Abstract
Acute kidney injury (AKI) is characterized by injury to the tubular epithelium that leads to the sudden loss of renal function. Proper tubular regeneration is essential to prevent progression to chronic kidney disease. In this study, we examined the role of FoxM1, a forkhead box family member transcription factor in tubular repair after AKI. Renal FoxM1 expression increased after renal ischemia/reperfusion (I/R) induced AKI in mouse kidneys. Treatment with thiostrepton, a FoxM1 inhibitor, reduced FoxM1 regulated pro-proliferative factors and cell proliferation in vitro, and tubular regeneration in mouse kidneys after AKI. Glycogen synthase kinase-3 (GSK3) was found to be an upstream regulator of FoxM1 because GSK3 inhibition or renal tubular GSK3β gene deletion significantly increased FoxM1 expression, and improved tubular repair and renal function. GSK3 inactivation increased β-catenin, Cyclin D1 and c-Myc, and reduced cell cycle inhibitors p21 and p27. Importantly, thiostrepton treatment abolished the improved tubular repair in GSK3β knockout mice following AKI. These results demonstrate that FoxM1 is important for renal tubular regeneration following AKI and that GSK3β suppresses tubular repair by inhibiting FoxM1.
Keywords: FoxM1, GSK3β, acute kidney injury, tubular regeneration, ischemia/reperfusion, cell proliferation, thiostrepton
INTRODUCTION
AKI is the sudden loss of renal function commonly caused by nephrotoxic agents, infection, drug toxicity or ischemia-reperfusion, and causes 1.7 million deaths per year, worldwide (1). AKI involves apoptotic or necrotic cell death of renal tubular epithelial cells, especially of the proximal tubules (2). The renal tubular epithelium has an inherent ability to regenerate after AKI, mainly by proliferation of surviving epithelial cells (3). However, tubular repair is often incomplete which can lead to chronic kidney disease (CKD) and end stage renal disease (ESRD) (2, 4). Faulty tubular repair after AKI have been attributed to activation of cell cycle inhibitors, suppression of pro-proliferative factors and G2/M cell cycle arrest (4, 5). Since AKI is often detected in patients after injury has occurred, a better understanding of the pathophysiology of repair following AKI will help to design delayed pharmacological interventions to accelerate repair and minimize the chance of AKI progressing to CKD.
FoxM1 is a forkhead box family transcription factor that regulates apoptosis, cell cycle and oxidative stress (6, 7). FoxM1 regulates S-phase kinase associated protein 2 (Skp2), an E3 ubiquitin protein ligase that degrades the cyclin-dependent kinase (CDK) inhibitors, p27Kip1 and p21waf1/cip1 which control G1/S and G2/M cell cycle transition. Hence FoxM1 inactivation prolongs the G2 phase, delays mitosis and induces mitotic catastrophe which leads to cell death (7–9). FoxM1 global gene knockout in mice is embryonic lethal (10). FoxM1 promotes nuclear recruitment of β-catenin, transcription of Cyclin-B1 and c-Myc (8) and reduces oxidative stress induced premature senescence in tumor cells by regulating reactive oxygen species (ROS) scavengers (11). FoxM1 is upregulated in various cancers and controls tumor growth and metastasis by regulating cell proliferation, apoptosis, oxidative stress and epithelial-to-mesenchymal transition (6, 7). FoxM1 also promotes tissue regeneration of the lungs, liver, pancreas and blood vessels (9, 12–15). In a recent study, siRNA suppression of FoxM1 in vitro was found to reduce cell proliferation of human renal proximal tubular epithelial cells (16). The role of FoxM1 in renal tubular regeneration after AKI is unclear.
GSK3 is a serine-threonine protein kinase consisting of α and β isoforms which play an important role in tubular regeneration in AKI. Multiple studies including our own have demonstrated that systemic pharmacological GSK3 inhibition, or proximal tubule specific GSK3β gene deletion can significantly reduce tubular injury, accelerate regeneration and suppress renal fibrosis following AKI in mouse models (17–23). However, the mechanism by which GSK3β impedes tubular repair after AKI is unclear. We postulated GSKβ could be a regulator of FoxM1 in the AKI kidney because in glioblastoma cell lines, GSKβ phosphorylates FoxM1, which led to ubiquitination and proteolytic degradation of FoxM1 in vitro (24). The current studies examine whether FoxM1 plays an essential role in renal tubular regeneration after AKI, and if GSKβ acts as an upstream regulator of FoxM1.
MATERIALS AND METHODS
In vivo studies:
Study 1: Ischemia/Reperfusion (I/R) induced AKI in wild type mice:
Male, 8–10 week old C57Bl/6J mice (from The Jackson Laboratory) were subjected to bilateral I/R or sham surgeries as we have described before (21). Briefly, mice were anesthetized using xylazine (10 mg/kg, IP) and ketamine (90–120 mg/kg, IP), and injected with analgesic Buprenorphine-Sustained Release (0.5 −1 mg/kg, SC). Ocular lubrication was applied. The anesthetized mouse was maintained at a body temperature of 37°C ± 0.5°C, as measured by a rectal thermometer during the procedure. One kidney at a time, the renal artery and vein were exposed with forceps and a micro-aneurysm clamp was used to clamp the renal pedicle to block blood flow to the kidney. Ischemia was thus induced for 30 minutes followed by release of the clamp for reperfusion. In the sham group, the kidneys were exposed, but renal pedicles were not clamped. Mice were sacrificed on days 3, 6 and 9 after I/R. Kidneys were weighed and flash frozen to determine RNA and protein expression or fixed in freshly prepared 4% paraformaldehyde and blocked in paraffin.
Study 2: Thiostrepton treatment in wild type mice subjected to I/R:
Male C57Bl/6J mice were subjected to sham or I/R surgery as shown above. Mice in the I/R group were treated with vehicle or thiostrepton (#598226; MilliporeSigma MO, USA) administered at a dose of 250mg/Kg BWt. by IP route on days 2, 5 and 8 after I/R. Thiostrepton is stable for 72h (25). The dose for thiostrepton was chosen based on previously published studies in mice (25) and our preliminary studies to confirm FoxM1 inhibition at this dose (data not shown). All mice were sacrificed on day 9
Study 3: TDZD treatment in wild type mice subjected to I/R:
TDZD (TDZD-8 #T8325; Millipore Sigma, MO, USA) was administered daily at a dose of 1mg/Kg by IP route starting on day 2 after I/R till day 9 when mice were sacrificed. This dose of TDZD was selected based on our previous studies which used this dose in mouse AKI studies (19, 21).
Study 4: I/R induced AKI in renal tubule-specific GSK3β gene knockout mice:
GSK3βf/fPax8TetOcre mice (GSK3β-KO) were generated by breeding GSK3βf/f (26) and Pax8TetOcre mice (27). Doxycycline at 1mg/ml dose in 3% sucrose was administered in drinking water, protected from light for 7 days to induce GSK3β gene deletion in 10 weeks old male mice. This dose of doxycycline was sufficient to induce cre in mice as per previous reports (28). Wild type (WT) mice were doxycycline treated GSK3βf/f mice. Male WT and GSK3β-KO mouse littermates were subjected to sham or I/R and sacrificed on day 3, 6 and 9 after I/R.
Study 5: Thiostrepton treatment in GSK3β-KO mice subjected to I/R:
Male WT and GSK3β-KO mouse littermates were subjected to I/R followed by vehicle or thiostrepton (250mg/Kg BWt. by IP) treatments on days 2, 5 and 8. Mice were sacrificed on day 9 after I/R.
All protocols were approved by the IACUC Committee of University of Kansas Medical Center.
Blood Urea Nitrogen (BUN) measurement:
BUN levels were measured in plasma samples following protocol of QuantiChrom Urea Assay Kit (BioAssay Systems, Hayward, CA, USA).
Quantitative real-time PCR (QRT-PCR):
QRT-PCR using cDNA prepared from RNA isolated from whole kidney and cultured cells were carried out as described previously (21). Primer sequences are provided in Supplemental Table-1. The mRNA levels were calculated relative to GAPDH levels for each sample.
Immunoblotting:
Mouse whole kidneys were homogenized, and cells were lysed in SDS Laemmli buffer and loaded onto 10% or 4–20% gradient SDS-polyacrylamide agarose electrophoresis gels as described before (29, 30). Primary antibodies for FoxM1 (sc376471), c-Myc (sc764), p21 (sc6246) and GAPDH (sc32233) from Santa Cruz Biotechnology, TX, USA, and β-catenin (#9582S), Cyclin-D1 (#2978S), p27 (#3686S) and GSK3β (#9832S) from Cell Signaling Technology, Inc., MA, USA and β-actin (#A5441) from MilliporeSigma, Mo, USA; secondary antibodies from Dako (CA, USA) and ECL reagent from PerkinElmer (Netherlands) were used.
Immunostaining:
Fixed and paraffin tissue sections were processed as described before (31, 32). FoxM1 and Ki-67 (cat # 12202S Cell Signaling Technology, Inc., MA, USA) antibodies were used for immunofluorescence (IF) co-staining. For immunohistochemistry, secondary antibodies were applied, followed by incubation with Streptavidin-HRP conjugate (Invitrogen, NY, USA) and slides were developed with DAB (Vector Laboratories) and counterstained with Harris Haematoxylin, dehydrated, and mounted with Permount (Fisher Scientific, NJ, USA). For IF, goat anti-Rabbit IgG fluor and Goat anti-mouse IgG Texas Red (Invitrogen, NY, USA), secondary antibodies were applied, stained with DAPI, and mounted with Flour-G (Invitrogen, NY, USA). All images were captured using a Nikon 80i upright microscope (Tokyo, Japan) in the KUMC imaging center.
TUNEL assay for cell death:
Cell death was determined by in situ Cell Death Detection Kit for TUNEL assay (Roche Applied Science, IN, USA) following the kit protocol.
Morphologic analysis:
Haematoxylin & Eosin (H&E) staining was performed on mouse kidney sections to detect renal injury characterized by tubular dilation, cellular necrosis, and loss of brush border of the proximal tubules. A renal pathologist blinded to the identity of the samples scored the H&E stained sections for tubular damage as follows: 0 = no damage; 1 = 0–25% damaged tubules; 2 = 25–50% damaged tubules; 3= 50–75% damaged tubules; and 4= >75% damaged tubules.
In vitro studies:
Human proximal tubular (HK2) cells (ATCC, VA, USA) were cultured in DME-F12 medium containing 10% fetal bovine serum, 0.5% penicillin and streptomycin. Cells were grown in 0.2% FBS containing media 16 h followed by thiostrepton treatment in the same media. For hypoxia-re-oxygenation studies (33), cells were exposed to hypoxic conditions to 0.5% O2 and 5% CO2 in a hypoxia chamber and for reoxygenation, cells were moved back to normoxic conditions having 21% O2 and 5% CO2. Primary culture normal human kidney (NHK) cells from de-identified patients were from the PKD Biomaterials Core, University of Kansas Medical Center. Cell viability was measured by MTT assay as described before (30). Cell proliferation was measured using BrdU incorporation assay. HK2 cells plated on glass coverslips were treated with BrdU (#10280879001, Millipore sigma) (3μg/ml) for the final 3 hours of incubation, fixed and immunostained for BrdU using anti-BrdU antibody (#5292) as per above mentioned (immunofluorescence) procedure. For quantitation, entire glass coverslips were imaged, from three separate in vitro studies. The number of BrdU positive nuclei/ total nuclei (DAPI stained) were calculated.
Statistical analysis:
Values are expressed as mean ± standard error and analyzed using Graphpad Prism software (Version 5.0d). Two-tailed unpaired t-test with Welch’s correction and One-Way Analysis of Variance (ANOVA) or repeated measures ANOVA followed by Tukey’s multiple comparison tests were used as shown in figure legends. A probability level of 0.05 (p ≤ 0.05) was considered significant.
RESULTS
Increase in renal FoxM1 expression after I/R induced AKI in mice.
To determine renal FoxM1 expression following AKI, time-dependent changes in its mRNA and protein levels were examined. In C57BL/6J mice subjected to bilateral renal I/R, FoxM1 protein levels increased by day 6, reaching significantly high levels (over 2-fold increase) by day 9 after I/R (Fig 1A, B). FoxM1 mRNA levels increased 6-fold by day 3 and 10-fold by days 6 and 9 after I/R, compared to sham and corresponded to increases in pro-proliferative factors, c-Myc, Cyclin-B1, and Skp2 (Fig 1C, Supplemental 1A–D). Skp2 and Cyclin-B1 are known transcriptional targets of FoxM1, while c-Myc is a regulator of FoxM1 expression (34). FoxM1 mRNA levels were reduced significantly by days 12 and 28 but remained significantly higher than sham (Fig 1C, Supplemental-1A). FoxM1 protein was also localized to the nucleus of epithelial cells of some renal tubules in day-9 I/R kidneys (Fig 1D).
Fig 1: FoxM1 inhibition increases renal injury after AKI:

(A) Western blot of kidney tissue lysate of male C57Bl/6J mice which underwent bilateral ischemia by clamping renal pedicles for 30 minutes at 37°C, followed by release for reperfusion for the indicated number of days. (B) Quantitation of band density in kidney tissue lysate. (C) QRTPCR for FoxM1, c-Myc, Skp2 and Cyclin-B1 mRNA relative to GAPDH mRNA n=4 each. (D) Immunofluorescence co-staining for FoxM1(green), E-Cadherin (red) and DAPI (blue) in kidney tissues on day 9 after I/R. (E) H&E staining of kidney tissue of C57/Bl6J mice which underwent bilateral I/R and were treated with vehicle or thiostrepton (Thio −250 mg/Kg BWt., IP on days 2,5 and 8 after I/R) and sacrificed on day 9. ‘G’ and ‘*’ represent glomerulus and dilated tubules respectively. (F) Injury score of H&E stained tissue sections by a pathologist. (G) Kim-1 mRNA relative to GAPDH mRNA in kidney tissue, (H) Ki-67 immunostaining in kidney tissue sections and (I) Quantitation of Ki-67 stained nuclei per kidney section. Scale bar=50μm. *P<0.05, **P<0.01, ***P<0.001 vs sham or vehicle group by t-test.
Pharmacological inhibition of FoxM1 suppresses tubular repair after AKI in mice and epithelial cell proliferation in vitro.
To determine the effect of systemic pharmacological FoxM1 inhibition on tubular regeneration following AKI, C57Bl/6J mice were subjected to bilateral I/R or sham surgeries, followed by treatment with vehicle or thiostrepton, a FoxM1 inhibitor (35). When compared to I/R + vehicle group, The I/R + thiostrepton group showed reduced renal tubular repair suggested by the presence of dilated tubules with flattened brush-borders in day 9 kidney tissue sections (Fig 1E), higher injury score (Fig 1F), and increased kidney injury molecule-1 (Kim-1) mRNA levels (Fig 1G). The I/R + thiostrepton group also showed reduced cell proliferation as suggested by a 50% reduction in Ki-67 positive tubular epithelial cells compared to I/R + vehicle group (Fig 1H, I). Examination of pro-proliferative factors showed significantly reduced protein levels of FoxM1, c-Myc and β-catenin in I/R + thiostrepton group when compared to I/R + vehicle group (Fig 2A–D). On the other hand, the CDK inhibitor p21 was significantly increased in I/R + thiostrepton group (Fig 2A, and E). Although Cyclin-D1 levels showed a decreasing trend in the thiostrepton group, this did not reach significance (Fig 2A and F). mRNA levels of c-Myc, FoxM1 and FoxM1- regulated pro-proliferative genes such as aurora kinase-B (AURKB), polo-like kinase (PLK) and Skp2, were also reduced in thiostrepton group compared to the vehicle group (Fig 2G–K). While BUN levels increased in both I/R + vehicle and I/R + thiostrepton groups compared to sham. The BUN levels were 35% higher in I/R + thiostrepton group than I/R + vehicle group on day 3 and remained higher until day 9 (Supplemental 1E). Thiostrepton treatment also significantly reduced renal mRNA levels of antioxidant enzymes, SOD, catalase, Trx2, GPx2 and Prdx3 (Supplemental 1F), known transcriptional targets of FoxM1 (11).
Fig 2: FoxM1 inhibition reduces renal tubular repair after AKI:

(A) Western blot of kidney tissue lysate of C57/Bl6J mice subjected to bilateral I/R and were treated with vehicle or thiostrepton as in Fig 1. (B), (C), (D), (E) and (F) quantitation of band density. (G) QRTPCR for mRNA relative to GAPDH in kidneys for c-Myc, (H) FoxM1, (I) AURKB, (J) PLK and (K) Skp2. *P<0.05, **P<0.01, ***P<0.001 vs vehicle group by t-test.
To further confirm the role of FoxM1 in cell proliferation, we tested the effect of thiostrepton on human proximal tubular HK2 cells and primary culture normal human kidney (NHK) renal epithelial cells. When HK2 cells were exposed to hypoxia for 24h followed by re-oxygenation for 48h, FoxM1, c-Myc and Cyclin-D1 protein levels increased, while β-catenin levels remained unchanged (Fig 3A, B). Thiostrepton treatment during re-oxygenation reduced FoxM1, c-Myc, Cyclin-D1 and β-catenin expression (Fig 3A, B). Thiostrepton treatment also reduced cell viability of HK2 cells exposed to hypoxia/re-oxygenation by 45% (Fig 3C). Thiostrepton treatment also significantly reduced BrdU incorporation in HK2 cells by 50% at 1μM dose of thiostrepton (Fig 3D, E) suggesting reduced number of cells in the S-phase of cell cycle and hence reduced cell proliferation. In HK2 cells treated with 1.5μM dose of thiostrepton, no cell death was observed by TUNEL assay (Supplemental 3). Furthermore, in primary culture control normal human kidney tubular epithelial cells (NHK cells), FoxM1 expression was detected especially in dividing cells as suggested by FoxM1 and Ki-67 co-staining (Fig 3F). Moreover, thiostrepton dose-dependently reduced cell viability of NHK cells with 50% reduction at 2.5μM dose (Fig 3G). The above in vitro and in vivo data suggest that FoxM1 activity is important for repair and that its inactivation can suppress tubular regeneration following AKI.
Fig 3: Effect of FoxM1 inhibition on cell proliferation of HK2 cells and primary culture normal human kidney (NHK) cells:

(A) Western blot of HK2 cells never exposed to hypoxia (normoxia), or Hy/ReOx- hypoxia 0.5% O2 for 24h followed by reoxygenation (normoxia) for 48h in the presence of vehicle or thiostrepton, and (B) quantitation of band density relative to GAPDH. (C) Cell viability of HK2 cells exposed to Hy/ReOx- as above by MTT assay (thiostrepton= 2.5μM for 48h). N=3 biological replicates and 3 or 4 technical replicates each. (D) Immunostaining for BrdU in HK2 cells treated with thiostrepton at the indicated doses for 48h. cells were treated with BrdU for the last 3 hours of incubation BrdU (green) and DAPI (blue), X 100 original magnification. (E) Quantitation of BrdU staining expressed as % BrdU positive nuclei/ total DAPI stained nuclei. (F) Immunostaining for FoxM1 (green), Ki-67 (red) and DAPI (blue) in NHK cells, Scale bar=25μm, and (G) Cell viability by MTT assay of NHK cells treated with thiostrepton. N= 2 biological replicates and 4 technical replicates each. *P<0.05, *** P<0.001 vs normoxia in B, vs vehicle in C & E and vs 0μM thiostrepton in G by t-test. # # P<0.01, # # # P<0.001 vs Hy/ReOx by t-test in B.
GSK3β is an upstream regulator of renal tubular FoxM1 expression after AKI.
To determine if GSK3 is a regulator of FoxM1 in AKI kidney, wildtype C57Bl/6J mice that had been subjected to I/R were treated with vehicle, or a GSK3 pharmacologic inhibitor, TDZD, starting on day 2 after I/R. By day 9 after I/R, significant increases were noted in the renal FoxM1 and β-catenin protein levels (Fig 4A, B, C) and Ki-67 staining in the TDZD group when compared to the vehicle group (Fig 4D, E). To further examine whether GSK3β regulates FoxM1-dependent tubular repair, we generated a renal tubule-specific and inducible GSK3β gene knockout GSK3βf/fPax8TetOcre (GSK3βKO) mouse with a reverse tetracycline regulated transactivator controlled by Pax8, which is expressed in proximal tubules, distal tubules, and collecting ducts (Supplemental 2A, B). When subjected to I/R, protein levels of FoxM1 increased by day 6 and 9 in WT mice, consistent with Fig 1; with parallel increase in β-catenin and Cyclin-D1 and decrease in p21 and p27 (Fig 4F). Compared to WT littermates, FoxM1 levels in the GSK3βKO mouse kidneys were dramatically increased as early as day 3 after I/R, accompanied by increases in Cyclin-D1 and β-catenin (Fig 4F, Supplemental 4). FoxM1, Skp2 and Cyclin-B1 mRNA levels were also increased in GSK3βKO mice compared to WT mice (Fig 4G). When HK2 cells were treated with TDZD in vitro, FoxM1 protein levels increased (Fig 4H), and was accompanied by increase in HK2 cell viability (Fig 4I), consistent with earlier studies in glioma cells which showed that Wnt signaling mediated GSK3 inhibition leads to increased deubiquitination and stabilization of FoxM1 (24). These results together demonstrate that the increase in renal FoxM1 and pro-proliferative factors after AKI can be enhanced by GSK3β inactivation.
Fig 4: GSK3 inhibition or renal tubule specific GSK3β gene deletion improves repair following I/R induced AKI in mice:
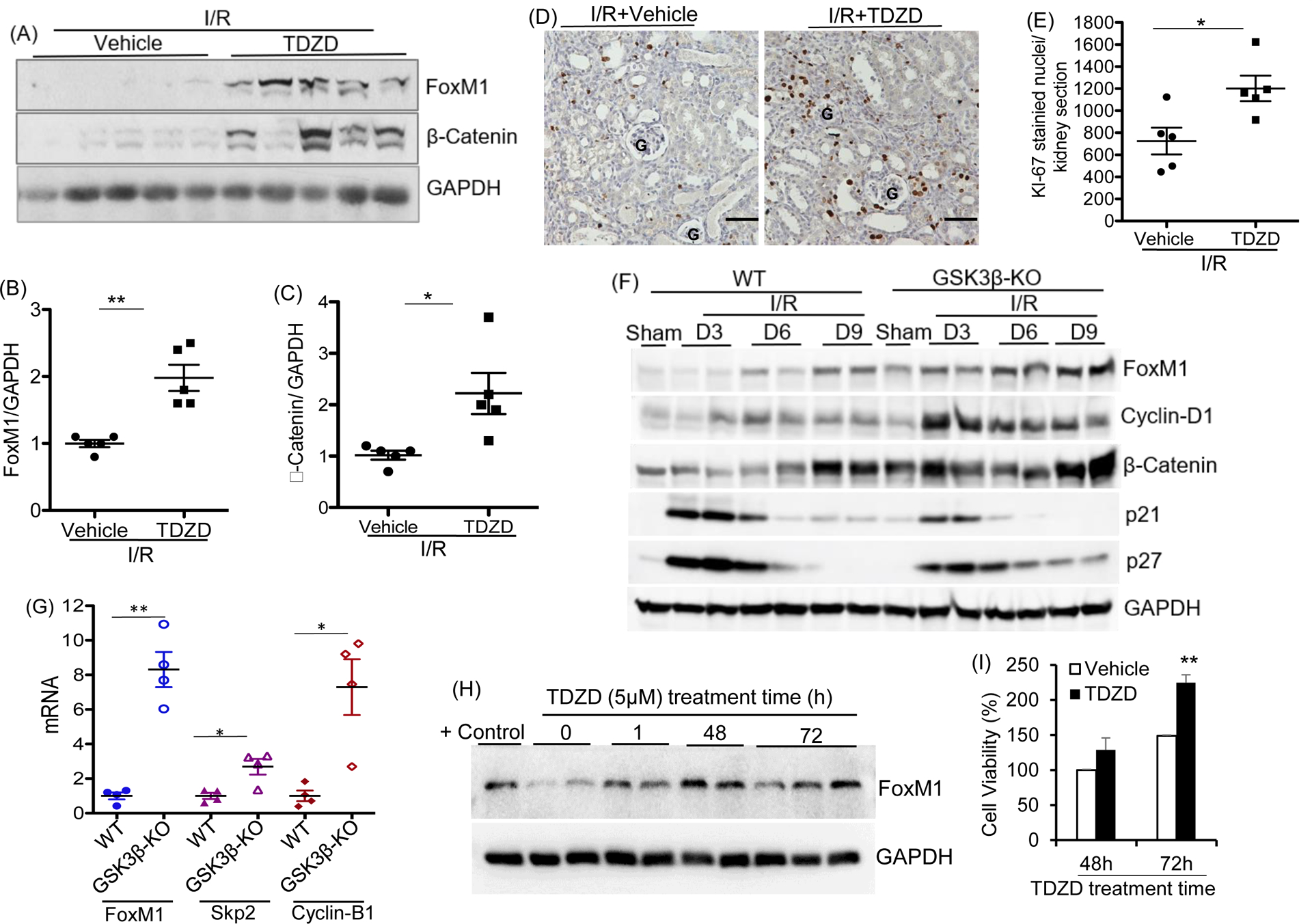
(A) Western blot of kidney tissue lysate of C57/Bl6J mice which underwent bilateral I/R followed by vehicle or GSK3 inhibitor- TDZD (1mg/Kg, IP) treatment starting from day 2 after surgery until sacrifice on day 9. (B) and (C), Densitometry relative to GAPDH. (D) Ki-67 staining of kidney tissue sections. G represents glomerulus Scale bar =50μm. (E) Quantitation of Ki-67 positive stained nuclei per kidney section. (F) Western blot of kidney tissue lysate of GSK3βf/fPax8TetOcre (GSK3β-KO) and WT littermates which underwent bilateral I/R and were sacrificed on D0, D3, D6 and D9. (G) mRNA relative to GAPDH mRNA in whole kidney tissue lysates. (H) Western blot showing time-dependent changes in FoxM1 expression in HK2 cells treated with TDZD-8. MCF7 tumor cell lysate is used as positive control. (I) Cell viability by MTT assay of HK2 cells treated with vehicle or TDZD (5μM for 48 or 72h). n= 6 technical replicates each. *P<0.05, **P<0.01, ***P<0.001 vs vehicle treated at 48h or WT group by t-test.
To further determine if FoxM1 inhibition can abolish the improved repair in renal tubule-specific GSK3β gene knockout mice after AKI, the effect of thiostrepton was tested on GSK3βKO mice. Thiostrepton treatment, starting on day 2 after I/R, abolished the improved repair observed in GSK3βKO mice as demonstrated by reduced pro-proliferative factors (Fig 5A and B), increased injury indicated by H&E staining, injury score and higher Kim-1 expression (Fig 5C, D and E), and reduced KI-67 staining (Fig 5F and G) compared to the I/R + vehicle group. Similarly, the I/R + thiostrepton group showed higher BUN values compared to I/R + vehicle group (Fig 5H, Supplemental 2 C, D and E). These results demonstrate that FoxM1 activity is critical for proper tubular regeneration and that the pro-regenerative effects of GSK3β gene deletion in renal tubules is mediated by FoxM1.
Fig 5: Improved repair in tubule specific GSK3β gene deleted mice is abolished by FoxM1 inhibition:

(A) Western blot of kidney tissue lysate of WT and GSK3β-KO mice were subjected to I/R and treated with vehicle or thiostrepton (250 mg/Kg BWt., IP on days 2,5 and 8 after I/R). (B) quantitation of band density. (C) H&E staining of kidney tissue. ‘G’ and ‘*’ represent glomerulus and dilated tubules respectively. and (D) Injury score of H&E stained tissue sections by a renal pathologist. (E) Kim-1 mRNA relative to GAPDH mRNA in kidney tissue and (F) Ki-67 staining in kidney tissue and (G) Quantitation of Ki-67 positively stained nuclei per kidney section. (H) Plasma BUN levels. Scale bar =50μm. *P<0.05, **P<0.01 ***P<0.001 vs sham by one-way ANOVA for H, vs vehicle, WT or GSK3β-KO by t-test in B, E, D and G.
DISCUSSION
The current study demonstrates that FoxM1 is a key regulator of renal tubular regeneration in AKI, and that GSK3β negatively regulates FoxM1, thereby inhibiting cell proliferation and increasing oxidative stress. We found that: (a) renal FoxM1 expression and activity increase after AKI and correlate with renal cell proliferation, (b) pharmacological FoxM1 inhibition using thiostrepton reduces cell proliferation in vitro, and renal tubular regeneration after I/R induced AKI in mice, and (c) GSK3β is an upstream regulator of FoxM1 since its inactivation increases renal FoxM1 and tubular cell proliferation, and FoxM1 inhibition can abolish the pro-regenerative effects of GSK3β inactivation.
The present study highlights the significant role of FoxM1 in renal tubular epithelial regeneration and its regulation by GSK3β in response to AKI. In a recent study, Chang-Panesso et al. identified FoxM1 as a differentially expressed gene in the proximal tubular cells of Kim1-GFPCreERt2 mice, a Kim1 knock-in mouse, when subjected to I/R (16). They demonstrated that in vitro gene silencing of FoxM1 in proximal tubular epithelial cells inhibited cell viability as determined by MTT assay (16). Our study shows for the first time that FoxM1 activity is essential for tubular repair following AKI because systemic pharmacologic FoxM1 inhibition using thiostrepton inhibited tubular regeneration and reduced renal function after renal I/R in mice. Thiostrepton is a specific inhibitor of FoxM1, which directly binds to its DNA-binding domain, as demonstrated by molecular docking, molecular simulation, affinity pull down assays and isothermal titration calorimetry assays (35, 36). Thiostrepton is a natural cyclic oligopeptide antibiotic that inhibits protein synthesis in prokaryotes by binding to the 23S subunit of ribosomal RNA (37) and can also act as a proteasome inhibitor, inhibiting 20S proteasome (6). However, it is to be considered that thiostrepton’s general properties as an inhibitor of the proteasome and protein synthesis could stabilize positive or negative regulators of FoxM1 as well as other pro- and anti- proliferative factors.
We demonstrate in vitro that FoxM1 inhibition can reduce cell proliferation of primary culture renal tubular epithelial cells and HK2 human proximal tubular cells (Fig 3). FoxM1 is a well-known regulator of cell proliferation. The Costa group first reported embryonic FoxM1 expression including in the renal cortex, with expression limited in adults to routinely proliferative cells of the intestine, colon, testes and thymus (38). Extensive studies in cancer biology have shown that FoxM1 controls transcription of cell cycle regulators such as Cyclin-B1, β-catenin, Skp2, MSH6 and Cyclin-E2. FoxM1 also binds to β-catenin, assisting its nuclear translocation making it a useful target in cancer therapy(6–9, 39–41). FoxM1 also regulates tissue regeneration of the lungs, vasculature, pancreas, intestine and liver (9, 12–15). Multiple in vitro studies have shown that gene silencing of FoxM1 using SiRNA inhibits cell proliferation (42), including reduction of cell viability of renal tubular epithelial cells (16).
In the current study we have determined the role of FoxM1 in epithelial tubular regeneration in response to AKI. FoxM1 is generally not expressed in adult tissues with low cell turnover (34). We found detectable, albeit low levels of FoxM1 mRNA and protein in normal mouse kidneys, which increased significantly after I/R induced AKI. Upregulation of FoxM1 after AKI also correlated with a rise in renal mRNA levels of FoxM1 target genes and other pro-proliferative factors. Moreover, FoxM1 was detected in dividing renal tubular epithelial cells. These findings show that expression and activation of FoxM1 is associated with renal cell proliferation during the injury/repair process in AKI. FoxM1 expression is regulated by KRAS, c-Myc and E2F1 (34), and FoxM1 itself (8). FoxM1 expression occurs in the G1 and S cell-cycle phases, with peak expression during G2-M phase. Post-translation, FoxM1 is sequentially phosphorylated by CDK4/6 and PLK1 to facilitate expression of G1/S phase genes or Cyclin-E-CDK2 / Raf-MEK-ERK for FoxM1 nuclear translocation (43). FoxM1 is repressed by tumor suppressors such as CHK2 and TP53 (34, 43). We found that FoxM1 inhibition suppresses HK2 or NHK cell proliferation in vitro, and reduced tubular repair in mouse kidneys following AKI, indicated by reduced cell proliferation and sustained injury in mice treated with thiostrepton. These studies demonstrate that FoxM1 is important for cell proliferation and repair of tubular epithelial cells in response to AKI.
Tubular injury in AKI is accompanied by endothelial injury, which results in an imbalance in oxygen supply, hypoxia and oxidative stress. Oxidative stress in renal injury occurs due to low antioxidant levels and high free radicals, which induces abnormal cell growth, growth-arrest, apoptosis or necrosis (44). FoxM1 senses ROS levels and reduces oxidative stress by regulating expression of anti-oxidants in tumors and during embryogenesis (11). Consistently, we found that antioxidant associated gene mRNA levels were significantly reduced in the thiostrepton treatment group, which could have contributed to the slower recovery from the I/R induced AKI in these mice.
FoxM1 transcriptional activity is highly dependent on its level, which is a function of a balance between its expression and degradation (34). Embryonic cell signaling pathways such as Wnt, Sonic Hedgehog and Hippo upregulate FoxM1 or reduce its degradation (34). These signaling pathways are generally inactive in differentiated cells of adult kidneys but become active during injury/repair after AKI (2). We found that GSK3β is an important upstream regulator of FoxM1 in AKI. Multiple studies have demonstrated that pharmacological inhibition of GSK3 using isoform non-selective inhibitors can suppress apoptosis and promote renal tubular epithelial regeneration (17–23, 45–48). Our previous studies demonstrated that proximal tubule-specific gene deletion of GSK3β or systemic GSK3 inhibition post-AKI improves regeneration (19) and reduces renal fibrosis in mice (21). However, the molecular pathway by which GSK3β inhibits cell proliferation was unclear.
In vitro studies in glioblastoma cells showed that GSK3β phosphorylates FoxM1 at S474 which promotes its ubiquitination and proteolytic degradation and GSK3 inactivation increases FoxM1 levels (24). Similarly, in human gastric carcinoma cell lines, high glucose and elevated O-GlcNAcylation inactivates GSK3β and stabilizes FoxM1 by reducing its degradation (49). We found a similar effect whereby, treatment of HK2 cells with TDZD increased FoxM1 protein levels within 30 minutes of treatment. Hence, to test if GSK3β regulates cell proliferation by controlling FoxM1, a master cell-cycle regulator, we tested the effect of GSK3β inactivation on regeneration after AKI. We found higher FoxM1 mRNA and protein levels in mice or cells treated with TDZD. Since FoxM1 regulates its own transcription (6), TDZD mediated increase in FoxM1 protein levels could also increase FoxM1 mRNA levels as we found in the kidneys of GSK3βKO mice subjected to I/R.
GSK3 inhibition or gene knockout was also accompanied by increased pro-proliferative factors and cell division. Unlike in WT mice, GSK3β gene knockout mice displayed increased FoxM1 expression as early as day 3, accompanied by increases in Cyclin-D1, c-Myc and β-catenin, and reduced cell cycle inhibitors p21 and p27. Cyclin-D1, c-Myc and β-catenin are also direct substrates of GSK3β that promotes their proteolytic degradation (50, 51). Importantly, this improved condition for cell proliferation afforded by GSK3β gene knockout was abolished by FoxM1 inhibitor treatment, evidenced by higher BUN, Kim-1 and kidney morphology, as well as reduced proliferative factors in thiostrepton-treated GSK3β knockout mice. Thus, during renal tubular repair, cells undergoing proliferation induce signals to re-express FoxM1 which is naturally inhibited by GSK3β, leading to delayed cell proliferation.
The EGFR and Wnt signaling pathways are well-known regulators of renal tubular regeneration after AKI (52–55) . These pathways also independently modulate GSK3 activity (51, 56) . Chang-Panesso et al. showed that FoxM1 expression could be regulated by EGFR in AKI kidneys because an EGFR inhibitor reduced FoxM1 mRNA levels (16). The FoxM1/ ADAM17 axis is known to drive EGFR/AKT/GSK3 signaling and maintain FoxM1 stability, leading to mesenchymal transition of glioma cell lines (57). Moreover, gene silencing of FoxM1 or ADAM17 reduce pEGFR, pAKT and pGSK3, while overexpression of FoxM1 or ADAM17 can increase pEGFR, pAKT and pGSK3. Thus, while GSK3 inhibits FoxM1 (24), FoxM1 can promote EGFR and AKT activity, and inhibit GSK3 (57). In conclusion, our study reveals a novel and critical role of FoxM1 in renal epithelial tubular regeneration following an episode of AKI, by stimulating cell proliferation and suppressing oxidative stress. We also identified that GSK3β is a natural inhibitor of FoxM1, and the GSK3β-FoxM1 axis can be a potential therapeutic target in treating AKI.
Supplementary Material
ACKNOWLEDGMENTS:
We thank the University of Kansas PKD Biomarkers and Biomaterials Core (P30 DK106912) for providing primary culture normal kidney tubular epithelial cells. This study was supported by American Heart Association’s Grant in Aid and NIH R01-DK083525 to RR. ND was supported by American Heart Association’s Postdoctoral Fellowship Grant.
ABBREVIATIONS:
- AKI
Acute kidney injury
- AURKB
Aurora kinase-B
- BUN
Blood urea nitrogen
- CKD
Chronic kidney disease
- CDK
Cyclin-dependent kinase
- ESRD
End stage renal disease
- FoxM1
Forkhead box family member transcription factor
- GSK3
Glycogen synthase kinase-3
- I/R
Ischemia/reperfusion (I/R)
- Kim-1
kidney injury molecule-1
- PLK
Polo-like kinase
- ROS
Reactive oxygen species
- Skp2
S-phase kinase associated protein 2
REFERENCES
- 1.Abd ElHafeez S, Tripepi G, Quinn R, Naga Y, Abdelmonem S, AbdelHady M, Liu P, James M, Zoccali C, and Ravani P (2017) Risk, Predictors, and Outcomes of Acute Kidney Injury in Patients Admitted to Intensive Care Units in Egypt. Scientific reports 7, 17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, Sun L, Peng Y, Liu F, Venkatachalam MA, and Dong Z (2017) AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney international 92, 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, and Bonventre JV (2008) Intrinsic epithelial cells repair the kidney after injury. Cell stem cell 2, 284–291 [DOI] [PubMed] [Google Scholar]
- 4.Ferenbach DA, and Bonventre JV (2015) Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nature reviews. Nephrology 11, 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Besschetnova TY, Brooks CR, Shah JV, and Bonventre JV (2010) Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine 16, 535–543, 531p following 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halasi M, and Gartel AL (2013) Targeting FOXM1 in cancer. Biochem Pharmacol 85, 644–652 [DOI] [PubMed] [Google Scholar]
- 7.Liao GB, Li XZ, Zeng S, Liu C, Yang SM, Yang L, Hu CJ, and Bai JY (2018) Regulation of the master regulator FOXM1 in cancer. Cell Commun Signal 16, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Wu F, Tan Q, Guo M, Ma P, Wang X, Zhang S, Xu J, Luo P, and Jin Y (2019) The multifaceted roles of FOXM1 in pulmonary disease. Cell Commun Signal 17, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Quail E, Hung NJ, Tan Y, Ye H, and Costa RH (2001) Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proceedings of the National Academy of Sciences of the United States of America 98, 11468–11473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, Yoder HM, Kiyokawa H, Kaestner KH, and Costa RH (2004) The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Developmental biology 276, 74–88 [DOI] [PubMed] [Google Scholar]
- 11.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, and Raychaudhuri P (2009) FoxM1, a critical regulator of oxidative stress during oncogenesis. The EMBO journal 28, 2908–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann Misfeldt A, Costa RH, and Gannon M (2008) Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 57, 3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalinichenko VV, Lim L, Shin B, and Costa RH (2001) Differential expression of forkhead box transcription factors following butylated hydroxytoluene lung injury. American journal of physiology. Lung cellular and molecular physiology 280, L695–704 [DOI] [PubMed] [Google Scholar]
- 14.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, and Malik AB (2006) Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. The Journal of clinical investigation 116, 2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zu G, Guo J, Zhou T, Che N, Liu B, Wang D, and Zhang X (2019) The transcription factor FoxM1 activates Nurr1 to promote intestinal regeneration after ischemia/reperfusion injury. Exp Mol Med 51, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang-Panesso M, Kadyrov FF, Lalli M, Wu H, Ikeda S, Kefaloyianni E, Abdelmageed MM, Herrlich A, Kobayashi A, and Humphreys BD (2019) FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. The Journal of clinical investigation 129, 5501–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao H, Ge Y, Wang Z, Zhuang S, Dworkin L, Peng A, and Gong R (2014) Delayed administration of a single dose of lithium promotes recovery from AKI. Journal of the American Society of Nephrology : JASN 25, 488–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao H, Ge Y, Zhuang S, Dworkin LD, Liu Z, and Gong R (2012) Inhibition of glycogen synthase kinase-3beta prevents NSAID-induced acute kidney injury. Kidney international 81, 662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard C, Tao S, Yang HC, Fogo AB, Woodgett JR, Harris RC, and Rao R (2012) Specific deletion of glycogen synthase kinase-3beta in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney international 82, 1000–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plotnikov EY, Grebenchikov OA, Babenko VA, Pevzner IB, Zorova LD, Likhvantsev VV, and Zorov DB (2013) Nephroprotective effect of GSK-3beta inhibition by lithium ions and delta-opioid receptor agonist dalargin on gentamicin-induced nephrotoxicity. Toxicology letters 220, 303–308 [DOI] [PubMed] [Google Scholar]
- 21.Singh SP, Tao S, Fields TA, Webb S, Harris RC, and Rao R (2015) Glycogen synthase kinase-3 inhibition attenuates fibroblast activation and development of fibrosis following renal ischemia-reperfusion in mice. Disease models & mechanisms 8, 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Huang WC, Wang CY, Tsai CC, Chen CL, Chang YT, Kai JI, and Lin CF (2009) Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. British journal of pharmacology 157, 1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Ge Y, Bao H, Dworkin L, Peng A, and Gong R (2013) Redox-sensitive glycogen synthase kinase 3beta-directed control of mitochondrial permeability transition: rheostatic regulation of acute kidney injury. Free radical biology & medicine 65, 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A, Lin K, Zhang S, Zhang N, Gottardi CJ, and Huang S (2016) Wnt-induced deubiquitination FoxM1 ensures nucleus beta-catenin transactivation. The EMBO journal 35, 668–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed M, Uddin S, Hussain AR, Alyan A, Jehan Z, Al-Dayel F, Al-Nuaim A, Al-Sobhi S, Amin T, Bavi P, and Al-Kuraya KS (2012) FoxM1 and its association with matrix metalloproteinases (MMP) signaling pathway in papillary thyroid carcinoma. J Clin Endocrinol Metab 97, E1–E13 [DOI] [PubMed] [Google Scholar]
- 26.Rao R, Patel S, Hao C, Woodgett J, and Harris R (2010) GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. Journal of the American Society of Nephrology : JASN 21, 428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma M, Tian X, Igarashi P, Pazour GJ, and Somlo S (2013) Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nature genetics 45, 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merentie M, Rissanen R, Lottonen-Raikaslehto L, Huusko J, Gurzeler E, Turunen MP, Holappa L, Makinen P, and Yla-Herttuala S (2018) Doxycycline modulates VEGF-A expression: Failure of doxycycline-inducible lentivirus shRNA vector to knockdown VEGF-A expression in transgenic mice. PloS one 13, e0190981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakade VR, Tao S, Rajagopal M, Zhou X, Li X, Yu AS, Calvet JP, Pandey P, and Rao R (2016) A cAMP and CREB-mediated feed-forward mechanism regulates GSK3beta in polycystic kidney disease. Journal of molecular cell biology 8, 464–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Dwivedi N, Tao S, Jamadar A, Kakade VR, Neil MO, Weiss RH, Enders J, Calvet JP, Thomas SM, and Rao R (2019) Targeting the vasopressin type-2 receptor for renal cell carcinoma therapy. Oncogene [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norregaard R, Tao S, Nilsson L, Woodgett JR, Kakade V, Yu AS, Howard C, and Rao R (2015) Glycogen synthase kinase 3alpha regulates urine concentrating mechanism in mice. American journal of physiology. Renal physiology 308, F650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, and Rao R (2015) Glycogen synthase kinase-3beta promotes cyst expansion in polycystic kidney disease. Kidney international 87, 1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Xun L, Jin G, and Shi L (2018) Salidroside protects renal tubular epithelial cells from hypoxia/reoxygenation injury in vitro. J Pharmacol Sci 137, 170–176 [DOI] [PubMed] [Google Scholar]
- 34.Kelleher FC, and O’Sullivan H (2016) FOXM1 in sarcoma: role in cell cycle, pluripotency genes and stem cell pathways. Oncotarget 7, 42792–42804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegde NS, Sanders DA, Rodriguez R, and Balasubramanian S (2011) The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem 3, 725–731 [DOI] [PubMed] [Google Scholar]
- 36.Kongsema M, Wongkhieo S, Khongkow M, Lam EW, Boonnoy P, Vongsangnak W, and Wong-Ekkabut J (2019) Molecular mechanism of Forkhead box M1 inhibition by thiostrepton in breast cancer cells. Oncol Rep 42, 953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonker HR, Ilin S, Grimm SK, Wohnert J, and Schwalbe H (2007) L11 domain rearrangement upon binding to RNA and thiostrepton studied by NMR spectroscopy. Nucleic Acids Res 35, 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye H, Kelly TF, Samadani U, Lim L, Rubio S, Overdier DG, Roebuck KA, and Costa RH (1997) Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Molecular and cellular biology 17, 1626–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez-Fernandez M, and Medema RH (2013) Novel functions of FoxM1: from molecular mechanisms to cancer therapy. Front Oncol 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, and Costa RH (2005) Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Molecular and cellular biology 25, 10875–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu C, Chen L, Yie L, Wei L, Wen T, Liu Y, and Chen H (2015) Targeting FoxM1 inhibits proliferation, invasion and migration of nasopharyngeal carcinoma through the epithelialto-mesenchymal transition pathway. Oncol Rep 33, 2402–2410 [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Shi Y, Yan J, and Fan H (2018) Downregulation of FoxM1 inhibits cell growth and migration and invasion in bladder cancer cells. Am J Transl Res 10, 629–638 [PMC free article] [PubMed] [Google Scholar]
- 43.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P, and Sicinski P (2011) A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer cell 20, 620–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratliff BB, Abdulmahdi W, Pawar R, and Wolin MS (2016) Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox Signal 25, 119–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T, Fang Y, Liu S, Yu X, Zhang H, Liang M, and Ding X (2014) Limb ischemic preconditioning protects against contrast-induced acute kidney injury in rats via phosphorylation of GSK-3beta. Free radical biology & medicine [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Zhu Y, Wang L, Hou J, Gao Y, Shen L, and Zhang J (2017) Effects of chronic alcohol exposure on ischemia-reperfusion-induced acute kidney injury in mice: the role of beta-arrestin 2 and glycogen synthase kinase 3. Exp Mol Med 49, e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, and Borkan SC (2010) GSK3{beta} promotes apoptosis after renal ischemic injury. Journal of the American Society of Nephrology : JASN 21, 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao K, Chen C, Shi Q, Deng W, Zuo T, He X, Liu T, Zhao L, and Wang W (2014) Inhibition of glycogen synthase kinase-3beta attenuates acute kidney injury in sodium taurocholateinduced severe acute pancreatitis in rats. Mol Med Rep 10, 3185–3192 [DOI] [PubMed] [Google Scholar]
- 49.Inoue Y, Moriwaki K, Ueda Y, Takeuchi T, Higuchi K, and Asahi M (2018) Elevated O-GlcNAcylation stabilizes FOXM1 by its reduced degradation through GSK-3beta inactivation in a human gastric carcinoma cell line, MKN45 cells. Biochem Biophys Res Commun 495, 1681–1687 [DOI] [PubMed] [Google Scholar]
- 50.Cormier KW, and Woodgett JR (2017) Recent advances in understanding the cellular roles of GSK-3. F1000Res 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel P, and Woodgett JR (2017) Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr Top Dev Biol 123, 277–302 [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Chen JK, and Harris RC (2012) Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney international 82, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, and Harris RC (2016) Interaction of the EGF Receptor and the Hippo Pathway in the Diabetic Kidney. Journal of the American Society of Nephrology : JASN 27, 1689–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, You H, Li Y, Xu Y, He Q, and Harris RC (2018) EGF Receptor-Dependent YAP Activation Is Important for Renal Recovery from AKI. Journal of the American Society of Nephrology : JASN 29, 2372–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou D, Tan RJ, Fu H, and Liu Y (2016) Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Laboratory investigation; a journal of technical methods and pathology 96, 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H, Ding Q, and Xia J (2016) Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion via the EGFR/GSK-3beta/Foxp3 Axis. The Journal of biological chemistry 291, 21085–21095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Han X, Xu X, Zhou Z, Chen X, Tang Y, Cheng J, Moazzam NF, Liu F, Xu J, Peng W, Du F, Zhang B, Song Z, Zeng J, and Gong A (2018) FoxM1 drives ADAM17/EGFR activation loop to promote mesenchymal transition in glioblastoma. Cell Death Dis 9, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


