Abstract
Background
Parabens are widely used to prevent organism growth and increase the shelf life of foods, medicines and personal care products (PCPs). Recent studies indicate their potentially harmful effects on human health. There is no information on the extent of exposure to parabens among Iranians.
Methods
We measured the concentration of urinary methylparaben (MP), ethylparaben (EP), propylparaben (PP) and butylparaben (BP) among Iranian adults and calculated their estimated daily intake (EDI). Also, association between the level of urinary parabens with socio-demographic and lifestyle variables were investigated.
Results
Detection frequencies of MP, EP, PP, and BP were 98.9, 91, 94.3, and 88.2%, and their median urinary concentrations were 69.06, 9.10, 12.4, and 9.87 µg/l, respectively. Urinary parabens were higher in females, and the difference in the concentration of MP and PP was significant. A significantly positive correlation between MP and PP (r = 0.638) and a moderate to a weak correlation between other parabens were observed. There was a significantly negative weak correlation between age and MP, BP and PP. There was also a significant association between different age groups and MP, BP and PP as well as different BMI values and MP. The highest EDI value belonged to MP in the female group. Despite being lower than the acceptable daily intake (ADI), its value was higher than that reported in other countries (except the US).
Conclusions
Our findings indicated that Iranians are widely exposed to the parabens and the range of exposure was associated with socio-demographic factors. These results could serve as a basis for assessing the risk of exposure to parabens amongst Iranians.
Electronic supplementary material
The online version of this article (10.1007/s40201-020-00540-6) contains supplementary material, which is available to authorized users.
Keywords: Parabens, Urine, Biomonitoring, Adults, Estimated daily intake
Introduction
Parabens are a family of alkyl esters of para-hydroxybenzoic acid. These compounds are the most widely used preservatives and antimicrobials used to prevent the growth of organisms and increase the shelf life of foods, medicines, and PCPs [1–4]. Parabens are widely used by companies producing these products worldwide because of their optimal stability, high water solubility, and relatively low cost. Methylparaben (MP), ethylparaben (EP), propylparaben (PP), and butylparaben (BP) are among the most commonly used paraben derivatives [1, 5]. Recent studies have reported that parabens exposure leads to endocrine disruption and potentially harmful effects on human health. However, there are public concerns in recent decades regarding human exposure to these compounds, and epidemiological studies have reported the association between parabens exposure and potentially harmful consequences on human health. There were also reports on their potential harmful impacts on efficacy of the endocrine system, estrogenic [6, 7] and anti-estrogenic activity [8, 9], lead to impairment of male reproductive system [5, 8], breast cancer [7, 10, 11], and the association between birth outcomes and maternal exposure to parabens during pregnancy [12–15]. Human exposure to paraben compounds often occurs through the consumption of products containing these compounds, including foodstuffs, cosmetics, and pharmaceuticals [2, 4, 16, 17]. The urinary excretion is the major route of eliminating parabens and their metabolites. These compounds are excreted from the body in a mixed manner shortly after exposure and are therefore known as biomarkers in biomonitoring studies [18–23]. Biomonitoring can be used to estimate the cumulative exposure of humans to such pollutants [24]. Results of previous studies indicated widespread exposure and different patterns of distribution of urinary parabens among the population of different countries. These differences may be due to demographic and lifestyle differences as well as different patterns of exposure to parabens [25–27]. Therefore, it is important to understand the profiles of environmental exposure to parabens in vulnerable populations, especially in developing countries where exposures remain largely uncharacterized and the relationship of these compounds with various factors are unclear [28–30]. By now, only limited study exist in Iran reporting the urinary parabens level in association with socio-demographic and lifestyle factors, as well as their estimated daily intake (EDI). Therefore, further studies are needed to provide epidemiological information and shed light on the rate of exposure to parabens in Iranian population. According to the mentioned notes, the main goal of this study was to investigate urinary concentrations of four common parabens (MP, EP, PP and BP) in adult volunteers from Iran. Besides, we aimed to identify some socio-demographic and lifestyle determinants of exposure to parabens in order to set the basis for a large-scale biomonitoring study in Iran.
Materials and methods
Study population
This cross-sectional study was performed on people referring to health centres covered by Isfahan University of Medical Sciences. Participants were selected from health centres located in different areas of Isfahan city by multistage cluster random sampling with age and sex stratification. This study was approved by the Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.RESEARCH.REC.1397.339). All participants completed the consent form before recruitment in the study. A total of 200 questionnaire containing demographic data, anthropometric factors and lifestyle information, and also zone and date/hour of sampling and other required parameters were completed for the participants by a trained expert in a face-to-face manner. Inclusion criteria were a minimum age of 20 years, the residence of more than one year in the current place, no history of chronic diseases, and no long-term medication. Lack of these criteria, incomplete checklists, and failure to deliver urine samples were considered as exclusion criteria. Anthropometric data, including weight, height, and waist circumference of participants were measured using calibrated instruments and following the standard protocols. Body mass index (BMI) was calculated by division of weight (kg) to squared height (m2). BMI categorized as underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 23.0), overweight (23.0 ≤ BMI < 25.0) and obese (BMI ≥ 25.0) (25). Sampling was carried out from five regions (centre, east, west, north, and south) in the city based on different seasons of a year. Participants were categorized into three levels of education namely; lower than high school diploma, high school diploma, and upper than a high school diploma. The economic condition was categorized into low income (< 300$), middle income (300–1000$), and high income (> 1000$ ) based on their monthly income level. Data on tobacco use (cigarettes and hookah) were also categorized as current smokers, former smokers, and non-smokers.
Sampling and analysis
In this study, single-point urine samples were collected in pre-cleaned polypropylene containers in the early morning. The samples were coded, and transferred to the laboratory of Isfahan University of Medical Sciences under standard conditions. To determine the strength of urine samples and compensate any error of spot sampling, 5 ml of each sample was isolated for creatinine analysis. Hitachi 704 auto-analyzer (Jaffe Methodology) was used to measure the urinary creatinine levels of the samples. After analysis, the parabens concentrations were adjusted per unit mass of creatinine (µg per g of creatinine) [31]. All samples were kept at -70 °C until analysis of urinary concentrations of parabens. The standard solutions of MP, EP, PP, and BP were prepared in methanol and stored in dark glass containers at temperatures below zero. To determine MP, EP, PP, and BP levels, the urine samples were extracted using dispersive liquid-liquid micro-extraction (DLLME) and analyzed using GC/MS [32–35]. Briefly, we added 50 µL of the beta-glucuronidase solution to 1 ml of the urine sample, and incubated at 37 °C for 24 hours. After incubation, 0.1 g NaCl was added to each sample and centrifuged for 5 minutes. Then, the supernatant was transferred into a new 10 ml falcon. To form a cloudy state, chlorobenzene (30 µl) and acetone (500 µl) were rapidly injected into the falcon containing the urine sample. The formed cloud solution was then centrifuged for 5 minutes at 5000 rpm. Afterwards, a syringe was used to remove the 50 µl droplet formed at the end of the falcon tube and pour it into a vial. Then, it was dried with a slow flow of nitrogen gas. Later, the parabens were derivatized using N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA) to render them to be sufficiently volatile decreasing their elution temperature and adsorption in the GC column. 20 µl of MSTFA was injected into the vial and a vortex was used to prepare the complete mixture. Finally, 2µL of the mixture was injected into the GC/MS for analysis. The GC/MS settings and other parameters optimization were carried out based on similar previous studies [33, 34, 36, 37].
In this study, the paraben concentration range of 0.01–1000 ng/ml was used to draw the calibration curve of the device. The linear regression gave a good fit (R2: 0.994 for MP, 0.989 for EP, 0.992 for PP, and 0.999 for BP) with high precision (≤ 8.4% RSD). The recovery of spiked samples was > 89% for all the analyzed compounds. Limit of detection (LOD) and limit of quantification (LOQ) of parabens were determined based on the lowest acceptable point on the calibration curve with a signal-to-noise ratio of 3 and 10, respectively, from the replicate analysis (n = 05) of standard solution at a concentration of 10 ng/ml. The LOD and LOQ values for MP, EP, and PP were 0.014 and 0.046, respectively, and those for BP were 0.049 and 0.154 ng/ml, respectively.
Calculation and statistical analysis
To evaluate the extent of exposure to parabens, EDI for MP, EP, PP, and BP was calculated based on previous studies and Eq. 1 [38].
| 1 |
where, EDI (µg /kg-bw/day) is estimated daily intake of parabens, C (µg/L) denotes the urinary concentration of parabens, R (L/day) is the total volume of urine of an adult per day, F (dimensionless) is the paraben excretion factor, and BW (kg) is the bodyweight which was considered as 72.4 kg for the whole population, and 68 kg and 78.4 kg for females and males, respectively (Table 1). F for MP, EP, PP, and BP was equal to 17.4, 13.7, 10.2 and 6.8, respectively, according to previous studies [38, 39]. In this study, the R for our target population was calculated at 1.7 L/day based on similar studies [39, 40].
Table 1.
Socio-demographic characteristics of a population of Iranian adults participating in this study
| Characteristics | Subgroup | Number | Percent | Mean(S.D) |
|---|---|---|---|---|
| Sex | Female | 103 | 57.9 | - |
| Male | 75 | 42.1 | - | |
| weight (kg) | 178 | 100 | 72.4 ± 13.7 | |
| height(m) | 178 | 100 | 1.66 ± 0.09 | |
| waist(cm) | 178 | 100 | 89.6 ± 12.1 | |
| Pregnancy | 34 | - | - | |
| Age(year) | All | 178 | 178 | 43.7 ± 11.8 |
| 21–35 | 48 | 27 | 30.65 ± 3.3 | |
| 36–50 | 88 | 49.4 | 42.81 ± 4.5 | |
| > 50 | 42 | 23.6 | 60.57 ± 7.3 | |
| BMI (kg/m2) | All | 178 | 100 | 26.2 ± 4.3 |
| ≤ 18.5 | 7 | 3.9 | 18.4 ± 0.7 | |
| 18.5-22.99 | 34 | 19.1 | 21.54 ± 1.0 | |
| 23-24.99 | 31 | 17.4 | 23.9 ± 0.7 | |
| ≥ 25 | 106 | 59.6 | 28.9 ± 3.3 | |
| Education | < High school diploma | 48 | 27 | - |
| High school diploma | 61 | 34.3 | - | |
| >High school diploma | 69 | 38.7 | - | |
| Occupational class | housewives | 54 | 30.3 | - |
| Outside workers | 89 | 50 | - | |
| Students | 35 | 19.7 | - | |
| Household income (US$/month) | Low (< 299 ) | 39 | 21.9 | - |
| Moderate (299–999) | 123 | 69.1 | - | |
| High (> 1000 ) | 16 | 9 | - | |
| Time of sampling | Spring | 44 | 24.7 | - |
| Summer | 45 | 25.3 | - | |
| Autumn | 45 | 25.3 | - | |
| Winter | 44 | 24.7 | - | |
| Location | North | 35 | 19.7 | - |
| South | 36 | 20.2 | - | |
| East | 36 | 20.2 | - | |
| West | 36 | 20.2 | - | |
| Center | 35 | 19.7 | - | |
| Smoking status (Cigarette &Hookah) | Current | 22 | 12.4 | - |
| Former | 18 | 10.1 | - | |
| Never | 138 | 77.5 | - | |
| Physical Activity | Low | 109 | 61.2 | - |
| Moderate | 48 | 27 | - | |
| High | 21 | 11.8 | - |
Data analysis was performed using SPSS software (SPSS, Inc., Chicago IL, USA; version 16). Sample size in the current study was determined based on the formula for estimating the mean difference of parabens levels in different categories of demographic and lifestyle variables; by considering type one error rate 0.05, statistical power 80% for detecting a standardized mean difference 0.3 (based on previous studies) led to 220 samples [41, 42]. To adjust the dilution difference in urine samples, urinary concentrations of parabens was adjusted with urine creatinine level. Kolmogorov-Smirnov test and Q-Q plot were used to determine the normal distribution of continuous variables. The distribution of parabens in the present study was positively skewed; accordingly logarithmic transformation was used to normalize the data. Continuous and categorical data were reported as mean, standard deviation, (and median, geometric mean (GM), minimum, maximum and some specific percentiles for non-normally distributed continuous variables), and frequency (percentage), respectively. Comparison of numerical variables at the level of qualitative variables was performed using independent sample t-test, and Multivariate Analysis of Covariance (MANCOVA). Spearman correlation coefficient was also used to investigate the relationship between numerical variables. All tests were two-tailed tests and p < 0.05 was considered as the statistical significance level.
Results
Of the 200 volunteers in this study, 22 of them lacked complete data or optimum urine samples so that laboratory and statistical analysis were performed for 178 participants. Table 1 presents the anthropometric and socio-demographic characteristics of the study population. Out of 178 participants, 103 (57.9%) were female and 75 (42.1%) were male. The mean age of participants was 43.7 ± 11.8 years, and their mean BMI was 26.4 ± 2.3 kg / m2.
Table 2 presents both unadjusted and creatinine-adjusted urinary concentrations of parabens in Iranian adults. All four parabens were identified in most of the samples collected in this study. The detection frequency (DF) of MP, EP, PP, and BP in urine samples was 98.9%, 91%, 94.3%, and 88.2%, respectively. The highest unadjusted concentration of urinary parabens in this study belonged to MP (median = 69.06 µg / L). The median unadjusted concentrations of PP, EP, and BP were 9.10, 12.40, and 9.87 µg/L, respectively.
Table 2.
Parabens concentrations in urine samples of Iranian adults
| Paraben | Concentration type | LOD | DF(%) | Mean (SD) | GM | Min | 25P | 50P | 75P | 95P | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MP | Unadjusted ( µg/L) | 0.01 | 98.9 | 192.05(299.64) | <LOD | 27.46 | 69.06 | 227.47 | 927.03 | 1588.2 | |
| Creatinine adjusted (µg/g) | 190.28(245.20) | 80.61 | <LOD | 28.02 | 94.12 | 257.57 | 760.02 | 1430.60 | |||
| EP | Unadjusted ( µg/L) | 0.02 | 91 | 27.51 (52.25) | <LOD | 2.36 | 9.10 | 26.96 | 146.70 | 431.3 | |
| Creatinine adjusted (µg/g) | 29.24 (55.26) | 4.22 | <LOD | 2.48 | 9.82 | 29.00 | 172.20 | 418.74 | |||
| PP | Unadjusted ( µg/L) | 0.02 | 94.3 | 31.68 (41.45) | <LOD | 3.21 | 12.40 | 46.85 | 129.07 | 190.7 | |
| Creatinine adjusted (µg/g) | 32.24 (39.71) | 7.95 | <LOD | 3.93 | 17.00 | 48.19 | 121.90 | 244.07 | |||
| BP | Unadjusted ( µg/L) | 0.05 | 88.2 | 16.73 (24.66) | <LOD | 1.28 | 9.87 | 20.72 | 59.04 | 195.8 | |
| Creatinine adjusted (µg/g) | 19.86 (33.38) | 4.57 | <LOD | 1.45 | 8.54 | 25.79 | 74.70 | 281.51 |
DF: Detection Frequency (%); GM: Geometric Mean; P: Percentile.
The urinary concentrations of parabens in the two sex groups and their comparative results are reported in Table 3. As can be observed, the concentrations of all parabens in males are lower than those in females. For example, the median urinary MP concentration was 60.12 µg/L in males and 83.85 µg/L in females. The statistical analysis showed a significant difference between both sexes in terms of MP and PP concentrations (P = 0.029 and P = 0.004, respectively).
Table 3.
Comparison of urinary concentrations of parabens by sex
| Unadjusted ( µg/L) | Creatinine adjusted (µg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parabens | Mean ± SD | GM | Median(min-max) | Mean ± SD | GM | Median(min-max) | Mean Dif. | P | |
| MP | Female | 215.08 ± 317.83 | 86.72 | 83.85(< LOD-1588.17) | 218.67 ± 270.24 | 101.54 | 124.19(0.01-1430.60) | 0.54 | 0.029* |
| Male | 160.42 ± 271.59 | 56.14 | 60.12((< LOD-1455.99) | 151.28 ± 201.20 | 58.71 | 56.72(0.01-887.97) | |||
| EP | Female | 28.28 ± 57.68 | 3.83 | 9.31(< LOD-431.30) | 30.87 ± 62.29 | 4.48 | 10.11(< LOD-196.39) | 0.14 | 0.754 |
| Male | 26.44 ± 44.07 | 3.71 | 8.89(< LOD-197.56) | 27.00 ± 44.13 | 3.88 | 9.31(< LOD-196.39) | |||
| PP | Female | 37.34 ± 44.48 | 10.94 | 16.07(< LOD-183.43) | 38.97 ± 44.99 | 12.81 | 19.25(0.01-244.07) | 1.13 | 0.004* |
| Male | 23.91 ± 35.73 | 3.95 | 8.35(< LOD-190.67) | 23.01 ± 28.86 | 4.13 | 11.27(< LOD-149.57) | |||
| BP | Female | 17.10 ± 25.66 | 4.72 | 11.06(< LOD-195.78) | 21.59 ± 39.17 | 5.53 | 12.72(0.02-281.51) | 0.45 | 0.216 |
| Male | 16.22 ± 23.37 | 3.37 | 8.03(< LOD-141.77) | 17.50 ± 23.27 | 3.52 | 7.21(0.01-105.01) | |||
*Mean differences are significant at the 0.05 level (2-tailed). P-values resulted from independent samples t-test after normalizing the distribution of parabens
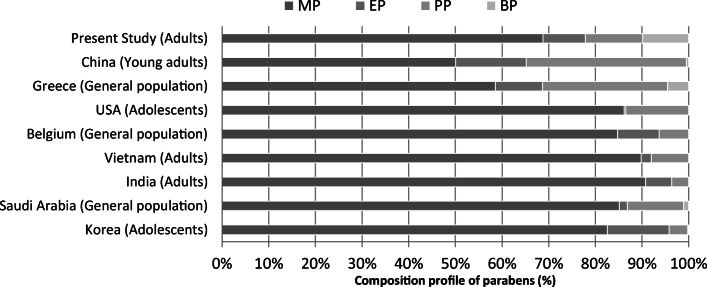
The results presented in Table 4 show that the urinary parabens measured in the study participants differ from that reported in other countries. The highest urinary concentration of MP and EP has been reported in a study performed in Korea [41] while the highest urinary concentration of PP and BP was observed in the present study (Table 4). Also in this study, the median urinary concentrations of MP, PP, EP, and BP were higher than those reported in the US, Belgium, China, and Denmark [21, 22, 43, 44]. Figure 1 presents the composition profile of parabens (median concentrations) amongst the population of different countries. The results presented in Fig. 1 show that the distribution pattern of urinary parabens was different in Iranian population compared to other countries. The proportion urinary of MP, PP, EP, and BP were 68.8, 9.1, 12.3 and 9.8% among the Iranian adults respectively. The proportion urinary of BP in Iranian adults was much higher than in other countries.
Table 4.
Urinary concentration of parabens measured in Iran in comparison with those reported from other countries
| Country | Year | N | Population | Median urinary paraben concentration µg/L (%>LOD) | Ref | |||
|---|---|---|---|---|---|---|---|---|
| MP | EP | PP | BP | |||||
| Belgium | 2013 | 261 | General population | 16.1 (100%) | 1.7 (96.6%) | 1.2 (83.1%) | <LOD (41.8%) | [43] |
| Spain | 2011 | 251 | Young Men | 17 (92%) | 1.8 (75%) | 0.7 (60%) | <LOD (9%) | [47] |
| USA | 2005–2006 | 702 | Adolescents | 53.5 (99.1%) | 0.20 (92.7%) | 8.40 (42.4%) | <LOD (47%) | [21] |
| Saudi Arabia | 2014 | 130 | General population | 11.7 (100%) | 0.23 (87.7) | 1.66 (85.4%) | 0.15 (11.5%) | [45] |
| Korea | 2010 | 359 | Adolescents | 135 (97.7%) | 21.7(97.2%) | 6.54 (96.7%) | 0.33 (93.5%) | [41] |
| China | 2010 | 109 | young adults | 4.63 (100%) | 1.4(100%) | 3.17(100%) | 0.05(60%) | [22] |
| Greece | 212 | 100 | General population | 11.6 (100%) | 2.0(87%) | 5.3(72%) | 0.9(46%) | [46] |
| Denmark | 2012 | 129 | Children &Adolescent | 7.7 (95.3%) | 0.58(59.7%) | 1.02(64.3%) | - | [44] |
| Tunisia | 2012 | 34 | women | 34.94 (94.1%) | 1.77 (67.6%) | 3.06 (70.6%) | < 0.2 (38.2%) | [30] |
| Iran | 2018 | 178 | Adults | 69.06(98.9%) | 9.10(91%) | 12.40 (94.3%) | 9.87 (88.2%) | This study |
Fig. 1.
Composition profile of parabens (median concentrations) amongst population of different countries derived from: [21, 22, 39, 43, 45, 46]
The results of the bivariate correlation analysis showed a significant positive correlation between the parabens (Table 5). The results of the present study revealed a strong positive significant correlation between MP and PP (r = 0.638). BP had a statistically moderate significant correlation with MP, PP and EP, and a slightly lower correlation between EP with MP and PP was observed. The urinary concentrations of parabens were not significantly correlated with BMI and waist circumference, however, there was a significant negative correlation between age and urinary concentrations of MP, PP, and BP (r = -0.223,-0.288, -0.277).
Table 5.
Correlation coefficient between urinary parabens and demographic and anthropometric variables of Iranian adults
| MP | EP | PP | BP | |
|---|---|---|---|---|
| Age | -0.223** | -0.095 | -0.288** | -0.277** |
| BMI | -0.034 | -0.089 | -0.042 | -0.004 |
| Waist | -0.082 | -0.071 | -0.071 | -0.115 |
| MP | 1 | 0.357** | 0.638** | 0.482** |
| EP | 1 | 0.306** | 0.403** | |
| PP | 1 | 0.524** | ||
| BP | 1 |
**Correlation is significant at the 0.01 level (2-tailed),
*Correlation is significant at the 0.05 level (2-tailed)
The Spearman rank correlation coefficient was reported.
The urinary concentrations of parabens based on different sociodemographic and lifestyle categories of the study population and season and location of sampling are presented in Table 6. The results showed a significant difference between age groups in terms of urinary concentrations of parabens. The present study showed significantly higher urinary concentrations of MP, PP, and BP in the younger age group (21–35 years) than those in the older age groups (p < 0.05). The median concentrations of MP, PP and BP in the age group of 21–35 years were approximately 5 times higher than those in the age group above 50 years. Concerning BMI, there was a statistically significant difference between different BMI groups in terms of urinary MP concentrations (p = 0.026) so that the highest urinary MP concentration was observed in the lower BMI group. Comparing urinary concentration of parabens during different seasons indicated that urinary concentrations of MP, PP, and BP were significantly higher in spring than in other seasons. There was no significant difference between educational level, smoking, place of residence, and physical activity in terms of urinary concentrations of parabens. However, Urinary PP and BP concentrations were slightly higher (marginally significant: p < 0.1) in the student occupational category than in other occupational categories. Moreover, urinary concentrations of MP, EP, and PP were higher in individuals with higher household income than other income groups, which was marginally significant (p < 0.1).
Table 6.
Urinary concentration of parabens (µg/g) based on demographic characteristics and lifestyle in the study population
| characteristics | N | MP | EP | PP | BP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median(Min-Max) | P | Mean ± SD | Median(Min-Max) | P | Mean ± SD | Median(Min-Max) | P | Mean ± SD | Median(Min-Max) | P | ||
| Age | |||||||||||||
| 21–35 | 48 | 273.66 ± 321.10 | 151.35(2.18-1430.60) | 0.012* | 29.44 ± 45.08 | 13.51(< LOD-232.63) | 0.535 | 50.14 ± 51.06 | 38.76(0.02-244.07) | 0.001* | 30.65 ± 52.63) | 16.07(0.042–281.51) | 0.000* |
| 36–50 | 88 | 178.75 ± 226.66 | 76.60(0.01-1040.80) | 22.79 ± 36.33 | 8.48(< LOD-225.29) | 27.42 ± 32.49 | 11.82(< LOD-127.94) | 17.50 ± 21.47 | 12.81(< LOD-123.04) | ||||
| > 50 | 42 | 119.14 ± 136.90 | 51.19(0.01-614.28) | 42.50 ± 88.23 | 10.43(< LOD-418.74) | 21.90 ± 32.33 | 6.40(0.01-149.57) | 12.49 ± 21.30 | 2.54(0.02-105.01) | ||||
| BMI | |||||||||||||
| ≤ 18.5 | 7 | 642.04 ± 580.88 | 386.70(106.74-1430.60) | 0.026* | 29.88 ± 25.20 | 28.24(5.09–64.76) | 0.188 | 62.78 ± 58.27 | 31.66(4.64-144.43) | 0.091 | 21.79 ± 13.26 | 17.65(6.83–41.78) | 0.427 |
| 18.5–22.9 | 34 | 199.26 ± 199.52 | 123.59(11.66-189.22) | 29.12 ± 36.17 | 20.53(0.01-189.22) | 43.49 ± 44.46 | 31.55(0.02-150.84) | 30.78 ± 61.19 | 6.13(0.03-281.51) | ||||
| 23-24.9 | 31 | 215.32 ± 290.49 | 96.71(3.53-1040.80) | 28.22 ± 51.76 | 9.19(< LOD-225.29) | 33.45 ± 38.51 | 24.45(0.01-149.57) | 16.95 ± 18.41 | 12.89(0.02–75.97) | ||||
| > 25 | 106 | 150.24 ± 173.24 | 71.76(0.02-801.81) | 29.53 ± 62.72 | 7.81(< LOD-418.74) | 26.27 ± 35.77 | 11.20(< LOD-244.07) | 17.09 ± 23.37 | 7.75(< LOD-123.04) | ||||
| Education | |||||||||||||
| <High school diploma | 48 | 156.02 ± 175.65 | 90.85(0.01-801.81) | 0.350 | 39.06 ± 84.99 | 9.19(< LOD-418.74) | 0.963 | 29.70 ± 45.41 | 11.39(0.01-244.07) | 0.130 | 16.78 ± 25.01 | 6.79(0.02-123.04) | 0.777 |
| High school diploma | 61 | 238.49 ± 290.95 | 145.09(2.18-1430.60) | 28.85 ± 44.64 | 11.70(< LOD-225.29) | 32.64 ± 37.92 | 21.51(< LOD-144.43) | 23.33 ± 36.01 | 13.58(< LOD-227.65) | ||||
| > High school diploma | 69 | 171.48 ± 239.19 | 66.25(0.01-1402.42) | 22.75 ± 32.98 | 9.78(< LOD-189.22) | 33.67 ± 37.47 | 17.98(0.01-149.91) | 18.95 ± 36.14 | 8.20(0.02-281.51) | ||||
| Occupational class | |||||||||||||
| Homemakers | 71 | 213.17 ± 260.56 | 131.04(0.01-1430.60) | 0.380 | 35.07 ± 72.46 | 10.11(< LOD-418.74) | 0.940 | 38.77 ± 46.40 | 19.83(0.01-244.07) | 0.085 | 21.49 ± 33.89 | 12.72(0.03-227.65) | 0.071 |
| Outside workers | 98 | 167.82 ± 234.60 | 58.57(0.01-1402.42) | 24.89 ± 38.60 | 9.70(< LOD-196.39) | 27.01 ± 34.32 | 12.68(< LOD-149.91) | 18.29 ± 33.38 | 7.81(< LOD-281.51) | ||||
| Students | 9 | 254.21 ± 231.17 | 255.51(2.18–712.00) | 30.57 ± 55.02 | 8.00(0.01-171.79) | 37.75 ± 32.07 | 33.97(0.10–95.70) | 24.15 ± 31.98 | 19.46(0.45–103.50) | ||||
| Physical Activity | |||||||||||||
| Low | 109 | 195.63 ± 239.63 | 96.71(0.01-1430.60) | 0.912 | 29.52 ± 59.54 | 9.87(< LOD-418.74) | 0.996 | 36.38 ± 44.03 | 19.83(< LOD-244.07) | 0.510 | 18.50 ± 24.55 | 11.49(< LOD-123.04) | 0.602 |
| Moderate | 48 | 185.23 ± 269.93 | 98.01(0.01-1402.42 | 30.81 ± 51.41 | 9.71(< LOD-225.29) | 28.01 ± 34.82 | 9.32(0.01-123.01) | 27.29 ± 51.58 | 11.45(0.03-281.51) | ||||
| High | 21 | 174.01 ± 224.26 | 73.76(14.21-887.97) | 24.19 ± 40.48 | 9.62(0.01-171.79) | 20.43 ± 18.84 | 15.88(0.01–55.62) | 9.97 ± 10.58 | 5.25(0.02–33.71) | ||||
| Period of sampling (Seasons) | |||||||||||||
| Spring | 44 | 255.85 ± 199.91 | 221.27(17.88-887.97) | 0.001* | 44.45 ± 84.02 | 17.05(< LOD-418.74) | 0.162 | 53.58 ± 50.68 | 44.17(0.01-244.07) | 0.001* | 28.06 ± 23.20 | 24.64(0.03-113.99) | 0.001* |
| Summer | 45 | 217.69 ± 345.52 | 53.52(2.21-1430.60) | 29.05 ± 49.89 | 8.16(< LOD-225.29) | 24.47 ± 28.10 | 13.00(0.01-110.45) | 10.11 ± 17.86 | 4.78(0.02-105.01) | ||||
| Autumn | 45 | 147.80 ± 199.87 | 69.77(11.36-817.97) | 21.61 ± 36.97 | 8.76(0.01-196.39) | 24.46 ± 29.60 | 11.14(< LOD-114.09) | 15.16 ± 21.61 | 8.20(< LOD-123.04) | ||||
| Winter | 44 | 140.11 ± 186.63 | 53.98(0.01-739.47) | 22.01 ± 34.87 | 9.92(< LOD-189.22) | 26.81 ± 39.95 | 6.27(0.01-150.84) | 26.45 ± 54.86 | 6.74(< LOD-281.51) | ||||
| Location | |||||||||||||
| North | 36 | 172.30 ± 188.55 | 111.33(2.21-762.56) | 0.964 | 23.09 ± 71.10 | 5.43(< LOD-418.74) | 0.060 | 31.20 ± 37.03 | 19.83(< LOD-150.84) | 0.952 | 18.59 ± 38.86 | 5.98(< LOD-227.65) | 0.976 |
| South | 35 | 158.52 ± 151.77 | 69.21(11.74-550.39) | 29.04 ± 49.47 | 10.62(< LOD-189.22) | 29.80 ± 38.29 | 12.72(0.01-149.57) | 12.93 ± 16.41 | 6.09(0.02–75.97) | ||||
| East | 35 | 249.08 ± 289.09 | 146.67(0.011040.80) | 34.64 ± 66.00 | 12.55(0.01–312.60) | 39.48 ± 43.76 | 17.59(0.01-149.91) | 23.31 ± 28.58 | 17.77(0.02-123.04) | ||||
| West | 35 | 161.98 ± 214.07 | 86.26(2.18-801.81) | 22.99 ± 43.70 | 6.99(< LOD-232.63) | 31.41 ± 44.80 | 21.55(0.01-244.07) | 26.21 ± 51.78 | 7.94(0.02-281.51) | ||||
| Center | 36 | 209.53 ± 337.98 | 70.16(3.53-1430.60) | 36.47 ± 41.34 | 23.25(0.01-196.39) | 29.21 ± 34.92 | 8.91(0.01-124.66) | 18.21 ± 17.41 | 15.57(0.01–73.43) | ||||
| Family Monthly income | |||||||||||||
| Low(< 299 US$/month) | 39 | 130.41 ± 168.01 | 66.40(2.18-887.97) | 0.096 | 10.65 ± 14.93 | 5.43(< LOD-69.89) | 0.081 | 27.29 ± 29.32 | 15.91(0.01-109.23) | 0.085 | 17.75 ± 23.28 | 8.86(0.02-123.04) | 0.536 |
| Moderate(299-999US/month) | 123 | 188.71 ± 238.71 | 91.52(0.01-1402.42) | 33.24 ± 60.64 | 10.32(< LOD-418.74) | 29.84 ± 35.62 | 13.00(< LOD-149.91) | 19.09 ± 35.55 | 7.42(< LOD-281.51) | ||||
| High (> 1000 US$/month) | 16 | 348.21 ± 372.21 | 268.33(19.20-1430.60) | 43.75 ± 67.01 | 25.06(0.01-232.63) | 62.84 ± 70.74 | 26.58(2.64-244.07) | 30.96 ± 36.79 | 14.23(0.03-113.99) | ||||
| Smoking status(Cigarette & Hookah) | |||||||||||||
| Current | 22 | 325.28 ± 397.79 | 73.29(2.18-1402.42) | 0.912 | 46.12 ± 60.37 | 22.02(< LOD-181.08) | 0.473 | 27.68 ± 34.91 | 14.13(< LOD-110.45) | 0.178 | 14.57 ± 14.50 | 12.53(< LOD-45.40) | 0.603 |
| Former | 18 | 200.52 ± 336.44 | 90.85(17.03-1430.60) | 18.51 ± 21.54 | 7.81(< LOD-66.74) | 35.82 ± 43.26 | 25.18(0.02-127.94) | 16.48 ± 16.60 | 12.81(0.03–40.45) | ||||
| Never | 138 | 167.42 ± 189.50 | 100.01(0.01-1040.80) | 27.94 ± 57.15 | 9.66(< LOD-418.74) | 32.51 ± 40.17 | 17.00(0.01-244.07) | 21.15 ± 36.96 | 8.20(0.02-281.51) | ||||
*Mean differences are significant at the 0.05 level (2-tailed) (p < 0.05). The p-values resulted from MANCOVA, the adjustment was made for age and gender as a covariate
In this study, we determined the extent of exposure to parabens using EDI equation [38]. The EDI values determined for this study and those for other countries are reported in Table 7. According to the results, the highest and the lowest EDI values calculated in this study belonged to MP and EP, respectively. Mean EDI values for MP in females, males and the whole population were 25.9, 30.9, and 20.1 µg/kg-bw/day, respectively, and for EP were 4.72, 5.16, and 4.19 µg/kg-bw/day, respectively. The median EDI calculated for the whole population in Iran was higher than that in Germany [38], India, China, and Saudi Arabia [39], and less than that in the US [21] and South Korea [39]. In this study, the median EDI in males was higher than that reported in Germany[38] and China [48]. Inconsistent with our results, the median EDI of parabens in females was higher in Yu study than in our study, and the highest median EDI (24.6 µg/kg-bw/day) was reported for MP [48] (Table 7). The Acceptable Daily Intake (ADI) value was determined 10,000 µg /kg-bw/day for MP and EP [49] and 100 µg/kg-bw/day for PP alone based on the Non-Observable Effect Level (NOEL) [39].
Table 7.
Estimated daily intake (EDI) (µg/kg-bw/day) of parabens in Iranian people and comparison with other countries
| Country | year | Gender | MP | EP | PP | BP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | P.95 | Mean | Median | P.95 | Mean | Median | P.95 | Mean | Median | P.95 | Ref | |||
| Iran | 2018 | Male &Female | 25.9 | 9.32 | 125 | 4.72 | 1.56 | 25.1 | 7.30 | 2.85 | 29.7 | 5.78 | 3.42 | 20.4 |
This study |
| Female | 30.9 | 12.1 | 148 | 5.16 | 1.70 | 29.1 | 9.15 | 3.94 | 32.4 | 6.29 | 4.07 | 20.35 | |||
| Male | 20.1 | 7.49 | 99.7 | 4.19 | 1.41 | 23.6 | 5.08 | 1.77 | 22.9 | 5.17 | 2.56 | 19.4 | |||
| China | 2013–2015 | Female | 11.4 | 24.6 | 66.6 | 18 | 38.4 | 134 | 9.03 | 28.2 | 145 | - | - | - | [48] |
| Male | 6.67 | 1.64 | 30.1 | 7.55 | 1.07 | 77.8 | 12.2 | 0.26 | 56.4 | - | - | - | |||
| Germany | 1995–2012 | Male &Female | - | 3.0 | 31.7 | - | 0.2 | 3.4 | - | 0.3 a | 11.9 a | - | 0.1 b | 2.2 b | [38] |
| 2012 | Female | - | 9.7 | 43.5 | - | 1.3 | 19.8 | - | 2.7 a | 21 a | - | 0.2 b | 10.1 b | ||
| Male | - | 6.3 | 21 | - | NA | 1.5 | - | NA a | 6.5 a | - | 0.1 b | 0.9 b | |||
| USA | 2006 | Male &Female | 64.8 | 75.8 | - | - | - | - | 9.6 | 12.0 | - | - | - | - | [21] |
| Korea | 2010–2012 | Male &Female | - | 21.5 | - | - | 7.97 | - | - | 15.5 | - | - | - | - | [39] |
| China | 2010–2012 | Male &Female | - | 1.93 | - | - | 0.67 | - | - | 3.24 | - | - | - | - | |
| Saudi | 2010–2012 | Male &Female | - | 1.30 | - | - | 0.05 | - | - | 0.19 | - | - | - | - | |
| USA | 2010–2012 | Male &Female | - | 0.81 | - | - | 0.07 | - | - | 0.23 | - | - | - | - | |
| India | 2010–2012 | Male &Female | - | 1.20 | - | - | 0.06 | - | - | 0.13 | - | - | - | - | |
Discussion
Due to the lack of research and information on urinary concentrations of parabens in the Iranian adults as well as the extent of their exposure to parabens, the present cross-sectional study measured for the first time the urinary concentrations of parabens and calculated exposure levels in a population of Iranian adults. Furthermore, some factors affecting the parabens concentration including socio-demographic and lifestyle were investigated. In this study, significant concentrations of parabens were measured in most the urine samples and MP was higher than the other urinary paraben compounds. The high concentration and detection frequency of urinary parabens in the present study indicate a wide exposure to the parabens from various sources. In similar studies, high urinary concentrations and detection frequency of MP have been reported [21, 22, 50]. According to the comparative table (Table 4), the overall composition of urinary parabens measured in adults in this study was different from the results reported by some other studies. The urinary concentrations of MP and EP reported in South Korea [41] were approximately 2 folds of the amounts obtained in the present study. On the contrary, the PP and BP concentrations in our study were approximately 2 and 20 times higher than those reported in the South Korean, respectively. Also, the urinary MP and PP concentrations measured in the present study were higher than those reported in Tunisia, Greece, China, Saudi Arabia, Belgium and Denmark [22, 30, 43–46]. The urinary BP concentrations (Median = 9.87 µg/L) in the present study was higher than those reported in other countries [30, 41, 45, 46]. This may be due to the highest frequency of detection of BP in this study. Besides, several factors, e.g. the methodologies used in collecting urine samples and the time of sample collection may influence the concentrations in different studies. The distribution pattern of urinary parabens was different in Iranian population compared to other countries, indicating different patterns of exposure in Iran. The variation of reported levels may be due to differences in analytical methods or study designs. Also, different socio-economic and geographical characteristics of societies may influence the change in patterns of exposure to the compounds [35]. Another reason for such differences might be due to the significant differences in lifestyle, including the frequency of makeup and PCPs use and exposure through food sources containing paraben preservatives. Furthermore, chemical legislation and temporal trends vary between countries.
The results showed a significant positive correlation between all the measured parabens (Table 5). Similar to previous studies, we observed strong correlations between the concentrations of MP and PP (r = 0.638), most likely due to the simultaneous use of MP and PP in many consumer products owing to they antimicrobial effects [5]. In the Ma study, no significant correlation between MP and EP, as well as between PP and EP has been reported [22]. Similar to the results reported in the study in the US [21], our study also showed a statistically moderate significant correlation between BP with MP, PP and EP. Moreover, a slightly lower correlation between EP with MP and PP was observed.. These findings suggest concomitant exposure of the Iranian population to several parabens, as these compounds are used in combination with various consumer products.
The results of this study showed a higher urinary concentration of parabens in females. Also, statistically significant differences were observed between the two sexes in terms of urinary levels of MP and PP. Similarly, several studies have reported different urinary concentrations of parabens for males and females [22, 44]. This was relatively consistent with the level of PP and MP in the previous study for the US population [21]. Another study showed no statistically significant difference between the two sexes in terms of urinary levels of parabens [51]. The differences in urinary parabens between the sexes were not the same in different societies. These differences may be related to varying exposure to paraben compounds because of lifestyle factors or behavioural differences [38]. Since PCPs are more commonly used by females than males, and these products are one of the most important sources of exposure to parabens, females are more likely exposed to parabens [5, 21, 22].
In this study, a negative significant association was found between age and the urinary concentrations of MP, PP, and BP (p > 0.05). The results also demonstrated a negative significantly weak correlation between age and concentrations of MP, PP, and BP (Table 5). Similarly, in Denmark, urinary levels of parabens were higher in younger individuals and a significantly negative relationship has been reported [44]. In the other study, Honda et al. (2018) showed no significant relationship between urinary concentrations of parabens and age [39]. Contrary to our results, in South Korea and the United States, urinary concentrations of parabens were higher in older individuals and the concentration differences were statistically significant at different age groups [21, 41, 52]. A change in the urinary concentration of parabens with age is predictable due to the different extent of exposure to parabens. The change in the extent of exposure to paraben compounds is probably due to a change in lifestyle, followed by a change in the amount and type of PCPs consumed throughout a person’s life. Accordingly, the higher urinary concentration of parabens in younger individuals in the Iranian population is likely due to their greater exposure to paraben compounds in a variety of ways, including higher consumption of PCPs or foods containing parabens. Besides, individual differences and metabolic pathways of parabens in the human body can be other causes of differences in the urinary concentrations of parabens in different age groups [41].
The present study showed a statistically significant difference (p < 0.05) between people with different BMI values in terms of urinary concentrations of MP. Similar results also have been reported by the other studies conducted on adolescents in Iran [50] and in the US population [52, 53]. In other studies, some opposite results have also been reported. In this regard, Smith et al. (2012) and Kang et al. (2016) have reported a significant negative association between the urinary levels of parabens (MP and PP) and BMI among obese subjects compared to those had normal weight [41, 54]. Differences in the concentration of parabens between different BMI categories may be due to different exposure patterns to the sources of parabens such as PCPs, medications or foods containing parabens. This may also be due to different pharmacokinetics and metabolic pathways in the body of individuals with different BMI values.
We also examined the association between tobacco use and urinary concentrations of parabens. Despite the higher urinary concentrations of parabens in tobacco users, no significant association was found. These results were in agreement with the results of Yu’ study in China [48]. The results of another study in China showed a significant negative association between urinary MP concentrations and smoking [55]. In contrast, the results of Geer’s study showed that MP and PP concentrations were higher in smokers than in non-smokers [13]. It has been hypothesized that smoking may induce enzymes that accelerate the clearance rate of parabens [56]. Further epidemiological studies are needed to determine the precise effect of smoking on the urinary parabens concentrations. Our results showed that the urinary concentrations of MP, PP, and BP were significantly higher in spring than other seasons. Similarly, some previous studies, have shown the higher levels of urinary MP in spring than the other seasons [27, 35]. Results of another study showed a lower urinary level of MP in autumn and higher urinary level of BP in summer [57]. As usage patterns might differ by country, the association among variables may also vary. These differences can be due to the changes in the rate of use of cosmetics and foodstuffs as well as probably the changes in lifestyle habits. In Iran, it might be due to higher consumption of cosmetics and other PCPs during Iranian New Year celebrations (Nowrooz) held in early spring.
In this study, participants from the high-income group had higher urinary concentrations of parabens, but no significant association between family income and the urinary parabens concentrations was observed (p > 0.05). In agreement with our results, In South Korea [41] and China [55], no significant association was found between income level and urinary concentration of parabens. On contrast to our results, Calafat et al. (2010) found significantly higher urinary MP and PP concentrations among the high-income group [21]. The probable reason for the lack of this relationship in our study may be due to the larger number of people in the middle-income class (70%) and the uneven distribution of participants in different income groups.
There was no significant association between the urinary concentration of parabens and the level of participants’ education. Similarly, In another study in Shanghai, the urinary concentration of parabens was not significantly associated with income and education levels of the study population [55]. The results of a study in China showed that people with tertiary education had a lower urinary concentration of PP than people with a lower level of education [48]. In contrast, in a study on the US population, people with a higher level of education had higher urinary concentrations of MP and PP. This is in agreement with the results of other studies conducted in South Korea [41]. However, there is no consensus regarding the effect of level of education on the urinary parabens concentration. Based on our results, most likely, the level of education are interlinked with other lifestyle factors and there is no significant association between educational level and urinary parabens concentration.
In this study, we observed no significant association between the intensity of physical activity and the urinary concentration of parabens (p > 0.05). Results of another study showed a significant negative association of physical activity with urinary concentrations of parabens [27]. However, positive associations have been reported between urinary MP and BP concentrations and the physical activity level [58]. The mechanism of the effect of intensity of physical activity on the increase or decrease of paraben excretion from the body is not well known. Physical activity increases sweat rates in the body, and likely a considerable portion of parabens are excreted in this way.
Our findings revealed no significant association between occupational class and the urinary concentration of parabens (p > 0.05). This is in line with the findings of other studies conducted in Iran. On contrast to our findings, Jimenez-Dıaz et al. (2016) have reported that housewives have significantly lower urinary concentrations of parabens compared to women who work outdoors. This probably might be because those women work outside use higher levels of personal care products and processed foods [30]. The possible reason for the lack of this association in our study may be due to the lower number of subjects in the students class (5%) and the non-uniform distribution of participants in different occupational classes. In summary, the findings of our study reveal a relationship between urinary parabens concentrations with some sociodemographic characteristics and lifestyle factors of adults in Iran.
Consistent with previous studies in other countries, the present study reported the highest EDI for MP, indicating higher exposure to MP in the Iranian population. Comparison of the median EDI reported in different countries (Table 7), the median EDI calculated in Iran is higher than in Germany [38], China [48], and lower than in the US [21] and South Korea [39]. EDI values calculated for a population in India, Saudi Arabia, Japan and Kuwait reported in HONDA’s study were lower than values calculated in the present study [39]. In this study, the EDI value was higher in the female sex group than the male sex group so that the EDI calculated for MP and PP in the female sex group is more than 1.5 times the EDI calculated for the male sex group, which were consistent with previous studies in Germany and China [22, 38, 48]. Although the EDI of parabens was higher in the Iranian population than many other countries, the EDI value for MP and PP was lower than the ADI value. However, to further evaluate the daily exposure of the Iranian population to parabens and identify sources of exposure and metabolism of parabens, further studies on the larger target population using 24-h urine samples are needed. Overall, the urinary concentration and detection of parabens in the Iranian population is higher than in most countries. These results indicate widespread exposure to parabens in the Iranian population as well as a difference in distribution pattern of parabens compare with other countries. This differences may be due to racial and lifestyle differences, different concentrations of parabens in consumables as well as different patterns in the consumption of PCPs.
Study applications and limitations
The present study, as a population-based study, has several limitations. First, an adult population from Isfahan was investigated in the present study, so, caution must be exercised while generalizing the results to the whole population of Iran. Second, demographic characteristics and lifestyle checklists were completed in a self-reported manner in the presence of the study expert, and some information may be written in the checklist inaccurately due to forgetfulness or lack of accuracy. Third, in the present study, the paraben levels were measured in spot urine samples. The timing of sample collection may affected the.
urinary parabens concentrations. Forth, we did not investigate the relationship between the different sources of exposure and the concentrations of parabens due to the lack of access to information on the sources of paraben exposure, including the use of PCPs, foods, and medicines. Fifth, although the sample size was calculated according to the statistical criteria, we considered the minimum but reasonably acceptable size due to funding limitation. However, the present study evaluated for the first times the urinary concentrations and extent of exposure of parabens in a population of Iranian adults. The present study also showed an association between urinary concentrations of parabens and sociodemographic and lifestyle factors in Iranian adults. Accordingly, the results can serve as a basis for assessing the risk of exposure to parabens in future studies on the Iranian population. It is also suggested to carefully evaluate the sources of paraben exposure, including cosmetics, and investigate their relationship with concentrations of parabens in future studies.
Conclusion
In the present study, we measured the concentration of parabens in a population of Iranian adult, calculated EDI of parabens, and investigated the relationship between urinary concentrations of parabens with socio-demographic characteristics and lifestyle factors. As far as we know, this is the first study reporting the concentration and extent of exposure to urinary parabens in Iranian adults. The results indicate high urinary concentrations of parabens in Iranian adults, especially BP is several times higher than that in other countries with different patterns of exposure. Despite that, the EDI value calculated in this study was not exceeded from the recommended ADI value. Urinary concentrations of parabens are higher in females than males and there is a statistically significant difference between the two sexes in terms of urinary concentrations of MP and PP. Besides, there is a negative correlation between age with MP, PP and BP concentrations as well as between BMI with the urinary concentration of MP. The results can serve as a basis for assessing the risk of exposure to parabens in future studies on the Iranian population.
Electronic supplementary material
(DOCX 69.3 KB)
(PDF 98.5 KB)
(PDF 207 KB)
Acknowledgements
This research was financially supported by the Isfahan University of Medical Sciences. The authors are grateful to Isfahan University of Medical Sciences for their cooperation in this study. (Grant No: 397471, 2018)
Funding
This paper was extracted from a PhD thesis funded by the Isfahan University of Medical Science (Grant No: 397471).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ghasem Kiani Feizabadi, Email: ghasem_kia@yahoo.com.
Yaghoub Hajizadeh, Email: y_hajizadeh@hlth.mui.ac.ir.
Awat Feizi, Email: awat_feiz@hlth.mui.ac.ir.
Karim Ebrahimpour, Email: ebrahimpour@hlth.mui.ac.ir.
References
- 1.Andersen FA. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27:1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Wang L, Kannan K. Phthalates and parabens in personal care products from China: concentrations and human exposure. Arch Environ Contam Toxicol. 2014;66(1):113–9. doi: 10.1007/s00244-013-9937-x. [DOI] [PubMed] [Google Scholar]
- 3.Karthikraj R, Kannan K, Human Biomonitoring of Select Ingredients in Cosmetics, in Analysis of Cosmetic Products. 2nd ed. Cambridge: Elsevier Science; 2017. p. 387–434.
- 4.Moreta C, Tena M-T, Kannan K. Analytical method for the determination and a survey of parabens and their derivatives in pharmaceuticals. Environ Res. 2015;142:452–60. doi: 10.1016/j.envres.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Soni M, Carabin I, Burdock G. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem Toxicol. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008;28(5):561–78. doi: 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- 7.Charles AK, Darbre PD. Combinations of parabens at concentrations measured in human breast tissue can increase proliferation of MCF-7 human breast cancer cells. J Appl Toxicol. 2013;33(5):390–8. doi: 10.1002/jat.2850. [DOI] [PubMed] [Google Scholar]
- 8.Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem Toxicol. 2002;40(12):1807–13. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, et al. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmcol. 2007;221(3):278–84. doi: 10.1016/j.taap.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan S, et al. Parabens and human epidermal growth factor receptor ligand cross-talk in breast cancer cells. Environ Health Perspect. 2015;124(5):563–9. doi: 10.1289/ehp.1409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng X, et al. Occurrence and ecological potential of pharmaceuticals and personal care products in groundwater and reservoirs in the vicinity of municipal landfills in China. Sci Total Environ. 2014;490:889–98. doi: 10.1016/j.scitotenv.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 12.Philippat C, et al. Prenatal exposure to phenols and growth in boys. Epidemiology (Cambridge, Mass.). 2014;25(5):625. [DOI] [PMC free article] [PubMed]
- 13.Geer LA, et al. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater. 2017;323:177–83. doi: 10.1016/j.jhazmat.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamal A, et al. Prenatal urinary concentrations of environmental phenols and birth outcomes in the mother-infant pairs of Tehran Environment and Neurodevelopmental Disorders (TEND) cohort study. Environ Res. 2020:109331. [DOI] [PubMed]
- 15.Messerlian C, et al. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ Int. 2018;114:60–8. doi: 10.1016/j.envint.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol. 2013;47(24):14442–14449. doi: 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- 17.Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47(8):3918–3925. doi: 10.1021/es400724s. [DOI] [PubMed] [Google Scholar]
- 18.Aubert N, Ameller T, Legrand J-J. Systemic exposure to parabens: pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl-and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem Toxicol. 2012;50(3–4):445–54. doi: 10.1016/j.fct.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Kannan K. Alkyl protocatechuates as novel urinary biomarkers of exposure to p-hydroxybenzoic acid esters (parabens) Environ Int. 2013;59:27–32. doi: 10.1016/j.envint.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Ye X, et al. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006;114(12):1843. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calafat AM, et al. Urinary concentrations of four parabens in the US population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma W-L, et al. Urinary concentrations of parabens in Chinese young adults: implications for human exposure. Arch Environ Contam Toxicol. 2013;65(3):611–8. doi: 10.1007/s00244-013-9924-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, et al. Characteristic profiles of urinary p-hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ Sci Technol. 2013;47(4):2069–2076. doi: 10.1021/es304659r. [DOI] [PubMed] [Google Scholar]
- 24.Cowan-Ellsberry CE, Robison SH. Refining aggregate exposure: example using parabens. Regul Toxicol Pharmacol. 2009;55(3):321–9. doi: 10.1016/j.yrtph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, et al. Repeated measurements of paraben exposure during pregnancy in relation to fetal and early childhood growth. Environ Sci Technol. 2018;53(1):422–433. doi: 10.1021/acs.est.8b01857. [DOI] [PubMed] [Google Scholar]
- 26.Feizabadi GK, et al. Urinary concentrations of parabens in a population of Iranian adolescent and their association with sociodemographic indicators. Arch Environ Contam Toxicol. 2020:1–13. [DOI] [PubMed]
- 27.Fadaei S, et al. Investigating determinants of parabens concentration in maternal urine. Hum Ecol Risk Assess. 2020:1–19.
- 28.Kim K, et al. Urinary concentrations of bisphenol A and triclosan and associations with demographic factors in the Korean population. Environ Res. 2011;111(8):1280–5. doi: 10.1016/j.envres.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, et al. Urinary concentrations of acrylamide (AA) and N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA) and associations with demographic factors in the South Korean population. Int J Hyg Environ Health. 2014;217(7):751–7. doi: 10.1016/j.ijheh.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez-Díaz I, et al. Urinary levels of bisphenol A, benzophenones and parabens in Tunisian women: A pilot study. Sci Total Environ. 2016;562:81–8. doi: 10.1016/j.scitotenv.2016.03.203. [DOI] [PubMed] [Google Scholar]
- 31.Khalil N, Chen A, Lee M. Endocrine disruptive compounds and cardio-metabolic risk factors in children. Curr Opin Pharmacol. 2014;19:120–4. doi: 10.1016/j.coph.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, et al. DLLME combined with GC–MS for the determination of methylparaben, ethylparaben, propylparaben and butylparaben in beverage samples. Chromatographia. 2010;72(3–4):351–5. [Google Scholar]
- 33.Vela-Soria F, et al. A multiclass method for the analysis of endocrine disrupting chemicals in human urine samples. Sample treatment by dispersive liquid–liquid microextraction. Talanta. 2014;129:209–18. doi: 10.1016/j.talanta.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahim K, Poursafa P, Amin MM. Development of a simple and valid method for the trace determination of phthalate esters in human plasma using dispersive liquid–liquid microextraction coupled with gas chromatography–mass spectrometry. J Sep Sci. 2017;40(22):4403–10. doi: 10.1002/jssc.201700589. [DOI] [PubMed] [Google Scholar]
- 35.Hajizadeh Y, Ebrahimpour KFG, Shoshtari-Yeganeh K, Fadaei B, Darvishmotevalli S, Karimi MH. Urinary paraben concentrations and their implications for human exposure in Iranian pregnant women. Environ Sci Pollut Res. 2020. [DOI] [PubMed]
- 36.Azzouz A, Rascón AJ, Ballesteros E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry. J Pharm Biomed Anal. 2016;119:16–26. doi: 10.1016/j.jpba.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Jiménez-Díaz I, et al. Analytical methods for the determination of personal care products in human samples: an overview. Talanta. 2014;129:448–58. doi: 10.1016/j.talanta.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 38.Moos RK, et al. Daily intake and hazard index of parabens based upon 24 h urine samples of the German Environmental Specimen Bank from 1995 to 2012. J Expo Sci Environ Epidemiol. 2017;27(6):591. doi: 10.1038/jes.2016.65. [DOI] [PubMed] [Google Scholar]
- 39.Honda M, Robinson M, Kannan K. Parabens in human urine from several Asian countries, Greece, and the United States. Chemosphere. 2018;201:13–9. doi: 10.1016/j.chemosphere.2018.02.165. [DOI] [PubMed] [Google Scholar]
- 40.Perucca J, et al. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Reg Integr Comp Physiol. 2007;292(2):R700–5. doi: 10.1152/ajpregu.00500.2006. [DOI] [PubMed] [Google Scholar]
- 41.Kang H-S, et al. Urinary concentrations of parabens and their association with demographic factors: a population-based cross-sectional study. Environ Res. 2016;146:245–51. doi: 10.1016/j.envres.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Dewalque L, et al. Simultaneous determination of some phthalate metabolites, parabens and benzophenone-3 in urine by ultra high pressure liquid chromatography tandem mass spectrometry. J Chromatogr B. 2014;949:37–47. doi: 10.1016/j.jchromb.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Dewalque L, Pirard C, Charlier C. Measurement of urinary biomarkers of parabens, benzophenone-3, and phthalates in a Belgian population. BioMed Res Int. 2014;2014. [DOI] [PMC free article] [PubMed]
- 44.Frederiksen H, et al. Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected 2006–2012. Reproduction. 2014:REP-13-0522. [DOI] [PubMed]
- 45.Asimakopoulos AG, et al. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ Res. 2016;150:573–81. doi: 10.1016/j.envres.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Asimakopoulos AG, Thomaidis NS, Kannan K. Widespread occurrence of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters (parabens), benzophenone type-UV filters, triclosan, and triclocarban in human urine from Athens, Greece. Sci Total Environ. 2014;470:1243–9. doi: 10.1016/j.scitotenv.2013.10.089. [DOI] [PubMed] [Google Scholar]
- 47.Adoamnei E, et al. Urinary concentrations of parabens and reproductive parameters in young men. Sci Total Environ. 2018;621:201–9. doi: 10.1016/j.scitotenv.2017.11.256. [DOI] [PubMed] [Google Scholar]
- 48.Yu Y, et al. Urinary parabens in adults from South China: Implications for human exposure and health risks. Ecotoxicol Environ Saf. 2019;182:109419. doi: 10.1016/j.ecoenv.2019.109419. [DOI] [PubMed] [Google Scholar]
- 49.WHO. Seventeenth report of the joint FAO/WHO expert committee on food additives. WHO Technical Report Series; 2009(539). [PubMed]
- 50.Kiani Feizabadi G, et al. Urinary concentrations of parabens in a population of Iranian adolescent and their association with sociodemographic indicators. Arch Environ Contam Toxicol; 2020. [DOI] [PubMed]
- 51.Frederiksen H, et al. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother–child pairs. Int J Hyg Environ Health. 2013;216(6):772–83. doi: 10.1016/j.ijheh.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Koeppe ES, et al. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013;445:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meeker JD, et al. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect. 2010;119(2):252–7. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith KW, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120(11):1538–43. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engel LS, et al. Predictors and variability of repeat measurements of urinary phenols and parabens in a cohort of Shanghai women and men. Environ Health Perspect. 2014;122(7):733–40. doi: 10.1289/ehp.1306830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain RB. Impact of pregnancy on the levels of parabens and bisphenol A: Data from NHANES 2005–2010. J Chem. 2016;2016.
- 57.Bethea TN, et al. Correlates of exposure to phenols, parabens, and triclocarban in the Study of Environment, Lifestyle and Fibroids. J Expo Sci Environ Epidemiol. 2020;30(1):117–36. doi: 10.1038/s41370-019-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, et al. Variations, determinants, and coexposure patterns of personal care product chemicals among Chinese pregnant women: a longitudinal study. Environ Scie Technol. 2019;53(11):6546–6555. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 69.3 KB)
(PDF 98.5 KB)
(PDF 207 KB)