Abstract
Propose
Among antibiotic resistance cases, resistance to β-lactam antibiotics is a major concern for the treatment of microbial infections. Furthermore, the prevalence of extended-spectrum β-lactamases (ESBL) Escherichia coli (E. coli) in environment, food, and human resources of Iran has increased over the past few years. This study aimed to predict the relationship between the prevalence of ESBL E. coli in the environment and the food chains with the presence of this infection in people suspected of septicemia using fuzzy set qualitative comparative analysis model.
Methods
In this analytical cross sectional study samples were collected from the environment (hospital sewage, downstream and upstream urban sewage, and slaughterhouse sewage), food (chicken), and human chains (people suspected of septicemia) in Tehran province, Iran. This study was conducted from September to February 2019 and the prevalence of ESBL E. coli was calculated in each resource. Then, the relationship between the prevalence of ESBL E. coli in the environment and food chains and its prevalence in the human chain was predicted using the fuzzy set qualitative comparative analysis.
Results
The results showed the prevalence of ESBL E. coli in those suspected of septicemia in September, October, November, December, January and February was 58.1%, 60%, 33.3%, 100%, 43%, and 57.8%, respectively. Also, the results of the fuzzy set qualitative comparative analysis indicated hospital wastewater and chicken contamination with ESBL E. coli were the main causes of contamination with ESBL E. coli in people suspected of septicemia.
Conclusions
According to the results of this study, if there is a contamination of hospital wastewater and chickens in an area, it can be claimed that people suspected of septicemia are infected with ESBL E. coli, and the percentage of this contamination can be high. On the other hand, controlling ESBL E. coli in hospital wastewater (environmental chain) and chickens (food chain) can prevent contamination in people with suspected septicemia.
Keywords: Extended spectrum β-lactamase producing, Escherichia coli, Food chain, Human chain, Environment chain, Fuzzy set qualitative comparative analysis
Introduction
Antibiotics are the main and important treatment for infectious diseases, and such treatments have played a significant role in the dramatic development of global health in the past. Millions of people have now survived what was once a life-threatening infection. However, overuse of antibiotics has led to antibiotic resistance and reduced effectiveness over the past few years [1, 2]. The presence of antibiotic-resistant bacteria in aquatic environments has raised concerns about public health through food and human chains [3]. In most cases the genetic code for antibiotic resistance lies on R plasmids, which can rapidly spread to sensitive species in aquatic environments and spread antibiotic resistance among bacteria [4]. Resistance to β-lactam antibiotics is a major concern for the treatment of microbial infections. The main mechanism of bacterial resistance to β-lactam antibiotics is the production of β-lactamase enzymes [5]. These enzymes hydrolyze and inactivate β-lactam antibiotics before they reach the penicillin-binding proteins (PBPs) in the cytoplasmic membrane. The PBPs are located in the cell envelope (cytoplasmic membrane and cell wall) of bacteria [6]. Extended-spectrum β-lactamases (ESBL) are a class of β-lactamase enzymes that are of particular importance in antimicrobial therapy and are able to completely hydrolyze Oximino-β-lactams, such as third generation and fourth generation cephalosporins [7]. The production of ESBL varies among Enterobacteriaceae worldwide. In a recent study (tigecycline evaluation and surveillance trial (TEST)), the highest production rate of ESBL was by Klebsiella pneumonia and E. coli, respectively [8]. The incidence of human urinary tract infections induced by Escherichia coli (E. coli) is on the rise worldwide, especially in developing countries [9]. β-lactamases are classified into 2 types: molecular (Ambler) and functional (Bush Jacoby Medeiros). Functional classification begins when cephalosporins are distinguished from penicillin [10]. The prevalence of ESBL E. coli in environmental and food resources of Iran has increased over the past few years and has led to some hypotheses indicating the prevalence of ESBL E. coli in these resources has had an effect on the increased prevalence of ESBL E. coli in the human chain [11]. One favorable approach to event analysis (EV) that involves various factors or a combination of factors is qualitative comparative analysis (QCA) [12–14]. This method is a combination of quantitative and qualitative analysis that identifies and tests hypotheses related to independent conditions that lead to outcomes in different groups. QCA analytical method determines causality by inductive logic using medium to low sample size without using complex analysis or software. The approach is built on principles of configurational causality and algorithms that determine whether a contributing factor in a pattern leading to an outcome is necessary (always present but with other factors), sufficient (always present, sole factor), or irrelevant/noncontributory. Therefore, this study aimed to determine the relationship between the prevalence of ESBL E. coli in the environment (hospital wastewater, downstream and upstream urban wastewater, slaughterhouses’ wastewater), and the food chains (chicken samples) on the one side and the spread of this bacterium in the human chain (people suspected of septicemia) on the other.
Methods
This was an analytical cross sectional study conducted on samples collected from food, environment, and human chains in Tehran province, Iran, from September to February 2019. The ethics committee of Iran University of Medical Sciences approved the study and issued the code of ethics. Samples were collected from the chicken cecum (food chain), communal waste, downstream and upstream surface water, and slaughterhouse sewage (environment chain), and hospitalized patients who were suspected of septicemia (human chain) and cultured in MacConkey agar with and without cefotaxime. After initial isolation, lactose positive strains were subjected to conventional biochemical tests to identify E. coli and ESBL E. coli. Isolation methods were set separately for different samples.
Food chain
Samples of the food chain were prepared from chicken cecum and stored at 2 °C -8 °C during the transfer to the laboratory. In the laboratory the samples were kept at 4 °C until the experiment was performed. The experiments were performed on the cecum samples within 24 hours. The suspected E. coli strains were isolated from the chicken sample by streaking 10 µL from the contents of the chicken cecum to the MacConkey agar containing 4 µg/mL cefotaxime (MAC-CEF) using a sterile loop. The plates were then incubated at 37 °C for 18–24 hours. On the second day, suspected ESBL E. coli strains appeared in MAC-CEF media as red to pink colonies. The 3 different lactose positive colonies from the plates were selected and subcultured separately on other MAC-CEF plates. The plates were incubated at 37 °C for 18–24 hours. On the third day, a single colony was removed from the colonies isolated on each plate, cultured linearly on the blood agar medium, and incubated at 37 °C for 18–22 hours, which was done to ensure the creation of single colonies that could be used in the blood agar plates for identification. On the fourth day, all 3 prepared colonies were coded and subjected to conventional biochemical tests, including oxidase, sulfur indole motility media (SIM), triple sugar iron (TSI), methyl red and Voges-Proskauer (MR-VP), urease producing, and Simmon citrate, to identify E. coli strains. Phenotypic confirmation of E. coli was performed by citrate negative, indole positive, TSI; glucose and lactose/ sucrose fermented, MR positive, VP negative, and urease and oxidase negative. If sample A was E. coli, the rest of the samples were excluded, but if sample A was not E. coli, sample B and sample C were examined. Each of the samples that matched E. coli diagnostic reactions was stored and the rest of the plates were removed from the study. If none of the colonies were E. coli, the corresponding chicken sample was considered as a negative sample for the presence of ESBL E. coli.
Environment chain
Environmental samples were collected from 4 different sources, including communal waste, downstream and upstream surface water, and poultry slaughterhouses waste in Tehran province, Iran. Upstream samples indicate the pollution index before reaching Tehran, Iran, and downstream samples indicate the pollution index for Tehran itself. The pollution index related to agricultural and human products and wet market were considered as the animal pollution index. Some experts were trained to collect samples from these 4 sources of environmental pollution (upstream and downstream, hospitals and slaughterhouses sewage). Samples were tested in less than 2 hours after transfer to the laboratory. Also, 1 mL of each wastewater sample was added to 9 mL of sterile normal saline and mixed well. To reduce the number of bacteria, samples were serially diluted 10 folds with sterile normal saline from 10− 1 to 10− 6. Moreover, 100 microliter of each diluted and undiluted samples was cultured on MacConkey (Mac) and MacConkey + 4 µg/mL cefotaxime (Mac + cef), separately and spread by sterile L-shaped hockey stick cell spreaders. All media were incubated at 37 °C for 18 to 24 hours.
Enumeration of bacterial population in environmental samples
Three different populations were detected among samples, including suspected E. coli (flat, dry, pink colonies with a surrounding darker pink area of precipitated bile salts), non- E. coli coliform or other lactose fermenters (pink to brick red colonies with or without a zone of precipitated bile), non- E. coli, and non-other coliforms (colorless or clear colonies). The enumeration was done according to the “BS EN ISO 8199:2007” [15]. The total counts of bacteria and E. coli were calculated on the MacConkey plates and the total ESBL E. coli and total ESBL population were counted on the plates with cefotaxime. Uncountable plates with > 200 colonies were excluded from the study and identification and enumeration were done on plates with countable bacterial count. To calculate the total number of colonies used as colony forming units (CFUS), E. coli and ESBL E. coli were determined according to the formula provided in BS EN ISO 8199:2007” guideline [15].
Identification of presumptive E. coli
Five representative colonies from all countable MacConkey plates (E. coli) and MacConkey + cefotaxime plates (ESBL E. coli) were selected and streaked to purification. To obtain pure isolates, colonies were streaked a minimum of 2 times on blood agar. all suspected colonies were subjected to conventional biochemical test after purification.
Identification of Presumptive ESBL-E. coli
Five representative colonies from all countable MacConkey + cefotaxime plates were selected and streaked for purification. To obtain pure isolates, colonies were streaked a minimum of 2 times on blood agar. All suspected colonies were subjected to conventional biochemical tests after purification.
Human Chain
Human samples were collected from the blood sample of hospitalized patients suspected to septicemia. Bacteria isolated from blood cultures on MacConkey agar containing 4 µg/mL of cefotaxime (MAC-CEF) were subcultured on all the sample plate streaks by a sterile loop. They were then incubated at 37 °C for 18–24 hours to obtain isolated colonies. According to the guideline and protocol, potential strains of ESBL E. coli appear red, purple, or pink in the culture medium [16, 17]. Therefore, if we saw these colonies, we removed 3 different colonies from the plates and cultured them separately on 3 MacConkey agars containing 4 µg/mL of cefotaxime (MAC-CEF) plates. The plates were incubated at 37 °C for 18–24 hours. Then, a single colony was removed from the isolated colonies on each plate and cultured linearly on blood agar for 18–22 hours and incubated at 37 °C. Finally, the isolated colonies from these blood agar plates were subjected to biochemical tests to identify E. coli strains.
Confirmation of producing extended-spectrum B-lactamases in presumptive ESBL-E. coli
After identifying E. coli, all confirmed isolates from all sources were analyzed for producing ESBL using double disc method according to the CLSI 2018 guideline [18, 19]. All confirmed E. coli strains suspected as ESBL E. coli were streaked on Mac + Cef media and incubated at 37 °C for 24 hours. In brief, bacterial suspensions were prepared using sterile normal saline to achieve a turbidity equivalent to a 0.5 McFarland standard. All confirmed E. coli isolates were cultured on Muler- Hinton agar plates using sterile swabs. Two sets of antibiotics were used to detect the ESBL producing isolates, including cefotaxime/cefotaxime-clavulanate and ceftazidime/ceftazidime-clavulanate. At least 5-mm increase in a zone diameter for either antimicrobial agent tested in combination with clavulanate vs. the zone diameter of the agent when tested alone confirmed ESBL producing isolates (e.g., ceftazidime zone = 16; ceftazidime-clavulanate zone = 21).
The reference strain E. coli ATCC 25,922 and Klebsiella pneumoniae ATCC 70,063 were used as nonproducing ESBL and producing ESBL controls, respectively.
Qualitative comparative analysis model
The qualitative comparison model is a combination of quantitative and qualitative methods that help to determine the necessary and sufficient causes in a causal relationship [20]. In this study this model was used to determine and predict the relationship between the prevalence of ESBL E. coli in the food and environment chains with its prevalence in the human chain (people suspected of septicemia). In this study the fuzzy set qualitative comparative analysis (fsQCA) was used to determine this relationship [21]. After determining the prevalence of ESBL E. coli in food, the environment, and human chains, a direct calibration method was used to convert ESBL E. coli prevalence to numbers between 0 and 1 [22]. Data were calibrated using the following formula [23]:
X is the desired variable; is maximum value (full membership in a causal relationship); indicates the average value (incomplete membership in a causal relationship), and indicates the minimum value (nonmembership in a causal relationship) [23]. In this study independent variables were the prevalence of ESBL E. coli infection in the food chain (chicken cecum) and environmental chain (hospital, slaughterhouse, downstream and upstream urban wastewater). The dependent variable or the desired outcome was the prevalence of ESBL E. coli infection in people suspected of septicemia. After calibrating the data, the truth table was prepared and the condition of the necessary and sufficient cause was examined based on Boolean algebra and inductive logic. In the qualitative comparison model an independent variable can be a sufficient cause for an outcome when all its values are equal or smaller than those of the outcome [23].
In addition, all the values obtained for the independent variables must be above or on the diagonal line in the XY graph. Necessary condition analysis and subset and superset analysis were used to investigate the required cause in this model, which was examined while considering the consistency and coverage indexes [23].
If the average percentage of consistency and coverage are 1 and above 0.20, respectively, it can be argued that the independent variable is a necessary cause for creating the desired outcome [23]. FsQCA software (version 3.1) was used for data analysis.
Results
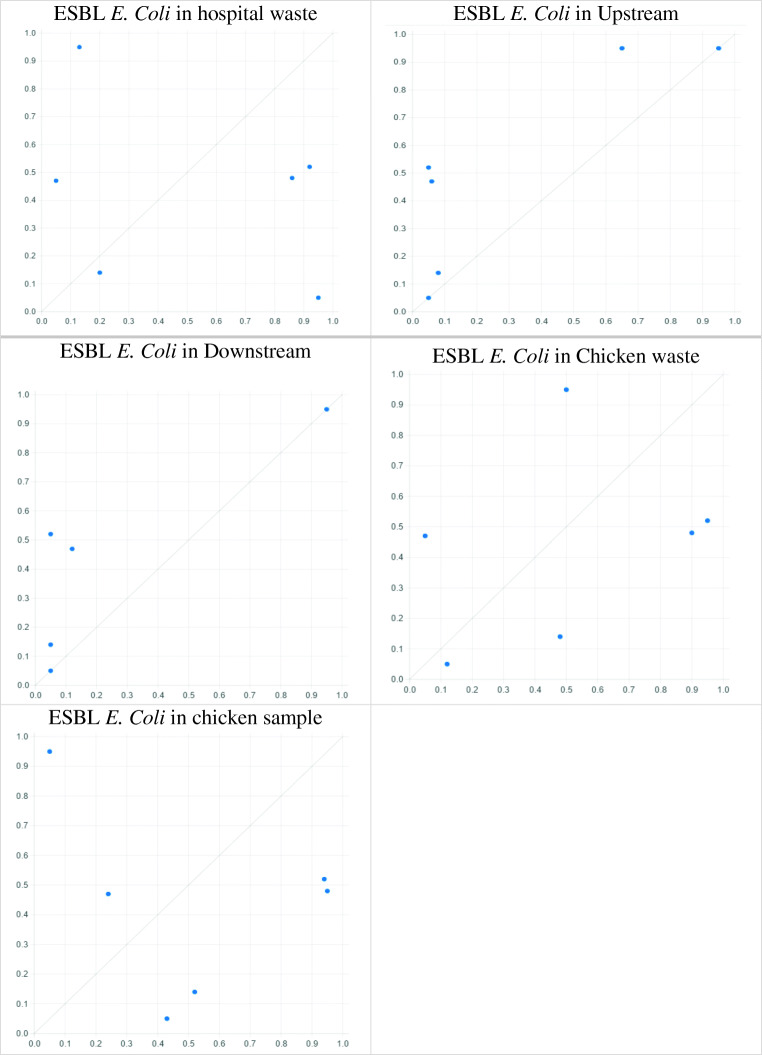
Initially, the results showed the prevalence of ESBL E. coli infection in the food chain (chicken cecum) during 6 months (September, October, November, December, January and February) was 57.4%, 55.5%, 29.4%, 0%, 33.3%, and 20%, respectively. Also, there was a higher prevalence in September compared to other months. The prevalence of ESBL E. coli in hospitals’ wastewater during 6 months (September, October, November, December, January and February) was 67.1%, 71.4%, 75%, 41.7%, 45.3%, and 33.2%, respectively. Also, the prevalence in downstream and upstream sewers of urban areas in different months is presented in Table 1; Fig. 1. The prevalence of ESBL E. coli in slaughterhouse sewage was close to 100% in October. Moreover, the prevalence of ESBL E. coli in people suspected of septicemia during 6 months (September, October, November, December, January and February) was 58.1%, 60%, 33.3%, 100%, 43%, and 57.8%, respectively. As observed, the prevalence was higher in December than in other months (Table 1; Fig. 1).
Table 1.
The prevalence of ESBL E. coli in the environment (hospital waste, upstream and downstream waste, chicken waste), food (chicken sample), and human chains (people suspected of septicemia)
| Environment Chain | Food Chain | Human Chain | ||||
|---|---|---|---|---|---|---|
| Month | Hospital Waste | Downstream Waste | Upstream Waste | Chicken Waste | Chicken Sample | Patients with Septicemia Suspected |
| Sep | 0.671 | 0.02 | 0.01 | 0.893 | 0.574 | 0.581 |
| Oct | 0.714 | 0 | 0 | 1 | 0.555 | 0.600 |
| Nov | 0.75 | 0 | 0 | 0.333 | 0.294 | 0.333 |
| Dec | 0.417 | 0.05 | 0.01 | 0.6 | 0 | 1 |
| Jan | 0.453 | 0.002 | 0 | 0.59 | 0.333 | 0.430 |
| Feb | 0.332 | 0.001 | 0.001 | 0.2 | 0.2 | 0.578 |
Fig. 1.
XY plot of sufficiency causes on occurrence ESBL E. Coli in people suspected of septicemia
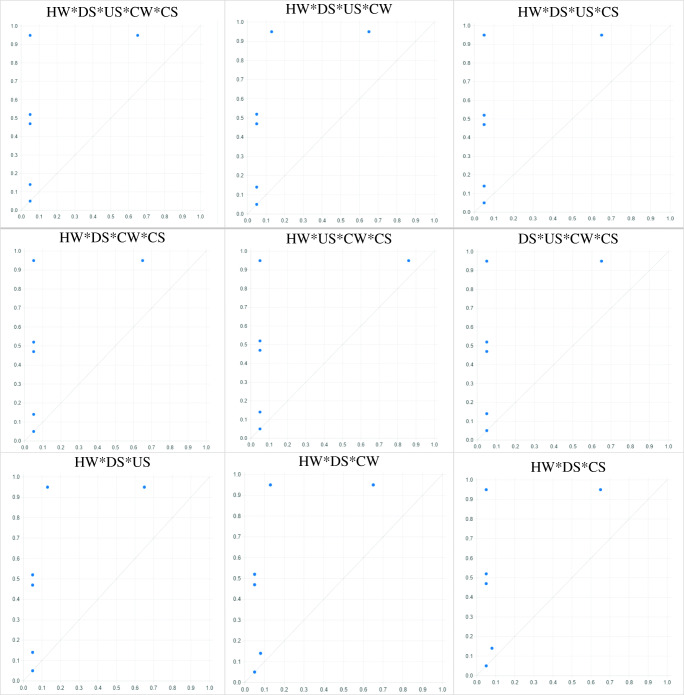
Calibration was performed directly and the results of truth table are presented in Table 2. The results showed the presence of ESBL E. coli infection in urban downstream and upstream sewers alone was a sufficient cause for the presence of ESBL E. coli infection in people suspected of septicemia, as in these 2 independent variables, all X values were less than or equal to the prevalence values of ESBL E. coli in individuals suspected of septicemia (dependent variable) (Fig. 2; Table 2). Moreover, the results of the necessary condition showed that the consistency and coverage for hospital wastewater and chicken samples were near 1 and above 0.20, respectively. Therefore, it can be argued that hospital wastewater and chicken contamination with ESBL E. coli were the necessary cause for the presence of ESBL E. coli infection in patients suspected of septicemia (Table 2).
Table 2.
The truth table of ESBL E. coli in the environment (hospital waste, upstream and downstream waste, chicken waste), food (chicken sample), and human chains (people suspected of septicemia) after direct calibration
| Environments Chain | Food Chain | Human Chain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | Hospital Waste | Downstream Waste | Upstream Waste | Chicken Waste | Chicken Sample | Patients with Septicemia Suspected | |||||
| Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | ||
| Sep | 0.86 | 0.86 | 0.65 | 0.65 | 0.95 | 0.90 | 0.90 | 0.90 | 0.95 | 0.90 | 0.95 |
| Oct | 0.92 | 0.92 | 0.05 | 0.05 | 0.05 | 0.05 | 0.95 | 0.93 | 0.94 | 0.93 | 0.52 |
| Nov | 0.95 | 0.95 | 0.05 | 0.05 | 0.05 | 0.05 | 0.12 | 0.12 | 0.43 | 0.43 | 0.05 |
| Dec | 0.13 | 0.05 | 0.95 | 0.05 | 0.95 | 0.05 | 0.50 | 0.05 | 0.05 | 0.05 | 0.95 |
| Jan | 0.20 | 0.09 | 0.08 | 0.08 | 0.05 | 0.05 | 0.48 | 0.09 | 0.52 | 0.09 | 0.14 |
| Feb | 0.05 | 0.05 | 0.06 | 0.06 | 0.12 | 0.10 | 0.05 | 0.05 | 0.24 | 0.10 | 0.47 |
| SUM Fuzzy Membership | 3.11 | 2.92 | 1.84 | 0.94 | 2.17 | 1.20 | 3 | 2.14 | 3.13 | 2.5 | 3.08 |
| Coverage | 0.948 | 0.305 | 0.389 | 0.694 | 0.798 | ||||||
| Consistency | 0.938 | 0.510 | 0.552 | 0.713 | 0.811 | ||||||
Fig. 2.
XY plot of all sufficiency causes on occurrence of ESBL E. coli in people suspected of septicemia (A)
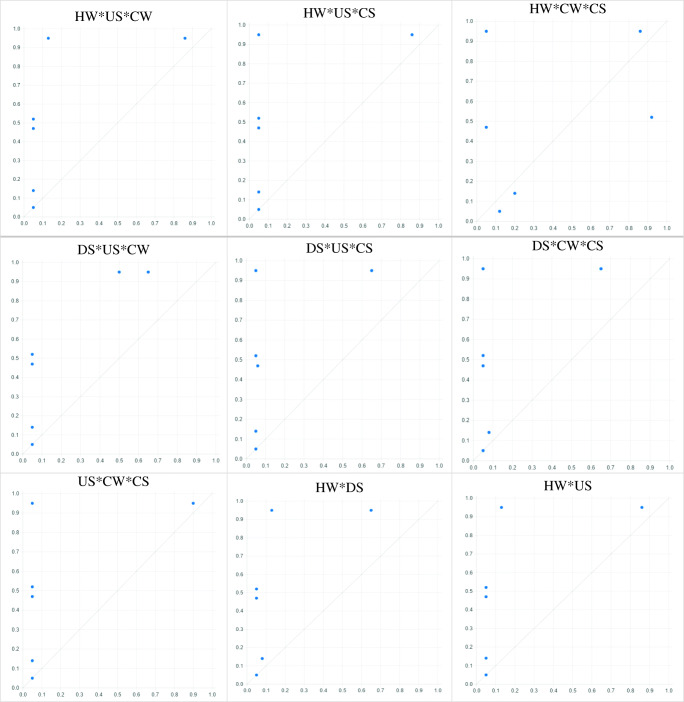
In this study considering the 5 independent modes for studying the desired outcome, there will be 32 independent conditions when these 5 modes are combined. The relationship of each of these conditions with the induction of ESBL E. coli infection in people suspected of septicemia was investigated and analyzed using the fsQCA model. All possible modes have been created and calibrated separately in Table 3. The results showed the simultaneous presence of ESBL E. coli infection in hospital wastewater, downstream urban sewage, upstream urban sewage, slaughterhouse sewage, and chicken samples was a sufficient cause for the presence of this infection in people suspected of septicemia (Fig. 2; Table 3). Also, simultaneous presence of ESBL E. coli infection in hospital wastewater, downstream urban sewage, upstream urban sewage, and slaughterhouse sewage, simultaneous presence of ESBL E. coli infection in hospital wastewater, downstream urban sewage, upstream urban sewage, and chicken samples, and simultaneous presence of ESBL E. coli infection in hospital wastewater, downstream urban sewage, slaughterhouse sewage, and chicken samples were sufficient causes for the presence of this infection in people suspected of septicemia (Fig. 2; Table 3).
Table 3.
The truth table of all conditions of ESBL E. coli in the environment (hospital waste, upstream and downstream waste, chicken waste), food (chicken sample), and human chains (people suspected of septicemia) after direct calibration
| Months /Conditions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | PSS | |||||||||
| Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | ||
| Sep | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.86 | 0.86 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.95 |
| Oct | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.52 |
| Nov | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Dec | 0.05 | 0.05 | 0.13 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.13 | 0.05 | 0.13 | 0.05 | 0.05 | 0.05 | 0.95 |
| Jan | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.08 | 0.08 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.08 | 0.08 | 0.08 | 0.08 | 0.14 |
| Feb | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.47 |
| SUM Fuzzy Membership | 0.90 | 0.90 | 0.98 | 0.90 | 0.90 | 0.90 | 0.93 | 0.93 | 1.11 | 1.11 | 0.90 | 0.90 | 0.98 | 0.90 | 1.01 | 0.93 | 0.93 | 0.93 | 3.08 |
| Coverage | 0.292 | 0.292 | 0.292 | 0.301 | 0.360 | 0.292 | 0.292 | 0.301 | 0.301 | ||||||||||
| Consistency | 1 | 0.918 | 1 | 1 | 1 | 1 | 0.918 | 0.920 | 1 | ||||||||||
| Months /Conditions | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | PSS | |||||||||
| Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | ||
| Sep | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.90 | 0.90 | 0.65 | 0.65 | 0.86 | 0.86 | 0.95 |
| Oct | 0.05 | 0.05 | 0.05 | 0.05 | 0.92 | 0.92 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.52 |
| Nov | 0.05 | 0.05 | 0.05 | 0.05 | 0.12 | 0.12 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Dec | 0.13 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.50 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.13 | 0.05 | 0.13 | 0.05 | 0.95 |
| Jan | 0.05 | 0.05 | 0.05 | 0.05 | 0.20 | 0.09 | 0.05 | 0.05 | 0.05 | 0.05 | 0.08 | 0.08 | 0.05 | 0.05 | 0.08 | 0.08 | 0.05 | 0.05 | 0.14 |
| Feb | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.47 |
| SUM Fuzzy Membership | 1.19 | 1.11 | 1.11 | 1.11 | 2.2 | 2.09 | 1.35 | 0.90 | 0.91 | 0.91 | 0.93 | 0.93 | 1.15 | 1.15 | 1.01 | 0.93 | 1.19 | 1.11 | 3.08 |
| Coverage | 0.360 | 0.360 | 0.678 | 0.292 | 0.295 | 0.301 | 0.373 | 0.301 | 0.360 | ||||||||||
| Consistency | 0.932 | 1 | 0.95 | 0.666 | 1 | 1 | 1 | 0.920 | 0.932 | ||||||||||
| Months /Conditions | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | PSS | ||||||||||
| Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | Callib. | Min | ||||
| Sep | 0.86 | 0.86 | 0.86 | 0.86 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.95 | 0.90 | 0.90 | 0.90 | 0.95 | ||
| Oct | 0.92 | 0.92 | 0.92 | 0.92 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.94 | 0.93 | 0.52 | ||
| Nov | 0.12 | 0.12 | 0.43 | 0.43 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.12 | 0.12 | 0.05 | ||
| Dec | 0.13 | 0.05 | 0.05 | 0.05 | 0.95 | 0.05 | 0.50 | 0.05 | 0.05 | 0.05 | 0.13 | 0.05 | 0.50 | 0.05 | 0.05 | 0.05 | 0.95 | ||
| Jan | 0.20 | 0.09 | 0.20 | 0.09 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.48 | 0.09 | 0.14 | ||
| Feb | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.05 | 0.05 | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.47 | ||
| SUM Fuzzy Membership | 2.28 | 2.09 | 2.51 | 2.40 | 1.84 | 0.94 | 1.38 | 0.93 | 0.94 | 0.94 | 1.01 | 0.93 | 1.68 | 1.18 | 2.54 | 2.14 | 3.08 | ||
| Coverage | 0.678 | 0.779 | 0.305 | 0.301 | 0.305 | 0.301 | 0.383 | 0.694 | |||||||||||
| Consistency | 0.916 | 0.956 | 0.510 | 0.673 | 1 | 0.920 | 0.702 | 0.842 | |||||||||||
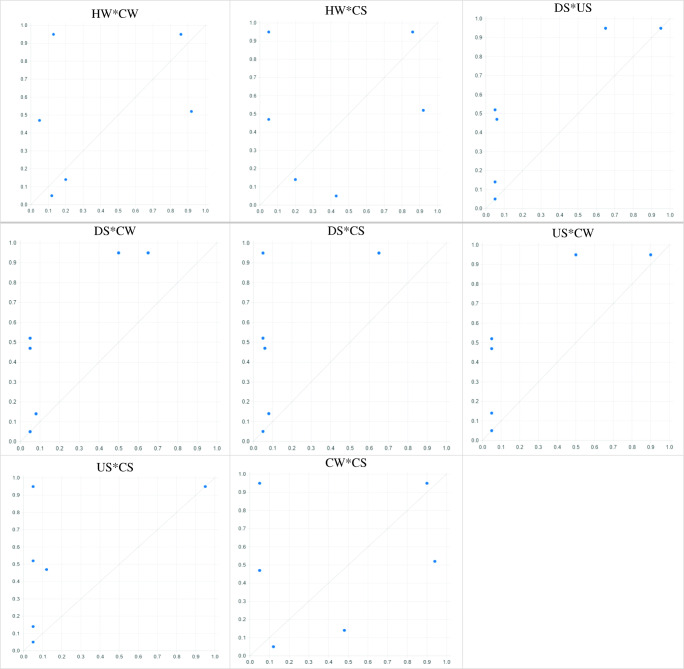
(1) HW*DS*US*CW*CS, (2) HW*DS*US*CW, (3) HW*DS*US*CS, (4) HW*DS*CW*CS, (5) HW*US*CW*CS, (6) DS*US*CW*CS, (7) HW*DS*US, (8) HW*DS*CW, (9) HW*DS*CS, (10) HW*US*CW, 11. HW*US*CS, 12. HW*CW*CS, 13. DS*US*CW, 14. DS*US*CS, 15. DS*CW*CS, 16. US*CW*CS, 17. HW*DS, 18. HW*US, 19. HW*CW, 20. HW*CS, 21. DS*US, 22. DS*CW, 23. DS*CS, 24. US*CW, 25. US*CS, 26. CW*CS
HW [Hospital Waste], US [Upstream Waste], DS [Downstream Waste], CW [Chicken Waste], CS [Chicken Sample], and PSS [People Suspected of Septicemia]
In addition, the results of necessary condition and subset and superset analysis showed that in any arrangement of independent variables, if there is a contamination in hospital sewage and chicken samples, there will also be contamination with ESBL E. coli in people suspected of septicemia, the consistency and coverage of which is 1 and above 0.20, respectively. Therefore, hospital sewage and chicken sample contamination with ESBL E. coli is a necessary cause for the presence of this infection in people suspected of septicemia. After combining different modes of independent variables with each other and examining the results of the truth table, the results showed that contamination of hospital wastewater and contamination of chicken samples with ESBL E.coli alone at the same time in a community can cause the outcome of ESBL E. coli infection in people suspected of septicemia (Figs. 3 and 4; Tables 3 and 4). For example, in the cases of or , consistency was close to 1 and the coverage was higher than 0.20, while in the cases of or consistency was less than 1, due to the non-contamination of the 2 necessary causes (hospital sewage, chicken sample) (Tables 3 and 4).
Fig. 3.
XY plot of all sufficiency causes on occurrence of ESBL E. coli in people suspected of septicemia (B)
Fig. 4.
XY plot of all sufficiency causes on occurrence of ESBL E. coli in people suspected of septicemia (C)
Table 4.
The sufficiency causes, necessary, and sufficiency causes on ESBL E. coli in people suspected of septicemia
| Outcome | Sufficiency Causes | Necessary and Sufficiency Causes |
|---|---|---|
| ESBL E. coli in Patients with Septicemia Suspected | DS | HW*DS*US*CW*CS |
| US | HW*DS*US*CS | |
| HW*DS*US*CW | HW*DS*CW*CS | |
| HW*DS*CW*CS | HW*US*CW*CS | |
| HW*DS*US | DS*US*CW*CS | |
| HW*DS*CW | HW*DS*CS | |
| HW*DS*CS | HW*US*CS | |
| HW*US*CW | DS*US*CS | |
| HW*US*CS | DS*CW*CS | |
| US*CW*CS | ||
| DS*CS |
HW [Hospital Waste], US [Upstream Waste], DS [Downstream Waste], CW [Chicken Waste], CS [Chicken Sample], and PSS [People Suspected of Septicemia]
Discussion
E. coli is a gram-negative bacterium that plays a clinically important role in hospital and urinary tract infections. The overuse of antibiotics in recent decades has increased the emergence of resistant strains with multiple antibiotic resistance in gram-negative intestinal bacteria, especially E. coli, so that these bacteria produce ESBLs and develop resistance to antibiotics such as third- generation and fourth-generation cephalosporins, penicillin, ciprofloxacin, and cefotaxime. The presence of β-lactamase-encoding gene and its transmission among gram-negative intestinal bacteria is a major threat to consumers of extended-spectrum cephalosporins. The aim of this study was to determine the relationship between the prevalence of ESBL E. coli in the environment (hospital wastewater, downstream and upstream urban wastewater, slaughterhouse wastewater) and the food chains (chicken sample) on the one side and its prevalence in people suspected of septicemia based on fsQCA on the other. fsQCA is one of the techniques of causal analysis that uses Boolean algebra and inductive logic to determine the existing causal relationships [24, 25]. Drawing on Boolean algebra and inductive logic, the model help to determine the necessary causes, sufficiency causes, or necessary and sufficiency causes affecting the outcome [23, 25]. It can also determine causal relationships with an average sample size at the lowest cost and time in medical and health sciences. In medical and clinical research, researchers are more interested in investigating the effect of independent variables or causes on the occurrence of different outcomes, such as the incidence of a disease or its complications by using a balanced sample size and low cost at the most appropriate time, which makes this model highly effective. This model has often been used in social sciences but less in medical sciences. The results of this study can help to further use this model in the field of medicine to determine the necessary and sufficient causes in the occurrence of various diseases. The results of this study showed the contamination of downstream and upstream urban sewage with ESBL E. coli is a sufficient cause for predicting its prevalence in people suspected of septicemia. This means that contamination of downstream or upstream urban sewers with ESBL E. coli alone is a sufficient cause to predict ESBL E. coli spreading to people suspected of septicemia. Even without this contamination, people suspected of septicemia may get infected with ESBL E. coli from other contaminated sources. Aquatic environments, especially wastewater, are the main recipients of intestinal bacteria, especially E. coli. On the other hand, they are a good place for many of these bacteria to become resistant to various types of antibiotics [26]. In such an environment, the resistant genes transfer well between different bacterial species due to higher levels of food and microbial loads [27]. In this study, the results showed hospital sewage and chicken sample contamination with ESBL E. coli is a necessary cause of this infection in people suspected of septicemia. The reason for hospital wastewater being a necessary cause but urban wastewater being a sufficient cause for this infection in people suspected of septicemia is the higher antibiotic resistance in hospital wastewater compared to urban sewage [28]. Also, the high concentration and diversity of antibiotics in hospital wastewater increases the chances of bacteria contacting antibiotics and creating resistant species, which increases the transmission of resistant genes between bacteria, especially E. coli [29]. Natural water environments provide a better selective advantage in making fecal coliforms resistant to antibiotics than soil, sand, and sewage. On the other hand, most wastewater treatment plants with conventional biological processes are not designed to remove highly polar pollutants, such as antibiotics, so they can easily spread to water resources [30]. Even wastewater treatment processes play a role in the selective increase of antibiotic-resistant bacteria [31]. In a study on bacteria found in hospital and urban wastewater, it was found that bacteria resistant to vancomycin and ciprofloxacin were significantly more common in hospital wastewater than in urban wastewater. It was also found that the range of antibiotic resistance bacteria in hospital and urban wastewater corresponds with clinical isolates. High consumption of antibiotics in hospitals and their entry into the sewer can be a factor in selective pressure on bacteria and their resistance [32]. Many studies have shown that the concentration of various antibiotics in hospital wastewater can reach more than 100 µg/L, which is a good reason for hospital wastewater contamination with ESBL E. coli being necessary to induce infection in people suspected of septicemia [33]. Also, hospital wastewater is the result of sanitary water use in hospital wards, operating rooms, laboratories, office units, laundry rooms, and hospital restaurants [34]. It is usually discharged directly or through incomplete treatment to the municipal sewer system, which contains a wide diversity of toxic pollutants, such as antibiotic, solvents, disinfectants, and radioactive materials [35]. These sewers are 5 to 15 times more toxic than municipal sewers. In addition to toxic chemical compounds, a variety of antibiotic-resistant pathogenic microbial agents are discharged into water ecosystems through hospital wastewater, which can contribute to the diversity of antibiotic-resistant strains in this source [36]. Mohammadi Mehr et al. conducted a study on gram-negative bacteria responsible for hospital infections in several hospitals in Tehran, with the highest frequency being related to E. coli (36%) [37]. Also, E. coli isolates isolated from the samples showed more than 70% resistance to 4 antibiotics. The important point of this study was that more than 70% of the isolates were resistant to at least 3 types of antibiotics: amoxiclav, ampicillin, and methicillin [37]. In a 2012 study by Dehghanzadeh et al., the results showed that the resistance of E. coli in wastewater isolates to β-lactam antibiotics was 50–72% [38]. In another study on clinical samples from a hospital in 2011, this resistance was about 40%. A study conducted by Hadi et al. showed that the rate of antibiotic resistance in bacteria isolated from hospital wastewater was higher than those isolated from municipal wastewater [39].
Conclusions
The results of this study showed that the contamination of hospital wastewater in the environmental chain and the contamination of chicken samples in the food chain are a necessary cause for existing the ESBL E. coli infection in people suspected of septicemia. If these infections are present in any of the independent cases, that case has a high consistency percentage for the creation of the desired outcome, i.e., the induction of ESBL E. coli infection in people suspected of septicemia. Therefore, if there is contamination of hospital wastewater and chicken in an area, it can be claimed that people suspected of septicemia are almost infected with ESBL E. coli and the percentage of this contamination can be high. Controlling ESBL E. coli in hospital wastewater (environmental chain) and chicken (food chain) can prevent contamination in people with suspected septicemia.
Acknowledgements
This paper was derived from a PhD thesis in the Department of Epidemiology, School of Public Health, Iran Medical Sciences University.
Author contributions
YM, BE, and HB contributed to the conception and design of the study, interpretation of data, literature review, modeling, and writing and revising the manuscript. SAM and AM contributed to the acquisition of data. MB and SSM performed all the microbial assays.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest. This study was supported by the Deputy of Research and Technology of Iran University of Medical Sciences, Tehran, Iran (Grant no. 15,396 and ethical code: IR.IUMS.REC.1398.679).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamid Reza Baradaran, Email: baradaran.hr@iums.ac.ir.
Yousef Moradi, Email: Yousefmoradi211@yahoo.com.
References
- 1.Wall S. Prevention of antibiotic resistance–an epidemiological scoping review to identify research categories and knowledge gaps. Glob Health Action. 2019;12(sup1):1756191. doi: 10.1080/16549716.2020.1756191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RA, M’ikanatha NM, Read AF. Antibiotic resistance: a primer and call to action. Health Commun. 2015;30(3):309–14. doi: 10.1080/10410236.2014.943634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J-S, Ni C-F, Liang C-P, Chiang C-C. Analytical power series solution for contaminant transport with hyperbolic asymptotic distance-dependent dispersivity. J Hydrol. 2008;362(1–2):142–9. doi: 10.1016/j.jhydrol.2008.08.020. [DOI] [Google Scholar]
- 4.Chen J-S, Chen J-T, Liu C-W, Liang C-P, Lin C-W. Analytical solutions to two-dimensional advection–dispersion equation in cylindrical coordinates in finite domain subject to first-and third-type inlet boundary conditions. J Hydrol. 2011;405(3–4):522–31. doi: 10.1016/j.jhydrol.2011.06.002. [DOI] [Google Scholar]
- 5.Escudero E, Vinue L, Teshager T, Torres C, Moreno M. Resistance mechanisms and farm-level distribution of fecal Escherichia coli isolates resistant to extended-spectrum cephalosporins in pigs in Spain. Res Vet Sci. 2010;88(1):83–7. doi: 10.1016/j.rvsc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Blanc V, Mesa R, Saco M, Lavilla S, Prats G, Miró E, et al. ESBL-and plasmidic class C β-lactamase-producing E. coli strains isolated from poultry, pig and rabbit farms. Vet Microbiol. 2006;118(3–4):299–304. [DOI] [PubMed]
- 7.Bortolaia V, Guardabassi L, Trevisani M, Bisgaard M, Venturi L, Bojesen AM. High diversity of extended-spectrum β-lactamases in Escherichia coli isolates from Italian broiler flocks. Antimicrob Agents Chemother. 2010;54(4):1623–6. doi: 10.1128/AAC.01361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randall L, Clouting C, Horton R, Coldham N, Wu G, Clifton-Hadley F, et al. Prevalence of Escherichia coli carrying extended-spectrum β-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J Antimicrob Chemother. 2011;66(1):86–95. doi: 10.1093/jac/dkq396. [DOI] [PubMed] [Google Scholar]
- 9.Girlich D, Poirel L, Carattoli A, Kempf I, Lartigue M-F, Bertini A, et al. Extended-spectrum β-lactamase CTX-M-1 in Escherichia coli isolates from healthy poultry in France. Appl Environ Microbiol. 2007;73(14):4681–5. doi: 10.1128/AEM.02491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves A, Torres C, Silva N, Carneiro C, Radhouani H, Coelho C, et al. Genetic characterization of extended-spectrum beta-lactamases in Escherichia coli isolates of pigs from a Portuguese intensive swine farm. Foodborne Pathog Dis. 2010;7(12):1569–73. doi: 10.1089/fpd.2010.0598. [DOI] [PubMed] [Google Scholar]
- 11.Aminzadeh Z, Kashi MS, Shaabani M. Bacteriuria by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates in a governmental hospital in south of Tehran, Iran. 2008. [PubMed]
- 12.Maxwell JA. Using qualitative methods for causal explanation. Field Methods. 2004;16(3):243–64. doi: 10.1177/1525822X04266831. [DOI] [Google Scholar]
- 13.Miethe TD, Drass KA. Exploring the social context of instrumental and expressive homicides: An application of qualitative comparative analysis. J Quant Criminol. 1999;15(1):1–21. doi: 10.1023/A:1007591025837. [DOI] [Google Scholar]
- 14.Schweizer T. Actor and event orderings across time: Lattice representation and Boolean analysis of the political disputes in Chen Village, China. Soc Netw. 1996;18(3):247–66. doi: 10.1016/0378-8733(95)00276-6. [DOI] [Google Scholar]
- 15.Aspinall LJ, Kilsby DC. A microbiological quality control procedure based on tube counts. J Appl Bacteriol. 1979;46(2):325–9.
- 16.Zhi S, Banting G, Stothard P, Ashbolt NJ, Checkley S, Meyer K, et al. Evidence for the evolution, clonal expansion and global dissemination of water treatment-resistant naturalized strains of Escherichia coli in wastewater. Water Res. 2019;156:208–22. doi: 10.1016/j.watres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Uzoechi SC, Abu-Lail NI. Changes in cellular elasticities and conformational properties of bacterial surface biopolymers of multidrug-resistant Escherichia coli (MDR-E. coli) strains in response to ampicillin. Cell Surf. 2019;5:100019. [DOI] [PMC free article] [PubMed]
- 18.Sahu C, Jain V, Mishra P, Prasad KN. Clinical and laboratory standards institute versus European committee for antimicrobial susceptibility testing guidelines for interpretation of carbapenem antimicrobial susceptibility results for Escherichia coli in urinary tract infection (UTI) J Lab Phys. 2018;10(3):289. doi: 10.4103/JLP.JLP_176_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez VP, Carvalho JK, de Oliveira MS, Rossato AM, Dani C, Corção G, d’Azevedo PA. Coagulase-negative staphylococci in outpatient routines: the implications of switching from CLSI to BrCAST/EUCAST guidelines. Braz J Microbiol. 2020;23:1–8. [DOI] [PMC free article] [PubMed]
- 20.Ragin CC, Berg-Schlosser D, De Meur G. Political methodology: Qualitative methods. A new handbook of political science. 1996;10:749–68.
- 21.Douglas EJ, Shepherd DA, Prentice C. Using fuzzy-set qualitative comparative analysis for a finer-grained understanding of entrepreneurship. J Bus Ventur. 2020;35(1):105970. doi: 10.1016/j.jbusvent.2019.105970. [DOI] [Google Scholar]
- 22.de Guinea AO, Raymond L. Enabling innovation in the face of uncertainty through IT ambidexterity: A fuzzy set qualitative comparative analysis of industrial service SMEs. Int J Inf Manag. 2020;50:244–60. doi: 10.1016/j.ijinfomgt.2019.05.007. [DOI] [Google Scholar]
- 23.Ragin CC. Qualitative comparative analysis using fuzzy sets (fsQCA). Configurational comparative methods: Qualitative comparative analysis (QCA) and related techniques. 2009;51:87–121.
- 24.Berg-Schlosser D, De Meur G, Rihoux B, Ragin CC. Qualitative comparative analysis (QCA) as an approach. Configurational comparative methods: Qualitative comparative analysis (QCA) and related techniques. 2009;1:18.
- 25.Ragin CC. The comparative method: Moving beyond qualitative and quantitative strategies. Berkeley: Univ of California Press; 2014.
- 26.Mudryk ZJ. Occurrence and distribution antibiotic resistance of heterotrophic bacteria isolated from a marine beach. Mar Pollut Bull. 2005;50(1):80–6. doi: 10.1016/j.marpolbul.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Iversen A, Kühn I, Franklin A, Möllby R. High prevalence of vancomycin-resistant enterococci in Swedish sewage. Appl Environ Microbiol. 2002;68(6):2838–42. doi: 10.1128/AEM.68.6.2838-2842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices–a review. J Environ Manag. 2011;92(10):2304–47. doi: 10.1016/j.jenvman.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Thomas KV, Bijlsma L, Castiglioni S, Covaci A, Emke E, Grabic R, et al. Comparing illicit drug use in 19 European cities through sewage analysis. Sci Total Environ. 2012;432:432–9. doi: 10.1016/j.scitotenv.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 30.Varela AR, Ferro G, Vredenburg J, Yanık M, Vieira L, Rizzo L, et al. Vancomycin resistant enterococci: from the hospital effluent to the urban wastewater treatment plant. Sci Total Environ. 2013;450:155–61. doi: 10.1016/j.scitotenv.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Al-Ahmad A, Daschner F, Kümmerer K. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch Environ Contam Toxicol. 1999;37(2):158–63. doi: 10.1007/s002449900501. [DOI] [PubMed] [Google Scholar]
- 32.Kümmerer K. Significance of antibiotics in the environment. J Antimicrob Chemother. 2003;52(1):5–7. doi: 10.1093/jac/dkg293. [DOI] [PubMed] [Google Scholar]
- 33.Kümmerer K. Resistance in the environment. J Antimicrob Chemother. 2004;54(2):311–20. doi: 10.1093/jac/dkh325. [DOI] [PubMed] [Google Scholar]
- 34.Kümmerer K, Alexy R, Hüttig J, Schöll A. Standardized tests fail to assess the effects of antibiotics on environmental bacteria. Water Res. 2004;38(8):2111–6. doi: 10.1016/j.watres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Guerrero JP, Skaggs T. Analytical solution for one-dimensional advection–dispersion transport equation with distance-dependent coefficients. J Hydrol. 2010;390(1–2):57–65. doi: 10.1016/j.jhydrol.2010.06.030. [DOI] [Google Scholar]
- 36.Alexy R, Sommer A, Lange FT, Kümmerer K. Local use of antibiotics and their input and fate in a small sewage treatment plant–significance of balancing and analysis on a local scale vs. nationwide scale. Acta Hydrochim Hydrobiol. 2006;34(6):587–92.
- 37.Dehghanzadeh RR, Roshani M. Determination of antibiotic farshchianm. resistance spectrum in enterobacteriaceae andstaphylococcus bacteria isolated from hospital wastewaters in Tabriz, 2015. 2018.
- 38.Reihani RD, Roshani M, Farshchian MR. Determination of Antibiotic Resistance Spectrum in Enterobacteriaceae and Staphylococcus Bacteria Isolated from Hospital Wastewaters in Tabriz, 2015. Majallah-i pizishki-i Danishgah-i Ulum-i Pizishki va Khadamat-i Bihdashti-i Darmani-i Tabriz. 2018;40(4):24–30.
- 39.Hadi M, Shokoohi R, Ebrahimzadeh Namvar A, Karimi M, Solaimany Aminabad M. Antibiotic resistance of isolated bacteria from urban and hospital wastewaters in Hamadan City. Iran J Health Environ. 2011;4(1):105–14. [Google Scholar]