Abstract
It has been proven that exposure to bioaerosols is associated with several health effects, such as pulmonary diseases and allergies. The present cross-sectional study was aimed to investigate fungal contamination in indoor air and on the surfaces of four traditional baths in Shiraz, Iran, one of the most historical cities in the world. Samples were taken from indoor air, using a microbial air sampler, as well as the surfaces of the shower, hallway, and dressing rooms of studied baths for 3 months. Totally 180 samples, including 45 air and 135 surfaces samples, were collected from studied baths. The concentrations of fungi collected from the air of studied baths were ranged from 22.6 to 34.6 CFU/m3. Besides, the levels of fungi collected from the surface samples of studied baths were ranged from 21.2 to 60 CFU/m2. The highest and lowest fungi species detected both in air and surfaces samples of the studied baths were Penicillium spp. and Mucor spp. respectively. Although the levels of fungi in the studied baths were lower than the levels recommended by the World Health Organization, some environmental health measures such as washing and disinfecting surfaces and tools after each working shift and periodic inspections are recommended ensuring the safety of costumers who are visiting such places.
Keywords: Bioaerosol, Bath, Contamination, Fungi, Indoor air
Introduction
In recent decades, several studies have shown the relationship between air pollution and various adverse effects on human health such as cardiovascular disease, cancers, and lung impairments [1, 2]. Nowadays, people spend approximately 80–90% of their time in indoor environments. As a result, indoor air quality is of paramount importance for human health. Many different sources such as the type of building materials, air conditioning, air exchange rate and activity of people affect indoor air quality. In addition, indoor air is affected by other factors such as outdoor air and the existence of chemical, physical and biological contaminants [3]. A mixture of living and non-living particles can be found in indoor air as pollutants. The non-living particles contain organic and inorganic constitutes, including various chemical compounds, and metals and living particles are the ones that can grow under the desirable conditions, like fungi, bacteria, and all other microorganisms [4].
Fungi can usually cause indoor air pollution through the heating system, air conditioning, windows and doors [5]. Humidity, ventilation, temperature, organic matters in building materials, and outdoor fungal loads are factors influencing the growth of indoor fungal contamination [6–8]. Today, more than 300 species of pathogenic fungi have been identified [9].
Some fungal species, including Aspergillus, Penicillium, and Fusarium produce mycotoxins that can enter the human body through inhalation and dermal contact, causing various reactions and symptoms in human [4]. Each fungus species has a unique combination of antigenic and allergy components that can cause cough, wheezing, eye and throat irritation, skin rash, diarrhea, nasal obstruction, headache, and vomiting [10, 11], increased risk of asthma [12] and exacerbated asthma [10, 13]. Besides, fungal pathogens are one of the known causes of skin infections. It has been reported that more than 25% of the world population is affected by these factors [14]. Studies have shown that the presence of fungal species such as Alternaria, Aspergillus, and Cladosporium in the indoor environment can cause rhinitis and allergic asthma [15].
Since most types of fungi are widely found in humid places such as pools, saunas, baths and especially public baths [16–18], so skin fungal infections can occur due to contact of the body with the surfaces and different facilities of these places [19]. In addition, high temperature and humidity in these places are major factors in fungal growth. Therefore, it is more likely for fungal infections to be transferred in traditional and public baths [14, 20, 21].
Traditional baths are among the indoor environments that have been important in terms of fungal contamination. These places are not only used for personal hygiene but also have been regarded as cultural heritage for long periods, so considering health issues in these unique places is important. Traditional bathrooms consist of a several rooms including dressing room, shower room and hallway room [19, 22–24].
Previous studies have investigated fungal air quality in various indoor environments. For example, the average concentration of airborne fungi in the indoor air of 10 hospitals were reported 19 ± 19 CFU/m3 [25]. Another study that assessed indoor air of a historical museum, the concentrations of fungi varied from 47 to 784 CFU/m3 [26]. In addition, The results of a study conducted to investigate the levels of fungal species on surfaces in traditional baths in Algeria showed that the predominant fungi were Penicillium spp. and Aspergillus spp., respectively [27]. Numerous studies have also been conducted on fungal contamination in different public places, such as swimming pools [28], student dorm bath [29], and subway stations [30] in Iran. Concerning the health effects of exposure to fungi in traditional baths, this study was aimed to identify the types of fungal species in the air and surfaces of traditional baths in Shiraz, Iran.
Materials and methods
Characteristics and location of the study area
Shiraz, with a population of more than 1.8 million and the capital of Fars province, is the fifth most populous city of Iran. The city located in the southwest part of the country and has a moderate climate. It is also one of the oldest and the most historical cities in Iran and even the world. Petrochemical and electronics industries are among the most important economic activities in Shiraz, but tourism also contributes to the economy of this city [31, 32]. Samples from air and surfaces of four traditional baths located in areas with similar geographical conditions were collected for 6 months (from December to June 2017). In this study, we selected four baths with codes A, B, C, and D.
Surface sample collection
Samples were collected from surfaces of shower rooms, dressing rooms and hallway s by using cotton-tipped applicators pre-moistened with sterile saline. In each investigated point, three samples were collected with sterile swabs and a 10 × 10 cm adhesive template. Samples were placed in a test tube containing 10 ml normal saline and transported to the laboratory for examination. After blending, 0.1 ml of each sample were directly transferred to plates containing Sabouraud Dextrose Agar (SDA) plus 50 μg/mL of chloramphenicol, to suppress any bacterial growth [33, 34].
Air sampling
Air samples collection were carried out in respiratory height (about 1.5 m above the floor of bathrooms) for 5 min using a microbial air sampler (Quick Take-30, SKC, USA) at a flow rate of 28.3 L/min for 3 months. Sampling was performed between 10 a.m. and 2 p.m., and each point was sampled twice. Before sampling, the internal part of the sampler was cleaned with 70% alcohol. In order to examine airborne fungi, plates contained Sabouraud Dextrose Agar (SDA) supplemented with 50 μg/mL of chloramphenicol (QUELAB, USA) [30, 35–37].
Fungal identification
After sampling both air and surfaces, the samples were immediately transferred to the laboratory in cold boxes. The plates were incubated at 20–25 °C for 3–7 days, and the number of colonies on each plate were counted and reported as colony-forming units per cubic meter (CFU/m3) and per square centimeter (CFU/cm2) for air and surface samples respectively. The identification of fungal species was performed by using the method of slide culturing [38–40]. Fungal genera were then identified based upon their micro- and macro morphological characteristics according to the methods provided by Ripon [41] and [42] Soleimani.
Survey and questionnaire
During sampling, direct interviews were conducted with the owners of traditional baths, in order to gather information about cleaning frequency and products used for cleaning and disinfection.
Statistical analysis
Data were analyzed using SPSS version 16. The normality of the distribution of fungal concentrations was tested using the Shapiro–Wilk test. The differences in the mean values of the concentrations of fungi species among different baths as well as in different sampling points in the studied baths were tested using non-parametric Kruskal-Wallis H test. P-Values <0.05 were considered as statistically significant.
Results and discussion
Fungal concentrations in surface samples
Table 1 shows the mean concentrations of fungal species that were identified on the surfaces of different parts of the studied baths. All cultured samples were positive for fungal growth and contained at least one colony. The highest and lowest concentrations of fungal contamination on the surfaces of dressing rooms were determined in the baths C (60 ± 29.7 CFU/cm2) and B (22.5 ± 12.8 CFU/cm2), respectively. Regarding the shower rooms, the maximum fungal concentration was measured in the bath A (43.7 ± 30.2 CFU/cm2), and the minimum was recorded in bath B (26.2 ± 13 CFU/cm2). The highest and lowest fungal concentrations on the surfaces of hallway s were observed in baths D (56.2 ± 23.8 CFU/cm2( and C (21.2 ± 11.2 CFU/cm2) respectively. The highest and lowest fungal concentrations on the different surfaces of the studied baths were found in the dressing room and hallway of bathroom C, respectively.
Table 1.
Concentrations of fungi as CFU/cm2 on the surfaces of different parts of the baths
| Section | A | B | C | D | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | S.D | Max | Min | Mean | S.D | Max | Min | Mean | S.D | Max | Min | Mean | S.D | |
| Dressing room | 60 | 10 | 33.7 | 15.9 | 40 | 10 | 22.5 | 12.8 | 90 | 10 | 60 | 29.7 | 90 | 10 | 42.5 | 32.4 |
| Shower room | 90 | 10 | 43.7 | 30.2 | 50 | 10 | 26.2 | 13 | 70 | 10 | 31.2 | 23.5 | 70 | 10 | 31.2 | 22.9 |
| Hall | 50 | 10 | 30 | 16 | 50 | 10 | 26.2 | 13 | 40 | 10 | 21.2 | 11.2 | 80 | 10 | 56.2 | 23.8 |
Baths B and D were the cleanest and the most contaminated baths, respectively. There was a significant difference between the mean fungal levels in different baths (p < 0.05); however, the significant difference was not observed between the fungal levels in different parts of the studied baths (p > 0.05).
Traditional baths like other relatively similar places such as swimming pools and saunas are suitable environments for the growth of microorganisms, especially fungi, due to the temperature and humidity. In addition, the lack of hygiene of the visitors of these baths can lead to transmission of fungi from the human body to the surfaces of the bath, which increase the contamination of the surfaces of such places [27, 43, 44]. In most of the studied baths, fungal contamination was higher in the hallway than in the other parts of the bathrooms. Although daily cleaning and weekly disinfection were performed in these areas, these sections showed significant levels of fungal contamination that could be due to skin scraping and massage, which decrease the effect of disinfection. So, these places provide a suitable environment for the growth of fungal species [21, 27, 45]. Nevertheless, in this study, according to the information gathered by interview as well as the findings, except the bath D, the other three bathrooms had a low fungal level in their hallway, which can be due to regular washing, proper disinfection, hygiene inspections, and the low number of visitors.
A similar study conducted in Turkey showed that none of the samples collected from floors of the baths were contaminated with fungi and only 25, 12.5 and 6.2% of the samples collected from the surfaces of windows, walls and dressing rooms of the studied baths showed fungal contamination, respectively [19]. Some previous studies reported that the most widely identified fungi in baths were Scolecobasidium, Cladophialophora and Phoma species, which use soap and shampoo as nutrients [46]. In the present study; however, these types of fungi were not detected in the samples collected from the traditional baths. The predominant genera identified on the surfaces of the studied baths were the same as dominant species found in the air. Since the highest fungal contamination was observed on the surface of the dressing rooms, therefore, the fungi observed are expected to enter the baths through the clothing of people or air infiltration.
Fungal concentrations in air samples
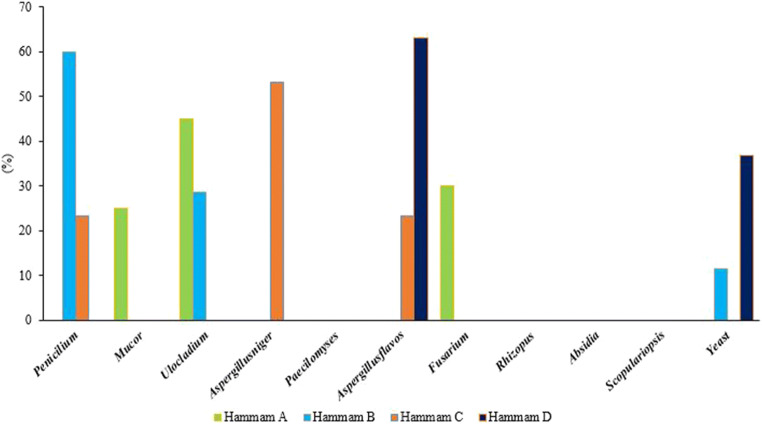
Levels of fungi in the air of the studied baths are shown in Fig. 1. The highest and lowest mean concentrations of airborne fungi were found in baths C (15.4 ± 8.8 CFU/m3) and D (8.4 ± 5.3 CFU/m3), respectively. According to the WHO recommendations, the mean concentration for any pathogenic and toxic gender of fungus in the indoor air should not exceed 50 CFU/m3. The corresponding value for a combination of fungal species is less than or equal to 150 CFU/m3 [47]. The average concentrations of fungi observed in the indoor air of the present study were lower than the level recommended by the WHO.
Fig. 1.
Fungal concentrations in the air of different baths
Several studies have been carried out on indoor fungal contamination in various settings, such as swimming pools [28], subway stations and public places. There are; however, limited studies on traditional public baths. So, the results of this study were compared with studies conducted in other places like swimming pools and other public places. The results of the present study showed that the highest mean fungal concentration in the air (15.4 ± 8.8 CFU/m3) was observed in the bath C. Results of a study conducted in a swimming pool revealed that total fungal loads in indoor air and the locker rooms of a swimming pool were 32.7 and 48.2 CFU/m3, respectively [16]. Another study reported the highest fungal concentrations on the surfaces of seven indoor swimming pools [48]. The results of the present study were not consistent with any of the similar studies. The differences between the detected fungus levels in the present study compared to those reported previously could be due to the type of disinfectant, the variation in the times of baths washing and disinfection, the differences in the number of visitors, the presence or absence of air conditioning and the differences in sampling condition. The levels of fungi species observed in the present study were lower than the WHO recommended concentrations. In addition, our findings were less than the levels of the fungi reported by the previous studies in the subway stations [30], public buildings [45], subway stations [30, 49] and a pediatric hospital [50]. Higher fungi levels in the previous studies compared to the present study could be due to high population density, poor ventilation and lack of proper hygiene.
Detection of fungal species on the surfaces and in the air of studied baths
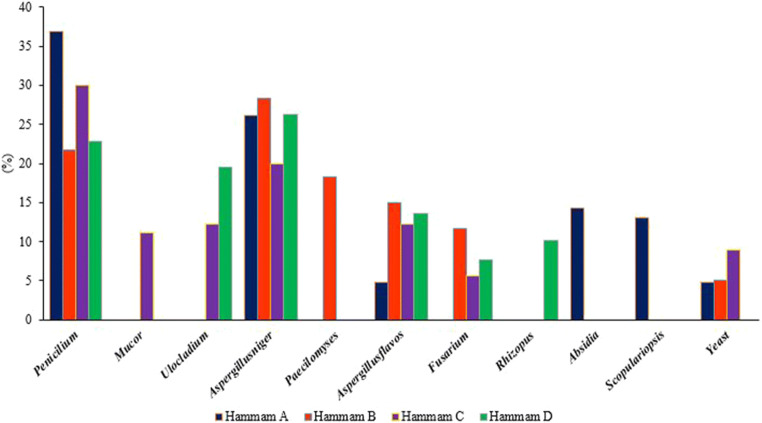
Tables 2 and 3 show the mean concentrations of different fungal species in the air and on the surfaces of different parts of the studied baths. In addition, the relative abundance of fungal species in the air and on the surfaces of the studied baths are presented in Figs. 2 and 3. The order of fungal species on surfaces of the studied baths was as follows: Penicillium (29%), Aspergillus niger (25.2%), respectively. In addition, the most dominant fungal species in the samples collected from the air of the studied baths were Penicillium (27%), Ulocladium (18.4%), and Aspergillus flavus (18.1%), respectively. Besides, unlike surfaces, fungal species such as Paecilomyces, Rhizopus, Absidia and Scopulariopsis were not found in the air samples. Moreover, the highest relative frequency of fungi was observed for Penicillium spp., which was detected in all collected samples from the studied baths. Also, 50% of the detected fungal species in the baths C and A belonged to this fungal. The lowest of fungi was found Mucor spp. had the lowest relative abundance among the fungal species, which found only in the shower room of the bath C and the indoor air of the bath A.
Table 2.
The mean concentrations of different fungal species CFU/m3 in the air of the studied bath
| Genus | Baths | |||
|---|---|---|---|---|
| A | B | C | D | |
| Penicillium spp. | 0 | 9.2 | 3 | 0 |
| Paecilomyces | 0 | 0 | 0 | 0 |
| Aspergillus niger. | 0 | 0 | 7 | 0 |
| Aspergillus flavus | 0 | 0 | 3 | 5.2 |
| Fusarium | 2.6 | 0 | 0 | 0 |
| Rhizopus | 0 | 0 | 0 | 0 |
| Absidia | 0 | 0 | 0 | 0 |
| Mucor | 2.2 | 0 | 0 | 0 |
| Scopulariopsis | 0 | 0 | 0 | 0 |
| Ulocladium | 3.9 | 4.4 | 0 | 0 |
| Yeasts | 0 | 1.7 | 0 | 3 |
Table 3.
The mean concentrations of different fungal species CFU/cm2 on the surfaces of the studied bath
| Baths | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | A | B | C | D | ||||||||
| Dressing room | Shower room | Hall | Dressing room | Shower room | Hall | Dressing room | Shower room | Hall | Dressing room | Shower room | Hall | |
| Penicillium spp. | 17.5 | 15 | 8.7 | 7.5 | 8.7 | 0 | 23.7 | 0 | 10 | 15 | 0 | 18.7 |
| Paecilomyces | 0 | 0 | 0 | 0 | 13.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus niger. | 15 | 0 | 11.2 | 10 | 0 | 11.2 | 22.5 | 0 | 0 | 18.7 | 20 | 0 |
| Aspergillus flavus | 0 | 0 | 5 | 5 | 0 | 6.2 | 12.5 | 0 | 0 | 20 | 0 | 0 |
| Fusarium | 0 | 0 | 0 | 0 | 0 | 8.7 | 0 | 0 | 6.2 | 0 | 11.2 | 0 |
| Rhizopus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
| Absidia | 0 | 11.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.2 | 0 | 0 | 0 | 0 |
| Scopulariopsis | 0 | 13.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ulocladium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13.7 | 0 | 0 | 0 | 30 |
| Yeasts | 0 | 0 | 5 | 0 | 3.7 | 0 | 0 | 5 | 5 | 0 | 0 | 0 |
Fig. 2.
Frequency of fungal species in the air of different baths
Fig. 3.
Frequency of fungal species in the samples collected from surfaces in different baths
In the bath C, the highest and lowest fungal species found in the surfaces were Penicillium spp. (30%) and Fusarium (5.5%), respectively. Also, the highest and lowest fungal species in the air of this bath were Aspergillus niger, Aspergillus flavus, and Penicillium, respectively. Paecilomyces, Rhizopus, Absidia and Scopulariopsis species were not found in the samples collected from this bath. In the bath B, Penicillium (60%) and Aspergillus niger (28.3%) were the most dominate fungal genera in the air and on the surfaces, respectively.
The most abundant molds in Algerian traditional baths were Penicillium (45.1%) and Aspergillus (28.8%) species, respectively [27]. In addition, in a study conducted in baths in Japan, the most common fungal species were Cladosporium, Penicillium and Aspergillus, respectively. They also reported a significant relationship between the fungal flora in the air and the surfaces of the studied baths [20]. Penicillium and Aspergillus were found in most baths because its spores could be transmitted through the air [51]. In addition, studies conducted in Japan and Algerian reported penicillium as the most abundant fungi species in the baths [20, 27]. Other major species found in previous studies were Mucor, Fusarium, Rhizopus, Ulocladium and yeast, which are similar to the results of our study [27, 28]. The lower concentration of yeast observed in the present study may be due to the difficulty of sampling and isolating the yeast from the sample. Another reason for this can be the antifungal activity of some species such as Aspergillus flavus, which has to present a significant activity against the growth of yeasts [27].
In a study conducted in Italian indoor swimming pool, Fusarium was the most dominant isolated genus from surfaces samples [16], which is in contradicts with our findings. This can be due to differences in the number of collected samples, the number of locations and period of investigation [52]. In another study performed in the bathroom of student dormitories in Iran, the most common isolated fungal species were Cladosporium (28.9%), Exophiala (20.3%) and Rhodotorula (13.7%), respectively [29], which is inconsistent with the results of the present study. This difference may be due to the higher number of people in the dormitories and the more frequent use of these baths. Although Penicillium and Aspergillus were the most prevalent fungal species in the present study, in another study performed in the swimming pools, the fungi such as Alternaria, Cladosporium, Phytophthora and Trichophyton mentagrophytes were reported as the most prevalent fungal species [53]. In fact, Aspergillus fungi species are thermophilic and grow well at 20–50 °C. However, temperature and humidity are not the only determinants of fungal growth in places like bathrooms, as there are other factors such as cleaning methods, type of used disinfectants, times of washing and disinfection, natural or artificial ventilation, and the density of the clients that can influence the growth of fungal colonies in such places [19]. In addition, previous studies reported Cladosporium. spp as the most common fungi species in indoor environments after Penicillium. Spp and Aspergillus. Spp, which is inconsistent with our findings. Previous studies reported Cladosporium in wet building material such as gypsum board, acrylic painted wood, walls, wallpaper, carpet and mattress dust which are not presented in the baths. In addition, Cladosporium can be easily treated with strong disinfectants such as vinegar or bleach [54]. Therefore, the possible reason for not detecting Cladosporium in this study could be due to the frequent disinfection of baths and the absence of carpets, wallpaper and wood. Although this study provides the first set of ranges of the concentration of fungal contamination in air and surfaces reported at the indoor of traditional baths of Shiraz which can be used for comparative purposes in future studies, however, this has some limitations. Since outdoor fungi sources could impact significantly on the indoor fungi levels, future studies can be conducted to investigate the effect of outdoor sources on the fungi levels in places like public baths [55, 56].
Conclusions
This study investigated the levels of fungal species in the air and surfaces of traditional baths in Shiraz, Iran. Although all samples taken from the air and surfaces were positive in this study, the observed fungi levels were lower than the recommended values provided by the WHO. The highest frequency of fungal related to Penicillium and Aspergillus species which were ranged from 31 to 50%. These species can cause allergies in susceptible individuals, especially those with asthma. However, in this study, fungal contamination in baths was lower than the levels recommended by WHO and lower than in other public places such as pools. In bathrooms with poor sanitation, people are at risk for fungal contamination. Therefore, to prevent fungal infections among the individuals who visit the baths, some personal hygiene and environmental health measures should be applied such as the use of personal or disposable slippers, washing and disinfecting surfaces and tools after each working shift. In addition, periodic inspections of the public bathrooms along with regular disinfection, are recommended ensuring the safety of costumers who are visiting such places. More studies are needed to investigate other relevant parameters that influence fungal diversity in baths and to suggest control methods against fungal contamination in such indoor environment which can impose adverse health effects.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neisi A, Vosoughi M, Idani E, Goudarzi G, Takdastan A, Babaei AA, Ankali KA, Hazrati S, Shoshtari MH, Mirr I, Maleki H. Comparison of normal and dusty day impacts on fractional exhaled nitric oxide and lung function in healthy children in Ahvaz, Iran. Environ Sci Pollut Res. 2017;24(13):12360–12371. doi: 10.1007/s11356-017-8853-4. [DOI] [PubMed] [Google Scholar]
- 2.Dianat M, Radmanesh E, Badavi M, Goudarzi G, Mard SA. The effects of PM 10 on electrocardiogram parameters, blood pressure and oxidative stress in healthy rats: the protective effects of vanillic acid. Environ Sci Pollut Res. 2016;23(19):19551–19560. doi: 10.1007/s11356-016-7168-1. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein JA, Alexis N, Bacchus H, Bernstein IL, Fritz P, Horner E, Li N, Mason S, Nel A, Oullette J, Reijula K, Reponen T, Seltzer J, Smith A, Tarlo SM. The health effects of non-industrial indoor air pollution. J Allergy Clin Immunol. 2008;121(3):585–591. doi: 10.1016/j.jaci.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 4.Ramos CA, Viegas C, Verde SC, Wolterbeek HT, Almeida SM. Characterizing the fungal and bacterial microflora and concentrations in fitness centres. Indoor Built Environ. 2015;25(6):872–882. [Google Scholar]
- 5.Brągoszewska E. Exposure to bacterial and fungal aerosols: microorganism indices in a waste-sorting Plant in Poland. Int J Environ Res Public Health. 2019;16(18):3308. doi: 10.3390/ijerph16183308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medrela-Kuder E. Seasonal variations in the occurrence of culturable airborne fungi in outdoor and indoor air in Crac??w. Int Biodeterior Biodegradation. 2003;52:203–205. [Google Scholar]
- 7.Oliveira M, Ribeiro H, Abreu I. Annual variation of fungal spores in atmosphere of Porto: 2003. Ann Agric Environ Med. 2005;12(2):309–315. [PubMed] [Google Scholar]
- 8.Sepahvand A, Azimi F, Hashemi SY, Rashidi R, Safari M, Zeidali S. General hospitals indoor air quality in Lorestan, Iran. J Air Pollut Health. 2017;2(1).
- 9.Garcia-Solache MA, Casadevall A. Global warming will bring new fungal diseases for mammals. mBio. 2010;1(1):e00061–e00010. doi: 10.1128/mBio.00061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect. 2011;119(6):748–756. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahkali P. Fungal flora in house dust in Riyadh. Saudi Arabia Mycoses. 1999;42(4):339–343. doi: 10.1046/j.1439-0507.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 12.Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS One. 2012;7(11):e47526. doi: 10.1371/journal.pone.0047526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17(4):284–296. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 14.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 15.Menezes EA, Carvalho PG, Trindade ECPM, Madeira Sobrinho G, Cunha FA, Castro FFM. Airborne fungi causing respiratory allergy in patients from Fortaleza, Ceará, Brazil. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2004;40(2):79–84. [Google Scholar]
- 16.Brandi G, Sisti M, Paparini A, Gianfranceschi G, Schiavano GF, De Santi M, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17(3):197–206. doi: 10.1080/09603120701254862. [DOI] [PubMed] [Google Scholar]
- 17.Chabasse D, Pihet M, Bouchara J-P. Émergence de nouveaux champignons pathogènes en médecine : revue générale. Revue Francophone des Laboratoires. 2009;2009(416):71–86. [Google Scholar]
- 18.Matos T, De Hoog G, De Boer A, De Crom I, Haase G. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses. 2002;45(9–10):373–377. doi: 10.1046/j.1439-0507.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 19.Goksugur N, Karabay O, Kocoglu E. Mycological flora of the Hammams, traditional Turkish bath. Mycoses. 2006;49(5):411–414. doi: 10.1111/j.1439-0507.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 20.Moriyama Y, Nawata N, Tsuda T, Nitta M. Occurrence of moulds in Japanese bathrooms. Int Biodeterior Biodegradation. 1992;30(1):47–55. [Google Scholar]
- 21.Hamada N, Abe N. Physiological characteristics of 13 common fungal species in bathrooms. Mycoscience. 2009;50(6):421–429. [Google Scholar]
- 22.Cherif-Seffadj N. Medieval and ottoman Hammams of Algeria: elements for a historical study of baths architecture in North Africa. Archnet-IJAR: International Journal of Architectural Research; 2009.
- 23.Ouaffak Z. Zaim, Lyagoubi, Laboratoire de Mycologie. Département de Parasitologie. Institut national d'Hygiène MAR. Flore fongique pathogene des bains maures de Rabat (Maroc).; pathogenic fungal flora in Moorish baths of Rabat (Morroco) J Mycol Med. 2003;13(1):19–23. [Google Scholar]
- 24.Bastuji-Garin S, Turki H, Mokhtar I, Nouira R, Fazaa B, Jomaa B, Zahaf A, Osman AB, Souissi R, Hémon D, Roujeau JC, Kamoun MR. Possible relation of Tunisian pemphigus with traditional cosmetics: a multicenter case-control study. Am J Epidemiol. 2002;155(3):249–256. doi: 10.1093/aje/155.3.249. [DOI] [PubMed] [Google Scholar]
- 25.Perdelli F, Cristina M, Sartini M, Spagnolo A, Dallera M, Ottria G, et al. Fungal contamination in hospital environments. Infect Control Hosp Epidemiol. 2006;27(1):44–47. doi: 10.1086/499149. [DOI] [PubMed] [Google Scholar]
- 26.Awad AHA, Saeed Y, Shakour AA, Abdellatif NM, Ibrahim YH, Elghanam M, et al. Indoor air fungal pollution of a historical museum, Egypt: a case study. Aerobiologia. 2020:1–13.
- 27.Benammar L, Menasria T, Chergui A, Benfiala S, Ayachi A. Indoor fungal contamination of traditional public baths (Hammams) Int Biodeterior Biodegradation. 2017;117:115–122. [Google Scholar]
- 28.Rafiei A, Amirrajab N. Fungal contamination of indoor public swimming pools, Ahwaz, South-West of Iran. Iran J Public Health. 2010;39(3):124–128. [PMC free article] [PubMed] [Google Scholar]
- 29.Khodaveisi S, Ghahremani E, Abdolahi P, Soori S, Moradzadeh S, Shamdi A, et al. Investigation of mycological flora in Kurdistan University of Medical Sciences bath hostels in 2011. Sci J Kurdistan Univ Med Sci. 2014;19(3):123–129. [Google Scholar]
- 30.Hoseini M, Jabbari H, Naddafi K, Nabizadeh R, Rahbar M, Yunesian M, Jaafari J. Concentration and distribution characteristics of airborne fungi in indoor and outdoor air of Tehran subway stations. Aerobiologia. 2013;29(3):355–363. [Google Scholar]
- 31.Shahsavani S, Dehghani M, Hoseini M, Fararouei M. Biological monitoring of urinary 1-hydroxypyrene by PAHs exposure among primary school students in shiraz, Iran. Int Arch Occup Environ Health. 2017;90(2):179–187. doi: 10.1007/s00420-016-1184-9. [DOI] [PubMed] [Google Scholar]
- 32.Shahsavani S, Hoseini M, Dehghani M, Fararouei M. Characterisation and potential source identification of polycyclic aromatic hydrocarbons in atmospheric particles (PM10) from urban and suburban residential areas in shiraz. Iran Chemosphere. 2017;183:557–564. doi: 10.1016/j.chemosphere.2017.05.101. [DOI] [PubMed] [Google Scholar]
- 33.Pastor C, Cruz G, Josefina M, Aguilar N, Arroyo O. Fungal and bacterial contamination on indoor surfaces of a Hospital in Mexico. Jundishapur J Microbiol. 2012;5.
- 34.Nanbakhsh H, Diba K, Hazarti K. Study of fungal contamination of indoor public swimming pools in Uromia, Iran. Iranian J Publ Health. 2004;33:60–6560. [Google Scholar]
- 35.Naddafi K, Jabbari H, Hoseini M, Nabizade R, Rahbar M, Yunesian M. Investigation of indoor and outdoor air bacterial density in Tehran subway system. Iran. J. Environ. Health. Sci. Eng. 2011;8(4):381–386. [Google Scholar]
- 36.Azimi F, Naddafi K, Nabizadeh R, Hassanvand MS, Alimohammadi M, Afhami S, Musavi SN. Fungal air quality in hospital rooms: a case study in Tehran, Iran. J Environ Health Sci Eng. 2013;11(1):30. doi: 10.1186/2052-336X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallawicha K, Chao HJ, Kotchasatan N. Bioaerosol levels and the indoor air quality of laboratories in Bangkok metropolis. Aerobiologia. 2019;35(1):1–14. [Google Scholar]
- 38.Pitt J, Hocking A. Fungi and food spoilage. London, United Kingdom: Blackie Academic and professional; 1997. [Google Scholar]
- 39.Carmichael J, Kendrick WB, Conners I, Sigler L. Genera of hyphomycetes. Univ. Alberta Press; 1980.
- 40.Samson R, Hoekstra E, Frisvad J, Filtenborg O. Introduction to food and air borne fungi. 7. Utrecht, the Netherlands: Fungal Biodiversity Centre CBS; 2000. p. 389. [Google Scholar]
- 41.Ripon J. Medical mycology; chapter 5: Mycetoma. 3. New York: WB Saunders Company; 1988. [Google Scholar]
- 42.Soleimani Z, Goudarzi G, Naddafi K, Sadeghinejad B, Latifi SM, Parhizgari N, Alavi N, Babaei AA, Akhoond MR, Khaefi M, Rad HD, Mohammadi MJ, Shahsavani A. Determination of culturable indoor airborne fungi during normal and dust event days in Ahvaz. Iran. Aerobiologia. 2013;29(2):279–290. [Google Scholar]
- 43.Nunes I, Mesquita N, Verde SC, Bandeira AML, Carolino MM, Portugal A, et al. Characterization of an airborne microbial community: a case study in the archive of the University of Coimbra. Portugal Int Biodeterior Biodegradation. 2013;79:36–41. [Google Scholar]
- 44.Picco AM, Rodolfi M. Airborne fungi as biocontaminants at two Milan underground stations. Int Biodeterior Biodegradation. 2000;45(1–2):43–47. [Google Scholar]
- 45.Kim K-Y, Park J-B, Jang G-Y, Kim C-N, Lee K-J. Assessment of bioaerosols in the public buildings of Korea. Indoor Built Environ. 2007;16(5):465–471. [Google Scholar]
- 46.Hamada N. Effect of materials on mold contamination in bathrooms. J Antibac Antifungal Agents (Japan). 2008.
- 47.Organization WH. Indoor air quality: biological contaminants: report on a WHO meeting, Rautavaara, 29 August–2 September 1988: World Health Organization. Regional Office for Europe; 1990.
- 48.Ekowati Y, Ferrero G, Kennedy MD, de Roda Husman AM, Schets FM. Potential transmission pathways of clinically relevant fungi in indoor swimming pool facilities. Int J Hyg Environ Health. 2018;221(8):1107–1115. doi: 10.1016/j.ijheh.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Kim KY, Kim YS, Kim D, Kim HT. Exposure level and distribution characteristics of airborne bacteria and fungi in Seoul metropolitan subway stations. Ind Health. 2011;49(2):242–248. doi: 10.2486/indhealth.ms1199. [DOI] [PubMed] [Google Scholar]
- 50.Mirhoseini SH, Didehdar M, Akbari M, Moradzadeh R, Jamshidi R, Torabi S. Indoor exposure to airborne bacteria and fungi in sensitive wards of an academic pediatric hospital. Aerobiologia. 2020:1–8.
- 51.Atya AK, Alyasiri MH, Altamimy R, Ethaib S. Assessment of airborne Fungi in indoor environment for biological lab rooms. J Pure Appl Microbiol. 2019;13(4):2281–2286. [Google Scholar]
- 52.Leviã JT, Stanković S, Krnjaja V, Bočarov-Stančić A. Fusarium species: the occurrence and the importance in agriculture of Serbia. Zbornik Matice Srpske za Prirodne Nauke. 2009;116:33–48. [Google Scholar]
- 53.Nanbakhsh H, Diba K, Hazarti K. Study of fungal contamination of indoor public swimming pools in Uromia. Iran Environmental Health. 2004;4(4):68. [Google Scholar]
- 54.Peternel R, Culig J, Hrga I. Atmospheric concentrations of Cladosporium spp. and Alternaria spp. sporesin Zagreb (Croatia) and effects of some meteorological factors. Ann Agr Environ Med. 2004;11(2):303–307. [PubMed] [Google Scholar]
- 55.Cho E-M, Hong HJ, Park SH, Yoon DK, Nam Goung SJ, Lee CM. Distribution and influencing factors of airborne Bacteria in public facilities used by pollution-sensitive population: a meta-analysis. Int J Environ Res Public Health. 2019;16(9):1483. doi: 10.3390/ijerph16091483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soleimani Z, Parhizgari N, Dehdari Rad H, Akhoond MR, Kermani M, Marzouni MB, Goudarzi H, Goudarzi G. Normal and dusty days comparison of culturable indoor airborne bacteria in Ahvaz. Iran Aerobiologia. 2015;31(2):127–141. [Google Scholar]