Abstract
Background:
Chronic, excessive alcohol drinkers, even without dependence, can exhibit changes in behavior and neurochemical systems. Identifying these changes and their relationship with one another could provide novel avenues for the prevention and treatment of alcohol use disorder. We recently demonstrated, in rats, that neurotensin (NTS) in the paraventricular thalamus (PVT) regulates excessive ethanol drinking. Here, we investigate the effects of chronic ethanol drinking on the PVT-NTS system and its contribution to ethanol-induced behavioral changes.
Methods:
We gave adult male Long-Evans rats 20% ethanol under the intermittent-access two-bottle-choice paradigm or maintained them on chow and water for up to 11 weeks. Prior to ethanol exposure and following several weeks of access, during acute abstinence, we tested these groups for multiple behaviors. In the 12th week, during acute abstinence, we examined gene expression and peptide levels of NTS and its receptors in the anterior and posterior subregions of the PVT. Finally, in chronic ethanol drinkers, during acute abstinence, we microinjected the NTS receptor type 2 (NTS2R) agonist, JMV-431, in the anterior PVT (aPVT), and examined subsequent ethanol intake and behavior.
Results:
Following chronic intermittent ethanol access, rats were classified by cluster analysis as high or low ethanol drinkers. High ethanol drinkers spent more time in the light chamber of a light-dark box and open arms of an elevated plus maze and entered fewer familiar holes in a hole-board apparatus. These differences were absent prior to ethanol exposure but were detectable as early as 4 weeks into drinking. Time in the light chamber following chronic drinking also predicted level of subsequent drinking. High ethanol drinkers also showed elevated protein levels of NTS2R in the aPVT, and pharmacological stimulation of aPVT NTS2R in low drinkers mimicked the increased time spent in the light chamber that was observed in high drinkers.
Conclusions:
Our findings suggest that chronic, excessive, but not lower level, ethanol drinking induces heightened or flexible exploratory behavior, which predicts future ethanol drinking and is partly mediated by elevated NTS2R signaling in the aPVT. These ethanol-induced alterations represent adaptations that could perpetuate excessive drinking and lead to the development of ethanol dependence.
Keywords: acute abstinence, elevated plus maze, individual differences, JMV-431, light-dark box
Excessive alcohol drinking is widely prevalent in the United States, with 26.5% of adults engaging in binge drinking, involving four or more drinks on a single occasion, and 6.6% engaging in heavy drinking, involving five or more binge drinking days in a month (Substance_Abuse_and_Mental_Health_Services_Administration, 2019). Excessive drinkers are at higher risk for developing several diseases, including cardiovascular disease, certain cancers, and alcohol dependence, highlighting the importance of understanding the characteristics that define these individuals (Kimbrough et al., 2017, World_Health_Organization, 2018). Clinical evidence suggests that even prior to alcohol dependence, chronic excessive drinkers exhibit changes in emotion and motivation (Field et al., 2008, Leone et al., 2016, Montgomery et al., 2012). Understanding these alcohol-induced changes and their underlying neurobiology may be useful for the prevention and treatment of alcohol dependence.
Preclinical studies have begun to identify behavioral changes that emerge after chronic excessive drinking, during acute abstinence (24 – 48 hours after ethanol access). Following 6–7 weeks of drinking under a modified intermittent-access two-bottle-choice (IA2BC) paradigm, high compared to low ethanol drinking rats showed increased sign tracking in a Pavlovian conditioned approach test (Spoelder et al., 2017), suggesting enhanced cue driven behavior, and lower anxiety and shelter seeking in a multivariate concentric square field (Momeni and Roman, 2014), suggesting enhanced exploration and risk assessment (Meyerson et al., 2006); and following 6 weeks in a drinking-in-the-dark paradigm, ethanol compared to water drinking mice traveled more in the light side of a light-dark box (Lee et al., 2015), suggesting lower anxiety and enhanced exploratory drive (Crawley, 1985). Thus, in rodents, repeated excessive ethanol drinking for six or more weeks induces behaviors that, in part, reflect enhanced exploratory motivation.
Studies in non-dependent excessive drinkers have suggested that alcohol-induced changes in behavior are linked to changes in the corticolimbic system (Arienzo et al., 2019, Cservenka and Brumback, 2017, de Guglielmo et al., 2016, Hu et al., 2018, Klenowski et al., 2016), with rodent studies establishing this with neurotrophic factors, neurotransmitters, and neuropeptides (Darcq et al., 2015, George et al., 2012, Ketchesin et al., 2016, Lee et al., 2018). Although these studies used drinking models that yielded overall high ethanol consumption, it remains unclear how these neurobiological changes may manifest differently with the higher compared to lower drinking that occurs within a heterogenous population. Like humans, among adult Long-Evans rats with access to 20% ethanol under the IA2BC paradigm, about 30% consume ethanol at binge levels and do so at each of the three weekly exposures. This heterogeneity allows investigation of characteristics of chronic excessive drinkers, separate from lower drinkers (Barson and Leibowitz, 2015, Pandey et al., 2019, Simms et al., 2008), which could ultimately lead to a mechanistic understanding of individual differences in the predisposition for development of dependence. Using this model, we recently discovered that excessive ethanol drinking is regulated by the neuropeptide neurotensin (NTS) in the paraventricular thalamus (PVT), a limbic region that also expresses the two major NTS receptors (NTS1R and NTSR2R) (Pandey et al., 2019). Thus, a change in the PVT-NTS system could represent one neuroadaptation that mediates behavioral changes induced by chronic excessive drinking.
In this study, we sought to identify behavioral changes that emerge following chronic ethanol drinking and determine if the PVT-NTS system is involved in these changes. We gave Long-Evans rats access to 20% ethanol under the IA2BC paradigm or maintained them on water and chow for up to 11 weeks, and we examined their behaviors and neurochemicals at different time points during this period. We hypothesized that chronic high but not low ethanol drinkers would show changes in exploratory behavior and that this would be mediated by neuroadaptations in the PVT-NTS system, which may be restricted to either the anterior PVT (aPVT), involved in arousal (Cheng et al., 2018, Flagel et al., 2011), or posterior PVT (pPVT), involved in stress (Barson and Leibowitz, 2015, Li et al., 2014, Meffre et al., 2019).
MATERIALS AND METHODS
Subjects
Adult, male Long-Evans rats (N = 117; 225 – 250 g, Charles River Laboratories International, Inc., Malvern, PA, USA) were individually housed in an AAALAC-accredited facility, on a 12-hour reversed light/dark cycle (lights off at 0900 h). They were given one week to acclimate to the facility and were handled daily prior to the start of experiments. Rats received ad libitum chow (Laboratory Rodent Diet 5001, Lab Diet, St. Louis, MO, USA) and water, unless otherwise specified. Experiments were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine and followed the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Protocols
Experiment 1:
To identify characteristics of high and low chronic ethanol drinkers, rats with access to 20% ethanol under the IA2BC paradigm (ethanol drinkers, n = 18) and those maintained on chow and water (water drinkers, n = 8) were examined in behavioral tests, each on separate days, 24–27 hours after the last ethanol access (acute abstinence), starting at a time when they would normally receive ethanol. During the 9th week of access, animals were tested in a locomotor activity chamber (AC), light-dark box (LDB), and elevated plus maze (EPM), and during the 10th week, in a hole-board apparatus (HBA). The LDB, EPM, and HBA are considered unconditioned tests of anxiety that involve elements of exploration, while the AC provides a control measure of locomotor activity (Bourin, 2015, Sousa et al., 2006, van der Staay et al., 2012). On days of behavioral testing, scheduled ethanol access was provided after each test.
Experiment 2:
To determine if behaviors characteristic of high ethanol drinkers exist prior to ethanol exposure or emerge over the course of drinking, new ethanol drinkers (n = 24) and water drinkers (n = 8) were examined in behavioral tests. While ethanol-naïve (Week 0), they were tested in an AC, LDB, EPM, and HBA; during the 4th week of ethanol (or water) access during acute abstinence they were tested in an AC and LDB; and during the 9th week during acute abstinence they were tested in an AC and EPM. Data from the HBA could not be used due to a technical error.
Experiment 3:
To determine the effects of chronic ethanol drinking on the PVT-NTS system, rats from Experiment 1 (n = 19 ethanol drinkers, 8 water drinkers) were sacrificed on the 12th week of ethanol access, during acute abstinence, and their aPVT and pPVT were examined for mRNA levels of NTS, NTS1R, and NTS2R, using quantitative real-time polymerase chain reaction (qRT-PCR).
Experiment 4:
Following up on the observed effects on NTS2R mRNA, rats from Experiment 2 (n = 17 ethanol drinkers, 6 water drinkers) were sacrificed on the 12th week of ethanol access, during acute abstinence, and their aPVT and pPVT were examined for protein levels of NTS2R, using Western blotting.
Experiment 5:
To determine if NTS2R signaling in the aPVT influences behavior, new rats (N = 25), with a history of IA2BC ethanol drinking, were injected in the aPVT with an NTS2R agonist prior to behavioral assessments. During the 6th week of ethanol access, animals were cannulated in the aPVT. During the 8th week, they were injected in a within-subject Latin-square design across three ethanol access days, with saline vehicle (0.3 μl) counterbalanced against the NTS2R agonist JMV-431 (0.1 and 1.0 μg; NIMH J-901; RTI International, Research Triangle Park, NC, USA) and their subsequent ethanol, food, and water intake was monitored. During the 10th week, they were tested during acute abstinence in the LDB, first for baseline performance and then, over the next two days, following counter-balanced within-subject injection with vehicle (0.3 μl) or JMV-431 (1.0 μg). During the 12th week, animals were sacrificed, and their brains harvested to verify cannula placement. Fluorescent histochemistry was used to determine the localization of NTS2R within the PVT subregions.
Ethanol Drinking
Rats were given unsweetened 20% v/v ethanol under the IA2BC paradigm adapted from Wise (1973) and Simms (2008) and previously used in our laboratory (Gupta et al., 2018, Pandey et al., 2019). They had three 24-hour ethanol access sessions each week (Monday, Wednesday, and Friday or Tuesday, Thursday, and Saturday), starting one hour into the dark cycle. Animals were weighed twice per week. Ethanol intake was calculated as: (weight ethanol solution consumed (g) * (density ethanol * 0.20)) / rat body weight (kg). Ethanol preference was calculated as: volume ethanol solution consumed (ml) / (volume ethanol solution consumed (ml) + volume water consumed (ml)). To determine blood ethanol concentration (BEC) during acute abstinence, trunk blood was obtained 24–27 hours after the last ethanol access and analyzed with an Analox AM1 Alcohol Analyzer (Lunenburg, MA, USA).
Behavioral Testing
All behavioral tests were conducted in a sound- and light-attenuated room (< 5 lux), starting one hour into the dark cycle, and 24–27 hours into abstinence. One day prior to testing, animals in their home cage were acclimated to the testing room for 30 minutes, and on test days, they were acclimated for 5 minutes prior to testing.
Activity Chamber (AC):
Locomotor activity was assessed in an automated activity chamber with an area of 43.2 cm x 43.2 cm and 42 cm high walls (Med Associates, Inc., St. Albans, VT, USA). Animals were placed at the center of the chamber and allowed to explore for 10 minutes, while ambulatory time and distance were measured, to ensure that locomotor ability was not a confound for the other tests. Research suggests that behavior in an AC does not significantly change on repeat testing (Bronstein, 1972).
Light-Dark Box (LDB):
A light/dark insert (Med Associates, Inc., St. Albans, VT, USA) was placed in the same chamber used for locomotor activity testing, to create a two-chamber light-dark box. A lamp was placed directly above the light chamber, creating a luminous intensity of approximately 700 lux in the center and 450 lux in the corners. Animals were placed in a corner of the box and allowed to explore for 10 minutes, while time spent in the light chamber and number of entries into the two chambers, both measures of anxiety and exploration (Crawley, 1985), were measured. In Experiment 1, they were placed in the light chamber, but because one (identified as a statistical outlier) demonstrated total freezing, animals in Experiments 2 and 5 were placed in the dark chamber. Research suggests that behavior in the LDB does not significantly change on repeat testing (Holmes et al., 2001, Onaivi and Martin, 1989).
Elevated Plus Maze (EPM):
The automated elevated plus maze (Med Associates, Inc., St. Albans, VT, USA) consisted of four arms (each 50 cm long and 10 cm wide), separated by a central junction (10 cm x 10 cm), and standing 74 cm above the ground. The two open arms had 1 cm high ledges and the two closed arms had 43 cm-high opaque walls. Each animal was placed in the junction and left to explore for 5 minutes, while time spent in and number of entries into the open arms were measured as indicators of anxiety and exploration, and number of entries into the closed arms and both arms together were measured as indicators of locomotor activity (Bourin, 2015). While animals generally spend less time in the open arms on retest in an EPM, this can be circumvented by waiting > 3 weeks and testing in a different environment (Adamec and Shallow, 2000, Adamec et al., 2005, Schneider et al., 2011). In Experiment 2, for retest (9 weeks after the first test), the EPM was reoriented and placed in a different corner of the room.
Hole Board Apparatus (HBA):
A hole-board insert (Med Associates, Inc., St. Albans, VT, USA), comprised of 16 equidistant receptacle holes (3 cm in diameter) on a 43 cm x 43 cm metal platform, was placed in the same chamber used for locomotor activity testing. The hole-board stood 5 cm above the floor of the chamber and was not baited. Each animal was placed in the corner of the chamber and allowed to explore for 5 minutes, while head dipping into novel and total holes were recorded as indicators of exploration and repeat holes were recorded as an indicator of working memory error (van der Staay et al., 2012).
Quantitative Real-Time -Polymerase Chain Reaction (qRT-PCR)
The aPVT (Bregma −1.5 to −2.5 mm) and pPVT (Bregma −2.5 to −3.5 mm) (Paxinos and Watson, 2005) were harvested and processed for mRNA analysis using qRT-PCR, as described (Pandey et al., 2019). Samples showed A260/A280 ratios between 1.78 and 2.20, indicating high purity. Target mRNA expression was quantified relative to cyclophilin-A using the relative quantification method (ΔΔCT), as in our prior investigation of the PVT-NTS system (Pandey et al., 2019). Primers were designed with the NCBI Primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Ye et al., 2012), and acquired from Invitrogen at ThermoFisher Scientific (Pittsburgh, PA, USA) (Pandey et al., 2019) (Table 1).
Table 1.
Primer sequences and concentrations used for quantitative real-time polymerase chain reaction in Experiment 3.
| Primer | Sequence | Concentration |
|---|---|---|
| Cyclophilin-A | 5′-GTGTTCTTCGACATCACGGCT-3′ (forward) 5′-CTGTCTTTGGAACTTTGTCTGCA-3′ (reverse) |
200 nM |
| Neurotensin | 5′-CATCGAAGGTCAGCAAAGGAA-3′ (forward) 5′-GGTCGTCATCACGCATTTCTC-3′ (reverse) |
100 nM |
| Neurotensin Receptor Type 1 | 5′-AAGCAGGCACCCTTCATCT-3′ (forward) 5′-GGAGGCTGGATGGTTCTGT-3′ (reverse) |
100 nM |
| Neurotensin Receptor Type 2 | 5′-GAATGTGCTGGTGTCCTTCGC-3′ (forward) 5′-ACTTGTATTTCTCCCAGGCTG-3′ (reverse) |
100 nM |
Primers used for quantitative real-time polymerase chain reaction
Western Blotting
The aPVT and pPVT were dissected out after rapid decapitation, immediately frozen on dry ice, and stored at −80 °C, until protein quantification using Western blotting. Each sample was homogenized in ice-cold 1X RIPA buffer (ThermoFisher Scientific) containing a phosphatase/protease inhibitor cocktail (Pierce™ Protease and Phosphatase Inhibitor Mini Tablets, ThermoFisher Scientific), and lysed briefly with a sonicator. Samples were then centrifuged at 20,000 rpm for 20 minutes at 4 °C and the supernatant (lysate) was used for measuring total protein concentration, using a standard BCA assay (Pierce™ BCA Protein Assay Kit, ThermoFisher Scientific).
The proteins of interest (NTS2R and GAPDH) were separated using a standard SDS-PAGE method, by running 60 μg of tissue lysate on 4 –15% Mini-PROTEAN® TGX™ Precast Protein Gels (BioRad, Hercules, CA, USA) for two hours at 90 mV. Samples for each PVT subregion were randomly distributed across 4 blots, such that each blot contained an approximately equal number of samples from each group. Two samples were omitted due to issues with tissue extraction. Proteins in the gel were transferred to a polyvinylidene difluoride (PVDF) membrane (BioRad) using the wet transfer system, running overnight at 30 mV at 4 °C. The membrane was then blocked in a blocking solution (5% w/v non-fat dry milk (LabScientific, Highlands, NJ, USA) in 1X TBS containing 0.2% Tween-20 (TBST)), for 1 hour at room temperature and then incubated with primary antibodies (rabbit polyclonal anti-NTS2R (1:500, Cat # AP01326PU-N, Origene, Rockville, MD, USA) and mouse monoclonal anti-GAPDH (1:10,000, Cat # ab8245, Abcam, Cambridge, MA, USA)) for 24 hours at 4 °C. It was then washed in 1X TBST (three 5 min washes) with vigorous shaking on an orbital shaker, incubated in the IRDye® 800CW goat anti-rabbit secondary antibody and IRDye 680RD goat anti-mouse secondary antibody (1:15,000, LI-COR, Lincoln, NE, USA) for 1 hour at room temperature, and washed again in 1X TBST (three 5 min washes) and 1X TBS (one 5 min wash).
The antibody for NTS2R was raised against a synthetic peptide corresponding to amino acids (aa) 151–200 of human NTS2R and specifically bound the region surrounding Asp184. To validate its specificity in rats, a protein-protein BLAST was run with the human aa151–200 as a “query sequence”, against the rat (Rattus norvegicus) RefSeq protein database (NIH, NCBI pBLAST). The BLAST found an 88.64% identity match with the rat NTS2R protein sequence ranging aa157–200 (E value 6e-21). The only other protein showing any identity match was rat NPAT, with a 50% identity match and E value of 8.5. For additional validation, the sample lysates were run alongside HeLa cell lysate (negative control), which showed no band at 45 kDa, and rat whole brain lysate (post-natal day 21) (positive control), which showed a strong band in that region.
Western blots were imaged using Image Studio software and analyzed using Empiria Studio software (LI-COR Odyssey Clx). For each blot, the lane normalization factor was determined using the highest GAPDH signal intensity for the blot and used to calculate the normalized NTS2R signal intensity for each sample.
Fluorescent Histochemistry
Rats were anesthetized under isoflurane and then decapitated. Brains were extracted, fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 5 days at 4 °C, cryoprotected in 30% sucrose for 4 days at 4 °C, and then frozen at −80 °C until sectioning. Coronal slices at 30 μm were processed for fluorescent histochemistry as described (Gupta et al., 2018, Pandey et al., 2019). Tissue was immunotagged with polyclonal rabbit anti-NTS2R antibody (1:200, Cat # AP01326PU-N, Origene) and monoclonal mouse anti-NeuN antibody (1:1000, Cat # ab104224, Abcam) and labeled with fluorescent goat-anti-rabbit conjugated to AF488 (1:10,000, Cat # ab8245, Abcam) and goat-anti-mouse conjugated to AF594 (1:500, Cat # A11005, ThermoFisher Scientific). Antibodies were diluted in a blocking solution made of 5% w/v non-fat dry milk (LabScientific) in 0.1 M PBS containing 0.5% Triton X-100. The tissue was mounted on glass slides and coverslipped with ProLong® Diamond Antifade Mountant with DAPI (Life Technologies at ThermoFisher Scientific). Images were taken under a Leica DM5500 automated microscope (Buffalo Grove, IL, USA) using an Olympus DP71 high resolution digital color camera (Waltham, MA, USA) and Slidebook V6 image acquisition and analysis software (3i, Denver, CO, USA).
Drugs
The selective NTS2R agonist, JMV-431 (NIMH J-901), was generously donated by RTI International (Research Triangle Park, NC, USA), through the NIMH Chemical Synthesis and Drug Supply Program (NIMH, Bethesda, MD, USA). It was dissolved in 0.9% saline (Baxter International Inc., Deerfield, IL, USA) at doses of 0.1 and 1.0 nmol per 0.3 μl of solution, based on prior studies that used central injections to affect behavior (Lafrance et al., 2010, Roussy et al., 2009, Sarret et al., 2005, Steele et al., 2017). The preferential binding of JMV-431 to NTS2R has been established (Doulut et al., 1992, Dubuc et al., 1999, Richard et al., 2001).
Cannulation and Microinjections
Rats were cannulated in the aPVT (1.7 mm posterior to bregma, ±0.0 mm lateral to midline, 4.6 mm ventral to the level skull) as described (Barson et al., 2015, Pandey et al., 2019). They were initially anesthetized with 5% isoflurane in 2 L/min oxygen and were then maintained under continuous anesthesia with 2 − 3% isoflurane in 1 L/min oxygen. Warm saline (5 ml s.c., Baxter) was injected to prevent dehydration and bupivicaine (2 mg/kg s.c., Hospira Worldwide, Lake Forest, IL, USA) was injected into the scalp prior to incision. Buprenorphine hydrocholoride (0.03 mg/kg s.c., Reckitt & Colman Inc, Slough, UK) was administered for post-operative analgesia. A stainless steel stylet was left in the guide shaft between injections. During the week of recovery from surgery, animals were handled daily, and their stylet was removed and replaced to acclimate them to the microinjection procedure. On injection days, freshly prepared drugs were injected 1.5 mm ventral to the guide cannula as described (Pandey et al., 2019). Injections of 0.3 μl remain localized to the aPVT (Barson et al., 2015, Barson and Leibowitz, 2015).
For the experiment monitoring the effect of drug injection on ethanol intake, water and chow were removed from the cage at the time of injection and returned, along with ethanol, 20 minutes after the completion of the injection. For the experiment monitoring the effect of drug on behavior in the LDB, testing began 30 minutes after the completion of the injection. Prior studies show peak behavioral effects of JMV-431 20–30 minutes after intracerebroventricular or intrathecal injection (Lafrance et al., 2010, Roussy et al., 2009, Sarret et al., 2005, Steele et al., 2017). Intake measurements were taken at 30 minutes, 1 hour, 2 hours, 4 hours, and 24 hours following the start of ethanol access. Ethanol, food, and water intake was measured by briefly removing and weighing the containers, one subject at a time.
To verify injection location, slide-mounted 30 μm coronal brain slices were examined under a microscope.
Data Analysis
Hierarchical cluster analysis (centroid method) was used to identify sub-groups of ethanol drinkers based on their average daily intake. Linear regression was used to examine the relationship between ethanol intake, behavior, mRNA, and protein. Mixed ANOVA was used to compare groups across time points. Differences between multiple groups were analyzed by one-way ANOVA, or Kruskall Wallis test for non-gaussian distribution, followed up by Tukey multiple comparison tests when appropriate. To examine the effects of JMV-431, repeated measure ANOVAs were used, followed up by Sidak pairwise comparisons when appropriate. Statistical outliers were identified using IBM SPSS Statistics version 24 (IBM, Armonk, NY, USA) and removed from analysis. Significance was determined at p < 0.05. Data are reported as mean ± standard error of the mean (S.E.M.).
RESULTS
Experiment 1: Chronic high ethanol drinkers demonstrate heightened or more flexible exploratory behavior
To identify behavioral characteristics of chronic ethanol drinkers, rats drinking ethanol under the IA2BC paradigm or those maintained on water and chow were examined in behavioral tests after at least eight weeks of drinking during acute abstinence. Hierarchical cluster analysis of average ethanol intake across 11 weeks revealed two groups of ethanol drinkers: low drinkers (58% of ethanol drinkers, n = 11) and high drinkers (42%, n = 8). Low drinkers consumed < 4.9 g/kg/day of ethanol (average 2.6 ± 1.1 g/kg/day) and showed an ethanol preference < 31.1% (average 19.6 ± 7.7%), whereas high drinkers consumed > 5.4 g/kg/day (average 6.3 ± 0.7 g/kg/day) and showed an ethanol preference > 33.7% (average 36.1 ± 5.2%). For ethanol intake, a two-way repeated measures ANOVA showed significant main effects of group [F(1, 16) = 31.88, p < 0.0001] and time [F(3.85, 61.58) = 9.26, p < 0.0001] and a significant interaction between group and time [F(10, 160) = 2.98, p < 0.01]. Pairwise comparisons revealed that high drinkers drank significantly more than low drinkers beginning at Week 6, until the end of the experiment (p < 0.05) (Figure 1A). For ethanol preference, there were also significant main effects of group [F(1, 16) = 24.91, p < 0.0001] and time [F(3.86, 61.8) = 18.66, p < 0.0001] and a significant interaction between group and time [F(10, 160) = 2.56, p < 0.01]. Ethanol preference was significantly higher in high compared to low drinkers at Weeks 6, 7, and 10 (p < 0.05), and marginally higher at Weeks 8, 9, and 11 (p = 0.06, 0.09, and 0.09). For water intake, there were also significant main effects of group [F(2, 23) = 14.19, p < 0.0001] and time [F(5.14, 118.2) = 8.53, p < 0.0001] and a significant interaction between group and time [F(20, 230) = 6.07, p < 0.01]. High drinkers drank significantly less water than low drinkers at Week 7 (p < 0.05) and less than water drinkers at Weeks 4 – 7 and 11 (p < 0.05). There were no group differences in food intake [F(20, 230) = 1.57, not significant, ns] or body weight [F(20, 230) = 1.35, ns]. During acute abstinence, blood ethanol concentration for all ethanol drinkers averaged 1.78 ± 1.05 mg/dL.
Fig 1.
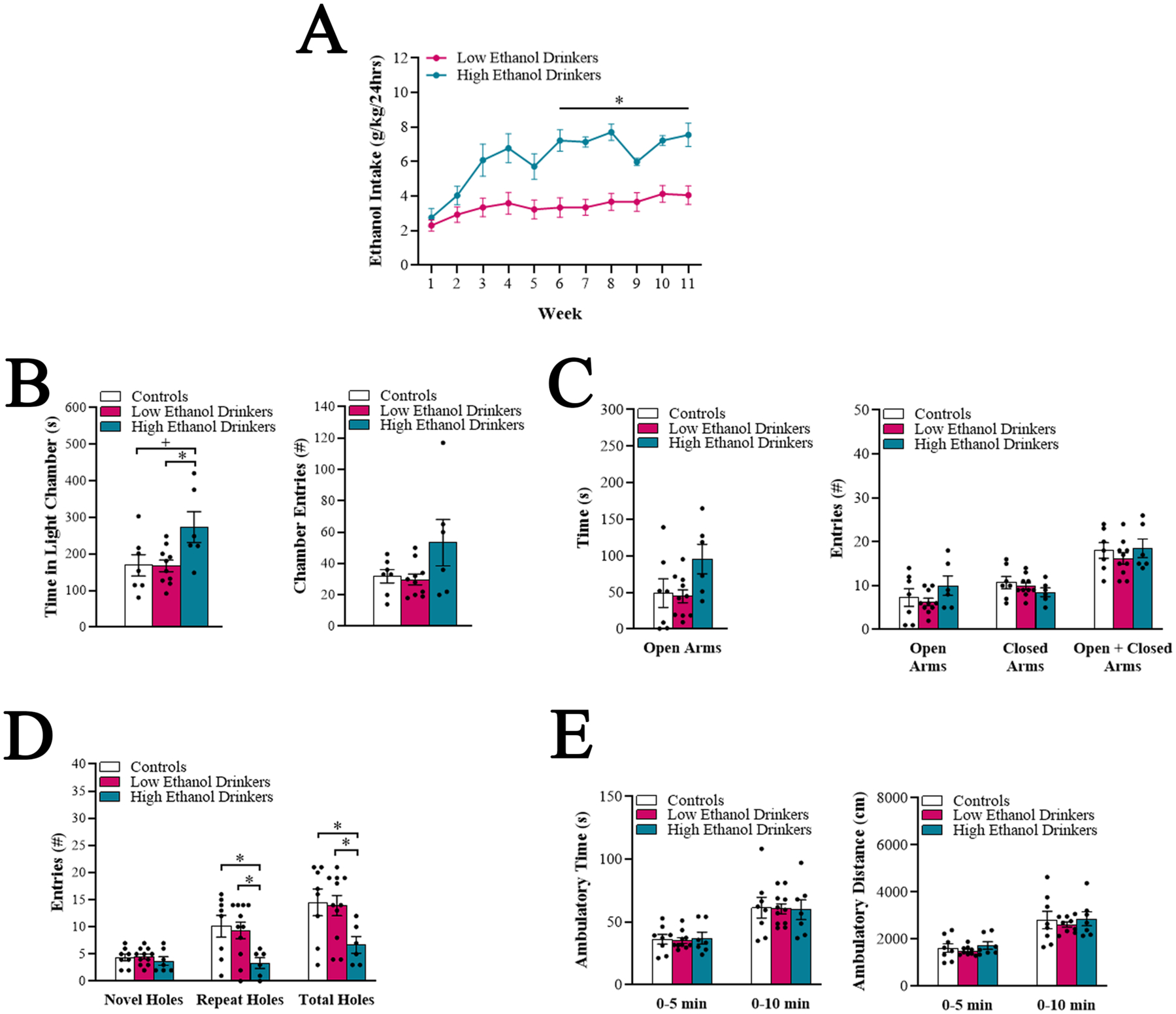
Chronic high ethanol drinkers demonstrate heightened and flexible exploratory behavior (Experiment 1). A. Average daily ethanol intake for low ethanol drinkers (n = 11) compared to high ethanol drinkers (n = 8) was significantly different at each week starting at Week 6. B-D. Comparison of the high ethanol drinkers (n = 6 – 7), low ethanol drinkers (n = 10 – 11), and water drinkers (controls) (n = 7 – 8) for different behavioral measures after 8 weeks (Weeks 9 and 10) of ethanol access, during acute abstinence. B. In the light-dark box, the high ethanol drinkers spent more time spent in the light chamber, compared to the low ethanol drinkers and water drinkers. C. In the elevated plus maze, the high ethanol drinkers showed a strong trend for more time spent in the open arms, compared to the low ethanol drinkers. D. In the hole board apparatus, the high ethanol drinkers explored fewer repeat and total holes, compared to the low ethanol drinkers and water drinkers. E. In the activity chamber, there were no differences in locomotor measures between groups. Data are mean ± S.E.M., *p < 0.05, +p = 0.05.
One-way ANOVAs revealed that high drinkers differed from low drinkers and water drinkers in specific behavioral measures during acute abstinence. For the LDB, there was a significant group difference in time spent in the light chamber [F(2, 20) = 4.37, p < 0.05], and multiple comparisons revealed that high drinkers spent significantly more time in the light than low drinkers (p < 0.05) and showed a strong trend for spending more time in the light than water drinkers (p = 0.05) (Figure 1B). Linear regression revealed that average level of ethanol intake in Weeks 1 – 8 significantly predicted time spent in the light (R2 = 0.26, p < 0.05). Notably, time spent in the light predicted subsequent level of ethanol intake, at Weeks 9 (R2 = 0.28, p < 0.05), 10 (R2 = 0.32, p < 0.05), and 11 (R2 = 0.36, p < 0.05). There was no group difference in number of entries into the chambers [K-statistic = 2.68, ns] (Figure 1B). For the EPM, there was a trend for a group difference in time spent in the open arms [F(2, 20) = 3.03, p = 0.07], with high drinkers tending to spend more time in the open arms than low drinkers (p = 0.07) (Figure 1C). There were no differences in the number of open arm entries [F(2, 20) = 1.38, ns], closed arm entries [F(2, 20) = 0.98, ns], or total arm entries [F(2, 20) = 0.58, ns] (Figure 1C). For the HBA, there was no group difference in the number of novel hole entries [F(2, 23) = 0.51, ns]; rather, there was a significant group difference in the number of familiar (repeat) hole entries [F(2, 22) = 3.03, p < 0.05] and total hole entries [F(2, 20) = 5.03, p < 0.05]. High drinkers made significantly fewer repeat and, as a consequence, total hole entries than low drinkers and water drinkers (p < 0.05) (Figure 1D). Linear regression revealed that average level of ethanol intake in Weeks 1 – 8 significantly predicted the number of repeat hole entries (R2 = 0.44, p < 0.01) and total hole entries (R2 = 0.39, p < 0.01). For the AC, there were no significant group differences in ambulatory time [F(2, 23) = 0.02, ns] or distance [F(2, 21) = 0.23, ns] (Figure 1E). Together, these findings suggest that, during acute abstinence following chronic ethanol drinking, high drinkers but not low drinkers, compared to water drinkers, exhibit behaviors that overall reflect greater or more flexible exploration.
Experiment 2: Heightened exploratory behavior in high ethanol drinkers develops after weeks of drinking
To determine if behavioral differences between groups exist prior to ethanol exposure or if they emerge as a consequence of chronic drinking, a new group of rats was examined in behavioral tests prior to ethanol access and at different time points during IA2BC drinking. Hierarchical cluster analysis of their average ethanol intake across 11 weeks again revealed two groups: low drinkers (63%, n = 15) and high drinkers (37%, n = 9). Low drinkers consumed < 3.2 g/kg/day of ethanol (average 1.9 ± 0.6 g/kg/day) and showed an ethanol preference < 22.4% (average 13.3 ± 1.3%), whereas high drinkers consumed > 4.2 g/kg/day (average 5.3 ± 0.7 g/kg/day) and showed a preference > 26% (average 37.2 ± 2.5%). Two-way repeated measures ANOVA for ethanol intake showed significant main effects of group [F(1, 22) = 99.40, p < 0.001] and time [F(2.87, 63.12) = 12.09, p < 0.001] and a significant interaction between group and time [F(10, 220) = 11.11, p < 0.001]. Pairwise comparisons revealed that high drinkers drank significantly more than low drinkers starting at Week 3, until the end of the experiment (p < 0.05) (Figure 2A). For ethanol preference, there were also significant main effects of group [F(1, 16) = 24.91, p < 0.0001] and time [F(3.86, 61.8) = 18.66, p < 0.0001] and a significant interaction between group and time [F(10, 160) = 2.56, p < 0.01], with high compared to low drinkers showing a significantly higher preference starting at Week 3, until the end of the experiment (p < 0.05).
Fig 2.
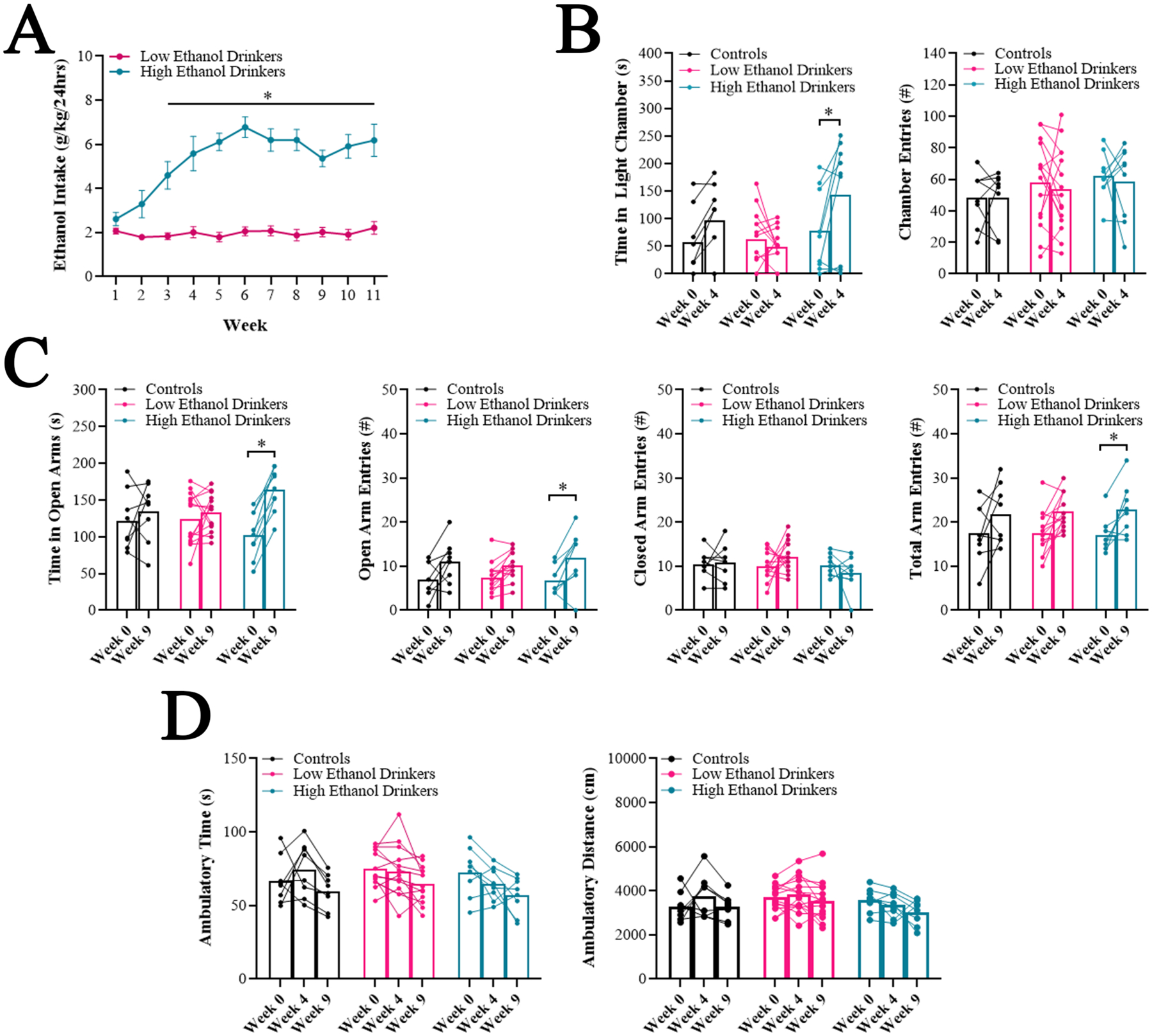
Heightened exploratory behavior in high ethanol drinkers develops after weeks of drinking (Experiment 2). A. Average daily ethanol intake for low ethanol drinkers (n = 15) compared to high ethanol drinkers (n = 9) was significantly different at each week starting at Week 3. B-D. Comparison of the high ethanol drinkers (n = 8 – 9), low ethanol drinkers (n = 13–14), and water drinkers (controls) (n = 8) for different behavioral measures following 0 weeks (Week 0), 3 weeks (Week 4) and/or 8 weeks (Week 9) of ethanol access, during acute abstinence. B. In the light-dark box, the high ethanol drinkers, but not the water drinkers or the low ethanol drinkers, increased the time they spent in the light chamber at Week 4 compared to Week 0. C. In the elevated plus maze, the high ethanol drinkers, but not the water drinkers or the low ethanol drinkers, increased the time they spent in the open arms at Week 9 compared to Week 0. D. In the activity chamber, there were no changes in locomotor measures across time for any group. Data are mean ± S.E.M., *p < 0.05.
Mixed ANOVAs revealed that behavioral differences between groups did not exist prior to ethanol drinking but developed across time, with the high drinkers exhibiting changes. For the LDB, for time spent in the light chamber, there was no significant main effect of group [F(2, 27) = 2.26, ns], but there was a significant main effect of time [F(1, 27) = 7.66, p < 0.05] and a significant interaction between group and time [F(2, 27) = 5.44, p < 0.05]. Pairwise comparisons revealed that, while there were no significant differences between groups at Week 0 (ns), high drinkers at Week 4 spent significantly more time in the light chamber compared to low drinkers (p < 0.01), and high drinkers, but not low or water drinkers, spent more time in the light chamber at Week 4 compared to Week 0 (p < 0.01) (Figure 2B). For number of entries into the chambers, there were no significant main effects of group [F(2, 29) = 0.88, ns] or time [F(1, 29) = 0.33, ns] (Figure 2B). For the EPM, for time spent in the open arms, there was again no significant main effect of group [F(2, 28) = 0.09, ns], but a significant main effect of time [F(1, 28) = 18.06, p < 0.001] and a significant interaction between group and time [F(2,28) = 6.84, p < 0.01]. Pairwise comparisons revealed that, while there were no significant differences between groups at Week 0 (ns), high drinkers at Week 9 tended to spend more time in the open arms compared to low drinkers (p = 0.09), and high drinkers, but not low or water drinkers, spent more time in the open arms at Week 9 compared to Week 0 (p < 0.0001) (Figure 2C). There were no significant main effects of group for open arm entries [F(2, 28) = 0.05, ns], closed arm entries [F(2, 28) = 1.64, ns], or total arm entries [F(2, 27) = 0.02, ns] and, while there were significant main effects of time for open arm entries [F(1, 28) = 30.33, p < 0.001] and total arm entries [F(1, 27) = 20.76, p < 0.001] (but not closed arm entries [F(1, 28) = 0.06, ns]), the interaction effects were not significant (open arms: [F(2, 28) = 1.07, ns], total arms: [F(2, 27) = 0.13, ns]) (Figure 2C). For the AC, for ambulatory time and distance, there was no significant main effect of group (time: [F(2, 26) = 0.67, ns], distance: [F(2, 27) = 1.10, ns]), a significant main effect of time (time: [F(1.83, 47.7) = 10.56, p < 0.05], distance: [F(1.83, 49.4) = 4.05, p < 0.05]), but no significant interaction effect (time: [F(4, 52) = 1.27, ns], distance: [F(4, 54) = 1.17, ns]) (Figure 2D). Together, these findings show that behaviors representing heightened exploration emerge in high ethanol drinkers after only four weeks of drinking and are not accounted for by changes in locomotor activity.
Experiment 3: Chronic high ethanol drinkers exhibit reduced mRNA expression of NTS2R in the aPVT
To determine if the NTS system in the PVT is altered with chronic ethanol drinking, the aPVT and pPVT of rats from Experiment 1, obtained during acute abstinence, were examined using qRT-PCR. There were no significant group differences in mRNA levels in either PVT subregion for NTS [aPVT: F(2, 21) = 0.46, ns; pPVT: F(2, 12.83) = 0.83, ns] or NTS1R [aPVT: K statistic = 0.98, ns; pPVT: K statistic = 0.76, ns], nor were there differences for NTS2R in the pPVT [F(2, 22) = 0.43, ns]; however, there was a significant group difference in mRNA levels of NTS2R in the aPVT [F(2, 20) = 5.56, p < 0.05] (Figure 3A, 3B). Multiple comparisons revealed that high drinkers had significantly lower levels of NTS2R in the aPVT compared to low drinkers (−26.8%, p < 0.05) and water drinkers (−28.1%, p < 0.05). Linear regression revealed that average level of ethanol intake significantly predicted mRNA level of NTS2R in the aPVT (R2 = 0.41, p < 0.01). These findings suggest that, in addition to specific behavioral characteristics, chronic high ethanol drinkers exhibit distinct gene expression in the aPVT.
Fig 3.
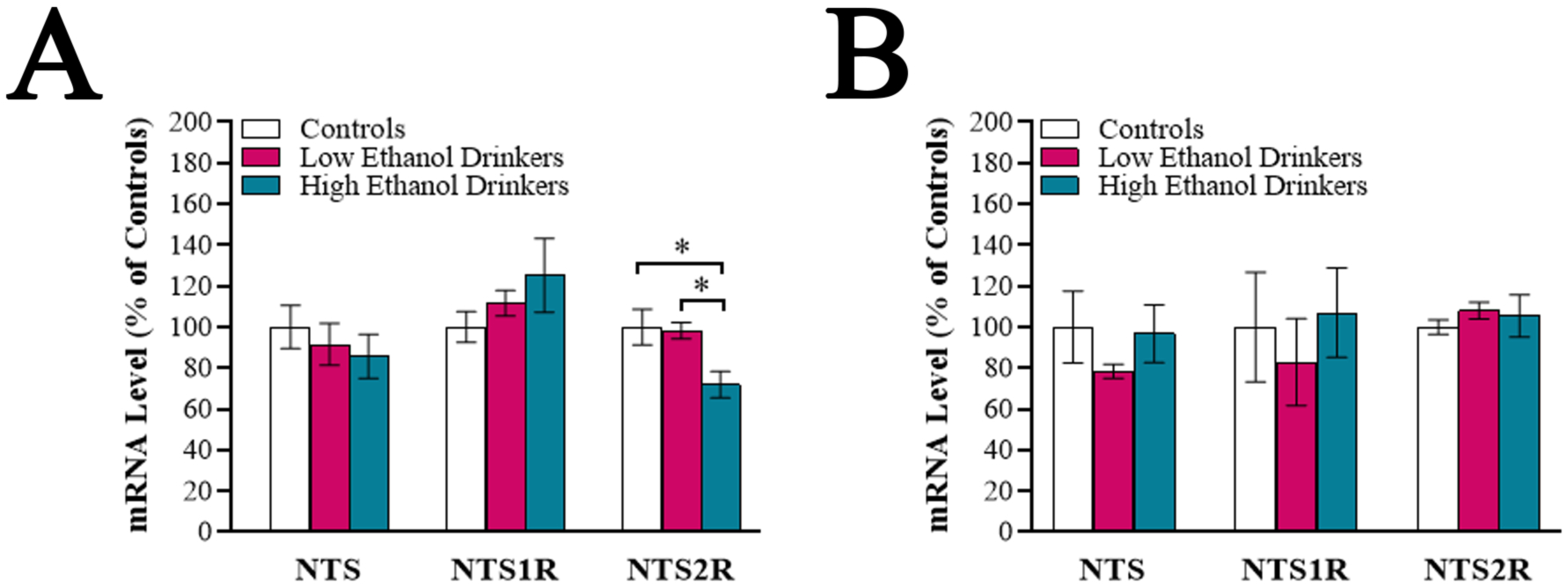
Chronic high ethanol drinkers exhibit reduced mRNA expression of neurotensin receptor type 2 (NTS2R) in the anterior paraventricular thalamus (aPVT), as assessed using quantitative real-time PCR (Experiment 3). A. Comparison of the high ethanol drinkers (n = 6), low ethanol drinkers (n = 9) and water drinkers (controls) (n = 8) for mRNA levels of neurotensin (NTS), neurotensin receptor type 1 (NTS1R), and NTS2R in the aPVT following 11 weeks of ethanol access (Week 12) during acute abstinence. Compared to low ethanol drinkers and water drinkers, the high ethanol drinkers showed significantly reduced expression of NTS2R mRNA in the aPVT. B. Comparison of these same groups for the same measures in the posterior paraventricular thalamus revealed no group differences. Data are mean ± S.E.M., *p < 0.05.
Experiment 4: Chronic high ethanol drinkers exhibit elevated protein levels of NTS2R in the aPVT
To determine if protein levels of NTS2R in the PVT are also altered with chronic ethanol drinking, the aPVT and pPVT of rats from Experiment 2, obtained during acute abstinence, were examined using Western blotting. Consistent with mRNA results, there was no significant group difference in NTS2R protein levels in the pPVT [F(2, 23) = 0.64, ns] (Figure 4B), but there was a significant group difference in the aPVT [K statistic = 8.76, p < 0.01] (Figure 4A, 4C). Multiple comparisons this time revealed that high drinkers had significantly higher levels of NTS2R protein in the aPVT compared to low drinkers (+28%, p < 0.05) and water drinkers (+39%, p < 0.05). Linear regression revealed that average level of ethanol intake significantly predicted protein level of NTS2R in the aPVT (R2 = 0.65, p < 0.001). These findings suggest that aPVT-NTS2R signaling may in fact be elevated in chronic high ethanol drinkers.
Fig 4.
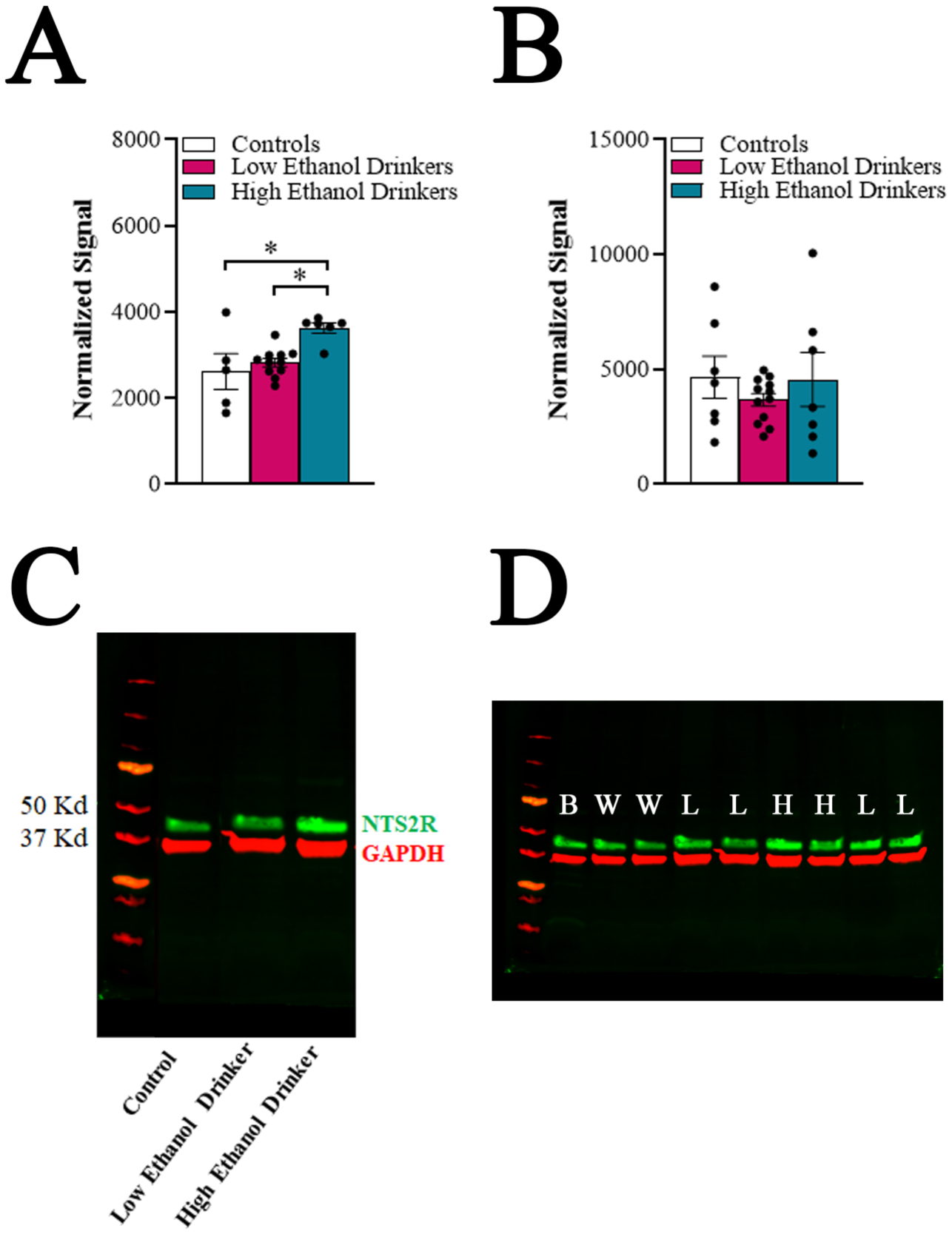
Chronic high ethanol drinkers exhibit elevated protein levels of neurotensin receptor type 2 (NTS2R) in the anterior paraventricular thalamus (aPVT), as analyzed using Western blot (Experiment 4). A. Comparison of the high ethanol drinkers (n = 6), low ethanol drinkers (n = 11) and water drinkers (controls) (n = 5) for protein levels of NTS2R in the aPVT following 11 weeks of ethanol access (Week 12) during acute abstinence. Compared to low ethanol drinkers and water drinkers, the high ethanol drinkers showed significantly elevated expression of NTS2R protein in the aPVT. B. Comparison of these same groups for the same measures in the posterior paraventricular thalamus revealed no group differences. C. Representative Western blot showing protein bands of NTS2R (green) and loading control GAPDH (red) in a water drinker (control), a low ethanol drinker, and a high ethanol drinker. D. Original, full-size version of the Western blot, showing protein bands of NTS2R (green) and loading control GAPDH (red) in adult whole brain lysate for control (B), water drinkers (W), low ethanol drinkers (L), and high ethanol drinkers (H). Data are mean ± S.E.M., *p < 0.05.
Experiment 5: Elevated NTS2R signaling in the aPVT stimulates exploratory behavior
To determine if elevated NTS2R signaling in the aPVT is related to exploratory behavior and ethanol intake, a new group of rats with a history of IA2BC ethanol drinking was injected in the aPVT with the NTS2R agonist JMV-431 and their subsequent behavior was assessed. Hierarchical cluster analysis of their average ethanol intake across 7 weeks revealed two groups: low drinkers (64%, n = 16) and high drinkers (36%, n = 9). Low drinkers consumed < 3.7 g/kg/day of ethanol (average 2.6 ± 0.8 g/kg/day) and showed an ethanol preference < 22.9% (average 14.4 ± 1.23%), whereas high drinkers consumed > 5.3 g/kg/day (average 6.8 ± 1.2 g/kg/day) and showed a preference > 27.6% (average 41.3 ± 3.42%). Mixed ANOVA of ethanol intake showed significant main effects of group [F(1, 23) = 108.40, p < 0.001] and time [F(3.95, 90.4) = 20.45, p < 0.001] and a significant interaction between group and time [F(10, 229) = 5.99, p < 0.001], and pairwise comparisons revealed that high drinkers drank significantly more than low drinkers starting at Week 3, until the end of the experiment (p < 0.05) (Figure 5A). For ethanol preference, there were significant main effects of group [F(1, 23) = 78.55, p < 0.0001] and time [F(4.37, 100.1) = 46.16, p < 0.0001] and a significant interaction between group and time [F(10, 229) = 15.29, p < 0.0001], with high compared to low drinkers showing a significantly higher preference starting at Week 3, until the end of the experiment (p < 0.05).
Fig 5.
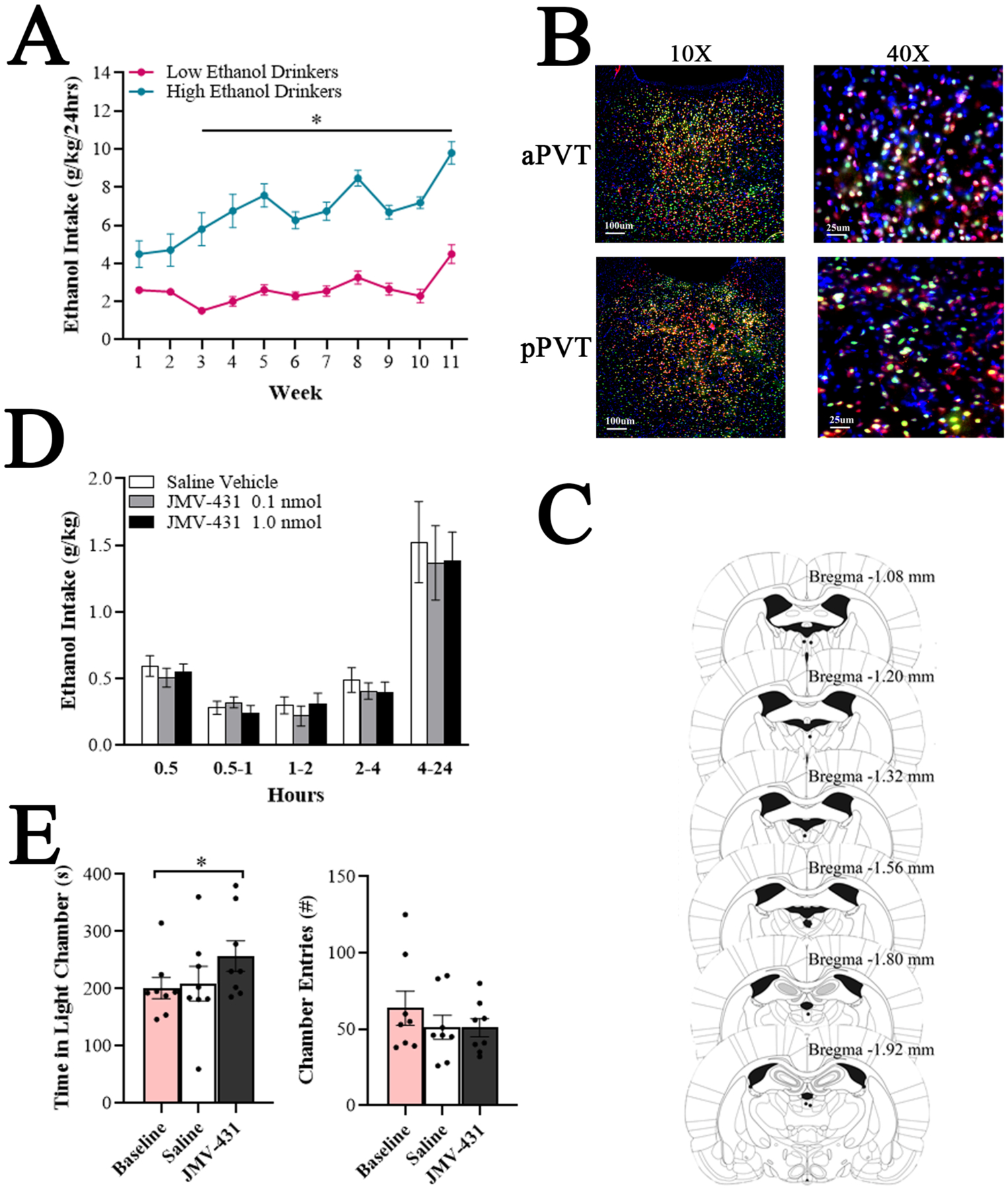
Elevated neurotensin receptor type 2 (NTS2R) signaling in the anterior paraventricular thalamus (aPVT) stimulates exploratory behavior (Experiment 5). A. Average daily ethanol intake for low ethanol drinkers (n = 16) compared to high ethanol drinkers (n = 9) was significantly different at each week starting at Week 3. B. Immunolabeling of NTS2R (green) and NeuN (neuronal nuclei; red), and nuclear staining by 4′,6-diamidino-2-phenylindole (DAPI; blue) in the aPVT and posterior paraventricular thalamus (pPVT) shows expression of NTS2R in both neuronal cells (yellow) and non-neuronal cells (green). Images were taken at 10X magnification (left; scale bar: 100 μm) and 40X magnification (right; scale bar: 25 μm). C. Schematic showing injection sites (black dots) for animals that received injections in the aPVT. Animals with a misplaced cannula, resulting in injections outside of the aPVT, were removed from analysis. Adapted from The Rat Brain, 5th edition, G. Paxinos and C. Watson, Copyright 2005, with permission from Elsevier. B = bregma. D. Stimulating the NTS2R in the aPVT with the selective NTS2R agonist, JMV-431 (0.1 nmol or 1.0 nmol), compared to saline vehicle (0.3 μl), at Week 8 of ethanol access in low ethanol drinkers did not alter ethanol intake at any of the time periods measured during a 24-hour drinking session. E. Stimulating the NTS2R in the aPVT with JMV-431 (1.0 nmol), compared to baseline or saline vehicle (0.3 μl), at Week 10 of ethanol access in low ethanol drinkers stimulated exploratory behavior in the light-dark box. Data are mean ± S.E.M., *p < 0.05.
Using fluorescent histochemistry, NTS2R was identified within both the aPVT and pPVT, primarily on neurons, but also on non-neuronal cells (Figure 5B).
Histological examination revealed that several cannulations were off-target, which left the high ethanol drinking group statistically underpowered. Thus, only data from the low ethanol drinking group were analyzed. In the low drinkers with on-target cannulation, injection sites ranged between bregma −1.08 and −1.92 mm (Figure 5C).
Examining effects of the NTS2R agonist, JMV-431, on ethanol intake in low drinkers, a repeated measures ANOVA revealed no significant main effect of treatment on ethanol intake [F(1, 31) = 0.92, ns] and, despite a significant main effect of time [F(2.37, 73.6) = 177.69, p < 0.0001], no significant interaction between treatment and time [F(4, 28) = 0.55, ns] (Figure 5D). Similarly, there were no main effects on water [F(1, 31) = 0.46, ns] or food intake [F(1, 31) = 0.92, ns].
Examining effects of JMV-431 on behavior in an LDB in low drinkers, a repeated measures ANOVA on time in the light chamber revealed a significant main effect of treatment [F(2, 14) = 4.22, p < 0.05, ηp = 0.38]. Pairwise comparisons revealed that JMV-431 led to a significant increase in time in the light chamber compared to baseline (+35%, p < 0.05) and a non-significant increase compared to saline (+28%, ns, Figure 5E). There was no significant main effect on number of entries into the chambers [F(2, 14) = 1.80, ns] (Figure 5E). These results suggest that elevated NTS2R signaling in the aPVT stimulates exploratory behavior.
DISCUSSION
In this study, we found that, after several weeks of ethanol drinking under the IA2BC paradigm, excessive (high) but not low drinkers develop heightened or more flexible exploratory behavior and elevated protein levels of the NTS2R in the aPVT. Moreover, pharmacological elevation of NTS2R signaling in the aPVT stimulates exploratory behavior in low drinkers, mimicking the ethanol-induced changes in high drinkers. Together, the results suggest that chronic, excessive ethanol drinking leads to heightened exploratory behavior, mediated in part by enhanced NTS2R signaling in the aPVT.
One major finding was that chronic, excessive ethanol drinking precipitates specific behavioral changes. Excessive drinkers spent more time in the light chamber of an LDB and the open arms of an EPM, reflecting lower anxiety and higher exploratory behavior. In the HBA, excessive drinkers reduced exploration of the familiar holes and, as a consequence, the total holes, suggesting that they cease exploration once they learn about an environment. While all three tasks measure exploratory drive in an approach-avoidance conflict environment (Calhoon and Tye, 2015, Cryan and Holmes, 2005), the LDB and EPM are not believed to measure working memory like the HBA (van der Staay et al., 2012). Behavioral flexibility could be a characteristic of high ethanol drinkers, as high compared to low ethanol drinking rats after 8 weeks of drinking have been found in a gambling task to show a higher percentage choice for an optimal option (Spoelder et al., 2017). Thus, the ethanol-induced behavioral changes observed in our excessive drinkers may represent an increase in flexible exploratory motivation, likely related to reward seeking (Koob, 2013, Wise and Koob, 2014).
The increased exploratory behavior of excessive drinkers emerged only after ethanol exposure. This confirms our previous work, which showed that rats that drink at high levels in the IA2BC paradigm could not be identified by their behaviors in an EPM or HBA while ethanol-naïve (Pandey et al., 2019). It is also consistent with prior literature, which identified increased exploratory behaviors after intermittent access excessive ethanol drinking (Lee et al., 2015, Momeni and Roman, 2014, Spoelder et al., 2017). However, while these studies reported changes with 6–7 weeks of drinking, we observed changes with as few as 4 weeks of drinking. This four-week time point coincides with when animals drinking under the IA2BC paradigm generally shift from an escalation to a stable-intake phase (Pandey et al., 2019) indicating that it may be an important time point in the drinking history. While we assessed behavior after an abstinence period of approximately 24 hours, the prior studies also reported changes up to 21 days into abstinence (Lee et al., 2015, Momeni and Roman, 2014). Thus, we hypothesize that ethanol-induced heightened exploratory drive continues long after cessation of drinking.
Another major finding was that chronic excessive drinking affects levels of NTS2R but not NTS1R in the aPVT but not pPVT. A subregion-specific response of the NTS system to ethanol has been reported in the amygdala (Torruella-Suarez et al., 2020). The involvement of the aPVT is consistent with its role in incentive-salience and novelty-related reward pursuit (Cheng et al., 2018, Flagel et al., 2011). The specific change in NTS2R may be due, in part, to its localization in soma of the PVT, unlike NTS1R which is primarily present in axons and axon terminals in this region (Boudin et al., 1996, Sarret et al., 2003). Our own analysis revealed dense expression of NTS2R in both neuronal and glial soma in both PVT subregions. Functionally, the involvement of NTS2R may be related to the higher levels of ethanol intake, as suggested by systemic knockdown studies that implicate NTS1R in the behavioral effects of lower doses of ethanol and NTS2R in higher doses (Lee et al., 2010, Lee et al., 2011).
One curious finding was that chronic high drinkers had lower levels of NTS2R mRNA but higher levels of NTS2R protein in the aPVT. These opposite results may be due to a negative feedback relationship between protein levels and gene expression of NTS2R, similar to the transcription-translation feedback loop of clock genes (Takahashi, 2017). This possibility requires further examination, with assessment of mRNA and protein within the same animals at multiple time points. Considering the results with pharmacological manipulation, it appears that the major effect is an increase in functionality of the NTS2R in the aPVT.
The final, major finding was that pharmacological activation of NTS2R in the aPVT augments exploratory behavior in the LDB. This supports our hypothesis that the elevated NTS2R in high drinkers is causally related to their heightened exploratory behavior. Although we could not corroborate this idea with a reliable NTS2R antagonist, due to the lack of availability, this finding is consistent with known anxiolytic and analgesic properties of centrally administered JMV-431 and the NTS2R agonist, β-lactotensin (Hou et al., 2011, Roussy et al., 2009, Steele et al., 2017). Future research should use shRNA to determine if reducing activity at NTS2R can block exploratory behaviors in high drinkers. Regarding the lack of effect on ethanol drinking, it appears that aPVT NTS2R signaling is not involved in the consummatory aspect of ethanol use, although it may influence appetitive aspects.
A noteworthy aspect of all of our findings is that alcohol-induced changes were identified in high drinkers but not low drinkers. These changes were not apparent when comparing overall alcohol drinkers with water drinkers (data not shown), similar to prior work (Momeni and Roman, 2014). This highlights the importance in alcohol research of screening a population for possible subgroups, and also the utility of heterogenous outbred rodents in revealing important individual differences in alcohol-related neurobiology.
Together, our results reveal unique behavioral and neurochemical changes in chronic excessive drinkers, which may influence future excessive drinking and, ultimately, the development of alcohol use disorder.
ACKNOWLEDGMENTS
This research was supported by the National Institute on Alcohol Abuse and Alcoholism under Award Numbers R01AA028218 and R00AA021782 (J.R.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Dr. Courtney A. Miller (The Scripps Research Institute) for her thoughtful feedback on an early version of the manuscript.
REFERENCES
- Adamec R & Shallow T 2000. Effects of baseline anxiety on response to kindling of the right medial amygdala. Physiol Behav, 70: 67–80. [DOI] [PubMed] [Google Scholar]
- Adamec R, Shallow T & Burton P 2005. Anxiolytic and anxiogenic effects of kindling--role of baseline anxiety and anatomical location of the kindling electrode in response to kindling of the right and left basolateral amygdala. Behav Brain Res, 159: 73–88. [DOI] [PubMed] [Google Scholar]
- Arienzo D, Happer JP, Molnar SM, Alderson-Myers A & Marinkovic K 2019. Binge drinking is associated with altered resting state functional connectivity of reward-salience and top down control networks. Brain Imaging Behav. [DOI] [PMC free article] [PubMed]
- Barson JR, Ho HT & Leibowitz SF 2015. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol, 20: 469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR & Leibowitz SF 2015. GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett, 609: 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W & Beaudet A 1996. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol, 373: 76–89. [DOI] [PubMed] [Google Scholar]
- Bourin M 2015. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin Neurosci, 17: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein PM 1972. Repeated trials with the albino rat in the open field as a function of age and deprivation. J Comp Physiol Psychol, 81: 84–93. [DOI] [PubMed] [Google Scholar]
- Calhoon GG & Tye KM 2015. Resolving the neural circuits of anxiety. Nat Neurosci, 18: 1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Wang J, Ma X, Ullah R, Shen Y & Zhou YD 2018. Anterior paraventricular thalamus to nucleus accumbens projection is involved in feeding behavior in a novel environment. Front Mol Neurosci, 11: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN 1985. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev, 9: 37–44. [DOI] [PubMed] [Google Scholar]
- Cryan JF & Holmes A 2005. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov, 4: 775–90. [DOI] [PubMed] [Google Scholar]
- Cservenka A & Brumback T 2017. The burden of binge and heavy drinking on the brain: Effects on adolescent and young adult neural structure and function. Front Psychol, 8: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F & Ron D 2015. MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry, 20: 1219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF & George O 2016. Recruitment of a neuronal ensemble in the central nucleus of the amygdala is required for alcohol dependence. J Neurosci, 36: 9446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulut S, Rodriguez M, Lugrin D, Vecchini F, Kitabgi P, Aumelas A & Martinez J 1992. Reduced peptide bond pseudopeptide analogues of neurotensin. Pept Res, 5: 30–8. [PubMed] [Google Scholar]
- Dubuc I, Remande S & Costentin J 1999. The partial agonist properties of levocabastine in neurotensin-induced analgesia. Eur J Pharmacol, 381: 9–12. [DOI] [PubMed] [Google Scholar]
- Field M, Kiernan A, Eastwood B & Child R 2008. Rapid approach responses to alcohol cues in heavy drinkers. J Behav Ther Exp Psychiatry, 39: 209–18. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H & Robinson TE 2011. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience, 196: 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD & Koob GF 2012. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A, 109: 18156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Gargiulo AT, Curtis GR, Badve PS, Pandey S & Barson JR 2018. Pituitary adenylate cyclase-activating polypeptide-27 (PACAP-27) in the thalamic paraventricular nucleus is stimulated by ethanol drinking. Alcohol Clin Exp Res, 42: 1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Iles JP, Mayell SJ & Rodgers RJ 2001. Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res, 122: 159–67. [DOI] [PubMed] [Google Scholar]
- Hou IC, Suzuki C, Kanegawa N, Oda A, Yamada A, Yoshikawa M, Yamada D, Sekiguchi M, Wada E, Wada K & Ohinata K 2011. beta-Lactotensin derived from bovine beta-lactoglobulin exhibits anxiolytic-like activity as an agonist for neurotensin NTS(2) receptor via activation of dopamine D(1) receptor in mice. J Neurochem, 119: 785–90. [DOI] [PubMed] [Google Scholar]
- Hu S, Ide JS, Chao HH, Zhornitsky S, Fischer KA, Wang W, Zhang S & Li CR 2018. Resting state functional connectivity of the amygdala and problem drinking in non-dependent alcohol drinkers. Drug Alcohol Depend, 185: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchesin KD, Stinnett GS & Seasholtz AF 2016. Binge drinking decreases corticotropin-releasing factor-binding protein expression in the medial prefrontal cortex of mice. Alcohol Clin Exp Res, 40: 1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kim S, Cole M, Brennan M & George O 2017. Intermittent access to ethanol drinking facilitates the transition to excessive drinking after chronic intermittent ethanol vapor exposure. Alcohol Clin Exp Res, 41: 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM, Fogarty MJ, Shariff M, Belmer A, Bellingham MC & Bartlett SE 2016. Increased synaptic excitation and abnormal dendritic structure of prefrontal cortex layer V pyramidal neurons following prolonged binge-like consumption of ethanol. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF 2013. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry, 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrance M, Roussy G, Belleville K, Maeno H, Beaudet N, Wada K & Sarret P 2010. Involvement of NTS2 receptors in stress-induced analgesia. Neuroscience, 166: 639–52. [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, Mcgregor HA, Waltermire RS & Szumlinski KK 2015. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res, 291: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, Sern KR, Bocz MD & Szumlinski KK 2018. mGlu5 receptor blockade within the nucleus accumbens shell reduces behavioral indices of alcohol withdrawal-induced anxiety in mice. Front Pharmacol, 9: 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E & Choi DS 2010. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharmacol Biochem Behav, 95: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Unal SS, Richelson E & Choi DS 2011. Increased ethanol consumption and preference in mice lacking neurotensin receptor type 2. Alcohol Clin Exp Res, 35: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone RM, Crane CA, Parrott DJ & Eckhardt CI 2016. Problematic drinking, impulsivity, and physical IPV perpetration: A dyadic analysis. Psychol Addict Behav, 30: 356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dong X, Li S & Kirouac GJ 2014. Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front Behav Neurosci, 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre J, Sicre M, Diarra M, Marchessaux F, Paleressompoulle D & Ambroggi F 2019. Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens core. Curr Biol, 29: 3298–3306 e4. [DOI] [PubMed] [Google Scholar]
- Meyerson BJ, Augustsson H, Berg M & Roman E 2006. The Concentric Square Field: a multivariate test arena for analysis of explorative strategies. Behav Brain Res, 168: 100–13. [DOI] [PubMed] [Google Scholar]
- Momeni S & Roman E 2014. Subgroup-dependent effects of voluntary alcohol intake on behavioral profiles in outbred Wistar rats. Behav Brain Res, 275: 288–96. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Fisk JE, Murphy PN, Ryland I & Hilton J 2012. The effects of heavy social drinking on executive function: a systematic review and meta-analytic study of existing literature and new empirical findings. Hum Psychopharmacol, 27: 187–99. [DOI] [PubMed] [Google Scholar]
- Onaivi ES & Martin BR 1989. Neuropharmacological and physiological validation of a computer-controlled two-compartment black and white box for the assessment of anxiety. Prog Neuropsychopharmacol Biol Psychiatry, 13: 963–76. [DOI] [PubMed] [Google Scholar]
- Pandey S, Badve PS, Curtis GR, Leibowitz SF & Barson JR 2019. Neurotensin in the posterior thalamic paraventricular nucleus: inhibitor of pharmacologically relevant ethanol drinking. Addict Biol, 24: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Watson C 2005. The Rat Brain in Stereotaxic Coordinates (5th Edition), San Diego, CA, Elsevier Academic Press. [Google Scholar]
- Richard F, Barroso S, Martinez J, Labbe-Jullie C & Kitabgi P 2001. Agonism, inverse agonism, and neutral antagonism at the constitutively active human neurotensin receptor 2. Mol Pharmacol, 60: 1392–8. [DOI] [PubMed] [Google Scholar]
- Roussy G, Dansereau MA, Baudisson S, Ezzoubaa F, Belleville K, Beaudet N, Martinez J, Richelson E & Sarret P 2009. Evidence for a role of NTS2 receptors in the modulation of tonic pain sensitivity. Mol Pain, 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Esdaile MJ, Perron A, Martinez J, Stroh T & Beaudet A 2005. Potent spinal analgesia elicited through stimulation of NTS2 neurotensin receptors. J Neurosci, 25: 8188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T & Beaudet A 2003. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol, 461: 520–38. [DOI] [PubMed] [Google Scholar]
- Schneider P, Ho YJ, Spanagel R & Pawlak CR 2011. A novel elevated plus-maze procedure to avoid the one-trial tolerance problem. Front Behav Neurosci, 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R & Bartlett SE 2008. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res, 32: 1816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Almeida OF & Wotjak CT 2006. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav, 5 Suppl 2: 5–24. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Flores Dourojeanni JP, De Git KCG, Baars AM, Lesscher HMB & Vanderschuren L 2017. Individual differences in voluntary alcohol intake in rats: relationship with impulsivity, decision making and Pavlovian conditioned approach. Psychopharmacology (Berl), 234: 2177–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele FF 3rd, Whitehouse SC, Aday JS & Prus AJ 2017. Neurotensin NTS1 and NTS2 receptor agonists produce anxiolytic-like effects in the 22-kHz ultrasonic vocalization model in rats. Brain Res, 1658: 31–35. [DOI] [PubMed] [Google Scholar]
- Substance_Abuse_and_Mental_Health_Services_Administration 2019. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54) In: SERVICES, U. S. D. O. H. A. H (ed.). [Google Scholar]
- Takahashi JS 2017. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet, 18: 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruella-Suarez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, Patel GK, Mchenry JA, Hardaway JA, Kantak PA, Crowley NA, Diberto JF, Faccidomo SP, Hodge CW, Stuber GD & Mcelligott ZA 2020. Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J Neurosci, 40: 632–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Staay FJ, Gieling ET, Pinzon NE, Nordquist RE & Ohl F 2012. The appetitively motivated “cognitive” holeboard: a family of complex spatial discrimination tasks for assessing learning and memory. Neurosci Biobehav Rev, 36: 379–403. [DOI] [PubMed] [Google Scholar]
- Wise RA 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia, 29: 203–10. [DOI] [PubMed] [Google Scholar]
- Wise RA & Koob GF 2014. The development and maintenance of drug addiction. Neuropsychopharmacology, 39: 254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World_Health_Organization 2018. Fact sheet: Alcohol.
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S & Madden TL 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics, 13: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]


