A new role for myosin-binding protein C in damping sarcomere force oscillations is proposed that has implications for mechanical feedback and cell-to-cell communication in the heart.
Abstract
Myosin-binding protein C (MyBP-C) is a critical regulator of muscle performance that was first identified through its strong binding interactions with myosin, the force-generating protein of muscle. Almost simultaneously with its discovery, MyBP-C was soon found to bind to actin, the physiological catalyst for myosin’s activity. However, the two observations posed an apparent paradox, in part because interactions of MyBP-C with myosin were on the thick filament, whereas MyBP-C interactions with actin were on the thin filament. Despite the intervening decades since these initial discoveries, it is only recently that the dual binding modes of MyBP-C are becoming reconciled in models that place MyBP-C at a central position between actin and myosin, where MyBP-C alternately stabilizes a newly discovered super-relaxed state (SRX) of myosin on thick filaments in resting muscle and then prolongs the “on” state of actin on thin filaments in active muscle. Recognition of these dual, alternating functions of MyBP-C reveals how it is central to the regulation of both muscle contraction and relaxation. The purpose of this Viewpoint is to briefly summarize the roles of MyBP-C in binding to myosin and actin and then to highlight a possible new role for MyBP-C in inducing and damping oscillatory waves of contraction and relaxation. Because the contractile waves bear similarity to cycles of contraction and relaxation in insect flight muscles, which evolved for fast, energetically efficient contraction, the ability of MyBP-C to damp so-called spontaneous oscillatory contractions (SPOCs) has broad implications for previously unrecognized regulatory mechanisms in vertebrate striated muscle. While the molecular mechanisms by which MyBP-C can function as a wave maker or a wave breaker are just beginning to be explored, it is likely that MyBP-C dual interactions with both myosin and actin will continue to be important for understanding the new functions of this enigmatic protein.
Introduction
Myosin-binding protein C (MyBP-C) is an important regulator of striated muscle contraction. First identified through its strong interactions with myosin (Offer et al., 1973), the force-generating protein of muscle, it was soon discovered that MyBP-C also binds to actin (Moos, 1981; Moos et al., 1978; Yamamoto, 1986), the protein catalyst that activates myosin to exert force. Because myosin and actin are the main constituent proteins of sarcomere thick and thin filaments, respectively, and because the two filament systems occupy distinct but overlapping spatial distributions, it had been an ongoing debate whether MyBP-C regulates contraction primarily through inhibitory effects on myosin or through its more recently discovered activating effects on the thin filament. While the controversy is not yet entirely settled, it is now more widely accepted that MyBP-C affects both thick and thin filaments and that it may provide a direct line of communication between the two filament systems. Current research efforts are thus focused on resolving the dynamics of how MyBP-C influences each filament system and how it relays mechanical information between thick and thin filaments.
This review provides a short synopsis of historic and current views on how cMyBP-C regulates contraction through its dual interactions with myosin and actin. The review then highlights evidence for a newly emerging role of MyBP-C to regulate spontaneous oscillatory contractions, so-called SPOCs, in vertebrate striated muscles. It is argued that SPOCs are analogous to stretch-activated contractions in insect flight muscles (IFMs) where Ca2+ is permissive for cycles of contraction and relaxation to occur, but the cycles are initiated and terminated by mechanical signals originating from sarcomeres rather than from chemical signals such as a rise and fall in intracellular Ca2+. Because acute removal of MyBP-C from sarcomeres using a novel cut-and-paste approach was recently found to induce SPOCs in cardiac muscle, while replacement of MyBP-C abolished SPOCs (Napierski et al., 2020), it is proposed that a previously unrecognized role of MyBP-C is to damp stretch-activated mechanical oscillations of muscle sarcomeres in vertebrate striated muscles. It is further argued that the results have broad implications for mechanisms of contractile regulation, energetic efficiency, and excitation–contraction coupling in vertebrate striated muscles. Because evidence for these ideas comes primarily from recent studies using cardiac muscle, the cardiac isoform (cMyBP-C) will be emphasized throughout the discussion. However, because it is likely that there are overall similarities between skeletal and cardiac MyBP-C function, cardiac and skeletal MyBP-C proteins will be considered interchangeably unless otherwise noted.
MyBP-C interactions with myosin
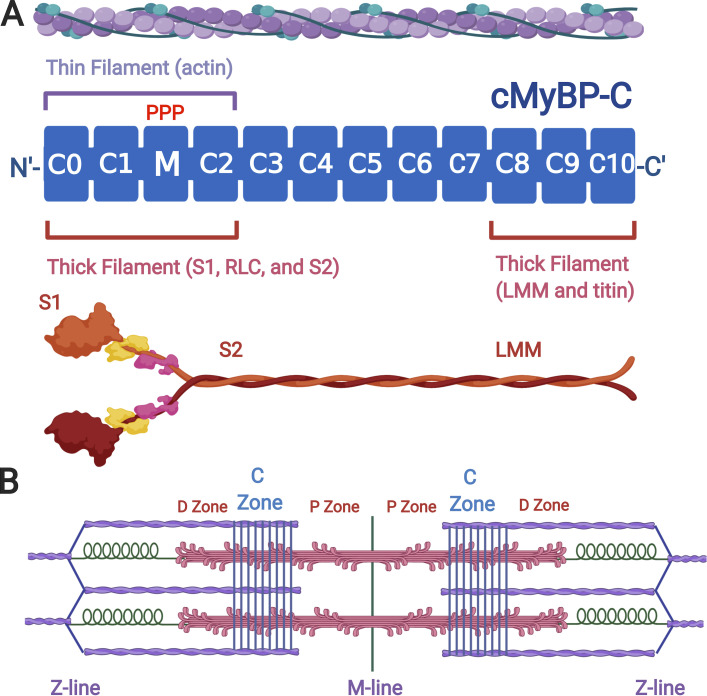
cMyBP-C is composed of a series of domains with structural similarity to folded Ig-like and fibronectin-like domains. As indicated in Fig. 1, cMyBP-C, encoded by the MYBPC3 gene, has 11 folded domains numbered C0–C10 beginning at the N terminus of the protein (Carrier et al., 1997), whereas the slow and fast skeletal isoforms (encoded by MYBPC1 and MYBPC2, respectively) each lack the C0 domain and have only domains C1–C10 (Weber et al., 1993). For all three proteins, the last three C-terminal domains, C8, C9, and C10, mediate tight binding to the light meromyosin (rod) portion of the myosin thick filament (Flashman et al., 2007; Gilbert et al., 1999, 1996), with binding to this segment in the A-band reinforced by a trimeric complex formed by myosin, MyBP-C, and the super repeats of titin (Freiburg and Gautel, 1996). The additional interactions of MyBP-C with titin confer its characteristic localization to a series of “stripes” spaced ∼43 nm apart that occur in the middle third of each half-thick filament (Tonino et al., 2019). The position of the MyBP-C stripes defines the C-zone of each half sarcomere, with the flanking A-band segments that lack MyBP-C referred to as proximal and distal zones (P- and D-zones, respectively) that lie nearest to the M-line and Z-line, respectively (Fig. 1). Thus, although MyBP-C binds tightly to myosin, its distribution within the sarcomere is narrowly restricted and corresponds to approximately every third crown of myosin heads that emerge from the thick filament only within the C-zone.
Figure 1.
cMyBP-C domain structure and binding interactions. (A) cMyBP-C (MYBPC3 gene product) is depicted as a series of blue rectangles representing either Ig- or fibronectin-like folded domains. Domains are numbered C0–C10 beginning from the N terminus to the C terminus. The position of the regulatory M-domain with three serines (PPP) that are phosphorylated by protein kinase A is shown between domains C1 and C2. Domains C0–C2 bind to thin (actin) filaments (purple) and to thick (myosin) filaments (red). Domains C8–C10 anchor MyBP-C to the thick filament by binding to the light-meromyosin (LMM) segment of myosin and to titin. (B) Cartoon of a sarcomere showing thin filaments (purple), thick filaments (red), and titin (green). MyBP-C localization is shown as a series of nine regularly spaced stripes (blue) in the C-zones of each half sarcomere. D- and P-zones of the thick filament are also indicated. Figure created with Biorender.com.
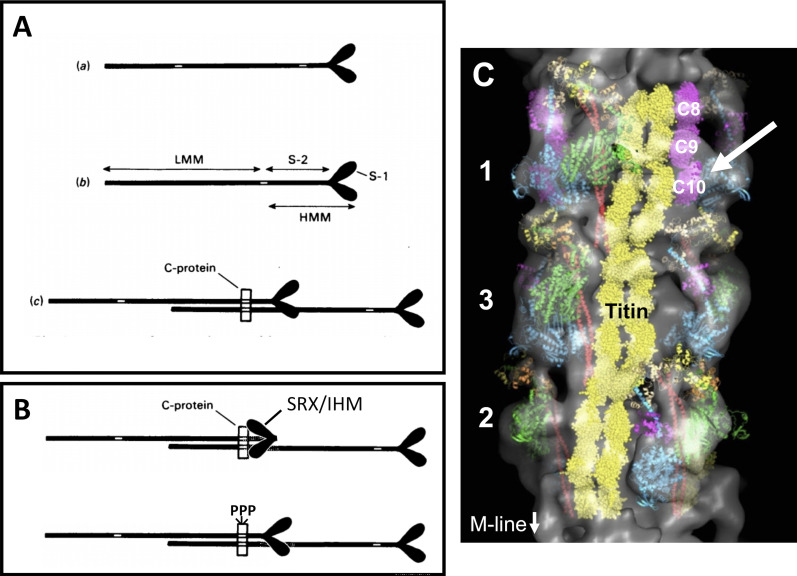
Additional lower-affinity myosin binding sites are contained within N-terminal domains of MyBP-C that span C0–C2. For instance, the cardiac-specific C0 domain interacts with myosin regulatory light chain (Ratti et al., 2011), while C1, C2, and the regulatory M-domain bind to the S1 or S2 segments of myosin (Gruen and Gautel, 1999; Ababou et al., 2007, 2008; Nag et al., 2017). The discovery that MyBP-C binds to myosin at multiple positions prompted the early hypothesis that MyBP-C (or C-protein, as it was first named) could restrict the movement of myosin heads away from the thick filament by simultaneously binding to both the myosin rod and myosin S2 segments of myosin (Fig. 2).
Figure 2.
Diagrams and 3-D reconstruction showing possible arrangements of binding sites for C-protein on myosin and the thick filament. (A) Diagram from Starr and Offer (1978) showing how MyBP-C binding sites could restrict myosin heads. Binding sites are indicated by the white areas in myosin tails. (A a) Separate binding sites in the light meromyosin and subfragment-2 regions. (A b) Binding site shared by the light-meromyosin (LMM) and subfragment-2 (S-2) regions. (A c) Diagram showing how in the thick filament one C-protein molecule could interact with the heavy-meromyosin (HMM) region of one myosin molecule and the LMM region of another. S-1, subfragment-1. Figure and legend reprinted from Starr and Offer (1978) with permission from Biochemical Journal. (B) Modification of myosin heads redrawn from A to represent myosin heads stabilized by MyBP-C and folded back against the thick filament in the IHM/SRX state (top) and effects of cMyBP-C phosphorylation to disrupt the IHM/SRX state (bottom). PPP, regulatory M-domain with three serines (see legend to Fig. 1). (C) 3-D reconstruction of a cardiac thick filament showing three crowns of myosin heads (1, 3, and 2), myosin S2 (red), myosin free heads (cyan), myosin blocked heads (green), titin (yellow), and MyBP-C C8-C10 domains (magenta). Possible interaction of the C10 domain of MyBP-C with a myosin free head is indicated by white arrow. Figure modified from Al-Khayat et al. (2013) with permission from Proceedings of the National Academy of Sciences of the United States of America.
According to Starr and Offer (1978): “If, in resting muscle, the subfragment-2 regions of the myosin molecules making up the thick filaments were attached to the light-meromyosin part of the filament shaft by C-protein, the outward movement of the cross-bridges of heavy meromyosin thought to occur during contraction (Huxley, 1969) would be prevented. It is an intriguing possibility that, if the binding of C-protein to the subfragment-2 region or to light meromyosin were regulated, this could form the basis of a mechanism to regulate the interaction of myosin heads with actin.”
This hypothesis (later referred to as the “tether” hypothesis) foreshadowed how MyBP-C could regulate contraction by limiting myosin head motion away from the thick filament such that unbinding the S2 portion of myosin, for instance through phosphorylation, would release the tether (Calaghan et al., 2000). Evidence for regulation of MyBP-C by phosphorylation in response to β-adrenergic stimuli or by dephosphorylation in response to acetylcholine was obtained shortly thereafter (Jeacocke and England, 1980; Hartzell and Titus, 1982). Phosphorylation of serine residues in the M-domain of cMyBP-C was indeed found to abolish binding to S2 (Gruen et al., 1999; Calaghan et al., 2000) and cause myosin heads to move away from the thick filament (Kensler et al., 2017; Colson et al., 2008), consistent with the idea that unbinding of cMyBP-C to myosin S2 mediates inotropic responses in the heart.
More recent studies have furnished additional details of MyBP-C interactions with myosin S2. In particular, there is increasing evidence that MyBP-C stabilizes a newly described super-relaxed state (SRX) of myosin characterized by significantly reduced myosin ATPase activity (McNamara et al., 2019, 2017; Stewart et al., 2010). The SRX state is associated with myosin heads adopting a bent-back conformation (the so-called interacting heads motif [IHM]), where they are folded back against the myosin rod (Fig. 2). The conformation is evolutionarily conserved across the entire myosin II family (Lee et al., 2018), but MyBP-C stabilizes myosin heads in a way that provides additional regulatory control in vertebrate striated muscles in a manner similar to the original mechanism proposed by Starr and Offer (1978). Consistent with the idea that MyBP-C confers additional regulatory control over myosin activity, phosphorylation reduces the number of myosin heads in the SRX state (McNamara et al., 2019). Additional interactions of MyBP-C with myosin S1 along a surface on S1 referred to as the “myosin mesa” also appear important for stabilization of the inhibited, folded state of myosin such that reciprocal mutations in either the myosin mesa or cMyBP-C lead to dysregulation of contraction and cause hypertrophic cardiomyopathy (HCM; Nag et al., 2017; Trivedi et al., 2018; Toepfer et al., 2020).
Stabilization of the SRX state may also be an integral part of thick filament regulation via a mechanosensing mechanism. Recent studies suggest that thick filaments undergo a change in activation state from “off” to “on” during contraction, similar to thin filaments (Linari et al., 2015). However, unlike thin filament activation, thick filament activation is independent of Ca2+ and is instead controlled by a mechanism in which stress on the thick filament increases the number of myosin heads available for force generation (Irving, 2017; Fusi et al., 2016). MyBP-C could directly contribute to thick filament activation via force sensing if load alters MyBP-C interactions with titin or myosin in such a way that it disrupts the SRX state (Linari et al., 2015). An atomic model of cardiac thick filaments (Al-Khayat et al., 2013) revealed that a C-terminal domain of MyBP-C (most likely C10; Lee et al., 2015) appears to interact with titin and with the free S1 head of myosin when it is folded in the IHM (Fig. 2). It is therefore plausible that stress on the thick filament may be sensed by titin and MyBP-C C-terminal domains and then communicated to the thin filament, as previously suggested for length-dependent activation (Ait-Mou et al., 2016; McDonald, 2011). Alternatively, changes in MyBP-C layer lines occurred coincident with Ca2+ activation but before thick filament activation in response to load (Reconditi et al., 2011). Therefore, it is possible that Ca2+ loosens MyBP-C connections with myosin that otherwise stabilize the interacting heads conformation, thereby freeing heads from the SRX state before contraction. MyBP-C effects would therefore be analogous to a parking brake whose removal is necessary for subsequent motion of myosin heads. Lastly, MyBP-C may accelerate the return of thick filaments to a relaxed state by sequestering myosin heads in the SRX state as MyBP-C returns to its preactivated conformation during the late phase of relaxation (Reconditi et al., 2011).
MyBP-C interactions with actin
Along with early discoveries that MyBP-C interacted with myosin, MyBP-C was also found to bind to F-actin and to regulated thin filaments (F-actin plus the regulatory proteins troponin and tropomyosin) in muscle sarcomeres (Moos, 1981; Moos et al., 1978; Yamamoto, 1986). Importantly, binding was increased in the presence of Ca2+, suggesting that binding could be regulated during contraction (Moos, 1981; Yamamoto, 1986). At the time, however, it was difficult to reconcile the ability of MyBP-C to bind to actin with the elegant tether hypothesis described above that seemed to fully account for the ability of MyBP-C to inhibit myosin cross-bridge kinetics. As a result, findings that MyBP-C could bind to actin were largely overlooked or considered to be nonspecific due to charge interactions of MyBP-C with the highly negatively charged actin filament. Binding to actin was later rediscovered in studies showing that recombinant N-terminal domains C0–C2 bound to F-actin and thin filaments (Kensler et al., 2011; Whitten et al., 2008; Shaffer et al., 2009). Binding was indeed sensitive to charge, as noted for full-length MyBP-C, but was also stereospecific, saturable, and sensitive to phosphorylation, arguing against purely nonspecific effects. Additional actin binding sites were also localized to the C5–C10 domains (Rybakova et al., 2011).
Functional studies with recombinant N-terminal domains (e.g., C0–C2) next demonstrated both potent activating and inhibitory effects of MyBP-C in a number of assay systems such as force measurements in detergent-permeabilized myocytes, sliding velocity in in vitro motility assays, and actin-activated myosin ATPase assays (Razumova et al., 2006, 2008; Belknap et al., 2014). Notably, the activating effects of cMyBP-C N-terminal domains were similar to those elicited by Ca2+ and rigor (strongly bound) myosin cross-bridges, i.e., the only other known activators of striated muscle contraction. Indeed, in some cases N-terminal domains activated contraction in fully relaxed muscle even in the absence of Ca2+ (Herron et al., 2006; Razumova et al., 2008). However, the physiological significance of these effects was still questioned, in part because it was difficult to reconcile how activating effects of N-terminal domains observed in force assays related to apparently opposite results obtained in cMyBP-C knockout mice, in which cross-bridge cycling rates were increased in the absence of cMyBP-C (Korte et al., 2003). Moreover, it was still difficult to reconcile how the N-terminal domains of MyBP-C could physically interact with both myosin and actin when the two filaments occupy overlapping but distinct positions in muscle sarcomeres.
Nonetheless, a structural basis for the activating effects of cMyBP-C N-terminal domains was shown by EM in studies that revealed that the N-terminal domains shifted the position of tropomyosin toward the “open” or “on” state on the thin filament (Mun et al., 2014). Remarkably, a shift in tropomyosin toward the open state was predicted 30 yr previously as a mechanism to explain the strong correlation of cMyBP-C phosphorylation with the rate of relaxation. Specifically, Hartzell (1984) proposed that cMyBP-C binding to the thin filament could “prevent or slow the return of tropomyosin to its blocking position as intracellular Ca declines and thus would prolong the active state.”
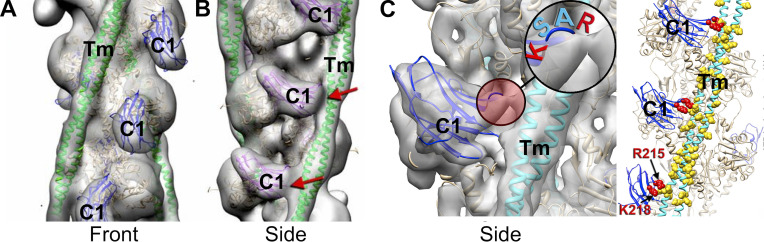
In further support of an effect on tropomyosin, a MyBP-C missense mutation (L348P) was identified in the trihelix bundle of the M-domain that increased the activating effects of the N-terminal domains by moving tropomyosin even further past its open state on the thin filament (Mun et al., 2016). The exaggerated movement of tropomyosin was greater than that induced by both Ca2+ and rigor cross-bridges combined and could thus account for the ability of recombinant proteins with this mutation to activate force even in the absence of Ca2+ (Bezold et al., 2013). Cryo-EM and single-particle analyses next revealed molecular details of individual domain interactions with actin and tropomyosin (Harris et al., 2016; Risi et al., 2018). Somewhat unexpectedly, these studies revealed complex polymorphic binding of the individual domains to F-actin and the thin filament. For instance, C0 and C1 each could bind to the thin filament in multiple stereospecific positions. However, only C1 affected the position of tropomyosin (Fig. 3). The latter accounted for the ability of C1, but not C0, to augment actin-activated myosin ATPase activity and increase force in contracting sarcomeres (Harris et al., 2016). As shown in Fig. 3, additional cryo-EM studies showed that the activating effects of C1 were due to charge–charge interactions of a 4-amino acid (RASK) loop that trapped tropomyosin in its open position (Risi et al., 2018).
Figure 3.
Polymorphic binding of the C1 domain to two positions (front and side) on the thin filament. The C1 Ig-like domain binds to the thin filament in multiple positions. (A and B) C1 is shown binding to the front of the thin filament (A) and to the side of the thin filament (B). C1 displaces tropomyosin from its closed position on F-actin when bound in the front mode and traps tropomyosin in its open state when bound to the thin filament in the side mode. Reconstructions are shown as gray transparent surfaces. Actin molecules are shown as tan ribbons; tropomyosin (Tm) in the open state is shown as green ribbons. The crystal structure of the C1 Ig-like domain (PDB accession no. 2V6H) is shown in blue (front mode) or purple (side mode). In the side mode, C1 makes a prominent contact with tropomyosin (red arrows). All complexes were formed at low Ca2+ (pCa > 8). A and B modified from Harris et al. (2016) with permission from Proceedings of the National Academy of Sciences of the United States of America. (C) Higher-resolution 3-D reconstruction showing the interface of C1 on the thin filament when bound in the side mode and interacting with tropomyosin. Highlighted region shows a 4-amino acid loop (RASK) of C1 that contacts tropomyosin and traps it in the open position. C modified from Risi et al. (2018) with permission from Structure.
While initially surprising, the finding that N-terminal domains of cMyBP-C can bind to the thin filament in multiple configurations implies a structural plasticity that may be important to allow cMyBP-C to interact dynamically with both filament systems. For example, multiple binding configurations might be permissive for maintaining contact with both thick and thin filaments as they slide past one another without impeding the movement of myosin cross-bridges. It is therefore plausible that changes in [Ca2+], phosphorylation, mechanical load, or strongly bound myosin cross-bridges dynamically reposition domains and thereby modulate MyBP-C effects on contraction or relaxation. Cryo-EM studies to visualize different domain configurations under different conditions should be valuable in testing these hypotheses and are ongoing.
Although the significance of MyBP-C activation of the thin filament is still not completely understood, it was proposed that thin filament activation by MyBP-C might augment contraction at a time when the influence of other activators of the thin filament, i.e., Ca2+ and strongly bound cross-bridges, is declining (Hinken and Solaro, 2007; Hartzell, 1984). Consistent with this idea, loss of thin filament activation by cMyBP-C could account for the profound shortening of the ejection phase of systole in cMyBP-C knockout mice compared with WT mice (Palmer et al., 2004). To directly test whether activating effects of cMyBP-C maintain the thin filament in an open conformation at the end of systole, systolic ejection time was measured either in transgenic mice expressing the L348P mutation, which increases thin filament activation as mentioned above, or in transgenic mice expressing a separate mutation (E330K) that decreases binding affinity for actin. Consistent with the hypothesis that the activating effects of cMyBP-C are important at a time when [Ca2+]i is falling and when shortening-induced activation of the thin filament should promote relaxation (Hinken and Solaro, 2007), systolic ejection time was prolonged in L348P mice and shortened in E330K mice (van Dijk et al., 2018). Of course, these results do not exclude the possibility that activating effects of cMyBP-C N-terminal domains may be relevant at other times during the contractile cycle too. For instance, cMyBP-C may help promote uniform thin filament activation in the C-zone to compensate for Ca2+ gradients upon release from the SR that would be expected to activate myosin cross-bridges in the D-zones first, before those in C-zones (Previs et al., 2015; Brunello et al., 2020).
Dual inhibitory and activating effects of MyBP-C
The significant role played by MyBP-C in affecting regulation of both thick and thin filaments was recently highlighted in time-resolved x-ray diffraction studies showing that cross-bridges in the C-zone bear peak force during a twitch and that detachment of cross-bridges in the C-zone is rate limiting in relaxation (Brunello et al., 2020). However, despite increasing evidence for the physiological significance of interactions of MyBP-C with both actin and myosin, it is only relatively recently that attempts have been made to consolidate the inhibitory and activating effects of MyBP-C into integrated models of MyBP-C function. Most models broadly propose that the activating effects of cMyBP-C augment force during systole and slow relaxation as mentioned above (Brunello et al., 2020; Irving, 2017; van Dijk et al., 2018), whereas inhibitory effects of MyBP-C are most apparent during diastole (or at rest in skeletal muscle), where there is increasing evidence to support a role for MyBP-C in stabilizing the SRX state of myosin (McNamara et al., 2016, 2019).
However, molecular details are still limited, and it is yet unclear whether individual MyBP-C molecules cycle between thick and thin filaments during a single twitch or if different MyBP-C populations remain bound to either actin or myosin. In support of the former, Ca2+ activation of skeletal muscle in x-ray diffraction studies induced a rapid decrease in the intensity of layer lines associated with MyBP-C that preceded force generation (Reconditi et al., 2011). The decrease in MyBP-C layer line intensity could reflect formation of “C-links” between thick and thin filaments (Irving, 2017; Luther et al., 2011), consistent with increased binding of MyBP-C to the thin filament in the presence of Ca2+ (Yamamoto, 1986; Moos, 1981). Because MyBP-C layer lines increase in intensity again during the late phase of relaxation, results support the idea that MyBP-C likely undergoes fast, dynamic binding within the time course of a single twitch. However, evidence in favor of discrete populations of MyBP-C molecules comes from studies of MyBP-C phosphorylation, which differentially affects binding to actin or myosin (Ponnam et al., 2019) and occurs on timescales slower than a twitch. Therefore, binding equilibria may be shifted preferentially toward either actin or myosin by phosphorylation, resulting in distinct functional populations of MyBP-C. Additional studies, for instance using FRET probes attached to MyBP-C and its binding partners on thick or thin filaments, are needed to distinguish these possibilities.
A cut-and-paste approach for modifying cMyBP-C in sarcomeres in situ
A major obstacle to progress in understanding the molecular mechanisms of how MyBP-C affects contraction and relaxation is a dearth of experimental systems that allow rapid manipulation of MyBP-C while still preserving its localization at its native position in the C-zones of muscle sarcomeres. Stoichiometric exchange of smaller thick and thin filament proteins such as myosin regulatory light chain and troponin has been possible for decades using biochemical approaches in detergent-permeabilized (“skinned”) muscle preparations (Moss, 1992). These methods are powerful because they allow mutations or probes of interest to be rapidly introduced and tested in functional sarcomeres. However, larger integral proteins of the thick filament, such as myosin, MyBP-C, and titin, have proven difficult or impossible to manipulate with such methods. Most studies on these proteins therefore have by necessity resorted either to creation of transgenic animals, which can be time consuming and costly to produce, or to in vitro systems that typically use smaller proteolytic or recombinant fragments of the protein of interest. In the case of MyBP-C, recombinant proteins containing the first several N-terminal domains of MyBP-C (e.g., C0–C2 or C1–C2) are commonly used because these domains contain binding sites for actin or myosin along with the regulatory M-domain that is phosphorylated in response to inotropic stimuli. However, information regarding the function of the whole protein is lost. Other simplified in vitro systems such as ATPase and motility assays are often used that lack sarcomeres altogether.
An exception is detergent-permeabilized muscle preparations, in which cell membranes and organelles are extracted with detergent, leaving functional sarcomeres intact. Force is then measured by fixing the two ends of a skinned preparation between a force transducer and positional motor and submersing the preparations in precisely buffered solutions to achieve a desired free [Ca2+] to activate contraction. Advantages of so-called skinned muscle experiments are that they allow complete control over the buffer compositions that surround the sarcomeres, while muscle functional parameters including sarcomere length and load can be set or varied as desired. For instance, the potent activating effects of cMyBP-C N-terminal domains such as C0–C2 or C1–C2 on force were demonstrated by adding recombinant proteins to solutions bathing skinned muscle preparations (Razumova et al., 2008; Herron et al., 2006; Kunst et al., 2000; Shaffer et al., 2007). However, limitations of simply adding recombinant proteins to bath solutions include uncertainty as to whether added recombinant proteins are restricted exclusively to the positions normally occupied by endogenous cMyBP-C in C-zones and the potential for competition with endogenous cMyBP-C. Although issues related to competition can be overcome using myocytes from cMyBP-C knockout mice (Harris et al., 2004, 2002), other compensatory effects can still confound data interpretation in studies using knockout myocytes. For these reasons, until recently it had remained an open question whether cMyBP-C could augment or activate contraction when localized at its native position in muscle sarcomeres in situ.
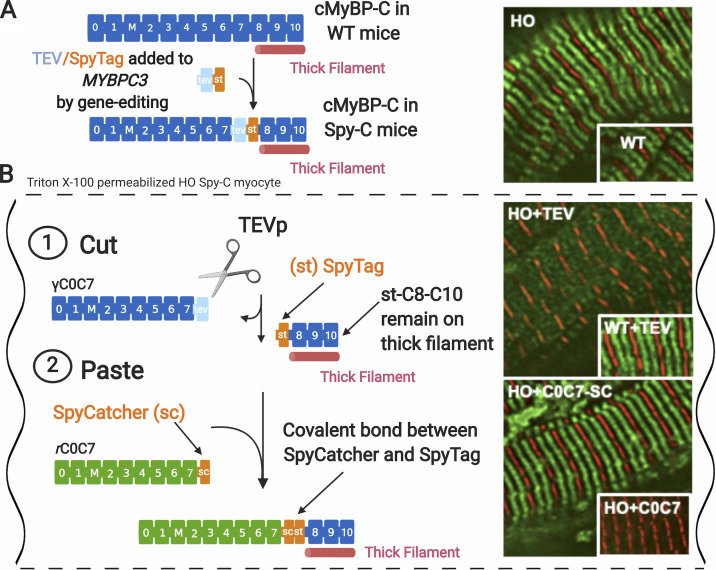
To overcome these limitations, we recently designed a novel hybrid approach that combines genetic and protein engineering to selectively remove and replace N-terminal domains of cMyBP-C at their native positions in skinned cardiac muscle (Napierski et al., 2020). As shown in Fig. 4, the method uses detergent-permeabilized cardiac muscle from gene edited Spy-C mice that express a modified cMyBP-C protein with a 20-amino acid cassette inserted between domains C7 and C8. The first 7 amino acids of the cassette encode a tobacco etch virus protease (TEVp) recognition site, and the last 13 encode a SpyTag sequence (Zakeri et al., 2012). Treatment of detergent-permeabilized myocytes with TEVp in the bath solutions first causes efficient cleavage of the endogenous, genetically (γ) encoded C0–C7 domains, which are soluble and can be removed from the sarcomeres by gentle washing. Next, new recombinant (r) C0–C7 domains with a SpyCatcher sequence encoded at their C-termini are added to bath solutions, where they become covalently joined to SpyTag (exposed at the N terminus of genetically encoded domains C8–C10) via a spontaneous isopeptide bond formed between SpyCatcher and SpyTag (Zakeri et al., 2012). The entire process is rapid, requiring only minutes, and has a replacement efficiency of ∼90%. The cut-and-paste method thus achieves rapid and efficient exchange of domains C0–C7 with virtually any recombinant protein containing any desired point mutation, deletion, insertion, biosensor, enzyme, etc. Replacement of cMyBP-C with recombinant variants carrying FRET sensors should be especially valuable for determining dynamic interactions of cMyBP-C N-terminal domains with thin and thick filaments, as mentioned above.
Figure 4.
Cut-and-paste approach for removal and replacement of cMyBP-C N-terminal domains in cardiomyocytes from homozygous Spy-C mice. (A) Gene-edited Spy-C mice express modified cMyBP-C with a TEVp recognition site (light blue rectangle) and a SpyTag (orange rectangle) inserted between domains C7 and C8. Inset: Immunofluorescence localization of cMyBP-C in homozygous (HO) and WT Spy-C myocytes showing expected localization (green) in each half sarcomere. Z-lines are shown stained with α-actinin (red). (B) Cut and paste of cMyBP-C in Triton X-100–permeabilized cardiomyocytes from HO Spy-C mice. (B 1) Cut: TEVp treatment releases genetically encoded (γ) C0–C7 domains, which are soluble and can be removed from sarcomeres through gentle rinsing. Inset: Immunofluorescence showing loss of cMyBP-C stripes after TEVp treatment in HO myocytes but not WT myocytes. (B 2) Paste: New recombinant rC0C7-sc domains (green; encoding any desired modification such as mutations, deletions, and fluorescent probes) are added to the bath where they become covalently attached to st-C8C10 on the thick filament via a spontaneous bond formed between SpyCatcher and SpyTag. Inset: Immunofluorescence localization showing reappearance of cMyBP-C stripes (green) after addition of rC0C7-sc in HO myocytes but not rC0C7 lacking encoded SpyCatcher in HO myocytes. Data and figure modified from Napierski et al. (2020). Figure created with BioRender.com.
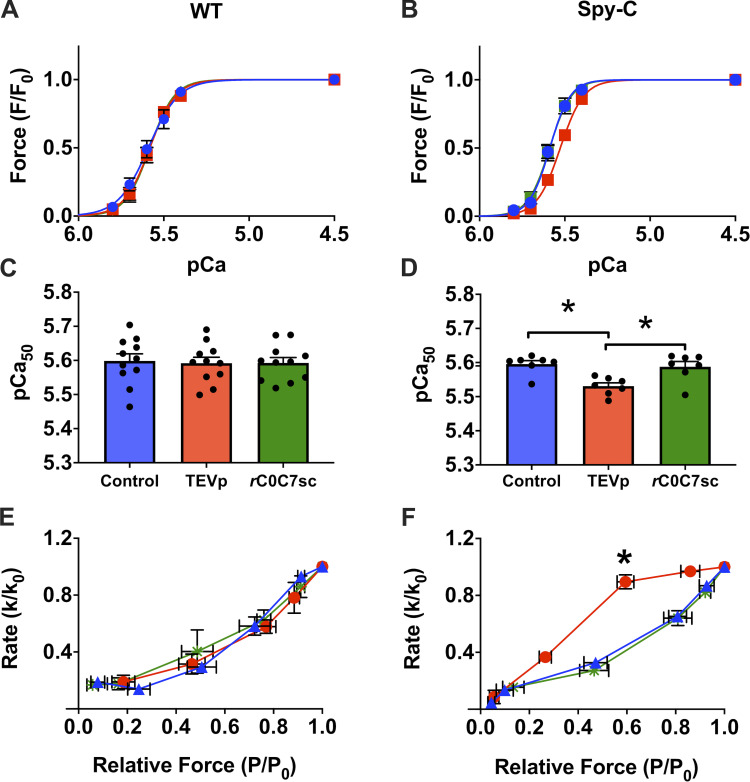
Using the cut-and-paste approach, we first confirmed that cMyBP-C N-terminal domains can indeed augment Ca2+-activated force when they are restricted to their native positions in muscle sarcomeres in situ (Fig. 5). This was evident by a decrease in Ca2+-activated force (rightward shift in the tension–pCa relationship) after TEVp treatment, whereas ligation of new recombinant C0–C7 domains via the SpyCatcher/SpyTag bond caused a leftward shift in Ca2+ sensitivity of force. The magnitude of the effect was modest compared with effects observed when recombinant domains are added free in solution to skinned muscle preparations, but was nonetheless anticipated because fewer sites are occupied by cMyBP-C when restricted to sarcomere C-zones. Results are thus in good agreement with structural and functional studies showing that N-terminal domains can activate force by repositioning tropomyosin on the thin filament (Mun et al., 2014; Harris et al., 2016). In addition, because replacement with phosphorylated C0–C7 did not induce a leftward shift in Ca2+ sensitivity (Napierski et al., 2020), results suggest that phosphorylated cMyBP-C induces a decrease in Ca2+ sensitivity that acts in concert with troponin I phosphorylation, which decreases Ca2+ sensitivity following inotropic stimuli (Kögler and Rüegg, 1997; Garvey et al., 1988).
Figure 5.
Effects of cut and paste of cMyBP-C N-terminal domains on force in skinned myocytes. (A and B) Normalized tension–pCa relationships in WT myocytes (A) or HO Spy-C myocytes (B); before TEVp treatment (control, blue), after TEVp treatment (red), and after incubation with rC0C7-sc (green). (C and D) Summary data showing average pCa50 values for each condition in WT (C) and HO Spy-C myocytes (D). Loss of C0–C7 domains after TEVp treatment caused a decrease in Ca2+ sensitivity to tension that was reversed by ligation of rC0-C7-sc in HO but not WT myocytes. (E and F) ktr data from WT (E) and HO (F) myocytes showing an increase in relative ktr at intermediate [Ca2+] after TEVp treatment that was reversed by ligation of rC0-C7-sc in HO but not WT myocytes. Data reprinted from Napierski et al. (2020).
The new cut-and-paste method also recapitulated the canonical increase in cross-bridge kinetics following loss of cMyBP-C in knockout mice (Korte et al., 2003; Stelzer et al., 2006a). As shown in Fig. 5, rates of cross-bridge cycling (ktr) assessed using a release and restretch maneuver (to break and reform cross-bridge attachments) were significantly increased at –log10 of the [Ca2+] (pCa) values near the midpoint (pCa50) of force development following treatment with TEVp to remove domains C0–C7 of cMyBP-C. These results are in good agreement with force measurements in skinned myocytes from cMyBP-C knockout mice showing that cMyBP-C slows cross-bridge kinetics, but only at middle ranges of Ca2+ activation. Importantly, ligation with recombinant C0–C7 fully reversed these effects and again reduced ktr, consistent with previous conclusions from knockout mice that cMyBP-C slows cross-bridge cycling rates. Taken together, these results validate the cut-and-paste method by recapitulating both the inhibitory effects of cMyBP-C N-terminal domains on cross-bridge kinetics and the activating effects that increase myofilament Ca2+ sensitivity.
SPOCs appear after loss of cMyBP-C N-terminal domains
An unanticipated change in the contractile properties of skinned muscle not predicted based on previous experiments became obvious immediately following loss of genetically encoded C0–C7 domains using the cut-and-paste method. The change was the appearance of vigorous oscillatory contractile waves that were visible upon activation of force by Ca2+ (Video 1; Napierski et al., 2020). The oscillatory contractions propagated from sarcomere to sarcomere throughout the entire length of the skinned muscle preparations (∼200–300 µm) and continued back and forth for as long as preparations were activated by Ca2+ (≤60 min). The contractile waves occurred most frequently at intermediate [Ca2+] but could also sometimes be observed at maximal Ca2+ activation at saturating [Ca2+] (pCa4.5). The oscillatory waves were due specifically to the loss of cMyBP-C, because waves ceased immediately upon ligation of new recombinant C0–C7 domains. As is discussed in more detail below, the significance of these discoveries is that they suggest a previously unrecognized role of cMyBP-C to damp oscillatory contractions that originate at the sarcomere, and further suggest that MyBP-C has the potential to modulate excitation–contraction coupling at the level of the sarcomere.
Video 1.
SPOCs in detergent-skinned cardiomyocytes from a homozygous Spy-C mouse. Three activations are shown for the same permeabilized homozygous Spy-C myocyte preparation in a submaximal pCa solution near the pCa50 for force development. Left: Myocyte preparation is shown actively contracting at steady-state force before treatment with TEVp (control). Middle: The same myocyte preparation is shown contracting at steady-state force after treatment with TEVp to remove domains C0–C7 of cMyBP-C. Note the vigorous and continuous oscillatory wave behavior (SPOCs) after removal of C0–C7. Right: The same myocyte preparation is shown contracting at steady-state force after ligation with recombinant (r) C0C7-sc. Note that force oscillations (SPOCs) are completely damped after C0–C7 replacement with rC0-C7-sc.
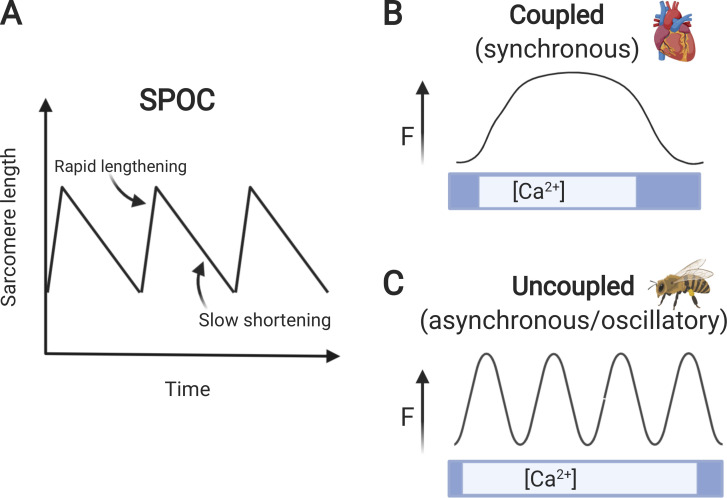
The first detailed descriptions of SPOCs (Fig. 6) were in skinned skeletal muscles, where the phenomenon was characterized as alternating cycles of sarcomere contraction and relaxation (Fabiato and Fabiato, 1978). Importantly, the oscillations were shown to occur independently of other cyclic changes in Ca2+ signaling, such as those arising from either extracellular or intracellular sources, including the SR, because the SR and other membranous organelles are removed from the preparations during skinning with detergent. In addition, inhibition of the SR with ryanodine does not block SPOCs, further demonstrating that the cycles of contraction and relaxation do not originate from the SR. Since its initial description, SPOC has been described in a wide variety of skinned skeletal and cardiac muscles (Wolfe et al., 2011; Sasaki et al., 2005; Ishiwata et al., 2011 and video therein). More recently, it has been observed in living myocardial cells where the SR and sarcolemma remain intact (Serizawa et al., 2011).
Figure 6.
SPOC: synchronous and asynchronous modes of contraction. (A) SPOCs are depicted as a sawtooth pattern of rapid sarcomere lengthening followed by slow sarcomere shortening. Figure redrawn from Wolfe et al. (2011) with permission from Biophysical Reviews. (B) Synchronous twitch contraction typically found in vertebrate striated muscles such as heart is depicted as an increase and decrease in force (F) coupled to a corresponding increase and decrease in [Ca2+]. (C) Asynchronous twitch contractions representative of IFMs are shown as multiple cycles of contraction and relaxation in the presence of Ca2+. Note that during cycles of asynchronous contractions, relaxation is uncoupled from a decrease in [Ca2+]. Figure created with BioRender.com.
Several models have been proposed to explain SPOCs, with variables ranging from the intrinsic oscillatory properties of motor proteins to emergent properties of muscle sarcomeres (Ishiwata et al., 2011; Kagemoto et al., 2015; Wolfe et al., 2011). At a minimum, ensembles of actin and myosin molecules coupled elastically to a surface can generate force oscillations (Jülicher and Prost, 1997). Dependence on underlying myosin motor properties also explains observations that the period of SPOC correlates with heart rate, because cross-bridge cycling rates are known to scale with both muscle speed and myosin isoform expression (Sasaki et al., 2006). However, other factors contribute to SPOC properties, including sarcomere lattice spacing, viscoelastic properties of cross-bridges and Z- and M-lines, length-dependent activation of contraction, and shortening-induced deactivation of thin filaments. According to one model, a balance of feed-forward changes in these variables explains the cycles of slow contraction followed by rapid relaxation that are characteristic of SPOC (Sato et al., 2011).
Loss of cMyBP-C could in principle induce SPOCs by affecting any one of these interrelated mechanisms, because MyBP-C affects apparent cross-bridge cycling rates (attachment/detachment rates; Korte et al., 2003; Stelzer et al., 2006b; Moss et al., 2015), lattice spacing (Colson et al., 2008; Palmer et al., 2011), length-dependent activation (Mamidi et al., 2014, 2016; Ait-Mou et al., 2016), and shortening-induced deactivation (Palmer et al., 2004). For instance, binding of cMyBP-C N-terminal domains to actin damps torsional flexibility of F-actin in solution (Colson et al., 2012; Bunch et al., 2019), which could reduce propagation of SPOCs across Z-lines (Nishizaka et al., 1993; Linke et al., 1993). MyBP-C can also act as a strut that stabilizes lattice spacing (Palmer et al., 2011), thereby minimizing structural changes thought to drive SPOCs (Sato et al., 2011; Ishiwata et al., 2011). Electrical damping is also a possibility, as the highly basic M-domain of MyBP-C slides past fixed electrostatic charges between negatively charged actin and myosin filaments (Millman, 1998).
Synchronous and asynchronous modes of muscle contraction
Despite the widespread occurrence of SPOCs in different muscles, the physiological relevance of contractile oscillations that originate from sarcomeres is not well understood. Because SPOCs occurs most commonly at intermediate [Ca2+], it was proposed that SPOC constitutes a third state of muscle poised between full activation and complete relaxation (Ishiwata et al., 2011). However, the alternating cycles of contraction and relaxation of SPOCs bear striking similarity to the specialized type of contraction that occurs in some IFMs (Fig. 6). Indeed, descriptions of contractile oscillations in rabbit psoas muscle were initially compared with oscillations in bumblebee wing muscle (Goodall, 1956; Lorand and Moos, 1956). In the latter, contraction is referred to as asynchronous because multiple cycles of contraction and relaxation ensue for as long as Ca2+ remains above a threshold value (Fig. 6; Iwamoto, 2011). Importantly, the primary stimulus for each contraction in asynchronous IFM is not an electrical or chemical stimulus per se but a mechanical stretch (“stretch activation”) caused by contraction of an antagonist muscle (Pringle, 1967, 1978). Alternating activities of paired agonist and antagonist muscles thus lead to contractile oscillations in both. Although stretch activation occurs to a limited extent in vertebrate striated muscles, including cardiac and skeletal muscle, these muscles are typically considered synchronous, because each twitch is initiated by a single action potential that gives rise to a single Ca2+ transient through excitation–contraction coupling (Bers, 2002). The result is that contraction and relaxation are tightly coupled to the rise and fall of intracellular [Ca2+] in synchronous muscles.
The comparison of SPOCs in vertebrate muscles to asynchronous IFM is useful because it sheds light on what may be the essential mechanistic difference between SPOCs and the synchronous modes of contraction that are typical of cardiac and skeletal muscles. As mentioned above, unlike synchronous contraction, in which contraction and relaxation are strictly coupled to the rise and fall of intracellular Ca2+, relaxation in asynchronous muscles or in muscles exhibiting SPOCs can be considered uncoupled from a decrease in intracellular Ca2+. That is, relaxation during SPOCs occurs despite the continued presence of sufficient Ca2+ to initiate and maintain force generation (Fig. 6). Because loss of cMyBP-C induces SPOCs during contraction when skinned muscle preparations are submersed in constant Ca2+, it follows that a major effect of cMyBP-C is to prevent premature relaxation before a decrease in intracellular Ca2+. This conclusion is consistent with previous suggestions that cMyBP-C is a coupling factor that matches rates of contraction and relaxation (Janssen, 2010) and with effects of cMyBP-C to slow relaxation and influence cardiac diastolic function (Tong et al., 2014; Nagayama et al., 2007; Gresham and Stelzer, 2016; Hartzell, 1984). Recent evidence that detachment of myosin cross-bridges in the C-zones of thick filaments is rate limiting in relaxation of the thick filament also supports the critical role of MyBP-C in this process (Brunello et al., 2020).
Sarcomere relaxation is typically described as proceeding in two phases. The first is a slow, isometric phase limited by cross-bridge detachment from the thin filament, followed by a second faster and more chaotic phase. The latter occurs once cross-bridge detachment proceeds to the point that sarcomeres suddenly “yield” and rapidly relengthen (Stehle et al., 2002, 2009). MyBP-C could thus slow relaxation by affecting either or both phases (Fig. 7). For instance, by shifting the position of tropomyosin and maintaining the thin filament in an activated state, MyBP-C could prolong the initial slow phase of relaxation. This may account for the ability of cMyBP-C to extend the duration of systole at a time when Ca2+ is falling as proposed earlier (van Dijk et al., 2018; Palmer et al., 2004; Hartzell, 1984). A similar slowing of relaxation was achieved by treating skinned muscle with NEM-S1, a strong binding derivative of myosin S1, which like MyBP-C, presumably maintains the thin filament in an open state, thus allowing force-generating cross-bridges to cycle and maintain force (Fitzsimons et al., 2001). However, because SPOC was occasionally observed even under conditions of maximal Ca2+ activation, reduced thin filament activation alone following loss of cMyBP-C may not fully account for the appearance of SPOCs. Another possibility is that MyBP-C directly slows cross-bridge detachment rates independently of thin filament activation. The ability of MyBP-C to slow filament sliding in in vitro motility assays is consistent with slowed cross-bridge detachment rates (Razumova et al., 2006). However, under these conditions, MyBP-C may also act as a viscous drag that slows filament sliding (Weith et al., 2012b; Previs et al., 2012). Inhibitory effects of MyBP-C in myosin ATPase assays have also been attributed to competition with myosin S1 rather than to direct effects on cross-bridge cycling (Belknap et al., 2014). Nonetheless, accelerated rates of cross-bridge detachment can induce SPOC, as shown by the ability of ADP and inorganic phosphate (Pi) to elicit SPOCs in the absence of Ca2+ (ADP-SPOC; Ishiwata et al., 2007; Kagemoto et al., 2015). Creatine phosphate and phosphoenolpyruvate added to skinned muscle solutions presumably induce contractile oscillations in the same way, by increasing cross-bridge detachment and accelerating relaxation (Goodall, 1956; Lorand and Moos, 1956).
Figure 7.
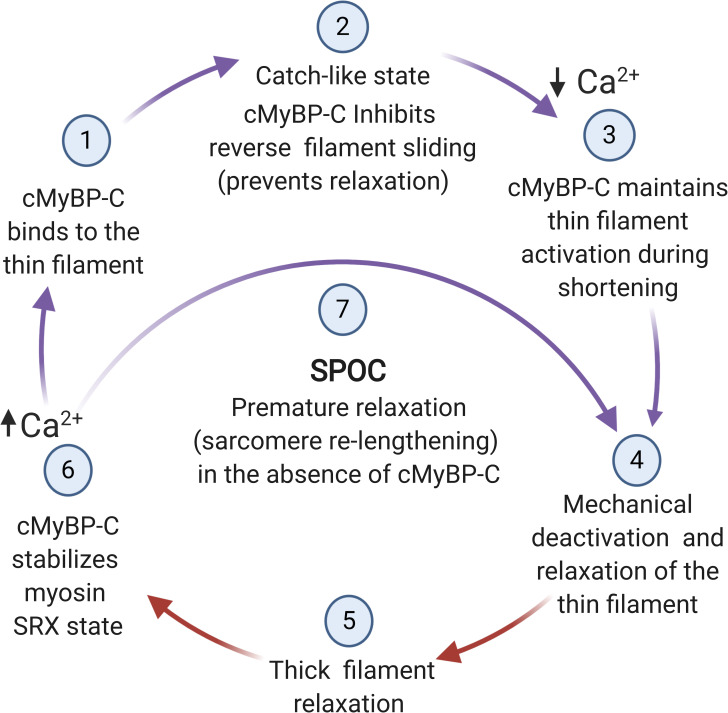
Hypothetical scheme showing proposed alternating interactions of cMyBP-C with thin and thick filaments regulated by Ca2+ and mechanical signaling. (1) MyBP-C N-terminal domains undergo Ca2+-dependent binding to the thin filament and/or become repositioned on the thin filament in response to Ca2+. (2) Ca2+-activated contraction induces sarcomere shortening, whereas MyBP-C prevents sarcomere relengthening but not shortening. (3) As shortening proceeds, MyBP-C N-terminal domains maintain thin filament activation by shifting tropomyosin toward its on state. (4) Sarcomere relaxation and relengthening occurs when [Ca2+] falls below a threshold value and when mechanical control of relaxation causes MyBP-C N-terminal domains to unbind or become repositioned on the thin filament. (5) MyBP-C interactions with myosin filament promote relaxation. (6) MyBP-C stabilizes the SRX state by promoting the interacting head conformation of myosin under conditions of low load. (7) In the absence of MyBP-C, relaxation occurs prematurely because sarcomere relengthening is not prevented by MyBP-C and the activating effects of N-terminal domains are absent. Steps in the cycle where cMyBP-C N-terminal domains interact with actin are indicated by purple arrows; interactions with myosin are indicated by red arrows. Figure created with BioRender.com.
A third possibility is that MyBP-C slows the transition to the second, chaotic phase of relaxation by preventing sarcomere relengthening. For instance, MyBP-C could cross-link thick and thin filaments in a way that slows or prevents reverse filament sliding (i.e., sarcomere lengthening). Simultaneous binding of MyBP-C to both thick and thin filaments is widely thought to produce a drag force that slows sarcomere shortening (Hofmann et al., 1991; Previs et al., 2012; Robinett et al., 2019). If MyBP-C also limits filament sliding in the opposite direction, i.e., in the direction of sarcomere lengthening, then it could prevent relaxation by selectively slowing the transition into the rapid chaotic phase of relaxation. Such directional binding could essentially permit cMyBP-C to function as a catch bond between the two filament systems in which force in one direction preferentially reinforces binding and limits filament back sliding, whereas force in the opposite direction does not (Thomas et al., 2008). Consistent with the idea of directional binding of MyBP-C to actin, laser trap assays showed that cMyBP-C N-terminal domains bound to actin and preferentially displaced filaments in one direction only (Weith et al., 2012a). We also previously hypothesized that mechanical force could cause opening of the conserved trihelix bundle, which would function as a hinge within the M-domain to expose an additional conserved actin binding sequence (Bezold et al., 2013). Notably, the sequence is conserved across all MyBP-C isoforms from fish skeletal to human cardiac muscle (Shaffer and Gillis, 2010) and was originally identified through sequence homology with an actin binding sequence in twitchin, the protein responsible for the long-lived catch state of molluscan smooth muscle (Funabara et al., 2005; Butler et al., 2010).
Although the precise mechanisms by which MyBP-C slows relaxation are yet to be determined, the observation that MyBP-C prevents relaxation before a fall in Ca2+ also implies that MyBP-C effects are either directly or indirectly sensitive to Ca2+. In support of the former, Ca2+ induced a conformational change resulting in extension of cMyBP-C N-terminal domains in solution (Previs et al., 2016), as well as increased MyBP-C binding to sarcomere thin filaments (Yamamoto, 1986; Moos, 1981). X-ray diffraction studies also showed changes in the intensity of MyBP-C layer lines following Ca2+activation but before changes in myosin cross-bridge movements (Linari et al., 2015). It is therefore plausible that a rise in [Ca2+]i directly alters the conformation of MyBP-C and promotes its binding to the thin filament, where it then can slow relaxation via one or more of the mechanisms proposed above. A fall in [Ca2+]i would reverse these effects, albeit with markedly slower dissociation kinetics to account for sustained activation of the thin filament at the end of systole, when [Ca2+]i has already returned to near-diastolic values (Bers, 2002; Hinken and Solaro, 2007). However, a fall in Ca2+ may be necessary but not sufficient for relaxation to occur (Irving, 2017). In this case, other mechanical signals such as a quick stretch or fast relengthening may also be required to accelerate relaxation by speeding cross-bridge detachment (Chung et al., 2017; Tesi et al., 2002; Stehle et al., 2002). It is plausible that mechanical control of relaxation via a quick stretch or release also promotes unbinding of MyBP-C from the thin filament in a manner similar to unbinding of other catch bonds (Chen et al., 2012; Prezhdo and Pereverzev, 2009). It is also possible that differential strains exerted by the D- and P-zones of the thick filament that relax faster than cross-bridges in the C-zone could also provide mechanical input, causing unbinding of MyBP-C that drives faster sarcomere relengthening (Brunello et al., 2020).
Regulation of SPOCs in health and disease
The finding that loss of MyBP-C can induce SPOCs in vertebrate nonflight muscles raises the intriguing possibility that asynchronous oscillatory contractions are not an exclusive property of highly adapted IFMs but rather represent a more fundamental characteristic of all striated muscles. Lorand and Moos (1956) proposed that the difference between skeletal and insect muscle types is the presence of a damping factor in vertebrate skeletal muscle that was absent in IFM: “Indeed, one wonders if the oscillation observed in the insect muscles might not be due to the absence of a ‘damping factor’ similar to that shown … to exist in the rabbit psoas.”
Because MyBP-C is not expressed in IFM, it is an excellent candidate for the damping factor predicted by Lorand and Moos (1956). Consistent with this idea, transgenic expression of MyBP-C in Drosophila melanogaster resulted in a flightless phenotype, although MyBP-C in that study was not restricted to C-zones (Vu Manh et al., 2005). Nonetheless, the lack of MyBP-C in IFM may also help explain observations that the folded interacting heads conformation of myosin heads appears less stable in these muscle types (Lee et al., 2018). Still, it is surprising that a single protein can effectively toggle between contractile modes, because asynchronous contraction is thought to have evolved multiple times in different insect species, each by expressing a variety of specializations. For instance, a specialized troponin C isoform (TnC-F1) with increased Ca2+ sensitivity is critical for stretch activation at low priming levels of Ca2+ in the water bug, Lethocerus americanus (Bullard and Pastore, 2019). Other IFM adaptations include modifications to the structure, periodicity, and enzyme activities of myosin and actin in thick and thin filaments; sarcomeres with minimal I-band segments; and the presence of variety of additional accessory proteins (e.g., kettin, flightin, and paramyosin; Chakravorty et al., 2017; Perz-Edwards et al., 2011; Taylor et al., 2019; Wray, 1979). While functional similarities between MyBP-C and various proteins or structures in IFM have been proposed (Bullard and Pastore, 2019), a functional analogue in IFM for the damping effects of MyBP-C is not obvious.
Expression of MyBP-C in vertebrate striated muscles therefore suggests that induction and damping of oscillatory contractile activity may be a regulated property in these muscles. Because unphosphorylated cMyBP-C completely damped SPOCs in cardiac muscle but phosphorylated cMyBP-C did not (Napierski et al., 2020), inotropic pathways that phosphorylate cMyBP-C, such as β-adrenergic signaling, may induce or increase the occurrence of oscillatory contractions in cardiac muscle. If so, it is worth considering how SPOCs could provide adaptive advantages under conditions of increased inotropic or lusitropic stimulation. Comparison with IFM is again instructive, because the process of moving Ca2+ into and out of the cytosol for each contraction is both time consuming and energetically expensive at the high wingbeat frequencies necessary for flight (∼200–1,000 Hz). Asynchronous stretch activation was therefore thought to have evolved in IFM to achieve high-frequency contraction while at the same time minimizing the high energetic costs associated with Ca2+ transport during each wingbeat (Josephson et al., 2000). Similar considerations may apply to vertebrate striated muscles, especially heart muscle, in which oscillatory or uncoupled asynchronous contraction could allow for greater contraction and relaxation speeds at improved energetic efficiency. Indeed, reduced energetic efficiency is thought to be a major factor contributing to or exacerbating disease etiologies such as HCMs and heart failure (Yotti et al., 2019).
Other potential advantages of SPOCs include the ability of oscillations to propagate rapidly across multiple sarcomeres and across multiple cells, as SPOC waves travel in coherent patterns (Sato et al., 2013). Such coordinated intersarcomere and multicellular activity again may be energy sparing, especially in the heart under conditions of increased inotropic and lusitropic drive, where coordination across cardiac regions could augment the heart’s conduction system to produce more powerful and coordinated heartbeats (Viner et al., 2019; Nitsan et al., 2016). Synchronized mechanical control of relaxation could account for observations that diastolic vibration shortens the duration of late systole and speeds relaxation (Janssen et al., 1996; Takagi et al., 1992). If so, then sarcomere elastic properties, especially those of the Z-line and thin filaments, are likely to be important in storing and relaying mechanical energy from cross-bridges in one sarcomere to those in adjacent sarcomeres (Linke et al., 1993). Rhythmic storage and release of mechanical energy across Z-lines could also provide mechanical-chemo-electrical feedback that impacts Ca2+ handling and action potential properties (ter Keurs et al., 2006a, 2006b; Izu et al., 2020).
Conversely, dysregulation of SPOCs may cause or exacerbate the clinical course of diseases involving MyBP-C, because MyBP-C protein expression and phosphorylation are commonly reduced in cardiac diseases (van Dijk et al., 2009; Glazier et al., 2019; Kraft et al., 2016). Inherited mutations in the slow skeletal isoform of MyBP-C (MYBPC1) are also increasingly linked to distal arthrogryposes and muscle tremor (Shashi et al., 2019; Stavusis et al., 2019). In the case of cardiac muscle, mutations in MYBPC3 are among the most frequent causes of HCM, where most mutations lead to reduced cMyBP-C protein expression (haploinsufficiency; Yotti et al., 2019; Helms et al., 2014). Cardiac stress also results in proteolytic cleavage of cMyBP-C at a calpain site located within the M-domain (Govindan et al., 2012, 2013; Witayavanitkul et al., 2014). Therefore, cMyBP-C haploinsufficiency in HCM patients or in response to cardiac stress could result in increased SPOCs and dysregulation of myofilament relaxation similar to that seen in Spy-C myocytes following TEVp treatment. Chronic dysregulation of SPOC activity could then alter contractile kinetics, energetics, excitation–contraction coupling, or Ca2+ homeostasis (Lehman et al., 2019; Solaro, 2008; Yotti et al., 2019; Vitale et al., 2019; Toepfer et al., 2020). Allelic and cell-to-cell imbalances in cMyBP-C expression in myocytes of HCM patients (Kraft et al., 2016) could further exacerbate dysregulation, leading to changes in source-sink dynamics between neighboring cells that create ectopic or reentry foci for arrhythmia (Viner et al., 2019; Nitsan et al., 2016). The opposite effects, i.e., reduced SPOCs and slowed contraction and relaxation kinetics, may occur if cMyBP-C is chronically hypophosphorylated, as in heart failure patients (van Dijk et al., 2009; Jacques et al., 2008).
Conclusions
MyBP-C was discovered as an intimate binding partner of myosin nearly 50 yr ago (Offer et al., 1973). Almost simultaneously with its discovery, MyBP-C was also found to interact with actin (Moos, 1981; Moos et al., 1978; Yamamoto, 1986). Yet, it is only recently that these seemingly contradictory observations are becoming integrated into a cohesive picture that places MyBP-C at a central position within the sarcomere, poised to sense, communicate, and regulate contraction and relaxation through interactions with both thick and thin filaments (Shaffer et al., 2009; Irving, 2017; Brunello et al., 2020). Like the initial observations that MyBP-C interacts with both actin and myosin, the recent discovery that MyBP-C modulates SPOCs (Napierski et al., 2020) also does not fit neatly into existing paradigms. However, the idea that MyBP-C regulates SPOCs hints at new, previously unrecognized modes of regulation that have the potential for broad functional impact (Fig. 8). The similarity between SPOCs and asynchronous contraction found in IFMs is especially striking and suggests that excitation–contraction coupling may be more variable in vertebrate striated muscles than previously thought. These ideas have yet to be rigorously tested, but one implication is that the range of contractile responsiveness to Ca2+ is greater than changes in either intracellular Ca2+ or myofilament Ca2+ sensitivity alone would predict. At an extreme, Ca2+ may be merely permissive to initiate oscillations of an otherwise independent contractile apparatus. Similarly, a fall in [Ca2+]i may be necessary but not sufficient for relaxation that is ultimately dependent on mechanical events rather than chemical signals (Chung et al., 2017).
Figure 8.
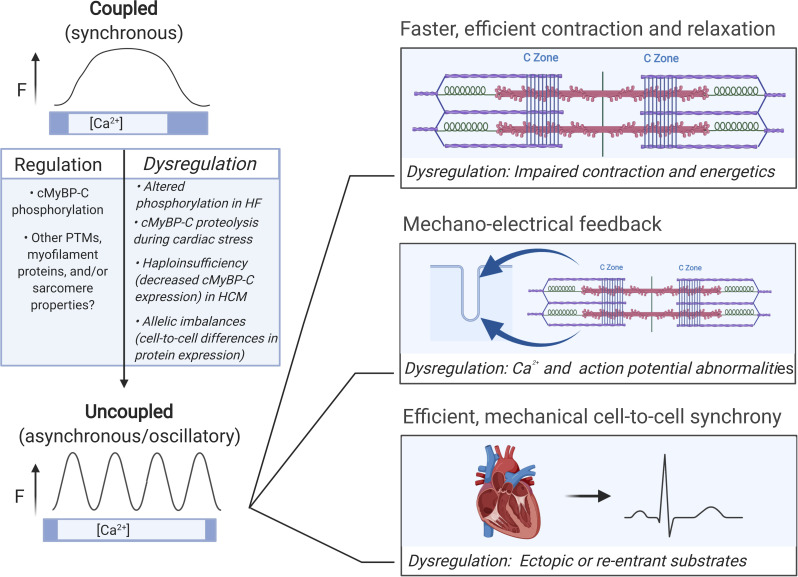
Proposed impact of SPOCs in health and disease. Potentially adaptive responses of SPOC include faster, more efficient contractile kinetics, enhanced mechanoelectrical feedback, and more coordinated contraction and relaxation across multiple cells or cardiac regions. Potential for SPOC dysregulation may occur under conditions that chronically affect cMyBP-C phosphorylation or that reduce cMyBP-C expression such as haploinsufficiency in HCM patients or proteolytic degradation of cMyBP-C during cardiac stress. Dysregulation may include negative impacts on contractile properties and efficiency; deranged mechanoelectrical feedback that disrupts Ca2+ signaling, or action potential characteristics (e.g., delayed afterdepolarizations, early afterdepolarizations, or slowed repolarization); and regional foci of hyper- or hypoexcitability. HF, heart failure. Figure created with BioRender.com.
Finally, a new conceptualization of MyBP-C as a wave maker (when phosphorylated) or a wave breaker (when unphosphorylated) offers fresh perspectives on the function of this enigmatic protein. For instance, by playing on heart “strings,” MyBP-C may tune oscillations that constructively harmonize to produce stronger, more resonant heartbeats, whereas dysregulation may lead to discordant beats and arrhythmogenesis (ter Keurs et al., 2006a, 2006b; Viner et al., 2019; Nitsan et al., 2016). Accordingly, it is tempting to speculate that the longstanding mystery of MyBP-C localization at 43-nm intervals across thick filaments corresponds to critical damping nodes for SPOC waves, analogous to the position of frets on a musical instrument. It will be exciting to systematically test these and other new hypotheses of MyBP-C function and its effects on heart rhythms using the cut-and-paste method that allows rapid exchange MyBP-C in both cardiac and skeletal muscles. These experiments are in progress.
Acknowledgments
Richard L. Moss served as guest editor.
This work was supported by American Heart Association grant 17IRG33411051 to S.P. Harris and National Institutes of Health grants HL080367 to S.P Harris and HL140925 (m-PI) to S.P. Harris, H. White, and V. Galkin.
The author declares no competing financial interests.
References
- Ababou, A., Gautel M., and Pfuhl M.. 2007. Dissecting the N-terminal myosin binding site of human cardiac myosin-binding protein C. Structure and myosin binding of domain C2. J. Biol. Chem. 282:9204–9215. 10.1074/jbc.M610899200 [DOI] [PubMed] [Google Scholar]
- Ababou, A., Rostkova E., Mistry S., Le Masurier C., Gautel M., and Pfuhl M.. 2008. Myosin binding protein C positioned to play a key role in regulation of muscle contraction: structure and interactions of domain C1. J. Mol. Biol. 384:615–630. 10.1016/j.jmb.2008.09.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Mou, Y., Hsu K., Farman G.P., Kumar M., Greaser M.L., Irving T.C., and de Tombe P.P.. 2016. Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc. Natl. Acad. Sci. USA. 113:2306–2311. 10.1073/pnas.1516732113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khayat, H.A., Kensler R.W., Squire J.M., Marston S.B., and Morris E.P.. 2013. Atomic model of the human cardiac muscle myosin filament. Proc. Natl. Acad. Sci. USA. 110:318–323. 10.1073/pnas.1212708110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap, B., Harris S.P., and White H.D.. 2014. Modulation of thin filament activation of myosin ATP hydrolysis by N-terminal domains of cardiac myosin binding protein-C. Biochemistry. 53:6717–6724. 10.1021/bi500787f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers, D.M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Bezold, K.L., Shaffer J.F., Khosa J.K., Hoye E.R., and Harris S.P.. 2013. A gain-of-function mutation in the M-domain of cardiac myosin-binding protein-C increases binding to actin. J. Biol. Chem. 288:21496–21505. 10.1074/jbc.M113.474346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello, E., Fusi L., Ghisleni A., Park-Holohan S.-J., Ovejero J.G., Narayanan T., and Irving M.. 2020. Myosin filament-based regulation of the dynamics of contraction in heart muscle. Proc. Natl. Acad. Sci. USA. 117:8177–8186. 10.1073/pnas.1920632117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard, B., and Pastore A.. 2019. Through thick and thin: dual regulation of insect flight muscle and cardiac muscle compared. J. Muscle Res. Cell Motil. 40:99–110. 10.1007/s10974-019-09536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch, T.A., Kanassatega R.-S., Lepak V.C., and Colson B.A.. 2019. Human cardiac myosin-binding protein C restricts actin structural dynamics in a cooperative and phosphorylation-sensitive manner. J. Biol. Chem. 294:16228–16240. 10.1074/jbc.RA119.009543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, T.M., Mooers S.U., Narayan S.R., and Siegman M.J.. 2010. The N-terminal region of twitchin binds thick and thin contractile filaments: redundant mechanisms of catch force maintenance. J. Biol. Chem. 285:40654–40665. 10.1074/jbc.M110.166041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaghan, S.C., Trinick J., Knight P.J., and White E.. 2000. A role for C-protein in the regulation of contraction and intracellular Ca2+ in intact rat ventricular myocytes. J. Physiol. 528:151–156. 10.1111/j.1469-7793.2000.00151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier, L., Bonne G., Bährend E., Yu B., Richard P., Niel F., Hainque B., Cruaud C., Gary F., Labeit S., et al. 1997. Organization and sequence of human cardiac myosin binding protein C gene (MYBPC3) and identification of mutations predicted to produce truncated proteins in familial hypertrophic cardiomyopathy. Circ. Res. 80:427–434. 10.1161/01.res.0000435859.24609.b3 [DOI] [PubMed] [Google Scholar]
- Chakravorty, S., Tanner B.C.W., Foelber V.L., Vu H., Rosenthal M., Ruiz T., and Vigoreaux J.O.. 2017. Flightin maintains myofilament lattice organization required for optimal flight power and courtship song quality in Drosophila. Proc. Biol. Sci. 284:20170431. 10.1098/rspb.2017.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B., Kemkemer R., Deibler M., Spatz J., and Gao H.. 2012. Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS One. 7:e48346. 10.1371/journal.pone.0048346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C.S., Hoopes C.W., and Campbell K.S.. 2017. Myocardial relaxation is accelerated by fast stretch, not reduced afterload. J. Mol. Cell. Cardiol. 103:65–73. 10.1016/j.yjmcc.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson, B.A., Bekyarova T., Locher M.R., Fitzsimons D.P., Irving T.C., and Moss R.L.. 2008. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ. Res. 103:244–251. 10.1161/CIRCRESAHA.108.178996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson, B.A., Rybakova I.N., Prochniewicz E., Moss R.L., and Thomas D.D.. 2012. Cardiac myosin binding protein-C restricts intrafilament torsional dynamics of actin in a phosphorylation-dependent manner. Proc. Natl. Acad. Sci. USA. 109:20437–20442. 10.1073/pnas.1213027109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato, A., and Fabiato F.. 1978. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J. Gen. Physiol. 72:667–699. 10.1085/jgp.72.5.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons, D.P., Patel J.R., and Moss R.L.. 2001. Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filament. J. Physiol. 530:263–272. 10.1111/j.1469-7793.2001.0263l.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flashman, E., Watkins H., and Redwood C.. 2007. Localization of the binding site of the C-terminal domain of cardiac myosin-binding protein-C on the myosin rod. Biochem. J. 401:97–102. 10.1042/BJ20060500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiburg, A., and Gautel M.. 1996. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur. J. Biochem. 235:317–323. 10.1111/j.1432-1033.1996.00317.x [DOI] [PubMed] [Google Scholar]
- Funabara, D., Kanoh S., Siegman M.J., Butler T.M., Hartshorne D.J., and Watabe S.. 2005. Twitchin as a regulator of catch contraction in molluscan smooth muscle. J. Muscle Res. Cell Motil. 26:455–460. 10.1007/s10974-005-9029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusi, L., Brunello E., Yan Z., and Irving M.. 2016. Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat. Commun. 7:13281. 10.1038/ncomms13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey, J.L., Kranias E.G., and Solaro R.J.. 1988. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem. J. 249:709–714. 10.1042/bj2490709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, R., Cohen J.A., Pardo S., Basu A., and Fischman D.A.. 1999. Identification of the A-band localization domain of myosin binding proteins C and H (MyBP-C, MyBP-H) in skeletal muscle. J. Cell Sci. 112:69–79. [DOI] [PubMed] [Google Scholar]
- Gilbert, R., Kelly M.G., Mikawa T., and Fischman D.A.. 1996. The carboxyl terminus of myosin binding protein C (MyBP-C, C-protein) specifies incorporation into the A-band of striated muscle. J. Cell Sci. 109:101–111. [DOI] [PubMed] [Google Scholar]
- Glazier, A.A., Thompson A., and Day S.M.. 2019. Allelic imbalance and haploinsufficiency in MYBPC3-linked hypertrophic cardiomyopathy. Pflugers Arch. 471:781–793. 10.1007/s00424-018-2226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall, M.C. 1956. Auto-oscillations in extracted muscle fibre systems. Nature. 177:1238–1239. 10.1038/1771238b0 [DOI] [PubMed] [Google Scholar]
- Govindan, S., Kuster D.W., Lin B., Kahn D.J., Jeske W.P., Walenga J.M., Leya F., Hoppensteadt D., Fareed J., and Sadayappan S.. 2013. Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction. Am. J. Cardiovasc. Dis. 3:60–70. [PMC free article] [PubMed] [Google Scholar]
- Govindan, S., McElligott A., Muthusamy S., Nair N., Barefield D., Martin J.L., Gongora E., Greis K.D., Luther P.K., Winegrad S., et al. 2012. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J. Mol. Cell. Cardiol. 52:154–164. 10.1016/j.yjmcc.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham, K.S., and Stelzer J.E.. 2016. The contributions of cardiac myosin binding protein C and troponin I phosphorylation to β-adrenergic enhancement of in vivo cardiac function. J. Physiol. 594:669–686. 10.1113/JP270959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen, M., and Gautel M.. 1999. Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J. Mol. Biol. 286:933–949. 10.1006/jmbi.1998.2522 [DOI] [PubMed] [Google Scholar]
- Gruen, M., Prinz H., and Gautel M.. 1999. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett. 453:254–259. 10.1016/S0014-5793(99)00727-9 [DOI] [PubMed] [Google Scholar]
- Harris, S.P., Bartley C.R., Hacker T.A., McDonald K.S., Douglas P.S., Greaser M.L., Powers P.A., and Moss R.L.. 2002. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ. Res. 90:594–601. 10.1161/01.RES.0000012222.70819.64 [DOI] [PubMed] [Google Scholar]
- Harris, S.P., Belknap B., Van Sciver R.E., White H.D., and Galkin V.E.. 2016. C0 and C1 N-terminal Ig domains of myosin binding protein C exert different effects on thin filament activation. Proc. Natl. Acad. Sci. USA. 113:1558–1563. 10.1073/pnas.1518891113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S.P., Rostkova E., Gautel M., and Moss R.L.. 2004. Binding of myosin binding protein-C to myosin subfragment S2 affects contractility independent of a tether mechanism. Circ. Res. 95:930–936. 10.1161/01.RES.0000147312.02673.56 [DOI] [PubMed] [Google Scholar]
- Hartzell, H.C. 1984. Phosphorylation of C-protein in intact amphibian cardiac muscle. Correlation between 32P incorporation and twitch relaxation. J. Gen. Physiol. 83:563–588. 10.1085/jgp.83.4.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell, H.C., and Titus L.. 1982. Effects of cholinergic and adrenergic agonists on phosphorylation of a 165,000-dalton myofibrillar protein in intact cardiac muscle. J. Biol. Chem. 257:2111–2120. [PubMed] [Google Scholar]
- Helms, A.S., Davis F.M., Coleman D., Bartolone S.N., Glazier A.A., Pagani F., Yob J.M., Sadayappan S., Pedersen E., Lyons R., et al. 2014. Sarcomere mutation-specific expression patterns in human hypertrophic cardiomyopathy. Circ. Cardiovasc. Genet. 7:434–443. 10.1161/CIRCGENETICS.113.000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron, T.J., Rostkova E., Kunst G., Chaturvedi R., Gautel M., and Kentish J.C.. 2006. Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ. Res. 98:1290–1298. 10.1161/01.RES.0000222059.54917.ef [DOI] [PubMed] [Google Scholar]
- Hinken, A.C., and Solaro R.J.. 2007. A dominant role of cardiac molecular motors in the intrinsic regulation of ventricular ejection and relaxation. Physiology (Bethesda). 22:73–80. 10.1152/physiol.00043.2006 [DOI] [PubMed] [Google Scholar]
- Hofmann, P.A., Greaser M.L., and Moss R.L.. 1991. C-protein limits shortening velocity of rabbit skeletal muscle fibres at low levels of Ca2+ activation. J. Physiol. 439:701–715. 10.1113/jphysiol.1991.sp018689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley, H.E. 1969. The mechanism of muscular contraction. Science. 164:1356–1365. 10.1126/science.164.3886.1356 [DOI] [PubMed] [Google Scholar]
- Irving, M. 2017. Regulation of Contraction by the Thick Filaments in Skeletal Muscle. Biophys. J. 113:2579–2594. 10.1016/j.bpj.2017.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata, S., Shimamoto Y., Suzuki M., and Sasaki D.. 2007. Regulation of muscle contraction by Ca2+ and ADP: focusing on the auto-oscillation (SPOC). Adv. Exp. Med. Biol. 592:341–358. 10.1007/978-4-431-38453-3_29 [DOI] [PubMed] [Google Scholar]
- Ishiwata, S., Shimamoto Y., and Fukuda N.. 2011. Contractile system of muscle as an auto-oscillator. Prog. Biophys. Mol. Biol. 105:187–198. 10.1016/j.pbiomolbio.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Iwamoto, H. 2011. Structure, function and evolution of insect flight muscle. Biophysics (Nagoya-Shi). 7:21–28. 10.2142/biophysics.7.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu, L.T., Kohl P., Boyden P.A., Miura M., Banyasz T., Chiamvimonvat N., Trayanova N., Bers D.M., and Chen-Izu Y.. 2020. Mechano-electric and mechano-chemo-transduction in cardiomyocytes. J Physiol. 598:1285–1305. 10.1113/JP276494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques, A.M., Copeland O., Messer A.E., Gallon C.E., King K., McKenna W.J., Tsang V.T., and Marston S.B.. 2008. Myosin binding protein C phosphorylation in normal, hypertrophic and failing human heart muscle. J. Mol. Cell. Cardiol. 45:209–216. 10.1016/j.yjmcc.2008.05.020 [DOI] [PubMed] [Google Scholar]
- Janssen, P.M.L. 2010. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am. J. Physiol. Heart Circ. Physiol. 299:H1092–H1099. 10.1152/ajpheart.00417.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen, P.M., Honda H., Koiwa Y., and Shirato K.. 1996. The effect of diastolic vibration on the relaxation of rat papillary muscle. Cardiovasc. Res. 32:344–350. 10.1016/0008-6363(96)00094-6 [DOI] [PubMed] [Google Scholar]
- Jeacocke, S.A., and England P.J.. 1980. Phosphorylation of a myofibrillar protein of Mr 150 000 in perfused rat heart, and the tentative indentification of this as C-protein. FEBS Lett. 122:129–132. 10.1016/0014-5793(80)80418-2 [DOI] [PubMed] [Google Scholar]
- Josephson, R.K., Malamud J.G., and Stokes D.R.. 2000. Asynchronous muscle: a primer. J. Exp. Biol. 203:2713–2722. [DOI] [PubMed] [Google Scholar]
- Jülicher, F., and Prost J.. 1997. Spontaneous Oscillations of Collective Molecular Motors. Phys. Rev. Lett. 78:4510–4513. 10.1103/PhysRevLett.78.4510 [DOI] [Google Scholar]
- Kagemoto, T., Li A., Dos Remedios C., and Ishiwata S.. 2015. Spontaneous oscillatory contraction (SPOC) in cardiomyocytes. Biophys. Rev. 7:15–24. 10.1007/s12551-015-0165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler, R.W., Craig R., and Moss R.L.. 2017. Phosphorylation of cardiac myosin binding protein C releases myosin heads from the surface of cardiac thick filaments. Proc. Natl. Acad. Sci. USA. 114:E1355–E1364. 10.1073/pnas.1614020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler, R.W., Shaffer J.F., and Harris S.P.. 2011. Binding of the N-terminal fragment C0-C2 of cardiac MyBP-C to cardiac F-actin. J. Struct. Biol. 174:44–51. 10.1016/j.jsb.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögler, H., and Rüegg J.C.. 1997. Cardiac contractility: modulation of myofibrillar calcium sensitivity by beta-adrenergic stimulation. Isr. J. Med. Sci. 33:1–7. [PubMed] [Google Scholar]
- Korte, F.S., McDonald K.S., Harris S.P., and Moss R.L.. 2003. Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ. Res. 93:752–758. 10.1161/01.RES.0000096363.85588.9A [DOI] [PubMed] [Google Scholar]
- Kraft, T., Montag J., Radocaj A., and Brenner B.. 2016. Hypertrophic Cardiomyopathy: Cell-to-Cell Imbalance in Gene Expression and Contraction Force as Trigger for Disease Phenotype Development. Circ. Res. 119:992–995. 10.1161/CIRCRESAHA.116.309804 [DOI] [PubMed] [Google Scholar]
- Kunst, G., Kress K.R., Gruen M., Uttenweiler D., Gautel M., and Fink R.H.. 2000. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ. Res. 86:51–58. 10.1161/01.RES.86.1.51 [DOI] [PubMed] [Google Scholar]
- Lee, K., Harris S.P., Sadayappan S., and Craig R.. 2015. Orientation of myosin binding protein C in the cardiac muscle sarcomere determined by domain-specific immuno-EM. J. Mol. Biol. 427:274–286. 10.1016/j.jmb.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.H., Sulbarán G., Yang S., Mun J.Y., Alamo L., Pinto A., Sato O., Ikebe M., Liu X., Korn E.D., et al. 2018. Interacting-heads motif has been conserved as a mechanism of myosin II inhibition since before the origin of animals. Proc. Natl. Acad. Sci. USA. 115:E1991–E2000. 10.1073/pnas.1715247115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman, S.J., Tal-Grinspan L., Lynn M.L., Strom J., Benitez G.E., Anderson M.E., and Tardiff J.C.. 2019. Chronic Calmodulin-Kinase II Activation Drives Disease Progression in Mutation-Specific Hypertrophic Cardiomyopathy. Circulation. 139:1517–1529. 10.1161/CIRCULATIONAHA.118.034549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari, M., Brunello E., Reconditi M., Fusi L., Caremani M., Narayanan T., Piazzesi G., Lombardi V., and Irving M.. 2015. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 528:276–279. 10.1038/nature15727 [DOI] [PubMed] [Google Scholar]
- Linke, W.A., Bartoo M.L., and Pollack G.H.. 1993. Spontaneous sarcomeric oscillations at intermediate activation levels in single isolated cardiac myofibrils. Circ. Res. 73:724–734. 10.1161/01.RES.73.4.724 [DOI] [PubMed] [Google Scholar]
- Lorand, L., and Moos C.. 1956. Auto-oscillations in extracted muscle fibre systems. Nature. 177:1239. 10.1038/1771239a0 [DOI] [PubMed] [Google Scholar]
- Luther, P.K., Winkler H., Taylor K., Zoghbi M.E., Craig R., Padrón R., Squire J.M., and Liu J.. 2011. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc. Natl. Acad. Sci. USA. 108:11423–11428. 10.1073/pnas.1103216108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi, R., Gresham K.S., and Stelzer J.E.. 2014. Length-dependent changes in contractile dynamics are blunted due to cardiac myosin binding protein-C ablation. Front. Physiol. 5:461. 10.3389/fphys.2014.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi, R., Gresham K.S., Verma S., and Stelzer J.E.. 2016. Cardiac Myosin Binding Protein-C Phosphorylation Modulates Myofilament Length-Dependent Activation. Front. Physiol. 7:38. 10.3389/fphys.2016.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, K.S. 2011. The interdependence of Ca2+ activation, sarcomere length, and power output in the heart. Pflugers Arch. 462:61–67. 10.1007/s00424-011-0949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, J.W., Li A., Smith N.J., Lal S., Graham R.M., Kooiker K.B., van Dijk S.J., Remedios C.G.D., Harris S.P., and Cooke R.. 2016. Ablation of cardiac myosin binding protein-C disrupts the super-relaxed state of myosin in murine cardiomyocytes. J. Mol. Cell. Cardiol. 94:65–71. 10.1016/j.yjmcc.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, J.W., Li A., Lal S., Bos J.M., Harris S.P., van der Velden J., Ackerman M.J., Cooke R., and Dos Remedios C.G.. 2017. MYBPC3 mutations are associated with a reduced super-relaxed state in patients with hypertrophic cardiomyopathy. PLoS One. 12:e0180064. 10.1371/journal.pone.0180064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, J.W., Singh R.R., and Sadayappan S.. 2019. Cardiac myosin binding protein-C phosphorylation regulates the super-relaxed state of myosin. Proc. Natl. Acad. Sci. USA. 116:11731–11736. 10.1073/pnas.1821660116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman, B.M. 1998. The filament lattice of striated muscle. Physiol. Rev. 78:359–391. 10.1152/physrev.1998.78.2.359 [DOI] [PubMed] [Google Scholar]
- Moos, C. 1981. Fluorescence microscope study of the binding of added C protein to skeletal muscle myofibrils. J. Cell Biol. 90:25–31. 10.1083/jcb.90.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos, C., Mason C.M., Besterman J.M., Feng I.N., and Dubin J.H.. 1978. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J. Mol. Biol. 124:571–586. 10.1016/0022-2836(78)90172-9 [DOI] [PubMed] [Google Scholar]
- Moss, R.L. 1992. Ca2+ regulation of mechanical properties of striated muscle. Mechanistic studies using extraction and replacement of regulatory proteins. Circ. Res. 70:865–884. 10.1161/01.RES.70.5.865 [DOI] [PubMed] [Google Scholar]
- Moss, R.L., Fitzsimons D.P., and Ralphe J.C.. 2015. Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium. Circ. Res. 116:183–192. 10.1161/CIRCRESAHA.116.300561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun, J.Y., Kensler R.W., Harris S.P., and Craig R.. 2016. The cMyBP-C HCM variant L348P enhances thin filament activation through an increased shift in tropomyosin position. J. Mol. Cell. Cardiol. 91:141–147. 10.1016/j.yjmcc.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun, J.Y., Previs M.J., Yu H.Y., Gulick J., Tobacman L.S., Beck Previs S., Robbins J., Warshaw D.M., and Craig R.. 2014. Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism. Proc. Natl. Acad. Sci. USA. 111:2170–2175. 10.1073/pnas.1316001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag, S., Trivedi D.V., Sarkar S.S., Adhikari A.S., Sunitha M.S., Sutton S., Ruppel K.M., and Spudich J.A.. 2017. The myosin mesa and the basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Nat. Struct. Mol. Biol. 24:525–533. 10.1038/nsmb.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama, T., Takimoto E., Sadayappan S., Mudd J.O., Seidman J.G., Robbins J., and Kass D.A.. 2007. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation. 116:2399–2408. 10.1161/CIRCULATIONAHA.107.706523 [DOI] [PubMed] [Google Scholar]
- Napierski, N.C., Granger K., Langlais P.R., Moran H.R., Strom J., Touma K., and Harris S.P.. 2020. A Novel “Cut and Paste” Method for In Situ Replacement of cMyBP-C Reveals a New Role for cMyBP-C in the Regulation of Contractile Oscillations. Circ. Res. 126:737–749. 10.1161/CIRCRESAHA.119.315760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaka, T., Yagi T., Tanaka Y., and Ishiwata S.. 1993. Right-handed rotation of an actin filament in an in vitro motile system. Nature. 361:269–271. 10.1038/361269a0 [DOI] [PubMed] [Google Scholar]
- Nitsan, I., Drori S., Lewis Y.E., Cohen S., and Tzlil S.. 2016. Mechanical communication in cardiac cell synchronized beating. Nat. Phys. 12:472–477. 10.1038/nphys3619 [DOI] [Google Scholar]
- Offer, G., Moos C., and Starr R.. 1973. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J. Mol. Biol. 74:653–676. 10.1016/0022-2836(73)90055-7 [DOI] [PubMed] [Google Scholar]
- Palmer, B.M., Georgakopoulos D., Janssen P.M., Wang Y., Alpert N.R., Belardi D.F., Harris S.P., Moss R.L., Burgon P.G., Seidman C.E., et al. 2004. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ. Res. 94:1249–1255. 10.1161/01.RES.0000126898.95550.31 [DOI] [PubMed] [Google Scholar]
- Palmer, B.M., Sadayappan S., Wang Y., Weith A.E., Previs M.J., Bekyarova T., Irving T.C., Robbins J., and Maughan D.W.. 2011. Roles for cardiac MyBP-C in maintaining myofilament lattice rigidity and prolonging myosin cross-bridge lifetime. Biophys. J. 101:1661–1669. 10.1016/j.bpj.2011.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perz-Edwards, R.J., Irving T.C., Baumann B.A.J., Gore D., Hutchinson D.C., Kržič U., Porter R.L., Ward A.B., and Reedy M.K.. 2011. X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc. Natl. Acad. Sci. USA. 108:120–125. 10.1073/pnas.1014599107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnam, S., Sevrieva I., Sun Y.-B., Irving M., and Kampourakis T.. 2019. Site-specific phosphorylation of myosin binding protein-C coordinates thin and thick filament activation in cardiac muscle. Proc. Natl. Acad. Sci. USA. 116:15485–15494. 10.1073/pnas.1903033116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs, M.J., Beck Previs S., Gulick J., Robbins J., and Warshaw D.M.. 2012. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science. 337:1215–1218. 10.1126/science.1223602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs, M.J., Mun J.Y., Michalek A.J., Previs S.B., Gulick J., Robbins J., Warshaw D.M., and Craig R.. 2016. Phosphorylation and calcium antagonistically tune myosin-binding protein C’s structure and function. Proc. Natl. Acad. Sci. USA. 113:3239–3244. 10.1073/pnas.1522236113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs, M.J., Prosser B.L., Mun J.Y., Previs S.B., Gulick J., Lee K., Robbins J., Craig R., Lederer W.J., and Warshaw D.M.. 2015. Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling. Sci. Adv. 1:e1400205–e1400205. 10.1126/sciadv.1400205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezhdo, O.V., and Pereverzev Y.V.. 2009. Theoretical aspects of the biological catch bond. Acc. Chem. Res. 42:693–703. 10.1021/ar800202z [DOI] [PubMed] [Google Scholar]
- Pringle, J.W.S. 1967. The contractile mechanism of insect fibrillar muscle. Prog. Biophys. Mol. Biol. 17:1–12. 10.1016/0079-6107(67)90003-X [DOI] [PubMed] [Google Scholar]
- Pringle, J.W. 1978. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc. R.Soc. Lond. B Biol. Sci. 201:107–130. [DOI] [PubMed] [Google Scholar]
- Ratti, J., Rostkova E., Gautel M., and Pfuhl M.. 2011. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J. Biol. Chem. 286:12650–12658. 10.1074/jbc.M110.156646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razumova, M.V., Bezold K.L., Tu A.-Y., Regnier M., and Harris S.P.. 2008. Contribution of the myosin binding protein C motif to functional effects in permeabilized rat trabeculae. J. Gen. Physiol. 132:575–585. 10.1085/jgp.200810013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razumova, M.V., Shaffer J.F., Tu A.-Y., Flint G.V., Regnier M., and Harris S.P.. 2006. Effects of the N-terminal domains of myosin binding protein-C in an in vitro motility assay: Evidence for long-lived cross-bridges. J. Biol. Chem. 281:35846–35854. 10.1074/jbc.M606949200 [DOI] [PubMed] [Google Scholar]
- Reconditi, M., Brunello E., Linari M., Bianco P., Narayanan T., Panine P., Piazzesi G., Lombardi V., and Irving M.. 2011. Motion of myosin head domains during activation and force development in skeletal muscle. Proc. Natl. Acad. Sci. USA. 108:7236–7240. 10.1073/pnas.1018330108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi, C., Belknap B., Forgacs-Lonart E., Harris S.P., Schröder G.F., White H.D., and Galkin V.E.. 2018. N-Terminal Domains of Cardiac Myosin Binding Protein C Cooperatively Activate the Thin Filament. Structure. 26:1604–1611.e4. 10.1016/j.str.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett, J.C., Hanft L.M., Geist J., Kontrogianni-Konstantopoulos A., and McDonald K.S.. 2019. Regulation of myofilament force and loaded shortening by skeletal myosin binding protein C. J. Gen. Physiol. 151:645–659. 10.1085/jgp.201812200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova, I.N., Greaser M.L., and Moss R.L.. 2011. Myosin binding protein C interaction with actin: characterization and mapping of the binding site. J. Biol. Chem. 286:2008–2016. 10.1074/jbc.M110.170605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, D., Fujita H., Fukuda N., Kurihara S., and Ishiwata S.. 2005. Auto-oscillations of skinned myocardium correlating with heartbeat. J. Muscle Res. Cell Motil. 26:93–101. 10.1007/s10974-005-0249-2 [DOI] [PubMed] [Google Scholar]
- Sasaki, D., Fukuda N., and Ishiwata S.. 2006. Myocardial sarcomeres spontaneously oscillate with the period of heartbeat under physiological conditions. Biochem. Biophys. Res. Commun. 343:1146–1152. 10.1016/j.bbrc.2006.03.070 [DOI] [PubMed] [Google Scholar]
- Sato, K., Kuramoto Y., Ohtaki M., Shimamoto Y., and Ishiwata S.. 2013. Locally and globally coupled oscillators in muscle. Phys. Rev. Lett. 111:108104. 10.1103/PhysRevLett.111.108104 [DOI] [PubMed] [Google Scholar]
- Sato, K., Ohtaki M., Shimamoto Y., and Ishiwata S.. 2011. A theory on auto-oscillation and contraction in striated muscle. Prog. Biophys. Mol. Biol. 105:199–207. 10.1016/j.pbiomolbio.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Serizawa, T., Terui T., Kagemoto T., Mizuno A., Shimozawa T., Kobirumaki F., Ishiwata S., Kurihara S., and Fukuda N.. 2011. Real-time measurement of the length of a single sarcomere in rat ventricular myocytes: a novel analysis with quantum dots. Am. J. Physiol. Cell Physiol. 301:C1116–C1127. 10.1152/ajpcell.00161.2011 [DOI] [PubMed] [Google Scholar]
- Shaffer, J.F., and Gillis T.E.. 2010. Evolution of the regulatory control of vertebrate striated muscle: the roles of troponin I and myosin binding protein-C. Physiol. Genomics. 42:406–419. 10.1152/physiolgenomics.00055.2010 [DOI] [PubMed] [Google Scholar]
- Shaffer, J.F., Kensler R.W., and Harris S.P.. 2009. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J. Biol. Chem. 284:12318–12327. 10.1074/jbc.M808850200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, J.F., Razumova M.V., Tu A.-Y., Regnier M., and Harris S.P.. 2007. Myosin S2 is not required for effects of myosin binding protein-C on motility. FEBS Lett. 581:1501–1504. 10.1016/j.febslet.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Shashi, V., Geist J., Lee Y., Yoo Y., Shin U., Schoch K., Sullivan J., Stong N., Smith E., Jasien J., et al. Undiagnosed Diseases Network . 2019. Heterozygous variants in MYBPC1 are associated with an expanded neuromuscular phenotype beyond arthrogryposis. Hum. Mutat. 40:1115–1126. 10.1002/humu.23760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro, R.J. 2008. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J. Biol. Chem. 283:26829–26833. 10.1074/jbc.R800037200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, R., and Offer G.. 1978. The interaction of C-protein with heavy meromyosin and subfragment-2. Biochem. J. 171:813–816. 10.1042/bj1710813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavusis, J., Lace B., Schäfer J., Geist J., Inashkina I., Kidere D., Pajusalu S., Wright N.T., Saak A., Weinhold M., et al. 2019. Novel mutations in MYBPC1 are associated with myogenic tremor and mild myopathy. Ann. Neurol. 86:129–142. 10.1002/ana.25494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle, R., Krüger M., and Pfitzer G.. 2002. Force kinetics and individual sarcomere dynamics in cardiac myofibrils after rapid ca(2+) changes. Biophys. J. 83:2152–2161. 10.1016/S0006-3495(02)73975-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle, R., Solzin J., Iorga B., and Poggesi C.. 2009. Insights into the kinetics of Ca2+-regulated contraction and relaxation from myofibril studies. Pflugers Arch. 458:337–357. 10.1007/s00424-008-0630-2 [DOI] [PubMed] [Google Scholar]
- Stelzer, J.E., Dunning S.B., and Moss R.L.. 2006a. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ. Res. 98:1212–1218. 10.1161/01.RES.0000219863.94390.ce [DOI] [PubMed] [Google Scholar]
- Stelzer, J.E., Fitzsimons D.P., and Moss R.L.. 2006b. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys. J. 90:4119–4127. 10.1529/biophysj.105.078147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, M.A., Franks-Skiba K., Chen S., and Cooke R.. 2010. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA. 107:430–435. 10.1073/pnas.0909468107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi, T., Koiwa Y., Kikuchi J., Honda H., Hoshi N., Butler J.P., and Takishima T.. 1992. Diastolic vibration improves systolic function in cases of incomplete relaxation. Circulation. 86:1955–1964. 10.1161/01.CIR.86.6.1955 [DOI] [PubMed] [Google Scholar]
- Taylor, K.A., Rahmani H., Edwards R.J., and Reedy M.K.. 2019. Insights into Actin-Myosin Interactions within Muscle from 3D Electron Microscopy. Int. J. Mol. Sci. 20:1703. 10.3390/ijms20071703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs, H.E.D.J., Wakayama Y., Miura M., Shinozaki T., Stuyvers B.D., Boyden P.A., and Landesberg A.. 2006a. Arrhythmogenic Ca(2+) release from cardiac myofilaments. Prog. Biophys. Mol. Biol. 90:151–171. 10.1016/j.pbiomolbio.2005.07.002 [DOI] [PubMed] [Google Scholar]
- ter Keurs, H.E.D.J., Wakayama Y., Sugai Y., Price G., Kagaya Y., Boyden P.A., Miura M., and Stuyvers B.D.M.. 2006b. Role of sarcomere mechanics and Ca2+ overload in Ca2+ waves and arrhythmias in rat cardiac muscle. Ann. N. Y. Acad. Sci. 1080:248–267. 10.1196/annals.1380.020 [DOI] [PubMed] [Google Scholar]
- Tesi, C., Piroddi N., Colomo F., and Poggesi C.. 2002. Relaxation kinetics following sudden Ca(2+) reduction in single myofibrils from skeletal muscle. Biophys. J. 83:2142–2151. 10.1016/S0006-3495(02)73974-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, W.E., Vogel V., and Sokurenko E.. 2008. Biophysics of catch bonds. Annu. Rev. Biophys. 37:399–416. 10.1146/annurev.biophys.37.032807.125804 [DOI] [PubMed] [Google Scholar]
- Toepfer, C.N., Garfinkel A.C., Venturini G., Wakimoto H., Repetti G., Alamo L., Sharma A., Agarwal R., Ewoldt J.F., Cloonan P., et al. 2020. Myosin Sequestration Regulates Sarcomere Function, Cardiomyocyte Energetics, and Metabolism, Informing the Pathogenesis of Hypertrophic Cardiomyopathy. Circulation. 141:828–842. 10.1161/CIRCULATIONAHA.119.042339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, C.W., Nair N.A., Doersch K.M., Liu Y., and Rosas P.C.. 2014. Cardiac myosin-binding protein-C is a critical mediator of diastolic function. Pflugers Arch. 466:451–457. 10.1007/s00424-014-1442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonino, P., Kiss B., Gohlke J., Smith J.E. III, and Granzier H.. 2019. Fine mapping titin’s C-zone: Matching cardiac myosin-binding protein C stripes with titin’s super-repeats. J. Mol. Cell. Cardiol. 133:47–56. 10.1016/j.yjmcc.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi, D.V., Adhikari A.S., Sarkar S.S., Ruppel K.M., and Spudich J.A.. 2018. Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light. Biophys. Rev. 10:27–48. 10.1007/s12551-017-0274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk, S.J., Dooijes D., dos Remedios C., Michels M., Lamers J.M.J., Winegrad S., Schlossarek S., Carrier L., ten Cate F.J., Stienen G.J.M., and van der Velden J.. 2009. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 119:1473–1483. 10.1161/CIRCULATIONAHA.108.838672 [DOI] [PubMed] [Google Scholar]
- van Dijk, S.J., Kooiker K.B., Napierski N.C., Touma K.D., Mazzalupo S., and Harris S.P.. 2018. Point mutations in the tri-helix bundle of the M-domain of cardiac myosin binding protein-C influence systolic duration and delay cardiac relaxation. J. Mol. Cell. Cardiol. 119:116–124. 10.1016/j.yjmcc.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner, H., Nitsan I., Sapir L., Drori S., and Tzlil S.. 2019. Mechanical Communication Acts as a Noise Filter. iScience. 14:58–68. 10.1016/j.isci.2019.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, G., Ferrantini C., Piroddi N., Scellini B., Pioner J.M., Colombini B., Tesi C., and Poggesi C.. 2019. The relation between sarcomere energetics and the rate of isometric tension relaxation in healthy and diseased cardiac muscle. J. Muscle Res. Cell Motil. 19:111. 10.1007/s10974-019-09566-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu Manh, T.P., Mokrane M., Georgenthum E., Flavigny J., Carrier L., Sémériva M., Piovant M., and Röder L.. 2005. Expression of cardiac myosin-binding protein-C (cMyBP-C) in Drosophila as a model for the study of human cardiomyopathies. Hum. Mol. Genet. 14:7–17. 10.1093/hmg/ddi002 [DOI] [PubMed] [Google Scholar]
- Weber, F.E., Vaughan K.T., Reinach F.C., and Fischman D.A.. 1993. Complete sequence of human fast-type and slow-type muscle myosin-binding-protein C (MyBP-C). Differential expression, conserved domain structure and chromosome assignment. Eur. J. Biochem. 216:661–669. 10.1111/j.1432-1033.1993.tb18186.x [DOI] [PubMed] [Google Scholar]
- Weith, A., Sadayappan S., Gulick J., Previs M.J., Vanburen P., Robbins J., and Warshaw D.M.. 2012a. Unique single molecule binding of cardiac myosin binding protein-C to actin and phosphorylation-dependent inhibition of actomyosin motility requires 17 amino acids of the motif domain. J. Mol. Cell. Cardiol. 52:219–227. 10.1016/j.yjmcc.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weith, A.E., Previs M.J., Hoeprich G.J., Previs S.B., Gulick J., Robbins J., and Warshaw D.M.. 2012b. The extent of cardiac myosin binding protein-C phosphorylation modulates actomyosin function in a graded manner. J. Muscle Res. Cell Motil. 33:449–459. 10.1007/s10974-012-9312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten, A.E., Jeffries C.M., Harris S.P., and Trewhella J.. 2008. Cardiac myosin-binding protein C decorates F-actin: implications for cardiac function. Proc. Natl. Acad. Sci. USA. 105:18360–18365. 10.1073/pnas.0808903105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witayavanitkul, N., Ait Mou Y., Kuster D.W.D., Khairallah R.J., Sarkey J., Govindan S., Chen X., Ge Y., Rajan S., Wieczorek D.F., et al. 2014. Myocardial infarction-induced N-terminal fragment of cardiac myosin-binding protein C (cMyBP-C) impairs myofilament function in human myocardium. J. Biol. Chem. 289:8818–8827. 10.1074/jbc.M113.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, J.E., Ishiwata S., Braet F., Whan R., Su Y., Lal S., and Dos Remedios C.G.. 2011. SPontaneous Oscillatory Contraction (SPOC): auto-oscillations observed in striated muscle at partial activation. Biophys. Rev. 3:53–62. 10.1007/s12551-011-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray, J.S. 1979. Filament geometry and the activation of insect flight muscles. Nature. 280:325–326. 10.1038/280325a0 [DOI] [Google Scholar]
- Yamamoto, K. 1986. The binding of skeletal muscle C-protein to regulated actin. FEBS Lett. 208:123–127. 10.1016/0014-5793(86)81545-9 [DOI] [PubMed] [Google Scholar]
- Yotti, R., Seidman C.E., and Seidman J.G.. 2019. Advances in the Genetic Basis and Pathogenesis of Sarcomere Cardiomyopathies. Annu. Rev. Genomics Hum. Genet. 20:129–153. 10.1146/annurev-genom-083118-015306 [DOI] [PubMed] [Google Scholar]
- Zakeri, B., Fierer J.O., Celik E., Chittock E.C., Schwarz-Linek U., Moy V.T., and Howarth M.. 2012. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA. 109:E690–E697. 10.1073/pnas.1115485109 [DOI] [PMC free article] [PubMed] [Google Scholar]