Abstract
Immune exhaustion in T cells significantly impacts their ability to control malignancies and infections, and its discovery has led to revolutionary therapies for cancer in the form of checkpoint blockade. Natural killer (NK) cells, like T cells, are lymphocytes that recognize virally infected and malignantly transformed cells. However, it remains unclear if NK cells are similarly susceptible to exhaustion. In this review we seek to summarize what is currently known and to identify key areas of variability that skew the scientific literature on NK cell exhaustion. A lack of consensus on the defining features of NK cell dysfunctional states such as senescence, suppression, and exhaustion has made comparison between studies difficult. There are also significant differences in the biology of NK cell subsets with long-lived, adaptive NK cells sharing an epigenetic signature closer to memory CD8+ T cells than to conventional NK cells. We have shown very different checkpoint receptor expression and effector functions in adaptive versus conventional NK cells chronically exposed to activating signals. Adaptive NK cells develop in individuals with cytomegalovirus (CMV) infection and well over half of the human population worldwide is CMV seropositive by adulthood. Despite this high prevalence, most studies do not account or control for this population. This may contribute to some of the variability reported in the literature on checkpoint receptor expression on NK cells. In this review we will also discuss the protective role that exhaustion plays in T cells and examine evidence for a similar phenomenon in NK cells.
Graphical Abstract

INTRODUCTION
Immune exhaustion is a dysfunctional state, characterized by decreased effector functions, that was originally described in T cells in the setting of chronic lymphocytic choriomeningitis virus (LCMV) infection [1–3]. Exhaustion in T cells is driven by persistent antigen exposure and T cell receptor stimulation [4] and has been described in chronic infections and cancer [5, 6]. Exhausted T cells have unique transcriptional and epigenetic signatures that include overexpression of several checkpoint inhibitory receptors, dysregulated intracellular signaling pathways, and metabolic alterations [7–10]. Reversal of T cell exhaustion with therapies targeting checkpoint inhibitory receptors has led to dramatic improvements in cancer treatment through reinvigoration of the patient’s own immune system. Despite the clinical importance of T cell exhaustion, a recent review of experts in the field revealed a lack of consensus on its exact definition [11].
Natural killer (NK) cells, like T cells, are lymphocytes that play a role in the recognition and elimination of virally infected or malignantly transformed cells. Unlike T cells, NK cells lyse their targets in a major histocompatibility complex (MHC)-unrestricted manner that does not require antigen priming. NK cells are now recognized as members of the innate lymphoid cell (ILC) family [12]. ILCs do not express antigen-specific receptors and are considered a part of the innate immune system. However, it is now well established that NK cells can develop traits of adaptive immunity in certain circumstances such as cytomegalovirus (CMV) infection [13].
Exposure to cancer and chronic viral infection clearly induces a state of reduced effector function in NK cells, though it is not yet clear if this state is equivalent to T cell exhaustion. Given the efficacy of checkpoint blockade therapies targeting T cell exhaustion, there is intense interest in blocking NK cell ‘exhaustion’. NK cells have been reported to express many of the same checkpoint inhibitory receptors as T cells, but it is unclear to what extent or under which circumstances, as published studies have provided conflicting results. Even less is known about the intracellular signaling and metabolic changes that occur concomitant with loss of functionality, though there is evidence that both obesity and cancer perturb NK cell metabolism [14, 15]. In this review we will discuss the evolutionarily conserved role of T exhaustion and its relation to adaptive NK cells that help control CMV viremia. We will compare NK cell dysfunctional states and discuss the current findings on checkpoint inhibitory receptor expression.
THE PROTECTIVE ROLE OF EXHAUSTION
Despite the negative effects of immune exhaustion in cancerous and infectious states, it plays an important role in attenuating immune responses. Human T cells can activate telomerase [16] but clonal analysis of T cells maintained in long-term culture by intermittent reactivation of the antigen receptor and exogenous interleukin 2 (IL-2) show that with each generation, an increasing fraction of progeny cells undergo activation-induced cell death until the clone is lost [17]. Clonal attrition of T cells specific for cytomegalovirus has been demonstrated in the elderly and is associated with mortality [18]. These findings indicate that exhaustion is a mechanism to prevent activation- induced cell death and retain specific T cell clones.
Conventional NK cells lack antigen specificity and live less than 10 days in humans [19] or 7–10 days in mice [20]. Control of NK cell activity is regulated by the integration of activating and inhibitory signals from germ-line encoded cell surface receptors. Given their short lifespan and lack of antigen specificity, exhaustion in conventional NK cells might not be worth the energetic costs. On the other hand, adaptive NK cells are long-lived, arise in response to CMV infection, possess memory recall, and exhibit some degree of antigen specificity [21–24]. Their expansion can be driven through CD94/NKG2C interactions with CMV-encoded UL40 peptides presented by HLA-E in humans [25]. However, adaptive NK cells also arise in individuals who are homozygous for deletion of NKG2C [26]. The human NK cell response to CMV infection is heterogenous and includes decreased expression of the signaling molecules FcεR1γ, SYK, and EAT-2 and the transcription factor PLZF as part of their signature [27]. In mice, the activating receptor Ly49H specifically recognizes the murine CMV-encoded glycoprotein m157 [21] and contributes to control of murine CMV infection [28–30]. Adaptive NK cells persist for several months in the absence of detectable CMV viremia and are stably maintained at elevated frequencies for years in some individuals [31, 32]. Given that adaptive NK cells are important for control of CMV throughout the life of the host, exhaustion may play a role in retaining this subset.
Other important roles of exhaustion include attenuating immune responses to persistent viral pathogens and preventing autoimmunity. In mice infected with LCMV, exhaustion of T cells prevents tissue damage, whereas ongoing T cell activation leads to necrosis of the lungs and liver [33]. Knockout of the checkpoint receptor programmed death receptor (PD-1) in mice leads to lupus-like glomerulonephritis and arthritis [34]. A study of murine CMV infection shows that endogenous glucocorticoids induce tissue-specific expression of PD-1 on the surface of splenic NK cells, and an NK cell-specific PD-1 gene knockout causes splenic necrosis despite similar viral loads compared to wild type mice [35]. Tissue damage has also been seen in the context of therapeutic checkpoint blockade in both mice [36] and humans [37]. Moderate to severe, immune-mediated side effects occur in at least 10–20% of patients who receive anti-PD-1 therapy with the most common side-effects being colitis, hepatitis, adrenocorticotropic hormone insufficiency, hypothyroidism, type 1 diabetes, acute kidney injury, and myocarditis [38]. These findings highlight the importance of checkpoint inhibitory receptors and exhaustion in preventing self-harm by the immune system.
DYSFUNCTIONAL STATES IN NK CELLS
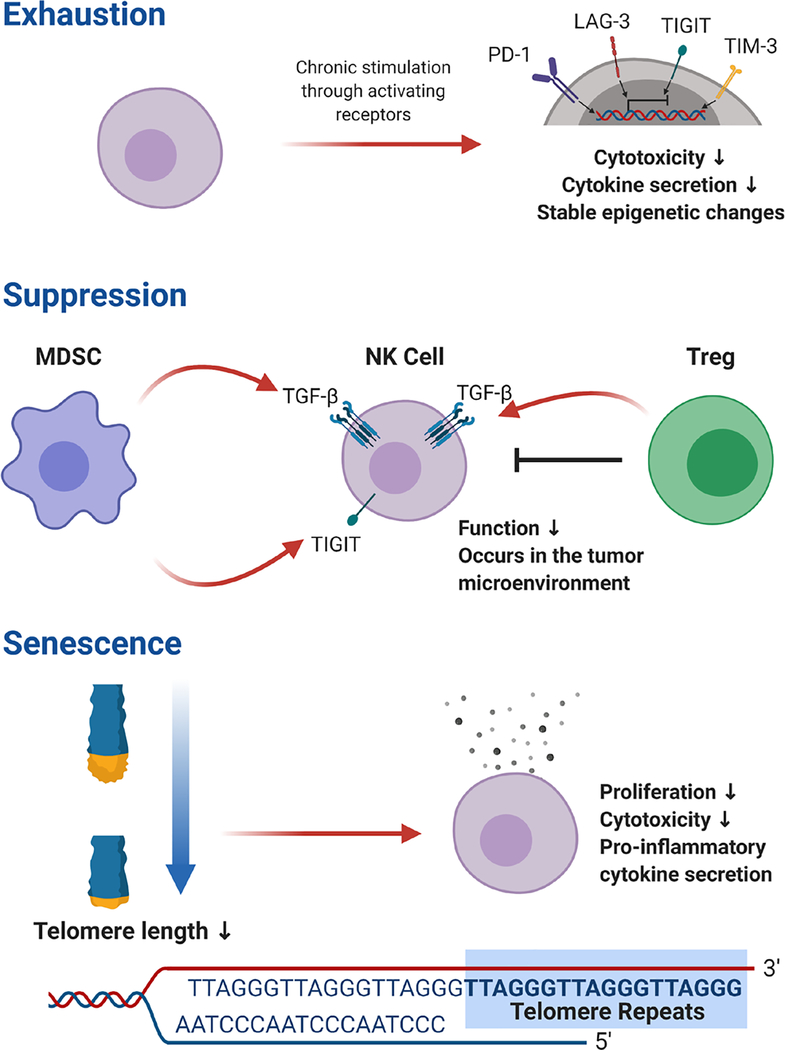
Exhaustion is typified by attenuation of effector function via ligation of inhibitory receptors on the cellular surface, referred to as checkpoint receptors (Figure 1). In common parlance, the term ‘exhaustion’ suggests that necessary resources have been completely utilized but also implies a temporary state that allows for potential regeneration. Studies of T cells in tumor bearing mice indicate that immune exhaustion does follow a continuum of checkpoint receptor expression and epigenetic remodeling. In the early stages, T cell exhaustion can be reversed by blockade of programmed death receptor 1 (PD-1), an inhibitory checkpoint receptor that is expressed by exhausted T cells. However, ongoing exposure to tumor conditions leads to stable epigenetic changes that cannot be reversed with checkpoint inhibitor therapy alone [13]. Although exhausted T cells are dysfunctional, they still retain some level of function as they can drive epitope mutations during chronic HIV infection [14].
Figure 1.
Comparison of Dysfunctional States in NK cells.
Senescence, a state of immune dysfunction commonly confused with exhaustion [4], is related to shortening of telomeres and DNA damage that ultimately leads to cell cycle arrest [5]. Senescent T cells are found in elderly adults and after prolonged culture in vitro [6]. In addition to decreased replicative ability, senescent T cells secrete high levels of pro-inflammatory cytokines that are caused by metabolic derangements and an accumulation of reactive oxygen species [7]. T cell numbers decrease with age, but NK cell numbers increase despite a decreased ability to proliferate in vitro compared to NK cells from younger individuals [8]. Like exhaustion in T cells, NK cell senescence may not be an aberrant immune phenomenon but rather have an evolutionarily conserved role in the host life cycle. NK cells in the gravid uterus undergo senescence when DNA damage response pathways are stimulated by HLA-G, causing them to exhibit a secretory phenotype that promotes the vascular remodeling required for a healthy pregnancy [39].
Another dysfunctional state, often confused with exhaustion, is suppression through extrinsic signaling. Suppression has been described in NK cells in the tumor microenvironment where T regulatory cells (Tregs), tumor-associated macrophages, tumor-associated fibroblasts, and myeloid derived suppressor cells (MDSC) have been shown to inhibit NK cell function [10] (Summarized Table 1). Tregs and MDSCs utilize TGF-β signaling to stop NK cells from killing tumor cells [11, 12]. Hypoxia and adenosine, both commonly found in the tumor microenvironment, also have suppressive effects on NK cell activity [15]. Suppression implies a reversible state with the withdrawal of suppressive signaling, but exhaustion leads to stable phenotypic and epigenetic changes. In addition to decreased cytotoxicity and cytokine secretion, NK cells from individuals with malignancies or chronic viral infections often show a loss of activating receptors that has been postulated to represent an ‘exhausted’ phenotype. NKG2D is an activating receptor that binds ligands up-regulated by cellular stress [62], and it has been shown to be down-regulated on NK cells from patients with digestive cancers [60], chronic lymphocytic leukemia [63], breast cancer [64], chronic hepatitis B virus [65], and hepatitis C virus [66]. CD16, an activating receptor that binds the Fc portion of antibodies, is down-regulated on NK cells from patients with breast cancer [64]. Since NK cell activation is dictated by the integration of activating and inhibitory signals from cell surface receptors, weakened signals from activating receptors lead to domination by inhibitory signals and decreased NK cell function. With ongoing research, we are likely to find that there is overlap between suppression and exhaustion with different types of stimuli predisposing to one or the other, and it is unlikely that these states can be differentiated from one another simply based on receptor expression.
Table 1.
Summary of selected studies in NK cell dysfunction in the cancer
| NK cell function | Context | Species | References |
|---|---|---|---|
| Decreased degranulation, decreased IFN-γ | Intratumoral NK cells from Non-small cell lung cancer | Human | [56, 57] |
| Decreased cytotoxicity | NK cells from peritoneal effusions in ovarian cancer patients | Human | [58] |
| Decreased cytotoxicity, increased expression of NKG2A, decreased IFN-γ | NK cells from hepatocellular carcinoma compared to liver derived NK cells from healthy donors | Human | [59] |
| Decreased cytotoxicity | Peripheral blood NK cells from pancreatic, gastric, and colorectal cancer patients compared to healthy donors | Human | [60] |
| Decreased degranulation, decreased IFN-γ | NK cells adoptively transferred into mice with leukemia | Murine | [61] |
| Decreased cytotoxicity, decreased activating receptors | Cancer associated fibroblasts co-cultured with NK cells | Human | [77–79] |
| Decreased cytotoxicity, decreased cytokine secretion | Myeloid derived suppressor cells from hepatocellular carcinoma co-cultured with NK cells | Human | [80] |
EXHAUSTION IN ADAPTIVE NK CELLS
Further complicating studies of NK cell dysfunction, most publications do not account for differences between conventional NK cells and long-lived, adaptive NK cells since both types will fall into the CD3-CD56dim flow cytometry gates frequently used to identify NK cells. Adaptive NK cells exhibit an epigenetic signature more comparable to effector CD8+ T cells than to conventional NK cells [26] and have a distinct metabolic profile [40]. Because they are also long-lived and contribute to control of CMV viremia [28, 41, 42] we hypothesized that adaptive NK cells might become exhausted with chronic activation. Many, but not all, adaptive NK cells express the lectin-like heterodimeric receptor CD94/NKG2C [43]. NKG2C+ NK cells rapidly acquire the maturation marker CD57 [31] and exhibit a skewed killer cell immunoglobulin-like receptor (KIR) profile with enrichment of self-inhibitory KIRs and activating KIRs [44]. CD94, can heterodimerize with NKG2A or NKG2C, and both of these receptors recognize the HLA-E molecule. Signaling through NKG2A is inhibitory and signaling through NKG2C is activating [45].
To test if chronic activation led to exhaustion in adaptive NK cells, we utilized antibodies against NKG2C in plate-bound assays with interleukin-15 (IL-15). NK cells were obtained from the peripheral blood of CMV seropositive donors. We found that agonist antibodies against NKG2C promoted proliferation of adaptive NK cells, but their expansion was also associated with expression of the checkpoint receptors lymphocyte activation gene (LAG-3) and PD-1. LAG-3 is an inhibitory receptor homologous to CD4 that is expressed on activated T and NK cells [46], and its ligands are HLA class II molecules and fibrinogen family protein (FGL1). FGL1 is normally released by the liver in low levels but can be expressed by tumor cells at high levels [47]. In our culture assays with anti-NKG2C antibodies, LAG-3 levels reached 65–85% on NKG2C+ cells, and PD-1 levels ranged from 0–45% by day seven of co-culture. Incubation with isotype control antibodies did not promote the high levels of LAG-3 and PD-1 seen with anti-NKG2C antibodies, indicating that chronic activation promotes exhaustion in adaptive NK cells. LAG-3 was virtually absent on NK cells prior to expansion, but up-regulation started as early as day 3 of culture. All PD-1+ NK cells co-expressed LAG-3, possibly indicating sequential up-regulation of checkpoint receptors with ongoing stimulation. We confirmed that checkpoint receptor expression was associated with decrements in NK cell function in killing assays with a leukemic target cell line (K562). LAG-3+ NK cells exhibited no defect in degranulation when cultured with K562 but produced significantly less IFN-γ. This is similar to findings in T cell exhaustion where the ability to secrete cytokines was the first function compromised [48].
Since NK cell activation is dependent on the balance of signaling through activating and inhibitory receptors, we hypothesized that simultaneous inhibition would rescue NK cells from becoming exhausted. To this end, we compared the effect of incubation with an anti-NKG2C antibody to an antibody that bound both NKG2C and the inhibitory receptor NKG2A. NKG2C+ NK cells incubated with anti-NKG2C antibody had significantly higher checkpoint expression than NKG2C+ NK cells incubated with an antibody that bound both NKG2A and NKG2C. These data indicate that signaling through inhibitory receptors may help mitigate exhaustion during chronic stimulation [49]. This is consistent with the known role of NKG2A during viral infections where it limits excessive activation, prevents apoptosis, and preserves CD8+ T cell responses [50].
Adaptive NK cells often express high levels of NKG2C, while conventional NK cells do not. Since we hypothesized that adaptive NK cells would be more likely to undergo exhaustion, we tested activation through other receptors expressed by both adaptive and conventional NK cells. We used agonist antibodies against the activating receptors NKp30 and NKG2D and found that adaptive NK cells (NKG2C+), but not conventional NK cells (NKG2C-), significantly up-regulated increased expression of LAG-3 and PD-1 [49]. These results show that adaptive NK cells differ from conventional NK cells in their propensity to express checkpoint inhibitory receptors with chronic activation.
Since adaptive NK cells share many transcriptional characteristics with CD8+ T cells [27], we hypothesized that adaptive NK cells would undergo similar epigenetic remodeling after chronic engagement of NKG2C. CD8+ T cells undergo extensive epigenetic remodeling during the process of exhaustion [4, 51, 52], and tumor-specific T cells in murine cancer models transition through discrete chromatin states where they can initially be rescued by PD-1 blockade but later develop a fixed dysfunctional state [52, 53]. We looked at whole genome epigenetic signatures with DNA methylation arrays in adaptive NK cells after incubation with interleukin-15 (IL-15) and plate-bound, agonist antibodies against NKG2C or NKG2A/C. Many of the same genes that are epigenetically remodeled in exhausted CD8+ T cells exhibited similar alterations in DNA methylation in NKG2C-stimulated adaptive NK cells. Hypermethylation in the promoter sequences of some transcription factors and epigenetic regulators (FOXO1, FOXP1, SATB1, TCF12) [54–59] and hypomethylation in other transcription factors and epigenetic regulators (IKZF3, NFATC, TOX, ZBTB38) [60–63] mirrored results seen in exhausted CD8+ T cells. These results suggest that adaptive NK cells and CD8+ T cells share a molecular pathway leading to exhaustion and that NK cell exhaustion is not a transient state.
There are significant differences in adaptive and conventional NK cell biology, and the global seroprevalence of CMV is 83% [64]. We have processed blood from over 2000 CMV-seropositive blood donors and 5–10% exhibit at least 20% circulating, adaptive NK cells by flow cytometry (NKG2C+). Our findings with adaptive NK cells indicate that inclusion of donors with unrecognized high levels of adaptive NK cells may lead to skewing of checkpoint receptor patterns and should be considered in study design.
THE ROLE OF CHECKPOINT RECEPTORS ON NK CELLS
It is unsurprising, given the functional similarities between NK cells and CD8+ T cells, that they share many checkpoint inhibitory receptors [65] and checkpoint receptor expression has frequently been cited as evidence that NK cells undergo exhaustion. Further evidence is needed to determine if checkpoint expression on conventional NK cells is actually associated with decreased function and whether or not the functional consequences require ligation of checkpoint ligands. Checkpoint receptors that have been implicated in NK cell exhaustion include PD-1, LAG-3, T-cell immunoglobulin and mucin domain-3 (TIM-3), T cell immunoreceptor with Ig and ITIM domains (TIGIT), and CD96. PD-1 has received the most attention because of the success of antibody-mediated PD-1 blockade in reinvigorating exhausted T cells, but results for all of these checkpoint receptors have been contradictory between studies (Summarized Table 2).
Table 2.
Summary of key studies of checkpoint inhibitory receptors on NK cells.
| Marker | Context | Species | References |
|---|---|---|---|
| Minimal PD-1 Increased TIGIT | Murine NK cells after CMV infection or acute leukemia; human NK cells incubated with cytokines or isolated from solid tumors | Human and Murine | [74] |
| Increased LAG-3 and PD-1 on adaptive but not conventional NK cells after seven days of stimulation | Checkpoint receptor expression compared on adaptive or conventional NK cells stimulated with activating antibodies for seven days | Human | [49] |
| Increased PD-1 on NK cells from spleen | Tissue specific NK cells examined in mice with Murine CMV infection | Murine | [35] |
| Increased TIGIT PD-1 < 10% expression | Intratumoral NK cells from mice with colon, breast, fibrosarcoma, and melanoma tumors | Murine | [81] |
| PD-1 expression on NK cells associated with more degranulation and IFN-γ secretion | Expression compared on tumor infiltrating NK cells | Murine | [82] |
| PD-1 expression increased after 12 days on expanded NK cells but not associated with functional deficits | Expression of PD-1 evaluated on NK cells after in vitro expansion with K562 expressing membrane bound IL-15 and 4-1BB ligand | Human | [83] |
| Increased PD-1 | Peripheral blood NK cells from patients with digestive cancers compared to healthy donors | Human | [84] |
| PD-1 expressed on 25% | Examination of peripheral blood NK cells from healthy donors seropositive for CMV | Human | [70] |
| Increased PD-1 | Comparison of NK cells from patients with Kaposi sarcoma compared to asymptomatic HHV-8 carriers | Human | [71] |
| Increased PD-1 on peripheral blood NK in patients with renal cell carcinoma | Compared PD-1 on NK cells from renal cancer patients before nephrectomy to healthy donors | Human | [68] |
| No increase in PD-1 TIM-3 expression associated with NK dysfunction | Compared NK cells from metastatic melanoma to healthy donors | Human | [85] |
| Increased PD-1 | NK cells compared in Post-Transplant Lymphoproliferative Disorder compared to transplant patients and healthy donors | Human | [72] |
| Increased PD-1 | NK cells from multiple myeloma patients compared to healthy donors | Human | [66] |
PD-1 has been reported on human NK cells from multiple myeloma [66], Hodgkin’s lymphoma and diffuse large B cell lymphoma [67], renal cell carcinoma [68], nasopharyngeal carcinoma [69, 70], ovarian cancer [70], and Kaposi’s sarcoma [71] as well as post-transplantation lymphoproliferative disorder (PTLD) [72]. Resting NK cells have intracellular stores of PD-1 mRNA and protein, even in the absence of surface expression [73], suggesting that they may express PD-1 under specific circumstances or in specific tissues. A recent study looked at both murine and human NK cells in in vitro and in vivo assays of cytokine stimulation, infection and tumor exposure. The authors observed minimal PD-1 expression on NK cells, and this was confirmed with qRT-PCR and RNA-Seq. However, they observed significantly increased TIGIT expression on murine NK cells after CMV infection or acute leukemia and on human NK cells incubated with cytokines or isolated from solid tumors. The authors did not test if TIGIT+ NK cells were hypofunctional compared to TIGIT- NK cells in these assays [74] so it is unclear if the TIGIT+ NK cells exhibited an exhausted phenotype.
There are several factors that likely contribute to the variation in checkpoint receptor expression seen in different studies. Technical issues include differences in flow cytometry gating strategies, the use of different antibodies for flow cytometry, and different NK cell stimulatory conditions. NK cells also perform trogocytosis, and this could be a source for false positive PD-1 staining in some models [75, 76]. Another possible source of variation that has yet to be explored is the contribution of genetic diversity between donors. Human NK cell activation is influenced by germline-encoded receptor polymorphisms such as those in the KIR family, and these genetic differences between donors may influence the propensity of NK cells to become exhausted and/or to express checkpoint receptors. Despite a paucity of data to show that blockade of checkpoint receptors enhances NK cell function, clinical trials with LAG-3, TIM-3 and TIGIT blocking antibodies are currently underway. Results from these studies may help further elucidate the role of these receptors in attenuating NK cell function.
CONCLUSION
A better understanding of NK cell dysfunctional states and the factors that promote them will continue to be important in the search for novel therapies for cancer and chronic infections. Future studies should account for both conventional and adaptive NK cell subsets since they possess distinct epigenetic and metabolic profiles and respond differently to chronic activating signals. Flow cytometric findings of checkpoint receptor expression should be confirmed with alternative methods. Care should be taken when defining NK cell ‘exhaustion’ in the literature, as it remains to be determined if the decreased functionality seen in conventional NK cells is associated with stable epigenetic and transcriptomic changes. We would suggest that ‘suppression’ would be a better descriptor of reversible NK cell dysfunction.
ACKNOWLEDGEMENTS
This work was supported by the NIH grant K99/R00 HL123638 (to FC), P01 CA111412 (to JSM), P01 CA65493 (to JSM), and T32 2T32HL007062 (AM). The graphics in this paper were generated with BioRender.
Abbreviations
- NK
Natural Killer
- CMV
cytomegalovirus
- LCMV
chronic lymphocytic choriomeningitis virus
- MHC
major histocompatibility complex
- ILC
innate lymphoid cell
- IL-2
interleukin 2
- PD-1
programmed death receptor
- Tregs
T regulatory cells
- MDSC
myeloid derived suppressor cells
- KIR
killer cell immunoglobulin-like receptor
- IL-15
interleukin-15
- LAG-3
lymphocyte activation gene
- FGL1
fibrinogen family protein
- TIM-3
T-cell immunoglobulin and mucin domain-3
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- PTLD
post-transplantation lymphoproliferative disorder
- IFN-γ
interferon-γ
REFERENCES
- 1.Moskophidis D, et al. , Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature, 1993. 362(6422): p. 758–61. [DOI] [PubMed] [Google Scholar]
- 2.Gallimore A, et al. , Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med, 1998. 187(9): p. 1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajac AJ, et al. , Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med, 1998. 188(12): p. 2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schietinger A, et al. , Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity, 2016. 45(2): p. 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, et al. , T Cell Dysfunction and Exhaustion in Cancer. Front Cell Dev Biol, 2020. 8: p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wherry EJ and Kurachi M, Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol, 2015. 15(8): p. 486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry EJ and Ahmed R, Memory CD8 T-cell differentiation during viral infection. J Virol, 2004. 78(11): p. 5535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauken KE, et al. , Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science, 2016. 354(6316): p. 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macian F, et al. , Transcriptional mechanisms underlying lymphocyte tolerance. Cell, 2002. 109(6): p. 719–31. [DOI] [PubMed] [Google Scholar]
- 10.Heissmeyer V, et al. , Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol, 2004. 5(3): p. 255–65. [DOI] [PubMed] [Google Scholar]
- 11.Blank CU, et al. , Defining ‘T cell exhaustion’. Nat Rev Immunol, 2019. 19(11): p. 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberl G, et al. , Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science, 2015. 348(6237): p. aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Sullivan TE, Sun JC, and Lanier LL, Natural Killer Cell Memory. Immunity, 2015. 43(4): p. 634–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien KL and Finlay DK, Immunometabolism and natural killer cell responses. Nat Rev Immunol, 2019. 19(5): p. 282–290. [DOI] [PubMed] [Google Scholar]
- 15.Chambers AM, Lupo KB, and Matosevic S, Tumor Microenvironment-Induced Immunometabolic Reprogramming of Natural Killer Cells. Front Immunol, 2018. 9: p. 2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi H and Sakaguchi N, Telomerase activity is induced by the stimulation to antigen receptor in human peripheral lymphocytes. Biochem Biophys Res Commun, 1996. 219(2): p. 649–55. [DOI] [PubMed] [Google Scholar]
- 17.Adibzadeh M, et al. , Long-term culture of monoclonal human T lymphocytes: models for immunosenescence? Mech Ageing Dev, 1995. 83(3): p. 171–83. [DOI] [PubMed] [Google Scholar]
- 18.Hadrup SR, et al. , Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol, 2006. 176(4): p. 2645–53. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. , In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology, 2007. 121(2): p. 258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JW, et al. , Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science, 2002. 295(5562): p. 2094–7. [DOI] [PubMed] [Google Scholar]
- 21.Sun JC, Beilke JN, and Lanier LL, Adaptive immune features of natural killer cells. Nature, 2009. 457(7229): p. 557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolle A, et al. , CD2-CD58 interactions are pivotal for the activation and function of adaptive natural killer cells in human cytomegalovirus infection. Eur J Immunol, 2016. 46(10): p. 2420–2425. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, et al. , Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity, 2015. 42(3): p. 431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun JC, et al. , Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med, 2011. 208(2): p. 357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer Q, et al. , Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol, 2018. 19(5): p. 453–463. [DOI] [PubMed] [Google Scholar]
- 26.Liu LL, et al. , Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep, 2016. 15(5): p. 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlums H, et al. , Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity, 2015. 42(3): p. 443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukowski JF, Woda BA, and Welsh RM, Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol, 1984. 52(1): p. 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MG, et al. , Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science, 2001. 292(5518): p. 934–7. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, et al. , Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet, 2001. 28(1): p. 42–5. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Verges S, et al. , Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A, 2011. 108(36): p. 14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley B, et al. , Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood, 2012. 119(11): p. 2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornberg M, et al. , Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front Immunol, 2013. 4: p. 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura H, et al. , Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity, 1999. 11(2): p. 141–51. [DOI] [PubMed] [Google Scholar]
- 35.Quatrini L, et al. , Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol, 2018. 19(9): p. 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Affolter T, et al. , Inhibition of immune checkpoints PD-1, CTLA-4, and IDO1 coordinately induces immune-mediated liver injury in mice. PLoS One, 2019. 14(5): p. e0217276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Refai SM, et al. , Immune Checkpoint Inhibition and the Prevalence of Autoimmune Disorders Among Patients With Lung and Renal Cancer . Cancer Inform, 2017. 16: p. 1176935117712520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajwa R, et al. , Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res, 2019. 11(4): p. 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajagopalan S and Long EO, Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proc Natl Acad Sci U S A, 2012. 109(50): p. 20596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cichocki F, et al. , ARID5B regulates metabolic programming in human adaptive NK cells. J Exp Med, 2018. 215(9): p. 2379–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels KA, et al. , Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med, 2001. 194(1): p. 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuijpers TW, et al. , Human NK cells can control CMV infection in the absence of T cells. Blood, 2008. 112(3): p. 914–5. [DOI] [PubMed] [Google Scholar]
- 43.Guma M, et al. , Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood, 2004. 104(12): p. 3664–71. [DOI] [PubMed] [Google Scholar]
- 44.Beziat V, et al. , NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood, 2013. 121(14): p. 2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser BK, et al. , Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci U S A, 2008. 105(18): p. 6696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Triebel F, et al. , LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med, 1990. 171(5): p. 1393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, et al. , Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell, 2019. 176(1–2): p. 334–347 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wherry EJ, et al. , Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol, 2003. 77(8): p. 4911–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merino A, et al. , Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J Clin Invest, 2019. 130: p. 3770–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapaport AS, et al. , The Inhibitory Receptor NKG2A Sustains Virus-Specific CD8(+) T Cells in Response to a Lethal Poxvirus Infection. Immunity, 2015. 43(6): p. 1112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bengsch B, et al. , Deep immune profiling by mass cytometry links human T and NK cell differentiation and cytotoxic molecule expression patterns. J Immunol Methods, 2018. 453: p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philip M, et al. , Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature, 2017. 545(7655): p. 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghoneim HE, et al. , De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell, 2017. 170(1): p. 142–157 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delpoux A, et al. , Continuous activity of Foxo1 is required to prevent anergy and maintain the memory state of CD8(+) T cells. J Exp Med, 2018. 215(2): p. 575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng X, et al. , Foxp1 is an essential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood, 2010. 115(3): p. 510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng X, et al. , Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol, 2011. 12(6): p. 544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duruisseaux M, et al. , Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir Med, 2018. 6(10): p. 771–781. [DOI] [PubMed] [Google Scholar]
- 58.Stephen TL, et al. , SATB1 Expression Governs Epigenetic Repression of PD-1 in Tumor-Reactive T Cells. Immunity, 2017. 46(1): p. 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Cruz LM, et al. , An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol, 2010. 11(3): p. 240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J, et al. , Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity, 1999. 10(3): p. 345–55. [DOI] [PubMed] [Google Scholar]
- 61.Martinez GJ, et al. , The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity, 2015. 42(2): p. 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aliahmad P, et al. , TOX provides a link between calcineurin activation and CD8 lineage commitment. J Exp Med, 2004. 199(8): p. 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pozner A, et al. , The C-Terminal Zinc Fingers of ZBTB38 are Novel Selective Readers of DNA Methylation. J Mol Biol, 2018. 430(3): p. 258–271. [DOI] [PubMed] [Google Scholar]
- 64.Zuhair M, et al. , Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol, 2019. 29(3): p. e2034. [DOI] [PubMed] [Google Scholar]
- 65.Bezman NA, et al. , Molecular definition of the identity and activation of natural killer cells. Nat Immunol, 2012. 13(10): p. 1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benson DM Jr., et al. , The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood, 2010. 116(13): p. 2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vari F, et al. , Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood, 2018. 131(16): p. 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacFarlane A.W.t., et al. , PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res, 2014. 2(4): p. 320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liou AK, et al. , Elevated IL18 levels in Nasopharyngeal carcinoma induced PD-1 expression on NK cells in TILS leading to poor prognosis. Oral Oncol, 2020. 104: p. 104616. [DOI] [PubMed] [Google Scholar]
- 70.Pesce S, et al. , Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol, 2017. 139(1): p. 335–346 e3. [DOI] [PubMed] [Google Scholar]
- 71.Beldi-Ferchiou A, et al. , PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget, 2016. 7(45): p. 72961–72977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiesmayr S, et al. , Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur J Immunol, 2012. 42(2): p. 541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mariotti FR, et al. , PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology, 2019. 8(3): p. 1557030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Judge SJ, et al. , Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J Clin Invest, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caumartin J, et al. , Trogocytosis-based generation of suppressive NK cells. EMBO J, 2007. 26(5): p. 1423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miner CA, et al. , Acquisition of activation receptor ligand by trogocytosis renders NK cells hyporesponsive. J Immunol, 2015. 194(4): p. 1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balsamo M, et al. , Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A, 2009. 106(49): p. 20847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li T, et al. , Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett, 2012. 318(2): p. 154–61. [DOI] [PubMed] [Google Scholar]
- 79.Li T, et al. , Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol, 2013. 30(3): p. 663. [DOI] [PubMed] [Google Scholar]
- 80.Hoechst B, et al. , Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology, 2009. 50(3): p. 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Q, et al. , Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol, 2018. 19(7): p. 723–732. [DOI] [PubMed] [Google Scholar]
- 82.Hsu J, et al. , Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest, 2018. 128(10): p. 4654–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lieberman NAP, et al. , An Uncoupling of Canonical Phenotypic Markers and Functional Potency of Ex Vivo-Expanded Natural Killer Cells. Front Immunol, 2018. 9: p. 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, et al. , Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene, 2017. 36(44): p. 6143–6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.da Silva IP, et al. , Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res, 2014. 2(5): p. 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]