Abstract
Single-tensor diffusion imaging (DTI) has traditionally been used to assess integrity of white matter. For example, we previously showed that integrity of limbic white matter tracts declines in healthy aging and relates to episodic memory performance. However, multi-compartment diffusion models may be more informative about microstructural properties of gray matter. The current study examined hippocampal gray matter integrity using both single-tensor and multi-compartment (neurite orientation dispersion and density imaging, NODDI) diffusion imaging. Younger (20–38 years) and older (59–84 years) adults also completed the Mnemonic Similarity Task to measure mnemonic discrimination performance. Results revealed age-related declines in both single-tensor (lower fractional anisotropy, higher mean diffusivity) and multi-compartment (higher restricted, hindered and free diffusion) measures of hippocampal gray matter integrity. As expected, NODDI measures (hindered and free diffusion) captured more age-related variance than DTI measures. Moreover, mnemonic discrimination of highly similar lure items in memory was related to hippocampal gray matter integrity in younger but not older adults. These findings support the notion that age-related differences in gray matter integrity are better captured by multi-compartment versus single-tensor diffusion models and show that the relationship between mnemonic discrimination and hippocampal gray matter integrity is moderated by age.
Keywords: Diffusion imaging, Aging, NODDI, Hippocampus
1. Introduction
The hippocampus, which is critical for episodic memory, is known to be affected in healthy aging (Lister and Barnes, 2009; Scahill et al., 2003), even in absence of dementia (Park and Reuter-Lorenz, 2009). Structural neuroimaging studies, for example, have shown age-related declines in hippocampal macrostructure, with decreased volume seen in whole hippocampus in older adults relative to younger adults (Doxey and Kirwan, 2015; Raz et al., 2005). In the last decade, diffusion imaging has allowed for in vivo examinations of neural microstructure, with numerous studies reporting age-related differences in the integrity of white matter (de Lange et al., 2016; Gunning-Dixon et al., 2009; Madden et al., 2012), including white matter tracts projecting to and from the hippocampus (e.g., fornix, cingulum; Bennett et al., 2015; Bennett and Stark, 2016). However, few studies have assessed whether diffusion imaging may also be a promising tool for evaluating microstructural properties of hippocampal gray matter in aging, especially as it relates to episodic memory performance.
Diffusion imaging data is traditionally modeled as a single tensor per voxel that summarizes the rate of molecular water diffusion along three axes (diffusion tensor imaging, DTI; Beaulieu, 2002; Hassan et al., 2014). This single-tensor DTI approach yields metrics, such as the degree of restricted diffusion (fractional anisotropy, FA) and average rate of diffusion (mean diffusivity, MD), from which the integrity of underlying tissue can be inferred. In white matter, for example, higher FA and lower MD would be seen in regions with highly aligned, densely packed, and tightly myelinated axonal fibers. Across the lifespan, decreases in FA and increases in MD (Bennett et al., 2015; Gunning-Dixon et al., 2009; Madden et al., 2012) are interpreted as declines in white matter integrity (e.g., age-related demyelination). In gray matter, however, the underlying tissue is relatively less organized (e.g., dendrites, cell bodies, glia), resulting in lower FA and higher MD than white matter. Owing to this microstructural complexity, the single-tensor approach alone may not be suited for accurately modeling diffusion in gray matter.
A potentially more accurate way to assess microstructural properties of gray matter is with multi-compartment diffusion approaches that separately model different sources (compartments or volume fractions) of the total diffusion signal (Fukutomi et al., 2018; Kaden et al., 2016; Rae et al., 2017). Neurite Orientation Dispersion and Density Imaging (NODDI; Zhang et al., 2012), for example, models restricted diffusion (also known as neurite density index; NDI) as a set of sticks, hindered diffusion (also known as orientation dispersion index; ODI) as the dispersion of the sticks, and unrestricted diffusion (also known as isotropic fraction; fISO) as an isotropic sphere (Fukutomi et al., 2018; Rae et al., 2017; Zhang et al., 2012). Differences in these metrics may result from microstructural properties that affect intracellular, extracellular, and free sources of diffusion (e.g., age-related increases in cell swelling, loss of spines or synaptic remodeling, and vascular permeability; Clarke et al., 2018; Dickstein et al., 2013, Szebenyi et al., 2005; Elahy et al., 2015, respectively). An additional advantage of this multi-compartment approach is that the free diffusion metric can be used to account for free diffusion contamination in remaining integrity metrics, which is prevalent in the aging brain (Chad et al., 2018; Metzler-Baddeley, O’Sullivan, Bells, Pasternak and Jones, 2012; Rathi et al., 2014).
Multiple diffusion imaging studies have examined the effect of aging on gray matter integrity using either single-tensor (Bhagat and Beaulieu, 2004; Càmara et al., 2007; Carlesimo et al., 2010; Cherubini et al., 2009; Den Heijer et al., 2012; Pereira et al., 2014; Pfefferbaum et al., 2010; Rathi et al., 2014; Salminen et al., 2016; Sasson et al., 2012) or multi-compartment (Fukutomi et al., 2018; Kaden et al., 2016; Nazeri et al., 2017) approaches, but only a handful have assessed aging of hippocampal gray matter integrity. Using the single-tensor approach, studies have reported age-related increases in hippocampal MD (Carlesimo et al., 2010; Pereira et al., 2014), no change (Cherubini et al., 2009) or mixed results depending on the region profiled (Pfefferbaum et al., 2010; Salminen et al., 2016). After excluding free diffusion (e.g., using cerebrospinal fluid [CSF]-suppression diffusion imaging or region of interest [ROI] based segmentation), DTI studies have found both age-related increases in hippocampal FA (Rathi et al., 2014) and age-related decrease in anterior hippocampal relative anisotropy (Càmara et al., 2007). Using the NODDI multi-compartment approach, at least one study demonstrated that hindered diffusion within bilateral hippocampus increased with age in adults across a lifespan sample (age 21–84; Nazeri et al., 2015). However, because none of these studies directly compared single-tensor and multi-compartment models, it remains unknown whether these age differences in DTI and NODDI metrics are capturing similar microstructural mechanisms within hippocampus.
The functional relevance of hippocampal gray matter integrity in non-demented older adults also remains understudied. Previous single-tensor DTI studies have reported that hippocampal MD was associated with impaired episodic memory assessed by a list learning (Den Heijer et al., 2012) and visuospatial task (Carlesimo et al., 2010). An important component of successful episodic memory is mnemonic discrimination, the ability to discriminate between highly similar events in memory. Using a modified recognition task, the Mnemonic Similarity Task (MST; Stark et al., 2013, Kirwan and Stark, 2007), our group has previously shown that mnemonic discrimination declines in healthy aging (Stark, Yassa, Lacy and Stark, 2013) and that worse discrimination performance is related to lower integrity of white matter tracts projecting to (perforant path; Bennett and Stark, 2016; Yassa et al., 2010) and emanating from (fornix; Bennett et al., 2015) the hippocampus in adults across the lifespan. However, these effects have not been assessed for hippocampal gray matter integrity using either single-tensor or multi-compartment diffusion metrics.
Building on this work, the current study examined hippocampal gray matter integrity using both single-tensor (DTI) and multi-compartment (NODDI) diffusion modeling of the same diffusion data in younger and older adults (20–38 and 59–84 years, respectively) who also completed the MST. Our primary aim was to assess age-related differences in hippocampal gray matter integrity and in particular whether the multi-compartment diffusion approach was more sensitive to hippocampal aging than the single-tensor approach. To assess whether free diffusion influences traditional integrity metrics (e.g., from partial volume effects with adjacent CSF), the effect of age on single-tensor integrity measures were examined before (unthresholded DTI) and after (thresholded DTI) accounting for the NODDI free diffusion compartment. Our secondary aim was to determine whether hippocampal gray matter integrity relates to mnemonic discrimination performance.
2. Materials and methods
2.1. Participants
Fifty-one adults were recruited from the University of California, Irvine and surrounding Orange County neighborhoods. One older participant was excluded for poor general cognition (Mini-Mental State Exam [MMSE] < 28; Folstein et al., 1975) and one young participant was excluded for neuroimaging segmentation errors. The final sample included 24 younger (20–38 years, 27.6 ± 5.1 years, 12 females) and 25 older (59–84 years, 69.9 ± 5.31 years, 14 females) adults. The final sample of 24 younger and 25 older adults were used for all analyses except the behavioral analysis as detailed below.
All individuals provided informed consent prior to participation in this study. The University of California, Irvine Institutional Review Board (IRB) approved the experimental procedures and participants were compensated for their time.
2.2. Neuropsychological battery
To characterize their cognitive profiles, participants underwent a battery of neuropsychological tests including the MMSE to assess general cognition; Rey Auditory Verbal Learning Test (RAVLT) to assess recall and recognition (Rey, 1941); Geriatric Depression Scale (GDS) and Beck Depression Index (BDI) to assess depression status (Yesavage et al., 1982, Beck et al., 1961); Trails A and B, Stroop test and Letter Number Sequencing to assess executive functioning (Reitan and Wolfson, 1985, Stroop, 1935, and Wechsler, 1997a); Digit Span to assess working memory (Wechsler, 1997a); and Physical Activity Scale for the Elderly (PASE) to assess overall activity level (Washburn et al., 1993). These data are presented in Table 1.
Table 1.
Demographic and neuropsychological data
| Younger | Older | Group Comparisons [t (p)] | |
|---|---|---|---|
| Demographics | |||
| N | 24 | 25 | |
| Mean Age | 27.6 ± 5.1 | 70.4 ± 5.9 | |
| Education | 16.3 ± 2.3 | 17.4 ± 1.7 | |
| Neuropsychological | |||
| Tests | |||
| MMSE | 29.6 ± 0.7 | 29.5 ± 0.7 | −0.34 (0.736) |
| RAVLT Total | 62.0 ± 6.4 | 55.3 ± 7.9 | −3.26 (0.002) |
| RAVLT Immediate | 13.8 ± 1.4 | 12.3 ± 2.4 | −2.76 (0.008) |
| RAVLT Delay | 13.9 ± 1.2 | 12.1 ± 2.6 | −3.12(0.003) |
| GDS | 2.0 ± 1.9 | 0.5 ± 1.0 | −3.45 (0.001) |
| BDI | 3.7 ± 3.9 | 2.4 ± 2.4 | −1.34 (0.185) |
| Trails A | 17.7 ± 5.1 | 25.0 ± 8.0 | 3.81 (0.001) |
| Trails B | 48.8 ± 13.0 | 66.9 ± 22.1 | 3.49 (0.001) |
| Stroop Raw | 112.8 ± 12.8 | 98.1 ± 18.1 | − 3.29 (0.002) |
| Digit Span Total | 20.4 ± 4.4 | 18.4 ± 3.7 | −1.73(0.091) |
| PASE | 180.9 ± 57.2 | 138.8 ± 48.4 | −2.78 (0.008) |
Note. Neuropsychological test scores (mean ± standard deviation) are presented separately for younger and older adults. Significant between-group differences (Bonferroni corrected for 11 comparisons, p < 0.005 are indicated in bolded text. Key: MMSE, Mini-Mental State Examination; RAVLT, Ray Auditory Verbal Learning Task; GDS, Geriatric Depression Scale; BDI, Beck Depression Inventory; PASE, physical activity scale for the elderly.
2.3. Mnemonic Similarity Task
Mnemonic discrimination was assessed using the Mnemonic Similarity Task (MST; see Stark et al., 2013 for additional details). In separate incidental study and test phases, participants viewed a series of common objects (e.g., rubber duck, piano) in color on a white background. During the study phase, participants judged whether each object belongs “indoors” or “outdoors” via button press. During the test phase, participants judged whether objects were repeated from the study phase (target), similar to objects from the study phase (lure), or completely new (novel) using “old”, “similar” or “new” responses, respectively. Mnemonic discrimination was assessed using the Lure Discrimination Index (LDI), calculated as the probability of correctly judging lures as “similar” after accounting for any bias in using the “similar” response: LDI = p(“similar”|lure) – p(“similar”|novel). Additionally, Recognition was calculated as the probability of correctly judging targets as “old” after accounting for any bias in using the “old” response: Recognition = p(“old”|target) – p(“old”|novel). Some participants were excluded from the MST analysis if they had a large number of omitted responses (>80% of trials; 4 younger adults) or poor Recognition (>2 SD from the overall mean; 3 younger, 1 older adult).
3. Neuroimaging protocol
3.1. Image acquisition
Participants were scanned using a Philips Achieva 3.0 Tesla MRI system at the University of California, Irvine using an 8-channel SENSE receive only head coil and fitted padding to minimize head movements.
A single T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) scan was acquired using the following parameters: time repetition (TR)/time echo (TE) = 11/4.6 ms, field of view (FOV) = 240 × 231 mm, flip angle = 18°, 200 sagittal slices, and 0.75 mm3 spatial resolution.
Three diffusion-weighted scans were acquired for each of four gradient values (b = 500,1000, 2000 and 2500 s/mm2). For each of the 12 scans, gradients were applied in 10 orthogonal directions, with one image having no diffusion weighting (b = 0). This yielded a total of 120 diffusion-weighted and 12 non-diffusion-weighted images. The following parameters were used for all 12 scans: TR/TE = 2174–2734/94 ms, FOV = 128 × 128 mm, 80 axial slices, 1.69 mm3 spatial resolution, and the total scan time was approximately 50minutes per subject.
3.2. Region of interest segmentation
The hippocampus was defined on each participant’s MP-RAGE using FMRIB Software Library (FSL) Integrated Registration and Segmentation Tool (FIRST; Patenaude et al., 2011), which automatically segmented bilateral hippocampus using shape/appearance models with default boundary correction. The 3-stage affine registration was used to improve segmentation compared to the default FIRST settings. In the first stage, the subject’s MP-RAGE image is aligned to standard space (Montreal Neurological Institute; MNI) using an affine transformation. In the second stage, this transformation is linearly aligned to a subcortical mask in MNI space. In the third stage, a dilated hippocampal mask is aligned to refine the registration. Quality control of this segmentation, which included checks for coverage limited to the hippocampal gray matter region and allowing no more than a 1–2 voxel shift of the mask into the surrounding areas, were done by a trained researcher blinded to participant age and did not yield notable age differences.
3.3. Diffusion data processing
For each participant, all diffusion data were pre-processed using Analysis of Functional NeuroImages (AFNI) to remove non-brain tissue and generate a whole brain mask from the first non-diffusion weighted image (b0 image), and Advanced Normalization Tools (ANTs, Avants et al., 2009) to correct for gross motion by aligning all diffusion images to the b0 image. This preprocessed data from all diffusion scans (30 orthogonal directions for each of four gradient values) were used as input for both the single-tensor and NODDI analyses. Although these data were not corrected for bias field distortions, we replicated the age effects of interest in a separate sample with this correction (see Supplementary Material).
Single-tensor DTI analyses were completed using FSL dtifit. A single diffusion tensor was estimated at each voxel within a whole brain mask. The output included voxel-wise images for FA and MD.
Multi-compartment NODDI analyses were completed using the default settings in the NODDI matlab toolbox (http://mig.cs.ucl.ac.uk/index.php?n=Tutorial.NODDImatlab). The diffusion signal for each voxel was separated into restricted, hindered, and free diffusion compartments using a two-stage approach (Tariq et al., 2016; Zhang et al., 2012). In the first stage, the total signal is separated into non-free and free diffusion sources of diffusion, with the latter being modeled as an isotropic sphere (also known as fISO). In the second stage, the remaining signal is then separated into restricted and hindered source of diffusion, modeled as a set of sticks (also known as NDI) and the dispersion of the sticks (also known as ODI), respectively. Restricted (or Gaussian) diffusion occurs when the movement of water molecules is constrained by the presence of impermeable barriers, whereas hindered (or non-Gaussian) diffusion occurs when their movement is constrained by the presence of partially permeable barriers (Martin, 2013; Morozov et al., 2020; Raja et al., 2019). Thus, modeling restricted diffusion as a set of sticks is intended to capture restricted diffusion within neurons and glia (i.e., intracellular diffusion). Modeling hindered diffusion as dispersion of those sticks is intended to capture hindered diffusion around those structures (i.e., extracellular diffusion). The output included voxel-wise images for restricted, hindered, and free diffusion. The scale of all NODDI measures range from 0–1.
To extract hippocampal gray matter integrity metrics for each participant, their MP-RAGE was linearly aligned to their b0 image using FSL’s flirt command. This transformation was then applied to align the FIRST segmented bilateral hippocampus to diffusion space. Quality control of this co-registration was completed as outlined above and did not yield notable age group differences. The aligned segmentations were binarized, creating a bilateral hippocampus mask. Unthresholded FA and MD metrics were obtained by multiplying the bilateral hippocampus mask by the corresponding voxel-wise DTI image and then taking the average across voxels. Free diffusion was obtained by multiplying the bilateral hippocampus mask by the corresponding voxel-wise NODDI image and then taking the average across voxels. Remaining diffusion metrics were limited to voxels with sufficient cellular fraction (>10%) by excluding voxels with free diffusion >0.9 from the bilateral hippocampus mask. Measures of restricted and hindered diffusion were obtained by multiplying this thresholded bilateral hippocampus mask by the corresponding voxel-wise NODDI images and then taking the average across voxels. Finally, to assess the effect of free water on DTI measures, thresholded FA and MD were obtained by multiplying the thresholded hippocampus mask by the corresponding voxel-wise DTI image and then taking the average across voxels.
3.4. Statistical analyses
All statistical analyses were run using Prism (Version 7.0 d; GraphPad Software, La Jolla California USA), except for the logistic regression run using SPSS (Version 24.0; IBM, Armonk, NY, USA). For all analyses, the significance threshold was set to p < 0.05.
Age group differences in single-tensor DTI (FA, MD) and multi-compartment NODDI (restricted, hindered, free diffusion) metrics were assessed using separate independent sample t-tests. The effect of free diffusion on each DTI metric was assessed using an Age Group (younger, older) × Thresholding (thresholded, unthresholded) ANOVA, with Age Group as a between-subject variable, and Thresholding as a within-subject variable. The ability of single-tensor and multi-compartment diffusion approaches to capture age-related variance were compared using a forward selection likelihood ratio (LR) logistic regression. The dependent variable was dichotomized age groups and independent variables were the unthresholded DTI and NODDI metrics. Variables were entered using the Forward Selection (LR) option in SPSS in which variables are entered in a step-wise manner based on the significance of the score statistic.
The moderating effect of age group on these relationships were assessed using separate linear regressions for each integrity metric (see Baron and Kenny, 1986). Relationships between hippocampal integrity and MST performance were assessed separately in each age group using linear regressions.
4. Results
4.1. Neuropsychological test performance
Age group differences in cognition were assessed using separate independent sample t-tests for each neuropsychological test (see Table 1). Results followed the expected pattern for healthy aging, with older adults performing worse than younger adults on measures of episodic memory (RAVLT, t(47) = −3.26, p < 0.002) and executive function (Trails B, t(47) = 3.49, p < 0.001; Stroop, t(47) = −3.29, p < 0.002), but not general cognition (MMSE). Although we screened for neurological conditions including Mild Cognitive Impairment (MCI) and excluded participants with low general cognitions (MMSE <28), we acknowledge that some older adults with preclinical dementia may be present in the sample.
4.2. Age group differences for unthresholded single-tensor metrics
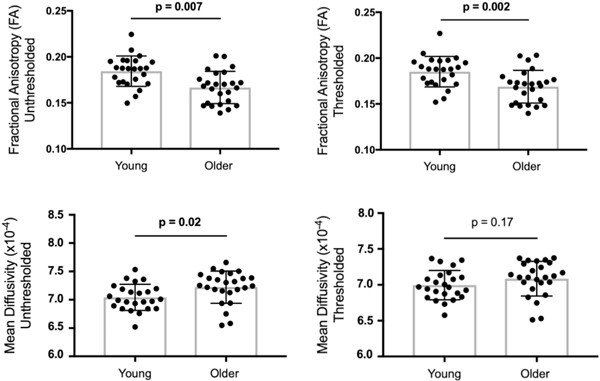
Age group differences in hippocampal gray matter integrity were first assessed for traditional single-tensor DTI measures (unthresholded FA, unthresholded MD). Across separate independent sample t-tests, significant age group differences were observed, with older adults (0.17 ± 0.02) showing lower unthresholded FA than younger adults (0.18 ± 0.02), t(47) = 3.64, p < 0.001, d = 0.5. Effects were also significant for unthresholded MD (younger: 0.0007 ± 0.00002, older: 0.0007 ± 0.00003), t(47) = 2.44, p < 0.02, d = 0.0 (see Fig. 1).
Fig. 1.
Age group differences in single-tensor DTI measures of hippocampal gray matter integrity shown separately for unthresholded (top row) and thresholded (bottom row) measures of fractional anisotropy and mean diffusivity. Group differences were significant for FA, thresholded FA and MD. Thresholded MD did not show significant differences.
4.3. Age group differences for thresholded single-tensor metrics
Age group differences in hippocampal gray matter integrity were next assessed for single-tensor DTI measures that were thresholded to exclude voxels with excessively high NODDI free diffusion (thresholded FA, thresholded MD). Across separate independent sample t-tests, significant age group differences were observed, with older adults (0.17 ± 0.02) showing lower FA than younger adults (0.19 ± 0.02), t(47) = 3.32, p < 0.002, d = 1.00. The difference for thresholded MD was not significant, t(47) = 1.40, p < 0.167, d = 0.0 (see Fig. 1).
4.4. Effect of thresholding single-tensor metrics
To assess whether accounting for free diffusion by thresholding the DTI metrics had an effect on the aforementioned age group differences in hippocampal gray matter integrity, separate Age Group (younger, older) × Thresholding (thresholded, unthresholded) ANOVAs were conducted for each metric. As expected, results revealed significant main effects of Age Group for FA, F(1, 47) = 12.11, p < 0.002, but not MD, p > 0.05. Significant main effects of Thresholding were seen for both FA, F(1, 47) = 31.92, p < 0.0001, and MD, F(1, 47) = 60.68, p < 0.0001. Most importantly, significant age group × thresholding interactions for FA, F(1, 47) = 7.18, p < 0.02, and MD, F(1, 47) = 14.96, p < 0.001, revealed that age group differences were larger for unthresholded versus thresholded DTI metrics.
4.5. Age group differences for multi-compartment metrics
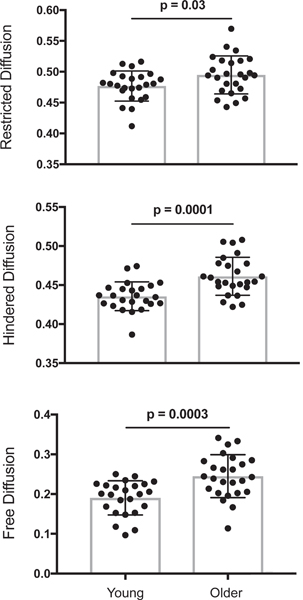
Finally, age group differences in hippocampal gray matter integrity were assessed for multi-compartment NODDI measures (restricted, hindered, and free diffusion). Across separate independent sample t-tests, significant age group differences were observed, with older adults showing higher restricted (0.49 ± 0.03), t(47) = 2.26, p < 0.03, d = 0.39, hindered (0.46 ± 0.02), t(47) = 4.14, p < 0.001, d = 1.00, and free (0.34 ± 0.06), t(47) = 3.24, p < 0.003, d = 1.27 diffusion compared to younger adults (0.48 ± 0.02 restricted, 0.44 ± 0.02 hindered, 0.27 ± 0.05 free diffusion; see Fig. 2).
Fig. 2.
Age group differences in multi-compartment NODDI measures of hippocampal gray matter integrity shown separately for restricted (top), hindered (middle) and free diffusion (bottom). Group differences were significant for restricted, hindered and free diffusion.
4.6. Multi-compartment integrity metrics account for more age-related variance than single-tensor metrics
To determine whether multi-compartment integrity measures account for more age-related variance in hippocampal gray matter integrity than traditional single-tensor measures, DTI (unthresholded FA and MD) and NODDI (restricted, hindered, free diffusion) integrity measures were entered into a forward (LR) logistic regression. Results revealed that the single best predictor of age group was hindered diffusion, Nagelkerke r2 = 0.366, p < 0.003. At the second step, adding free diffusion significantly increased the total explained variance, Nagelkerke r2 = 0.643, p < 0.002. Adding a third predictor did not lead to a significant increase in variance explained by the model. Thus, traditional DTI measures (FA, MD) were not included in the best model. These age effects were replicated in a separate dataset of younger and older adults (see Supplemental Materials).
4.7. Age effects for mnemonic discrimination
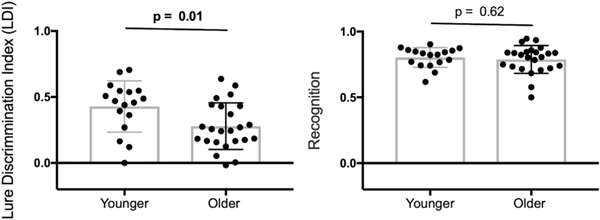
Age group differences in MST performance were assessed using separate independent sample t-tests for the LDI and recognition measures. Consistent with previous work (Stark, Yassa, Lacy and Stark, 2013), results revealed that LDI was significantly reduced in older (0.28 ± 0.05) compared to younger (0.43 ± 0.04) adults, t(39) = −2.58, p < 0.015, d = 3.31, whereas recognition did not significantly differ between age groups (younger: 0.81 ± 0.07, older: 0.79 ± 0.11,), t(39) = −0.51, p > 0.60, d = 0.22 (see Fig. 3).
Fig. 3.
Age group differences in Mnemonic Similarity Task performance shown separately for mnemonic discrimination (left) and recognition memory (right). Group differences were significant for mnemonic discrimination performance but not recognition.
4.8. Mnemonic discrimination-hippocampal gray matter integrity relationships
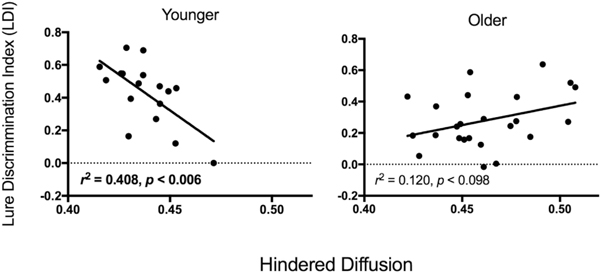
Regression analysis assessed whether age group was a moderator of the relationship between mnemonic discrimination and hippocampal integrity. Analyses were limited to the NODDI measures of hippocampal integrity that were most sensitive to age in the stepwise logistic regression analyses (hindered, free diffusion). Age group, diffusion, and the age group × diffusion interaction were used as predictor variables and LDI as the outcome variable (see Baron and Kenny, 1986). Results revealed that the interaction term was significant for hindered diffusion (β = 13.2, p < 0.002) indicating that this relationship was significantly stronger for younger compared to older adults. Follow-up regression analyses in each age group revealed that LDI was significantly related to hippocampal hindered diffusion in younger adults, such that lower hindered diffusion was correlated with better mnemonic discrimination performance, r2 = 0.41, p < 0.006 (see Fig. 4). In older adults, the relationship between hindered diffusion and mnemonic discrimination performance did not reach significance, r2 = 0.12, p < 0.09 (see Fig. 4). Relationships between recognition and hippocampal integrity also did not approach significance, ps > 0.3.
Fig. 4.
Relationships between mnemonic discrimination performance and NODDI hindered diffusion in hippocampus are shown separately for younger (left) and older (right) adults. For younger adults, hindered diffusion was significantly related to mnemonic discrimination (two-tailed).
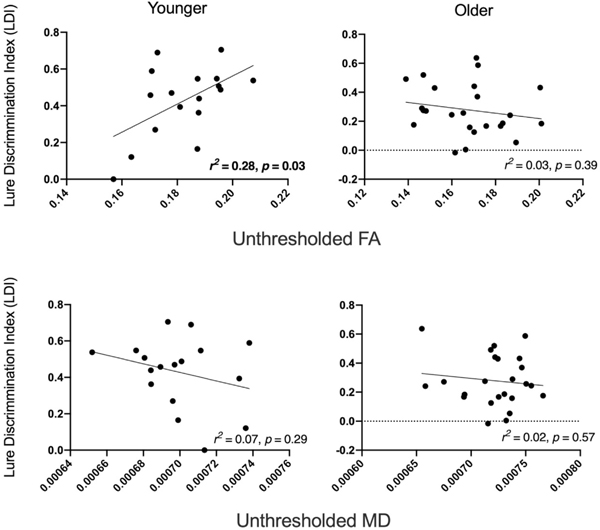
For comparison, analyses were also run to assess whether age group was a moderator of the relationship between mnemonic discrimination and hippocampal integrity for unthresholded DTI measures (FA, MD). Age group, diffusion, and the age group × diffusion interaction were used as predictor variables and LDI as the outcome variable. Results revealed that the interaction term was significant for FA (β = −4.1, p < 0.02) indicating that this relationship was significantly stronger for younger compared to older adults. The interaction term for MD and free diffusion did not approach significance (p > 0.41). Follow-up regression analyses in each age group revealed that LDI was significantly related to hippocampal FA in younger adults, such that higher FA was correlated with better mnemonic discrimination performance, r2 = 0.28, p < 0.03 (see Fig. 5). In older adults, the relationship between FA and mnemonic discrimination performance did not reach significance, r2 = 0.03, p < 0.40 (see Fig. 5). Relationships between MD and LDI also did not approach significance ps > 0.28.
Fig. 5.
Relationships between mnemonic discrimination performance and DTI measures in hippocampus are shown separately for younger (left) and older (right) adults. For younger adults, FA, the relationship integrity and mnemonic discrimination approached significance (two-tailed).
To compare whether NODDI (hindered diffusion) or DTI (FA) measures better captured mnemonic discrimination performance within younger adults, Steiger’s Z test (Steiger, 1980) was used to compare the previously mentioned significant relationships. Results revealed that the relationship between hippocampal hindered diffusion and LDI was significantly stronger than the relationship between hippocampal FA and LDI, Z = −2.92, p < 0.004.
5. Discussion
The current study aimed to directly compare the sensitivity of single-tensor (DTI) and multi-compartment (NODDI) diffusion measures as they relate to age within hippocampal gray matter and to assess whether these measures predict episodic memory performance. Results revealed several major findings, each of which will be discussed in more detail below. First, we demonstrated that thresholding DTI metrics (FA, MD) to account for free diffusion significantly attenuates the effect of age on hippocampal gray matter integrity. Second, we showed that NODDI metrics (hindered and free diffusion) account for more age-related variance in hippocampal gray matter integrity than DTI metrics. These findings were replicated in a separate dataset (see Supplementary Material), highlighting the robustness of these age effects in light of different diffusion acquisition parameters and preprocessing steps. Third, we found a moderating effect of age group on the relationship between hippocampal gray matter integrity and mnemonic discrimination, such that lower hindered diffusion was related to better discrimination performance in younger but not older adults.
Traditional, single-tensor DTI was sensitive to age-related differences in hippocampal gray matter integrity. Older adults had significantly lower unthresholded FA and higher unthresholded MD than younger adults in bilateral hippocampus, consistent with an earlier report of similar effects for MD, albeit within an older sample (age 55–90; Den Heijer et al., 2012). Whereas age-related increases in MD are consistently reported for white matter (Head et al., 2004, Hugenschmidt et al., 2008, Salat et al., 2005), the findings are more mixed for gray matter. That is, some gray matter studies find age-related increase in MD (Carlesimo et al., 2010; Den Heijer et al., 2012; Pereira et al., 2014), others find no age difference (Cherubini et al., 2009), and yet other find mixed results depending on the region (Pfefferbaum et al., 2010; Salminen et al., 2016). Inferences that can be drawn about the neural substrates underlying differences in these scalar measures are limited (e.g., see Wheeler-Kingshott and Cercignani, 2009). As in white matter, age-related decreases in hippocampal gray matter FA may result from degradation of the underlying tissue (e.g., loss of dendrites), reorganization of tissue (e.g., differences in dendritic layout), or some combination of the 2; whereas age-related increases in hippocampal gray matter MD may indicate a loss of underlying tissue or an expansion of non-tissue space. Relative to the largely aligned microstructure in white matter (e.g., axons, neurofilaments), the organization of gray matter microstructure (e.g., dendrites, cell bodies, glia) is less coherent. We argue that, although both FA and MD were sensitive to age effects, the microstructural complexity of gray matter is not adequately captured by single-tensor diffusion models.
Our results further revealed that age-related differences in hippocampal gray matter integrity measured using single-tensor DTI were attenuated after accounting for free diffusion. Consistent with an earlier report using relative anisotropy (Càmara et al., 2007), older adults showed significantly lower hippocampal FA than younger adults after accounting for the CSF fraction. We observed no significant difference in thresholded MD, in bilateral hippocampus. Importantly, age group differences for our thresholded DTI measures were significantly smaller than for the unthresholded measures. By directly comparing age effects for the unthresholded and thresholded measures, our study revealed that single-tensor DTI measures are significantly influenced by the presence of free diffusion, which may originate from partial volume effects with cerebrospinal fluid in nearby ventricles (Chad et al., 2018; Jeon et al., 2012; Metzler-Baddeley et al., 2012; Tohka, 2014) or cellular shrinkage or neurodegeneration (Ofori et al., 2015; Albi et al., 2016). Thus, rather than solely capturing the integrity of underlying gray matter tissue, MD in particular may be more sensitive to differences in free diffusion as evidenced by the lack of significant age-group differences after thresholding for free water.
Multi-compartment NODDI was also sensitive to age-related differences in hippocampal gray matter integrity, outperforming the ability of single-tensor DTI measures to capture these age effects. Older adults had significantly higher restricted, hindered and free diffusion in bilateral hippocampus relative to younger adults. Of note, these effects survived after controlling for volume (data not shown). A similar finding was previously reported for hindered diffusion using a lifespan sample (Nazeri et al., 2015). However, free diffusion was not assessed in that study, or controlled for in the other diffusion compartments, as was done here. Although speculative, potential mechanisms for these age-related increases in hindered and free diffusion are neurodegeneration (e.g., age-related loss of apical dendrites, Dickstein et al., 2013), loss of support cells like microglia (Robillard et al., 2016), and increases in blood-brain barrier permeability (Elahy et al., 2015; Oakley and Tharakan, 2014). Younger adult brains are less likely to be affected by neurodegeneration but may be impacted by other cellular mechanisms (e.g., remodeling of synapses; Szebenyi et al., 2005, astrocyte activity; Hansson and Rönnbäck, 1995) which may also impact measures of hindered and free diffusion. The lack of an age-related decline in restricted diffusion however is consistent with evidence that normal aging is not accompanied by a loss of hippocampal neurons (Freeman et al., 2008). Importantly, when single-tensor DTI and multi-compartment NODDI measures were included in the same regression model, hindered diffusion was the strongest predictor of age followed by free diffusion, whereas no DTI measures survived in the model. This direct comparison supports the notion that NODDI and DTI are capturing different properties of hippocampal aging, and that these complex gray matter microstructures are best modeled using multiple NODDI diffusion compartments. Hippocampal NODDI metrics used here may serve as important biomarkers for normal brain aging and cognitive aging. In particular, future studies could build on this work to parse out if specific NODDI measures are sensitive to pathological aging such as MCI and Alzheimer’s Disease.
The functional relevance of multi-compartment NODDI was further supported by finding that age moderated the relationship between hippocampal gray matter integrity and mnemonic discrimination performance. Results revealed that decreased hindered diffusion within bilateral hippocampus was a significant predictor of better mnemonic discrimination in younger adults, but not older adults. This relationship was significantly stronger for hindered diffusion than FA, suggesting NODDI measures may be more informative for tracking cognition across a lifespan. However, these results were not replicated in a separate dataset that used a slightly different version of the MST, a younger sample that had a very restricted age range, and differences in imaging acquisition and analysis (see Supplementary Material), suggesting that additional research is needed to explore these age-brain-behavior relationships. Nonetheless, one interpretation of the age moderation is that, as a result of age-related degradation of the hippocampus, older adults may be relying less on this brain region, or on different brain regions, to perform the task relative to younger adult (Madden et al., 2004; Reuter-Lorenz, 2002). Support for this view will require longitudinal studies, which have not yet shown how brain-behavior relationships evolve over the lifespan. However, they do find that cognition and integrity later in life is predicted by early life cognition (Deary et al., 2006; Valdés Hernández et al., 2013; Wardlaw et al., 2011), indicating that the significant relationship between hippocampus integrity and mnemonic discrimination in young adults may inform the absence of this relationship in aging.
It is worth noting that some limitations to this study may contribute to the current findings. Most importantly, some model parameters for NODDI may be better suited for modeling diffusion in white matter compared to gray matter. For example, if the intrinsic diffusivity measure used to estimate diffusion within neurites and extracellular space is assumed to be lower than the true value in gray matter, this could weaken age-group differences in hindered diffusion (for more discussion see Guerrero et al., 2019). However, other parameters of the NODDI model were specifically designed to model hindered and restricted diffusion in gray matter (e.g., mean orientation of Watson distribution (μ), and the axon diameter parameter, (α); Jespersen et al., 2007, Zhang et al., 2012). These measures may vary across brain regions and within individuals, which may affect estimates of diffusion reported here. Other limitations include having acquired these diffusion data in a single-phase encoding direction and only correcting for gross motion (not eddy current distortions). However, it is unlikely that these methodological approaches significantly impacted the current findings because we replicated all age effects in a separate dataset acquired in opposing phase encoding directions that allowed us to correct for bias distortions and preprocessed using more advanced eddy current corrections (see Supplementary Material).
In sum, the current study demonstrated that multi-compartment NODDI is more sensitive to age-related differences in hippocampal gray matter integrity than single-tensor DTI, likely due to its ability to more accurately model complex gray matter microstructure while accounting for free diffusion. Gray matter integrity as measured with NODDI hindered diffusion was also sensitive to mnemonic discrimination performance, particularly in younger adults. Taken together, our results suggest that caution should be taken when using DTI integrity measures to assess gray matter integrity as these measures are highly impacted by free diffusion. Instead, multi-compartment NODDI appears to be a more sensitive tool for assessing age-related decline of hippocampal gray matter integrity and episodic memory performance across the lifespan. Although we focus on the hippocampus in this study, based on its involvement in mnemonic discrimination, future studies are needed to determine whether these findings also extend to other gray matter regions (e.g., cortex, basal ganglia).
Supplementary Material
Table 2.
Performance of single-tensor and multi-compartment integrity measures to account for age-related variance were compared using forward likelihood ratio logistic regression. Hindered and free diffusion measures captured the most age-related variance. All other predictors were excluded from the model
| Model | Variable | B | S.E. | Wald | Sig. | Negelkerke R square |
|---|---|---|---|---|---|---|
| 1 | Hindered | 61.799 | 20.07 | 9.481 | 0.002 | 0.366 |
| 2 | Free | 37.101 | 12.511 | 8.794 | 0.003 | 0.643 |
| Diffusion | ||||||
| Hindered | 78.046 | 24.063 | 10.519 | 0.001 | ||
Predicted Variable: Age, dichotomized. B = Intercept, S.E.= Standard Error, Wald = Wald chi-square test, Sig.= Significance.
Acknowledgments
This work was supported by R00 AG047334 (Bennett, IJ) and R01 AG034613 (Stark, CEL). We would like to thank Chelsea Savina Evelyn, Nicole Huffman, Maryam Hanachi and Justino Joel Flores at UC Riverside for assistance with image acquisition and quality control of the supplemental data.
Footnotes
Uncited section
Table 2,Barnes et al., 2004,Kohama et al., 1995,Weston et al., 2015
CRediT authorship contribution statement
Anu Venkatesh: Data curation, Writing - original draft, Visualization, Investigation, Formal analysis. Shauna M. Stark: Data curation, Project administration, Writing - review & editing. Craig E.L. Stark: Funding acquisition, Writing - review & editing. Ilana J. Bennett: Conceptualization, Methodology, Funding acquisition, Supervision, Writing - review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2020.08.004.
The authors declare no competing financial interests.
References
- Albi A, Pasternak O, Minati L, Marizzoni M, Bartrés-Faz D, Bargalló N, Jovicich J, 2016. Free water elimination improves test-retest reproducibility of diffusion tensor imaging indices in the brain: a longitudinal multisite study of healthy elderly subjects. Hum. Brain Mapp. 38, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Scahill RI, Boyes RG, Frost C, Lewis EB, Rossor CL, Fox NC, 2004. Differentiating AD from aging using semiautomated measurement of hippocampal atrophy rates. Neuroimage 23, 574–581. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA, 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15, 435–455. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Stark CEL, 2016. Mnemonic discrimination relates to perforant path integrity: an ultra-high resolution diffusion tensor imaging study. Neurobiol. Learn. Mem 129, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Huffman DJ, Stark CEL, 2015. Limbic tract integrity contributes to pattern separation performance across the lifespan. Cereb. Cortex 25, 2988–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat YA, Christian Beaulieu P, 2004. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. J. Magn. Reson. Imaging 20, 216–227. [DOI] [PubMed] [Google Scholar]
- Càmara E, Bodammer N, Rodríguez-Fornells A, Tempelmann C, 2007. Age-related water diffusion changes in human brain: a voxel-based approach. Neuroimage 34, 1588–1599. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Cherubini A, Caltagirone C, Spalletta G, 2010. Hippocampal mean diffusivity and memory in healthy elderly individuals: a cross-sectional study. Neurology 74, 194–200. [DOI] [PubMed] [Google Scholar]
- Chad JA, Pasternak O, Salat DH, Chen JJ, 2018. Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol. Aging 71, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Péran P, Caltagirone C, Sabatini U, Spalletta G, 2009. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage 48, 29–36. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA, 2018. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci 115, E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange AMG, Bråthen ACS, Grydeland H, Sexton C, Johansen-Berg H, Andersson JLR, Walhovd KB, 2016. White-matter integrity as a marker for cognitive plasticity in aging. Neurobiol. Aging 47, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Heijer T, der Lijn, van F, Vernooij MW, de Groot M, Koudstaal PJ, van der Lugt A, Breteler MMB, 2012. Structural and diffusion MRI measures of the hippocampus and memory performance. Neuroimage 63, 1782–1789. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, Hof PR, 2013. Dendritic spine changes associated with normal aging. Neuroscience 251, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey CR, Kirwan CB, 2015. Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. Hippocampus 25, 524–533. [DOI] [PubMed] [Google Scholar]
- Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Takechi R, 2015. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hyman BT, 2008. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without alzheimer disease. J. Neuropathol. Exp. Neurol 67, 1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi H, Glasser MF, Zhang H, Autio JA, Coalson TS, Okada T, Hayashi T, 2018. Neurite imaging reveals microstructural variations in human cerebral cortical gray matter. Neuroimage 182, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero JM, Adluru N, Bendlin BB, Goldsmith HH, Schaefer SM, Davidson RJ, Alexander AL, 2019. Optimizing the intrinsic parallel diffusivity in NODDI: an extensive empirical evaluation. PLoS One 14, e0217118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS, 2009. Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psychiatry 24,109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E, Rönnbäck L, 1995. Astrocytes in glutamate neurotransmission. FASEB J. 9, 343–350. [DOI] [PubMed] [Google Scholar]
- Hassan MK, ul-Haq M, Amin M, Tahirullah, Nawaz A, Ullah H, 2014. Association between baseline parameters and end of treatment response to combination of conventional interferon & ribavirin in patients with chronic hepatitis C. J. Postgrad. Med. Inst 28, 149–153. [Google Scholar]
- Jeon T, Mishra V, Uh J, Weiner M, Hatanpaa KJ, White CL, Huang H, 2012. Regional changes of cortical mean diffusivities with aging after correction of partial volume effects. Neuroimage 62, 1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen SN, Kroenke CD, Østergaard L, Ackerman JJH, Yablonskiy DA, 2007. Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage 34, 1473–1486. [DOI] [PubMed] [Google Scholar]
- Kaden E, Kelm ND, Carson RP, Does MD, Alexander DC, 2016. Multi-compartment microscopic diffusion imaging. NeuroImage 139, 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama SG, Goss JR, Finch CE, McNeill TH, 1995. Increases of glial fibrillary acidic protein in the aging female mouse brain. Neurobiol. Aging 16, 59–67. [DOI] [PubMed] [Google Scholar]
- Lister JP, Barnes CA, 2009. Neurobiological changes in the hippocampus during normative aging. Arch. Neurol 66, 829–833. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM, 2004. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage 21, 1174–1181. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N, Song AW, 2012. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim. Biophys. Acta 1822, 386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, 2013. Measuring restriction sizes using diffusion weighted magnetic resonance imaging: a review. Magn. Reson. Insights 6, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, O’Sullivan MJ, Bells S, Pasternak O, Jones DK, 2012. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 59, 1394–1403. [DOI] [PubMed] [Google Scholar]
- Morozov S, Sergunova K, Petraikin A, Akhmad E, Kivasev S, Semenov D, Morozov A, 2020. Diffusion processes modeling in magnetic resonance imaging. Insights Imaging 11, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Mulsant BH, Rajji TK, Levesque ML, Pipitone J, Stefanik L, Voineskos AN, 2017. Gray matter neuritic microstructure deficits in schizo-phrenia and bipolar disorder. Biol. Psychiatry 82, 726–736. [DOI] [PubMed] [Google Scholar]
- Oakley R, Tharakan B, 2014. Vascular hyperpermeability and aging. Aging Dis. 5, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori E, Pasternak O, Planetta P, Burciu R, Snyder A, Febo M, Vaillancourt D, 2015. Increased free-water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol. Aging 36, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P, 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol 60, 173–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M, 2011. A Bayesian Model of Shape and Appearance for Subcortical Brain Segmentation. Neuroimage 56, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Valls-Pedret C, Ros E, Palacios E, Falcón C, Bargalló N, Junque C, 2014. Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus 24, 403–414. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV, 2010. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol. Aging 31, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Davies G, Garfinkel SN, Gabel MC, Dowell NG, Cercignani M, Critchley HD, 2017. Archival report deficits in neurite density underlie white matter structure abnormalities in first-episode psychosis. Biol. Psychiatry 82, 716–725. [DOI] [PubMed] [Google Scholar]
- Raja R, Rosenberg G, Caprihan A, 2019. Review of diffusion MRI studies in chronic white matter diseases. Neurosci. Lett 694, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi Y, Pasternak O, Savadjiev P, Michailovich O, Bouix S, Kubicki M, Shenton ME, 2014. Gray matter alterations in early aging: a diffusion magnetic resonance imaging study. Hum. Brain Mapp. 35, 3841–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD, 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex 15, 1676–1689. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, 2002. New visions of the aging mind and brain. Trends Cogn. Sci. 6, 394–400. [DOI] [PubMed] [Google Scholar]
- Robillard KN, Lee KM, Chiu KB, MacLean AG, 2016. Glial cell morphological and density changes through the lifespan of rhesus macaques. Brain Behav. Immun. 55, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen LE, Conturo TE, Laidlaw DH, Cabeen RP, Akbudak E, Lane EM, Paul RH, 2016. Regional age differences in gray matter diffusivity among healthy older adults. Brain Imaging Behav. 10, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y, 2012. Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain Struct. Funct. 217, 503–515. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC, 2003. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol 60, 989. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL, 2013. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia 51, 2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH, 1980. Tests for comparing elements of a correlation matrix. Psychol. Bull 87, 245–251. [Google Scholar]
- Szebenyi G, Bollati F, Bisbal M, Sheridan S, Faas L, Wray R, Brady ST, 2005. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr. Biol 15, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Tariq M, Schneider T, Alexander DC, Gandini Wheeler-Kingshott CA, Zhang H, 2016. Bingham-NODDI: mapping anisotropic orientation dispersion of neurites using diffusion MRI. Neuroimage 133, 207–223. [DOI] [PubMed] [Google Scholar]
- Tohka J, 2014. Partial volume effect modeling for segmentation and tissue classification of brain magnetic resonance images: a review. World J. Radiol. 6, 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston PSJ, Simpson IJA, Ryan NS, Ourselin S, Fox NC, 2015. Diffusion imaging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimers Res. Ther. 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M, 2009. About “axial” and “radial” diffusivities. Magn. Reson. Med 61, 1255–1260. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CEL, 2010. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc. Natl. Acad. Sci. U. S. A 107, 12687–12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC, 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.