Abstract
The Persian Gulf consider as the fundamental biological marine condition between the seas. There is a different assortment of marine life forms including corals, wipes, and fish in this marine condition. Mangrove timberlands are found all through this sea-going biological system. Sullying of the Persian Gulf to oil-based goods is the principle of danger to this marine condition and this contamination can effectively affect this differing marine condition. Numerous specialists examined the result of oil contamination on Persian Gulf marine creatures including corals sponges, bivalves, and fishes. These analysts affirmed this oil contamination on the Persian Gulf significantly diminished biodiversity. Diverse microorganisms fit to consume oil-based commodities detailed by various scientists from the Persian Gulf and their capacity to the debasement of unrefined petroleum has been examined. There has additionally been little exploration of cyanobacteria, yeast, and unrefined petroleum debasing organisms in this sea-going environment. Biosurfactants are amphipathic molecules that upgrade the disintegration of oil and increment their bioavailability to corrupt microscopic organisms. Additionally, biosurfactant-producing bacteria were discovered from the Persian Gulf, and the capability to degradation of crude oil in microscale was evaluated. The current review article aims to collect the finding of various researches performed in the Persian Gulf on oil pollution and crude-oil biodegradation. It is expected that by applying biological methods in combination with mechanical and chemical methods, the hazard consequences of crude-oil contamination on this important aquatic ecosystem at the world will be mitigated and a step towards preserving this diverse marine environment.
Keywords: Bacteria, Biodegradation, Marine environment, Oil pollution, Persian gulf
Introduction
Life has begun from the sea and continues to this day. About 70% of the Earth is surrounded by the seawater, and marine organisms include the primary organism to complex and the most diverse species. Increasing human populations have put pressure on many natural sources, and to cope with this growing need, we can take refuge in the resources of the sea, which occupy one-third of the land [1].
The oceans comprise one-third of the Earth. There are various forms of life in the oceans. In recent years, due to human activities, various pollutants have entered the oceans and marine environments, leading to the change of life in these important aquatic ecosystems [2].
Petroleum pollution can enter the seas in several ways, which are divided into two categories: natural oil spill and artificial oil spill. Natural ways such as oil spills from reservoirs and volcanic processes in the deep ocean. Artificial processes include oil tanker accidents, oil transportation processes, oil refineries, oil extraction processes, and petroleum loading processes. In most cases, the main pollution way for contamination of sea with crude oil represents artificial roads [3].
Crude oil has an extremely complex composition of hydrocarbons. In general, crude oil compounds are divided into four fundamental categories and accordingly, there are two types of crude oil: light crude oil and heavy crude oil. Crude oil that has saturated hydrocarbons and polycyclic aromatic hydrocarbons (PAH) called light petroleum, and crude oil that has intenser amounts of resins and asphaltenes compounds than saturated hydrocarbons consider as heavy petroleum. In general, the environmental impacts of heavy crude oil are greater than light crude oil. [4].
Diverse strategies have therefore extremely been employed to remove crude oil from marine environments. Like physical, chemical, and mechanical methods. Each of these methods has advantages and disadvantages for crude-oil removal. The main advantage of these strategies is that they rapidly remove oil contamination from the sea surface, but the disadvantages of these methods are the creation of hazardous chemical intermediates, some of which are more harmful to the initial contamination and on the other hand, these strategies only eliminate pollution from the surface of the sea. However, many petroleum compounds are heavy and sediment deep in the sea.
The use of biological strategies to eliminate oil pollution has been considered in recent years. Biological techniques are slower than the above methods, but they have better advantages to use. Their benefits can be attributed to cost-effectiveness, lack of intermediate products, and complete degradation of pollutants from the marine environment. Biological strategies like biodegradation and bioremediation are the best technologies for conserve marine environments in the future [5–7].
The Persian Gulf has 68% of the world’s oil reserves and also more than 40% of gas resources. Therefore, based on hydrocarbon resources, this marine environment is the richest in the world. As a result, the beaches and islands of southern Iran that are located in the Persian Gulf are constantly exposed to oil pollution. In this way, to study the oil spill in the Persian Gulf is important. Furthermore, the Persian Gulf has a vast diversity of marine invertebrates [8–10].
The Persian Gulf has experienced numerous and huge oil spills in recent years, each of which was enough to pollute any marine area, so the Persian Gulf can be considered the most pronounced victim of an oil spill. The most noticeable and paramount case of the Persian Gulf pollution and contamination is related to the Gulf War spill of 1991, which is considered the biggest oil spill in history. What has entered the Persian Gulf, which covers an area of approximately 239,000 km2 and an average depth of only 35 m [11] or 36 m [12], to be the most polluted marine basin in the world is that the water there is warm and in heated waters, the toxicity level of oil is higher, and weathering takes place much faster [13]. Wars in the past two decades may have increased the pollution loads in the area [14].
Contamination of oil in the marine ecosystems
The pollution of crude oil in the ocean remains a hazardous threat to the existence of the planet earth, which can cause major damage to marine ecosystems and coastal areas. Annually, crude oil typically enters the environment through natural and anthropogenic sources, the former would be sufficient to pollute all of the marine ecosystems. According to an investigation, the annual rate of entered crude-oil in the waters of the world is about 1.3 million tones [1].
For the Persian Gulf that is one of the world’s most significant water bodies, this rate represents 300 tones. Since approximately 60% of worldwide oil transportation takes place through this Gulf, oil contamination constitutes an acute and international threat in the Persian Gulf [15]. Since approximately 60% of worldwide oil transportation takes place through this Gulf, oil contamination constitutes an acute and international threat in the Persian Gulf [15]. An example of oil spill events caused by the human in this Gulf was the war between Iraq and the United States in 1991, known as the second Gulf war. In this conflict, the United States forced Iraq to release petroleum to the Persian Gulf from Mina Al-Ahmadi terminal for three successive days and burned about 700 oil wells. In a comparison between the Persian Gulf and the Gulf of Mexico, the rate of petroleum hydrocarbon concentrations was 1.2–542 (μg l−1) and 0.4–66.8 (μg.l−1) respectively [16–18].
Mixtures of crude oil
The petroleum is a natural, toxic, heterogeneous and complex organic compound and the mixture of hydrocarbons, its mass spectrometry showed more than 17,000 distinct chemical compounds including general alkanes with various chain lengths and branch points, cycloalkanes, mono-aromatic and polycyclic aromatic hydrocarbons. Sulfur, oxygen, and nitrogen can be excessively found in the structure of certain compounds, whereas phosphorus and heavy metals like vanadium and nickel are observed rarely [19]. Because of the considerable differences in the chemical and physical features of oil compounds (for instance solvability, viscosity, capacity to absorb and equally varying in its toxicity and bioavailability), their biodegradation potential and environmental fate are different.
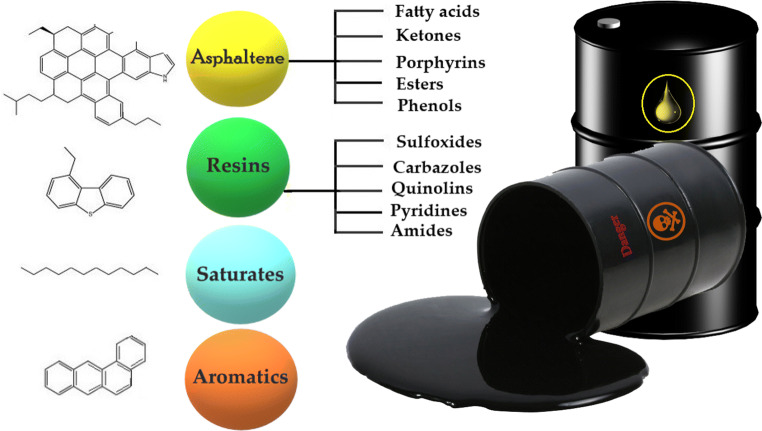
In a classification, petroleum hydrocarbons are categorized in four general classes: Saturated, Aromatics, Asphaltenes (Porphyrins, Esters, Ketones, fatty acids, Phenols), and Resins (Amides, Sulfoxides, Carbazoles, Quinolines, Pyridines). In Fig. 1 the crude oil combination was illustrated. The rate of more polar chemical compounds such as asphaltenes and resins and also saturated and aromatic hydrocarbons are higher in light oils. The resistance of crude oil hydrocarbons to microbial attacks is as follows: n-alkanes > branched alkanes > low-molecular-weight aromatics > cyclic alkanes [20]. Biodegradation process of large branched aliphatic chain and high-molecular-weight aromatic hydrocarbons due to their structural complexity is harder than elementary hydrocarbons. Saturated hydrocarbons remain the most significant group of petroleum compounds which their biodegradation is environmentally significant. Because of the more toxicity and persistence of the aromatic compounds and polar components, they can cause long-time effects on the ecosystem. [21].
Fig. 1.
The Chemical composition of crude oil
Sources of crude oil pollution and effect on marine ecosystems
The crude-oil hydrocarbons are the major pollutants to the environment (crude oil and intermediate products) for biological systems. Among of main biological sources that import hydrocarbons into the sea, can be mentioned to Wax materials of terrestrial organisms, degradation, burning of biological substances, hydrocarbons synthesis of plants, phytoplankton, bacteria, microscopic and large-scale algae. Anthropogenic sources of oil pollution are as follows: exploitation, transportation, refining, or damage to oil tankers. Damage to human health and ecological systems are recognized as the most destructive effects of the oil spill. Other destructive effects of oil pollution are influencing human lifestyle, carcinogenicity, mutagenicity, prevention of light diffusion, oxygen penetration, and consequently the death of organisms due to hypothermia [22–24]. For instance, in the Gulf of Mexico, the Deepwater Horizon explosion in April 2010 with a spill of about five million barrels of petroleum led to vertebrate and metazoan meiofauna reducing biodiversity [25–27].
At the same of an event in Spain over 66% of whole species (containing insects, crustaceans, mollusks, and polychaetes) was eradicated in oily coasts (Prestige, November 2002). Also in the Exxon Valdez spill in March 1989, nearly 41 million liters of petroleum were entered into the sea in the north of Alaska [28]. Hydrocarbons of crude oil can stick to the fur’s mammals and feathers of marine birds, and these organisms have perished when they clean themselves due to overdose hydrocarbon consumption. Detriments caused by hydrocarbons specifically polycyclic aromatic hydrocarbons (PAHs) to fisheries and wildlife can be long-term; for instance, the pollution caused by the Brear spill (Shetland Islands, United Kingdom, 1993) because of polluted fish and shellfish, remained in place for more than six years. Moreover, physiological, behavioral, and genetic damages, declines in both development and fertility in fishes occur as a result of chronic contamination at supra-lethal concentrations [29]. The most extensive oil spill in human history caused by the Gulf War in 1991, in which oil and tar-covered an extensive area more than 770 km of the shoreline from Abu Ali Island in the south of Kuwait (Saudi Arabia) and destroyed the local plants and animals [30].
In cases where natural oil seepages occur, they penetrate from faults and cracks in seabed into water depth [31, 32]. Proverbially, California’s coal oil point, the Timor Sea in Indonesia, and the north of the Persian Gulf are areas where such leakages occur. Coral reefs that are one of the most varied and complex systems in marine ecosystems, harbor an enormous variety of marine organisms and are extremely influenced by petroleum [33, 34]. Abnormalities in reproduction, produced few gonads and immature planulae, abortion, increase in the size of mucus cells (these cells are one of the remarkable defense agents against pathogens and stress which results in cell wall rupture and finally a decreased number of mucus cells can be attributed to petroleum. Numerous magnitude of oil can also have altered the primary composition of Vibrio with the hydrocarbon-degradation capacity of beneficial bacteria like Alteromonas, Pseudoalteromonas, and Pseudomonas [35, 36].
Persian Gulf and importance as a major marine environment in the world
The Persian Gulf is amongst the world’s most various aquatic environments. There is a diverse variety of marine organisms including corals, sponges, and fish in this marine environment. Mangrove forests are found throughout this aquatic ecosystem. In mangrove forests, some complex ecological relationships between organisms live in intertidal regions. Other marine organisms in the Persian Gulf are also widely diversified [37, 38].
Conservation of this aquatic ecosystem in the world is, therefore, more important. In addition to its wide diversity, this marine environment poses a vast range of ecological threats, like the development of the fishing industry and the construction of artificial islands. The critical threat to this marine ecosystem is oil pollution. Oil pollution from various sources can enter the Persian Gulf and threaten the life of this ecosystem and cause the loss of marine life. These sources of contaminants in the Gulf include oil spills from oil reservoirs, shipping incidents, maritime transfers, and oil extraction processes.
Since nearly 60% of the worldwide oil is transferred to the Persian Gulf, oil contamination is inevitable. This has constructed the marine environment the most polluted sea in the world. Oil pollution is unmanaged in the Persian Gulf, many corals, sponges. Mangrove forests will be destroyed soon, and biodiversity will be considerably reduced.
Oil and marine sediments interactions
Oil spill takes place in the marine environment after that alter complex biological, physical, and chemical parameters such as spreading, dispersal, weathering (processes including evaporation, biodegradation, dissolution, and emulsification), drifting and stranding. Despite the dispersion of all oil droplets in the water column, monocyclic compounds (e.g., benzene and alkyl-substituted benzenes) with the log Kow values between 2.1 and 3.7 and selected lower molecular weight, 2–3 ring polycyclic aromatic hydrocarbons (PAHs) with the log Kow values between 3.7 and 4.8 can be partially dissolved [39–41]. Interactions of soluble and diffused oil components and sediment particles extremely affect such environmental processes. After an oil spill in the sea, sediments are accredited as significant vectors for transporting oil from one phase to another [7]. Muschenheim and Lee’s [42] studies, has been shown the role of oil-sediments interactions in scattering and degradation of spilled oil. In nearshore waters, aggregations of oil droplets with suspended particulate material (SPM) are performed via connecting naturally dispersed oil droplets to SPM like organic matter and clay minerals. Following the oil spill, oil diffused in a mixture of sediments and seawater settled and is trapped on the bottom through adhering to sediments [7].
Several experiments have been shown the role of mineral-oil interactions in the natural cleaning of oiled shorelines in Prince William Sound, Alaska, after the Exxon Valdez spill [28, 43–46].
Diffused oil droplets and soluble oil components are two physical forms of oil in water. Also, sediments may come in two forms in water: SPM and settled aggregates. The reaction between diffused oil droplets or soluble oil components with sediments maybe happened in two forms of (1) direct aggregation to form OSAs, and (2) adsorption on or incorporation in the sediment phase [47, 48]. Oil-sediment interactions, one of the most important processes vis-à-vis oil spill, change the destiny and transport of petroleum from the aqueous phase by removing it [49]. It seems that the decrease in oil droplets size can be a factor for the formation of OSAs for oil droplets fixation and inhibition of their re-coalescing [50].
When the oil converts to droplets, increase its surface areas and increasing the availability of oil resulting in enhancement biodegradation rate. After the collision and adherence of oil adsorbed droplets or free phase liquid with solid particles in an aqueous suspension, form the oil-SPM aggregation which is called OSAs. Following electrical charge interactions between the polar oil compounds and particle surfaces, with the cations intermediary as electrical bridges form OSAs [49]. When an oil droplet attaches to solid particles with micrometer-sized, form the most recognizable and common type of OSAs and also sometimes with more than one droplet attachment form multi-droplet OSAs. Three forms of OSAs (droplets, solids, and flake aggregates) are recognized by UV Epi-fluorescence, bright-field microscopy, and scanning electron microscopy (SEM) techniques [51]. Thin sheets of flake aggregates are created by the ordered configuration of oil and solid particles.
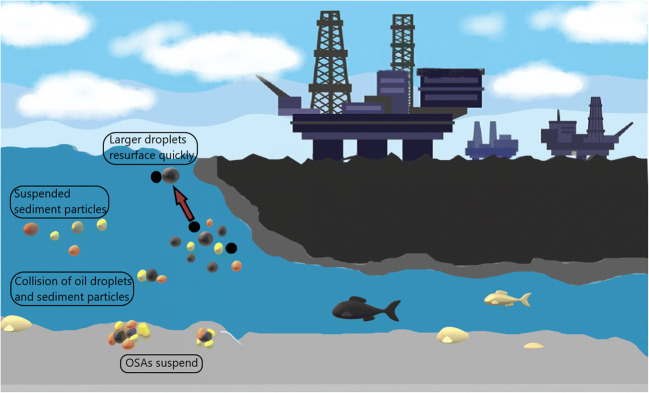
Using confocal laser imaging to evaluate the oil-mineral structures, Omotoso et al. [52] the OSAs are classified based on their buoyancy: (1) Negatively floating flocs caused by interactions of hydrophilic minerals and low-viscosity oils (kaolinite and quartz). The minerals stabilize oil droplets in a water-continuous phase (oil dissipated in water), and (2) positively floating flocs consist of the mineral stabilized oil droplets as well as calcite in an oil-continuous phase (water dissipated in oil) [7, 53]. Steps of OSAs formation are as followed: breaking the oil film into small oil droplets constitutes the first step in such process, which through the outside disturbing forces created by flow fields like inertia or viscous forces and inner restoring forces of the oil like the interfacial tension to can maintain the oil droplet form [7]. For this step, the particular effective variables include the mixing energy, oil viscosity, and interfacial tension between oil and seawater. In the second step, the interaction of SPM and polar compounds in oil droplets leads to OSAs formation. This step can affect by factors like saltiness (or ion power), sort and concentration of oil and sort, and concentration of sediment particle [7]. Figure 2 presents the schematic presentation of the interaction between oil and marine sediment.
Fig. 2.
The interaction between crude oil and marine sediments
Hassanshahian et al. [54] studied the reactions of the natural microbial community in polluted and unpolluted sediments of the Persian Gulf and the Caspian Sea through microcosm experiments. Their results have shown the pollution of crude-oil hydrocarbons maintained a diversity of effects on microbial activity in sediment belongs to the Persian Gulf and Caspian. The rate of Persian Gulf contamination is more rapid than the Caspian Sea. These marine ecosystems from Iran have the ability for bioremediation as well as several bioremediation strategies based on molecular and enzymatic conditions of these two ecosystems need to be selected in the future.
Marine crude oil-degrading bacteria isolated from the Persian Gulf
Crude oil-utilizing bacteria are distributed in marine environments. Many researchers isolated these bacteria in different oceans in the world. Some of these bacteria solely degraded crude oil and were known as Hydrocarbonoclastic bacteria (HCB). The term of Hydrocarbonclastic means eating, breaking, and decrease of hydrocarbon molecules. Diazotrophic bacteria that are scarcely capable of degrading hydrocarbon are called Hydrocarbonoclasticus. Such bacteria would be ideal for bioremediation. The marine hydrocarbon-degrading bacteria namely the genera: Alcanivorax, Cycloclasticus, Oleispira, Oleiphilus, and Thalassolituus can only inhabit on hydrocarbons in marine ecosystems [55].
Several investigations in the past two decades have been accomplished on non-symbiotic hydrocarbonoclasticus bacteria and their function in its self-cleaning in the Persian Gulf [56–59].
Picocyanobacteria exist in abundance in surface layers of the whole saltwater bodies such as the Persian Gulf and are associated with hydrocarbonoclasticus bacteria. Such communities are effectively involved in the bioremediation of oil spills. The initial hydrocarbon-utilizing bacteria were isolated nearly one century ago. In recent years, surveys revealed that 14 algal genera, 103 fungal genera, 79 bacterial genera, and nine Cyanobacterial genera demonstrate the potential of degrading and transform hydrocarbons [58, 59]. In anaerobic, oil-degrading sulfate-reducing bacteria carry out this role. To discover genetic, biochemistry basis, and arrangement of hydrocarbon-utilizing pathways some oil-utilizing bacteria have been applied as a model [60]. Such models (e.g. Alcanivorax borkumensis) degrade hydrocarbon during lack of nutrients like nitrogen and phosphorus. Various hydrocarbon-utilizing bacteria have been isolated from the Persian Gulf which Proteobacteria, for instance, Gamaproteobacterial genera (e.g. Acinetobacter, Marinobacter, and Alcanivorax) and Alphaproteobacterial genera (e.g. Tissrella and Zavarzina) and also Actinobacteria are the dominant cultivable marine bacteria [61–64].
Marine ecosystems all around the world such as the Persian Gulf are considered as appropriate habitat for native hydrocarbon-degrading bacteria with the potential of stick to animate and inanimate substrates and biofilms formation [65–67]. These bacteria belong to the genera Marinobacter, Microbacterium, Rhodococcus, Kocuria, Alcanivorax, Microbacterium, Pseudomonas, Pseudoalteromonas, Dietzia and some others which approximately all of them are found in the aerobic zone of marine sediments [56, 57, 68–70, 86].
In the past decade, various hydrocarbon-utilizing bacteria, for example, Thalassolituus spp., Oleiphilus spp., Oleispira spp., Cycloclasticus spp., Alcanivorax spp. and some genera belong to Planomicrobium have been isolated formerly known as Planococcus [55, 71]. Among the above-mentioned cases, Thalassolituus spp., Oleiphilus spp., Oleispira spp., Alcanivorax spp. possess the potential of degrading saturated hydrocarbons with straight-chain and or branched-chain whereas Cycloclasticus spp. degrade a variety of polycyclic aromatic hydrocarbons [21]. The alkaliphilic oil-utilizing bacteria include Marinobacter, Citricoccus, Oceanobacillus, Micrococcus, Bacillus and Dietzia and also the halophilic oil-degrading bacteria comprise Microbacterium, Cellulomonas, Stappia, Marinobacter, Isoptericola, Bacillus, and Georgia [72].
Such alkaliphilic and halophilic bacteria were isolated from the Persian Gulf and then recognized by their 16S ribonucleic acid sequences. Most of these bacteria are capable of developing an extensive range of aromatic compounds and pure n-alkanes. Quantitative Gas-Liquid Chromatographic analysis (GLC) indicated that individual isolated bacteria may attenuate petroleum and represent pure hydrocarbons in the culture medium [72]. In a review of marine sediments, incubation of sediments in the presence of phenanthrene and bromodeoxyuridine (BDU) followed by an analysis of BDU-labelled DNA determined considerable variety of PAH-degrading bacteria belong to the genera Bacteroides, Shewanella, Pseudomonas, Methylomonas, Exiguobacterium and also Deltaproteobacteria and Gammaproteobacteria that were nearly unaffiliated to cultivated organisms [73].
In the same way, stable-isotope probing (SIP) of DNA was applied to determine the involvement of a novel clade of Rhodobacteraceae in biodegradation of low molecular weight (LMW) PAHs in marine algal bloom [74]. In the course of investigation in the Persian Gulf reported slurry and microbial mat samples rich in filamentous Cyanobacteria, Picocyanobacteria, and cultivable oil-degrading bacteria, according to their 16S rRNA gene sequences were associated to Marinobacter hydrocarbonoclasticus, Halomonas aquamarina, Marinobacter sp.; Dietzia maris and Alcanivorax sp. [75, 76].
These bacteria are diazotrophic and consume a high variety of individual aliphatic and aromatic hydrocarbons. In a report illustrated Halomonas genetically close to Marinobacter [77] besides the bacteria referred above, certain uncultivable bacteria can also degrade crude oil. The predominant Halomonas bacteria couldn’t grow on all the aliphatic and aromatic sectors unlike other cultivable utilizing-oil bacteria in pristine slurry samples. Heretofore, various hydrocarbon-degrading bacteria namely Pseudomonas, Nocardia, Vibrio, Acinetobacter, Achromobacter, Alcanivorax, Marinobacter, Sphingomonas, Micrococcus and MS1 (Halomonas) have been caught from Kish Island in Iran [78]. The genus Halomonas, includes more than 20 species, are among the larger moderate halophilic bacterial groups with biodegradation potential of hydrocarbon pollutants for the first time proposed by Vreeland. The diverse arrangement of alkane hydroxylase systems in Acinetobacter spp., that generally isolates from oil-polluted marine environments enables them to metabolize both long and short-chain alkanes. Proverbially, the consumption of C32 and C36 n-alkanes by Acinetobacter strain DSM 17874 is due to a flavin-binding monooxygenase (AlmA). Also, this gene has been observed in Alcanivorax dieselolei B-5 which is caused by long-chain n-alkanes of C22 - C36 [79].
The genus of Alcanivorax can degrade branched and straight-alkanes. The species of Alcanivorax borkumensis despite the lack catabolic versatility, it has several alkane-catabolism pathways with key enzymes like alkane hydroxylases (a non-heam diiron monooxygenase, AlkB1, and AlkB2) and three cytochrome P450-associated alkane monooxygenases and almost exclusively utilizes alkanes as carbon and energy sources [80]. This bacterium has been adapted for the availability of oil through the synthesis of emulsifiers and producing of biofilm as well as for survival in distinct marine ecosystems (e.g. scavenging nutrients and ultraviolet resistance). Similarly, in oil-spill bioremediation experiments carried out in laboratory microcosms and in the field, 16S ribosomal RNA (rRNA)-gene sequences from Alcanivorax spp. were undetectable in control experiments in which samples were untreated with oil, but within 2 weeks of oil treatment, they consisted of more than 30% of the sequences in libraries of 16S-rRNA gene clones constructed from oil-treated sediments and more than 70% of the sequences recovered from sediment treated with oil and inorganic nutrients [81].
These results were mirrored by the detection of alkB genes, which encode the catalytic component of alkane hydroxylase, only in samples in which Alcanivorax spp. 16S-rRNA genes were abundant [21]. It is traditionally believed that these organisms are normally present in very small numbers, and utilizing of the hydrocarbons as a carbon and energy source causes their growth and reproduction rapidly. Alcanivorax-like bacteria have directly been detected in oil-impacted environments across the globe. They have been isolated or detected in culture-independent bacterial community surveys from the United States, Germany, the United Kingdom, Spain, Italy, Singapore, China, the West Philippines, Japan, the Mid-Atlantic Ridge near Antarctica, and from deep-sea sediments from the eastern Pacific Ocean [36, 81, 82]. Presumably, the importance of Alcanivorax in contrast to other hydrocarbon-degrading bacteria is due to its capability to branched-chain alkanes degradation efficiently. Because of Pristine entering the sea through various sources such as oil spills and marine plankton, Alcanivorax with the potential of consumption these hydrocarbons maintain extensive distribution. There is a similar status for Cycloclasticus spp., which carry out a worldwide and significant role in biodegradation through the consumption of oil spilled aromatic hydrocarbons in marine environments [21].
A study realized that Cycloclasticus increase by amendment of oil-contaminated gravel with inorganic nutrients. Likewise, culture-based researches have indicated an abundance of Cycloclasticus than other polycyclic aromatic hydrocarbon utilizing bacteria (PAHs) in the Gulf of Mexico and Puget Sound (United States), particularly in oily sites. In addition to Cycloclasticus that regarded as the most dominant marine PAH-degrading microbe, the genera of Vibrio, Pseudoalteromonas, Marinomonas, marinobacter, Cycloclasticus and Halomonas isolated from San Diego Bay sediments also can grow on chrysene or phenanthrene [83].
Hassanshahian et al. [58] isolated different petroleum utilizing bacteria from the Persian Gulf. They isolate 25 petroleum utilizing bacteria from the Persian Gulf and the Caspian Sea sediments in their research. The molecular recognition confirmed that these degrading bacteria belong to these genera: Acinetobacter, Cobetia, Gordonia, Rhodococcus, Pseudomonas, Halomonas, Microbacterium, Marinobacter, and Alcanivorax. Their results confirmed that petroleum degrading bacteria in Iran are capable of biodegradation at a high level. Using such bacteria for bioremediation goals can easily reduce the rate of oil contamination in the Persian Gulf and the Caspian Sea.
Hassanshahian et al. [8–10] screened fifteen petroleums utilizing bacteria from oil-polluted sites in the Persian Gulf at Khorramshahr provenance. One strain that shows high crude oil degradation belongs to the Corynebacterium variable. Their results indicate that effective petroleum degrading bacteria exist in the Persian Gulf. Besides, they found that petroleum biodegradation by C. variable strain PG-Z maintains a direct relationship with the production of biosurfactant. The Table 1 shows the crude oil-degrading bacteria isolated from the Persian Gulf.
Table 1.
The crude oil degrading bacteria that isolated from the Persian Gulf
| Bacteria name | Isolation source | Reference |
|---|---|---|
| Bacteria | ||
| Alcanivorax | Persian Gulf | Rosenberg et al. 2006 |
| Isoptericola | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Cellulomonas | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Bacillus | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Oceanobacillus | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Citricoccus | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Stappia | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Georgenia | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Marinobacter | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Micrococcus | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Microbacterium | Kuwait,Persian Gulf | Al-Awadhi et al. [57] |
| Psychrobacter | Kuwait,Persian Gulf | Radwan et al. [84] |
| Vibrio | Kuwait,Persian Gulf | Radwan et al. [84] |
| Planococcus | Kuwait,Persian Gulf | Radwan et al. [84] |
| Pseudomonas | Kuwait,Persian Gulf | Radwan et al. [84] |
| Actinobacterium | Kuwait,Persian Gulf | Radwan et al. [84] |
| Dietzia maris | Persian Gulf | Al-Mailem et al. [61, 85] |
| Halomonas aquamorina | Persian Gulf | Al-Mailem et al. [61, 85] |
| Gordonia bronchialis | Persian Gulf | Al-Mailem et al. [61, 85] |
| Zavarzinia | Persian Gulf | Al-Mailem et al. [61, 85] |
| Flavobacterium | Persian Gulf | Al-Mailem et al. [61, 85] |
| Gaetbulibacter | Persian Gulf | Al-Mailem et al. [61, 85] |
| Owenweeksia | Persian Gulf | Al-Mailem et al. [61, 85] |
| Tistrella | Persian Gulf | Al-Mailem et al. [61, 85] |
| Nocardia | Kish Island, Iran | Hassanshahian et al. [86] |
| Achromobacter | Kish Island, Iran | Hassanshahian et al. [86] |
| Sphingomonas | Kish Island, Iran | Hassanshahian et al. [86] |
| Halomonas sp. MS1 | Kish Island, Iran | Hassanshahian et al. [86] |
The marine cyanobacteria of the Persian Gulf and their importance in biodegradation
Cyanobacteria represent ancient photosynthetic prokaryotes, autotroph, anaerobic and often mobilizing, which also known as turquoise bacteria and cyanophytes. The effect on biodegradation of petroleum hydrocarbons considers as the most remarkable function of cyanobacteria. These organisms are both involved in hydrocarbon oxidation and in limiting the growth of oxygen-dependent oil-degrading bacteria [15, 87, 88]. In the intertidal zone, cyanobacteria colonized in the presence of oil and started growing on top of the oiled sediments [89]. Increasing the amount of cyanobacteria is offered as the first step of bioremediation [89]. The growth of cyanobacteria is limited through grazing pressure by benthic animals and where bioturbation caused by crabs and polychaetes leads to destabilization of sediment surface but after the resettlement of sediments their growth resumed and a deep layer was created [30, 90].
Such layers inhibit oil degradation and resettlement by macro-fauna through the attachment of sediments and producing an anaerobic environment. On the other hand, these mats carry out an indirect and significant role in biodegradation which amplifies the rate of activity and growth of aerobic oil-degrading bacteria. In a study, was examined the ability of ten non-axenic typical mat-forming cyanobacterial strains to attenuate dibenzothiophene, n-octadecane, pristane and phenanthrene. Five strains (Aphanothece halophyletica, Dactyolococcopsis salina, Halothece strain EPUS, Oscillatoria strain OSC, and Synechocystis strain UNIGA) had the potential to degrade n-alkanes. Whereas in the other five strains (Microcoleus chthonoplastes, Oscillatoria sp. MPI 95 OS 01, Halothece strain EPUG, Halomicronema excentric, and Phormidium strain UNITF) were not observed significant results [91].
Cyanobacterial mats that isolated from a bloom of Phormidium spp. and Oscillatoria spp. In oily sabkhas along the Gulf of Suez coasts (Africa) and the pristine solar lake (Sinai) indicated effective petroleum degradation in the light. Cyanobacteria in the light contribute to biodegradation of hydrocarbons by the production of oxygen for the aerobic heterotrophic bacteria such as Marinobacter, and in lack of light (anaerobic sulfide-rich habitat) with affecting on sulfate-degrading bacteria. Two picocyanobacterial strains related to Acaryochloris, isolated from the north and south shore of Kuwait, in three meters below the water surface. Both strains were ultrastructurally, morphologically and phylogenetically (to a lesser extend) similar to Acaryochloris. However, in both strains, chlorophyll a is the particular photosynthetic pigment and missed chlorophyll d.
Both picocyanobacterial isolates were related to oil-degrading bacteria in the magnitude of 105 cells g−1. Their 16S rRNA gene sequences indicated that related bacteria isolated from the north were associated with Paenibacillus sp., Bacillus pumilus, and Marinobacter aquaeolei, but those related that isolated from the south were associated to Bacillus asahii and Alcanivorax jadensis. These bacterial diversities occur presumably because of environmental variations [92].
The oil-degrading yeasts and fungi at the Persian Gulf
Fungi can adapt and resist extreme environments. Fungi are considered to remain notable factors in the process of bioremediation. The process of remediation by fungi is preferred to approaches microbial degradation due to their potential to cultivate large groups of substrates. Fungi nonspecifically action in PAHs degradation and can hydroxylate numerous xenobiotic. The process of biodegradation occurs more gradually by fungi in contrast to bacteria [93]. Also, the ability to the consumption of PAHs like Benzo[α] pyrene and biosurfactant production increases the importance of these organisms. Among oil hydrocarbons-degrading, fungi can imply to Aspergillus, Penicillium, Fusarium, Amorphotheca, Neosartoria, Paecilomyces, Talaromyces, Graphium Cunninghamella [94]. Such function can also observe in yeasts such as Candida, Clavispora, Debariomyces, Sporobolomyces, Leucosporidium, Lodderomyces, Rhodosporidium, Rhodotorul. Esporidiobolus, Trichosporium.
Fungal extracellular enzymes penetration into the contaminated soils is known as one of the removing pollutant approaches [95]. For fungi, the function of degrading enzymes depends on agents such as accessibility of the nutrient, oxygen, pH, temperature, and chemical structure [96]. In the past few years, ligninolytic fungi have been extremely investigated for their ability to degradation, hydrocarbons mineralization, and irregular structure of lignin [97]. Major extracellular enzymes in the lignin system containing lignin peroxidases, manganese peroxidases, phenol oxidases involve in the PAHs biodegradation. Manganese peroxidases oxidize PAHs using other enzymes whereas lignin peroxidases directly lead to PAHs oxidation [98].
In addition to the mentioned cases, fungi’s enzymes like epoxide hydrolases, proteases, monooxygenases, dioxygenases, and lipases are capable of PAHs degradation [99]. An experiment by Novotny et al. proved the importance of MnP and laccase in biodegradation some compounds such as pyrene and anthracene by Tramtes versicular, Pleurotus ostereatus and Phanerachaeta chrisosporium. Studies on Aspergillus niger, A. ochraceus and Penicillium chrisogenum isolated from the coasts of Oman (Persian Gulf) by Snellman et al. [100] revealed significant differences between these species in the consumption of C15, C16, C17, and C18 and also in magnitude of produced biomass on C13, C17, C18, and crude oil. Statistically the biomass and petroleum degradation coefficient for these species is as follows the correlation coefficient of biomass and oil utilization for these species is as follows: A. niger > A. terreus > P. chrysogenum [101].
Hassanshahian et al. [59] studied petroleum degrading yeast from the Persian Gulf. They isolated six degrading yeast from the petroleum polluted region at the Persian Gulf and after screening analysis two strains were selected because of show high oil degradation. These two strains belong to Yarrowia lipolytica. Their results confirmed there was a relationship was between both the cell the yeast strains hydrophobicity and their emulsification and petroleum degradation and a reduction in surface tension. Ultimately, we concluded that these 6 strains could be useful in the Persian Gulf bioremediation cycle and the reduction of petroleum contamination in this aquatic environment.
Isolation of oil-degrading bacteria from marine organisms at the Persian Gulf
Various marine organisms including aquatic fauna, blue-green algae [84, 89], epilithic algal biomass [102] contain the most levels of hydrocarbon-degrading bacteria. In studies on 10 species of fish isolated from the Persian Gulf and two species from Fish, farms confirmed that millions of hydrocarbon-degrading bacteria exist per square centimeter of Fish surface and per gram of fish, that these bacteria belong to Psychrobacter, Vibrio, Planococcus, Pseudomonas and Actinobacterium [103]. Actinobacteria have recently been thought to be endemic to the marine ecosystem and their existence is due to be washed into the sea from nearby territories [103]. Although, Bull et al. [104] find Actinobacteria (Rhodococcus and Dietzia), and Austin [105] finds Microbacterium to be marine microflora indigenous.
These three bacteria, which are Fish-associated, could degrade petroleum [106–108]. In addition to fish isolates, planktonic and benthic microflora also accommodated oil-utilizing bacteria. Phytoplankton provides a better-aerated environment for bacteria in contrast to fishes consequently, bacterial diversity of phytoplanktonic and fish samples differs because of various environmental conditions. Whole samples could grow on many aliphatic and aromatic hydrocarbons as single sources of carbon and energy [103].
Corals, one of the bacterial hosts, harbor fewer bacteria than marine plants and animals. In an experiment by [109], determined isolated coral samples from the petroleum-contaminated beaches of Qaro and Umm Al-Maradim Islands contained oil-utilizing bacteria. Oil-degrading bacteria in produced mucose by P. compressa and A. clathrata are more abundant than tissue samples because of their adhesion properties [109]. Grossart [110, 111] expressed that Thalassiosira rotula (diatom), and a solitary copepod can accommodate 108 and 109 hydrocarbons-degrading bacteria, respectively [112].
Mussels, oysters, and clams (filter-feeding bivalves) remain extremely significant components of the marine ecosystem bioremediation cycle. They are capable of collecting and storing a significant number of small particulate matter such as phytoplankton, zooplankton, microorganisms, and other organic particulate material. Otherwise, organic components in suspended cases may be snared and used by filter-feeding bivalves. Suspended matters can be trapped and applied by filter-feeding bivalves.
Bayat et al. [113, 114] analyzed relationships between mussels and bacteria in oil-utilizing aquatic ecosystems. They screen petroleum-utilizing bacteria from numbers of mussels that are isolated from hydrocarbon-polluted zones of Qeshm island in the Persian Gulf. 28 petroleum-utilizing bacteria were collected from three mussels isolated from the hydrocarbon-polluted region at Qeshm Island. According to more growth and degradation of petroleum, four samples were selected between these twenty-eight strains for research. These strains were Shewanella algae, Micrococcus luteus, Pseudoalteromonas sp., and Shewanella haliotis.
In another study, Bayat et al. [115] research on symbiosis relationship between petroleum-utilizing bacteria and Mactra stultorum (mussel). They select bivalves according to their importance for estuarine and coastal communities. Bivalves filter vast quantities of water to satisfy their food needs and collect dissolved oil components and hydrocarbon-containing particles found in hydrocarbon-contaminated water regions. Bivalves can be filtered and concentrate bacteria may lead to raising the concentration of bacteria in the marine environment. In the mussel samples, bacterial concentrations were more elevated than the seawater during the year, thereby carry out a critical role in the marine environment’s bioremediation cycle. They succeeded to isolate Alcanivorax dieselolei and Idiomarina baltica from this mussel at the Persian Gulf.
Isolation of crude-oil degrading microorganisms from a harsh environment
A harsh environment can universally be considered as a setting in which an organism’s survival is difficult or impossible. A harsh environment called the Persian Gulf, Owing to temperatures of up to 50 °C and high evaporation, the water salinity can exceed 16%. Various microbial mat systems coat the coastal flats of the Gulf [116], which These systems undergo varying environmental conditions daily from mild to an extreme due to tidal conditions [117].
Hypersaline offshore areas in Kuwait are considered the super tidal “Sabkhas”, where the rate of degradation hydrocarbon and growth of extremely halophilic and oil-degrading bacteria and archaea [61, 62, 118] increase using special modifications including salts (K+ and Mg2+) and pure vitamins [61, 62]. The variations in environmental conditions can lead to diversification of microorganisms. For instance, the differences in the Denaturing Gradient Gel Electrophoresis (DGGE) profiles at various tidal positions demonstrate that the desiccation effect on the mats bacterial composition is different from their wetting effect [91].
Harsh environmental conditions may be considered as a natural obstacle to hydrocarbon degradation. Proverbially, enhancing salinity more than 15% prevents hydrocarbon biodegradation. The cyanobacterium Microcoleus chthonoplastes isolated from hypersaline areas in the Persian Gulf [119] have been indicated that able to survive even at salinities 12% in culture media [120]. Also, a study on Deinococcus showed the presence of microbial communities that are UV resistant [121].
In a work, oil-utilizing bacterium Bacillus licheniformis collected from Dagang oilfield that would be ideal for biosurfactant production, indicated resistance to high salinity and temperature [122]. Hambrick et al. [123] and Peyton et al. [124] have been investigated the biodegradation of hydrocarbon in alkaline environments. Sarnaik and Kanekar [125], Maltseva et al. [126], Maltseva and Oriel [127], Kanekar et al. [128] and Yumoto et al. [129, 130] showed alkaliphilic hydrocarbon-utilizing bacteria are capable of biodegradation of organic compounds. In an experiment on two diazotrophic, halophilic and hydrocarbonoclasics bacteria, Marinobacter sedimentarum and M. flavimarsis, isolated from shores of Kuwait were found these strains didn’t survive without NaCl and illustrated the rate of the highest growth and hydrocarbon degradation at 5 M NaCl concentration. Further, these strains mineralized crude oil in hypersaline media in the absence of any azote (N) additive. Among halophilic microorganisms that are capable to degrade many types of hydrocarbons at high salinities can be mentioned to bacteria such as Marinobacter sedimentalis, Halomonas salina and Pseudomonas sp., Archaea like Halobacterium salinarum and Haloferax larsenii and also fungi [131].
Fakhrzadegan et al. [132] studied petroleum-utilizing bacteria in mangrove forests at the Persian Gulf. Mangroves forests consider a harsh marine environment because these forests are environments discovered in tropical and subtropical areas all over the world. There are these forests in the regions between terrestrial and aquatic environments; because of their geographical distribution, these ecosystems have been seen in the Americas, Africa, Asia, and Oceania. Mangroves are remarkably resistant to harsh conditions such as low nutrient and oxygen levels and uncommon temperatures.
These forests are placed along the Iranian shores and around Bahrain, Qatar, Saudi Arabia, and the United Arab Emirates. Mangrove forests in Iran are filled with two species of trees: Avicennia marina and Rhizophora mucronata. A. marina represents the most abundant species, that covering more than 90% of the mangrove ecosystems of the Oman Sea and the Persian Gulf. The production of R. mucronata is restricted and principally confined to the rivers of the Syriac region (including Hara and gas creeks).
They concluded that these forests are the most susceptible marine ecosystem against hydrocarbon pollution, and therefore preservation of these habitats is significant for protecting the marine animal and plant species. Researches confirmed that in this Persian Gulf ecosystem petroleum-using bacteria have ample diversity and density. These bacteria can reduce petroleum contamination in this significant marine environment by using some strategies such as biostimulation and bioaugmentation. They characterized these genera as petroleum-utilizing bacteria in the mangroves around the Persian Gulf: Vibrio sp, Idiomarina sp, Kangiella sp, Marinobacter, Halomonas sp, and Vibrio sp.
Biosurfactant and their role in biodegradation
Biosurfactants are amphiphilic and active compounds with biodegradation ability, good adaptation to the environment, higher foaming, lower toxicity, high selectivity, stability and specificity at excessive temperatures, pH and salinities, which are produced by microorganisms including bacteria, fungi, and yeasts [133]. 3 analyses namely Using nuclear magnetic resonance spectroscopy (NMR), X-ray diffraction (XRD), and thermal gravimetric (TG) respectively, indicated the functional groups, surface nature, and biosurfactant thermos-stability [134].
The produced biosurfactant by these microorganisms either binds to the cell surface or is sending to extracellular in the growing culture media. Such compounds are employed in food, pharmaceutical [135, 136] and cosmetic industries, also increasing spilled oil and aromatic hydrocarbons biodegradation by microorganisms [137]. Glycolipids consist of rhamnolipid, trehalose, and sophorolipids, also lipopeptides including Surfactin, Gramicidin S, and Polymyxin represent extensive groups of low molecular-weight biosurfactant [138–140].
Substantially, rhamnolipid leads to increasing hydrocarbon bioavailability and its degradation. But according to a report rhamnolipid inhibited only Sphingomonas sp. In pure culture, whereas limited phenanthrene degradation by a group of 2 species bacteria namely Sphingomonas and Paenibacillus sp. [141]. Stress enhanced due to the solubilized phenanthrene, or the rhamnolipid and phenanthrene together [1]. High molecular weight EPS is a heterogeneous polymer consist of polysaccharides, proteins, lipopolysaccharides, lipoprotein or such biopolymers ‘complex mixtures which can serve a role corresponded to biosurfactants. Biosurfactant production is responsible for enhancing the bioavailability of PAHs. Despite the inhibition of specific microbes, biosurfactants are benefiting others through increasing the hydrophobic compound bioavailability and consequently recognizing as “common goods” [142].
Biosurfactants could act as antagonists and are known in many pathogens as virulence factors. They alter the hydrophobic property of surface cells. The hydrophobic substrates surface via diminishing the culture surface tension enhances by biosurfactant results to increase bioavailability. Hydrocarbon-degrading bacteria including Bacillus licheniformis isolated from Dagang oilfield [122], Rhodococcus erythropolis M-25 and Alcanivorax borkumensis are capable of biosurfactant production [143].
In an investigation on hydrocarbon-degrading bacterium with the potential of biosurfactant production, Bacillus methylotrophicus USTBa, observed that produced biosurfactant by this strain could drastically reduce the water surface tension [134]. It displayed a 90% emulsification activity on petroleum and had caused antibiotic activity inhibition of many bacteria even though it is not an inhibitor for various vegetables [134].
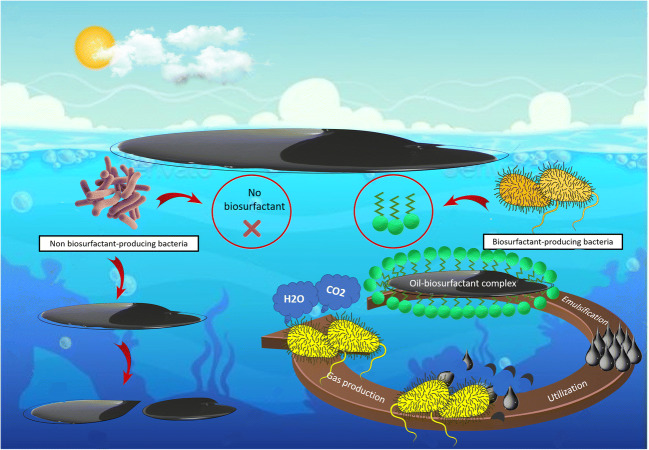
Pseudomonas aeruginosa sp. ZN, biosurfactant producing bacterium with high-ability, was selected from oil-contaminated soils at Ahvaz City (southern of Iran). The synthesis of biosurfactant in BH2 culture medium modified with 1% n-hexadecane occurred in the course of the exponential process causing a decrease in surface tension. This biosurfactant possesses distinct properties to other Pseudomonas strains. The created biosurfactant was unable to separate stable emulsion of span-80-kerosene: Tween80-distilled water within 1 day. The created biosurfactants could be enhancing the bacterial cell hydrophobicity [144]. In terms of Chirwa and Bezza, experiments occur an enhancing in the rate of hydrocarbon biodegradation when the biosurfactant was present. Investigation of a group of eight distinct species isolated from the French beach by [145] determined that biosurfactant only using single pure strain didn’t exhibit emulsification petroleum and rapid hydrocarbon degradation required the whole bacterial communities. The Schematic representation of biosurfactant function on enhancing crude-oil degradation was shown in Fig. 3.
Fig. 3.
The effect of biosurfactant on crude oil biodegradation
Hassanshahian et al. [8, 9] reported some of the bacteria that produce biosurfactants from the Persian Gulf. He used some screening techniques to select the best bacteria in terms of biosurfactant production from samples that were isolated from petroleum-contaminated areas of the Persian Gulf (coastline of Bushehr provenance). The genera that he named as best biosurfactant producers were Shewanella alga, Shewanella upenei, Vibrio furnissii, Gallaecimonas pentaromativorans, Brevibacterium epidermidis, Psychrobacter namhaensis, and Pseudomonas fluorescens bacteria.
Factors influencing the degradation of crude oil in marine environments
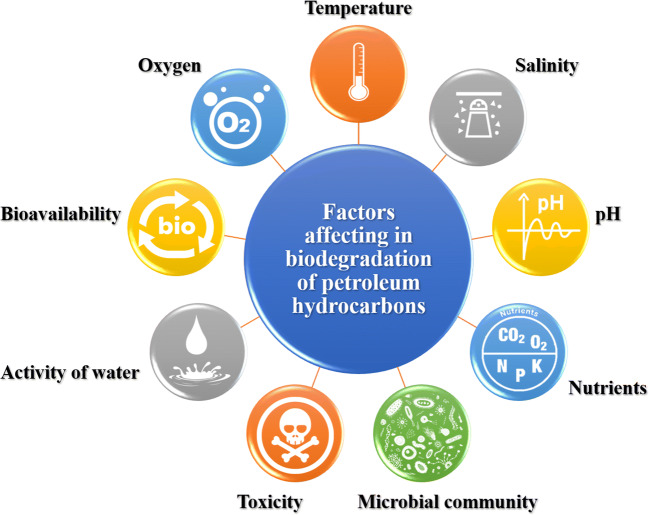
Several agents including temperature, oxygen, pH, and nutrients can dramatically be useful on microbial activity that is described as follows. Also in Fig. 4 as schematic representation, these factors were shown.
Fig. 4.
Some factors that affect crude oil biodegradation on marine environment
Temperature
Temperature results in alteration of the physical and chemical nature of oily compounds [94]. At low temperature, due to reducing the enzymatic activities notably decrease the degradation rate [146]. The maximum biodegradation in the different ecosystems (land, sea, and freshwater) happened at the range of 30–40°C, 20–30°C and 15–20°C [94] Development of metabolic capabilities through genetic modifications, infusion of different enzymes and eclectic enrichment of microorganisms can successfully lead to biodegradation. Reportedly, The degradation of Metula crude oil using mixed marine bacterial cultures is likely at 30°C [94]. Also, Hassanshahian et al. [147] expressed that the crude-oil degraded in the soil at 30°C. Also, the degradation of hydrocarbon in sediments is restricted at low temperatures in winter [94].
Oxygen
Hydroxylases (oxygenases), key enzymes in the biodegradation process, via oxygen production and oxidation of hydrocarbon substrates that are dependent on molecular oxygen for microbial mats involved in the decomposition of all hydrocarbons [148–150]. The rates of oxygen consumption by microorganisms, soil type, and the usable substrate presence affect the concentration of oxygen in the soil. In the molecular oxygen absence, alkyl-substituted aromatics and non-substituted metabolizing to benzene, 1,3-dimethyl benzene, and acenaphthene, naphthalene, toluene and xylene by soil microbial consortia [151]. In anaerobic conditions, hydrocarbons biodegradation is slower compared with aerobic conditions [152].
pH
The rate of pH can be extremely variable. The environmental pH change alters enzyme activities, Transport of cell membranes, and balancing catalytic reactions. Heterotrophic fungi and bacteria prefer the natural to alkaline acidity (in comparison with pH of the other aquatic environments) and fungi are more resistant than bacteria in acidic conditions. The rate of soil pH is 2.5–11 in alkaline lands [94]. The pH rate matters in improving biological treatment methods. The theory of biological treatment is to remove toxins and contaminants from enclosed sites by consuming microorganisms. Naphthalene and octadecane are mineralized by microorganisms at a pH of 6.5 [94]. It equally found that increasing in pH from 6.5 to 8.0 did not affect the mineralization rate of naphthalene whereas this parameter notably increased in octadecane [94]. Thavasi et al. [153] reported pH 8.0 was the most appropriate acidity for the highest petroleum biodegradation in water by Pseudomonas aeruginosa. Because of Pawar [154], the most suitable for pHs biodegradation was at pH 7.5. Burkholderia cocovenenas bacterium isolated from contaminated soils ranging from pH 6.5–7.0 shown the maximum rate of phenanthrene degradation in liquid media (Hassanshahian et al., 2014c).
The most effective nutrients in biodegradation include nitrogen, phosphorus, and iron (Al − Hawash et al. 2018). Furthermore, certain nutrients may become a limiting biodegradation factor. A remarkable enhance of carbon and reduction of phosphorus and nitrogen caused by oil spills can be effective in the biodegradation process. In wetlands, the biodegradation by microorganisms requires adding nutrients since nutrient consumption by plants. In other words, nutrient accumulation can limit the biodegradation (Al − Hawash et al. 2018).
Also, the abundance of PAHs limits the growth of microorganisms that developed a response to hydrocarbons concerning the cell membrane structure, sporulation alterations and pigmentation of mycelia [156]. A study on fungal strains including Penicillium chrysogenum, Lasiodiplodia, Theobromae, and Mucor racemosus determined that sucrose and cellulose include the best carbon sources for lipase production [99]. The best catalyst of high extent of lipase production in this fungy is yeast extract. Also, the degradation of hydrocarbons by Trichoderma hypocrea was investigated by pyrene as a carbon source [157].
After adding the extract of yeast, lactose or sucrose, the strain grows and degrading of pyrene interestingly increased after 1–2 weeks of incubation [157]. Fish peptides and amino acids can provide nitrogen for oil-utilizing bacteria [103].
Bioavailability
The effect of microbiological, physical and chemical agents on the level and rate of biodegradation defines as “bioavailability”.
The bioavailable part of the hydrocarbons in the area that can be access to microorganisms. Hydrophobic organic pollutants such as PHs have low bioavailability and their little water solubility leads to resistance to photolytic breakdown and chemical-biological [158]. Low local microbial biodiversity or the lack of local hydrocarbon-degrading microbes restrict biodegradation and presumably result in incomplete degradation of high molecular weight hydrocarbons [159].
Water activity
The growth and movement of microorganisms directly depend on water availability. Consequently, in terrestrial environments the biodegradation of hydrocarbons restricted since the scarcity of water. Illustrated the biodegradation was maximum when oil sludge saturate with 30–90% water [94].
Biodegradation mechanism of crude oil
The most organic pollutants quickly and completely degrade under aerobic conditions. Instantly, these pollutants take oxide and active states, and oxygen is formed through peroxidases and oxygenases. Peripheral degradation mechanisms of hydrocarbon pollutants include several steps which in the course of are produced intermediates like the tricarboxylic acid cycle [94]. The Key Precursor Metabolites, such as the pyruvate, succinate, and acetyl-CoA lead to the cell biomass biosynthesis.
The Gluconeogenesis process provides the saccharides necessary for growth and biosynthesis. The mechanisms like attachment of microbial cells to substrates and production of biosurfactant and also enzyme systems activities involve in PHs degradation [160]. Selectively metabolizing PHs may be possible both through a particular strain of microorganisms and through a microbial strain-relevant consortium of the same or dissimilar genera [161], but in the consortium occurs more degradation compare with the distinct cultures [162].
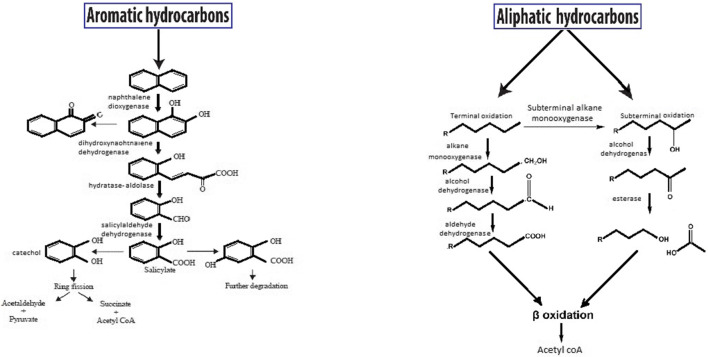
Enzymes are included as one of the efficient key factors for PHs degradation. The microbial degradation of many compounds such as chlorinated oil, PAHs and other hydrocarbons happen by cytochrome P450 hydroxylases [163]. Scheller et al. [164] extracted the cytochrome P450 enzymes from Candida species such as Candida apicola, C. maltose, and C. tropicalis. Among alkanes-degrading enzymes under aerobic conditions can be mentioned to alkane oxygenases, including cytochrome P450, membrane-bound copper-containing methane monooxygenases, soluble di-iron methane monooxygenases, and integral membrane di-iron alkane hydroxylase enzymes (e.g., alkB) which are various in prokaryotes and eukaryotes [165]. Aromatic and aliphatic degradation schematic were illustrated in Fig. 5.
Fig. 5.
The metabolic pathway of aromatic and aliphatic hydrocarbon biodegradation present in crude oil
The efficiency of biological approaches in crude oil removal
Oil spill takes place annually in the marine environment. Diverse techniques have been creating for crude-oil removal from the environment in which the user of these techniques requires an exact evaluation of several factors particularly, site characteristics, type of contaminant, and environmental conditions. Generally, preliminary phases of emergency plans including oil smudge limiting by afloat obstacles, eliminate pollution using absorbents material and skimmers using nanotechnology. Moreover, if the environmental condition is suitable surfactants can be applied that reduce the pollution damage [166].
The latter operation is performed using products with environmentally friendly properties and low toxicity, which has been confirmed by national authorities. Detergents agents increase the bioavailability to oil via their emulsification and fragmentation. Oil-diluting solvents that make possible elimination with physical strategies and surfactants with the potential of dispersing oil into the water are recognized as two major groups of these compounds [166].
In recent studies, the consequence of these materials has been investigated on the population and dynamic of seawater microorganisms, and bacterial isolated strains [167, 168]. The first reaction of surfactants remains the stimulation of the oil slick from the surface to the water which presumably increases the biodegradation by enhancing in microbial bioavailability [168–170].
The use of dispersant leads to an increase in growth rates and viability of non-hydrocarbon-degrading bacteria like Vibrio due to decreasing in the toxic effect of petroleum, whereas these parameters decrease in oil-degrading bacteria including Marinobacter acinetobacter because of diminishing carbon source. Obligate hydrocarbonoclastic bacteria (OHCB) are the major of the representative of hydrocarbon mineralization in the marine environment [70]. In recent years, using OHCB such as Marinobacter the concept of “autochthonous bioaugmentation” has been defined for oil bioremediation [171, 172]. Despite the increase in the biodegradation by the chemical dispersants, in an investigation on Rhodococcus erythropolis M-25 using the Corexit 9500A another result observed. This surfactant had not any enhancing effect on the degradation of crude oil, on the contrary, it reduced degradation from 60.7% to 32.8% [143].
The chemical dispersants do not solve the problem, but only resulting in oil transformation into a state that can’t be easily removed from the environment, so is not suitable economically, ecologically and technically [173, 174]. Consequently, innovative and sound technologies significantly have been regarded for the elimination of contamination. The technique of microbial remediation produces lower secondary pollution compare with the traditional methods and is extensively applied because it has better performance and environmental adaptation [175–184]. But low bioavailability of hydrocarbons to microorganisms limits the effectiveness of this technique. The success in biodegradation strategies related to some factors such as type of degrading microorganisms, bioavailability and optimize the environmental condition. In addition to the cases mentioned above, there are other strategies for bioremediation including bioaugmentation (i.e. inoculation) with exogenous hydrocabonoclastic microorganisms, biostimulation of microorganisms, laser technique and evaporation of oily compounds [85].
The efficiency of bio-stimulation and bio-augmentation strategies on the degradation of crude oil at field scale
Mesocosm is medium-sized and closed laboratory ecosystems that have been widely used as tools in ecological, applied, and developmental research. They have combined environmental management, technology, and live population change control. The mesocosm design can be in the form of tanks, cylinders, circulators, tubes, and so on. The type of material used is varied in structure and can be fiberglass, steel, and so on. The type and size of the reactor used to depend on the type of environment we intend to simulate and also depend on the chemical, physical, and biological processes studied [85].
Mesocosm reflects the development of microcosms. Their relatively massive size allows for more extensive use of ecological complexity. During experiments on mesocosms, it would be possible to allow interactions between chemical, physical, and biological parameters with a control. Mesocosm can largely mimic real-life conditions in aquatic ecosystems and serve as a bridge between the experimental scaling slopes that are difficult to control [185–187].
Numerous studies have shown the potential and power of these systems in mimicking and producing natural processes. Mesocosm systems are designed to examine the dynamics of the microbial community in marine environments, analyze diatom blooms, assess the effects of radioactive radiation, and study the effects of pollution on the ecosystem and so on [56, 57, 117, 188].
Hydrocarbon-degrading microorganisms maintain low distribution in marine environments. Contamination by petroleum hydrocarbons will stimulate the growth of such organisms and lead to changes in the structure of the microbial community in contaminated areas. Identification of key organisms that play a role in biodegradation of hydrocarbons is primary for understanding the evolution and development of biological treatment strategies. For this reason, numerous researches have been done to characterize bacterial communities to identify the responsible degrader and determine the catalytic potential of these degraders. In natural marine environments, nutrients, especially nitrogen and phosphorus, are inadequate to support the microbial needs for growth, especially after the sudden increase in the level of hydrocarbons with oil spills. Therefore, the addition of nitrogen and phosphorus to the contaminated media to stimulate the growth of hydrocarbon-degrading microorganisms results in increased biodegradation of the hydrocarbon pollutant [166].
Bioaugmentation is defined as a method to improve the ability of contaminated sites (soil, marine environments, etc.) to remove contaminants by introducing susceptible bacterial strains into a separate or mixed culture. The basis of this method is based on the increased metabolic capacity of the internal population by foreign microorganisms inoculated leading to a high biodegradation reaction. Bioaugmentation on a mesocosm scale comprises a combination of microbiology, microbial ecology, molecular biology, and bioengineering. Bioaugmentation has remained the subject of many biodegradation studies of crude oil and other pollutants in the last decade [23, 172, 189].
Ruberto et al. [190] designed mesocosm systems to analyze the biodegradation of oil-contaminated soils in the Antarctic region, which is a cold ecosystem. They inoculated the cold-tolerant bacterium Acinetobacter strain B22 into contaminated soils in the mesocosm for biological evaluation. They concluded that biodegradation with strain B22 removes 75% of the hydrocarbons from the contaminated soil and also increases the number of indigenous heterotrophic bacteria but reduces soil microbial diversity. They suggested biological evaluation as a way to improve biological treatment.
Maa et al. [191] investigated the bioaugmentation efficiency of an activated sludge system for petrochemical plant effluent analysis. They found that the impact of bioaugmentation of activated sludge on the degradation of toxic petrochemical compounds depends on several factors such as the pollutant’s chemical properties and the activity of the bacteria used to evaluate it. There is much evidence in the literature that the best way of bioaugmentation is the use of microorganisms derived from the similar ecological location of the contaminated environment.
Hassanshahian et al. [192] designed three types of mesosomes for biodegradation of oil in the marine environments. Two of these mesosomes had a bioassay and one bio-stimulation. In this section, we discuss the effects of these treatments on the typical microbial community, oil degradation, and comparison of their efficacy with each other. This is the first report on the design of bioaugmentation and biostimulation mesocosm systems for the evaluation of crude-oil biodegradation and ecological effects in marine environments by newly hydrocarbonoclasticus bacteria.
Flavia et al. [193] investigated the effects of oil pollution and biostimulation on soil biodiversity in the microbial community. They used both culture-dependent methods such as enumeration the number of heterotrophic bacteria and a molecular method like PCR-DGGE to perceive this effect. Their results showed that crude-oil contamination increased the number of hydrocarbon degraders in the soil that received the biostimulation treatment. Their DGGE pattern in soil with oil contamination and biostimulation showed biostimulation had a decreasing effect on the diversity of the natural microbial community. The number of phylogenetic groups and bands decreased sharply after 15 days of biostimulation treatment but increased from 15 days to 90 days and the band pattern remained constant until the end of incubation (360 days). They concluded the addition of mineral nutrients to oil-contaminated soil caused a more enormous effect on the bacterial community than on oil-only contaminated soil.
Hassanshahian et al. [192] studied the efficiency of two bioaugmentation mesocosms degradations of oil from marine environments. In the first mesocosm, bioaugmentation was studied by a unique culture of Alcanivorax and in the two mesocosms, the bioaugmentation by mixed culture of Alcanivorax and Thalassolituus bacteria was studied. The results showed that bioaugmentation by single culture was more effective in biodegradation of crude oil (95%) than bioaugmentation with mixed culture (70%). On the other hand, single cultures added to mesocosm caused less effect on indigenous marine microbial communities than mixed cultures, so that a remarkable decrease in biodiversity index and phylogenetic groups was observed in the bioaugmentation with mixed culture. However, biodiversity decreased in both cases, which is consistent with the results of other researchers. Adding food to contaminated sites to stimulate the growth of the indigenous microbial community is referred to as biostimulation. Biostimulation and application of nutrients for biodegradation purposes can be in three forms: (A) adding soluble mineral nutrients like nitrogen and phosphorus (B) adding organic nutrients such as acetate and fumarate (C) fertilizer additives. Slowly releasing minerals such as nitrogen and phosphorus are gradually released. Numerous studies have demonstrated the efficacy of using biostimulation as a method of conserve marine and onshore environments contaminated with oil and other pollutants, but our knowledge of the effects of this process on natural ecosystems is limited [10].
In a study by Hassanshahian et al. [192] the effect of biostimulation was investigated by including nitrogen and phosphorus nutrients on the oil-contaminated marine microbial community at the mesocosm level. The results showed biostimulation reduced biodiversity and reduced the number of DGGE bands by day 10 and then the phylogenetic groups returned to baseline at the terminal the incubation period (day 20) and according to with the results obtained by Flavia et al. [193] that the increase in the quantity of crude oil-degrading bacteria in a mesocosm.
Some researchers have compared the efficacy of two methods of biodegradation, including biostimulation and bioaugmentation, to develop a suitable strategy for removing crude oil from marine environments. Ruberto et al. [194] investigated the effect of adding nitrogen and phosphorus mineral nutrients (biostimulation) and inoculation with degrading bacteria such as Rhodococcus, Pseudomonas, Sphingomonas (bioaugmentation with mixed culture) on the removal of oil from contaminated soils (Antarctica). Their results showed that the number of heterotrophic bacteria and degrading bacteria increased and 86% of the oil degraded in the soil. But in the bioaugmentation, there was no significant difference between the soil that was added with the bacterium and the soil that was not bacterial inoculated, with only 56% of the oil degraded in the soil. They conclude that bio-stimulation is more effective than bioaugmentation when the soil has chronic oil pollution.
Hassanshahian et al. [195] evaluated two methods of bioaugmentation with a single bacterium and mixed culture, and compare it with the biostimulation method to degradation of crude oil in marine environments at mesocosm scale. The results of this study showed bioaugmentation with a single bacterium showed the best oil removal and biostimulation method is more efficient than bioaugmentation with a mixed culture, which is consistent with the above-mentioned results. Comparing the effect of these methods on the reduction of marine microbial community diversity, revealed the biostimulation method had the least decreasing effect on the diversity of the marine microbial community and the greatest reduction effect of biodiversity was related to bioaugmentation evaluation with mixed culture [86, 147, 155, 196–202].
Conclusion and future perspective
In this article, we tried collecting all the studies on crude-oil degrading microorganisms in the Persian Gulf. The sum of all these articles is that crude-oil degrading microorganisms, including bacteria, yeast, and fungi, maintain sufficient diversity in the Gulf. However, further studies are required, especially concerning marine organisms and bacteria. By developing field methods and evaluating bioaugmentation and biostimulation methods on a mesocosm scale and then extending it to the field experiment, these bacteria can be used to reduce oil pollution in the Persian Gulf and decrease the harmful effects of oil pollution on marine life in the Gulf. So one of the solutions to conserve the marine environment of the Persian Gulf is to develop biodegradation and bioremediation strategies.
Compliance with ethical standards
Conflicted of interest
There is not any conflicted of interest between the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGenity TJ, Folwell BD, McKew BA, Sanni GO. Marine crude-oil biodegradation: a central role for interspecies interactions. Aquat Biosyst. 2012;8:10. doi: 10.1186/2046-9063-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Q, Li J, Liu M, Sun H, Bao M. Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS One. 2017;12:e0174445. doi: 10.1371/journal.pone.0174445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simister R, Taylor MW, Rogers KM, Schupp PJ, Deines P. Temporal molecular and isotopic analysis of active bacterial communities in two New Zealand sponges. FEMS Microbiol Ecol. 2013;85:195–205. doi: 10.1111/1574-6941.12109. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev. 2007;71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappello S, Russo D, Santisi S, Calogero R, Gertler C, Crisafi F, et al. Presence of hydrocarbon-degrading bacteria in the gills of mussel Mytilus galloprovincialis in a contaminated environment: a mesoscale simulation study. Chem Ecol. 2012;28:239–52.
- 6.Ghanavati H, Emtiazi G, Hassanshahian M. Synergism effects of phenol-degrading yeast and ammonia-oxidizing bacteria for nitrification in coke wastewater of Esfahan steel company. Waste Manag Res. 2008;26:203–208. doi: 10.1177/0734242X07079874. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Zhao X, Cai Z, O’Reilly SE, Hao X, Zhao D. A review of oil, dispersed oil and sediment interactions in the aquatic environment: influence on the fate, transport and remediation of oil spills. Mar Pollut Bull. 2014;79:16–33. doi: 10.1016/j.marpolbul.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Hassanshahian M, Emtiazi G, Caruso G, Cappello S. Bioremediation (bioaugmentation/biostimulation) trials of oil polluted seawater: a mesocosm simulation study. Mar Environ Res. 2014;95:28–38. doi: 10.1016/j.marenvres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Hassanshahian M, Yakimov MM, Denaro R, Genovese M, Cappello S. Using real-time PCR to assess changes in the crude oil degrading microbial community in contaminated seawater mesocosms. Int Biodeterior Biodegrad. 2014;93:241–248. doi: 10.1016/j.ibiod.2014.06.006. [DOI] [Google Scholar]
- 10.Hassanshahian M, Zeynalipour MS, Musa FH. Isolation and characterization of crude oil degrading bacteria from the Persian Gulf (Khorramshahr provenance) Mar Pollut Bull. 2014;82:39–44. doi: 10.1016/j.marpolbul.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 11.de Mora S, Tolosa I, Fowler SW, Villeneuve JP, Cassi R, Cattini C. Distribution of petroleum hydrocarbons and organochlorinated contaminants in marine biota and coastal sediments from the ROPME Sea area during 2005. Mar Pollut Bull. 2010;60:2323–2349. doi: 10.1016/j.marpolbul.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Sale PF, Feary DA, Burt JA, Bauman AG, Cavalcante GH, Drouillard KG, et al. The growing need for sustainable ecological management of marine communities of the Persian Gulf. Ambio. 2011;40:4–17. [DOI] [PMC free article] [PubMed]
- 13.Ahmed M, El-Raey M, Nasr S, Frihy O. Socioeconomic impact of pollution on ecosystems of the Arabian gulf. Environ Int. 1998;24:229–237. doi: 10.1016/S0160-4120(97)00140-2. [DOI] [Google Scholar]
- 14.Al-Mailem D, Kansour M, Radwan S. Bacterial communities associated with biofouling materials used in bench-scale hydrocarbon bioremediation. Environ Sci Pollut Res. 2015;22:3570–3585. doi: 10.1007/s11356-014-3593-1. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hasan RH, Khanafer M, Eliyas M, Radwan SS. Hydrocarbon accumulation by picocyanobacteria from the Arabian gulf. J Appl Microbiol. 2001;91:533–540. doi: 10.1046/j.1365-2672.2001.01414.x. [DOI] [PubMed] [Google Scholar]
- 16.El Samra MI, Emara HI, Shunbo F. Dissolved petroleum hydrocarbon in the northwestern Arabian gulf. Mar Pollut Bull. 1986;17:65–68. doi: 10.1016/0025-326X(86)90293-6. [DOI] [Google Scholar]
- 17.Marchand M, Monfort J-P, Rubio A. Distribution of hydrocarbons in water and marine sediments after the Amoco Cadez and Istoc. 1. Oil spills. Energy Environ. 1982;1:487–509. [Google Scholar]
- 18.Sen Gupta R, Kureishy TW. Present state of oil pollution in the northern Indian Ocean. Mar Pollut Bull. 1981;12:295–301. doi: 10.1016/0025-326X(81)90079-5. [DOI] [Google Scholar]
- 19.Van Hamme JD, Singh A, Ward OP. Recent advances in petroleum microbiology. Microbiol Mol Biol Rev. 2003;67:503–549. doi: 10.1128/MMBR.67.4.503-549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leahy JG, Colwell RR. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/MR.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Head IM, Jones DM, Röling WFM. Marine microorganisms make a meal of oil. Nat Rev Microbiol. 2006;4:173–182. doi: 10.1038/nrmicro1348. [DOI] [PubMed] [Google Scholar]
- 22.Balachandran C, Duraipandiyan V, Balakrishna K, Ignacimuthu S. Petroleum and polycyclic aromatic hydrocarbons (PAHs) degradation and naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil. Bioresour Technol. 2012;112:83–90. doi: 10.1016/j.biortech.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 23.Bovio E, Gnavi G, Prigione V, Spina F, Denaro R, Yakimov M, et al. The culturable mycobiota of a Mediterranean marine site after an oil spill: isolation, identification and potential application in bioremediation. Sci Total Environ. 2017;576:310–8. [DOI] [PubMed]
- 24.Matsubara M, Lynch JM, De Leij FAAM. A simple screening procedure for selecting fungi with potential for use in the bioremediation of contaminated land. Enzym Microb Technol. 2006;39:1365–1372. doi: 10.1016/j.enzmictec.2005.04.025. [DOI] [Google Scholar]
- 25.Antonio FJ, Mendes RS, Thomaz SM. Identifying and modeling patterns of tetrapod vertebrate mortality rates in the Gulf of Mexico oil spill. Aquat Toxicol. 2011;105:177–179. doi: 10.1016/j.aquatox.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Baguley JG, Montagna PA, Cooksey C, Hyland JL, Bang HW, Morrison C, et al. Community response of deep-sea soft-sediment metazoan meiofauna to the Deepwater horizon blowout and oil spill. Mar Ecol Prog Ser. 2015;528:127–40.
- 27.King GM, Kostka JE, Hazen TC, Sobecky PA. Microbial responses to the deepwater horizon oil spill: from coastal wetlands to the deep sea. Annu Rev Mar Sci. 2015;7:377–401. doi: 10.1146/annurev-marine-010814-015543. [DOI] [PubMed] [Google Scholar]
- 28.Bragg JR, Prince RC, Harner EJ, Atlas RM. Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature. 1994;368:413–418. doi: 10.1038/368413a0. [DOI] [Google Scholar]
- 29.Carls MG, Rice SD, Hose JE. Sensitivity of fish embryos to weathered crude oil: part I. low-level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi) Environ Toxicol Chem. 1999;18:481–493. doi: 10.1002/etc.5620180317. [DOI] [Google Scholar]
- 30.Barth H-J. The influence of cyanobacteria on oil polluted intertidal soils at the Saudi Arabian gulf shores. Mar Pollut Bull. 2003;46:1245–1252. doi: 10.1016/S0025-326X(03)00374-6. [DOI] [PubMed] [Google Scholar]
- 31.Leifer I, Boles J. Measurement of marine hydrocarbon seep flow through fractured rock and unconsolidated sediment. Mar Pet Geol. 2005;22:551–568. doi: 10.1016/j.marpetgeo.2004.10.026. [DOI] [Google Scholar]
- 32.Logan GA, Jones AT, Kennard JM, Ryan GJ, Rollet N. Australian offshore natural hydrocarbon seepage studies, a review and re-evaluation. Mar Pet Geol. 2010;27:26–45. doi: 10.1016/j.marpetgeo.2009.07.002. [DOI] [Google Scholar]
- 33.Rosenberg E, Kellogg CA, Rohwer F. Coral Microbiology. Oceanography. 2007;20:146–154. doi: 10.5670/oceanog.2007.60. [DOI] [Google Scholar]
- 34.Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann CM, Marker A, Kurtböke DI. An overview on marine sponge-symbiotic bacteria as unexhausted sources for natural product discovery. Diversity. 2017;9:40. doi: 10.3390/d9040040. [DOI] [Google Scholar]
- 36.Yakimov MM, Denaro R, Genovese M, Cappello S, D’Auria G, Chernikova TN, et al. Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily) and community successions during microcosm enrichment with various hydrocarbons. Environ Microbiol. 2005;7:1426–41. [DOI] [PubMed]
- 37.Hassanshahian M, Emtiazi G. Investigation of alkane biodegradation using the microtiter plate method and correlation between biofilm formation, biosurfactant production and crude oil biodegradation. Int Biodeterior Biodegrad. 2008;62:170–178. doi: 10.1016/j.ibiod.2008.01.004. [DOI] [Google Scholar]
- 38.Hassanshahian M, Ahmadinejad M, Tebyanian H, Kariminik A. Isolation and characterization of alkane degrading bacteria from petroleum reservoir waste water in Iran (Kerman and Tehran provenances) Mar Pollut Bull. 2013;73:300–305. doi: 10.1016/j.marpolbul.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Council NR. Oil spill dispersants: efficacy and effects: National Academies Press; 2005.
- 40.Emtiazi G, Saleh T, Hassanshahian M. The effect of bacterial glutathione S-transferase on morpholine degradation. Biotechnol J. 2009;4:202–205. doi: 10.1002/biot.200800238. [DOI] [PubMed] [Google Scholar]
- 41.Payne JR, Clayton JR, Kirstein BE. Oil/suspended particulate material interactions and sedimentation. Spill Sci Technol Bull. 2003;8:201–221. doi: 10.1016/S1353-2561(03)00048-3. [DOI] [Google Scholar]
- 42.Muschenheim DK, Lee K. Removal of oil from the sea surface through particulate interactions: review and prospectus. Spill Sci Technol Bull. 2002;8:9–18. doi: 10.1016/S1353-2561(02)00129-9. [DOI] [Google Scholar]
- 43.Bragg J, Owens E. Clay-oil flocculation as a natural cleansing process following oil spills: part 1. Studies of shoreline sediments and residues from past spills. Environment Canada, Ottawa, ON(Canada) 1994;1:1–23. [Google Scholar]
- 44.Bragg JR, Owens EH. Shoreline cleansing by interactions between oil and fine mineral particles. Int Oil Spill Conf Proc. 1995;1995:219–227. doi: 10.7901/2169-3358-1995-1-219. [DOI] [Google Scholar]
- 45.Bragg J, Yang S. Clay-oil flocculation and its effects on the rate of natural cleansing in Prince William sound following the: Exxon Valdez; 1993.
- 46.Jahns HO, Bragg JR, Dash LC, Owens EH. Natural cleaning of shorelines following the Exxon Valdez spill. Int Oil Spill Conf Proc. 1991;1991:167–176. doi: 10.7901/2169-3358-1991-1-167. [DOI] [Google Scholar]
- 47.Lee K. Oil–particle interactions in aquatic environments: influence on the transport, fate, effect and remediation of oil spills. Spill Sci Technol Bull. 2002;8:3–8. doi: 10.1016/S1353-2561(03)00006-9. [DOI] [Google Scholar]
- 48.Sterling MC, Bonner JS, Ernest ANS, Page CA, Autenrieth RL. Application of fractal flocculation and vertical transport model to aquatic sol–sediment systems. Water Res. 2005;39:1818–1830. doi: 10.1016/j.watres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Bandara UC, Yapa PD, Xie H. Fate and transport of oil in sediment laden marine waters. J Hydro-environ Res. 2011;5:145–156. doi: 10.1016/j.jher.2011.03.002. [DOI] [Google Scholar]
- 50.Ajijolaiya LO, Hill PS, Khelifa A, Islam RM, Lee K. Laboratory investigation of the effects of mineral size and concentration on the formation of oil–mineral aggregates. Mar Pollut Bull. 2006;52:920–927. doi: 10.1016/j.marpolbul.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Stoffyn-Egli P, Lee K. Formation and characterization of oil–mineral aggregates. Spill Sci Technol Bull. 2002;8:31–44. doi: 10.1016/S1353-2561(02)00128-7. [DOI] [Google Scholar]
- 52.Omotoso OE, Munoz VA, Mikula RJ. Mechanisms of crude oil–mineral interactions. Spill Sci Technol Bull. 2002;8:45–54. doi: 10.1016/S1353-2561(02)00116-0. [DOI] [Google Scholar]
- 53.de la Huz R, Lastra M, Junoy J, Castellanos C, Viéitez JM. Biological impacts of oil pollution and cleaning in the intertidal zone of exposed sandy beaches: preliminary study of the “prestige” oil spill. Estuar Coast Shelf Sci. 2005;65:19–29. doi: 10.1016/j.ecss.2005.03.024. [DOI] [Google Scholar]
- 54.Hassanshahian M, Emtiazi G, Kermanshahi RK, Cappello S. Comparison of oil degrading microbial communities in sediments from the Persian Gulf and Caspian Sea. Soil Sediment Contam. 2010;19:277–291. doi: 10.1080/15320381003695215. [DOI] [Google Scholar]
- 55.Yakimov MM, Giuliano L, Denaro R, Crisafi E, Chernikova TN, Abraham W-R, et al. Thalassolituus oleivorans gen. Nov., sp. nov., a novel marine bacterium that obligately utilizes hydrocarbons. Int J Syst Evol Microbiol. 2004;54:141–8. [DOI] [PubMed]
- 56.Al-Awadhi H, Al-Mailem D, Dashti N, Khanafer M, Radwan S. Indigenous hydrocarbon-utilizing bacterioflora in oil-polluted habitats in Kuwait, two decades after the greatest man-made oil spill. Arch Microbiol. 2012;194:689–705. doi: 10.1007/s00203-012-0800-7. [DOI] [PubMed] [Google Scholar]
- 57.Al-Awadhi H, Dashti N, Kansour M, Sorkhoh N, Radwan S. Hydrocarbon-utilizing bacteria associated with biofouling materials from offshore waters of the Arabian gulf. Int Biodeterior Biodegradation. 2012;69:10–16. doi: 10.1016/j.ibiod.2011.12.008. [DOI] [Google Scholar]
- 58.Hassanshahian M, Emtiazi G, Cappello S. Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar Pollut Bull. 2012;64:7–12. doi: 10.1016/j.marpolbul.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Hassanshahian M, Tebyanian H, Cappello S. Isolation and characterization of two crude oil-degrading yeast strains, Yarrowia lipolytica PG-20 and PG-32, from the Persian Gulf. Mar Pollut Bull. 2012;64:1386–1391. doi: 10.1016/j.marpolbul.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 60.Ellis LBM, Hou BK, Kang W, Wackett LP. The University of Minnesota biocatalysis/biodegradation database: post-genomic data mining. Nucleic Acids Res. 2003;31:262–265. doi: 10.1093/nar/gkg048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Mailem D, Eliyas M, Khanafer M, Radwan S. Culture-dependent and culture-independent analysis of hydrocarbonoclastic microorganisms indigenous to hypersaline environments in Kuwait. Microb Ecol. 2014;67:857–865. doi: 10.1007/s00248-014-0386-5. [DOI] [PubMed] [Google Scholar]
- 62.Al-Mailem DM, Eliyas M, Radwan S. Enhanced bioremediation of oil-polluted, hypersaline, coastal areas in Kuwait via vitamin-fertilization. Environ Sci Pollut Res. 2014;21:3386–3394. doi: 10.1007/s11356-013-2293-6. [DOI] [PubMed] [Google Scholar]
- 63.Giovannoni SJ. Evolution, diversity and molecular ecology of marine prokaryotes. Microbial Ecol Oceans. 2000:47–84.
- 64.Karimi M, Hassanshahian M. Isolation and characterization of phenol degrading yeasts from wastewater in the coking plant of Zarand, Kerman. Braz J Microbiol. 2016;47:18–24. doi: 10.1016/j.bjm.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Church MJ, Björkman KM, Karl DM, Saito MA, Zehr JP. Regional distributions of nitrogen-fixing bacteria in the Pacific Ocean. Limnol Oceanogr. 2008;53:63–77. doi: 10.4319/lo.2008.53.1.0063. [DOI] [Google Scholar]
- 66.Jones KL, Rhodes-Roberts M. Physiological properties of nitrogen-scavenging Bacteria from the marine environment. J Appl Bacteriol. 1980;49:421–433. doi: 10.1111/j.1365-2672.1980.tb04717.x. [DOI] [Google Scholar]
- 67.Radwan S, Mahmoud H, Khanafer M, Al-Habib A, Al-Hasan R. Identities of Epilithic hydrocarbon-utilizing Diazotrophic Bacteria from the Arabian gulf coasts, and their potential for oil bioremediation without nitrogen supplementation. Microb Ecol. 2010;60:354–363. doi: 10.1007/s00248-010-9702-x. [DOI] [PubMed] [Google Scholar]
- 68.Harwati TU, Kasai Y, Kodama Y, Susilaningsih D, Watanabe K. Characterization of diverse hydrocarbon-degrading Bacteria isolated from Indonesian seawater. Microbes Environ. 2007;22:412–415. doi: 10.1264/jsme2.22.412. [DOI] [Google Scholar]
- 69.Radwan S. Microbiology of oil-contaminated desert soils and coastal areas in the Arabian gulf region. In: Dion P, Nautiyal CS, editors. Microbiology of extreme soils. Berlin Heidelberg, Berlin, Heidelberg: Springer; 2008. pp. 275–298. [Google Scholar]
- 70.Yakimov MM, Timmis KN, Golyshin PN. Obligate oil-degrading marine bacteria. Curr Opin Biotechnol. 2007;18:257–266. doi: 10.1016/j.copbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Engelhardt MA, Daly K, Swannell RPJ, Head IM. Isolation and characterization of a novel hydrocarbon-degrading, gram-positive bacterium, isolated from intertidal beach sediment, and description of Planococcus alkanoclasticus sp. nov. J Appl Microbiol. 2001;90:237–247. doi: 10.1046/j.1365-2672.2001.01241.x. [DOI] [PubMed] [Google Scholar]
- 72.Al-Awadhi H, Sulaiman RHD, Mahmoud HM, Radwan SS. Alkaliphilic and halophilic hydrocarbon-utilizing bacteria from Kuwaiti coasts of the Arabian gulf. Appl Microbiol Biotechnol. 2007;77:183–186. doi: 10.1007/s00253-007-1127-1. [DOI] [PubMed] [Google Scholar]
- 73.Edlund A, Jansson JK. Use of bromodeoxyuridine immunocapture to identify psychrotolerant phenanthrene-degrading bacteria in phenanthrene-enriched polluted Baltic Sea sediments. FEMS Microbiol Ecol. 2008;65:513–525. doi: 10.1111/j.1574-6941.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- 74.Gutierrez T, Singleton DR, Aitken MD, Semple KT. Stable isotope probing of an algal bloom to identify uncultivated members of the <span class="named-content genus-species" id="named-content-1">Rhodobacteraceae</span> associated with low-molecular-weight polycyclic aromatic hydrocarbon degradation. Appl Environ Microbiol. 2011;77:7856–7860. doi: 10.1128/AEM.06200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Mailem D, Sorkhoh N, Al-Awadhi H, Eliyas M, Radwan S. Biodegradation of crude oil and pure hydrocarbons by extreme halophilic archaea from hypersaline coasts of the Arabian gulf. Extremophiles. 2010;14:321–328. doi: 10.1007/s00792-010-0312-9. [DOI] [PubMed] [Google Scholar]
- 76.Al-Mailem DM, Sorkhoh NA, Salamah S, Eliyas M, Radwan SS. Oil-bioremediation potential of Arabian gulf mud flats rich in diazotrophic hydrocarbon-utilizing bacteria. Int Biodeterior Biodegradation. 2010;64:218–225. doi: 10.1016/j.ibiod.2010.01.007. [DOI] [Google Scholar]
- 77.Gauthier MJ, Lafay B, Christen R, Fernandez L, Acquaviva M, Bonin P, et al. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely Halotolerant, hydrocarbon-degrading marine bacterium. Int J Syst Evol Microbiol. 1992;42:568–76. [DOI] [PubMed]
- 78.Salleh AB, Ghazali FM, Rahman RNZA, Basri M. Bioremediation of petroleum hydrocarbon pollution. 2003. [Google Scholar]
- 79.Liu C, Wang W, Wu Y, Zhou Z, Lai Q, Shao Z. Multiple alkane hydroxylase systems in a marine alkane degrader, Alcanivorax dieselolei B-5. Environ Microbiol. 2011;13:1168–1178. doi: 10.1111/j.1462-2920.2010.02416.x. [DOI] [PubMed] [Google Scholar]
- 80.Schneiker S, dos Santos VAM, Bartels D, Bekel T, Brecht M, Buhrmester J, et al. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat Biotechnol. 2006;24:997–1004. [DOI] [PMC free article] [PubMed]
- 81.Röling WFM, Couto de Brito IR, Swannell RPJ, Head IM. Response of Archaeal communities in beach sediments to spilled oil and bioremediation. Appl Environ Microbiol. 2004;70:2614–2620. doi: 10.1128/AEM.70.5.2614-2620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang Y-J, Stephen JR, Richter AP, Venosa AD, Brüggemann J, Macnaughton SJ, et al. Phylogenetic analysis of aerobic freshwater and marine enrichment cultures efficient in hydrocarbon degradation: effect of profiling method. J Microbiol Methods. 2000;40:19–31. [DOI] [PubMed]
- 83.Melcher RJ, Apitz SE, Hemmingsen BB. Impact of irradiation and polycyclic aromatic hydrocarbon spiking on microbial populations in marine sediment for future aging and biodegradability studies. Appl Environ Microbiol. 2002;68:2858–2868. doi: 10.1128/AEM.68.6.2858-2868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radwan SS, Al-Hasan RH. Oil pollution and cyanobacteria, the ecology of cyanobacteria: Springer; 2000. p. 307–19.
- 85.Al-Mailem DM, Al-Deieg M, Eliyas M, Radwan SS. Biostimulation of indigenous microorganisms for bioremediation of oily hypersaline microcosms from the Arabian gulf Kuwaiti coasts. J Environ Manag. 2017;193:576–583. doi: 10.1016/j.jenvman.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 86.Hassanshahian M. The effects of crude oil on marine microbial communities in sediments from the Persian Gulf and the Caspian Sea: a microcosm experiment. Int J Adv Biol Biomed Res. 2014a;2(1):1–17.
- 87.Cerniglia CE, Gibson DT, Van Baalen C. Oxidation of naphthalene by cyanobacteria and microalgae. Microbiology. 1980;116:495–500. doi: 10.1099/00221287-116-2-495. [DOI] [Google Scholar]
- 88.Cerniglia CE, Freeman JP, Althaus JR, van Baalen C. Metabolism and toxicity of 1- and 2-methylnaphthalene and their derivatives in cyanobacteria. Arch Microbiol. 1983;136:177–183. doi: 10.1007/BF00409840. [DOI] [Google Scholar]
- 89.Sorkhoh N, Al-Hasan R, Radwan S, Höpner T. Self-cleaning of the Gulf. Nature. 1992;359:109–9.
- 90.Amini BN, Hassanshahian M, Khoshrou SMR. Isolation and characterization of phenol degrading bacteria from Persian gulf. Int J Adv Biol Biomed Res. 2014;2(2):408–416. [Google Scholar]
- 91.Abed RM, Al-Thukair A, De Beer D. Bacterial diversity of a cyanobacterial mat degrading petroleum compounds at elevated salinities and temperatures. FEMS Microbiol Ecol. 2006;57:290–301. doi: 10.1111/j.1574-6941.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- 92.Al-Bader D, Eliyas M, Rayan R, Radwan S. Subsurface associations of Acaryochloris-related picocyanobacteria with oil-utilizing bacteria in the Arabian gulf water body: promising consortia in oil sediment bioremediation. Microb Ecol. 2013;65:555–565. doi: 10.1007/s00248-012-0157-0. [DOI] [PubMed] [Google Scholar]
- 93.Novotný Č, Svobodová K, Erbanová P, Cajthaml T, Kasinath A, Lang E, et al. Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biol Biochem. 2004;36:1545–51.
- 94.Al-Hawash AB, Dragh MA, Li S, Alhujaily A, Abbood HA, Zhang X, et al. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquat Res. 2018;44:71–6.
- 95.Messias JM, da Costa BZ, de Lima VM, Dekker RF, Rezende MI, Krieger N, et al. Screening Botryosphaeria species for lipases: production of lipase by Botryosphaeria ribis EC-01 grown on soybean oil and other carbon sources. Enzym Microb Technol. 2009;45:426–31.
- 96.Singh A, Ward OP. Applied bioremediation and phytoremediation: Springer Science & Business Media; 2004.
- 97.Lee H, Yun SY, Jang S, Kim G-H, Kim J-J. Bioremediation of polycyclic aromatic hydrocarbons in creosote-contaminated soil by Peniophora incarnata KUC8836. Bioremediat J. 2015;19:1–8. doi: 10.1080/10889868.2014.939136. [DOI] [Google Scholar]
- 98.Li X, Wang Y, Wu S, Qiu L, Gu L, Li J, et al. Peculiarities of metabolism of anthracene and pyrene by laccase-producing fungus P ycnoporus sanguineus H 1. Biotechnol Appl Biochem. 2014;61:549–54. [DOI] [PubMed]
- 99.Balaji V, Arulazhagan P, Ebenezer P. Enzymatic bioremediation of polyaromatic hydrocarbons by fungal consortia enriched from petroleum contaminated soil and oil seeds. J Environ Biol. 2014;35:521–529. [PubMed] [Google Scholar]
- 100.Snellman E, Collins R, Cooke J. Utilization of fuel oils by fungi isolated from oceanic tar balls. Lett Appl Microbiol. 1988;6:105–107. doi: 10.1111/j.1472-765X.1988.tb01225.x. [DOI] [Google Scholar]
- 101.Elshafie A, AlKindi AY, Al-Busaidi S, Bakheit C, Albahry S. Biodegradation of crude oil and n-alkanes by fungi isolated from Oman. Mar Pollut Bull. 2007;54:1692–1696. doi: 10.1016/j.marpolbul.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 102.Radwan S, Al-Hasan R, Al-Awadhi H, Salamah S, Abdullah H. Higher oil biodegradation potential at the Arabian gulf coast than in the water body. Mar Biol. 1999;135:741–745. doi: 10.1007/s002270050675. [DOI] [Google Scholar]
- 103.Radwan S, Al-Hasan R, Mahmoud H, Eliyas M. Oil-utilizing bacteria associated with fish from the Arabian gulf. J Appl Microbiol. 2007;103:2160–2167. doi: 10.1111/j.1365-2672.2007.03454.x. [DOI] [PubMed] [Google Scholar]
- 104.Bull AT, Stach JE, Ward AC, Goodfellow M. Marine actinobacteria: perspectives, challenges, future directions. Antonie Van Leeuwenhoek. 2005;87:65–79. doi: 10.1007/s10482-004-6562-8. [DOI] [PubMed] [Google Scholar]
- 105.Austin B. The bacterial microflora of fish, revised. Sci World J. 2006;6:931–945. doi: 10.1100/tsw.2006.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leys NM, Ryngaert A, Bastiaens L, Wattiau P, Top EM, Verstraete W, Springael D. Occurrence and community composition of fast-growing Mycobacterium in soils contaminated with polycyclic aromatic hydrocarbons. FEMS Microbiol Ecol. 2005;51:375–388. doi: 10.1016/j.femsec.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 107.Sorkhoh N, Ghannoum M, Ibrahim A, Stretton R, Radwan S. Growth of Candida albicans in the presence of hydrocarbons: a correlation between sterol concentration and hydrocarbon uptake. Appl Microbiol Biotechnol. 1991;34:509–512. doi: 10.1007/BF00180579. [DOI] [Google Scholar]
- 108.Zhang H, Kallimanis A, Koukkou AI, Drainas C. Isolation and characterization of novel bacteria degrading polycyclic aromatic hydrocarbons from polluted Greek soils. Appl Microbiol Biotechnol. 2004;65:124–131. doi: 10.1007/s00253-004-1614-6. [DOI] [PubMed] [Google Scholar]
- 109.Al-Dahash LM, Mahmoud HM. Harboring oil-degrading bacteria: a potential mechanism of adaptation and survival in corals inhabiting oil-contaminated reefs. Mar Pollut Bull. 2013;72:364–374. doi: 10.1016/j.marpolbul.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 110.Grossart HP. Ecological consequences of bacterioplankton lifestyles: changes in concepts are needed. Environ Microbiol Rep. 2010;2:706–714. doi: 10.1111/j.1758-2229.2010.00179.x. [DOI] [PubMed] [Google Scholar]
- 111.Grossart HP, Levold F, Allgaier M, Simon M, Brinkhoff T. Marine diatom species harbour distinct bacterial communities. Environ Microbiol. 2005;7:860–873. doi: 10.1111/j.1462-2920.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 112.Carman KR, Dobbs FC. Epibiotic microorganisms on copepods and other marine crustaceans. Microsc Res Tech. 1997;37:116–135. doi: 10.1002/(SICI)1097-0029(19970415)37:2<116::AID-JEMT2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 113.Bayat Z, Hassanshahian M, Cappello S. Immobilization of microbes for bioremediation of crude oil polluted environments: A mini review. Open Microbiol J. 2015;9:48–54. doi: 10.2174/1874285801509010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bayat Z, Hassanshahian M, Hesni MA. Enrichment and isolation of crude oil degrading bacteria from some mussels collected from the Persian Gulf. Mar Pollut Bull. 2015;101:85–91. doi: 10.1016/j.marpolbul.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 115.Bayat Z, Hassanshahian M, Hesni MA. Study the symbiotic crude oil-degrading bacteria in the mussel Mactra stultorum collected from the Persian Gulf. Mar Pollut Bull. 2016;105:120–124. doi: 10.1016/j.marpolbul.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 116.Al-Thukair AA, Al-Hinai K. Preliminary damage assessment of algal mats sites located in the western gulf following the 1991 oil spill. Mar Pollut Bull. 1993;27:229–238. doi: 10.1016/0025-326X(93)90029-J. [DOI] [Google Scholar]
- 117.Abed RM, Köster J. The direct role of aerobic heterotrophic bacteria associated with cyanobacteria in the degradation of oil compounds. Int Biodeterior Biodegradation. 2005;55:29–37. doi: 10.1016/j.ibiod.2004.07.001. [DOI] [Google Scholar]
- 118.Al-Mailem D, Eliyas M, Radwan S. Enhanced haloarchaeal oil removal in hypersaline environments via organic nitrogen fertilization and illumination. Extremophiles. 2012;16:751–758. doi: 10.1007/s00792-012-0471-y. [DOI] [PubMed] [Google Scholar]
- 119.Prufert-Bebout L, Garcia-Pichel F. Field and cultivated Microcoleus chthonoplastes: the search for clues to its prevalence in marine microbial mats, microbial Mats: Springer; 1994. p. 111–6.
- 120.Karsten U. Growth and organic osmolytes of geographically different isolates of microcoleus chthonoplastes (cyanobacteria) from benthic microbial mats: response to salinity change 1. J Phycol. 1996;32:501–506. doi: 10.1111/j.0022-3646.1996.00501.x. [DOI] [Google Scholar]
- 121.Rainey FA, Nobre MF, Schumann P, Stackebrandt E, DA COSTA MS. Phylogenetic diversity of the deinococci as determined by 16S ribosomal DNA sequence comparison. Int J Syst Evol Microbiol. 1997;47:510–514. doi: 10.1099/00207713-47-2-510. [DOI] [PubMed] [Google Scholar]
- 122.Liu B, Ju M, Liu J, Wu W, Li X. Isolation, identification, and crude oil degradation characteristics of a high-temperature, hydrocarbon-degrading strain. Mar Pollut Bull. 2016;106:301–307. doi: 10.1016/j.marpolbul.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 123.Hambrick GA, DeLaune RD, Patrick W. Effect of estuarine sediment pH and oxidation-reduction potential on microbial hydrocarbon degradation. Appl Environ Microbiol. 1980;40:365–369. doi: 10.1128/AEM.40.2.365-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peyton BM, Yonge DR, Alva VA, Oie C, Aston J. Biodegradation of non-point source pollutants in soap Lake, Washington. Pullman: Project completion report state of Washington water research report WRR-11 state of Washington water research center; 2002. [Google Scholar]
- 125.Sarnaik S, Kanekar P. Bioremediation of colour of methyl violet and phenol from a dye-industry waste effluent using Pseudomonas spp. isolated from factory soil. J Appl Bacteriol. 1995;79:459–469. doi: 10.1111/j.1365-2672.1995.tb03162.x. [DOI] [Google Scholar]
- 126.Maltseva O, McGowan C, Fulthorpe R, Oriel P. Degradation of 2, 4-dichlorophenoxyacetic acid by haloalkaliphilic bacteria. Microbiology. 1996;142:1115–1122. doi: 10.1099/13500872-142-5-1115. [DOI] [PubMed] [Google Scholar]
- 127.Maltseva O, Oriel P. Monitoring of an alkaline 2, 4, 6-Trichlorophenol-degrading enrichment culture by DNA fingerprinting methods and isolation of the responsible organism, Haloalkaliphilic Nocardioides sp. strain M6. Appl Environ Microbiol. 1997;63:4145–4149. doi: 10.1128/AEM.63.11.4145-4149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kanekar P, Sarnaik S, Kelkar A. Bioremediation of phenol by alkaliphilic bacteria isolated from alkaline lake of Lonar, India. J Appl Microbiol. 1998;85:128S–133S. doi: 10.1111/j.1365-2672.1998.tb05291.x. [DOI] [PubMed] [Google Scholar]
- 129.Yumoto I, Nakamura A, Iwata H, Kojima K, Kusumoto K, Nodasaka Y, et al. Dietzia psychralcaliphila sp. nov., a novel, facultatively psychrophilic alkaliphile that grows on hydrocarbons. Int J Syst Evol Microbiol. 2002;52:85–90. [DOI] [PubMed]
- 130.Yumoto I, Yamaga S, Sogabe Y, Nodasaka Y, Matsuyama H, Nakajima K, et al. Bacillus krulwichiae sp. nov., a halotolerant obligate alkaliphile that utilizes benzoate and m-hydroxybenzoate. Int J Syst Evol Microbiol. 2003;53:1531–6. [DOI] [PubMed]
- 131.Oren A. Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechnol. 2002;28:56–63. doi: 10.1038/sj/jim/7000176. [DOI] [PubMed] [Google Scholar]
- 132.Fakhrzadegan I, Hassanshahian M, Askari H, Saadatfar M, A. A study of crude oil-degrading bacteria from mangrove forests in the Persian Gulf. Mar Ecol. 2019;40(2):1–10. doi: 10.1111/maec.12544. [DOI] [Google Scholar]
- 133.Lima TM, Procópio LC, Brandão FD, Carvalho AM, Tótola MR, Borges AC. Biodegradability of bacterial surfactants. Biodegradation. 2011;22:585–592. doi: 10.1007/s10532-010-9431-3. [DOI] [PubMed] [Google Scholar]
- 134.Chandankere R, Yao J, Cai M, Masakorala K, Jain A, Choi MM. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel. 2014;122:140–148. doi: 10.1016/j.fuel.2014.01.023. [DOI] [Google Scholar]
- 135.Anjum F, Gautam G, Edgard G, Negi S. Biosurfactant production through Bacillus sp. MTCC 5877 and its multifarious applications in food industry. Bioresour Technol. 2016;213:262–269. doi: 10.1016/j.biortech.2016.02.091. [DOI] [PubMed] [Google Scholar]
- 136.Zhang X, Fan X, Solaiman DK, Ashby RD, Liu Z, Mukhopadhyay S, et al. Inactivation of Escherichia coli O157: H7 in vitro and on the surface of spinach leaves by biobased antimicrobial surfactants. Food Control. 2016;60:158–65.
- 137.Bezza FA, Chirwa EMN. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil. Chemosphere. 2016;144:635–644. doi: 10.1016/j.chemosphere.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 138.Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS. Environmental applications of biosurfactants: recent advances. Int J Mol Sci. 2011;12:633–654. doi: 10.3390/ijms12010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perfumo A, Smyth T, Marchant R, Banat I. Production and roles of biosurfactants and bioemulsifiers in accessing hydrophobic substrates, Handbook of hydrocarbon and lipid microbiology. 2010. pp. 1501–1512. [Google Scholar]
- 140.Tebyanian H, Hassanshahian M, Kariminik A. Hexadecane-degradation by Teskumurella and Stenotrophomonas strains isolated from hydrocarbon contaminated soils. Jundishapur J Microbiol. 2013;6:1S. doi: 10.5812/jjm.9182. [DOI] [Google Scholar]
- 141.Shin KH, Ahn Y, Kim KW. Toxic effect of biosurfactant addition on the biodegradation of phenanthrene. Environ Toxicol Chem. 2005;24:2768–2774. doi: 10.1897/05-071R1.1. [DOI] [PubMed] [Google Scholar]
- 142.Diggle SP. Microbial communication and virulence: lessons from evolutionary theory. Microbiology. 2010;156:3503–3512. doi: 10.1099/mic.0.045179-0. [DOI] [PubMed] [Google Scholar]
- 143.Pi Y, Chen B, Bao M, Fan F, Cai Q, Ze L, et al. Microbial degradation of four crude oil by biosurfactant producing strain Rhodococcus sp. Bioresour Technol. 2017;232:263–9. [DOI] [PubMed]
- 144.Najmi Z, Ebrahimipour G, Franzetti A, Banat IM. Investigation of Physicho-chemical properties and characterization of produced biosurfactant by selected indigenous oil-degrading bacterium. Iran J Public Health. 2018;47:1151. [PMC free article] [PubMed] [Google Scholar]
- 145.Rambeloarisoa E, Rontani J, Giusti G, Duvnjak Z, Bertrand J. Degradation of crude oil by a mixed population of bacteria isolated from sea-surface foams. Mar Biol. 1984;83:69–81. doi: 10.1007/BF00393087. [DOI] [Google Scholar]
- 146.Bisht S, Pandey P, Bhargava B, Sharma S, Kumar V, Sharma KD. Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Braz J Microbiol. 2015;46:7–21. doi: 10.1590/S1517-838246120131354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hassanshahian M. Isolation and characterization of biosurfactant producing bacteria from Persian gulf (Bushehr provenance). Mar Pollut Bull. 2014b;86:361–6. [DOI] [PubMed]
- 148.Klug M, Markovetz A. Utilization of aliphatic hydrocarbons by micro-organisms. Adv Microb Physiol Elsevier. 1971:1–43. [DOI] [PubMed]
- 149.Meng L, Li H, Bao M, Sun P. Metabolic pathway for a new strain Pseudomonas synxantha LSH-7′: from chemotaxis to uptake of n-hexadecane. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rehm H, Reiff I. Mechanisms and occurrence of microbial oxidation of long-chain alkanes, reactors and reactions: Springer; 1981. p. 175–215.
- 151.Grbić-Galić D, Vogel TM. Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol. 1987;53:254–260. doi: 10.1128/AEM.53.2.254-260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grishchenkov V, Townsend R, McDonald T, Autenrieth R, Bonner J, Boronin A. Degradation of petroleum hydrocarbons by facultative anaerobic bacteria under aerobic and anaerobic conditions. Process Biochem. 2000;35:889–896. doi: 10.1016/S0032-9592(99)00145-4. [DOI] [Google Scholar]
- 153.Thavasi R, Jayalakshmi S, Balasubramanian T, Banat IM. Effect of salinity, temperature, pH and crude oil concentration on biodegradation of crude oil by Pseudomonas aeruginosa. J Biol Environ Sci. 2007;1:51–57. [Google Scholar]
- 154.Pawar RM. The effect of soil pH on bioremediation of polycyclic aromatic hydrocarbons (PAHS) J Bioremediat Biodegrad. 2015;6:291–304. [Google Scholar]
- 155.Hassanshahian M, Yaghoobi MM. Cloning and gene expression of cytochrome P450 gene from Alcanivorax borkumensis bacterium. Int J Adv Biol Biomed Res. 2014c;2(1):76–85.
- 156.Zafra G, Absalón AE, Cortés-Espinosa DV. Morphological changes and growth of filamentous fungi in the presence of high concentrations of PAHs. Braz J Microbiol. 2015;46:937–941. doi: 10.1590/S1517-838246320140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mineki S, Suzuki K, Iwata K, Nakajima D, Goto S. Degradation of polyaromatic hydrocarbons by fungi isolated from soil in Japan. Polycycl Aromat Compd. 2015;35:120–128. doi: 10.1080/10406638.2014.937007. [DOI] [Google Scholar]
- 158.Semple KT, Morriss A, Paton GI. Bioavailability of hydrophobic organic contaminants in soils: fundamental concepts and techniques for analysis. Eur J Soil Sci. 2003;54:809–818. doi: 10.1046/j.1351-0754.2003.0564.x. [DOI] [Google Scholar]
- 159.Ron EZ, Rosenberg E. Enhanced bioremediation of oil spills in the sea. Curr Opin Biotechnol. 2014;27:191–194. doi: 10.1016/j.copbio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 160.Rahman KS, Rahman TJ, Kourkoutas Y, Petsas I, Marchant R, Banat I. Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol. 2003;90:159–168. doi: 10.1016/S0960-8524(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 161.Varjani SJ, Upasani VN. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: production, characterization and surface active properties of biosurfactant. Bioresour Technol. 2016;221:510–516. doi: 10.1016/j.biortech.2016.09.080. [DOI] [PubMed] [Google Scholar]
- 162.Varjani SJ, Upasani VN. Core flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo-and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour Technol. 2016;220:175–182. doi: 10.1016/j.biortech.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 163.Van Beilen JB, Funhoff EG. Alkane hydroxylases involved in microbial alkane degradation. Appl Microbiol Biotechnol. 2007;74:13–21. doi: 10.1007/s00253-006-0748-0. [DOI] [PubMed] [Google Scholar]
- 164.Scheller U, Zimmer T, Becher D, Schauer F, Schunck W-H. Oxygenation cascade in conversion of n-alkanes to α, ω-dioic acids catalyzed by cytochrome P450 52A3. J Biol Chem. 1998;273:32528–32534. doi: 10.1074/jbc.273.49.32528. [DOI] [PubMed] [Google Scholar]
- 165.van Beilen JB, Funhoff EG. Expanding the alkane oxygenase toolbox: new enzymes and applications. Curr Opin Biotechnol. 2005;16:308–314. doi: 10.1016/j.copbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 166.Crisafi F, Genovese M, Smedile F, Russo D, Catalfamo M, Yakimov M, et al. Bioremediation technologies for polluted seawater sampled after an oil-spill in Taranto gulf (Italy): A comparison of biostimulation, bioaugmentation and use of a washing agent in microcosm studies. Mar Pollut Bull. 2016;106:119–26. [DOI] [PubMed]
- 167.Chakraborty R, Borglin SE, Dubinsky EA, Andersen GL, Hazen TC. Microbial response to the MC-252 oil and Corexit 9500 in the Gulf of Mexico. Front Microbiol. 2012;3:357. doi: 10.3389/fmicb.2012.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hamdan LJ, Fulmer PA. Effects of COREXIT® EC9500A on bacteria from a beach oiled by the Deepwater horizon spill. Aquat Microb Ecol. 2011;63:101–109. doi: 10.3354/ame01482. [DOI] [Google Scholar]
- 169.Kleindienst S, Seidel M, Ziervogel K, Grim S, Loftis K, Harrison S, et al. Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. PNAS. 2015;112:14900–5. [DOI] [PMC free article] [PubMed]
- 170.Zuijdgeest A, Huettel M. Dispersants as used in response to the MC252-spill lead to higher mobility of polycyclic aromatic hydrocarbons in oil-contaminated Gulf of Mexico sand. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed]
- 171.DiGregorio S, Ruffini Castglione M, Gentini A, Lorenzi R. Biostimulation of the autochthonous bacterial community and bioaugmentation of selected bacterial strains for the depletion of polycyclic aromatic hydrocarbons in a historically contaminated soil: EGU General Assembly Conference Abstracts; 2015.
- 172.Nikolopoulou M, Pasadakis N, Kalogerakis N. Evaluation of autochthonous bioaugmentation and biostimulation during microcosm-simulated oil spills. Mar Pollut Bull. 2013;72:165–173. doi: 10.1016/j.marpolbul.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 173.Schaum J, Cohen M, Perry S, Artz R, Draxler R, Frithsen JB, et al. Screening level assessment of risks due to dioxin emissions from burning oil from the BP Deepwater horizon Gulf of Mexico spill. Environ Sci Technol. 2010;44:9383–9. [DOI] [PubMed]
- 174.Zheng M, Ahuja M, Bhattacharya D, Clement TP, Hayworth JS, Dhanasekaran M. Evaluation of differential cytotoxic effects of the oil spill dispersant Corexit 9500. Life Sci. 2014;95:108–117. doi: 10.1016/j.lfs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 175.Beolchini F, Rocchetti L, Regoli F, Dell’Anno A. Bioremediation of marine sediments contaminated by hydrocarbons: experimental analysis and kinetic modeling. J Hazard Mater. 2010;182:403–407. doi: 10.1016/j.jhazmat.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 176.Brodkorb TS, Legge RL. Enhanced biodegradation of phenanthrene in oil tar-contaminated soils supplemented with Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3117–3121. doi: 10.1128/AEM.58.9.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hii YS, Law AT, Shazili N, Abdul-Rashid M, Lee CW. Biodegradation of Tapis blended crude oil in marine sediment by a consortium of symbiotic bacteria. Int Biodeterior Biodegradation. 2009;63:142–150. doi: 10.1016/j.ibiod.2008.08.003. [DOI] [Google Scholar]
- 178.Mohajeri L, Aziz HA, Isa MH, Zahed MA. A statistical experiment design approach for optimizing biodegradation of weathered crude oil in coastal sediments. Bioresour Technol. 2010;101:893–900. doi: 10.1016/j.biortech.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 179.Murado MA, Vázquez JA, Rial D, Beiras R. Dose–response modelling with two agents: application to the bioassay of oil and shoreline cleaning agents. J Hazard Mater. 2011;185:807–817. doi: 10.1016/j.jhazmat.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 180.Solano-Serena F, Marchal R, Heiss S, Vandecasteele JP. Degradation of isooctane by Mycobacterium austroafricanum IFP 2173: growth and catabolic pathway. J Appl Microbiol. 2004;97:629–639. doi: 10.1111/j.1365-2672.2004.02344.x. [DOI] [PubMed] [Google Scholar]
- 181.Townsend GT, Prince RC, Suflita JM. Anaerobic biodegradation of alicyclic constituents of gasoline and natural gas condensate by bacteria from an anoxic aquifer. FEMS Microbiol Ecol. 2004;49:129–135. doi: 10.1016/j.femsec.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 182.Vogel TM. Bioaugmentation as a soil bioremediation approach. Curr Opin Biotechnol. 1996;7:311–316. doi: 10.1016/S0958-1669(96)80036-X. [DOI] [PubMed] [Google Scholar]
- 183.Wang X-B, Chi C-Q, Nie Y, Tang Y-Q, Tan Y, Wu G, et al. Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel Dietzia strain. Bioresour Technol. 2011;102:7755–61. [DOI] [PubMed]
- 184.Wardlaw GD, Nelson RK, Reddy CM, Valentine DL. Biodegradation preference for isomers of alkylated naphthalenes and benzothiophenes in marine sediment contaminated with crude oil. Org Geochem. 2011;42:630–639. doi: 10.1016/j.orggeochem.2011.03.029. [DOI] [Google Scholar]
- 185.Bartha R. Biotechnology of petroleum pollutant biodegradation. Microb Ecol. 1986;12:155–172. doi: 10.1007/BF02153231. [DOI] [PubMed] [Google Scholar]
- 186.Radwan SS. Gulf oil spill. Nature. 1991;350:456–6.
- 187.Radwan S, Sorkhoh N, Fardoun F, Al-Hasan R. Soil management enhancing hydrocarbon biodegradation in the polluted Kuwaiti desert. Appl Microbiol Biotechnol. 1995;44:265–270. doi: 10.1007/BF00164513. [DOI] [PubMed] [Google Scholar]
- 188.Zavareh MSH, Ebrahimipour G, Moghadam MS, Fakhari J, Abdoli T. Bioremediation of crude oil using bacterium from the coastal sediments of Kish Island, Iran. Iran J Public Health. 2016;45:670. [PMC free article] [PubMed] [Google Scholar]
- 189.Bao M-t, Wang L-n, Sun P-y, Cao L-x, Zou J, Li Y-m. Biodegradation of crude oil using an efficient microbial consortium in a simulated marine environment. Mar Pollut Bull. 2012;64:1177–1185. doi: 10.1016/j.marpolbul.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 190.Ruberto L, Vazqueza SC, Walter P, Mac C. Effectiveness of the natural bacterial flora, biostimulationand and bioaugmentation on the bioremediation of a hydrocarbon contaminated Antarctic soil. Int Biodeterior Biodegradation. 2003;52:115–125. doi: 10.1016/S0964-8305(03)00048-9. [DOI] [Google Scholar]
- 191.Maa FB, Jing B, Guo L, Zhao C, Chein-chi C, Di C. Application of bioaugmentation to improve the activated sludge system into the contact oxidation system treating petrochemical wastewater. Bioresour Technol. 2009;100:597–602. doi: 10.1016/j.biortech.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 192.Hassanshahian M, Bayat Z, Cappello S, Smedile F, Yakimov M. Comparison the effects of bioaugmentation versus biostimulation on marine microbial community by PCR-DGGE: A mesocosm scale. J Environ Sci (China) 2016;43:136–146. doi: 10.1016/j.jes.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 193.Flavia F, Evans S, Rosado GV, Sebasti S, Renata C, Pedro LO, et al. Impact of oil contamination and biostimulation on the diversity of indigenous bacterial communities in soil microcosms. FEMS Microbiol Ecol. 2004;49:295–305. [DOI] [PubMed]
- 194.Ruberto LR, Dias A, Balbo SC, Vazquez EA, Mac W. Influence of nutrients addition and bioaugmentation on the hydrocarbon biodegradation of a chronically contaminated Antarctic soil. J Appl Microbiol. 2009;106:1101–1110. doi: 10.1111/j.1365-2672.2008.04073.x. [DOI] [PubMed] [Google Scholar]
- 195.Hassanshahian M, Boroujeni NA. Enrichment and identification of naphthalene-degrading bacteria from the Persian Gulf. Mar Pollut Bull. 2016;107:59–65. doi: 10.1016/j.marpolbul.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 196.Abarian M, Hassanshahian M, Esbah A. Degradation of phenol at high concentrations using immobilization of Pseudomonas putida P53 into sawdust entrapped in sodium-alginate beads. Water Sci Technol. 2019;79:1387–1396. doi: 10.2166/wst.2019.134. [DOI] [PubMed] [Google Scholar]
- 197.Al-Mailem D, Eliyas M, Radwan S. Bioremediation of oily hypersaline soil and water via potassium and magnesium amendment. Can J Microbiol. 2013;59:837–844. doi: 10.1139/cjm-2013-0698. [DOI] [PubMed] [Google Scholar]
- 198.Chaprão MJ, Ferreira IN, Correa PF, Rufino RD, Luna JM, Silva EJ, et al. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron J Biotechnol. 2015;18:471–9.
- 199.Chen Q, Bao M, Fan X, Liang S, Sun P. Rhamnolipids enhance marine oil spill bioremediation in laboratory system. Mar Pollut Bull. 2013;71:269–275. doi: 10.1016/j.marpolbul.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 200.Garcia-Pichel F, Prufert-Bebout L, Muyzer G. Phenotypic and phylogenetic analyses show Microcoleus chthonoplastes to be a cosmopolitan cyanobacterium. Appl Environ Microbiol. 1996;62:3284–3291. doi: 10.1128/AEM.62.9.3284-3291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khan NY, Al-Ajmi D. Post-war imperatives for the sustainable management of the Gulf ecosystem. Environ Int. 1998;24:239–248. doi: 10.1016/S0160-4120(97)00141-4. [DOI] [Google Scholar]
- 202.Sajna KV, Sukumaran RK, Gottumukkala LD, Pandey A. Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour Technol. 2015;191:133–139. doi: 10.1016/j.biortech.2015.04.126. [DOI] [PubMed] [Google Scholar]