Abstract
The present work seeks to investigate the kinetics and thermodynamic studies of ethidium bromide (EtBr) and eosin adsorption onto the synthesized Manganese (II) doped Zinc (II) Sulphide nanoparticles. A convenient scheme of co-precipitation was used for the synthesis of Manganese (II) doped Zinc (II) Sulphide nanoparticles. The Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FESEM) and X-ray diffractogram (XRD) techniques were used for the characterization of synthesized nanoparticles. The adsorption study was undertaken in a systematic manner. Effects of different experimental parameters were studied using batch adsorption method. It was evident from the results that EtBr and eosin removal was inversely proportional to the concentration of initial dye and directly proportional to contact time and adsorbent used. To study the adsorption equilibrium three different isotherm models like Langmuir, Freundlich and Flory-Huggins were used. It was observed that adsorption data synced most successfully with Langmuir isotherm model as compared to Freundlich and Flory-Huggins isotherm model. To fit the investigational statistics, the kinetic models pseudo 1st order, pseudo 2nd order and intra particle diffusion were taken onto consideration. The maximum dye removal of 98.19% and 97.16% for EtBr and eosin, was observed during the synthesis of nanoparticles.
Keywords: Zinc (II) sulphide nanoparticles, Ethidium bromide, Eosin, Adsorption, Kinetic
Introduction
Dyes are widely used in plethora of industries like textile, paints, leather, paper etc. These dyes, are mostly released as an unprocessed waste. Such releases damages the environment and ecosystem in numberless ways, by directly or indirectly contaminating the soil and water, thus adversely affecting all the forms of life. As is evident, most textile companies in developing countries are sited in rural areas, where waste water of the dyes is directly disposed into the municipal sewage system. There are numerous dyes used for the industrial and experimental purposes such as methylene blue, Eosin blue, EtBr, crystal violet, etc. [7, 8, 10, 36, 39, 40, 46].
Nanoparticles (NPs) are significant scientific tools that are being exploited in environmental, biotechnological and pharmacological sectors. NPs have countless magnetic, electronic, optical and chemical properties which are being used instrumentally in different fields such as diagnostics, biomedicine, sensors drug delivery systems, catalysis, antimicrobial activities and water treatment [7, 8, 23, 40, 49, 52].
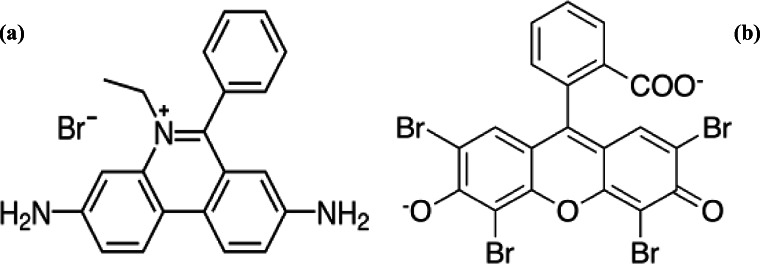
Ethidium bromide (Fig. 1a), an intercalating agent, and a fluorescent marker, is used in most of the molecular biology technique. It resembles to a DNA base pair, as it easily intercalate with the DNA strand. It is used as fluorescent tag in most of the nucleic acid experiments in the field of life science. Eosin (Fig. 1b) is a fluorescent acidic compound, which can easily bind with the protein molecules containing amino acid residue such as lysine and arginine. It is used to stain experimentally the collagen, muscle and protein present in the cytoplasm. It gives dark red or pink color, during the staining process. When exposed for longer period of time, it may lead to an eye irritation, skin, respiratory tract, gastrointestinal tract issues, etc. In the contemporary times the nanoparticles are being used to remove harmful dyes from the environment [14, 39, 53, 54, 56, 57, 60].
Figs. 1.
a, b: Structure of (a) ethidium bromide and (b) eosin
Nanoparticles (NPs) are significant scientific tools that are now contributing in environmental, biotechnological and pharmacological sectors. NPs have many magnetic, electronic, optical and chemical properties, which are being utilized in different fields such as diagnostics, biomedicine, sensors drug delivery systems, catalysis, antimicrobial activities and water treatment [7, 8, 15, 40, 49, 52, 58].
The wide range of industries like textile, plastic, paper, pulp, etc., use gigantic amount of water, dyes and chemicals in their manufacturing units during the process of coloring their products apart from that, these industrial units introduce alarmingly huge amount of pollutants in water. This irresponsible and untreated discharge of dyes in an environment is a grave concern from the toxicological and esthetical point of view. The removal treatment of synthetic dye from the environment is of crucial concern and need to be addressed as these dyes and their derivatives are highly carcinogenic and highly toxic to all the forms of life. Therefore, we cannot bank upon mere biodegradation for the management of this hazard. Therefore, the decolorization of the dye from the waste discharged is very important aspect of textile wastewater treatment [24, 34, 35, 37, 55].
Adsorption is one of best methods for the removal of various harmful waste materials. For the same purpose, different adsorbates like silica gel, activated carbon, nanoparticles, composite etc. are available. A chief problem with an activated carbon is the difficult segregation of powdered and granular carbon. Despite activated carbons of silica gel being the low cost adsorbent, they have low adsorption capacity, which limits their extensive utilization for the dye adsorption. Dyes removal through sunlight, UV light and different oxidizing agents is another serious concern, as the dye structure is complex aromatic and difficult to degrade with these methods [23, 59]. Nanoparticles are used for the adsorption of dyes, heavy metals pigments materials in a best way, because of their small size and higher surface area. Poly(methyl methacrylate) zinc oxide and Fe3O4 nanocomposite was successfully utilized for the removal of crystal violet and malachite green dyes, respectively ([19, 24]; Kahrizi et al., 2019; [41, 42]).
The present work aims to synthesize Manganese (II) doped Zinc (II) Sulphide nanoparticles by co-precipitation method. The synthesized ZnS NPs were used as a device for the removal of EtBr and eosin from water. To get the better results, all the experimental parameters were optimised with respect to dye adsorption. Adsorption kinetics of the dye removal was studied through different models like Langmuir, Freundlich and Flory-Huggins.
Experiment
Chemicals and reagents
Zinc sulphate Hepta-hydrate (ZnSO4.7H2O) (Sisco research laboratory Pvt., Ltd.) Manganese sulphate monohydrate, ethidium bromide (Mol. Weight = 394.29 g/mol, chemical formula = C21H20BrN3), NaOH, HCl (HiMedia Laboratories Pvt., Ltd.), Sodium sulphide, EDTA, Eosin blue dye (Mol. Weight = 647.89 g/mol, chemical formula = C20H6Br4Na2O5) (central Drug house (P) Ltd.). All the chemicals were of AR grade and were used without further purification.
UV-Visible spectrophotometer (Shimadzu UV-Spectrophotometer), hot air oven, glass beakers, volumetric flasks, magnetic stirrer, magnetic beads, Erlenmeyer flasks, measuring cylinder, pipette, weighing machine and Eppendorf tubes were used for the current study.
The manganese (II) doped ZnS NPs were characterized by FTIR, FESEM and XRD techniques. The analysis described the morphology of Mn2+ doped ZnS NPs. These characterizations gave insights about the morphology and crystalline nature of the synthesized NPs.
Synthesis of Mn2+ doped zinc sulphide nanoparticles (ZnS NPs)
Mn2+ doped ZnS NPs was synthesized by chemical co-precipitation method. All the chemical solutions were freshly prepared for the synthesis of ZnS NPs. These were synthesized by taking 50 mL of each 0.4 M zinc sulphate, 0.1 M manganese (II) sulphate, 0.5 M sodium sulphide in 80 mL distilled water at room temperature, then at the end EDTA was added with continuous stirring on magnetic stirrer. EDTA was used to stabilize the particles against aggregation. White color precipitates were formed, which were later separated from the reaction mixture by washing with distilled water followed by alcohol. An alcohol washing was used to eliminate the impurities and traces of EDTA along with original reactants, if either present. These wet precipitates (Fig. 2) were dried using hot air oven and then thoroughly grounded [4, 6, 12, 13, 18, 20, 31, 48].
Fig. 2.

White colour synthesised ZnS nanoparticles
Adsorption experiment
To study the adsorption process, 1000 mg/L of the EtBr and eosin solution was prepared. Different dye solutions of various concentrations were prepared by diluting the stock solution with deionised water. Adsorption kinetics and thermodynamics experiment were done using 0.05 g of ZnS NPS and 50 mL of EtBr and eosin dye solution with preliminary conc. of 50 mg/L. The flask was properly sealed, concentration of EtBr and eosin dye solution was studied using double beam UV-VIS spectrophotometer at a wavelength of 590 nm and 521 nm, respectively. Deionized water without EtBr and eosin was used as blank in the experiment. The adsorption of EtBr and eosin on synthesized ZnS NPs at different time interval was calculated from the concentration of EtBr and eosin in solution before and after the adsorption. An adsorption of EtBr and eosin (qe) (mg/g) onto ZnS NPs was calculated using the mass balance equation [9, 17, 26, 43, 44, 46, 47]:
Where, Ci and Ct is the conc. of the EtBr at the start of experiment and after time t (mg/L), respectively. V is the volume of the EtBr/eosin solution and w is the weight of the adsorbent. Error function was studied through ERRSQ. As the liquid-phase concentration increases at higher level, the magnitude of an error was increased which lead to a better fit for the isotherm parameter.
All the experiments were performed in triplicate so as to avoid error but to maintain reproducibility and accuracy.
Kinetic and isotherms
Adsorption isotherms demonstrated the interaction between adsorbate and adsorbent providing a comprehensive study about nature of interaction between the two. It is significant to optimise design of the adsorption process. The batch experimental kinetic data for the adsorption of EtBr and eosin onto ZnS NPs were studied through pseudo 1st – 2nd order and intra particle diffusion models. These models are given as [2, 3, 5, 21, 27, 45]:
The pseudo first order kinetic model is represented as:
Where, qe is the adsorption capacity of EtBr/Eosin (mg/g) at equilibrium and qt are adsorption capacity of EtBr/Eosin (mg/g) at time t. Rate constant of pseudo-1st order is given by K1 (min−1).
The pseudo second order kinetic is given as:
K2 is the rate constant for the pseudo second order (g/mg min.)
The equilibrium data obtained was investigated further using Freundlich, Langmuir and Flory-Huggins isotherm models and are represented as:
Langmuir isotherm model
Where qe is the amount of EtBr adsorbed on ZnS NPs at equilibrium (mg/g), qm is the theoretical maximum adsorption capacity, KL is the Langmuir isotherm constant (L/mg) and Ce is the EtBr concentration at equilibrium.
Freundlich isotherm model
The equation for Freundlich isotherm model is given as:
Where qe and Ce is the EtBr/Eosin concentration at equilibrium on ZnS NPS (mg/g) and solution (mg/L) respectively. Kf and n are the Freundlich constants.
Flory-Huggins isotherm model
Flory-Huggins isotherm model is represented as:
Where Ce is the concentration of EtBr/Eosin at equilibrium (mg/L), KFH is the equilibrium constant of adsorption, nFH is the number of EtBr molecules present in the adsorption site and θ is the degree of surface coverage.
Results and discussion
Adsorbent analysis
Field emission scanning Electron micrograph (FESEM) and dynamic light scattering (DLS)
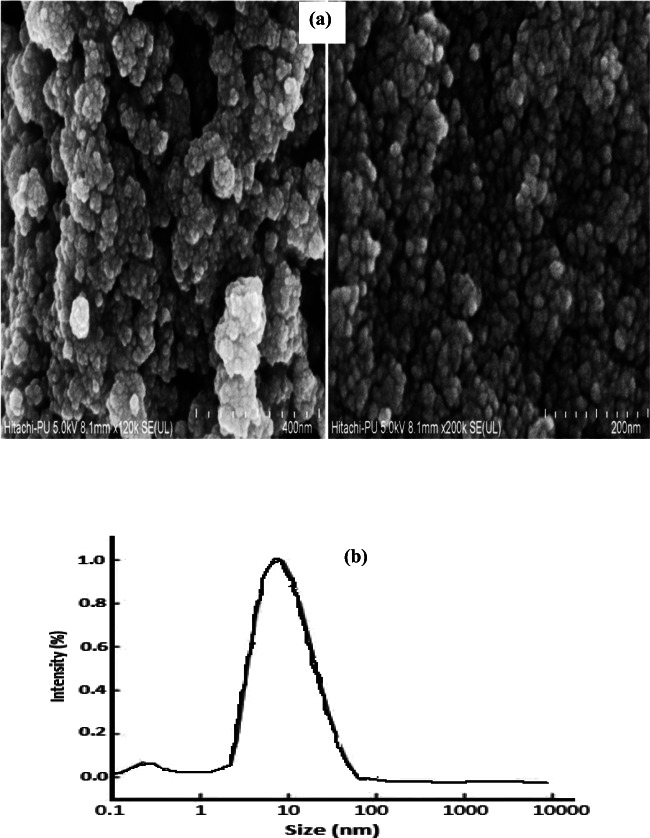
The FESEM of synthesized ZnS NPs was taken in electron 2 mode. The results showed homogenous size distribution with size range of approximately 15–28 nm (Fig. 3a). The average particle size of the synthesized Manganese (II) doped Zinc (II) sulphide nanoparticles were studied by DLS. DLS results clearly depicted the spherical narrow size distribution of synthesized ZnS nanoparticles and the diameter of ZnS NPs was found in the range 12–21 nm [14, 50, 60] (Fig. 3b).
Figs. 3.
a, b: (a) FESEM and (b) DLS image of Mn2+ doped ZnS nanoparticles
Fourier transform infrared spectroscopy (FTIR) analysis
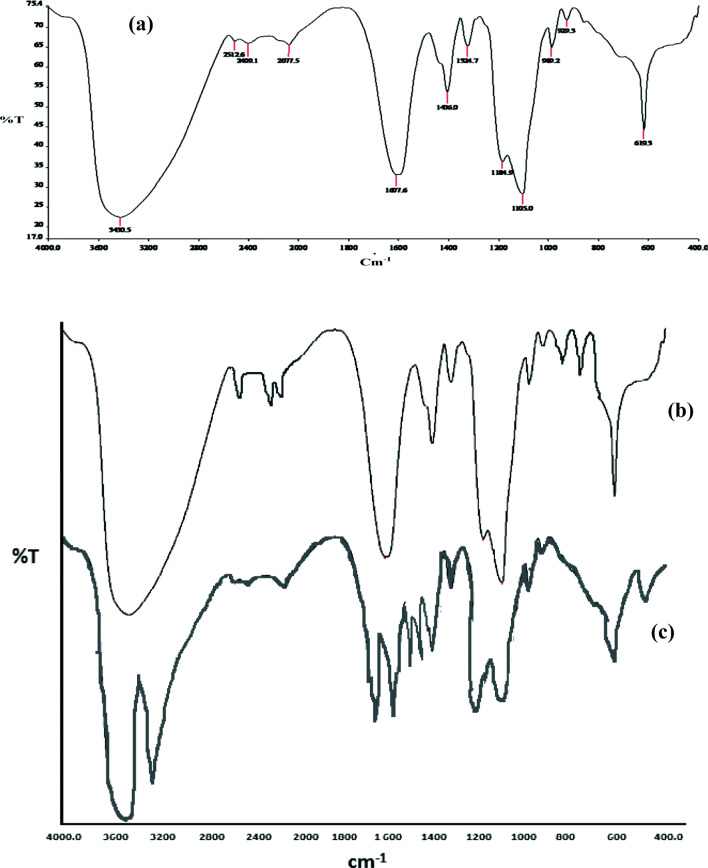
FTIR analysis of ZnS NPs are shown in Fig. 4a, ranging from 400 cm−1 to 4000 cm−1 wave number and producing peak between 3300 and 300 cm−1. Alkenyl C=C stretch at 1637.5 cm−1, aromatic C=C Bending at 1406 cm−1, polysaccharides at 1105 cm−1 and C-H Vibration at 619.3 cm−1. EtBr (Fig. 4b) shows additional peak in the range of 700-800 cm−1 and eosin (Fig. 4c) shows additional peaks at 1442, 1753 and 3468 cm−1. The additional peaks in case of eosin and EtBr depicts the presence of these dyes in the synthesized ZnS NPs.
Figs. 4.
a-c FTIR spectra of (a) Mn2+ doped ZnS NPs; (b) After EtBr adsorption and (c) eosin adsorption
XRD
X-ray diffractogram of the ZnS and Mangnese (II) doped Zinc (II) Sulphide NPs was used to study the crystalline aspects of the synthesized NPs. Fig. 5a shows the XRD pattern of ZnS and Fig. 5b shows the XRD pattern of Mangnese (II) doped ZnS NPs. Sharp and intense peaks were found in ZnS, this shows the polycrystalline structure of ZnS. The high intensity of the peak clearly indicates the crystalline nature of ZnS. The XRD pattern of Mn (II) doped ZnS NPs are similar to the ZnS. The Scherrar’s equation was used to calculate the crystalline size of ZnS and Mn (II) doped ZnS NPs. Crystalline size of both the samples are present in Table 1 and it is apparent from the results that crystalline size increased with the doping of Mn (II).
Figs. 5.
a,b: XRDs of (a) ZnS (b) manganese (II) doped ZnS NPs
Table 1.
Lattice parameter value of ZnS and Mn (II) doped ZnS nanoparticles
| Sample | Crystalline Size (nm) | a (A) | c (A) |
|---|---|---|---|
| ZnS | 12.76 | 2.89 | 4.76 |
| Mn doped ZnS | 16.21 | 2.93 | 4.81 |
Effect of pH on dye removal
The pH of the medium is an important parameter that influences the adsorption of EtBr and eosin onto the ZnS NPs. To study the pH effect on dye adsorption, pH was taken in the range of 2–10 with initial conc. of dye 0.5 mg/L and the ZnS NPs taken was 0.5 g. It was found from the results that at pH 2–7, the dye removal was far less, but it enhanced with an increase in the pH. The highest removal of 98.12% and 97.16% (Fig. 6) for EtBr and eosin, respectively occurred at pH 9. The surface of ZnS have same amount of zinc and sulfide ions in water, therefore, they are neutrally charged at pHpzc (point of zero charge). This shows that an alkaline medium enhanced the dye adsorption. High adsorption of dyes in alkaline medium is due to fact that the surface was alkaline, which lead to fall in the protonation on their surface because of neutralization of the positive charges, thus leading to faster and easier diffusion of dye particles. This can be explained on the basis that at alkaline pH no electrostatic repulsion takes place in non-ionized dyes and synthesized ZnS NPs, thus resulting in higher adsorption at alkaline medium. So for further study pH 9 was maintained throughout the experiment.
Fig. 6.

Effect of pH on the adsorption of EtBr and eosin onto synthesized ZnS NPs
Effect of contact time and temperature on adsorption
Effect of contact time was also studied to get the maximum adsorption of dyes from the solution. Equilibrium time is an important parameter, which help in designing the economic system for dyes treatment. Fig. 7ab shows the adsorption of EtBr and eosin on ZnS NPs, as a function of time. It is apparent from the results that adsorption is directly proportional to an increase in time. Rate of dye adsorption was fast initially and the saturation was observed after the adsorption of 98.09% in case of EtBr and 97.23% in case of eosin, after 14 h. The results shows that adsorption was faster at the initial stage and then it retarded to attain equilibrium after 14 h. Hence, these results shows that dye adsorption onto synthesized ZnS NPs is a two step process. Initially faster adsorption of dyes occurs on the surface of the adsorbents and later, a slow intracellular diffusion occurs in the interior of the adsorbent, thus, proving that the dye removal process is an adsorption process. Rate of adsorption do not increase with further increase in the contact time, this may be due to the fact that equilibrium was attained after 14 h of contact time and there was no vacant space for further adsorption, thus no adsorption occurred with further raise in contact time [16, 45, 50].
Figs. 7.
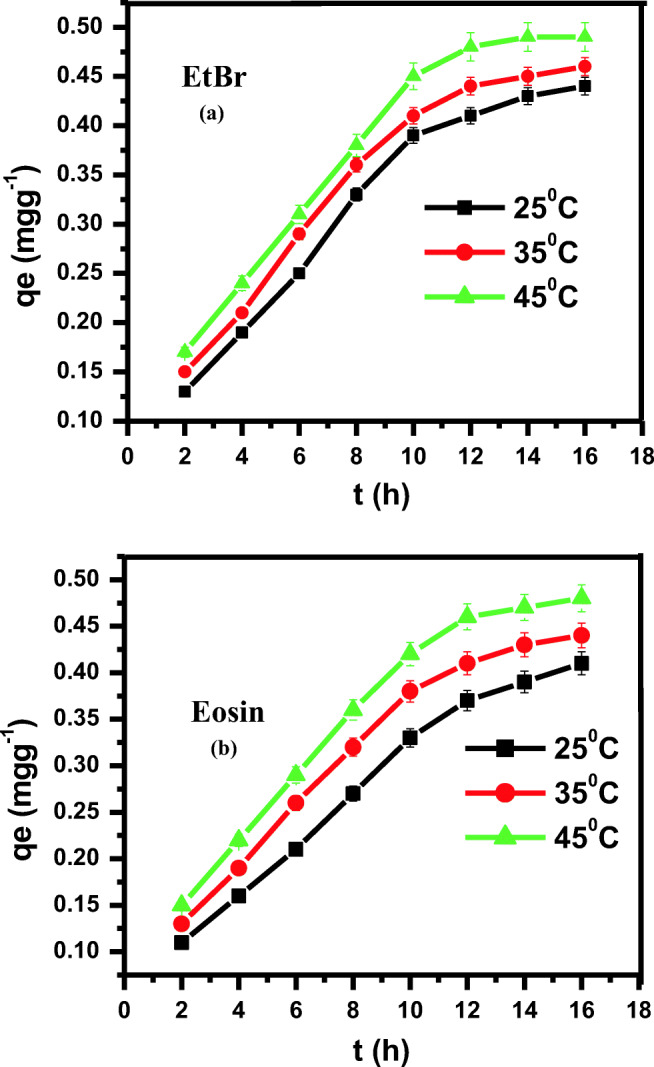
a,b: Effect of contact time and temperature on the adsorption of (a) EtBr and (b) eosin at ZnS NPs
The temperature at which adsorption occurred influenced the adsorption rate and the degree of adsorption, so it is deduced that the temperature is an important factor to study adsorption process. Temperature effect was studied by experimenting in the range of 25-45 °C. It was revealed from the Figs. 7a,b that adsorption of EtBr on ZnS nanoparticles is directly proportional to increase in temperature, and that the reaction was endothermic. This can be explained on the basis that as the temperature increases, the mobility of the EtBr and eosin molecules is also increases. It produces the swelling effect within the adsorbent structure, thus leading to the penetration of larger molecules of EtBr and eosin on the adsorbate surface. The larger removal of EtBr and eosin at high temperature shows that there is an increase in interaction between adsorbate and adsorbent with increase in temperature. The higher adsorption of EtBr as compared to the eosin onto synthesized ZnS NPs is due to the size of the EtBr is much less as compared to the eosin, so EtBr molecules can easily be adsorbed onto the ZnS NPs because of that its removal is higher than that of the eosin.
Effect of ZnS NPs dose
The amount of dye removal is directly proportional to increase in the ZnS NPs dose. This may be demonstrated on the basis of the availability of surface activities, which increased with an increase in the ZnS NPs dose and accumulation of the dyes. The enhancement of dyes adsorption is found to be inconsequential after the addition of 1.5 g/L of ZnS NPs (Fig. 8).
Fig. 8.

Effect of adsorbate dosage on adsorption of EtBr and eosin
h
Effect of initial dye concentration
The consequence of initial EtBr and eosin concentration (10–50 mg/L) onto ZnS NPs was given in the Fig. 9. It was revealed from the results that, there is faster dye removal in the beginning of process and then it proceeds leisurely until equilibrium was achieved. This can be explained on the basis that, initially there was presence of larger number of adsorption vacant sites, but as the adsorption increased the number of available site decreased. Equilibrium adsorption capacities of synthesized ZnS NPS decreased with an increase in the concentration of EtBr and eosin from 10 to 50 mg/L. Lesser the concentration of EtBr and eosin, higher the availability of the adsorption sites is. The EtBr and eosin molecules can easily find the available adsorption site on the adsorbate.
Fig. 9.
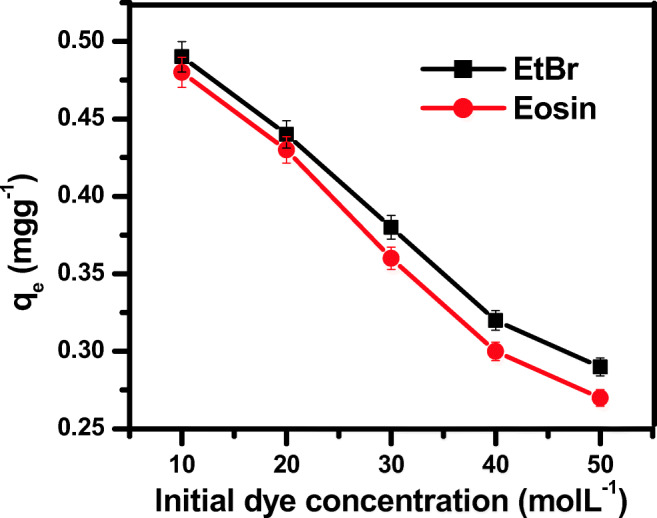
Effect of initial dye concentration on dye adsorption
Effect of agitation
The agitation speed was varied from 50 to 250 rpm for the adsorption of EtBr and eosin on ZnS NPs. It was clear from the results that no disparity occurred after 200 rpm (Fig. 10), it remained invariable with the adsorption of dyes.
Fig. 10.
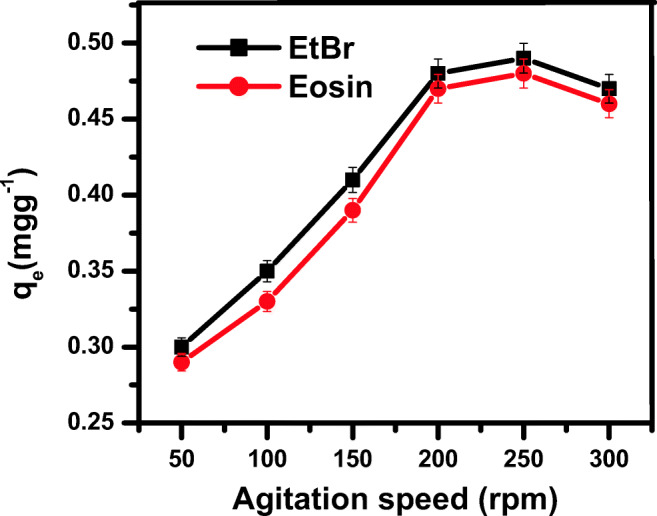
Effect of agitation speed in ETBr and eosin dye adsorption onto synthesized ZnS NPs
Desorption study
Desorption study of the synthesized ZnS NPs was analysed to find out the regeneration and reusability of ZnS NPs. Synthesized ZnS NPs were regenerated using ethanol with low concentration of alkali (1–5% v/v). The regeneration efficiency was found to be 91% for EtBr and 89% for eosin in first cycle and 62% and 54% for EtBr and eosin, respectively in second cycle. This shows that synthesized ZnS NPs are stable and easy to reuse efficiently, thus synthesized ZnS NPs is efficient and effective adsorbent for the removal of EtBr and eosin dyes [1, 28, 29].
Evaluation of adsorption isotherms
Langmuir adsorption isotherm model is following the monolayer adsorption on homogenous adsorbent, while Freundlich isotherm is an empirical isotherm and this is utilized in non-ideal adsorption and this generally involves heterogenous surface system. Flory-Huggins isotherms derive the surface coverage of the adsorbate on the adsorbent and tell about the viability and spontaneous nature of the process. Equilibrium adsorption plot of dyes onto synthesized ZnS NPs is present in Fig. (11) and all the related factors are given in Table 2. The results showed that correlation coefficient (R2) was higher than that of 0.99, which, demonstrated that dye adsorption onto synthesized ZnS NPs was best illustrated by Langmuir model. The value of R2 is higher than that of 0.99, so isotherm model is acceptable for the de adsorption onto synthesized ZnS NPs. There are four types of Langmuir isotherm model, but the type I of the Langmuir model is best fit to the isotherm as compared to Freundlich and Flory-Huggins isotherm models, so only type I is given in this manuscript.
Fig. 11.
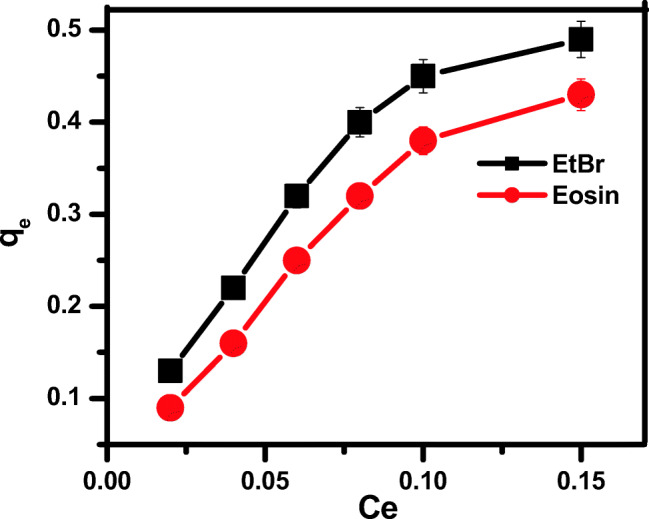
Equilibrium adsorption isotherm for EtBr and eosin removal through ZnS Nps
Table 2.
Langmuir, Freundlich and Flory-Huggins isotherm constant
| Langmuir model | |||
|---|---|---|---|
| KL (L/g) | qm (mg/g) | R2 | |
| EtBr | 13.178 | 0.892 | 0.9967 |
| Eosin | 13.056 | 0.789 | 0.9921 |
| Freundlich model | |||
| KF (mg/g)/(mg/L)1/n | N | R2 | |
| EtBr | 1.4872 | 1.9578 | 0.9912 |
| Eosin | 1.4695 | 1.7956 | 0.9908 |
| Flory-Huggins model | |||
| KFH | nFH | R2 | |
| EtBr | 21.018 | 1.9013 | 0.9781 |
| Eosin | 14.126 | 0.9157 | 0.9802 |
Favourability of the process is described by the separation factor RL. The value of RL =0 defined that the adsorption process is irreversible, if it is in between 0 and 1, then it is favourable and the value of RL = 1, showed that it is linear and greater than 1 defined that the process is unfavourable. RL is defined as:
The values of RL is found in range of 0.6–0.7 for EtBr and 0.7–0.8 for eosin, which confirms that two adsorption processes are favourable.
Adsorption kinetics
The value of k1 and qe was calculated from the slope and intercept of the plots of ln(qe - qt) for the pseudo first order kinetics (Table 2). The R2 value of pseudo first order was found in the lower range. Furthermore, there is a lot of disparity between the experimental (qe, exp) and calculated value (qe, cal) mg/g. The result suggests that dye adsorption onto synthesized ZnS NPs did not follow pseudo 1st order kinetics, thus in this case adsorption is not controlled by diffusion process [26–28, 32, 45].
In case of pseudo second order kinetics, the value of qe and k2 are determined from the slopes and intercepts of plots of t/qt versus t (Figs. 12a,b and Table 2). The value of R2 of linear plot for different concentration was found to be higher. The consistency of calculated and experimental data were also found, which suggests that dye adsorption on synthesized ZnS NPs follows pseudo second order kinetics.
Figs. 12.
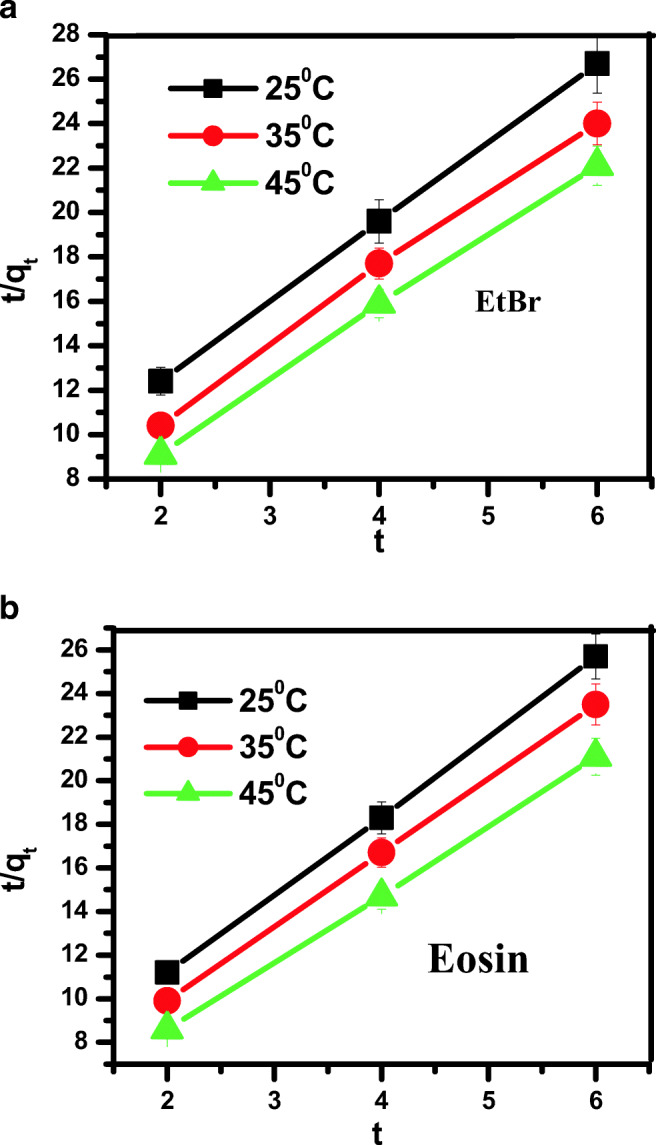
a,b: Pseudo second order kinetic for the removal of (a) EtBr and (b) eosin onto synthesized ZnS NPs
Intra-particle diffusion
The results obtained from the kinetic models were then analysed by intra particle diffusion model, so as to find out the diffusion process [21–23, 46]:
Where ki and C are the intra particle diffusion rate constant (mg/g min0.5), it intercept respectively.
Initially adsorption was fast, which may be due to the fact that adsorbate diffuses from the solution to the external surface of the ZnS NPs. Later on, there was gradual adsorption occurred, which is due to the diffusion of the various dye molecules within the ZnS NPs. This shows that ki was found to be higher in the first region as compared to the second region. Thus, it was evident from the results that rate of dye adsorption was faster in early stage as the larger vacant surface area was available to the adsorbent. This caused formation of thick layer on the external surface of the adsorbent, because of the interaction and molecular association. It causes hindrance of the adsorbent site for further adsorption of dyes molecules. The uptake rate of the dye was limited by the rate of transfer of adsorbate from the external to the internal site of the adsorbent (Table 3).
Table 3.
Kinetic parameter of EtBr and Eosin adsorption onto synthesized ZnS NPs
| Kinetic parameter | EtBr | Eosin | ||||
|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | |
| qe,exp (mg/g) | 0.271 | 0.284 | 0.291 | 0.248 | 0.259 | 0.268 |
| Pseudo first order | ||||||
| qe (mg/g) | 0.631 | 0.589 | 0.508 | 0.517 | 0.498 | 0.489 |
| k1 (min.−1) | 0.5686 | 0.5718 | 0.5791 | 0.5187 | 0.5264 | 0.5372 |
| R2 | 0.9891 | 0.9882 | 0.9873 | 0.9876 | 0.9871 | 0.9867 |
| Pseudo second order | ||||||
| qe (mg/g) | 0.293 | 0.302 | 0.309 | 0.265 | 0.278 | 0.289 |
| k2 (g/mg min.) | 4.4572 | 4.6718 | 4.7814 | 3.8761 | 3.9426 | 3.9863 |
| R2 | 1.000 | 0.9989 | 0.9976 | 0.9954 | 0.9932 | 0.992 |
Adsorption thermodynamics
Thermodynamic study of the adsorption is essential to study the spontaneity of the process. Gibbs free energy change (∆Go) designate about the spontaneity of any chemical reaction. It is significant to evaluate the energy and entropy factors so as to know about the Gibbs free energy of the process. Negative value of ∆G0 represents the spontaneity of the reaction at a particular temperature. The adsorption constant KL (L/mol) of the Langmuir isotherm, depends upon the temperature and is used to find the thermodynamic parameters. The free energy change (∆G0), entropy (∆S0) and enthalpy (∆H0) for the dye adsorption was studied by the equations as given [25, 30, 33–35, 46]:
Where T is the temperature and R is the universal gas constant. The slope and intercept of plot ∆Go versus T (Fig. 13) was used to calculate ∆Ho and ∆So of dyes. The results obtained are given in Table 4. The negative value of free energy change (∆Go) indicates that dyes adsorption on to ZnS NPs are feasible and spontaneous thermodynamically. As the temperature increases, there is decrease in the negative value of ∆Go, which suggests the high temperature is more favourable for the dyes adsorption onto synthesized ZnS NPs. The value of ∆Ho is positive, which shows that dye adsorption is an endothermic process. The positive value of ∆So indicates the affinity of adsorbent and adsorbate.
Fig. 13.

Gibbs free energy change with temperature for adsorption of EtBr and eosin onto synthesized ZnS Nps
Table 4.
Temperature effect on thermodynamic parameter
| Thermodynamic parameter | ∆Ho (KJ/mol) |
∆So (J/molK) |
∆Go (KJ/mol) | |||
|---|---|---|---|---|---|---|
| 290 K | 300 K | 310 K | 320 K | |||
| EtBr | 31.678 | 122.84 | −4.916 | −5.968 | 06.436 | −7.216 |
| Eosin | 33.192 | 127.56 | −3.617 | −4.712 | −4.784 | −5.153 |
Comparison of synthesized ZnS NPs with literature
It is clear from the Table 5 that synthesized Manganese (II) doped Zinc (II) sulphide nanoparticles are very effective adsorbent for the adsorption of harmful dyes ethidium bromide and eosin. There are many adsorbents mentioned in the literature, which were used for the removal of the dyes but the best result is shown by synthesized Zn NPs.
Table 5.
Comparative study of synthesized Manganese (II) doped Zinc (II) sulphide nanoparticles with other materials as mentioned in the literature for the removal of ethidium bromide and eosin dyes
| S.No. | Dye | Adsorbent | qe(mg/g) | References |
|---|---|---|---|---|
| 1. | Ethidium bromide | Graphene oxide | 0.584 mg/g | [41, 42] |
| 2. | Eosin yellow | Teak leaf litter powder | 31.64 mg/g | [11] |
| 3. | Eosin yellow | pineapple peels | 13.35 mg/g | [51] |
| 4. | Eosin yellow | pineapple peels | 89.80% | [38] |
| 5. | Ethidium btomide | Single-walled carbon nanotubes (SWCNTs) | 0.770 mg/g SWCNTs | [29] |
| 6. | Ethidium btomide |
Carboxylate functionalized single-walled carbon nanotube (SWCNT-COOH) |
0.830 mg/g, SWCNT-COOH, | [29] |
| 7. | Ethidium bromide | Manganese (II) doped Zinc (II) sulphide nanoparticles | 98.09% | Present work |
| 8. | Eosin | Manganese (II) doped Zinc (II) sulphide nanoparticles | 97.23% | Present work |
Comparison of EtBr and eosin removal from synthesized ZnS NPs
The results as shown in Table 6, clearly showed that synthesized ZnS NPs are best suited for the removal of EtBr.
Table 6.
Comparison of EtBr and eosin removal from the synthesized ZnS NPs
| EtBr | Eosin | |||||
| Langmuir | ||||||
| R2 | 0.9967 | 0.921 | ||||
| Freundlich | ||||||
| R2 | 0.9912 | 0.9908 | ||||
| Flory-Huggins | ||||||
| R2 | 0.9781 | 0.9802 | ||||
| Pseudo first order | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C |
| qe(mg/g) | 0.631 | 0.589 | 0.508 | 0.517 | 0.498 | 0.489 |
| R2 | 0.9891 | 0.9882 | 0.9873 | 0.9876 | 0.9871 | 0.9867 |
| Pseudo second order | ||||||
| qe(mg/g) | 0.293 | 0.302 | 0.309 | 0.265 | 0.278 | 0.289 |
| R2 | 1 | 0.9989 | 0.9976 | 0.9954 | 0.9932 | |
| 0.992 | ||||||
| Thermodynamic parameter | ||||||
| ∆Ho (KJ/mol) | 31.678 | 33.192 | ||||
| ∆So (J/molK) | 122.84 | 127.56 | ||||
Conclusion
The present study confirms the successful synthesis of Manganese (II) doped Zinc (II) Sulphide nanoparticles. The synthesized ZnS NPs acts as an effective material for the exclusion of EtBr and eosin. Various reaction parameters were optimized, so as to achieve maximum adsorption from the synthesized ZnS NPs and it was found that best results were obtained at Ph = 9, Contact time required is 14 h, temperature is 45 °C, Adsorbent dosage is 1.5 g/L with agitation speed of 200 rpm. The maximum dye removal was found to be 98.19% and 97.16% for EtBr and eosin, respectively. Different isotherm models were used to study the adsorption equilibrium, but the best fit model is Langmuir isotherm. The best kinetic model was found to be intra particle diffusion. The value of ∆Ho is positive, which shows that dye adsorption is an endothermic process. The negative value of ∆Go represents spontaneous nature of the reaction. This is one of the most effective methods for the removal of dyes from water.
Acknowledgements
Dr. Saruchi and Dr. Vaneet Kumar are thankful to CT Group of institutions, Jalandhar for carring out this research work. This work was also funded by researchers supporting project and one of the authors Asma A. ALOThman is grateful to the researchers supporting project number (RSP-2020/243), King Saud University, Riyadh, Saudi Arabia.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest regarding this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahamad T, Ruksana, Chaudhary AA, Naushad M, Alshehri SM, (2019) Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. International Journal of Biological Macromolecules 134:180–188 [DOI] [PubMed]
- 2.Akbari H, Gholami M, Akbari H, Adibzadeh A, Taghavi L, Hayati B, et al. Poly (amidoamine) generation 6 functionalized Fe 3O4@SiO2/GPTMS core–shell magnetic NPs as a new adsorbent for Arsenite adsorption: kinetic, isotherm and thermodynamic studies. J Environ Health Sci Eng. 2020;18:23–6. [DOI] [PMC free article] [PubMed]
- 3.Cao J, Yang J, Zhang Y, Yang L, Wang Y, Wei M, et al. Optimized doping concentration of manganese in zinc sulphide nanoparticles for yellow orange light emission. J Alloy Compd. 2009;486:890–4.
- 4.Chattopadhyay M, Kumbhakar P, Tiwary CS, Sarkar R, Mitra K, Chatterjee U. Multiphoton absorption and reflection in Mn+2 doped ZnS quantum dots. J Appl Phys. 2009;105:024313. [Google Scholar]
- 5.Chavoshan S, Khodadadi M, Nasseh N. Photocatalytic degradation of penicillin G from simulated wastewater using the UV/ZnO process: isotherm and kinetic study. J Environ Health Sci Eng. 2020;18:107–117. doi: 10.1007/s40201-020-00442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devi BSR, Raveendran R, Vaidyan AV. Synthesis and characterization of Mn+2 doped ZnS nanoparticles. Pramana. 2007;68(4):679–687. [Google Scholar]
- 7.Dogan M, Abak H, Alkan M. Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. J.Hazard.Mater. 2009;164:172–181. doi: 10.1016/j.jhazmat.2008.07.155. [DOI] [PubMed] [Google Scholar]
- 8.Dogan M, Karaoglu MH, Alkan M. Adsorption kinetics of maxilon yellow 4GL and maxilon red GRL dyes on kaolinite. J.Hazard.Mater. 2009;165:1142–1151. doi: 10.1016/j.jhazmat.2008.10.101. [DOI] [PubMed] [Google Scholar]
- 9.Dong B, Cao L, Su J, Liu W, Qu H, Zhai H. Water soluble ZnS:Mn/ZnS core/shell nanoparticles prepared by a novel two-step method. J Alloy Compd. 2010;492:363–367. [Google Scholar]
- 10.Elwakeel KZ, Elgarahy AM, Elshoubaky GA, Mohammad SH. Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J Environ Health Sci Eng. 2020;18:35–50. doi: 10.1007/s40201-019-00435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyelude EO, Awudza JAM, Twumasi SK. Equilibrium, kinetic and thermodynamic study of removal of eosin yellow from aqueous solution using teak leaf litter powder. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-12424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang X, Bando Y, Gautam UK, Zhai T, Zeng H, Xu X, et al. ZnO and ZnS nanostructures: ultraviolet-light emitters, lasers and sensors. Crit Rev Solid State. 2004;34:190–223.
- 13.Hu H, Zhang W. Synthesis and properties of transition metals and rare-earth metals doped ZnS nanoparticles. Opt Mater. 2006;28:536–550. [Google Scholar]
- 14.Naushad M, Mittal A, Rathore M, Gupta V. Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin. Water Treat. 2015;54:2883–90
- 15.Kahrizi P, Fatemeh S, Mohseni-Shahri MF. Adsorptive removal of cadmium from aqueous solutions using NiFe2O4/hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. J Nanostruct Chem. 2018;8(4):441–452. [Google Scholar]
- 16.Kaith BS, Saruchi JR, Bhatti MS. Screening and RSM optimization for synthesis of a gum tragacanth–acrylic acid based device for in situ controlled cetirizine dihydrochloride release. Soft Matter. 2012;8:2286–2293. [Google Scholar]
- 17.Kaur B, Chand S, Singh K, Malik AK. Detoxification of dye contaminated water by Mn+2 – doped ZnS nanostructures. Bull Mater Sci. 2019;42:61–70. [Google Scholar]
- 18.Kaur N, Kaur S, Singh J, Rawat M. A review on zinc sulphide nanoparticles: from synthesis, properties to applications. Journal of Bioelectronics and nanotechnology. 2016;1(1):1–5. [Google Scholar]
- 19.Khoshsang H, Ghaffarinejad A, Kazemi H, Wang Y. Hamidreza Arandiyan, one-pot synthesis of S-doped Fe2O3/C magnetic nanocomposite as an adsorbent for anionic dye removal: equilibrium and kinetic studies. J Nanostruct Chem. 2018;1(8):23–32. [Google Scholar]
- 20.Kole AK, Kumbhakar P. Effect of manganese doping on the photoluminescence characteristics of chemically synthesized zinc sulphide nanoparticles. Appl Nanosci. 2012;2(1):15–23. [Google Scholar]
- 21.Kumar V, Rehani V, Kaith BS, Saruchi. Synthesis of a biodegradable interpenetrating polymer network of Av-cl-poly(AA-ipn-AAm) for malachite green dye removal: kinetics and thermodynamic studies. RSC Adv. 2018;8:41920–38. [DOI] [PMC free article] [PubMed]
- 22.Li, X., Li, Y.. Adsorptive Removal of Dyes from Aqueous Solution by KMnO4-Modified Rice Husk and Rice Straw. 2019, Article ID 8359491, 9. 10.1155/2019/8359491, 9.
- 23.Liu J, Wang N, Zhang H, Baeyens J. Adsorption of Congo red dye on FexCo3-xO4 nanoparticles. J Environ Manag. 2019;238:473–483. doi: 10.1016/j.jenvman.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Liu QX, Zhou YR, Wang M, Zhang Q, Chen TY, Yu DC. Adsorption of methylene blue from aqueous solution onto viscose-based activated carbon fiber felts: kinetics and equilibrium studies. Adsorpt Sci Technol. 2019;37(3–4):312–332. [Google Scholar]
- 25.Lu C, Chiu H, Liu C. Removal of zinc(II) from aqueous solution by purified carbon nanotubes: kinetics and equilibrium studies. Ind Eng Chem Res. 2006;45:2850–2855. [Google Scholar]
- 26.Mittal H, Mishra SB. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydrate Polymer. 2014;101:1255–1264. doi: 10.1016/j.carbpol.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Mittal H, Kumar V, Saruchi RSS. Adsorption of methyl violet from aqueous solution using gum xanthan/Fe3O4 based nanocomposite hydrogel. Int J Biol Macromol. 2016;89:1–11. doi: 10.1016/j.ijbiomac.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed DN, Naji LA, Faisal AAH, Al-Ansari N, Naushad M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Scientific Reports. 2020;10 (1): 1–12. [DOI] [PMC free article] [PubMed]
- 29.Moradi O, Norouzi M, Fakhri A, Naddafi K. Interaction of removal Ethidium bromide with carbon nanotube: equilibrium and isotherm studies. J. Environ. Health Sci. Eng. 2014;2014:12:17. doi: 10.1186/2052-336X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moradi O, Zare K, Monajjemi M, Yari M, Aghaie H. The studies of equilibrium and thermodynamic adsorption of Pb(II), cd(II) and cu(II) ions from aqueous solution onto SWCNTs and SWCNT -COOH surfaces. Fullerenes, Nanotubes and Carbon Nanostructures. 2010;18:285–302. [Google Scholar]
- 31.Murugadoss G, Ramasamy V. Synthesis, effect of capping agents and optical properties of manganese-doped zinc sulphide nanoparticles. Luminescence. 2013;28(1):69–75. doi: 10.1002/bio.2346. [DOI] [PubMed] [Google Scholar]
- 32.Yari M, Rajabi M, Moradi O, Yari A, Asif M, Agarwal S, et al. Kinetics of the adsorption of Pb(II) ions from aqueous solutions by graphene oxide and thiol functionalized graphene oxide. Journal of Molecular Liquids. 2015;209:50–7.
- 33.Moradi O, Zare K. Adsorption of Pb(II), cd(II) and cu(II) ions from aqueous solution onto SWCNTs and SWCNT -COOH surfaces: kinetics study. Fullerenes, nanotubes and carbon nanostructures. 2011;19:628–652. [Google Scholar]
- 34.Najafi F, Norouzi M, Zare K, Fakhri A. Removal of ethidium bromide b carbon nanotube in aqueous solution: isotherms, equilibrium mechanism studies, and its comparison with nanoscale zero valent iron as adsorbent. Journal of Nanostructure in Chemistry. 2013;60(3):1–7. [Google Scholar]
- 35.Najafi F, Norouzi M, Zare K, Fakhri A. Removal of ethidium bromide by carbon nanotube in aqueous solution: isotherms, equilibrium mechanism studies, and its comparison with nanoscale of zero valent iron as adsorbent. Journal of Nanostructure in Chemistry. 2013;3:60. [Google Scholar]
- 36.Noradoun CE, Cheng IF. EDTA degradation induced by oxygen activation in a Zerovalent Iron/air/water system. Environ Part Sci Technol. 2005;39:7158–7163. doi: 10.1021/es050137v. [DOI] [PubMed] [Google Scholar]
- 37.Noubactep C. Characterizing the discoloration of methylene blue in FeO/H2O systems. J Hazard Mater. 2009;166:79–87. doi: 10.1016/j.jhazmat.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Okey-Onyesolu CF, Okoye CC, Chime DC. Removal of eosin yellow dye from aqueous solution using oil bean seed shells based activated carbons: equilibrium. Kinetics and thermodynamics studies International Journal of Scientific & Engineering Research. 2018;9(3):140. [Google Scholar]
- 39.Omid M, Ali F, Saeideh A, Sepideh A. Isotherm, thermodynamic, kinetics, and adsorption mechanism studies of Ethidium bromide by single-walled carbon nanotube and carboxylate group functionalized single-walled carbon nanotube. J Colloid Interface Sci. 2013;395:224–229. doi: 10.1016/j.jcis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Del Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 41.Rajabi M, Mahanpoor K, Moradi O. Thermodynamic and kinetic studies of crystal violet dye adsorption with poly(methyl methacrylate)-graphene oxide and poly(methyl methacrylate)-graphene oxide-zinc oxide nanocomposites. J Appl Polym Sci. 2019;136(22):47495. [Google Scholar]
- 42.Rajabi M, Mahanpoor K, Moradi O. Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: kinetic and thermodynamic studies. Compos Part B. 2019;167:544–555. [Google Scholar]
- 43.Saruchi KV. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb+2 ions from aqueous solutions by a hybrid ion-exchanger. Arab J Chem. 2019;12:316–329. [Google Scholar]
- 44.Saruchi K. V, Kaith BS. Synthesis of hybrid ion exchanger for rhodamine B dye removal: equilibrium, kinetic and thermodynamic studies. I & EC Research. 2016;55(39):10492–10499. [Google Scholar]
- 45.Saruchi KBS, Kumar V, Jindal R. Biodegradation study of enzymatically catalyzed interpenetrating polymer network: evaluation of agrochemical release and impact on soil fertility. Biotechnol. Rep. 2016;9:74–81. doi: 10.1016/j.btre.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saruchi, Kumar, Mittal H, Alhassan SM. Biodegradable hydrogels of tragacanth gum polysaccharide to improve water retention capacity of soil and environment-friendly controlled release of agrochemicals. International J Biol Macromol. 2019;132:1252–61. [DOI] [PubMed]
- 47.Saruchi TP. Kumar, V. Kinetics and thermodynamic studies for removal of methylene blue dye by biosynthesize copper oxide nanoparticles and its antibacterial activity, Journal of Environmental Health Science and Engineering. 2019;17:367–376. doi: 10.1007/s40201-019-00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa DM, Alves LC, Marques A, Gaspar G, Lima JC, Ferreira T. Facile microwave-assisted synthesis manganese doped zinc sulfide nanoparticles. Sci Rep. 2018;8:15992–16002. doi: 10.1038/s41598-018-34268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava R.K., Pandey N., Mishra S., 2013. Effect of cu concentration on the photoconductivity properties of ZnS nanoparticles synthesized by co-precipitation method. Material science in semiconductor processing, 16: 1659-1664.
- 50. Naushad M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium, Chem. Eng. J. 2014; 235: 100–8.
- 51.Ugbe FA, Ikudayisi VA. The kinetics of eosin yellow removal from aqueous solution using pineapple peels. Edorium J Waste Manag. 2017;2:5–11. [Google Scholar]
- 52.Venkatesha TG, Viswanatha R, Arthoba NY, Chethana BK. Kinetics and thermodynamics of reactive and vat dyes adsorption on MgO nanoparticles. Chem EngJ. 2012;198:1–10. [Google Scholar]
- 53.Vergis BR, Krishna RH, Kottam N, Nagabhushana BM, Sharath R, Darukaprasad B. Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. J Nanostruct Chem. 2018;8(1):1–12. [Google Scholar]
- 54.Koli PB, Kapadnis KH, Deshpande UG, Patil MR. Fabrication and characterization of pure and modified Co3O4 nanocatalyst and their application for photocatalytic degradation of eosine blue dye: a comparative study. J Nanostruct Chem. 2018;4(8):453–463. [Google Scholar]
- 55.Wang H, Zhou A, Peng F, Yu H, Yang J. Mechanism study on adsorption of acidified multi-walled carbon nanotubes to Pb(II) J Colloid Interface Sci. 2007;316:277–283. doi: 10.1016/j.jcis.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 56.Yao Y., Xu F., Chen M., Xu Z., Zhu Z.. Nano/micro engineered and molecular systems (NEMS), in: 5th IEEE international conference, 2010a 1083–1087.
- 57.Yao Y, Xu F, Chen V, Xu Z, Zhu Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol. 2010;101:3040–3046. doi: 10.1016/j.biortech.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, Y., Sun, Q., Zhang, X., Baeyens, J., Su, H., Self-assembled selenium nanoparticles and their application in the rapid diagnostic detection of small cell lung cancer biomarker. Soft Matter. 2017, 14(5) 10.1039/C7SM01687E [DOI] [PubMed]
- 59.Zhou M, Yang T, Hu W, He X, Kie J, Wang P, et al. Scalable fabrication of Metallopolymeric superstructures for highly efficient removal of methylene blue. Nanomaterials. 2019b;9:1–18. [DOI] [PMC free article] [PubMed]
- 60.Zhou Y, Lu J, Zhou Y, Liu Y. Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut. 2019;252A:32–365. doi: 10.1016/j.envpol.2019.05.072. [DOI] [PubMed] [Google Scholar]