Abstract
Introduction
Focal therapy can offer the middle ground for treatment between active surveillance and radical therapy in patients with low- and intermediate-risk prostate cancer. Factors that prohibit focal therapy from being standard of care are numerous. Several consensus projects have been conducted to position the utilization of imaging and trial design in focal therapy. However, the literature is still scarce on patient follow-up after focal therapy. For these reasons, an international multidisciplinary consensus project was established in order to reach consensus about a uniform follow-up protocol after focal therapy.
Objective
To standardize patient follow-up after focal therapy.
Materials and methods
A literature study was performed, and a questionnaire was constructed. The questionnaire was sent out to 76 participants (70 % urologists, 28 % radiologists and 2 % biomedical engineers) in three consecutive rounds according to the Delphi method. In each round, the panelists were presented with the results of the previous round. Participants each had the opportunity to adapt, delete or add questions. The topics discussed pertaining to follow-up after focal therapy were as follows: (1) general,(2) biopsies, (3) PSA, (4) digital rectal examination (DRE), (5) imaging, (6) quality of life (QoL) and (7) registration and pooling of data. The project was concluded with a face-to-face meeting in which final conclusions were formulated.
Results
The follow-up after focal therapy should be a minimum of 5 years. The following modalities should be included in assessing post-treatment outcomes: multiparametric MRI (mpMRI), biopsies, assessment of erectile function, QoL, urinary symptoms and incontinence. A systematic 12-core TRUS biopsy combined with 4–6 targeted biopsy cores of the treated area and any suspicious lesion(s) should be performed after 1 year, and thereafter only when there is suspicion on imaging. The ideal way to perform targeted biopsies is to use TRUS–MRI fusion technology. PSA should be performed for research purposes, in the first year, every 3 months, and after the first year, every 6 months. mpMRI is the optimal imaging modality for follow-up after focal therapy. On a 1.5T scanner, an endorectal coil is strongly advised by the panel, whereas on a 3T machine, it is optional, however, it will improve image quality. The following sequences should be included: T2WI, DWI including high b values of >1,000 and ADC maps of DWI, DCE and T1WI. Imaging should be performed at 6 months and at 1 year following treatment; after the first year post-treatment, it should be performed every year until 5 years following treatment. All data should ideally be pooled in a common global database.
Conclusion
Focal therapy is a relatively new form of treatment for prostate cancer. In order to include focal therapy as a standard of care treatment, consistent follow-up is necessary. By implementing the results of this consensus study, focal therapy users will be able to provide important and standardized outcome data.
Keywords: Focal therapy, Follow-up, Consensus, Prostate cancer
Introduction
Focal therapy offers a middle ground approach as a disease management strategy in a select group of men with low- and intermediate-risk prostate cancer (PCa), positioned between active surveillance on one side and radical treatment on the other side [1]. The factors that prohibit focal therapy from becoming standard of care are multiple: a lack of clear definitions of what constitutes focal therapy [2], a wide variety of ablative technologies available and lack of substantial standardized studies, and consequently reliable outcome data. Cryotherapy and high-intensity focused ultrasound (HIFU) are mentioned by the guidelines of the European Association of Urology as management options in patients with clinically localized PCa [3-5]. The following other technologies also belong to the armamentarium of focal therapies for prostate cancer: laser ablation therapy [6], radiofrequency ablation [7], irreversible electroporation [8], photodynamic therapy [9] and focal brachytherapy [10], among others. In order to advance the field and render focal therapy a standard of care, uniformly comparable outcome data have to be produced. Various consensus meetings among experts have led to protocols for both focal therapy trial design and the utilization of imaging in focal therapy [2, 11-14]. However, the literature on follow-up strategies following focal therapy is still scarce.
For these reasons, an international multidisciplinary consensus project was established to reach consensus about a uniform follow-up protocol following focal therapy. The project was executed according to an adapted Delphi method. The Delphi method has been used for many years to reach consensus on topics with minimum bias. By implementation of the results of this consensus project, scientific results can be generated which may allow focal therapy to evolve as an accepted means of prostate cancer care for the appropriate patient.
Materials and methods
The project was conducted according to the Delphi method, derived from the 1950s that proved to be effective in reaching consensus on economic and political issues. More recently, this method has been used regularly in the medical field [15-17].
A systematic literature search was performed on “prostate cancer” (and synonyms) and “focal therapy” (and synonyms) and “follow-up”(and synonyms) (see textbox below). This search yielded 378 articles, of which only a limited number was useful. The panel members were selected as authors of clinical trials from the literature study, or recommended as expert in the field by their peers. The systematic literature search was also used to select controversial topics in the field and to design a questionnaire to resolve these issues. BM and WvdB designed the questionnaire under the supervision of OU and TP.
(((“Prostatic Neoplasms”[Mesh] OR prostatic neoplasm*[tiab] OR prostate neoplasm*[tiab] OR prostate cancer*[tiab] OR prostatic cancer*[tiab] OR prostatic malignan*[tiab] OR prostate malignan*[tiab] OR prostatic tumor*[tiab] OR prostate tumour*[tiab] OR prostate tumor*[tiab] OR prostatic tumour*[tiab])) AND (Focal Therapy[tiab] OR focal therap*[tiab] OR “Cryotherapy”[Mesh] OR Cryotherapy[tiab] OR Cryotherap*[tiab] OR Cold Therapy[tiab] OR Cold therap*[tiab] OR Radiofrequency ablation[tiab] OR RFA[tiab] OR Radiofrequency[tiab] OR “Laser Therapy”[Mesh] OR laser therapy[tiab] OR laser therap*[tiab] OR laser ablation[tiab] OR ILT[tiab] OR interstitial laser thermotherapy[tiab] OR “Electroporation”[Mesh] OR Electroporation[tiab] OR irreversible electroporation[tiab] OR IRE[tiab] OR nanoknife[tiab] OR “High-Intensity Focused Ultrasound Ablation”[Mesh] OR High-Intensity Focused Ultrasound Ablation[tiab] OR High Intensity Focused Ultrasound Ablation[tiab] OR High-Intensity Focused Ultrasound[tiab] OR High Intensity Focused Ultrasound[tiab] OR HIFU[tiab] OR “Photochemotherapy”[Mesh] OR Photochemotherapy[tiab] OR Photochemotherap*[tiab] OR Photodynamic therapy[tiab] OR Photodynamic Therap*[tiab] OR Photo dynamic therapy[tiab] OR photo dynamic therap*[tiab] OR PDT[tiab] OR tookad[tiab])) AND ((((“Follow-Up Studies”[Mesh]) OR “Follow-Up Studies”) OR “follow-up”) OR “follow up”)
The web-based questionnaire was constructed by the use of www.surveymonkey.com (accessed between July 1, 2014, and August 15, 2014). The panel members were sent the questionnaire in three consecutive rounds. For each new round, the panelists were presented with the results of the previous rounds. They were encouraged to adapt the questions or indicate when they disagreed with a question/topic. In this way, a structured academic discussion was held in order to reach consensus about a variety of subtopics. The whole questionnaire can be found in Appendix 1 of supplementary material. The pertinent topics related to follow-up after focal therapy discussed were as follows: (1) general, (2) biopsies, (3) PSA, (4) DRE, (5) imaging, (6) QoL and (7) registration and pooling of data. Consensus was defined as 75 % agreement on a topic.
The project was concluded with a final meeting on August 21, 2014, for which every participant received an invitation. In this final meeting, the results of the questionnaires were presented to the panelists, and conclusions were formulated.1 The meeting was recorded for documentation purposes.
Results
Seventy-six experts were invited to participate in the project, 51 agreed, 3 declined and 22 did not reply. Questionnaires were sent out in 3 rounds, to each person who did not decline. Fifty-eight participants filled out at least 1 round, 38 participants filled out all rounds, 15 experts did not fill out any round and were excluded from the project. The response rate for round 1 was 86.2 % (50/58), the response rate for round 2 was 84.5 % (49/58), and the response rate for round 3 was 79.3 % (46/58). Seventy percent of the panel were urologists, 28 % radiologists and 2 % biomedical engineers. The total estimated number of patients treated by the panel on a yearly basis was 1931, with a minimum of 0, a maximum of 300, a mean of 39 patients per year and median of 20 patients per year.
Results from the consensus project
General
The follow-up period following focal therapy should be defined as the period needed to determine the efficacy of the treatment. It is reasonable to expect that after a period of 5 years, the chance that residual disease will become apparent is low and therefore the follow-up period following focal therapy should be defined as a minimal period of 5 years. Oncological treatment success has to be defined as negative biopsies of the treated area.
Functional treatment success should be defined as the absence of treatment-related functional change, after a period of healing. Therefore, the various functional parameters have to be assessed, each at their own intervals. Erections and ejaculations have to be assessed for 24 months, QoL for 24 months and urinary symptoms for 12 months. The most important parameters for functional treatment success are QoL and erections, continence and prostate symptoms.
The following modalities should be evaluated in the follow-up following focal therapy: histology (prostate biopsies), serum PSA, prostate imaging, and assessment of erectile function, QoL, urinary symptoms and incontinence. Although there was no consensus (71 %) about the inclusion of an objective assessment of incontinence using a 24-h pad weight test, it is advised by the panel. There was no consensus about assessment of bowel symptoms by a bowel symptom score. The panel agreed that assessment of ejaculation should be part of the follow-up, by the addition of Q9 and Q10 (orgasmic function) of the IIEF-15 to the shortened IIEF-5 score.
Biopsies
At 1 year after treatment, a biopsy of the treated area should be performed, combined with a systematic 12× TRUS biopsy of the whole prostate (also untreated areas). After the first biopsy at 1-year post-treatment, re-biopsy of the treated area should be done only when there is suspicion on imaging. The best way to take a biopsy of the treated area is to take 4–6 targeted cores from the margins of the ablation zone including at least one sample from within the area of ablated/fibrosed tissue. These 4–6 cores from the treated area are essential to account for: (1) fibrosis-related gland deformity and (2) the possible slight degree of misregistration even when using a fusion device. After the initial biopsy at 1 year, the panel advised only to perform re-biopsies if suspect imaging findings are demonstrated.
The untreated area of the prostate should be biopsied at 1 year after treatment for surveillance purposes. When the treated area is sampled, a 12-core systematic TRUS biopsy of the remainder of the prostate should be performed. Afterward, biopsy of the untreated area should be performed only when there is suspicion on imaging. When performing a targeted biopsy of a suspicious lesion, it is advised to also perform a systematic 12-core random TRUS biopsy. The optimal modality for follow-up biopsy of suspicious lesions is TRUS–MRI fusion guided biopsy technology.
With the rising awareness of molecular characterization being predictive with outcome of prostate biopsies, the biological effects of focal therapy could be documented by molecular analysis of post-therapy biopsies. The pathologist who reads the biopsy material should have an understanding of focal therapy and interpreting tissue after a particular ablative intervention.
PSA
PSA should be included in the follow-up following focal therapy. The first PSA should be taken 3-month post-treatment. After the first measurement, PSA should be taken every 3 months during the first year; after the first year, PSA should be taken every 6 months. Although PSA doubling time seems the most important parameter that could indicate treatment failure, no consensus could be reached about a definition of biochemical recurrence. Since there is no consensus on the role of PSA, the data should be collected for research purposes.
DRE
DRE should still be part of the follow-up after focal therapy. Although it does not have much added value for prostate evaluation, it may have some value in the evaluation of bowel-related symptoms, especially in ablative procedures performed by a transrectal approach.
Imaging
mpMRI is the optimal imaging modality for follow-up following focal therapy. mpMRI can be performed at 1.5T, but only with the use of an endorectal coil. When a 3T mpMRI is available, the endorectal coil is not a strict prerequisite, but it will enhance image quality by improving the signal-to-noise ratio. For optimal imaging, the following sequences should be included: T2WI, DWI including high b values of >1,000 and ADC maps of DWI, DCE and T1WI. T1WI is required for assessment of hemorrhage and lymph nodes. MRSI is not beneficial and should not be performed in the follow-up following focal therapy. The first imaging should be performed at 6-month post-treatment as a baseline to evaluate recurrence or residual disease. Before that time, imaging may still be distorted by treatment-related hemorrhage, artifact or the expected inflammatory process after an intervention. Follow-up imaging should be performed every year after the initial imaging at 6 and 12 months, or in the case of a biochemical suspicion of progression (although no consensus could be reached about a clear definition of this).
DCE is considered the most important sequence to detect a recurrence or residual disease. Suspicion for treatment failure is defined as one or a combination of the following features: early focal enhancement on DCE, focal area with diffusion restriction on ADC maps and focal area with high signal intensity on DWI with a b value >1,000 after 6 months. Treatment success on imaging is defined as the absence of treatment failure in the targeted area, after 1 year. Curve type analysis for DCE may also help following focal treatment, since fibrosis would show gradual increased enhancement rather than rapid wash-in. When mpMRI findings are positive, they always have to be confirmed by targeted biopsy of the suspicious area.
Quality of life and functional outcome
QoL, urinary symptoms, incontinence, erections and ejaculations should be assessed in the follow-up following focal therapy. Each aspect should be evaluated every 3 months in the first year and every 6 months in the second year. IPSS/AUA score combined with uroflowmetry/residual urine measurement should be used to evaluate urinary symptoms, incontinence should ideally be evaluated by a 24-h pad weight test, or a question about the number of pads used per day. IIEF-5 scores should be used to evaluate erections. Ejaculations should be evaluated by the addition of two questions to the IIEF-5 questionnaire that are pertinent to orgasmic function [Q9 (When you had sexual stimulation or intercourse, how often did you ejaculate?) and Q10 (When you had sexual stimulation or intercourse, how often did you have the feeling of orgasm or climax)] taken from the IIEF-15 questionnaire. However, no consensus was reached on the best overall QoL assessment tool, as several are available. Each individual question should be entered as a data point. For example, using the IIEF-5, the second question [With sexual stimulation, how often have your erections been sufficient for penetration (entering your partner)?] is the most important.
Data pooling and complication registration
100 % of the panel members agreed that adverse events and complications should be registered. Moreover, there was a strong consensus (84 %) that it is important to pool data on focal therapy in a common database, on the condition that all focal therapy data will be gathered in a standardized way. Based on the results of this meeting, we constructed a flow diagram for surveillance after focal therapy that aims to standardize follow-up (Fig. 1).
Fig. 1.
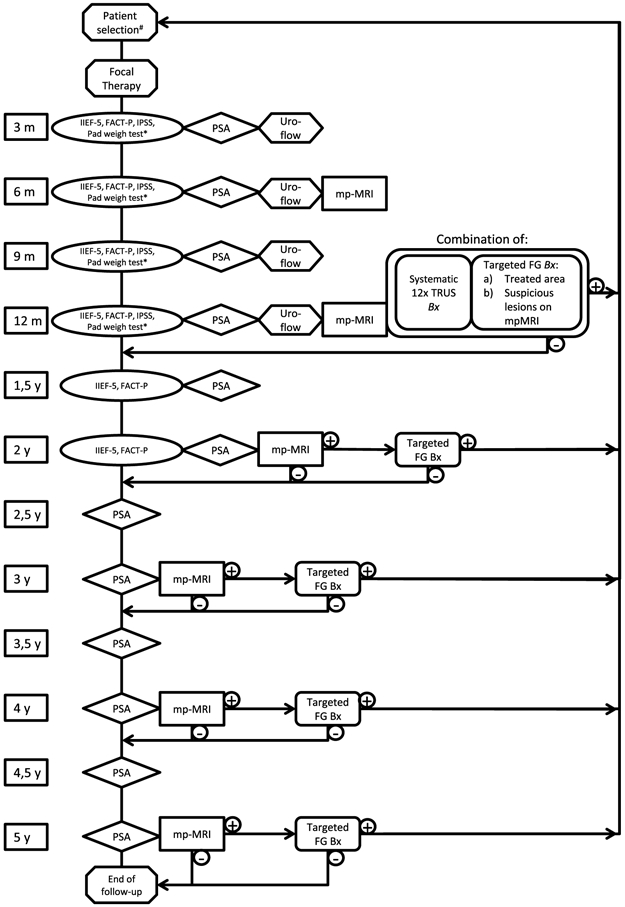
Follow-up scheme as proposed by the panel: #Patient selection for focal therapy is not a topic of this consensus project. When biopsies in the follow-up proof recurrent or residual disease, the treating physician should evaluate the treatment options again. *Assessment of incontinence by either 24-h pad weight test (most objective test and advised as optimum), or number of pads used per day
Discussion
The majority of panel members agreed that all findings on follow-up should be registered in a common database. It is important to establish such a database and clinicians who perform focal therapy should use it. An online platform would be most suitable. The Cryo On-Line Database is an example of such a registry that reports on outcome after cryotherapy for prostate cancer. When setting up a global registry for focal therapy, one needs to take into account the following possible pitfalls: funding of the project (ideally, the database should be independent of any public company), authorships for publication, monitoring/auditing of the data, quality control, selection bias (every patient needs to be registered) and different methods/energy sources of focal therapy that could be compared. Although constructing an international database could be costly and challenging, it is deemed essential. We demonstrated earlier that most centers with expertise currently only treat 10–30 patients a year. In order to form strong, uniform data, such a database is of paramount importance.
In the proposed follow-up diagram (Fig. 1), biopsies will be performed 1 year after treatment. After this initial pathological evaluation of the treatment, biopsies will only be performed when there is suspicion on imaging. Pitfalls of (repeated) prostate biopsies include: (1) infections appear in 2–5 % of patients [4], (2) after focal ablation, the treated area will contract and eventually will be partially reabsorbed and replaced by fibrotic tissue, surrounded by normal prostate tissue, therefore, the treated area might be challenging to target, (3) due to fibrosis, the prostate tissue can be firm and biopsies can be painful and (4) interpretation of PSA can be challenging because of biopsy induced damage/inflammation/infection of the prostate [18, 19]. A study which evaluated biopsies of the treated area as early as 6 months following HIFU showed no difficulty in diagnostics using microscopy or immunohistochemistry [20].
There is consensus that PSA does not currently offer any reliable, reproducible data in the follow-up following focal therapy. PSA is not cancer specific, it is volume dependent on glandular tissue and when the prostate is damaged or inflamed, serum PSA will increase. Since a significant amount of functional prostate tissue is still in situ after focal therapy, PSA could indicate a need for imaging, but as an indicator for biopsy, PSA is unreliable. PSA is expected to temporally rise right after treatment as a consequence of treatment-related damage to and inflammation of the prostate. Since the PSA half-life is approximately 2–3 days, values should normalize approximately within 1 month following treatment [21]. For this reason, it is advised to measure the first PSA 3 months after treatment and in the first year every 3 months. Although PSA doubling time might appear to be an important parameter that could indicate treatment failure, no consensus could be reached about a definition of biochemical recurrence.
For the evaluation of functional outcome, objective and standardized evaluation is very important. Although no consensus could be reached about the best QoL assessment tool, any validated instrument would be suitable. Various questionnaires have been published, each with their own purpose [22, 23]. The FACT-P questionnaire has been validated for assessment of QoL after radiotherapy for prostate cancer [24]. The panel agreed that this tool is suitable and advised its use in the follow-up following focal therapy. Preservation of ejaculation is an important reason why men may opt for focal therapy. The panel agreed it should be evaluated by the addition of Q9 and Q10 (orgasmic function) of the IIEF-15, to the shortened IIEF-5 version commonly used when evaluating focal therapy outcomes [25].
Limitations
By implementing an adapted Delphi method, objective results were reached without dominant personalities being able to influence the results. The questions were formulated from the literature and by the selected expert panel. A bias could be that the panelists were all experts in focal therapy and might therefore be enthusiastic about its abilities. A second limitation is that not every panelist completed all the questionnaires, and not every panelist was able to attend the face-to-face meeting.
Conclusion
Focal therapy is a relatively new form of localized prostate cancer treatment. In order to enable focal therapy becoming a standard of care, standardization of patient follow-up is essential. A specific roadmap is given for the middle- and long-term follow-up of currently available focal therapy approaches. By implementing the results of this Delphi-based consensus study, standardized literature may be generated that accelerates a broader implementation of focal therapy.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00345-014-1475-2) contains supplementary material, which is available to authorized users.
Conflict of interest The following authors declare no conflict of interest: B.G. Muller, W. van den Bos, M. Brausi, J.J. Fütterer, S. Ghai, P.A. Pinto, I.V. Popeneciu, T.M. de Reijke, B. Turkbey and H. Wijkstra. C. Robertson is consultant/PI at EDAP TMS Inc., S. Scionti is consultant at SonaCare Medical, T.J. Polascik receives a research grant from Endocare, O. Ukimura is advisory board member at SonaCare Medical, J.J.M.C.H. and de la Rosette is consultant to AngioDynamics.
Ethical standard The manuscript does not contain clinical studies or patient data.
Participants of the meeting B. G. Muller, M. Brausi, J. J. Fütterer, S. Ghai, P. A. Pinto, I. V. Popeneciu, T. M. de Reijke, C. Robertson, S. Scionti, H. Wijkstra, O. Ukimura and T. J. Polascik.
Contributor Information
B. G. Muller, Department of Urology, AMC University Hospital, Amsterdam, The Netherlands
W. van den Bos, Department of Urology, AMC University Hospital, Amsterdam, The Netherlands
M. Brausi, Department of Urology, Estense S.Agostino Hospital, Modena, Italy
J. J. Fütterer, Department of Radiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; MIRA Institute for Biomedical Technology and Technical Medicine, University of Twente, Enschede, Netherlands
S. Ghai, Joint Department of Medical Imaging, University Health Network – Mount Sinai Hospital – Women’s College Hospital, University of Toronto, Toronto, Canada
P. A. Pinto, Urologic Oncology Branch, National Cancer Institute, Bethesda, MD, USA
I. V. Popeneciu, Department of Urology, University Clinic Heidelberg, Heidelberg, Germany
T. M. de Reijke, Department of Urology, AMC University Hospital, Amsterdam, The Netherlands
C. Robertson, Department of Surgery, Duke University Medical Center, Durham, NC, USA; Department of Urology, Duke University Medical Center, Durham, NC, USA
J. J. M. C. H. de la Rosette, Department of Urology, AMC University Hospital, Amsterdam, The Netherlands
S. Scionti, Urology, Scionti Prostate Center, Sarasota, FL, USA
B. Turkbey, Molecular Imaging Program, National Cancer Institute, Bethesda, MD, USA
H. Wijkstra, Department of Urology, AMC University Hospital, Amsterdam, The Netherlands; Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, The Netherlands
O. Ukimura, Department of Urology, Norris Cancer Center, University of Southern California, Los Angeles, CA, USA
T. J. Polascik, Department of Surgery, Duke University Medical Center, Durham, NC, USA; Department of Urology, Duke University Medical Center, Durham, NC, USA
References
- 1.Valerio M et al. (2014) The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol 66(4):732–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed HU et al. (2012) Transatlantic Consensus Group on active surveillance and focal therapy for prostate cancer. BJU Int 109(11):1636–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordeiro ER et al. (2012) High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int 110(9):1228–1242 [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich A et al. (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1):124–137 [DOI] [PubMed] [Google Scholar]
- 5.Tsivian M, Polascik TJ (2010) Focal cryotherapy for prostate cancer. Curr Urol Rep 11(3):147–151 [DOI] [PubMed] [Google Scholar]
- 6.Oto A et al. (2013) MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology 267(3):932–940 [DOI] [PubMed] [Google Scholar]
- 7.Garcia MAB, Gimeno AV, Cruz JF (2007) Radiofrequency interstitial tumor ablation (RITA) for the treatment of localised prostate cancer. Actas Urol Esp 31(6):627–632 [DOI] [PubMed] [Google Scholar]
- 8.Valerio M et al. (2014) Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate Cancer Prostatic Dis 17(4):343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore CM et al. (2014) Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localized prostate cancer using WST11-vascular targeted photodynamic (VTP) therapy. BJU Int. doi: 10.1111/bju.12816 [DOI] [PubMed] [Google Scholar]
- 10.Kovacs G, Cosset JM, Carey B (2014) Focal radiotherapy as focal therapy of prostate cancer. Curr Opin Urol 24(3):231–235 [DOI] [PubMed] [Google Scholar]
- 11.de la Rosette J et al. (2010) Focal therapy in prostate cancer-report from a consensus panel. J Endourol 24(5):775–780 [DOI] [PubMed] [Google Scholar]
- 12.Dickinson L et al. (2011) Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 59(4):477–494 [DOI] [PubMed] [Google Scholar]
- 13.Muller BG et al. (2014) Role of multiparametric magnetic resonance imaging (MRI) in focal therapy for prostate cancer: a Delphi consensus project. BJU Int 114(5):698–707. doi: 10.1111/bju.12548 [DOI] [PubMed] [Google Scholar]
- 14.van den Bos W et al. (2014) Focal therapy in prostate cancer: international multidisciplinary consensus on trial design. Eur Urol 65(6):1078–1083 [DOI] [PubMed] [Google Scholar]
- 15.Linstone HA, Turoff M (1975) The Delphi method: techniques and applications. Addison-Wesley, London [Google Scholar]
- 16.Stewart J et al. (1999) Identifying appropriate tasks for the preregistration year: modified Delphi technique. BMJ 319(7204):224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams PL, Webb C (1994) The Delphi technique: a methodological discussion. J Adv Nurs 19:180–186. doi: 10.1111/j.1365-2648.1994.tb01066.x [DOI] [PubMed] [Google Scholar]
- 18.Collins GN et al. (1997) The effect of digital rectal examination, flexible cystoscopy and prostatic biopsy on free and total prostate specific antigen, and the free-to-total prostate specific antigen ratio in clinical practice. J Urol 157(5):1744–1747 [PubMed] [Google Scholar]
- 19.Yuan JJ et al. (1992) Effects of rectal examination, prostatic massage, ultrasonography and needle biopsy on serum prostate specific antigen levels. J Urol 147(3 Pt 2):810–814 [DOI] [PubMed] [Google Scholar]
- 20.Biermann K et al. (2010) Histopathological findings after treatment of prostate cancer using high-intensity focused ultrasound (HIFU). Prostate 70(11):1196–1200 [DOI] [PubMed] [Google Scholar]
- 21.Oesterling JE et al. (1993) Effect of cystoscopy, prostate biopsy, and transurethral resection of prostate on serum prostate-specific antigen concentration. Urology 42(3):276–282 [DOI] [PubMed] [Google Scholar]
- 22.Hamoen EH et al. (2014) Measuring health-related quality of life in men with prostate cancer: a systematic review of the most used questionnaires and their validity. Urol Oncol. doi: 10.1016/j.urolonc.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Penson DF, Litwin MS, Aaronson NK (2003) Health related quality of life in men with prostate cancer. J Urol 169(5):1653–1661 [DOI] [PubMed] [Google Scholar]
- 24.Esper P et al. (1997) Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 50(6):920–928 [DOI] [PubMed] [Google Scholar]
- 25.Rosen RC, Cappelleri JC, Gendrano N 3rd (2002) The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 14(4):226–244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


