Abstract
Background:
Desmoplastic small round cell tumor (DSRCT) is a rare aggressive sarcoma that affects children and young adults and portends poor outcomes despite intensive multimodal treatment approaches. We reporttoxicity, response and outcomes of patients with DSRCT treated with the addition of vincristine, irinotecan, and temozolomide (VIT) to interval-compressed chemotherapy as per Children’s Oncology GroupARST08P1.
Methods:
All newly diagnosed pediatric patients with DSRCT treated at Dana-Farber Cancer Institute and Boston Children’s Hospital between 2014 and 2019as per ARST08P1, Arm P2 with replacement of VAC cycles with VIT, were identified.Medical records were reviewed for clinical and disease characteristics, and treatment response and outcomes.
Results:
Six patients were treated as per the above regimen. Median age at diagnosis was15.1 years (range: 3.2-16.4) and five patients were male. Five patients had abdominal primary tumors, of which one had exclusivelyintra-abdominal and four hadextra-abdominal metastases. Two initial cycles of VIT were well tolerated with nausea, vomiting, diarrhea, and constipation as the most common adverse events. Overall response rate defined as partial or complete response after two initial cycles of VIT was 50%. For local control, all patients had surgical resection followed by radiotherapy, and two patients received hyperthemic intraperitoneal chemotherapy at the time of surgery. Of the four patients who have completed therapy to date, three remain disease-free with median follow-up time of 46.7 months.
Conclusions:
Theaddition of VIT to interval-compressed chemotherapy is tolerable and active in DSRCT, with activity warranting additional investigation.
Keywords: desmoplastic small round cell tumor, sarcoma, pediatric, adolescent, radiation, chemotherapy, ARST08P1
1. INTRODUCTION
Desmoplastic small round cell tumor (DSRCT) is a rare aggressive sarcoma affecting children and young adults. These tumors are defined by a translocation between the EWSR1 and WT1 genes.1Patientsoften present with a primary abdominal mass with diffuse intra-abdominal dissemination. Reported long-term outcomes areexceedingly poor with five-year overall survival (OS) rates of approximately 20%.2-11Current multimodal therapeutic approaches consist of intensive multi-agent chemotherapy and local control measures including surgery and whole abdomen radiotherapy (WART) with or without hyperthermic intraperitoneal chemotherapy (HIPEC). Aggressive local control with surgery and WART have been shown to be associated with improvements in overall survival.2-9,11,12
While multi-agent chemotherapy is also thought to be important for achieving long-term survival,an optimal regimen remains undetermined.3,4,7A wide range of chemotherapy regimens have been utilized for DSRCT, often based on treatment strategies for Ewing sarcoma.3,4Responses to vincristine and irinotecan in DSRCT have been reported in the published literature, and this combination is active in many sarcomas.13-18 Temozolomide has also been of interest in DSRCT given its radiosensitizing effects.8
The Children’s Oncology Group (COG) protocol ARST08P1, which was initially developed for children with high-risk rhabdomyosarcoma, evaluated interval-compressed vincristine/doxorubicin/cyclophosphamide (VDC) alternating with cycles of ifosfamide/etoposide (IE), cycles of vincristine/dactinomycin/cyclophosphamide (VAC) and either cycles of vincristine/irinotecan/temozolomide (VIT, Arm P2) or vincristine/irinotecan/cixutumumab (arm P1).19 This regimen was tolerable, but did not improve outcomes for patient with high-risk rhabdomyosarcoma.19Given the known efficacy of many of these agents in DSRCT, this regimen, utilizing the temozolomide-containing arm P2 withsubstitution of VAC for VIT cycles given lack of evidence for the use of dactinomycin in DSRCT, became the standard initial treatment regimen at our center in 2014. The tolerability and efficacy of Arm P2 when given in combination with the aggressive local control strategies utilized in DSRCT remains unknown.Herein, we describe a case series of pediatric patients with DSRCT and report clinical outcomes for patients receiving modified intensive multi-agent interval compressed chemotherapy as per ARST08P1 with VIT.
2. METHODS
Thisstudy was deemed exempt by the Dana-Farber Cancer Institute Institutional Review Board. We performed a retrospective chart review of pediatric patients diagnosed with DSRCT and treated as per ARST08P1 prior to Nov 1, 2019 at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. We report all six cases, who represent the most recent six consecutive patients to present to our center with newly diagnosed DSRCT. EWSR1-WT1 translocation was confirmed in all cases. Cycles containing dactinomycin were replaced with VIT and the modified treatment regimen as per ARST08P1 can be found in Supplemental Figure 1.
For each patient, we performed medical record reviews and extracted demographic, disease-related, treatment-related and outcome data, including age, sex, race, ethnicity, presentation, pathology, treatment course, acute and late toxicity, disease and survival status, and clinical follow-up. Imaging was reviewed, and response to two cycles of VIT and best overall response was assessed by two physicians according to RECIST 1.1 criteria for solid tumors.20Overall response rate after induction chemotherapy with two cycles of VIT was defined as the proportion of patients with complete or partial response as defined by RECIST 1.1 criteria for solid tumors.20Surgical resection status was determined using the residual tumor (R) classification where R0 is defined as resection with no cancer cells seen microscopically at the resection margins of the primary tumor bed and R1 is defined as resection with cancer cells present microscopically at the margins of the primary tumor bed.21Progression-free survival (PFS) was defined as the time from the date of initial diagnosis to the date of last follow-up time or progression of DSRCT. OS was defined as the time from the date of initial diagnosis to the date of last follow-up or death. Disease and patient characteristics, toxicity and outcomes were reported descriptively.
3. RESULTS
3.1. Clinical characteristics
Clinical and treatment characteristics are summarized in Table 1.Median age at diagnosis was 15.1 years (range: 3.2-16.4). Four patients (66.7%) presented with abdominal or flank pain. The median size of the largest tumor deposit was 10.25 cm (range: 4.6-13.5; Figure 1A,C,E). Four patients had extra-abdominal lymph node involvement and one had liver parenchymal metastaseswithout involvement of the porta hepatis at presentation.One patient had a primary tumor arising outside of the abdomen and pelvis. All patients had evidence of an EWSR1-WT1 translocation identified by FISH, RT-PCR or next-generation sequencing.
TABLE 1.
Clinical and disease characteristics
| Patient | Age at diagnosis (years) |
Sex | Race/ Ethnicity |
Presenting Symptoms |
Largest tumor deposit diameter at diagnosis (cm) |
Liver metastases at diagnosis |
Bone marrow involved at diagnosis |
Extra-abdominal sites at diagnosis (locations) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.3 | Male | NH White | Abdominal pain | 5.7 | Yes | No | No |
| 2 | 15.9 | Female | NH Black | Palpable abdominal mass | 13.5 | No | No | Yes (cardiac apex nodule, IMN) |
| 3 | 14.5 | Male | Hispanic | Abdominal pain | 13.5 | No | - | Yes (cardiophrenic nodule) |
| 4 | 3.2 | Male | NH White | Abdominal pain, fevers | 10.2 | No | No | Yes (cardiophrenic nodule, IMN) |
| 5 | 16.4 | Male | NH Black | Flank pain, vomiting, constipation | 10.3 | No | - | No |
| 6 | 15.7 | Male | Hispanic | Thigh mass, fevers | 4.9 | No | - | Yes (primary thigh mass, inguinal LN, hilar LN) |
Abbreviations: IMN, internal mammary nodes; LN, lymph node; NH, non-Hispanic
FIGURE 1.
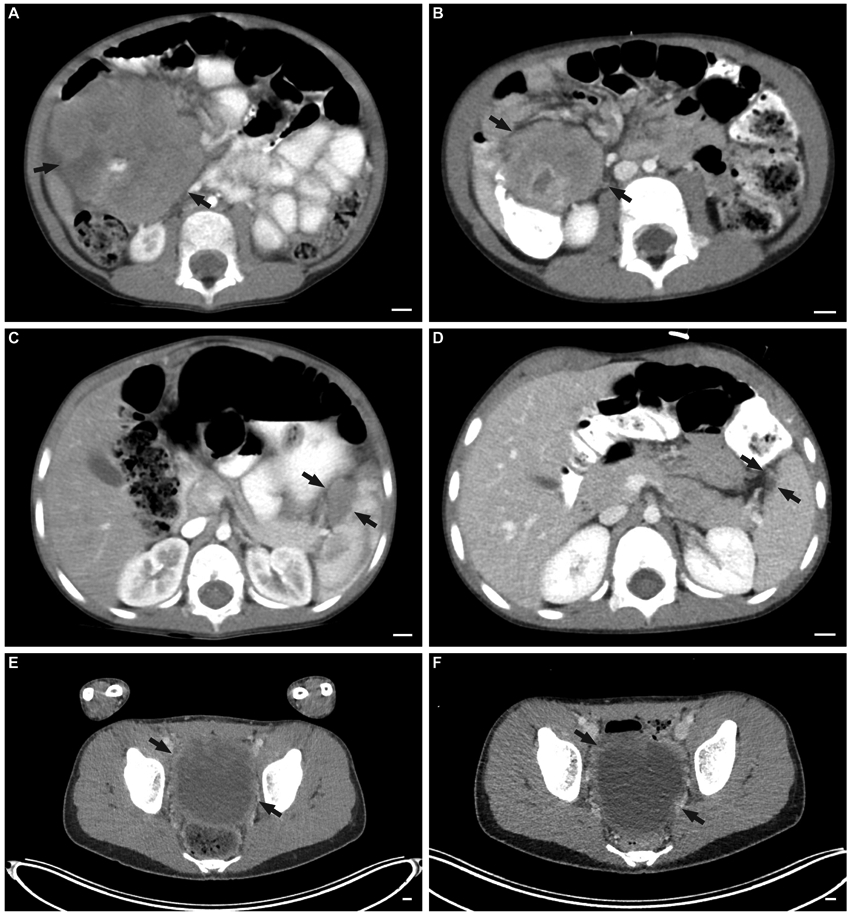
Tumor imaging at diagnosis and after two induction cycles of VIT as per ARST08P1. Representative images of abdominal tumor (A) before and (B) after two cycles of VIT in one of the patients with partial response. Representative images of splenic lesion (C) before and (D) after two cycles of VIT in one of the patients with partial response. Representative images of a large pelvic lesion (E) before and (F) after two cycles of VIT in one of the patients with stable disease.
3.2. Response to induction chemotherapy with two initial cycles of VIT
All six patients received two induction cycles of VIT followed by re-staging scans. The overall response rate was 50% with three patients having a partial response and three patients having stable disease (Figures 1-2). Patients tolerated the first two cycles of VIT well. Common symptoms were nausea, vomiting, constipation, and diarrhea.No patients were admitted for febrile neutropenia. Notably, one patient developed severe C. difficile colitis requiring intensive care unit admission during cycle 3 of chemotherapy, and had no prior use of proton pump inhibitors.
FIGURE 2.
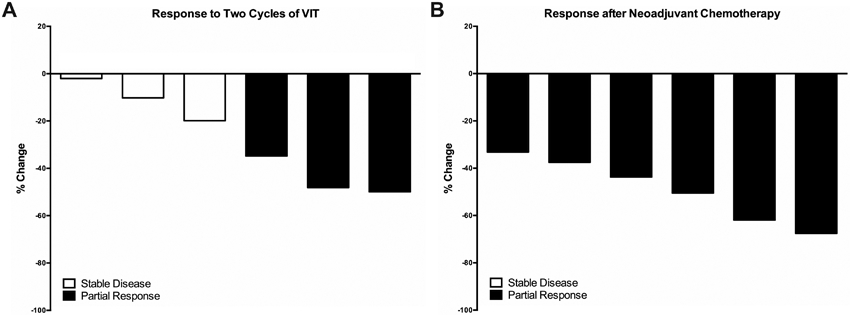
Response during and after neoadjuvant chemotherapy. (A) Percentage of response after two cycles of VIT as measured by RECIST 1.1 criteria for patients receiving chemotherapy as per ARST08P1. (B) Percentage of response after completion of neoadjuvant chemotherapy as measured by RECIST 1.1 criteria for patients receiving chemotherapy as per ARST08P1.
3.4. Treatment Outcomes
Of the six patients, two are actively still undergoing frontline treatment. Patient 5 has completed 8 neoadjuvant cycles of chemotherapy, surgical resection, WART, and 7 adjuvant cycles.Patient 6 has completed 5 neoadjuvant cycles of chemotherapy, surgical resection, 2 adjuvant cycles, and is undergoing chemoradiation to the primary site. Of the four patients who completed therapy, three remain disease-free at a median follow-up of 46.7 months (range: 20.7-60.3) (Table 2).Despite a 48.1% reduction in tumor burden per RECIST criteria after two initial cycles of VIT (Figure 2A) and an eventual complete response, patient 1 had liver metastases at diagnosis experienced distant relapse five months after completing therapy and ultimately died due to disease at 19.3 months from diagnosis. Two-year PFS and OS for the cohort are 75.0%.
TABLE 2.
Frontline treatment characteristics and outcomes
| Patient | Treatment (in order received) | Response after two cycles of VIT |
Best response after frontline therapy |
Recurrence (time after treatment completion) |
Toxicity | Disease status |
Time from diagnosis to last follow-up or death (months) |
|---|---|---|---|---|---|---|---|
| 1 | ARST08P1 (VIT, VDC, IE) with 9 NAJ cycles, surgical resection (20% necrosis, R1) with HIPEC, concurrent WART (30 Gy) and pelvic boost RT (14.4 Gy, total dose of 44.4 Gy) with VIT, 5 ADJ cycles | PR | CR | Y (5 months) | Nausea, vomiting, diarrhea, constipation, myelosuppression, C. difficile infection, SBO requiring lysis of adhesions, intussusception, ileus, post-operative wound complications | DOD | 19.3 |
| 2 | ARST08P1 (VIT, VDC, IE) with 8 NAJ cycles, surgical resection (<20% necrosis, R1) with HIPEC, concurrent WART (30 Gy)and RT to extra-abdominal nodes (36 Gy) with VIT, 12 ADJ cycles | SD | CR | N | Nausea, vomiting, diarrhea, constipation, myelosuppression, febrile neutropenia, prolonged GJ tube, malnutrition, abdominal pain, chylous ascites, C. difficile infection, partial SBO requiring lysis of adhesions, enterocutaneous fistula | NED | 46.7 |
| 3 | ARST08P1 (VIT, VDC, IE) with 5 NAJ cycles, surgical resection (<2% necrosis, R1), concurrent WART (24 Gyof planned 30 Gy) with VDC/IE, 9 ADJ cycles, transitioned to 6 cycles of pazopanib | PR | CR | N | Nausea, vomiting, diarrhea, constipation, myelosuppression, SOS s/p defibrotide and peritoneal drain, C. difficile infection | NED | 20.7 |
| 4 | ARST08P1 (VIT, VDC, IE) with 5 NAJ cycles, surgical resection (0% necrosis, R1, concurrent WART, including cardiophrenic nodule (19.5 Gy) and pelvic boost RT (21.9 Gy, total dose of 41.4 Gy) with VIT, 15 ADJ cycles | PR | CR | N | Nausea, vomiting, diarrhea, constipation, myelosuppression, diarrhea during RT | NED | 60.3 |
| 5 | ARST08P1 (VIT, VDC, IE) with 8 NAJ cycles, surgical resection (30% necrosis, R0), concurrent WART (30 Gy) and pelvic boost RT (14.4 Gy, total dose of 44.4 Gy) with VIT/IE, 7 ADJ cycles (ongoing treatment) | SD | N/A | N/A | Nausea, vomiting, diarrhea, constipation, myelosuppression, mucositis, weight loss, febrile neutropenia | On therapy | 10.1 |
| 6 | ARST08P1 (VIT, VDC, IE) with 5 NAJ cycles, surgical resection (5% necrosis in primary tumor, 100% necrosis, R0) in lymph node, plan for RT to primary, inguinal node and pelvis (55.8 Gy) with concurrent chemotherapy, 2 ADJ cycle (ongoing treatment) | SD | N/A | N/A | Nausea, vomiting, diarrhea, constipation, myelosuppression | On therapy | 4.4 |
Abbreviations: ADJ, adjuvant; CR, complete response; DOD, died of disease; GJ, gastrostomy-jejunostomy; HIPEC, Hyperthermic intraperitoneal chemotherapy; IE, ifosfamide/etoposide; N, no; N/A, not applicable; NAJ, neoadjuvant; NED, no evidence of disease; PD, progressive disease; PR, partial response; R0, resection with no cancer cells seen microscopically at the resection margins of the primary tumor bed; R1, resection with cancer cells present microscopically at the margins of the primary tumor bed; RD, recurrent disease; RT, radiotherapy; SBO, small bowel obstruction; SD, stable disease; SOS, sinusoidal obstruction syndrome; VDC, vincristine/doxorubicin/cyclophosphamide; VIT, vincristine/irinotecan/temozolomide; WART, whole abdomen radiotherapy; WD, with disease; Y, yes
At the end of neoadjuvant chemotherapy, all patients achieved a partial response (Figure 2B). The patients received a median of 6.5 neoadjuvant chemotherapy cycles (range: 5-9) followed by complete cytoreductive surgery (Table 2).All patients had surgical pathology showing residual viable tumor at time of surgical resection, and all patients had ≤30% tumor necrosis noted in the primary tumor. Interestingly, Patient 6 had 100% tumor necrosis in the involved lymph node after five neoadjuvant chemotherapy cycles.No patients had macroscopic residual disease after complete cytoreductive surgery.An R0 resection was achieved in two patients (33.3%) and R1 resection was achieved in four patients (66.7%) (Table 2). Two patients received HIPEC after surgical resectionwith complete cytoreduction as previously described.2Following post-operative recovery, all patients received consolidation with concurrent chemoradiationwith median total dose of 42.9 Gy (range: 24-55.8) (Table 2). For the five patients with intra-abdominal primary tumors, all received WART (median dose: 30 Gy, range: 19.5-30) with three patients receiving concurrent VIT, one patient with VIT/IE, and one patient with VDC/IE (Table 2). For Patient 3, doxorubicin was given prior to the start of radiation. Three patients received pelvic boost radiotherapy (median dose: 14.4 Gy, range: 14.4-21.9) given large burden of pelvic disease prior to surgery, and of these three patients receiving a radiation boost to the pelvis, two had R1 resections(Table 2).Patient 2 received a simultaneous integrated boost to her extra-abdominal lymph nodes for a total dose of 36 Gy.Due to his young age, Patient 4 received 19.5 Gy of WART followed by a 21.9 Gy pelvic boost.Because all patients underwent an R0 or R1 resection, no patients received boost radiotherapy for gross residual disease.Patient 6 is planned to receive concurrent chemoradiationwith a total dose of 55.8 Gy to his inguinal primary and lymph node (Table 2). Of the four patients who completed therapy, the median number of adjuvant chemotherapy cycles was 10.5 (range: 5-15) with replacement of dactinomycin-containing cycles with VIT cycles, and all patients achieved complete responses (Table 2).
The most common side effects throughout treatment, including the initial two cycles of VIT,were nausea, vomiting, diarrhea, constipation, myelosuppression requiring red blood cell or platelet transfusions, febrile neutropenia, and C. difficile infection. For irinotecan-associated diarrhea, patients received oral cephalosporin prophylaxis.22,23Only one of three patients who developed C. difficile infections had prior use of proton pump inhibitors before diagnosis of infection. Patients 1 and 2received HIPEC and had gastrointestinal toxicity during and after consolidation chemoradiation and adjuvant chemotherapy (Table 2). Due to complications with small bowel obstruction requiring exploratory laparotomy and lysis of adhesions, post-operative wound infection, intussusception, functional ileus, and C. difficile infection, Patient 1 only completed 5 adjuvant cycles following HIPEC and WART for a total of 14 cycles, and suffered a relapse five months after completion of therapy. Patient 2 completed WART, 20 planned cycles of therapy, andrequired prolonged gastrostomy-jejunostomy tube feeding with poor nutrition. She had delay of adjuvant cycles due to chylous ascites of unclear etiology, which ultimately resolved with diet modification. After completion of therapy, she underwent lysis of adhesions for partial small bowel obstruction with improvement of symptoms, butdeveloped an enterocutaneous fistula that resolved with non-operative management. She remains disease-free at 46.7 months. Patient 3 developed sinusoidal obstruction syndrome (SOS) requiring defibrotide and a peritoneal drain during concurrent WART with VIT. Radiation was stopped early at 24 Gy of the 30 Gy planned treatments, and chemotherapy was stopped early at 14 total cycles due to SOS and C. difficile infection. He was subsequently transitioned to and completed maintenance pazopanib for six planned cycles.The patient remains disease-free at 20.7 months of follow-up from diagnosis. Patient 4 was unique in their young age at diagnosis(3.2 years), completed all treatment and isdisease-free more than 5 years from diagnosis (Table 2).
4. DISCUSSION
This report provides the first case series of patients with DSRCT receiving VIT in addition to interval-compressed chemotherapy as per COG ARST08P1. We report an overall response rate of 50%after two induction cycles of VIT, and three of four patients who completed treatment as per ARST08P1 are without evidence of disease at median follow-up of 46.7 months. Our data suggest that the addition of VIT to an interval compressed VDC/IE backboneistolerable, albeit with notable toxicity when combining the full regimen with extensive local control measures,anddemonstrates a level of activity warranting further evaluation.
Our cohort included five male patients (83.3%), which is similar to the previously reported rates of male predilection of approximately 4:1 in DSRCT.5,10Abdominal pain was the most frequent presenting symptom. One patient had an extraabdominal primary tumor, which is uncommon.24In our cohort, no patients had bone metastases at diagnosis, which is a rare occurrence in the literature.10 Four patients had enlarged extraabdominal lymph nodes at the time of diagnosis. We reported one patient (16.7%) with liver metastases at the time of diagnosis, which is similar to the reported frequency for liver metastases in patients with DSRCT.10The patient with metastatic disease to the liver on presentation had distant recurrence five months after completing therapy and ultimately died due to his disease. Liver metastases have been previously associated with poor prognosis.2,6,18 Furthermore, data suggest that despite aggressive local therapy, including complete resection, HIPEC, and WART, patients with liver metastases have poor prognosis.2
While response to vincristine, irinotecan, and temozolomide has been reported in DSRCT,8,13-18use of all three agents has not been described for this disease in combination with interval-compressed therapy in the upfront setting. We found that that VIT has activity against DSRCT, andin our cohort no patients experienced disease progression while on therapy.This treatment strategy of initiating therapy with VIT may be particularly useful for patients presenting with extensive abdominal disease who may have difficulty tolerating initial cycles of VDC/IE. Furthermore, allpatients completed neoadjuvant chemotherapy witha partial response.In comparison, in one large cohort of patients receiving varied regimens (interval-compressed VDC/IE, standard VDC/IE, vincristine/ifosfamide/doxorubicin/etoposide [VIDE], vincristine/ifosfamide/dactinomycin [VIA] or P6), 51.9% of patients had a partial response or better prior to local control, and this was associated with improved outcomes.12Although the study was not powered to assess differences in response by chemotherapy regimen, response rates for interval-compressed VDC/IE and standard VDC/IE were 68.4% and 48.2%, respectively.12The P6 protocol from Memorial Sloan Kettering consists of cyclophosphamide, doxorubicin, and vincristine alternating with ifosfamide and etoposide,and the initial report found 100% response rate in 10 patients who received P6 as first-line therapy.3 Additionalstudies with larger cohorts using neoadjuvant P6 have not reported exact response rates;6,25,26 however, Lal et al. reported that a majority of patients in a subsequent update study had a good response to 3-4 cycles of chemotherapy, consisting of P6 protocol with addition of irinotecan, topotecan, carboplatin and cisplatin for selected patients.4Preliminary results from a new pilot study exploring the addition of induction irinotecan, temozolomide and bevacizumab (ITB) to a modified P6 regimen found response rates of 27% following an initial two cycles of ITB and 73% at the completion of five neoadjuvant chemotherapy cycles.27Furthermore, all patientsin our study underwent an R0/R1 surgical resection after neoadjuvant chemotherapy with VIT, and results from a recent abstract demonstrated achievement of R0/R1 resection to be a predictor of overall survival.28
After neoadjuvant chemotherapy, all patients went on to undergo complete cytoreductive surgery followed by concurrent chemoradiation with five patients receiving WART for intra-abdominal primary tumors and one planned to receive radiation to his extra-abdominal primary tumor and lymph node. Four patients were planned for 30 Gy of WART, which is a commonly used dose for WART in DSRCT,4,7,8 and only 1 patient was unable to complete the planned radiation treatment due to SOS, receiving only 24 Gy. Patient 4 was 3.2 years old at diagnosis, and given the young age, received 19.5 Gy WART, a dose previously used for patients with Wilms tumor and extracranial rhabdoid tumors.29,30Four of five patients with intra-abdominal primary tumors received at least one cycle of VIT during radiation treatment. A previous study exploring the radiosensitizing effects of irinotecan and temozolomide in DSRCT and described a patient who is without disease 20 months after completion of WART with concurrent irinotecan/temozolomide.8It is possible that theradiosensitizing properties of VIT when used concurrently with radiotherapy may also contribute to better local control. Given the small number of patients, it is difficult to determine whether WART or total radiation dose impacted disease outcomes.For patients with DSRCT, relapses most frequently occur in the first two years after diagnosis.2,5,6,10In our case series, two patients who completed 20 cycles of planned chemotherapy are disease-free at 46.7 and 60.3 months.
The first two cycles of VIT were generally well-tolerated. During neoadjuvant therapy, the most common side-effects were gastrointestinal and hematologic and did not result in significant treatment delays. Three patients developed C. difficile colitis with two patients developing this infection during adjuvant treatment, a side-effect previously described during VDC/IE or irinotecan/temozolomide for pediatric sarcomas.31,32 While proton pump inhibitors have been associated with increased risk of C. difficile infection,33 only one patient had prior use of proton pump inhibitors. However, cephalosporin prophylaxis of irinotecan-associated diarrhea may have been a contributing factor as cephalosporins have been associated with C. difficile infections.34Toxicity following extensive local control measures and adjuvant chemotherapy were more profound and included small bowel obstruction, prolonged gastrostomy-jejunostomy tube requirement, enterocutaneous fistula, and SOS. Thesetoxicities were similar to those previously reported using other chemotherapy regimens and extensive local surgery, HIPEC, and WART.2-4,6,7,35In this setting, these toxicities precludedsome patients from completing planned adjuvant chemotherapy regardless of whether HIPEC was utilized.There have been few studies examining the association of WART dose with toxicity; however, studies have found higher doses of radiation to the abdomen and pelvis correlates with greater long-term toxicity.36,37 Future studies are needed to understand the optimal WART dose for patients with DSRCT that balance toxicity with achieving disease control.In one instance, maintenance pazopanib was added for a patient with toxicity that prevented completion of planned cycles. Taken together, these findings suggest that the addition of VIT to interval compressed chemotherapyas per ARST08P1 is tolerable in this disease context.However, toxicity for the entire regimen, particularly following the extensive local control strategies utilized in this disease, are profound in comparison to those seen among patients with rhabdomyosarcoma treated with similar regimens (ARST08P1 and ARST0431) and may preclude completion of all planned cycles.19,38 Given these serious toxicities, striving to better understand the relative importance of aggressive local control and the need to complete all planned cycles of adjuvant chemotherapy in order to maximize the chances of long-term survival will be an important area for future study.
One patient who developed complications during adjuvant chemotherapy cycles was transitioned to six planned cycles of pazopanib, and is currently without evidence of disease at 20.7 months from initial diagnosis. Studies have demonstrated that pazopanib is active in DSRCT and well-tolerated.39,40 It remains unknown whether patients with DSRCT benefit from targeted agents, such as pazopanib in the upfront setting, or whether maintenance therapy may be beneficial. Further studies are needed to investigate the role of maintenance therapy, potentially with pazopanib, as part of first-line treatment for DSRCT.
There are several limitations to this study. As a small retrospective study, we are unable to make definitive claims about tolerability orefficacy. Similarly, we do not present a direct comparator group, but instead utilize historic controls. Nevertheless, we report all patients at our center with this very rare disease who were treated with a uniform chemotherapy regimen. Furthermore, other factors, such as extent of disease at presentation, may also confound our results as liver metastases or extra-abdominal involvement are known to influence outcomes.6Of note, two of six patients remain on therapy with 4.4 and 10.1 months follow-up from initial diagnosis.Longer follow-up is also needed as long-term survivors will be at risk of numerous late effects, including infertility; cardiac, genitourinary, and gastrointestinal toxicity; and second malignancies. Future prospective studies with larger cohorts are necessary to further examine the role of VIT in addition to interval-compressed chemotherapy in DSRCT.
In conclusion, DSRCT is a rare aggressive sarcoma with poor outcomes despite intensive multimodality treatment. Our data suggest that the addition of VIT to interval-compressed chemotherapy as per COG ARST08P1 is tolerable with a higher than expected disease control rate. Further prospective studies with larger cohorts are needed to examine the efficacy of treating patients with DSRCT using VIT-containing regimens such as per ARST08P1.
Supplementary Material
SUPPLEMENTAL FIGURE 1. Treatment schematic of modified intensive multi-agent interval compressed chemotherapy as per ARST08P1.
Acknowledgments
FUNDING: NIH Grant T32 CA136432-08 (DSS) and Alex’s Lemonade Stand Foundation Center of Excellence Grant (DSS, SGD).
Abbreviations:
- COG
Children’s Oncology Group
- DSRCT
desmoplastic small round cell tumor
- HIPEC
hyperthermic intraperitoneal chemotherapy
- IE
ifosfamide/etoposide
- ITB
irinotecan/temozolomide/bevacizumab
- SOS
sinusoidal obstruction syndrome
- VAC
vincristine/dactinomycin/cyclophosphamide
- VDC
vincristine/doxorubicin/cyclophosphamide
- VIA
vincristine/ifosfamide/dactinomycin
- VIDE
vincristine/ifosfamide/doxorubicin/etoposide
- VIT
vincristine/irinotecan/temozolomide
- WART
whole abdomen radiotherapy
Footnotes
CONFLICTS OF INTEREST: SGD reports consulting fee from Loxo Oncology and travel expenses from Loxo Oncology, Roche, and Salarius. ALF reports serving on the advisory board of Decibel Therapeutics.
DATA SHARING: The data that support the findings of this study are available in a limited format on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1.Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res, 1994;54(11):2837–2840. [PubMed] [Google Scholar]
- 2.Hayes-Jordan AA, Coakley BA, Green HL, et al. Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann Surg Oncol. 2018;25(4):872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14(5):1526–1531. [DOI] [PubMed] [Google Scholar]
- 4.Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005;40(1):251–255. [DOI] [PubMed] [Google Scholar]
- 5.Honore C, Atallah V, Mir O, et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS One. 2017;12(2):e0171639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai NB, Stein NF, LaQuaglia MP, et al. Reduced toxicity with intensity modulated radiation therapy (IMRT) for desmoplastic small round cell tumor (DSRCT): an update on the whole abdominopelvic radiation therapy (WAP-RT) experience. Int J Radiat Oncol Biol Phys. 2013;85(1):e67–72. [DOI] [PubMed] [Google Scholar]
- 7.Osborne EM, Briere TM, Hayes-Jordan A, et al. Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol. 2016;119(1):40–44. [DOI] [PubMed] [Google Scholar]
- 8.Pinnix CC, Fontanilla HP, Hayes-Jordan A, et al. Whole abdominopelvic intensity-modulated radiation therapy for desmoplastic small round cell tumor after surgery. Int J Radiat Oncol Biol Phys. 2012;83(1):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atallah V, Honore C, Orbach D, et al. Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys. 2016;95(4):1244–1253. [DOI] [PubMed] [Google Scholar]
- 10.Honore C, Delhorme JB, Nassif E, et al. Can we cure patients with abdominal Desmoplastic Small Round Cell Tumor? Results of a retrospective multicentric study on 100 patients. Surg Oncol. 2019;29:107–112. [DOI] [PubMed] [Google Scholar]
- 11.Bent MA, Padilla BE, Goldsby RE, DuBois SG. Clinical Characteristics and Outcomes of Pediatric Patients with Desmoplastic Small Round Cell Tumor. Rare Tumors. 2016;8(1):6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbiah V, Lamhamedi-Cherradi SE, Cuglievan B, et al. Multimodality Treatment of Desmoplastic Small Round Cell Tumor: Chemotherapy and Complete Cytoreductive Surgery Improve Patient Survival. Clin Cancer Res. 2018;24(19):4865–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera D, Hayes-Jordan A, Anderson P, Woo S, Pearson M, Green H. Outpatient and home chemotherapy with novel local control strategies in desmoplastic small round cell tumor. Sarcoma. 2008;2008:261589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambar NBD, de Seixas Alves MT, Lederman HM, Abib S, Duarte AAB, Caran EM. Irinotecan and vincristine for the treatment of refractory desmoplastic small round cell tumor in a developing country: a case report. J Med Case Rep. 2019;13(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angarita FA, Hassan S, Cannell AJ, et al. Clinical features and outcomes of 20 patients with abdominopelvic desmoplastic small round cell tumor. Eur J Surg Oncol. 2017;43(2):423–431. [DOI] [PubMed] [Google Scholar]
- 16.Bisogno G, Riccardi R, Ruggiero A, et al. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer. 2006;106(3):703–707. [DOI] [PubMed] [Google Scholar]
- 17.Rosoff PM, Bayliff S. Successful clinical response to irinotecan in desmoplastic round blue cell tumor. Med Pediatr Oncol. 1999;33(5):500–503. [DOI] [PubMed] [Google Scholar]
- 18.Scheer M, Vokuhl C, Blank B, et al. Desmoplastic small round cell tumors: Multimodality treatment and new risk factors. Cancer Med. 2019;8(2):527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malempati S, Weigel BJ, Chi YY, et al. The addition of cixutumumab or temozolomide to intensive multiagent chemotherapy is feasible but does not improve outcome for patients with metastatic rhabdomyosarcoma: A report from the Children's Oncology Group. Cancer. 2019;125(2):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 21.Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115(15):3483–3488. [DOI] [PubMed] [Google Scholar]
- 22.Furman WL, Crews KR, Billups C, et al. Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006;24(4):563–570. [DOI] [PubMed] [Google Scholar]
- 23.Wagner LM, Crews KR, Stewart CF, et al. Reducing irinotecan-associated diarrhea in children. Pediatr Blood Cancer. 2008;50(2):201–207. [DOI] [PubMed] [Google Scholar]
- 24.Arora VC, Price AP, Fleming S, et al. Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol. 2013;43(1):93–102. [DOI] [PubMed] [Google Scholar]
- 25.Goodman KA, Wolden SL, La Quaglia MP, Kushner BH. Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys. 2002;54(1):170–176. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz RE, Gerald WL, Kushner BH, Coit DG, Brennan MF, La Quaglia MP. Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol. 1998;5(5):416–422. [DOI] [PubMed] [Google Scholar]
- 27.Magnan HD, Price A, Chou AJ, et al. A pilot trial of irinotecan, temozolomide and bevacizumab (ITB) for treatment of newly diagnosed patients with desmoplastic small round cell tumor (DSRCT). Journal of Clinical Oncology. 2017;35(15_suppl):11050–11050. [Google Scholar]
- 28.Shoushtari AN, Qin L-X, Kuk D, et al. Predictors of overall survival in patients diagnosed with desmoplastic small round cell tumor (DSRCT). Journal of Clinical Oncology. 2014;32(15_suppl):10582–10582. [Google Scholar]
- 29.Brennan B, De Salvo GL, Orbach D, et al. Outcome of extracranial malignant rhabdoid tumours in children registered in the European Paediatric Soft Tissue Sarcoma Study Group Non-Rhabdomyosarcoma Soft Tissue Sarcoma 2005 Study-EpSSG NRSTS 2005. Eur J Cancer. 2016;60:69–82. [DOI] [PubMed] [Google Scholar]
- 30.Davidoff AM, Giel DW, Jones DP, et al. The feasibility and outcome of nephron-sparing surgery for children with bilateral Wilms tumor. The St Jude Children's Research Hospital experience: 1999-2006. Cancer. 2008;112(9):2060–2070. [DOI] [PubMed] [Google Scholar]
- 31.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53(6):1029–1034. [DOI] [PubMed] [Google Scholar]
- 32.Navid F, Santana VM, Billups CA, et al. Concomitant administration of vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide for high-risk sarcomas: the St. Jude Children's Research Hospital experience. Cancer. 2006;106(8):1846–1856. [DOI] [PubMed] [Google Scholar]
- 33.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilcox MH, Chalmers JD, Nord CE, Freeman J, Bouza E. Role of cephalosporins in the era of Clostridium difficile infection. J Antimicrob Chemother. 2017;72(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stiles ZE, Murphy AJ, Anghelescu DL, et al. Desmoplastic Small Round Cell Tumor: Long-Term Complications After Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2020;27(1):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolling T, Willich N, Ernst I. Late effects of abdominal irradiation in children: a review of the literature. Anticancer Res. 2010;30(1):227–231. [PubMed] [Google Scholar]
- 37.Madenci AL, Fisher S, Diller LR, et al. Intestinal Obstruction in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2015;33(26):2893–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigel BJ, Lyden E, Anderson JR, et al. Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children's Oncology Group. J Clin Oncol. 2016;34(2):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frezza AM, Benson C, Judson IR, et al. Pazopanib in advanced desmoplastic small round cell tumours: a multi-institutional experience. Clin Sarcoma Res. 2014;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menegaz BA, Cuglievan B, Benson J, et al. Clinical Activity of Pazopanib in Patients with Advanced Desmoplastic Small Round Cell Tumor. Oncologist. 2018;23(3):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1. Treatment schematic of modified intensive multi-agent interval compressed chemotherapy as per ARST08P1.


