Abstract
Objective:
To characterize the hospitalization and death rates among patients with inflammatory arthritis affected by COVID-19 and to analyze the associations between comorbidities and immunomodulatory medications and infection outcomes.
Methods:
Clinical, demographic, maintenance treatment, and disease course data and outcomes of individuals with inflammatory arthritis (IA; rheumatoid arthritis and spondylarthritis) with symptomatic COVID-19 infection were prospectively assessed via web-based questionnaire followed by individual phone calls and electronic medical record review. Baseline characteristics and medication use were summarized for hospitalized and ambulatory patients, and outcomes were compared for each medication class using multivariable logistic regression.
Results:
A total of 103 patients with IA were included in the study (n=80 confirmed and n=23 highly suspicious for COVID-19). Twenty-six percent of participants required hospitalization, and 4% died. Patients who warranted hospitalization were significantly more likely to be older (P<0.001) and have comorbid hypertension (P=0.001) and chronic obstructive pulmonary disease (P=0.022). IA patients taking oral glucocorticoids had a higher likelihood of being admitted for COVID-19 (P<0.001) while those on maintenance anti-cytokine biologic therapies did not.
Conclusion:
In patients with underlying IA, COVID-19 outcomes were worse in those receiving glucocorticoids but not in patients on maintenance anti-cytokine therapy. Further work is needed to understand whether immunomodulatory therapies affect COVID-19 incidence.
Keywords: COVID-19, psoriatic arthritis, rheumatoid arthritis, spondyloarthritis, csDMARDs, biologics, JAKi
INTRODUCTION
In December 2019, the first case of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), was reported in Wuhan, China [1]. Since then, the number of cases has increased exponentially to pandemic levels [2] and shifted geographically to Europe and North America, with New York City becoming the epicenter. Reporting on the clinical characteristics of affected patients in the general population has emerged at a relatively slow pace [3], largely due to the necessary reassignment of practitioners and investigators towards COVID-19 patient care [4]. Data have been even more scarce for populations at the highest risk of infection, most notably immunosuppressed patients with underlying chronic inflammatory diseases or chronic use of anti-cytokine biologics and/or other immunomodulatory therapies. Yet, several of these medications are being studied in randomized clinical trials (RCTs) [5–7] with the underlying rationale that severe adverse outcomes may be, at least in part, related to a pro-inflammatory cytokine storm syndrome (CSR) [8]. Therefore, we and others hypothesize that patients chronically receiving immunosuppression with anti-cytokine [9, 10] or small molecule therapies [11] for inflammatory arthritis (IA) may have partial protection against worse outcomes of COVID-19 (i.e., need for hospitalization, mechanical ventilation, or death). An ongoing investigation of the impact of clinical, demographic and pharmacological care in our population with immune-mediated-inflammatory diseases (IMID; including inflammatory arthritis) affected with COVID-19 is well underway [12].
Here, we report on an expanded cohort of patients with rheumatoid arthritis (RA) and spondyloarthritis (SpA) to further characterize the effects of patients’ comorbidities and chronic medications on the natural history of subjects with IA who contracted COVID-19 in the New York City metropolitan area during the first months of 2020.
PATIENTS AND METHODS
WARCOV Study:
Recruitment stemmed from a larger New York University (NYU) Langone Health study, namely Web-based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV). WARCOV is a prospective cohort study of patients with IMID, including IA, psoriasis and inflammatory bowel disease (IBD). It consists of two sequential phases. Phase 1 aims to characterize the symptomatology and disease course of patients with IMID who are infected with COVID-19. Phase 2 will determine the incidence of COVID-19 in patients with IMID as well as associated genetic factors and immune responses. Further details on the NYU WARCOV study (NYU IRB#i20–00389) are detailed in the supplementary methods section.
Study Population:
Between March 3, 2020 to May 4, 2020, WARCOV received reports of 126 cases of adult patients with IA [including RA, psoriatic arthritis (PsA), ankylosing spondylitis (AS), or IBD-associated arthritis] and confirmed or highly suspected COVID-19 infection. Referrals came from 46 rheumatologists and dermatologists associated with NYU Langone Health, spanning across the five New York City boroughs and involving both private and public healthcare systems.
Case Identification:
All non-hospitalized patients (n=99) were contacted via web-based questionnaire followed by a phone call to confirm their rheumatic disease and COVID-19 diagnosis; of these, 6 were determined not to be cases and 17 declined to participate (Supplementary Figure 1). Hospitalized patients (n=27) had their charts reviewed and were contacted via questionnaire and phone call upon discharge.
Subject evaluation and data collection:
We collected clinical, demographic, treatment and disease course data and outcomes using the REDCap-based [13] questionnaire. Participants were then contacted via phone and electronic medical records to confirm diagnosis, baseline medications, and presence of SARS-CoV-2 PCR or antibody testing. Hospital course details were obtained where available (23/27 hospitalized patients; the other four had incomplete datasets due to either death or continued inpatient admission). IA severity status was rated as in-remission, mild, moderate, or severe based on patient reported outcomes or, in case of currently hospitalized patients or death, chart review. Confirmed COVID-19 was defined as a positive SARS-CoV-2 PCR test using nasopharyngeal swabbing and/or a positive antibody test. High suspicion for COVID-19 was defined in accordance with CDC guidelines as any patient with new fever >99°F or a known positive contact plus one or more respiratory symptoms (dry cough, anosmia, sore throat, or shortness of breath [14]) that could not be confirmed given the initial limited availability of SARS-CoV-2 PCR or antibody testing in New York at the time of this study. We then compared the data on IA patients who required hospitalization (hospitalized) to those who did not (ambulatory) and analyzed the association of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologic disease-modifying antirheumatic drugs (bDMARDs), and small molecules with COVID-19 infection outcomes. bDMARDs and small molecules were assessed individually and after grouping by mechanism of action: tumor necrosis factor inhibitors (TNFis; infliximab, adalimumab, etanercept, certolizumab, and golimumab), interleukin (IL)-17 blockers (secukinumab, ixekizumab), IL-23 and IL-12/23 blockers (ustekinumab, guselkumab), and JAKis (tofacitinib).
Statistical analysis:
Baseline characteristics and medication use were summarized using mean (range and/or standard deviation) for continuous variables and frequency and proportions for categorical variables, overall and stratified by hospitalization status. Bivariate t-test, chi-squared tests, or two-proportion Z-tests with Yates continuity correction [15], as appropriate, were employed to assess the baseline characteristics and medication use between patients who were hospitalized and those who were not.
Relationship between each comorbidities and hospitalization was further assessed using separate logistic regression models, first adjusting for age and sex, and subsequently also for BMI. For each medication class, we analyzed the association of drug use and hospitalization status by fitting separate covariate adjusted multivariable logistic regression models. The adjustment was done in two stages. First, models were adjusted for age and sex. In the next stage, BMI and comorbidities (asthma, COPD, diabetes, and high blood pressure) were also added as covariates. Binary covariates with sample size less than 5 total, or sample size of 0 in either the ambulatory or hospitalized patient groups, were not included. Odds ratios and confidence intervals were reported. As a sensitivity analysis and to assess for residual confounding in the association between medication use and hospitalization, E-values were computed [16] for the limit of the confidence interval closest to the null. Two tailed statistical significance at alpha level 0.05 was considered. Statistical software R (3.6.1) [17] was used for the analysis.
RESULTS
Patient Population
A total of 103 patients with inflammatory arthritis with confirmed (n=80) or highly suspected (n=23) symptomatic COVID-19 infection were identified (Table 1). Participants were prospectively followed for a mean of 42 +/− 9 days from the time of symptom onset, through a combination of web-based assessments, telephone calls, review of electronic medical records and, if warranting hospitalization, examination of hospital course charts. The mean age was 53 years (range 28–88), 72% were women, 63% White, 15% Black, 10% Asian, and 17% identified as Hispanic. Patients had an IA diagnosis of RA (47/103, 46%) or SpA (56/103, 54%; psoriatic arthritis 68% and other 32%). With respect to disease state prior to onset of COVID-19 symptoms, 24 patients (23%) rated their arthritis as in remission, 39 (38%) as mild, 35 (34%) as moderate, and 3 (3%) as severe. A majority (94%) of the patients were on an immunomodulatory medication (including anti-cytokine biologics, JAKis, oral glucocorticoids, leflunomide, sulfasalazine, methotrexate, apremilast, or hydroxychloroquine) for their primary diagnosis with 60% on an anti-cytokine biologic and 11% on a JAKi. Preexisting conditions included hypertension (23% of patients), diabetes mellitus (10%), chronic obstructive pulmonary disease (COPD, 5%), and asthma (15%). The average BMI was 28 +/− 6. Fever (84%), cough (79%), and shortness of breath (63%) were the most common symptoms of COVID-19. Findings were similar when analysis was restricted only to patients with confirmed COVID infection by SARS-CoV-2 PCR testing (Supplementary Table 1).
Table 1.
Comparison of baseline characteristics, medication use, and disease course in ambulatory and hospitalized IA patients with confirmed or highly suspected COVID-19 infection*
| Characteristic | Total (n = 103) | Ambulatory (n = 76) | Hospitalized (n = 27) | p-value |
|---|---|---|---|---|
| Age- mean (range) | 52.7 [28–88] | 49.7 [28–82] | 61.0 [34–88] | <0.001 |
| Female- n (%) | 74 (71.8) | 56 (73.7) | 18 (66.7) | 0.65 |
| Race- n (%) | 0.88 | |||
| White | 65 (63.1) | 48 (63.2) | 17 (63.0) | |
| Black | 15 (14.6) | 10 (13.2) | 5 (18.5) | |
| Asian | 10 (9.7) | 7 (9.2) | 3 (11.1) | |
| Other | 11 (10.7) | 9 (11.8) | 2 (7.4) | |
| Hispanic ethnicity- n (%) | 17 (16.5) | 12 (15.8) | 5 (18.5) | 0.98 |
| COVID-19 positive- n (%) | 80 (77.7) | 53 (69.7) | 27 (100.0) | 0.003 |
| COVID-19 suspect- n (%) | 23 (22.3) | 23 (30.3) | 0 (0.0) | 0.003 |
| Primary Inflammatory Arthritis Diagnosis- n (%) | ||||
| Spondyloarthritis | 56 (54) | 47 (62) | 9 (33) | 0.02 |
| Rheumatoid Arthritis | 47 (46) | 29 (38) | 18 (67) | 0.02 |
| IA Disease Severity Prior to COVID-19 symptom onset | ||||
| In remission | 24 (23) | 18 (24) | 6 (22) | 1.00 |
| Mild | 39 (38) | 28 (37) | 11 (41) | 0.90 |
| Moderate | 35 (34) | 26 (34) | 9 (33) | 1.00 |
| Severe | 3 (3) | 2 (3) | 1 (4) | 1.00 |
| Body Mass Index- mean (SD) | 28.7 (6.2) | 28.1 (5.5) | 30.5 (7.6) | 0.08 |
| Comorbidities- n (%) | ||||
| Congestive heart failure | 4 (3.9) | 2 (2.6) | 2 (7.4) | 0.60 |
| Hypertension | 24 (23.3) | 11 (14.5) | 13 (48.) | 0.001 |
| Diabetes | 10 (9.7) | 5 (6.6%) | 5 (18.5) | 0.16 |
| Chronic obstructive pulmonary disease | 5 (4.9) | 1 (1.3) | 4 (14.8) | 0.02 |
| Asthma | 15 (14.6) | 12 (15.8) | 3 (11.1) | 0.78 |
| Severe kidney disease | 1 (1.0) | 0(0.0) | 1 (3.7) | 0.59 |
| Chronic medications- n (%) | ||||
| ACE inhibitor /ARB | 22 (21.4) | 12 (15.8) | 10 (37.0) | 0.04 |
| Any medication for primary IA diagnosis | 97 (94.2) | 70 (92.1) | 27 (100.0) | 0.31 |
| Methotrexate | 35 (34.0) | 24 (31.6) | 11 (40.7) | 0.53 |
| Hydroxychloroquine | 13 (12.6) | 7 (9.2) | 6 (22.2) | 0.16 |
| Oral glucocorticoids | 13 (12.6) | 3 (3.9) | 10 (37.0) | <0.001 |
| Any biologic or JAK inhibitor | 73 (70.9) | 56 (73.7) | 17 (63.0) | 0.42 |
| Tumor Necrosis Factor inhibitor | 40 (38.8) | 34 (44.7) | 6 (22.2) | 0.07 |
| IL-17 blocker | 18 (17.5) | 16 (21.1) | 2 (7.4) | 0.19 |
| IL-23 and IL12/23 blockers | 5 (4.9) | 5 (6.6) | 0 (0.0) | 0.40 |
| JAK inhibitor | 11 (10.7) | 4 (5.3) | 7 (25.9) | 0.009 |
| Relevant Medication Dose—mean (SD) | ||||
| Methotrexate | 16.4 (5.5) | 16.0 (5.9) | 17.2 (4.8) | 0.56 |
| Oral glucocorticoids | 10.0 (8.1) | 17.5 (13.9) | 7.7 (4.5) | 0.06 |
| COVID-19 Symptoms- n (%) | ||||
| Fever | 86 (83.5) | 64 (84.2) | 22 (81.5) | 0.98 |
| Cough | 81 (78.6) | 60 (78.9) | 21 (77.8) | 1.00 |
| Shortness of breath | 65 (63.1) | 42 (55.3) | 23 (85.2) | 0.01 |
| Rhinorrhea | 29 (28.2) | 26 (34.2) | 3 (11.1) | 0.04 |
| Sore throat | 31 (30.1) | 26 (34.2) | 5 (18.5) | 0.20 |
| Diarrhea | 147 (45.6) | 38 (50.0) | 9 (33.3) | 0.21 |
| Anosmia | 44 (42.7) | 38 (50.0) | 6 (22.2) | 0.02 |
| Ageusia | 50 (48.5) | 43 (56.6) | 7 (25.9) | 0.01 |
| Days from first symptom to hospital, mean (SD) | 6.1 (3.9) | |||
| Regular floor—n (%) | 22 (81.5) | |||
| Supplementary O2—n (%) | 18 (66.7) | |||
| Intensive care unit level care/mechanical ventilation—n (%)◊ | 4 (14.8) | |||
| Death—n (%)◊ | 4 (14.8) |
IA= inflammatory arthritis; ACE= angiotensin converting enzyme; ARB= angiotensin II receptor blockers; IL= interleukin; JAK= Janus Kinase; O2= oxygen.
Of the four patients in the ICU, there were three death and one complete recovery. One other death occurred in unknown levels of care while hospitalized.
COVID-19 Outcomes and Clinical Characteristics
Within our study population, 27/103 IA participants required hospitalization (26%, 95% confidence interval [CI] 18–36%) (Figure 1). In patients between the ages of 18 and 44, the hospitalization rate was 3% (95% CI 0–19%); between 45 and 64, 34% (95% CI 22–48%); and in those older than 65, 41% (95% CI 19–67%).
Figure 1.
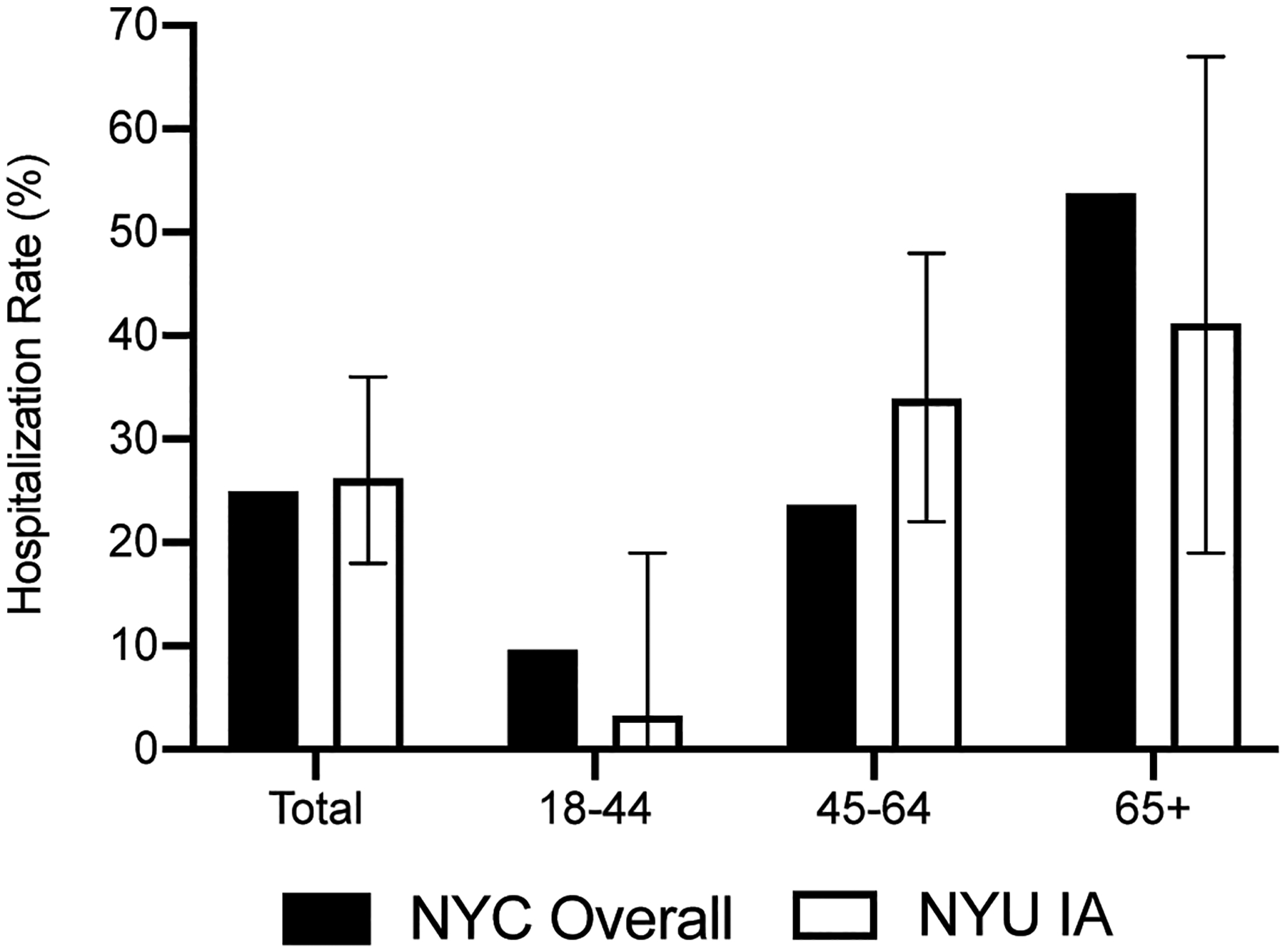
Hospitalization rates in the New York University Inflammatory Arthritis (NYU IA) population compared with the general New York City (NYC) population with COVID-19 infection.
The overall rate of COVID19-related death in our IA cohort was 4% (4/103). When stratified by age, the rate of death was 0% (0/30; 95% CI 0–14%) for those 18–44 years of age, 4% (2/56; 95% CI 1–13%) for those 45–64, and 12% (2/17; 95% CI 2–38%) for those older than 65. The four individuals who died had a median age of 70 (range 62–87). Of those, one patient had significant pre-existing pulmonary disease, and another had congestive heart failure, hypertension, diabetes, and severe chronic kidney disease. Of these four patients, three were on chronic methotrexate and/or glucocorticoids, one was being treated with a bDMARD, and none were on a JAKi. Detailed baseline characteristics and medications for participants with severe COVID-19 outcomes (i.e., ICU stay and/or death) are shown in Supplementary Table 2.
We then compared the characteristics of those patients who warranted hospitalization (hospitalized) with those who did not (ambulatory) (Table 1). The mean age was 61 and 50 for hospitalized and ambulatory patients, respectively (P<0.001) and there was no significant difference in gender. Hospitalized patients had higher BMI (31 vs. 28, P=0.08). Severity status of the underlying inflammatory disease at the time of COVID-19 did not affect the risk of hospitalization. Hospitalized patients were significantly more likely than ambulatory patients to have comorbid hypertension (48% vs. 15%, P=0.001) and COPD (15% vs. 1%, P=0.02). After adjusting for age and sex, the odds ratio of hospitalization for hypertension was 3.33 (95% CI 1.16 to 9.60, P=0.03) and for COPD, 6.75 (95% CI 0.66 to 68.98, P=0.11) (Figure 2). Fever, cough, and shortness of breath remained the most common COVID-19 symptomatic presentations in both ambulatory and hospitalized patients; shortness of breath was significantly more common in the hospitalized patients (85% vs. 63%, P= 0.01).
Figure 2.
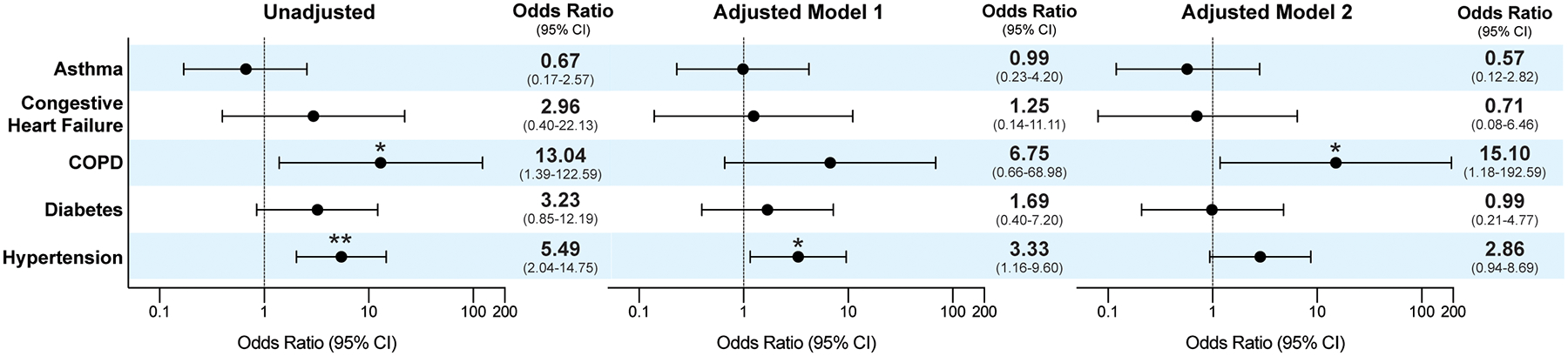
Risk of hospitalization for comorbid medical conditions in patients with known and highly suspected COVID-19 infection. Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, and body mass index. *Denotes P value less than 0.05, ** less than 0.005. CI= confidence interval; COPD= chronic obstructive pulmonary disease.
COVID-19 Outcomes and Immunomodulatory Medication Use
The overall baseline use of immunomodulatory medications was: anti-cytokine biologics (60%), JAKis (11%), oral glucocorticoids (13%), conventional synthetic (cs)DMARDS (55.4%). We further analyzed COVID-19 hospitalization based on maintenance medication use (Table 1 and Supplementary Table 3). When grouping medications by mechanism of action, the use of TNFi and IL-17 blockers was not statistically different between the two groups (22% hospitalized vs. 45% ambulatory, P=0.07 and 7% hospitalized vs. 21% ambulatory, P=0.19, respectively). While no patients on IL-23 and IL-12/23 blockers were hospitalized, only 5 participants were utilizing these medications. JAKi use was more common in those warranting hospitalization (26% in hospitalized vs. 5% in ambulatory, P=0.009). For csDMARDs, methotrexate and hydroxychloroquine use was similar (41% vs. 32%, P=0.53 and 22% vs. 9%, P=0.16 respectively). Chronic oral glucocorticoid use was significantly more common in those requiring hospitalization (37% hospitalized vs. 4% ambulatory, P<0.001). Although the average dose was higher in ambulatory patients (17.5 +/− 14 vs. 7.7 +/− 5, P = 0.06), the difference was not statistically significant.
When adjusting for age and sex using a multivariable logistic regression analysis, the odds ratio of hospitalization for those IA patients chronically on TNFis was 0.45 (95% CI 0.15 to 1.36, P=0.16); for IL-17 blockers, 0.42 (95% CI 0.08 to 2.09, P=0.29); for MTX, 0.82 (95% CI 0.30 to 2.28, P=0.71); and for hydroxychloroquine, 3.57 (95% CI 0.93 to 12.73, P=0.06) (Figure 3 and Supplementary Table 3). Oral glucocorticoids showed an odds ratio for hospitalization of 21.1 (95% CI 4.09 to 109.03, P<0.001, E-value =7.65). JAKis had an odds ratio of 6.24 (95% CI 1.49 to 26.24, P= 0.01, E-value=2.33). This relationship persisted for oral glucocorticoids (OR 26.22, 95% CI 3.82 to 180.19, P=0.001, E-value =7.09) and JAKis (OR 10.23, 95% CI 1.88 to 55.51, P= 0.007, E-value=3.18) after additional adjustment for BMI and comorbidities (asthma, COPD, diabetes mellitus, and hypertension). For JAKis, however, a significant OR was only maintained in the SpA group (OR 17.6, 95% CI 1.04 to 299.69, P= 0.047, E-value =1.23), but not for RA patients (OR 2.50, 95% CI 0.43 to 14.52, P= 0.31) (Supplementary Figure 2).
Figure 3.
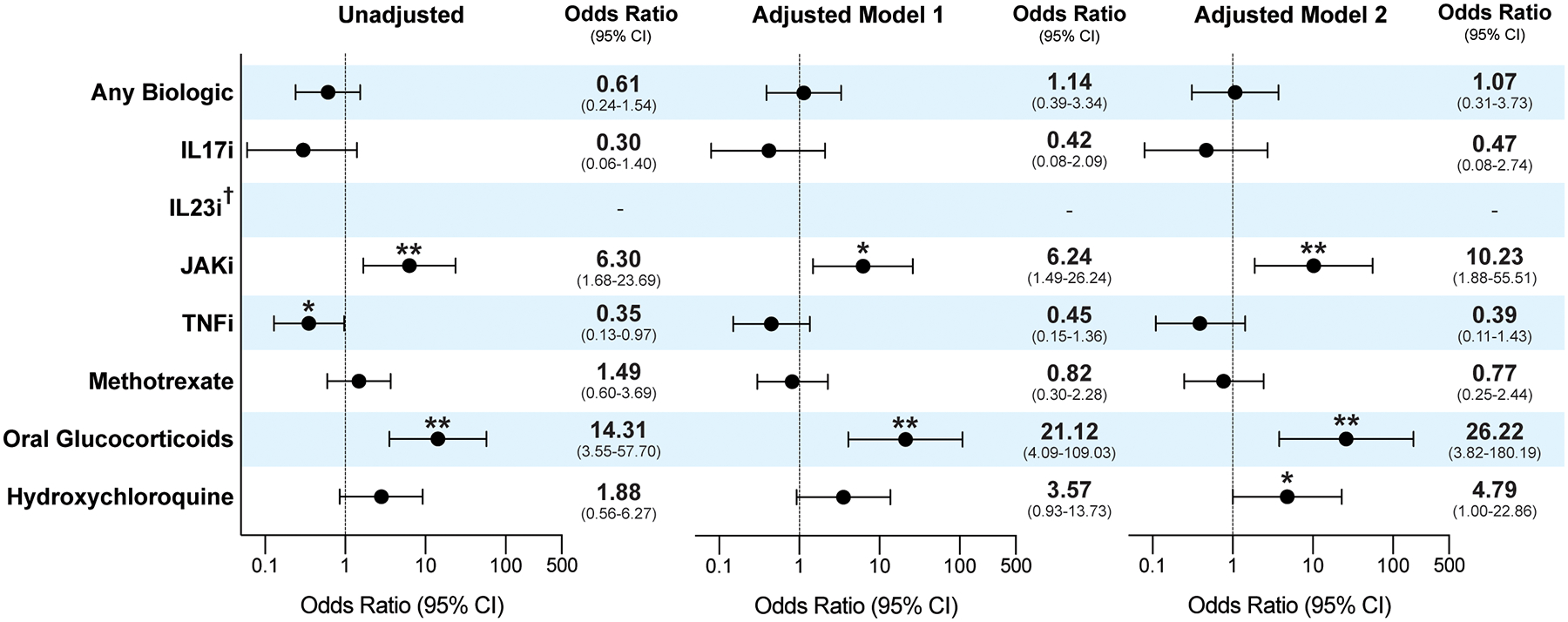
Risk of hospitalization by medication use in patients with known or highly suspected COVID-19 infections. Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, body mass index and comorbidities (asthma, chronic obstructive pulmonary disease, diabetes mellitus, and hypertension). †For medications with small occurrences, empty cell counts led to non-convergence in logistic regression, denoted by lack of odds ratios as in the case of IL-23 inhibitors. *Denotes P value less than 0.05, ** less than 0.005. CI= confidence interval; IL17i = interleukin 17 inhibitor; IL23i= interleukin 23 inhibitor; JAKi= janus kinase inhibitor; TNFi= tumor necrosis factor inhibitor. E-values for significant associations are presented in the body of the paper.
When comparing RA and SpA groups, we found that patients with RA had a higher hospitalization rate (38% vs. 16%, P=0.02) although both groups had a similar death rate (4.2% vs. 3.6%, P=1.0) (Supplementary Table 4). Patients with RA were older (56 vs. 49, P=0.005) and majority female (87% vs. 60%, P= 0.003). As expected, methotrexate (51% vs. 20%, P=0.002) and hydroxychloroquine (21% vs. 5%, P=0.03) use was significantly higher in RA. Patients with RA were also more likely to use chronic oral glucocorticoids compared to those with SpA (23% vs. 4%, P=0.007).
DISCUSSION
Since the early days of 2020, the COVID-19 pandemic has quickly emerged as the most challenging global health crisis in a generation. Affecting initially China, it shifted first to Europe only to hit the hardest in the U.S., and especially in New York City.
Multiple aspects of the pathogenesis, clinical presentation, natural history, and prognosis of COVID-19 have now been described [18–21] including severe forms of ARDS and hypercoagulable states leading to multi-organ failure due to immune-microthrombosis and hyperinflammatory responses [3, 22]. As RCTs of antiviral and anti-cytokine therapies are being conducted, one of the most relevant questions confronting rheumatologists relates to whether biologics, JAKis, or any other immunomodulatory medications could have deleterious (or beneficial) effects in the incidence and/or outcomes of COVID-19. In an attempt to contribute knowledge to the latter, we analyzed the clinical characteristics of patients with IA who contracted COVID-19 and the effects of comorbidities and chronic therapeutic modalities on outcomes of this infectious process. We characterized hospitalization and death rates in IA patients and confirmed risk factors for COVID-19 severity previously described for the general population, as well as identified features unique to patients with inflammatory arthritis.
Notably, and as in the case of patients with IMID [12], we found that the overall hospitalization rate in patients with inflammatory arthritis was consistent with that of the general New York City population (21% as of May 4, 2020) [23]. Given the low number of deaths in our study (n=4, 4%), extrapolating comparisons to the general NYC population is challenging, although data derived from our healthcare system showed a similar death or discharge to hospice rate (665/11544; 5.8%). Notably, all four deaths occurred in patients with other known risk factors (i.e., advanced age, hypertension, severe kidney disease, severe lung disease, or glucocorticoid use) and only one person in this group was on a biologic (TNFi) while none was being treated with a JAKi.
Of utmost importance is our finding that the chronic use of pathway-specific anti-cytokine biologic therapies was not associated with worse outcomes of COVID-19 in patients with IA. These results are in line with our previous work which included patients on biologics for the treatment of other IMID (i.e., psoriasis and IBD)[12]. This has also been observed in a recently published study looking at IBD patients in Italy, where active disease, age, and the presence of comorbidities were all associated with a negative COVID-19 outcome, whereas treatments for IBD, including biologics, were not [25]. Using a cross-sectional, physician-reported registry, the Global Rheumatology Alliance is also detecting similar outcomes (i.e., anti-TNF use is associated with a decreased odds of hospitalization) [26]. Whether the baseline use of biologic therapy, the prevention of acute IA flares, or a combination of those factors can help avoid worse COVID-19 in patients with IMID and IA is a matter of intense research, as both large population-based studies and RCTs are currently lacking.
Unsurprisingly, age, the presence of comorbidities (especially hypertension, COPD and possibly diabetes), and the use of oral corticosteroids were found to be significantly higher in patients with IA requiring hospitalization. In contrast to previous reports, and possibly due to relatively low number of patients, race and ethnicity did not appear to play a role in determining COVID-19 outcomes [23].
Like age [22, 27], comorbid conditions, including hypertension and COPD, have been known to increase the risk of worse viral syndromes outcomes in general, and of COVID-19 in particular [28]. Case series have shown that especially hypertension is more common in patients with severe COVID-19 than in those with milder illness [29], and predominantly so in the elderly [30]. Our own work further validates these observations. Although the underlying reasons for this association are still elusive, currently available data reveals that hypertension appears to be associated with more severe COVID-19 disease, a higher risk of ARDS, and increased mortality [31]. This is certainly applicable to RA and SpA, as hypertension and cardiovascular disease are frequent comorbid conditions [32, 33].
The use of corticosteroids as treatment for ARDS in the context of COVID-19 has been shown to be deleterious since the earliest reports of the pandemic [3]. The mechanisms by which IA patients on background corticosteroids appear to fare worse than those not on these medications are yet to be fully elucidated, but are certainly not surprising, as these medications are global suppressors of the host inflammatory response. Remarkably, though, we observed worse COVID-19 outcomes in our IA patients even when the average dose of corticosteroids was relatively low (i.e., less than 10mg/day), which is in line with other datasets [26].
Although hydroxychloroquine is being studied as a potential treatment for COVID-19, we did not find evidence of a protective effect from its use at standard doses in preventing COVID-19 hospitalization and associated outcomes in our IA cohort. This is in line with prior reports in patients with rheumatic disease [26, 34] including systemic lupus erythematosus [35].
Importantly, and although we are underpowered to analyze some of the medications given their low occurrence in our study population, the chronic use of JAKis may of be of potential interest. More IA patients with COVID-19 who required hospitalization were on JAKi treatment compared to ambulatory patients. However, this was only true when analyzing all IA patients, but not for those receiving these drugs for an RA indication, which were the majority of cases. The explanation for this finding is likely complex and will require validation as this may reflect unique prescription patterns in our healthcare system, which may not be representative of other practices. Moreover, the small E-values obtained in the adjusted models, an established metric for minimum strength of association needed for unmeasured confounders to explain away treatment-outcome association, indicate relatively strong evidence for confounding. It is quite possible that this observation relates to confounding by indication, because JAKi are typically prescribed as 2nd or 3rd choice for patients with more advanced disease who have not responded to other medications, especially in PsA. Importantly, while three of the four patients with PsA on a JAKi required hospitalization, they also had other reasons for the outcome, as two of them had severe, recalcitrant disease; and the third had comorbid common variable immunodeficiency. This may also explain why the association was not found when including other IMID patients (i.e., IBD) in the analysis [12] or when looking at RA patients alone, as the correlations may actually be phenotype-specific rather than unique to the mode of action or class. We also note that none of the patients with severe outcomes (i.e., intubation or death) were on maintenance JAKi. Another possibility is that these observations relate to intrinsic mechanisms of JAK/STAT pathway blockade, which impairs IFN-mediated anti-viral response [36] and, coupled with the known association of increased viral infections for JAKi as a class [37, 38], could at least theoretically impede adequate host responses to SARS-CoV2. Although a small, non-randomized study of baricitinib in COVID-19 patients showed potential benefit with no safety signals [6], the results of a large RCT are not yet available. These would help clarify the role of JAKi in COVID-19. Meanwhile, and given the low JAKi use in our study, these results should be interpreted with extreme caution.
Some differences between disease phenotypes are also of interest, as we observed a significantly higher rate of hospitalization in those with RA. However, this may be a reflection of the population’s demographic characteristics rather than the underlying pathophysiology. Patients with RA were older, had higher rates of hypertension and COPD, and were more likely to be on chronic glucocorticoids for their disease; each of these features on their own is associated with hospitalization.
Other inherent limitations of our study include relatively small sample size, and limited numbers of hospitalized cases which precludes the immediate generalization of our results. Further, the observational nature of the study prevents us from making any causal claims as there might exist unmeasured confounders, which is a known limitation of most such studies. Specifically, and despite attempts with several adjusted models, we cannot fully account for confounding by indication. We also recognize that not all of our patients who are highly suspicious for COVID-19 infection may ultimately have the diagnosis. However, at the time of disease, PCR testing was limited in New York City. Phase 2 of WARCOV is well underway and currently obtaining serologic testing for confirmation purposes which will address this gap. Furthermore, we acknowledge that our cohort is homogenous (subjects derived from a single healthcare system and geographic region) and biased towards patients with SpA (influenced by the presence of three distinct centers at NYU: the psoriatic arthritis center, the psoriasis and psoriatic arthritis clinic, and the inflammatory bowel disease center). Moreover, our work does not yet provide data on overall incidence of COVID-19 in inflammatory arthritis or translational understanding of reasons behind the differential clinical outcomes beyond medications or clinical variables. An ongoing effort from our group will characterize the immune response and host genomics as potential determinants of COVID-19 outcomes in patients with IMID. Other complementary initiatives are currently ongoing (i.e., Global Rheumatology Alliance [26]) and should shed light on some of these knowledge gaps.
Taken together, our results suggest that older patients with comorbidities and those receiving oral glucocorticoids, but not anti-cytokine biologic therapy, are at higher risk for requiring hospitalization after contracting COVID-19. Whether any immunomodulatory medication can eventually lead to prevention of COVID-19 or amelioration of its clinical outcomes will necessarily require further validation of our data coupled with the results of both ongoing randomized clinical trials/cohort studies and basic/translational mechanistic understanding of the interplay between SARS-CoV-2 pathogenesis and host response in patients with inflammatory arthritis and related conditions.
Supplementary Material
Acknowledgements
We would like to thank our patients and their families for participating in this study, especially during these challenging times and under such difficult circumstances. We would also like to recognize with profound admiration the moral courage and human sacrifices that many of the medical students, trainees, nurses and faculty have demonstrated during the COVID-19 pandemic, several of whom are co-authors in this study. We are grateful to Luz Alvarado, Rhina Medina and Parvathi Girija for coordinating and for data entry efforts. We would also like to thank Tania Moin and Ranit Shriky for helping with regulatory hurdles.
Supported by: NIH/NIAMS R01AR074500-01A, Bloomberg Philanthropies COVID-19 Response Initiative Grant; The Riley Family Foundation and The Snyder Family Foundation (Scher); NIH/NIAMS T32AR069515 and Bloomberg Philanthropies COVID-19 Response Initiative Grant (Haberman)
Footnotes
Conflicts of interest: UCB, Janssen, Abbvie, Pfizer, Novartis, Sanofi (Scher); Janssen (Haberman); Johnson & Johnson (Adhikari); Abbvie (Solomon); GSK (Izmirly); Abbvie, Amgen, BMS, Celgene, Janssen, Lilly, Novartis, Pfizer, Corrona (Ogdie)
Collaborators
The NYU WARCOV Investigators: Lily Cao, Brian Jaros MD, Keshav Mangalick, Joshua Novack, Connor Peterson, Lindsey Quintana, Amit Saxena MD, H. Michael Belmont MD, Ruth Fernandez Ruiz MD, Allison Guttmann MD, Mala Masson, Janine Sullivan NP, Miao Chang, Kimberly Robin, Philip Carlucci, Lauren Fried, Avani Kolla, Lauren Rangel, Shruthi Shankar, Alexa Steuer, Katerina Svigos, Kaitlyn (Lu) Yin, Trevor Young, David Hudesman MD, Jordan Axelrad MD, Shannon Chang MD, Simon Hong MD
REFERENCES
- 1.WHO. Pneumonia of unknown cause-China Disease Outbreak News. 2020. March 30, 2020]; Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.
- 2.WHO. Virtual press conference on COVI-19- 11 March 2020. 2020. [cited 2020 March 16]; Available from: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2.
- 3.Wang D, et al. , Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedford J, et al. , COVID-19: towards controlling of a pandemic. Lancet, 2020. 395(10229): p. 1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P, et al. , Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents, 2020: p. 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Cantini F, et al. , Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treatment of COVID-19 Patients With Anti-interleukin Drugs (COV-AID). [cited 2020 May 3, 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04330638.
- 8.Moore JB and June CH, Cytokine release syndrome in severe COVID-19. Science, 2020. 368(6490): p. 473–474. [DOI] [PubMed] [Google Scholar]
- 9.Mehta P, et al. , COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 2020. 395(10229): p. 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, et al. , Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res, 2007. 128(1–2): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson P, et al. , Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet, 2020. 395(10223): p. e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberman R, et al. , Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, et al. , Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009. 42(2): p. 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. Symptoms of Coronavirua. May 10, 2020]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- 15.Yates F, Contingency tables involving small numbers and the χ 2 test. Supplement to the Journal of the Royal Statistical Society 1934. 1(2): p. 217–235. [Google Scholar]
- 16.VanderWeele TJ and Ding P, Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med, 2017. 167(4): p. 268–274. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team, R: A language and environment for statistical computing. 2010, R Foundation of Statistical Computing: Vienna, Austria. [Google Scholar]
- 18.Fauver JR, et al. , Coast-to-Coast Spread of SARS-CoV-2 during the Early Epidemic in the United States. Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu R, et al. , Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 2020. 395(10224): p. 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan WJ, et al. , Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi M, Yokoe D, and Havlir D, Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control Covid-19. The New England journal of medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, et al. , Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 2020. 395(10229): p. 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NYCHealth. COVID-19: Data. 2020. [cited 2020 May 4]; Available from: https://www1.nyc.gov/site/doh/covid/covid-19-data.page.
- 24.Petrilli C, et al. , Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. The BMJ, 2020. [Google Scholar]
- 25.Bezzio C, et al. , Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut, 2020: p. gutjnl-2020–321411. [DOI] [PubMed] [Google Scholar]
- 26.Gianfrancesco M, et al. , Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koff WC and Williams MA, Covid-19 and Immunity in Aging Populations — A New Research Agenda. New England Journal of Medicine, 2020. [DOI] [PubMed] [Google Scholar]
- 28.Richardson S, et al. , Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan WJ, et al. , Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med, 2020. 382(18): p. 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg S, Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR. Morbidity and Mortality Weekly Report, 2020. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzik TJ, et al. , COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogdie A, et al. , Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis, 2015. 74(2): p. 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon DH, et al. , Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis, 2010. 69(11): p. 1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Silva KM, et al. , Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’ Ann Rheum Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathian A, et al. , Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Annals of the Rheumatic Diseases, 2020: p. annrheumdis-2020–217566. [DOI] [PubMed] [Google Scholar]
- 36.Fleming SB, Viral inhibition of the IFN-induced JAK/STAT signalling pathway: Development of live attenuated vaccines by mutation of viral-encoded IFN-antagonists. Vaccines, 2016. 4(3): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winthrop KL, The emerging safety profile of JAK inhibitors in rheumatic disease. Nature Reviews Rheumatology, 2017. 13(4): p. 234–243. [DOI] [PubMed] [Google Scholar]
- 38.Bechman K, et al. , A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology, 2019. 58(10): p. 1755–1766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


