Abstract
Acute and chronic pain delay recovery and impair outcomes after major pediatric surgery. Understanding unique risk factors for acute and chronic pain is critical to developing effective treatments for youth at risk. We aimed to identify adolescent and family psychosocial predictors of acute and chronic postsurgical pain following major surgery in adolescents. Participants included 119 youth age 10–18 years (Mage=14.9;78.2% white) undergoing major musculoskeletal surgery and their parents. Participants completed pre-surgery baseline questionnaires, with youth reporting on baseline pain, anxiety, depression, insomnia and sleep quality, and parents reporting on parental catastrophizing and family functioning. At baseline, 2-weeks, and 4-months post-surgery, youth completed 7-days of daily pain diaries and reported on health-related quality of life. Sequential logistic regression models examined pre-surgery predictors of acute and chronic postsurgical pain, defined as significant pain with impairment in health-related quality of life. Acute pain was experienced by 27.2% of youth at 2-weeks, while 19.8% of youth met criteria for chronic pain at 4-months. Baseline pain predicted acute pain (OR=1.96; 95%CI=1.32–2.90), while depressive symptoms (OR=1.22; 95%CI=1.01–1.47) and sleep quality (OR=0.26; 95%CI=0.08–0.83) predicted chronic pain. Tailored interventions need to be developed and incorporated into perioperative care to address risk factors for acute and chronic pain.
Keywords: adolescent, chronic postsurgical pain, spinal fusion, depression, insomnia
Introduction
Acute and chronic pain are common experiences after major surgery. Compared to younger children, adolescents are at elevated risk for both acute and chronic pain following surgery22,46. Negative consequences include delayed recovery and impaired health-related quality of life (HRQOL)45,47,49, making effective pain management a high priority. Despite high prevalence of acute pain (>50%)37,45 and chronic pain (20%)19,26,38,47,53, risk factors for postsurgical pain in youth remain largely unknown51. Given the influence of a large range of biopsychosocial factors on development of chronic pain14, it is possible that a different set of risk factors may predict acute versus chronic pain. It is important to understand the transition from acute to chronic postsurgical pain (CPSP) to develop effective treatments for youth at risk60.
Elman and Borsook’s model of the mechanisms of chronic pain and addiction14 highlights the roles of sensory (pain processing), psychological (emotions, cognitions), behavioral, and social (interpersonal) domains in the evolution of pain, from inciting tissue damage to chronification of pain. Although biopsychosocial models are often applied to pediatric chronic pain, much less attention has been directed toward understanding pediatric postsurgical pain51,60. Emerging research suggests that psychosocial factors play a role in persistence of pain after major surgery10,37,38,47,53,60. However, a recent systematic review examining risk factors for CPSP in youth identified a gap in well-designed studies that examine comprehensive risk factors in this population51. For example, several studies have examined adolescent anxiety (e.g., general anxiety, pain catastrophizing)4,3,6,50 but other emotional factors important in chronic pain (e.g., depressive symptoms)24 have not been assessed. Similarly, a few studies examined sleep quality as a predictive factor45,52, but more comprehensive assessment of sleep disturbances has not been conducted. Further, there has been minimal consideration of the social context, including parent and family factors27, in pediatric CPSP studies. Additional weaknesses in the literature include small sample sizes, inconsistency in measurement of both acute and CPSP to understand risk for transition, and differing definitions of CPSP based on presence of pain without consideration of severity and impact51, limiting conclusions that can be drawn.
To address these gaps, we conducted a prospective longitudinal cohort study in youth undergoing major musculoskeletal surgery. We aimed to examine adolescent psychological (emotional, cognitive) and behavioral factors (sleep quality, insomnia symptoms), and parent and family factors as predictors of risk for acute postsurgical pain (APSP) at 2-weeks and CPSP at 4-months following surgery. To begin to understand the sensory component of postoperative pain, we assessed pre-operative pain intensity and distribution. We defined postsurgical pain as pain that is associated with impairment in HRQOL59, and chose 2-weeks for the acute outcome period based on research demonstrating differentiation of recovery trajectories at 2-weeks post-surgery47. Based on prior perioperative as well as broader pediatric pain literature24,27,41,51, we expected baseline pain, adolescent emotional and behavioral factors, and parent and family factors to predict acute and CPSP. We hypothesized that higher baseline pain intensity6,10,45, wider pain extent48, higher levels of anxiety and depressive symptoms10,17,24,45, higher insomnia severity, poorer sleep quality52, higher parent pain catastrophizing37,47, and poor family functioning27, would significantly predict APSP, and subsequent CPSP.
Materials and Methods
Participants:
Participants included 119 youth undergoing major musculoskeletal surgery and their parent or guardian, recruited from a tertiary pediatric hospital in the Northwest United States between October 2014 and June 2018.
Youth were eligible for participation if they were 1) 10–18 years of age, 2) scheduled to undergo major musculoskeletal surgery (spinal fusion for idiopathic spinal deformity, Nuss procedure for pectus deformity, or hip or femur osteotomy), and 3) able to read and understand English. Youth were excluded from the study for 1) developmental delay affecting ability to complete study measures independently, 2) chronic medical condition (e.g. neuromuscular scoliosis, diabetes, cancer), 3) diagnosed chronic pain condition requiring treatment, or 4) prior major surgery (e.g. spine surgery, open thoracic or abdominal surgery). Eligible procedures were chosen as the most common major musculoskeletal procedures in adolescents without significant comorbidity.
Procedures:
The Institutional Review Board approved all study procedures. Potentially eligible youth were identified using an electronic screening tool selecting patients based on patient age and scheduled procedure type and indication from the electronic medical record. An informational study packet was mailed to potential participants, following which research assistants approached youth and their parents by phone, or in person at their pre-operative appointments. Of the 213 potentially eligible families approached, 88 declined, 6 were not eligible, and 119 enrolled into the study. Primary reasons for non-participation included not interested in research or not enough time. Five enrolled families dropped out of the study prior to the 4-month assessment, and 114 families completed the study through 4-month follow up. Long-term follow up of health outcomes in this sample is ongoing.
Eligible and interested families completed written consent (parents and youth≥18 years) and assent (youth<18 years). Youth completed 3 assessment timepoints: the week before surgery (pre-surgery baseline), 2-weeks after surgery (acute post-surgery outcomes), and 4-months post-surgery (chronic post-surgery outcomes) (Figure 1). Youth and their parents completed validated self-report questionnaire measures assessing pre-surgery psychosocial risk factors, once during the baseline assessment (the week before surgery). Youth reported on daily pain intensity using an online diary for 7 days at each timepoint, and completed additional pain and health outcomes measures once during each timepoint. Diaries and measures were collected using the Research Electronic Data Capture (REDCap) system which sends out an email to participants containing a link for completing measures online. Measures were sent separately to parents and youth, and entries were time-stamped. Youth and parents received a small gift card incentive ($20 and $10 respectively) on completion of each assessment.
Figure 1.
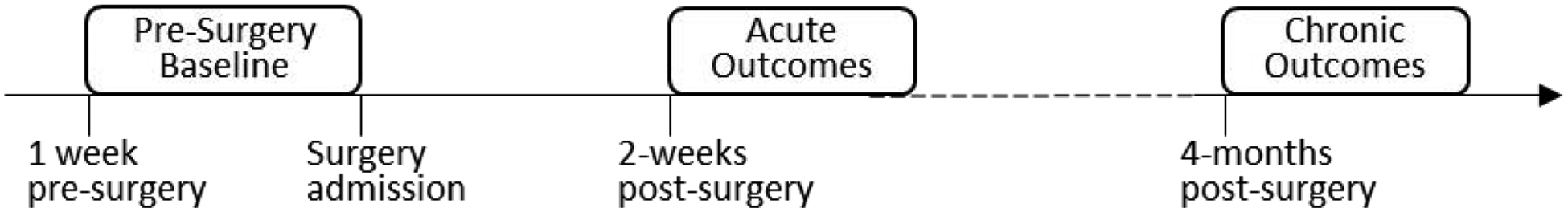
Study design
This is the first manuscript to report primary outcomes of this study. To date, a qualitative interview study with a small subset of participants has been published from this cohort49, and pain and quality of life data from the baseline assessment were used in a measure validation study13. Results from the long-term follow up will be reported on study completion.
Measures:
Youth measures
Pain intensity.
At each assessment, youth rated their daily pain intensity for seven days. They were prompted at the end of each day to respond on an 11-point numeric rating scale with anchors 0=“no pain” and 10=“worst pain possible”. Youth also self-reported daily medication use, indicating whether medication was taken (Yes/No) and listing the medication in a free text field. Self-report numeric rating scales are recommended for acute and chronic pain assessment in youth of this age range4,57 and have shown adequate validity and sensitivity to change in youth following surgery35.
Pediatric Quality of Life Inventory (PedsQL short form, acute version).
Youth completed the 15-item short form of the Pediatric Quality of Life Inventory assessing school, emotional, psychological, and physical functioning over the preceding 7 days5. The sum of items is transformed to yield a total score ranging from 0–100 reflecting both psychosocial and physical health, with lower scores indicating poorer HRQOL. The PedsQL has been extensively validated and is broadly used in youth of this age range5,12,30,54,55, and has demonstrated sensitivity to acute changes in HRQOL12. Values one standard deviation below the population mean, based on normative data from healthy populations, are an established as a cutoff indicating impairment in HRQOL5,55.
Pain characteristics.
Youth reported on extent to which pain limited activities in the prior 7 days on a 0–100 visual analogue scale, with anchors 0=“does not limit any activity” and 100=“limits all activities”40. Evidence of construct validity was shown in this sample through expected cross-sectional associations with pain intensity (r=0.4, p<0.001 at 2-weeks, r=0.5, p<0.001 at 4-months) and the physical health subscale of the PedsQL (r=−0.4, P<0.001 at 2-weeks, and r=−0.6, p<0.001). Youth rate level of emotional upset due to pain during the preceding 7 days on a 5-point Likert scale with response options ranging from “not at all” to “very much”, which are assigned values from 1 to 5.
Widespread Pain Index (WPI).
Youth indicate any locations where they have experienced pain or tenderness during the past 7 days from 19 pain locations. The number of pain locations is summed to describe pain distribution or pain extent, reflecting the degree to which pain is widespread. The WPI has shown good construct validity to assess pain distribution in youth of this age range, with both acute and chronic pain13.
Pain Catastrophizing Scale- Child version (PCS-C).
This 13-item measure assesses extent to which youth endorse thoughts and feelings of magnification, rumination, and helplessness in response to pain11. Response options range from 0 (“not at all”) to 4 (“extremely”), and are summed to yield a total score ranging from 0 to 52, with higher values indicating higher levels of pain catastrophizing. The measure is valid in this age range, and has been broadly used in youth undergoing major surgery17,36,45,47.
Revised Child Anxiety and Depression Scale (RCADS).
This 47-item self-report questionnaire assesses adolescent symptoms of anxiety and depression. The measure comprises 5 scales of anxiety (social phobia, panic disorder, separation anxiety, generalized anxiety, obsessive-compulsive), and 1 scale for major depression. Response options range from 0 (never) to 3 (always), and corresponding items are summed to yield subscale scores, with higher scores indicating greater anxiety and depressive symptoms. The total anxiety scale score (sum of the 5 anxiety subscales) ranges from 0 to 111, and the depression subscale score ranges from 0 to 30. This measure is valid, reliable, and sensitive to change in youth7,31,44, and has been broadly used in adolescents with pain8,21. Normative data (mean and SD) based on grade and sex can be used to calculate a T-score for subscale and total scores58 to aid interpretation of individual scores. A T-score > 65 is considered borderline symptoms, and a T score > 70 is above clinical threshold.
Insomnia Severity Index (ISI).
Youth provided self-report of insomnia severity and impact in the preceding 2 weeks on this 7-item measure with responses on a 5-point Likert scale. Items (range 0–4) are summed to yield a total score which can be classified into 4 levels of insomnia symptom severity: 1) as absence of insomnia (0–7), 2) sub-threshold insomnia (8–14), 3) moderate insomnia (15–21), or 4) severe insomnia (22–28). This valid and reliable measure2 has been used in research with adolescents9 including youth with pain43.
Adolescent Sleep Wake Scale (ASWS).
Youth completed the 10-item short form version of this measure assessing adolescent sleep quality in the preceding month, including going to bed, falling asleep, reinitiating sleep, and returning to wakefulness. Items are scored on a 6-point Likert scale ranging from 1 (always) to 6 (never), and are averaged to create a total score with higher scores indicating better sleep quality. The 10-item scale total score has shown adequate internal consistency and construct validity in adolescents with chronic pain16.
Parent Measures
Pain Catastrophizing Scale-Parent version (PCS-P).
The parent version of this measure contains 13 items assessing parent’s cognitive and emotional responses to their child experiencing pain20, using response options ranging from 0 (“not at all”) to 4 (“extremely”). Items are summed to yield a total score ranging from 0 to 52, with higher values indicating higher levels of pain catastrophizing. This measure is valid and reliable, and has been used with parents of adolescents in the perioperative setting6,37,45.
Family Assessment Device (FAD)- general functioning subscale.
Parents completed the 12-item general family functioning subscale of the McMaster Family Assessment Device15. Parents endorse items by selecting one of 4 response options: ‘strongly disagree’, ‘disagree’, ‘agree’, or ‘strongly agree’. The mean of item scores (range 1–4) is computed. Scores can be interpreted using an established cutoff score of 2.0 or greater to indicate unhealthy family functioning. The FAD has demonstrated adequate internal reliability, discriminant validity, concurrent validity, and predictive validity1,15,32,33 and has been used in parents of youth with pain27,33.
Demographic questionnaire.
Parents report on child race, ethnicity, and sex, and parent education and income.
Statistical Analysis:
Sample size calculations were performed using a target sample size for multivariable regression modeling, based on guidelines to include at least 10 subjects per degree of freedom to obtain reasonable estimates of regression coefficients23. Based on 95% retention rates in our prior studies, we aimed to enroll 120 participants to obtain a final sample size of over 110 participants, which was adequate for including the 11 continuous or binary baseline predictor variables of interest.
Bivariate analyses comparing study completers (n=114) versus study dropouts (n=5) did not identify any significant differences on participant demographic factors (age and sex) or baseline pain intensity or distribution (number of pain locations) (p’s>0.05). All available data from participants were included in the analyses.
Daily diary data were cleaned by deleting all entries with timestamps outside of the valid date and time frame of 7 pm on the same day to 5 am the following morning. On average, youth completed 5.4 valid days over the 7 day diary monitoring period at baseline, 2-week, and 4-month time points. Thus a total of 1,811 days of diary data were generated by the participants. Pre-surgery daily pain data were averaged for each participant to calculate a mean baseline pain intensity score. Post-surgery daily pain data at 2-weeks and 4-months after surgery were combined with HRQOL data to define binary outcome variables for APSP and CPSP. Daily medication use was manually coded into categories: over the counter analgesics (acetaminophen, non-steroidal anti-inflammatory drugs), opioid, or other medication. Medication use at 2-weeks and 4-months were described as the proportion of days (over the 7 day monitoring period) that medication use was reported for each medication category.
APSP and CPSP were defined by applying the modified International Association for the Study of Pain (IASP) definition of CPSP as pain that affects HRQOL59. Thus, we defined APSP as a binary variable indicating presence of moderate-severe pain and impaired HRQOL. Participants were classed as having APSP if at 2-weeks they reported, 1) moderate-severe pain (pain intensity ≥ 5) on more than 50% of daily diary days, and 2) impaired HRQOL based on PedsQL™ total score < 74.9. The cutoff of 74.9 is based on the established cutpoint for impaired quality of life of 1 SD (11.2 points) below the population mean (86.1 points)55 based on normative data for the PedsQL short form5. Participants were classed as having CPSP if at 4-months they experienced, 1) greater than minimal pain (pain intensity ≥ 3) on more than 50% of days during the daily monitoring period and 2) impairment in HRQOL (PedsQL™ < 74.9). The daily intensity cutoffs for acute and chronic pain were selected based on prior data supporting that pain above these thresholds represent a departure from the average experience after surgery10,37,38,47,51, as well as our own prior research demonstrating significant activity limitations and impairment in quality of life of acute postsurgical pain in the moderate-severe range46,5 and of chronic pain in the mild range47 after surgery. APSP and CPSP were defined independently, thus youth may meet criteria for CPSP even if they had not met criteria for APSP.
We conducted cross-sectional two-sided t-tests and Mann-Whitney U tests comparing pain characteristics (extent to which pain limits activities, and degree of emotional upset due to pain) at 2-weeks by APSP status. Similarly, we compared pain characteristics at 4-months by CPSP status. Further, for descriptive purposes, we applied two-sided t-tests and chi-squared tests to compare baseline demographic and psychosocial variables, with APSP status at 2-weeks, and with CPSP status at 4-months.
To examine psychosocial predictors of acute and chronic postsurgical pain, we took a sequential approach to examine 4 levels of a priori identified domains with the following ordering: demographic factors, baseline pain, adolescent psychological (emotional and behavioral), and parent/family risk factors. We conducted a sequence of multivariable logistic regression models to examine the associations between the domain factors and APSP or CPSP status. The base model (Model 1) included baseline demographic variables only. Then baseline pain intensity and pain distribution (Model 2), adolescent emotional and behavioral factors (Model 3), and parent and family factors (Model 4) were added into the models sequentially, leading to four nested regression models. The nested models were compared by Akaike’s Information Criterion (AIC) and likelihood ratio tests (LRTs). AIC is penalized log-likelihood statistic commonly used for model selection with smaller values indicating better model fit. Likelihood ratio tests comparing each successive model to the previous step (i.e. M2 vs. M1, M3 vs M2, and M4 vs. M3) were used to assess whether the domain specific factors as a whole had significant associations with APSP or CPSP status. All analyses were conducted using Stata version 14 (StataCorp, College Station, TX).
Results
Descriptive data
119 youth (75 girls, 44 boys), with a mean age of 14.9 years (range, 10.0–18.9 years), 78.2% white enrolled into the study (Table 1). APSP, as defined by moderate-severe pain on the majority of days and impaired HRQOL at 2-weeks, was experienced by 27.2% of youth. Youth who met criteria for APSP reported significantly higher pain interference on activities (73.5 vs. 53.7, p<0.001) and emotional upset from pain (3.5 vs. 2.6 vs., p<0.001) at 2-weeks as compared to those without APSP. On average, over the 7 day monitoring period, youth used an opioid on 21.4% of days at the 2-week post-surgery assessment. Use of over the counter analgesics was reported on 54.4% of days. Table 2 presents bivariate analyses for differences in baseline variables by APSP status.
Table 1.
Participant demographic and clinical characteristics
| Demographics | Mean (Range) / N (%) | |
|---|---|---|
| Age (years) | 14.9 (10.0 – 18.9) | |
| Sex | ||
| Female | 75 (63.0%) | |
| Male | 44 (37.0%) | |
| Child Race | ||
| White | 93 (78.2%) | |
| African American | 5 (4.2%) | |
| Asian | 4 (3.4%) | |
| Other/not reported | 17 (14.3%) | |
| Child Ethnicity | ||
| Hispanic or Latino | 10 (8.4%) | |
| Non-Hispanic or Latino | 103 (86.6%) | |
| Not reported | 6 (5.0%) | |
| Annual household income | ||
| < $29,999 | 8 (6.7%) | |
| $30,000 – $69,999 | 30 (25.2%) | |
| > $70,000 | 75 (63.0%) | |
| Not reported | 6 (5.0%) | |
| Parental educational level | ||
| High school or less | 16 (13.4%) | |
| Some college | 31 (26.1%) | |
| Bachelor’s Degree or higher | 69 (58.0%) | |
| Not reported | 3 (2.5%) | |
| Caregiver relationship (n = 119) | ||
| Biological Mother | 104 | |
| Biological Father | 7 | |
| Adoptive Mother | 5 | |
| Adoptive Father | 1 | |
| Stepmother | 1 | |
| Grandmother | 1 | |
| Surgery Type | ||
| Posterior Spinal Fusion | 81 (68.1%) | |
| Anterior Spinal Fusion | 3 (2.5%) | |
| Pectus | 31 (26.1%) | |
| Femur/Hip | 4 (3.4%) | |
Table 2.
Univariate analyses examining pre-surgery demographic and psychosocial variables by acute postsurgical pain (APSP) status at 2-weeks
| APSP-no | APSP-yes | |
|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Age | 14.8 (1.9) | 15.0 (1.8) |
| Sex* | ||
| male | 34 (41.0%) | 5 (16.1%) |
| female | 49 (59.0%) | 26 (83.9%) |
| Baseline pain** | 1.8 (1.6) | 3.5 (2.2) |
| Pain distribution | 2.2 (2.1) | 2.7 (2.2) |
| Pain catastrophizing | 14.2 (10.2) | 16.3 (9.4) |
| Anxiety symptoms | 23.8 (17.8) | 25.5 (15.9) |
| Depressive symptoms | 5.8 (5.1) | 7.9 (5.6) |
| Sleep quality* | 4.3 (0.7) | 3.9 (1.0) |
| Insomnia severity | ||
| absence of symptoms | 61 (74.4%) | 16 (51.6%) |
| sub-threshold | 15 (18.3%) | 9 (29.0%) |
| moderate | 5 (6.1%) | 6 (19.4%) |
| severe | 1 (1.2%) | 0 (0%) |
| Parent pain catastrophizing | 15.6 (10.1) | 19.7 (12.7) |
| Family functioning | ||
| healthy | 63 (77.8%) | 25 (83.3%) |
| unhealthy | 18 (22.2%) | 5 (16.7%) |
P<0.05;
P<0.001
At 4-months after surgery, 19.8% of youth met criteria for CPSP, as defined by more than minimal pain on the majority of diary days and impaired HRQOL at 4-months post-surgery. Youth with CPSP reported higher pain interference on activities (51.7 vs. 21.4, p<0.001) and emotional upset from pain (3.0 vs. 2.1, p<0.001) at 4-months as compared to those who did not have CPSP (i.e., minimal pain or normal HRQOL). The rate of CPSP was significantly higher amongst youth who met criteria for APSP as compared to those who did not (50.0% vs. 9.1%, p<0.001). No participants reported any opioid use over the 7 day diary monitoring period at 4-months; over the counter analgesic use was reported on 7.3% of days over the 7 day monitoring period at 4-months after surgery. Bivariate analyses comparing baseline variables by CPSP status are presented in Table 3.
Table 3.
Univariate analyses examining pre-surgery demographic and psychosocial variables by chronic postsurgical pain (CPSP) status at 4-months
| CPSP-no | CPSP-yes | |
|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Age* | 14.6 (1.9) | 15.7 (1.8) |
| Sex | ||
| male | 31 (36.5%) | 6 (28.6%) |
| female | 54 (63.5%) | 15 (71.4%) |
| Baseline pain* | 2.0 (1.7) | 3.2 (2.5) |
| Pain distribution* | 2.0 (1.8) | 3.1 (2.5) |
| Pain catastrophizing | 14.4 (10.2) | 17.8 (9.5) |
| Anxiety symptoms | 23.6 (17.9) | 31.7 (13.5) |
| Depressive symptoms** | 5.4 (4.8) | 10.8 (5.3) |
| Sleep quality** | 4.3 (0.7) | 3.6 (0.6) |
| Insomnia severity* | ||
| absence of symptoms | 63 (75%) | 8 (38.1%) |
| sub-threshold | 12 (14.3%) | 10 (47.6%) |
| moderate | 8 (9.5%) | 3 (14.3%) |
| severe | 1 (1.2%) | 0 (0%) |
| Parent pain catastrophizing* | 14.7 (9.8) | 19.9 (12.0%) |
| Family functioning | ||
| healthy | 68 (81.0%) | 16 (80%) |
| unhealthy | 16 (19.0%) | 4 (20%) |
P<0.05;
P<0.001
Before surgery, 5.1% of youth met the cutoff for clinical depression and 4.3% met the cutoff for clinical anxiety. 11.0% of youth had moderate to severe levels of insomnia before surgery, while the vast majority had absent or sub-threshold symptoms.
Predictors of APSP
A sequence of multivariable logistic regression models examined associations between demographic factors, baseline pain, adolescent psychological/behavioral, and parent/family factors before surgery with presence of APSP at 2-weeks post-surgery (Table 4). Results revealed that baseline pain variables were significantly associated with APSP, while psychological/behavioral and parent/family factors were not. Addition of baseline pain variables to the base model containing demographic factors significantly improved model fit (AIC reduced from 130 to 115), and increased the prediction of APSP (LR= 19.31 for M2 vs. M1, p<0.001). Sequential addition of psychological/behavioral factors and parent/family factors did not improve model fit (AIC=119 and 117 respectively) or increase prediction of APSP (LR= 5.81 for M3 vs. M2, and LR= 5.97 for M4 vs. M3, p’s>0.05). Of the baseline pain variables, mean baseline pain intensity was the only consistently significant independent predictor of APSP status (M4: OR=1.96; 95% CI 1.32 – 2.90). Thus, for each 1 point higher mean pain intensity rating during the week preceding surgery, the odds of APSP was 96% higher at 2-weeks following surgery (p<0.001).
Table 4.
Multi-variate logistic regression models examining pre-surgery risk factors for acute postsurgical pain at 2-weeks following surgery
| M1: Base model | M2: Clinical factors | M3: Emotional and behavioral factors | M4: FINAL MODEL Parent and family factors | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.21 | (0.95 – 1.55) | 1.14 | (0.87 – 1.49) | 1.12 | (0.84 – 1.49) | 1.17 | (0.86 – 1.58) |
| Sex | 4.75 | (1.55 – 14.56)* | 4.13 | (1.12 – 15.32)* | 3.79 | (0.99 – 14.56) | 3.86 | (0.93 – 16.07) |
| Baseline pain intensity | 1.81 | (1.29 – 2.54)** | 1.98 | (1.35 – 2.91)** | 1.96 | (1.32 – 2.90)** | ||
| Pain distribution | 0.77 | (0.58 – 1.04) | 0.71 | (0.51 – 0.99)* | 0.74 | (0.53 – 1.04) | ||
| Pain catastrophizing | 0.98 | (0.92 – 1.04) | 0.98 | (0.92 – 1.05) | ||||
| Anxiety symptoms | 0.99 | (0.96 – 1.03) | 0.99 | (0.96 – 1.03) | ||||
| Depressive symptoms | 0.95 | (0.80 – 1.12) | 0.94 | (0.80 – 1.12) | ||||
| Insomnia severity | 1.39 | (0.47 – 4.12) | 1.46 | (0.49 – 4.36) | ||||
| Sleep quality | 0.48 | (0.21 – 1.09) | 0.53 | (0.22 – 1.25) | ||||
| Parental pain catastrophizing | 1.02 | (0.97 – 1.07) | ||||||
| Family functioning | 0.45 | (0.10 – 2.01) | ||||||
| AIC | 130 | 115 | 119 | 117 | ||||
| LR | 19.3** | 5.8 | 6.0 | |||||
AIC, Akaike Information Criterion; LR, Likelihood Ratio test statistic;
P<0.05;
P<0.001
Predictors of CPSP
The same sequence of multivariable logistic regression models was used to examine the association between demographic factors, baseline pain, adolescent psychological/behavioral, and parent/family factors before surgery and CPSP status at 4-months after surgery. The 4 nested regression models are presented in Table 5. Only addition of adolescent psychological and behavioral factors increased model fit, as reflected by a reduction in AIC, and increased prediction of CPSP status (LR= 17.02, p=0.004). Addition of baseline pain variables, and of parent/family variables did not improve model fit, and was not associated with a significant increase in overall prediction of CPSP (LR=5.07 for M2 vs. M1, and LR=3.55 for M4 vs. M3, P’s>0.05). In the final model (M4), adolescent depressive symptoms (OR=1.22, 95% CI= 1.01–1.47), and sleep quality (OR=0.26, 95% CI= 0.08–0.83) were significantly associated with CPSP status at 4-months post-surgery. Each point higher level of depressive symptoms on the RCADs before surgery was associated with a 22% higher odds of CPSP at 4-months (p=0.03). Each 1 point higher sleep quality score on the ASWS before surgery was associated with 74% lower odds of developing CPSP at 4-months (p=0.02). Associations between these two factors and CPSP were also detected in Model 3.
Table 5.
Multi-variate logistic regression models examining pre-surgery risk factors for chronic postsurgical pain at 4-months following surgery
| M1: Base model | M2: Clinical factors | M3: Emotional and behavioral factors | M4: FINAL MODEL Parent and family factors | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.46 | (1.10 – 1.94)* | 1.34 | (1.00 – 1.80) | 1.15 | (0.81 – 1.63) | 1.18 | (0.82 – 1.70) |
| Sex | 2.31 | (0.75 – 7.12) | 1.22 | (0.35 – 4.32) | 1.22 | (0.28 – 5.27) | 1.29 | (0.29 – 5.72) |
| Baseline pain intensity | 1.26 | (0.92 – 1.72) | 1.18 | (0.80 – 1.75) | 1.31 | (0.86 – 1.99) | ||
| Pain distribution | 1.08 | (0.80 – 1.46) | 0.95 | (0.67 – 1.34) | 0.82 | (0.54 – 1.24) | ||
| Pain catastrophizing | 0.97 | (0.90 – 1.04) | 0.97 | (0.90 – 1.05) | ||||
| Anxiety symptoms | 1.00 | (0.95 – 1.04) | 1.00 | (0.96 – 1.04) | ||||
| Depressive symptoms | 1.24 | (1.03 – 1.49)* | 1.22 | (1.01 – 1.47)* | ||||
| Insomnia severity | 0.32 | (0.09 – 1.10) | 0.31 | (0.09 – 1.10) | ||||
| Sleep quality | 0.25 | (0.08 – 0.76)* | 0.26 | (0.08 – 0.83)* | ||||
| Parental pain catastrophizing | 1.01 | (0.96 – 1.08) | ||||||
| Family functioning | 1.38 | (0.32 – 6.03) | ||||||
| AIC | 104 | 103 | 96 | 96 | ||||
| LR | 5.1 | 17.0** | 3.6 | |||||
AIC, Akaike Information Criterion; LR, Likelihood Ratio test statistic;
P<0.05;
P<0.01
Discussion
This study comprehensively examined psychosocial risk factors for acute and chronic postsurgical pain in adolescents undergoing major musculoskeletal surgery. We examined demographic factors, baseline pain, adolescent psychological/behavioral, and parent/family risk factors as predictors of APSP at 2-weeks and of CPSP at 4-months following surgery. Our findings revealed that distinct domains predicted acute and chronic postsurgical pain. Pre-surgery pain intensity was the only significant predictor of APSP at 2-weeks post-surgery, with odds of developing APSP almost doubling with each 1-point increase in mean pain intensity during the week prior to surgery. As expected, pre-surgery adolescent emotional and behavioral factors were significant predictors associated with chronic pain at 4-months. Both depressive symptoms and sleep quality had a significant effect on chronic pain after surgery, with each 1-point difference on the scales assessing depressive symptoms and sleep quality before surgery being associated with a 22% and 74% difference in the odds of CPSP at 4-months, respectively. However, adolescent pain catastrophizing, anxiety symptoms, and insomnia symptoms were not predictive of CPSP.
This study builds on prior literature through assessing a comprehensive set of emotional, behavioral, and parent/family factors, allowing us to differentiate predictors of significant acute pain at 2-weeks and chronic pain at 4-months. Consistent with findings in prior studies in youth6,45 as well as adults39, baseline (pre-surgery) pain was a significant predictor of APSP. In adults, baseline pain severity has been identified as predictive of acute pain trajectories following major musculoskeletal surgery39. In addition, studies employing quantitative sensory testing before surgery in adults have found higher pre-surgery pain intensity is associated with higher pain sensitivity before surgery34. This suggests that altered sensory pain processing may potentially be one factor underlying the relationship between higher pre-operative pain and APSP. Further research is needed in pediatric populations to understand potential sensory mechanisms involved in acute postoperative pain in youth.
Prior research has demonstrated that pain trajectories become established during the first 2 weeks of recovery and pain may persist over the year following surgery in a subgroup of youth47. Similarly, we found that 50% of youth with APSP went on to develop CPSP at 4-months. This underscores the importance of developing and implementing interventions to target mechanisms underlying APSP, to improve recovery during the acute phase and promote resolution of pain before a pattern of persistence is established.
Our findings diverge from prior studies that identified psychosocial risk factors for acute postsurgical pain including pain catastrophizing17, sleep disturbance, parent pain catastrophizing45, and a cluster of high psychological symptoms56 to be associated with higher acute pain intensity. We believe there are several reasons for this difference in findings. Most prior studies have only accounted for baseline pain with a single retrospective pain rating17, while other studies did not assess pre-operative pain and only measured psychosocial risk factors28,36. In contrast, our study included a robust daily assessment of baseline pain intensity over a 7 day period and included multivariable modelling. Using this approach, psychosocial factors did not emerge as independent predictors of APSP.
However, as expected, psychosocial factors did predict CPSP. We found that emotional and behavioral factors including higher depressive symptoms and poorer sleep quality predicted CPSP. These findings mirror research in other chronic pain populations. For example, depressive symptoms have also predicted risk for transition from acute to chronic musculoskeletal pain in youth after injury24. Similarly, strong associations between pain, depression, and sleep disturbances have been found in youth with established chronic musculoskeletal pain25,29,42. Indeed, sleep is proposed as a risk factor for development of chronic pain18. However, once chronic pain is established it is difficult to distinguish whether emotional and behavioral symptoms represent a risk factor, a comorbidity, or a consequence of chronic pain. By studying elective musculoskeletal surgery as a discrete event, we extend prior research findings showing that depressive symptoms and sleep quality prior to the inciting injury influence subsequent development of chronic pain.
Notably, while higher depressive symptoms and poorer sleep quality impacted CPSP outcomes, the majority of youth in this population had subclinical levels of these symptoms before surgery suggesting that even minor alterations in mood and sleep quality may be important to address. However, subclinical symptoms could go undetected during routine clinical care. Both depressive symptoms and poor sleep quality can be screened for and modified with cognitive-behavioral treatments. Further research is needed to test screening procedures before surgery to identify youth at elevated risk and/or to closely monitor youth for significant APSP for early intervention to reduce risk of transition to chronic pain. Interventions targeting depressive symptoms and sleep quality before or after surgery may reduce risk for CPSP.
In our comprehensive assessment of psychosocial factors in youth, we found some differences from previous studies of chronic postsurgical pain. Prior studies identified parent pain catastrophizing as associated with CPSP in youth37,47,51. In the present study, higher parent pain catastrophizing was associated with CPSP in univariate analyses, however when accounting for a more comprehensive set of psychosocial predictors, the association between parent/family factors and CPSP status weakened. In addition, prior studies found associations between youth’s pre-surgery anxiety and pain catastrophizing with postsurgical pain6,10,47, which we did not replicate in the current study. Few studies have assessed both anxiety and depressive symptoms as independent predictors in youth, and when these variables were placed in the same multivariate model we found that depressive symptoms were predictive of CPSP. Further work is needed in this population to more fully understand the influence of affective factors. Other potential reasons for differences in findings may include differences in study design (e.g. timing of follow-up), and use of a definition of CPSP which includes pain and HRQOL, compared to prior perioperative studies that defined CPSP status by presence of pain or pain intensity alone.
A strength of our study is the use of multi-dimensional definitions of APSP and CPSP to include not only pain intensity, but also the impact of pain on HRQOL. Supporting the validity of this definition, we found large differences in activity interference and pain related bother in those who met our criteria for APSP and for CPSP. The prevalence of CPSP in the present study was similar to that described previously in the literature51, but goes beyond by demonstrating that this subset of youth experience CPSP characterized by pain and physical and psychosocial impact. Identification of those with the highest impact pain increases the strength of findings from our predictive models. Discrepant definitions of CPSP have hindered progress in postoperative pain research51. Research is needed to validate a multidimensional definition for CPSP which can be applied consistently across research studies, to strengthen the literature and facilitate comparison and synthesis of findings across studies.
Limitations and future directions.
The findings of our study should be interpreted in light of several limitations. Because our sample lacked sociodemographic diversity, we were limited in our ability to examine sociodemographic risk factors. Further, we enrolled low numbers of some surgery types and were therefore unable to stratify findings by procedure type. In addition, procedure type may be confounded by sex, i.e. spinal fusion patients predominantly female while pectus predominantly male. Future studies designed to examine sex differences in postoperative pain mechanisms and outcomes are needed. Our study was also not designed to examine opioid use following surgery, and future studies may consider using technologies such as medication adherence monitoring to objectively assess postoperative medication use. While we comprehensively characterized psychosocial risk factors before surgery, we did not assess resilience factors, such as effective coping and self-efficacy. The perioperative period provides a distinct opportunity to examine risk and resilience mechanisms underlying transition of acute to chronic pain. While our study was designed to identify baseline psychosocial risk factors, future studies should evaluate change in psychosocial factors during short- and longer-term recovery to examine the mechanistic contribution to pain persistence.
Conclusion.
In conclusion, a subgroup of adolescents undergoing major musculoskeletal surgery are at risk for experiencing acute and chronic postsurgical pain that reduces their HRQOL. Baseline pain predicted APSP, suggesting that altered sensory processing may play a role in acute pain. Emotional and behavioral factors including depressive symptoms and poor sleep predicted risk for chronic pain and impairment in quality of life, suggesting that after the acute phase, psychosocial processes influence development of chronic pain. Tailored intervention approaches need to be developed and incorporated into perioperative care to address risk for pain during the acute and chronic phases after surgery.
Highlights.
20% of youth experienced chronic pain at 4-months after major surgery
Distinct psychosocial domains predicted acute and chronic postsurgical pain
Higher baseline pain during the week before surgery predicted acute pain at 2-weeks
Worse depressive symptoms and sleep quality before surgery predicted chronic pain
Tailored interventions are needed address risk factors for acute and chronic pain
Perspective:
Longitudinal results demonstrate adolescents’ pre-surgery pain severity predicts acute postsurgical pain, while depressive symptoms and poor sleep quality predict chronic postsurgical pain. Tailored interventions should address separate risk factors for acute and chronic pain after adolescent surgery.
Acknowledgments:
The authors acknowledge the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (NIH) which funded this research. The authors thank the families who participated in this research study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (NIH) [grant number K23HD078239; PI: Jennifer Rabbitts]. The authors declare no competing interests.
Prior Presentations: This study was presented at the International Symposium on Pediatric Pain on Tuesday 18 June 2019 at 10:15 am in Basel, Switzerland. This is the meeting of the SIG on Pain in Childhood, of the International Association for the Study of Pain. This was an oral presentation; study findings have not been previously published.
References:
- 1.Alderfer MA, Fiese BH, Gold JI, Cutuli JJ, Holmbeck GN, Goldbeck L, Chambers CT, Abad M, Spetter D, Patterson J: Evidence-based assessment in pediatric psychology: family measures. J Pediatr Psychol 33: 1046–61; discussion 1062–4, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastien CH, Vallieres A, Morin CM: Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2: 297–307, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Birnie KA, Chorney J, El-Hawary R, Group PS: Child and parent pain catastrophizing and pain from pre-surgery to six weeks post-surgery: examination of cross-sectional and longitudinal actor-partner effects. PAIN 2017. [DOI] [PubMed] [Google Scholar]
- 4.Castarlenas E, Jensen MP, von Baeyer CL, Miro J: Psychometric Properties of the Numerical Rating Scale to Assess Self-Reported Pain Intensity in Children and Adolescents: A Systematic Review. Clin J Pain 2016. [DOI] [PubMed] [Google Scholar]
- 5.Chan KS, Mangione-Smith R, Burwinkle TM, Rosen M, Varni JW: The PedsQL: reliability and validity of the short-form generic core scales and Asthma Module. Med Care 43: 256–65, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Chidambaran V, Ding L, Moore DL, Spruance K, Cudilo EM, Pilipenko V, Hossain M, Sturm P, Kashikar-Zuck S, Martin LJ, Sadhasivam S: Predicting the pain continuum after adolescent idiopathic scoliosis surgery: A prospective cohort study. Eur J Pain 21: 1252–1265, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE: Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther 38: 835–55, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Chorpita BF, Moffitt CE, Gray J: Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther 43: 309–22, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Clarke G, McGlinchey EL, Hein K, Gullion CM, Dickerson JF, Leo MC, Harvey AG: Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behav Res Ther 69: 111–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly M, Fulmer RD, Prohaska J, Anson L, Dryer L, Thomas V, Ariagno JE, Price N, Schwend R: Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine 39: E174–81, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K: The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 104: 639–46, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM: Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr 168: 1114–21, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Dudeney J, Law EF, Meyyappan A, Palermo TM, Rabbitts JA: Evaluating the psychometric properties of the Widespread Pain Index and the Symptom Severity scale in youth with painful conditions. Canadian Journal of Pain Epub 2019/05/21. doi: 10.1080/24740527.2019.16200972019, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elman I, Borsook D: Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 89: 11–36, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Epstein NB, Baldwin LM, Bishop DS: The McMaster Family Assessment Device. Journal of Marital and Family Therapy 9: 171–180, 1983. [Google Scholar]
- 16.Essner B, Noel M, Myrvik M, Palermo T: Examination of the Factor Structure of the Adolescent Sleep-Wake Scale (ASWS). Behav Sleep Med 13: 296–307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteve R, Marquina-Aponte V, Ramirez-Maestre C: Postoperative pain in children: association between anxiety sensitivity, pain catastrophizing, and female caregivers’ responses to children’s pain. J Pain 15: 157–68 e1, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Finan PH, Goodin BR, Smith MT: The association of sleep and pain: an update and a path forward. J Pain 14: 1539–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortier MA, Chou J, Maurer EL, Kain ZN: Acute to chronic postoperative pain in children: preliminary findings. J Pediatr Surg 46: 1700–5, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G: Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain 123: 254–63, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Graves JK, Hodge C, Jacob E: Depression, Anxiety, and Quality of Life In Children and Adolescents With Sickle Cell Disease. Pediatr Nurs 42: 113–9, 144, 2016. [PubMed] [Google Scholar]
- 22.Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE: Prevalence of moderate-severe pain in hospitalized children. Paediatr Anaesth 22: 661–8, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE: Regression modeling strategies. New York, Springer, 2001 [Google Scholar]
- 24.Holley AL, Wilson AC, Palermo TM: Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain 158: 794–801, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD: Depression and functional disability in chronic pediatric pain. Clin J Pain 17: 341–9, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Landman Z, Oswald T, Sanders J, Diab M, Spinal Deformity Study G: Prevalence and predictors of pain in surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 36: 825–9, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT: Systematic review of family functioning in families of children and adolescents with chronic pain. J Pain 11: 1027–38, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan DE, Rose JB: Gender differences in post-operative pain and patient controlled analgesia use among adolescent surgical patients. Pain 109: 481–7, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Long AC, Krishnamurthy V, Palermo TM: Sleep disturbances in school-age children with chronic pain. J Pediatr Psychol 33: 258–68, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangione-Smith R, Solomon C, Limbers CAP, Zhou C, Varni JW: Assessing Outcomes of Care in the Pediatric Inpatient Setting: Feasibility, Validity, and Sensitivity to Change of the Pediatric Quality of Life Inventory (PedsQLTM) 4.0 Generic Core Scales and Infant Scales. E-PAS: 16452, 2011. [Google Scholar]
- 31.Mathyssek CM, Olino TM, Hartman CA, Ormel J, Verhulst FC, Van Oort FV: Does the Revised Child Anxiety and Depression Scale (RCADS) measure anxiety symptoms consistently across adolescence? The TRAILS study. Int J Methods Psychiatr Res 22: 27–35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller IW, Epstein NB, Bishop DS, Keitner GI: The McMaster Family Assessment Device: Reliability and validity. J Marital Fam Ther 11: 345–356, 1985. [Google Scholar]
- 33.Mitchell MJ, Lemanek K, Palermo TM, Crosby LE, Nichols A, Powers SW: Parent perspectives on pain management, coping, and family functioning in pediatric sickle cell disease. Clin Pediatr (Phila) 46: 311–9, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Neville SJ, Clauw AD, Moser SE, Urquhart AG, Clauw DJ, Brummett CM, Harte SE: Association Between the 2011 Fibromyalgia Survey Criteria and Multisite Pain Sensitivity in Knee Osteoarthritis. Clin J Pain 34: 909–917, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page MG, Katz J, Stinson J, Isaac L, Martin-Pichora AL, Campbell F: Validation of the numerical rating scale for pain intensity and unpleasantness in pediatric acute postoperative pain: sensitivity to change over time. J Pain 13: 359–69, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Page MG, Stinson J, Campbell F, Isaac L, Katz J: Pain-related psychological correlates of pediatric acute post-surgical pain. J Pain Res 5: 547–58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page MG, Campbell F, Isaac L, Stinson J, Katz J: Parental risk factors for the development of pediatric acute and chronic postsurgical pain: a longitudinal study. J Pain Res 6: 727–41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page MG, Stinson J, Campbell F, Isaac L, Katz J: Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res 6: 167–80, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page MG, Katz J, Curtis K, Lutzky-Cohen N, Escobar EM, Clarke HA: Acute pain trajectories and the persistence of post-surgical pain: a longitudinal study after total hip arthroplasty. J Anesth 30: 568–77, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Palermo TM, Witherspoon D, Valenzuela D, Drotar DD: Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain 109: 461–70, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Palermo TM, Chambers CT: Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain 119: 1–4, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Palermo TM, Kiska R: Subjective sleep disturbances in adolescents with chronic pain: relationship to daily functioning and quality of life. J Pain 6: 201–7, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Palermo TM, Beals-Erickson S, Bromberg M, Law E, Chen M: A Single Arm Pilot Trial of Brief Cognitive Behavioral Therapy for Insomnia in Adolescents with Physical and Psychiatric Comorbidities. J Clin Sleep Med 13: 401–410, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piqueras JA, Martin-Vivar M, Sandin B, San Luis C, Pineda D: The Revised Child Anxiety and Depression Scale: A systematic review and reliability generalization meta-analysis. J Affect Disord 218: 153–169, 2017. [DOI] [PubMed] [Google Scholar]
- 45.Rabbitts JA, Groenewald CB, Tai GG, Palermo TM: Presurgical psychosocial predictors of acute postsurgical pain and quality of life in children undergoing major surgery. J Pain 16: 226–34, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R: Pain and Health-Related Quality of Life After Pediatric Inpatient Surgery. J Pain 16: 1334–41, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM: Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain 156: 2383–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabbitts JA, Holley AL, Groenewald CB, Palermo TM: Association Between Widespread Pain Scores and Functional Impairment and Health-Related Quality of Life in Clinical Samples of Children. J Pain 17: 678–84, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabbitts JA, Aaron RV, Fisher E, Lang EA, Bridgwater C, Tai GG, Palermo TM: Long-Term Pain and Recovery After Major Pediatric Surgery: A Qualitative Study With Teens, Parents, and Perioperative Care Providers. J Pain 18: 778–786, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabbitts JA, Fisher E: Postsurgical pain in children: unraveling the interplay between child and parent psychosocial factors. Pain 158: 1847–1848, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabbitts JA, Fisher E, Rosenbloom BN, Palermo TM: Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J Pain 18: 605–614, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabbitts JA, Zhou C, Narayanan A, Palermo TM: Longitudinal and Temporal Associations Between Daily Pain and Sleep Patterns After Major Pediatric Surgery. J Pain 18: 656–663, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieberg CB, Simons LE, Edelstein MR, DeAngelis MR, Pielech M, Sethna N, Hresko MT: Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain 14: 1694–702, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varni JW, Seid M, Kurtin PS: PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 39: 800–12, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Varni JW, Burwinkle TM, Seid M, Skarr D: The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 3: 329–41, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Voepel-Lewis T, Caird MS, Tait AR, Malviya S, Farley FA, Li Y, Abbott MD, van Veen T, Hassett AL, Clauw DJ: A High Preoperative Pain and Symptom Profile Predicts Worse Pain Outcomes for Children After Spine Fusion Surgery. Anesth Analg 124: 1594–1602, 2017. [DOI] [PubMed] [Google Scholar]
- 57.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA: Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain 143: 223–7, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Weiss DC, Chorpita BF: Revised Children’s Anxiety and Depression Scale User’s Guide. Available at https://www.childfirst.ucla.edu/resources/ 2011.
- 59.Werner MU, Kongsgaard UE: I. Defining persistent post-surgical pain: is an update required? Br J Anaesth 113: 1–4, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Williams G, Howard RF, Liossi C: Persistent postsurgical pain in children and young people: prediction, prevention, and management. Pain Rep 2: e616, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


