkaya N, Aydın F. Identification and seasonal distribution of airborne fungi in urban outdoor air in an eastern Black Sea Turkish town. Turk J Med Sci. 2006;36:31–36. [Google Scholar]
- 20.Kalyoncu F. Indoor aeromycological study in Manisa. Turkey J Environ Sci Technol. 2008;1:85–89. [Google Scholar]
- 21.Bıçakçı A, Canıtez Y, Sapan N, Malyer H. Allergenic spores of Cladosporium spp. and Alternaria spp. in the atmosphere of İnegöl (Bursa) Allergy. 1999;54:46. [Google Scholar]
- 22.Erkara IP, İlhan S, Öner S. Monitoring and assessment of airborne Cladosporium link and Alternaria Nées spores in Sivrihisar (Eskişehir) Turkey Environ Monit Assess. 2009;148:477–484. doi: 10.1007/s10661-008-0177-x. [DOI] [PubMed] [Google Scholar]
- 23.Ceter T, Pinar NM. Atmospheric concentration of fungus spores in Ankara and the effect of meteorological factors in 2003 period. Mikrobiyol Bul. 2009;43:627–638. [PubMed] [Google Scholar]
- 24.Ataygül E, Çelenk S, Canıtez Y, Bıçakçı A, Malyer H, Sapan N. Allergenic fungal spore concentrations in theatmosphere of Bursa. Turkey J Biol Environ Sci. 2007;1:73–79. [Google Scholar]
- 25.Ceter T, Alan Ş, Pınar NM, Altıntaş DU. Airborne spore concentration in Adana Turkey, 2004. The 8th International Congress on Aerobiology, Neuchatel, Switzerland 2006; 211. (Abstract).
- 26.Akgül H, Yılmazkaya D, Akata I, Tosunoğlu A, Bıçakçı A. Determination of airborne fungal spores of Gaziantep (SE Turkey) Aerobiologia. 2016;32:441–452. [Google Scholar]
- 27.Ceter T, Pınar NM, Yildiz A, Güney K. Two year concentrations of allergen atmospheric fungal spores in Kastamonu, Turkey (2006-2007) Allergy. 2009;64:421. [Google Scholar]
- 28.Kalyoncu F. Relationship between airborne fungal allergens and meteorological factors in Manisa City. Turkey. Environ Monit Assess. 2010;165:553–558. doi: 10.1007/s10661-009-0966-x. [DOI] [PubMed] [Google Scholar]
- 29.Mezzari A, Perin C, Santos SA, Bernd LA. Airborne fungi in the city of Porto Alegre, Rio Grande do Sul, Brazil. Rev Inst Med Trop Sao Paulo. 2002;44:269–272. doi: 10.1590/s0036-46652002000500007. [DOI] [PubMed] [Google Scholar]
- 30.Hasnain SM, Akhter T, Waqar MA. Airborne and allergenic fungal spores of the Karachi environment and their correlation with meteorological factors. J Environ Monit. 2012;14:1006–1013. doi: 10.1039/c2em10545d. [DOI] [PubMed] [Google Scholar]
- 31.Almaguer-Chávez M, Aira MJ, Rojas TI, Fernández-González M, Rodríguez-Rajo FJ. New findings of airborne fungal spores in the atmosphere of Havana, Cuba, using aerobiological non-viable methodology. Ann Agric Environ Med. 2018;25:349–359. doi: 10.26444/aaem/89738. [DOI] [PubMed] [Google Scholar]
- 32.Kasprzyk I, Rzepowska B, Wasylów M. Fungal spores in the atmosphere of Rzeszów (south-East Poland) Ann Agric Environ Med. 2004;11:285–289. [PubMed] [Google Scholar]
- 33.Peternel R, Culig J, Hrga I. Atmospheric concentrations of Cladosporium spp. and Alternaria spp. spores in Zagreb (Croatia) and effects of some meteorological factors. Ann Agric Environ Med. 2004;11:303–307. [PubMed] [Google Scholar]
- 34.Chakrabarti HS, Das S, Gupta-Bhattacharya S. Outdoor airborne fungal spora load in a suburb of Kolkata, India: its variation, meteorological determinants and health impact. Int J Environ Health Res. 2012;22:37–50. doi: 10.1080/09603123.2011.588323. [DOI] [PubMed] [Google Scholar]
- 35.Stepalska D, Wolek J. Variation in fungal spore concentrations of selected taxa associated to weather conditions in Cracow, Poland, in 1997. Aerobiologia. 1997;21:43–52. [Google Scholar]
- 36.Oliveira M, Ribeiro H, Abreu I. Annual variation of fungal spores in atmosphere of Porto: 2003. Ann Agric Environ Med. 2005;12:309–315. [PubMed] [Google Scholar]
- 37.Celenk S, Bicakci A, Erkan P, Aybeke M. Cladosporium link ex Fr. And Alternaria Nees ex Fr. spores in the atmosphere of Edirne. J Biol Environ Sci. 2007;1:127–130. [Google Scholar]
- 38.Calderon C, Lacey J, Mc Cartney HA, Rosas I. Seasonal and diurnal variation of airborne basidiomycete spore concentrations in Mexico City. Grana. 1995;34:260–268. [Google Scholar]
- 39.Heinzerling LM, Burbach GJ, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, Bousquet J, Bousquet-Rouanet L, Bousquet PJ, Bresciani M, Bruno A, Burney P, Canonica GW, Darsow U, Demoly P, Durham S, Fokkens WJ, Giavi S, Gjomarkaj M, Gramiccioni C, Haahtela T, Kowalski ML, Magyar P, Muraközi G, Orosz M, Papadopoulos NG, Röhnelt C, Stingl G, Todo-Bom A, von Mutius E, Wiesner A, Wöhrl S, Zuberbier T. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–1506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez H, Bush RK. A review of Alternaria alternata sensitivity. Rev Iberoam Micol. 2001;18:56–59. [PubMed] [Google Scholar]
- 41.Güneser S, Atici A, Köksal F, Yaman A. Mold allergy in Adana, Turkey. Allergol Immunopathol (Madr) 1994;22:52–54. [PubMed] [Google Scholar]
- 42.Kilic M, Taskin E. Distribution of inhalant allergies in pediatric patients presenting with allergic complaints in the eastern Anatolia region. Minerva Pediatr. 2016;68:269–277. [PubMed] [Google Scholar]
- 43.Misirlioğlu ED, Cengizler MR. Skin prick test results of child patients diagnosed as bronchial asthma. Allergol Immunopathol (Madr). 2007;35:21–4. [DOI] [PubMed]
- 44.Yazicioglu M, Oner N, Celtik C, Okutan O, Pala O. Sensitization to common allergens, especially pollens, among children with respiratory allergy in the Trakya region of Turkey. Asian Pac J Allergy Immunol. 2004;22:183–190. [PubMed] [Google Scholar]
- 45.Corsico R, Cinti B, Feliziani V, Gallesio MT, Liccardi G, Loreti A, et al. Prevalence of sensitization to Alternaria in allergic patients in Italy. Ann Allergy Asthma Immunol. 1998;80:71–76. doi: 10.1016/s1081-1206(10)62943-2. [DOI] [PubMed] [Google Scholar]