Abstract
Background
Farm exposures may reduce the risk of atopic dermatitis (AD) in children, but this is controversial and US data are limited.
Objective
This study was conducted to identify patterns of farm exposure in Wisconsin family farms that modify AD incidence and prevalence in early childhood.
Methods
Environmental exposures, health history and clinical outcomes were prospectively recorded for 111 farm families and 129 non-farm families enrolled in the Wisconsin Infant Study Cohort birth cohort study. Exposures from the prenatal and early postnatal (2-month) visits were evaluated together with parental report of AD diagnosis by a healthcare provider through age 24 months. Latent class analysis was performed with prenatal and early postnatal farm-exposure variables to assign farm children to three classes.
Results
Overall, children of farm families had reduced AD incidence (P=0.03). Within farm families, exposures including poultry (3% vs 28%, P=0.003), pig (4% vs 25%, P=0.04), feed grain (13% vs 34%, P=0.02) and number of animal species were inversely associated with AD incidence. Among the latent class groups, children in families with diverse or more intense farm exposures (Classes A and B) had reduced AD incidence, while low-exposure (Class C) infants had AD incidence similar to non-farm children.
Conclusions
Infants in Wisconsin farm families had reduced AD incidence, and patterns of farm exposures further defined AD risk. These findings suggest that exposure to diverse farm animals, feed and bedding during the prenatal period and in early infancy reduce the risk of early-onset AD, a phenotype associated with multiple other atopic diseases.
Keywords: atopic dermatitis, farm effect, children, birth cohort, latent class analysis
Graphical Abstract
Capsule Summary
Wisconsin farm exposures reduce the risk for early onset atopic dermatitis, which is closely associated with subsequent food allergy and asthma. Understanding the mechanisms for this association could lead to prevention of the “atopic march”.
INTRODUCTION
Atopic dermatitis (AD) is a common chronic and relapsing inflammatory skin disease that is characterized by intense pruritus and recurrent flares.1 AD impacts more than 10% of children and may have a significant toll on quality of life for the patient and family.2, 3 AD is often the first clinical manifestation of allergic disease, and early onset and more severe diseases increases the risk for asthma or hay fever.4–7 The impaired skin barrier in AD allows for transcutaneous food allergen introduction, and can promote the development of food allergy.8–10 Thus, there are compelling reasons to identify risk factors and potential preventive strategies for AD, which could also have downstream effects to reduce the risk of other atopic diseases.
Early childhood environmental exposures, including pet ownership, sibling contact and skin care practices have been linked to the risk of AD.11–14 In addition, farm exposures during the prenatal period or during childhood have been inversely associated with to AD in some studies.14–17 Farm environments can be a rich source of biological and microbial exposures with the potential to promote immune development and thereby reduce the risk of AD. For example, animal and barn-related exposures during pregnancy have been positively related to the number and function of cord blood regulatory T cells and enhanced cord blood cell cytokine responses.18, 19
However, several observational studies have reported no effect of the farming environment on AD.20–27 The conflicting results could be related to variability in local farming characteristics and practices. The quality and intensity of environmental and microbial exposures can vary with characteristics such as the type of farm (e.g. arable [crops], pastoral, mixed), species of animals, and traditional vs. industrial farming practices.16, 28 In addition, mothers on farms have a range of duties that vary from farm to farm and even over time on the same farm, and the same is likely true for exposures of infants. Finally, while relationships between allergic diseases and traditional European farming exposures have been well studied,29 it is notable that data from the US are limited. Notably, US farming regions such as Wisconsin still have small family dairy farms where family members are embedded in the farm and its diverse environments. Farm size and characteristics in this region are still variable, however, and range from small family farms to larger mechanized operations. Importantly, dairy farms remain complex environmentally given the presence of crops, animals, and feed.
The Wisconsin Infant Cohort Study (WISC) was started in 2013 to identify environmental exposures that influence immune development, respiratory illnesses and allergic diseases in children from farming families compared to rural families with little or no farm contact.30 Our hypothesis for this analysis was that there are specific exposures or groups of interrelated exposures on farms in Central Wisconsin that are inversely associated with the incidence of AD. To test this hypothesis, we compared the incidence and cumulative prevalence of AD through 24 months of age for children growing up in farm vs. non-farm families, and for the farm-exposed children, we compared cumulative 2-year AD prevalence by presence and detailed type of farm exposures. Finally, given the diversity of Wisconsin farms and exposures, we used unsupervised clustering techniques to identify patterns of farm exposure and to test for associations between exposure classes and AD incidence through 24 months of age.
METHODS
Study Design
The WISC study is a birth cohort study based in rural Wisconsin with prenatal enrollment of two groups (farm and rural non-farm) of pregnant mothers and their babies to determine how farm exposures influence wheezing illnesses and allergic diseases in early childhood. All families provided written informed consent prior to study enrollment, and all study activities and procedures were approved by the Marshfield Clinic Health System Human Subjects Institutional Review Board. The majority of families enrolled in the WISC study live in the Marshfield Epidemiologic Study Area (MESA). MESA has approximately 85,000 residents, with about 19,000 living in the city of Marshfield and the rest living in rural areas – small towns, villages and countryside. The area has approximately 2,200 farms with a farm population of about 5,400. Farm mothers were defined as living on or within 1/8th mile of a farm, working on a farm, or having a household member who works on a farm. Farm mothers (or household members) were further defined as having regular exposure (≥4 days per week) with cattle (cows, calves, bulls, or steers), goats or pigs. Non-farm mothers were defined as not living on or within 1/8th mile of a farm, working on a farm, or having a household member who works on a farm. Non-farm mothers did not have regular exposure to farms or raise livestock animals as pets (e.g., cattle, goats, pigs, horses or chickens).
Inclusion criteria including meeting criteria listed above and were birth at ≥ 34 weeks gestation. Exclusion criteria included maternal use of antibiotics (except Group B Strep prophylaxis) or corticosteroids in the last trimester of pregnancy, perinatal infections or prolonged rupture of membranes, and the presence of significant respiratory distress after delivery or congenital anomalies. Between April 2013 and May 2018, screening pregnant women in the Marshfield area yielded 612 non-farm and 309 farm woman who met eligibility criteria, and 111 farm families and 129 non-farm families provided informed consent and were enrolled in WISC.30 Eleven of enrolled families were still awaiting delivery. For this analysis, 104 farm families (94%) and 120 non-farm families (93%) attended the 2-month study visit and were included in the AD and LCA analyses. Withdrawal rates for the farm and non-farm groups were similar (15% and 11%) through May 2018.
Questionnaires to assess environmental and farming exposures was administered prenatally and at postnatal timepoints (2, 9 and 24 months). Additional questionnaires were administered to mothers starting at 2 and 6 months of age and then every 3 months either by phone or in-person to assess child health information and clinical outcomes, including AD.
AD Definition
AD was defined as maternal report by questionnaire of a healthcare provider’s diagnosis of AD (collected at 2 and 6 months of age and then every 3 months). Children were labeled as having a healthcare provider’s diagnosis of AD if mothers responded positively at least one time to the following question, “Has a healthcare provider told you that your child had eczema (atopic dermatitis) since the last time we talked?”. Cumulative prevalence was defined as a “yes” answer to AD diagnosis over the first 2 years (up through the 24 month questionnaire), even if the condition subsequently resolved. The age at which the most recent questionnaire with a completed AD response (either yes or no) was noted, and used as a censoring age for children without a “yes” answer to the AD question.
Statistical Analysis
AD incidence (time in days from birth to date of first AD) was estimated using the Kaplan-Meier method, and was compared between groups using the log-rank test. Longitudinal AD prevalence was estimated using GEE logistic regression with an exchangeable correlation structure and a natural cubic spline term for time. Confidence intervals for prevalence were constructed using a subject-level bootstrap procedure with B=5000 replicates. A proportional hazard model31 that accounts for the timing of AD development and censoring for incomplete follow-up was used to examine associations between maternal, child, and household characteristics and farm status, and between maternal, prenatal, and early life characteristics and exposures and AD. A chi-square test for trend was used to examine the relationship between animal exposure diversity (number of species) and AD. Latent class analysis (LCA), with manifest variables including 22 specific farm-derived exposures evaluated prenatally and at 2 months of age, was used as a data reduction strategy to identify distinct farm cohort exposure classes. These exposures were evaluated as absent or present, with any unknown response considered absent. The number of latent classes was allowed to vary from 1 to 6, and a 3-class model was selected based on minimizing the Bayesian Information Criterion. All tables show unadjusted p-values, but models were also run with adjustment for the child’s sex. Any impact of this adjustment is noted in the text.
RESULTS
Study Population
Of the 104 farm families and 122 non-farm families included in this analysis (Table I), the last completed visit ranged from age 6 months to 48 months with median follow-up of 24 months. This analysis focused on the prenatal and 2 month data for exposures and the AD data up through the 24 month visit (since over half the population is complete up to that age). The farm group had significantly fewer female children (43% vs. 58%, P=0.03). Prenatal dog and cat ownership was lower in non-farm families (52% vs 73%, P=0.0009 and 32% vs 76%, P<0.0001, respectively). Non-farm mothers had a higher proportion of employment outside the home vs farm mothers (78% vs 60%) and lower consumption of raw farm milk during pregnancy (2% vs 15%, P<0.001). Otherwise, sociodemographic characteristics were similar between both groups and there were no group differences in household income, maternal smoking status, mode of delivery, or family history of atopic disease. These relationships persisted after adjustment for sex.
Table I.
Maternal, Child and Household Characteristics According to Farm Status
| Characteristic | Farm (n=111) | Non-farm (n=129) | P-value |
|---|---|---|---|
| Maternal | |||
| Maternal age (years) | 0.12 | ||
| ≥ 40 | 2% | 2% | |
| 35–39 | 20% | 9% | |
| 30–34 | 40% | 44% | |
| 25–29 | 32% | 40% | |
| 18–24 | 7% | 5% | |
| Education | 0.62 | ||
| High school or less | 6% | 6% | |
| Associate degree or some college | 29% | 28% | |
| Bachelor’s degree | 50% | 45% | |
| Graduate degree | 11% | 18% | |
| Unknown | 4% | 3% | |
| Employed outside the home | 60% | 78% | 0.005 |
| Annual household income | 0.24 | ||
| ≥ $100,000 | 18% | 22% | |
| $25,000-$99,999 | 63% | 68% | |
| < $25,000 | 9% | 4% | |
| Unknown | 10% | 6% | |
| Marital status | 0.37 | ||
| Married or living with a partner | 89% | 88% | |
| Single | 5% | 8% | |
| Unknown | 6% | 4% | |
| Maternal smoking | |||
| During year prior to pregnancy | 9% | 15% | 0.17 |
| During pregnancy | 2% | 4% | 0.33 |
| Maternal history of AD (ever) | 18% | 20% | 0.67 |
| Maternal history of allergic rhinitis (ever) | 11% | 18% | 0.10 |
| Maternal history of asthma (ever) | 16% | 21% | 0.37 |
| Consumption of raw farm milk during pregnancy | 15% | 2% | <0.001 |
| Child | |||
| Sex | 0.02 | ||
| Female | 43% | 58% | |
| Race/Ethnicity | 0.07 | ||
| White | 99% | 94% | |
| Black or African American | 1% | 2% | |
| Asian | 0% | 2% | |
| Other | 0% | 2% | |
| Mode of delivery | |||
| C-section (vs. vaginal) | 17% | 21% | 0.45 |
| Child daycare attendance at least one day per week (age 2 mo) | 14% | 21% | 0.13 |
| Exclusively breastmilk fed (age 2 mo) | 50% | 47% | 0.64 |
| Household | |||
| Number of children in household | |||
| ≥ 4 | 19% | 12% | 0.08 |
| 3 | 23% | 15% | |
| 2 | 26% | 42% | |
| 1 | 25% | 23% | |
| Unknown | 7% | 8% | |
| Dog ownership (prenatal) | 73% | 52% | <0.001 |
| Dog spends time indoors | 47% | 52% | 0.43 |
| Cat ownership (prenatal) | 76% | 32% | <0.001 |
| Cat spends time indoors | 37% | 29% | 0.22 |
Abbreviations: AD, atopic dermatitis; NS, not significant.
Within the farm cohort, 80% of mothers lived and worked on farms while 16% worked on the farm but lived elsewhere, as previously described.30 Cows (77%) were the predominant animal kept on farms, followed by bulls and steers (32%), poultry (32%), pigs (19%), and goats (13%). Many farms (43%) reared farm animals of a single species. During pregnancy, two-thirds of farm mothers reported at least weekly, direct contact with cattle (cows, calves, bulls, or steers), followed by 25% with poultry, 10% with pigs, and 7% with goats. Most farms (88%) grew and harvested crops. There were high rates of regular, direct contact between pregnant mothers and hay (76%), feed grain (66%), straw (63%) and silage (58%).
Associations Between Exposures Shared by Farm and Non-Farm Families and AD Development
We first tested whether common prenatal and infancy exposures were associated with AD risk in the farm and non-farm families (Table II). Among children in the farm group, cumulative prevalence of AD was positively related to delivery mode (vaginal delivery 16%, C-section 41%, P=0.01) and inversely related to exclusive breastfeeding (12% vs. 28%, p = 0.05), and there were nonsignificant trends for positive associations with maternal history of AD and asthma. These associations were not present within the non-farm group, and were not altered by adjustment for sex.
Table II.
Cumulative Prevalence of Atopic Dermatitis up to Age 2 Years According to Maternal and Early Life Characteristics in the Farm and Non-farm Groups*
| Characteristic | Cumulative Prevalence of Atopic Dermatitis | P-value† | ||||
|---|---|---|---|---|---|---|
| Farm Group (n=104) Has Characteristic | Non-Farm Group (n=122) Has Characteristic | |||||
| Yes | No | P-value | Yes | No | ||
| Maternal | ||||||
| History of AD (ever) | 33% (6/18) | 15% (12/81) | 0.10 | 35% (8/23) | 35% (32/94) | 0.91 |
| History of AR (ever) | 20% (2/10) | 18% (16/88) | 0.98 | 43% (9/21) | 33% (31/93) | 0.38 |
| History of asthma (ever) | 6% (1/17) | 21% (17/80) | 0.08 | 40% (10/25) | 34% (31/92) | 0.57 |
| Smoking during pregnancy | 0% (0/2) | 21% (21/102) | 0.38 | 40% (2/5) | 34% (40/117) | 0.99 |
| Raw farm milk consumed (prenatal) | 7% (1/15) | 20% (17/83) | 0.21 | 0% (0/2) | 36% (40/116) | 0.23 |
| Dog ownership (prenatal) | 17% (13/75) | 28% (8/29) | 0.22 | 33% (21/64) | 36% (21/58) | 0.60 |
| Cat ownership (prenatal) | 19% (15/78) | 23% (6/26) | 0.50 | 26% (10/38) | 39% (32/84) | 0.29 |
| Child | ||||||
| Sex (% male) | 20% (12/60) | 20% (9/44) | 0.94 | 37% (19/51) | 32% (23/71) | 0.49 |
| Vaginal Delivery | 16% (14/87) | 41% (7/17) | 0.02 | 36% (35/96) | 27% (7/26) | 0.35 |
| Daycare | 20% (3/15) | 20% (18/89) | 0.82 | 37% (10/27) | 34% (32/95) | 0.55 |
| Exclusively breastfed | 12% (5/43) | 28% (12/43) | 0.05 | 40% (19/48) | 35% (19/55) | 0.30 |
| Dog ownership (2 month) | 18% (13/73) | 26% (8/29) | 0.30 | 31% (19/61) | 39% (23/59) | 0.34 |
| Cat ownership (2 month) | 23% (15/65) | 15% (5/36) | 0.24 | 25% (10/40) | 41% (32/79) | 0.22 |
Abbreviations: AD, atopic dermatitis; AR, allergic rhinitis.
P values were calculated with the likelihood ratio test.
Farm Exposures and AD Development
AD incidence was significantly reduced in the farm group compared to the non-farm (P=0.03, Fig. 1A). This relationship was age-dependent with differences evident by the second six months of life. Farm exposure was also associated with reduced cumulative prevalence of AD through age 2 years (P=0.002, Fig. 1B). Both of these relationships persisted after adjustment for sex.
Figure 1: Atopic dermatitis incidence and prevalence by farm status.
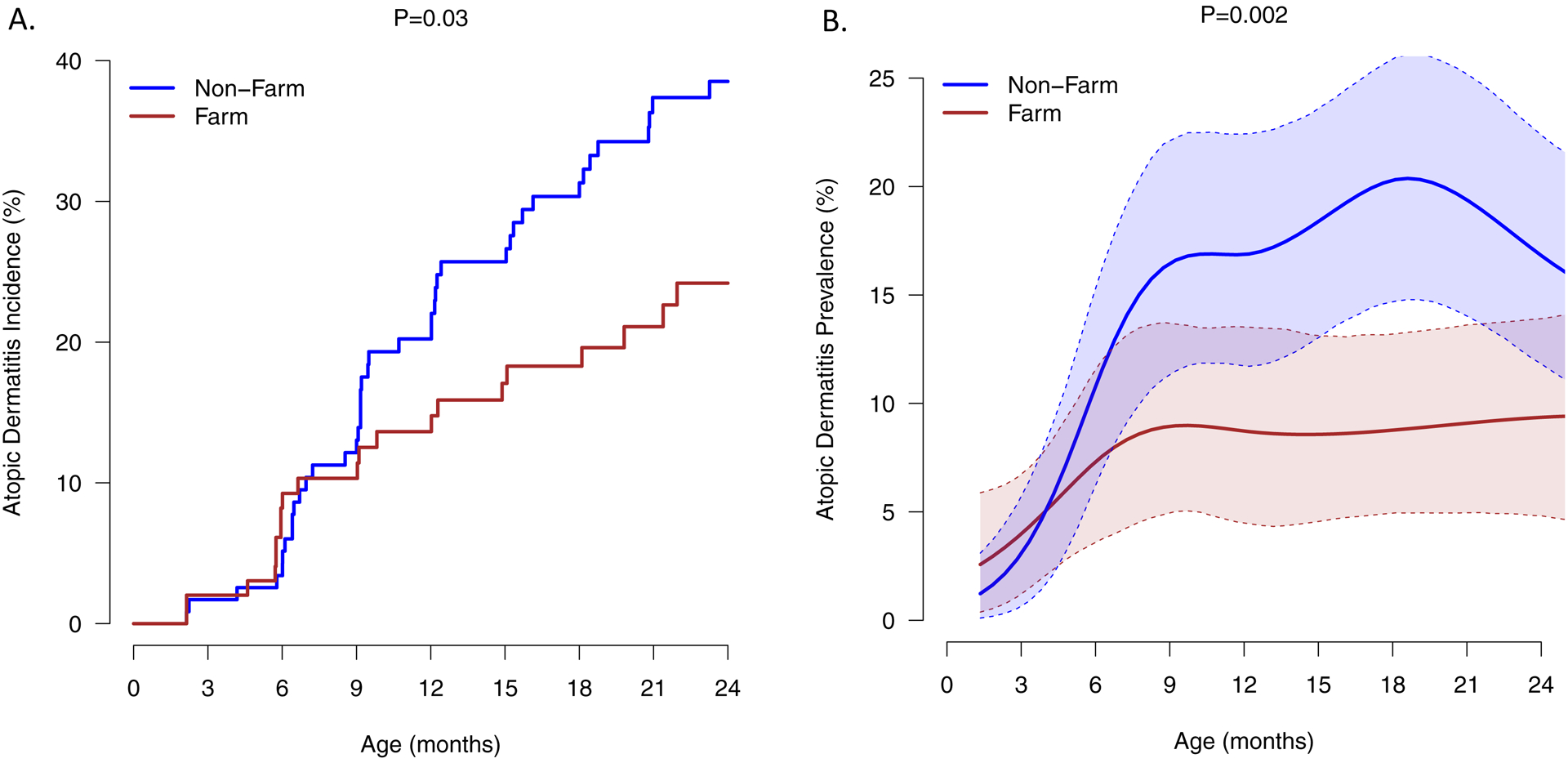
Exposure to a farm environment was associated with reduced AD incidence (A) and cumulative prevalence (B) during the first two years of life. The lines represent means and shaded areas are 90% (5th – 95th percentile) bootstrap confidence intervals for those means. The duration of follow-up for the 226 children included in the study is as follows: 24 mo, n=135; 21 mo, n=26; 18 mo, n= 17; 15 mo, n = 15; 12 mo, n = 2; 9 mo, n = 8; 6 mo, n = 11; 2 mo, n = 12).
Several specific prenatal and postnatal (2 months) farm-derived exposures were associated with AD risk among children in the farm cohort (Table III). Rates of AD were inversely associated with prenatal exposures to pigs (4% vs 25%, P=0.01), poultry (3% vs 28%, P<0.01) and feed grain (13% vs 34%, P=0.02). Prenatal contact with cattle, goats, pets (dog and cat), forage (hay, straw, and silage), manure, and raw milk consumption were not significantly associated with AD outcomes. Of the farm-derived exposures assessed at age 2 months, only poultry was significantly associated with AD development (0% vs 22%, p=0.03).
Table III.
Associations between Farm-Specific Prenatal and Early Life Exposures with Atopic Dermatitis in the Farm Cohort (n = 104).
| Atopic Dermatitis (Cumulative Prevalence*) | |||
|---|---|---|---|
| Exposure | Exposed | Non-Exposed | P-value† |
| Maternal Prenatal Contact | |||
| Hay | 16% (13/79) | 32% (8/25) | 0.14 |
| Straw | 18% (12/65) | 23% (9/39) | 0.56 |
| Feed grain | 13% (9/69) | 34% (12/35) | 0.02 |
| Silage | 17% (10/60) | 25% (11/44) | 0.28 |
| Manure | 13% (4/31) | 23% (17/73) | 0.23 |
| Cows or Cattle | 18% (16/91) | 38% (5/13) | 0.15 |
| Goats | 13% (2/16) | 22% (19/88) | 0.33 |
| Pigs | 4% (1/23) | 25% (20/81) | 0.01 |
| Poultry | 3% (1/33) | 28% (20/71) | <0.01 |
| Children Contact at 2 months | |||
| Forage | 16% (9/55) | 24% (12/49) | 0.27 |
| Cows or Cattle | 17% (12/70) | 26% (9/34) | 0.24 |
| Pigs | 9% (1/11) | 22% (20/93) | 0.31 |
| Poultry | 0% (0/9) | 22% (21/95) | 0.03 |
| Goats | 0% (0/4) | 21% (21/100) | 0.16 |
| Sheep | 33% (1/3) | 20% (20/100) | 0.67 |
| Horses | 0% (0/8) | 22% (21/96) | 0.10 |
Numerators represent the number of children with atopic dermatitis (cumulative prevalence up to age 2 years) and denominators represent the number of mothers or infants who were either exposed (column 2) or not exposed (column 3).
P values were calculated with the likelihood ratio test.
We also tested whether maternal prenatal exposure to a diversity of animal species was associated with AD risk. The number of animal species that the pregnant mothers had contact with was inversely associated with rates of AD (0 animals 43%, 1–2 animals 31%, 3–4 animals 16%, 5–6 animals 6%, P=0.01; Fig. 2). These findings suggested an additive effect of farm animal exposures on the risk for AD in children.
Figure 2: Atopic dermatitis by diversity of animal exposure.
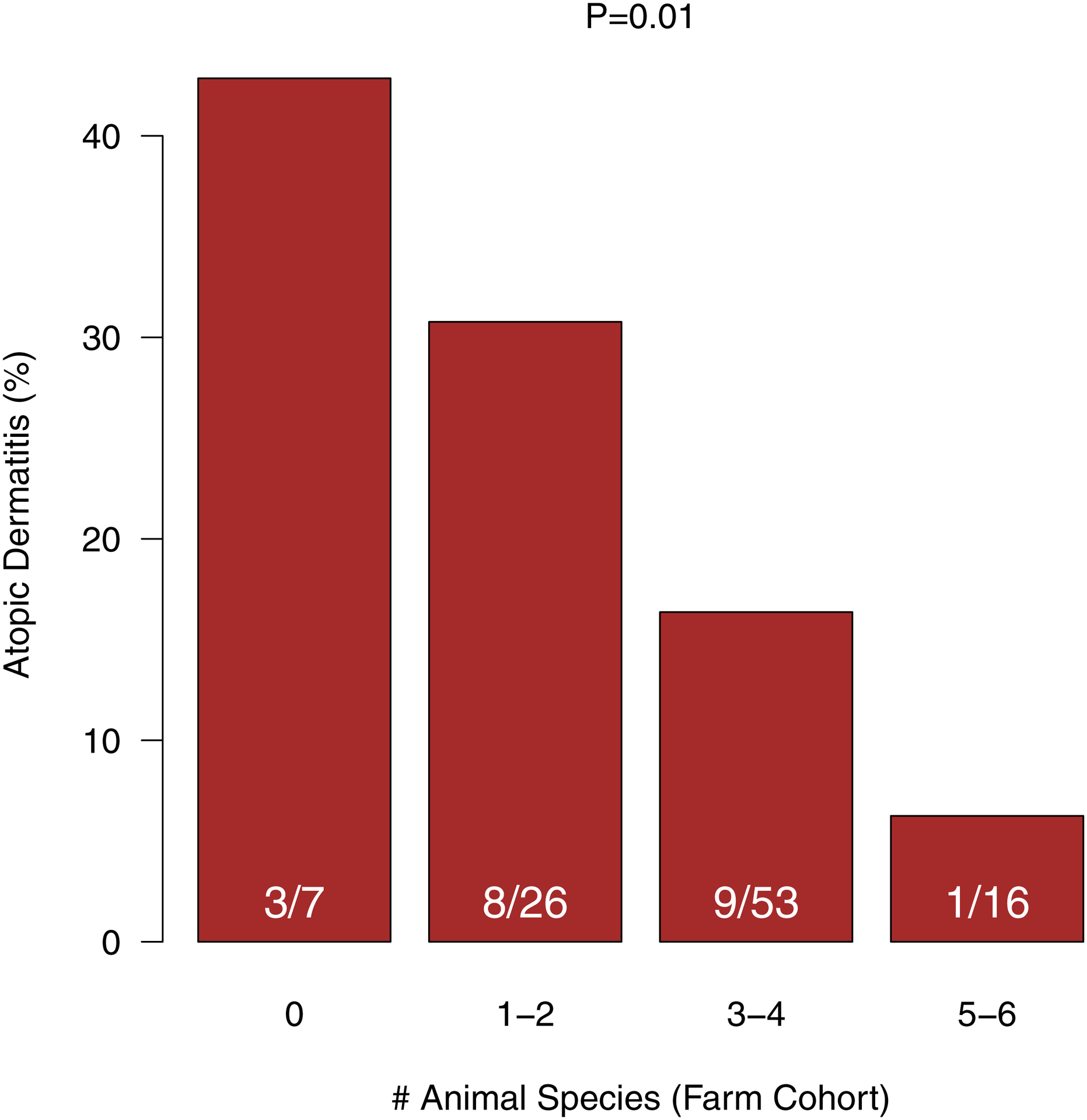
Cumulative prevalence of AD up to age 2 years was inversely related to the number of animal species that the mother had contact with during pregnancy.
Farm Cohort Exposure Classes
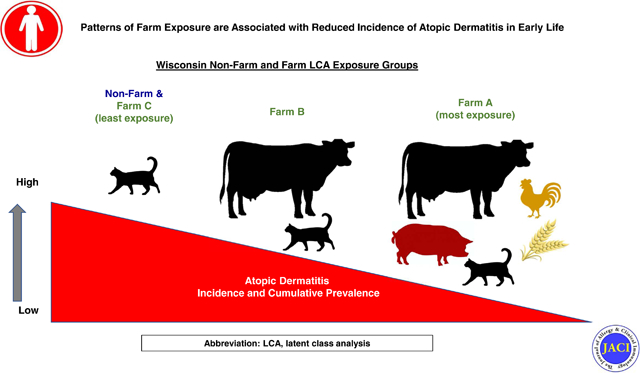
Many of the individual farm exposure were interrelated, and we used LCA to group farm families according to patterns of environmental exposures (see Supplemental Fig 1 for selected covariates). The analysis identified 3 distinct exposure classes (Class A, B, and C; descriptive analysis in Supplemental Tables I–II) with high probabilities of class assignment (Fig 3A). Farm Class A included 21% (22/104) of farm families, and was notable for prenatal and early life contact with a variety of farm animal species and a high rate of exposure to both indoor and outdoor dogs. Just over half (54/104) of the farm cohort was classified in Class B, which was remarkable for increased contact with cows or cattle and crops and a high prevalence of cat ownership. Last, Class C included 27% (28/104) of farm families, and had the lowest rate of maternal contact with farm animal species, farm animal feed (silage, feed grain), bedding (hay, straw) and manure (Supplemental Table I), and lower rates of pet ownership (Supplemental Table III).
Figure 3: Atopic dermatitis incidence by farm cohort exposure class.
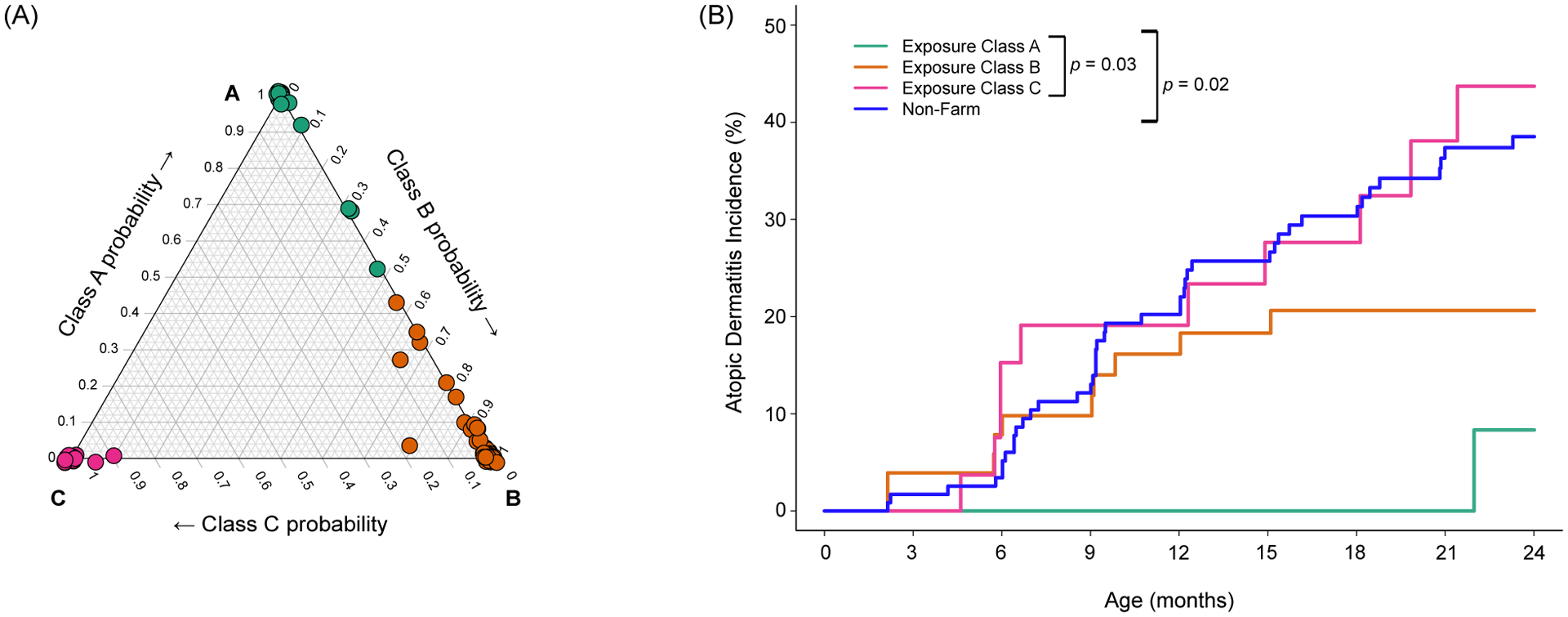
Three distinct patterns of farm exposure were identified by LCA, and the ternary probability plot (A) illustrates the probability of class assignment for each mother and child pair. The three classes of farm exposure were associated with distinct rates of AD incidence in the first two years (B). Group comparisons represent differences in overall AD indicence among the three farm exposure classes (p=0.03) and among the 3 classes considered together with the non-farm children (p=0.02).
Maternal history of allergic diseases and asthma, mode of delivery, and day care attendance were not significantly different among the classes (Supplemental Table III). Other than the diversity of animals on the farm, farm characteristics among the classes were similar (Supplemental Table IV), except for small differences in small vegetable farming on home farms (Class A: 14% vs Class B: 0% vs Class C: 7%, P=0.019) and the number of cows milked on the farm (fewest in Class A).
Farm Cohort Exposure Classes and AD Outcome
Farm cohort exposure classes were differentially related to AD incidence (Class C > B > A, P=0.03; Fig 3B), and these differences persisted after adjusting for sex. Notably, the incidence of AD was lowest in children born to mothers with regular contact with multiple animal species and diverse exposures in the barn (Class A). Notably, AD incidence among children with minimal prenatal and postnatal contact with animals (Class C) was similar to that of nonfarm children (Fig 3B).
DISCUSSION
This study was conducted to identify patterns of exposure on Wisconsin farms that are associated with reduced risk of AD. We found that farm exposures both prenatally and in the first year of life were associated with a reduced incidence and cumulative prevalence of AD in farm children when compared to non-farm children. The reduction in AD incidence was evident within the first year of life. Unlike prevalence, incidence is independent of disease duration and remission and may therefore be more valuable in identifying associations with factors related to disease onset. We identified 3 patterns of farm exposures on Wisconsin farms related to maternal and infant exposures: Class A had the most diverse animal and environmental exposures, Class B had less complex animal exposures, and Class C had the least exposures. These patterns differed in their association with AD incidence, and underscore the concept that diverse animal and barn exposures, especially during the prenatal period, reduce the risk of AD. These findings also suggest that the quality and quantity of personal exposure rather than the physical properties of the farm are the most important determinant of AD risk.
AD is similar to asthma in that several natural history phenotypes have been identified with distinct risk factors, and importantly, different prognoses and associations with other diseases. For example, in the Childhood Origins of Asthma birth cohort study, AD that began in the first year of life and persisted was associated with increased numbers of wheezing illnesses and a higher risk of allergic sensitization compared AD that was late onset (after age 3 years) or none/transient.32 Similarly, in the Protection Against Allergy Study in Rural Environments (PASTURE) European birth cohort, two early onset AD phenotypes (persistent and transient) were associated with increased risk of food allergy, and early persistent AD was also associated with increased risk of asthma compared to children with late onset or no AD.33 These studies provide evidence that there are different phenotypes of AD, and demonstrate that early onset AD is most strongly linked to subsequent allergic diseases, suggested a possible causal pathway. Thus, understanding mechanisms between farm exposures and reduced rates of early onset AD could also provide insights into the pathogenesis of wheezing illnesses, food allergy and asthma in children.
Notably, studies of farm exposure and AD development have been conflicting, and several previous studies did not demonstrate significant associations between farm exposures and AD development.20–27 These studies were cross-sectional and AD was ascertained in young adults or in families with school-aged children. Given the age of assessment, AD in these studies was likely of mixed phenotypes that included late onset AD, which we postulate may not be responsive to farming exposures in early life. Furthermore, in some of these studies, the farm environments and maternal/child contact with farm animals were not characterized in detail. In contrast, the two birth cohort studies (PASTURE and WISC [current analysis]) that have assessed early onset AD both have reported inverse relationships between specific farm exposures and AD risk. In the PASTURE/Mechanism of Early Protective Exposures on Allergy Development study, maternal contact with farm animals and cats during pregnancy were inversely related to AD in early life.14 This study also demonstrated that maternal exposure to multiple farm animal species during pregnancy was inversely related to the probability of AD in children, a relationship that was corroborated by the current study.
Previous studies have identified farm exposures including livestock, animal feed, and the consumption of unprocessed cow’s milk that may protect against development of asthma, hay fever, and AD during childhood.17, 21, 22, 24, 28, 34 Wisconsin farms are quite diverse in terms of size, animals and farming practices, and individual, maternal and child exposures. In our study population we identified multiple animal and barnyard (animal feed, bedding and manure) exposures that were inversely related to AD. Since these factors are interrelated, we used LCA to identify 3 unique exposure classes within the farm cohort. Two of the groups had more barn and farm animal exposures; Group A were more likely to report prenatal and postnatal contacts with a variety of animals, while Group B reported exposures primarily to cows and cattle. Group C reported the least contact with barn and animal exposures and were also least likely to have dogs. Accordingly, the AD risk was lowest in group A and intermediate in group B, while AD risk in Class C (low exposure) closely mirrored that of the non-farm cohort. Notably, patterns of exposure did not link cleanly to physical characteristics of the farm or practices such as milking style. Thus, farm exposures are diverse and are differentially related to AD risk. Notably, an LCA analysis of European farm exposure groups in the GABRIEL Surveys identified three exposure groups (summarized as “no cows”, “cows, no cultivation” and “cows and cultivation”) and the group with the least exposure to cows had the highest risk for AD.16 Both studies support the theory that frequent and diverse exposures beginning during the prenatal period have the strongest effect on risk of AD.
In 1989, David Strachan suggested the “hygiene hypothesis” and speculated that infections may protect against allergic disease.35 More recent iterations of this theory have linked microbial exposures to the risk of inflammatory diseases. Microbial stimuli in early life may help to mold immune development, and dysregulation may initiate and sustain an inflammatory cycle that leads to pathological effects.36, 37 Traditional farm environments provide rich biological diversity,28 and in addition to influencing immune development, farm-related microbes could help to inhibit skin colonization with Staphylococcus aureus, which has been closely linked to the pathogenesis of AD. Thus, farm-related microbial exposures may account for the lower risk of AD development. Farm-related exposures that could reduce the risk of allergic diseases through mechanisms in addition to those related to microbes, including arabinogalactans (plant-derived polysaccharide), N-glycolylneuraminic acid (Neu5Gc, expressed on mammalian cells) and raw cow milk consumption28, 38–42 also warrant further investigation with respect to AD.
The strengths of this study include the prospective design and populations of rural children with repeated exposure assessments. Central Wisconsin is a major producer within the U.S. dairy industry and many Wisconsin dairy farms in this region are small- to medium-sized and family owned and operated. Thus, rural Wisconsin is an ideal location to study effects of farm exposures in the US. Study limitations include AD defined by parental report of a healthcare provider’s diagnosis of AD. While farm and non-farm families could have different perceptions of medical problems, it is notable that health care utilization as measured by attendance at well child visits was similar between the two cohorts (data not shown). There are also several study limitations to consider. The diagnosis of AD was based on maternal report of diagnosis by a healthcare provider, which could have been affected by recall bias. WISC also has a modest sample size that limits ability to identify single or combinations of specific farm-related exposures related to AD. The prevalence of raw farm milk consumption is considerably lower in Wisconsin compared to Central Europe, which limited our ability to assess associations with AD. In addition, the WISC study is still enrolling, and the analysis of farm exposure patterns will require revision with increased data collection and the addition of more postnatal data.
In conclusion, WISC is the first birth cohort study in the US to study relationships between farm exposure and the risk of AD. Findings in the WISC birth cohort confirm observations from studies in Europe that link prenatal or early-life exposures to barns and diverse animals to reduced risk for AD. In addition, this analysis conducted in the heart of Wisconsin farm country provides new data relating farm-related patterns of exposures in the US to reduced incidence of AD. Finally, we observed that farm exposures are associated with reduced AD incidence beginning in the first year of life. Given the association between early onset AD and allergic outcomes such as food allergy and asthma, identification of farm-related microbiota or other exposures that positively influence early immunobiology and reduce AD development could lead to future preventative strategies for multiple atopic diseases.
Supplementary Material
Clinial Implications.
Prenatal exposure to a Wisconsin farm environment decreases AD development in offspring, particularly among mothers with diverse exposures to farm animals, feed and bedding.
Acknowledgements
This study was funded by NIH-NIAID grant U19 AI104317 and additional support was provided by NIH-NCATS grant UL1TR000427 and NIH-NIAID grant 5T32AI007635-18. We are grateful to the WISC study families for their participation and commitment. We thank the WISC study coordinators and research staff for their many contributions to this research. We thank Dr. Erika von Mutius University of Munich) and the GABRIEL and Prevention of Allergy—Risk Factors for Sensitization Related to Farming and Anthroposophic Lifestyle (PARSIFAL) study teams for generously sharing questionnaires and providing many helpful suggestions that were incorporated into the WISC study design.
Funding
This study was funded by NIH-NIAID grant U19 AI104317, NIH-NCATS grant UL1TR000427, NIH-NIAID grant 5T32AI007635–18, NIH Office of the Director UG3/UH3 OD023282 and the Marshfield Clinic Research Institute.
Abbreviations
- AD
Atopic dermatits
- LCA
latent class analysis
- MESA
Marshfield Epidemiologic Study Area
- PASTURE
Protection Against Allergy Study in Rural Environments European birth cohort
- WISC
Wisconsin Infant Study Cohort
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Approval and Consent to Participate
All study activities and procedures were approved by the Marshfield Clinic Research Institute (KEI10613) and University of Wisconsin-Madison (2012–1056) Human Subjects Institutional Review Boards.
Availability of Data and Material
The WISC study detailed manual of procedures and specific standard operating procedure source documents are available on reasonable request.
Conflicts of Interest
The authors declare that they have no financial conflicts of interest to report.
References
- 1.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 2011; 242:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol 2013; 131:295–9 e1–27. [DOI] [PubMed] [Google Scholar]
- 3.Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol 2006; 155:145–51. [DOI] [PubMed] [Google Scholar]
- 4.Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years. A prospective study of 543 newborns. Allergy 1983; 38:339–46. [DOI] [PubMed] [Google Scholar]
- 5.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest 2004; 113:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol 2010; 125:4–13; quiz 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol 2007; 120:565–9. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 9.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol 2012; 132:949–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsakok T, Marrs T, Mohsin M, Baron S, du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol 2016; 137:1071–8. [DOI] [PubMed] [Google Scholar]
- 11.Gern JE, Reardon CL, Hoffjan S, Nicolae D, Li Z, Roberg KA, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol 2004; 113:307–14. [DOI] [PubMed] [Google Scholar]
- 12.Benn CS, Melbye M, Wohlfahrt J, Bjorksten B, Aaby P. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. BMJ 2004; 328:1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strachan DP, Ait-Khaled N, Foliaki S, Mallol J, Odhiambo J, Pearce N, et al. Siblings, asthma, rhinoconjunctivitis and eczema: a worldwide perspective from the International Study of Asthma and Allergies in Childhood. Clin Exp Allergy 2015; 45:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roduit C, Wohlgensinger J, Frei R, Bitter S, Bieli C, Loeliger S, et al. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol 2011; 127:179–85, 85 e1. [DOI] [PubMed] [Google Scholar]
- 15.Ludka-Gaulke T, Ghera P, Waring SC, Keifer M, Seroogy C, Gern JE, et al. Farm exposure in early childhood is associated with a lower risk of severe respiratory illnesses. J Allergy Clin Immunol 2018; 141:454–6 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J Allergy Clin Immunol 2012; 129:1470–7 e6. [DOI] [PubMed] [Google Scholar]
- 17.Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J 2008; 32:603–11. [DOI] [PubMed] [Google Scholar]
- 18.Schaub B, Liu J, Hoppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009; 123:774–82 e5. [DOI] [PubMed] [Google Scholar]
- 19.Pfefferle PI, Buchele G, Blumer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol 2010; 125:108–15 e1–3. [DOI] [PubMed] [Google Scholar]
- 20.Horak E, Morass B, Ulmer H, Genuneit J, Braun-Fahrlander C, von Mutius E, et al. Prevalence of wheezing and atopic diseases in Austrian schoolchildren in conjunction with urban, rural or farm residence. Wien Klin Wochenschr 2014; 126:532–6. [DOI] [PubMed] [Google Scholar]
- 21.Riedler J, Eder W, Oberfeld G, Schreuer M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy 2000; 30:194–200. [DOI] [PubMed] [Google Scholar]
- 22.Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy 2000; 30:187–93. [DOI] [PubMed] [Google Scholar]
- 23.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non-farming environments. A nationwide study over three decades. Clin Exp Allergy 2004; 34:38–43. [DOI] [PubMed] [Google Scholar]
- 24.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001; 358:1129–33. [DOI] [PubMed] [Google Scholar]
- 25.Kilpelainen M, Terho EO, Helenius H, Koskenvuo M. Farm environment in childhood prevents the development of allergies. Clin Exp Allergy 2000; 30:201–8. [DOI] [PubMed] [Google Scholar]
- 26.Braun-Fahrlander C, Gassner M, Grize L, Neu U, Sennhauser FH, Varonier HS, et al. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clin Exp Allergy 1999; 29:28–34. [DOI] [PubMed] [Google Scholar]
- 27.Wickens K, Lane JM, Fitzharris P, Siebers R, Riley G, Douwes J, et al. Farm residence and exposures and the risk of allergic diseases in New Zealand children. Allergy 2002; 57:1171–9. [DOI] [PubMed] [Google Scholar]
- 28.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 2010; 10:861–8. [DOI] [PubMed] [Google Scholar]
- 29.von Mutius E 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: farm lifestyles and the hygiene hypothesis. Clin Exp Immunol 2010; 160:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seroogy CM, VanWormer JJ, Olson BF, Evans MD, Johnson T, Cole D, et al. Respiratory health, allergies, and the farm environment: design, methods and enrollment in the observational Wisconsin Infant Study Cohort (WISC). BMC Research Notes 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society, Series B 1972; 34:187–220. [Google Scholar]
- 32.Singh AM, Evans MD, Gangnon R, Roberg KA, Tisler C, DaSilva D, et al. Expression patterns of atopic eczema and respiratory illnesses in a high-risk birth cohort. Journal of Allergy & Clinical Immunology 2010; 125:491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roduit C, Frei R, Depner M, Karvonen AM, Renz H, Braun-Fahrlander C, et al. Phenotypes of Atopic Dermatitis Depending on the Timing of Onset and Progression in Childhood. JAMA Pediatr 2017; 171:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ege MJ, Frei R, Bieli C, Schram-Bijkerk D, Waser M, Benz MR, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol 2007; 119:1140–7. [DOI] [PubMed] [Google Scholar]
- 35.Strachan DP. Hay fever, hygiene, and household size. BMJ 1989; 299:1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep 2015; 15:65. [DOI] [PubMed] [Google Scholar]
- 37.Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DY, Muraro A, et al. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 2017; 139:1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters M, Kauth M, Scherner O, Gehlhar K, Steffen I, Wentker P, et al. Arabinogalactan isolated from cowshed dust extract protects mice from allergic airway inflammation and sensitization. J Allergy Clin Immunol 2010; 126:648–56 e1–4. [DOI] [PubMed] [Google Scholar]
- 39.Waser M, Michels KB, Bieli C, Floistrup H, Pershagen G, von Mutius E, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy 2007; 37:661–70. [DOI] [PubMed] [Google Scholar]
- 40.Loss G, Bitter S, Wohlgensinger J, Frei R, Roduit C, Genuneit J, et al. Prenatal and early-life exposures alter expression of innate immunity genes: the PASTURE cohort study. J Allergy Clin Immunol 2012; 130:523–30 e9. [DOI] [PubMed] [Google Scholar]
- 41.Frei R, Ferstl R, Roduit C, Ziegler M, Schiavi E, Barcik W, et al. Exposure to nonmicrobial N-glycolylneuraminic acid protects farmers’ children against airway inflammation and colitis. J Allergy Clin Immunol 2018; 141:382–90 e7. [DOI] [PubMed] [Google Scholar]
- 42.Brick T, Schober Y, Bocking C, Pekkanen J, Genuneit J, Loss G, et al. omega-3 fatty acids contribute to the asthma-protective effect of unprocessed cow’s milk. J Allergy Clin Immunol 2016; 137:1699–706 e13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


