Abstract
Estradiol effects on skeletal muscle are multifactorial including the preservation of mass, contractility and regeneration. Here, we aimed to determine the extent to which estradiol deficiency affects strength recovery when muscle is challenged by multiple BaCl2-induced injuries and to assess how satellite cell number is influenced by the combination of estradiol deficiency and repetitive skeletal muscle injuries. A longitudinal study was designed, using an in vivo anesthetized mouse approach to precisely and repeatedly measure maximal isometric torque, coupled with endpoint fluorescent-activated cell sorting to quantify satellite cells. Isometric torque and strength gains were lower in ovariectomized mice at several time points after the injuries compared to those treated with 17β-estradiol. Satellite cell number was 41-43% lower in placebo- than estradiol-treated ovariectomized mice, regardless of injury status or number of injuries. Together, these results indicate that the loss of estradiol blunts adaptive strength gains and that the number of satellite cells likely contributes to the impairment.
Keywords: estrogen, muscle stem cells, ovariectomy, recovery, skeletal muscle
Estradiol, the primary female sex hormone, is classically known to regulate reproductive organ development and function. It is also recognized that estradiol plays an important role in regulating skeletal muscle strength (Greising et al., 2009). For example, muscle strength of women declines at a time corresponding to menopause (i.e., the cessation of estradiol production), and estradiol-based hormone therapy in post-menopausal women has been shown to maintain muscle strength (Phillips et al., 1993; Greising et al., 2009). A common approach to study estradiol in rodent models is the surgical removal of the ovaries (ovariectomy; Ovx) and subsequent treatment with or without 17β-estradiol (E2), the most biologically active form of estrogen. Using this approach, strength loss has been measured in Ovx mice (Moran et al., 2007; Greising et al., 2009), similar to post-menopausal women (Phillips et al., 1993; Greising et al., 2009). Loss of muscle strength in females due to E2 deficiency is attributed to inadequate preservation of skeletal muscle mass (Kamanga-Sollo et al., 2017) and reduced quality of the remaining skeletal muscle (Moran et al., 2007; Qaisar et al., 2013; Lai et al., 2016). Although E2 likely works through various mechanisms, leading candidates contributing to strength loss are apoptotic-induced reductions in muscle mass (La Colla et al., 2017; Laakkonen et al., 2017; Collins et al., 2019), modifications to myosin heavy chain function (Moran et al., 2007; Qaisar et al., 2013) through phosphorylation of the regulatory light chain (Lai et al., 2016; Collins et al., 2018), abnormal inflammation (Tiidus et al., 2001), and impaired mitochondrial function (Ribas et al., 2016; Valencia et al., 2016).
Estradiol also affects recovery of muscle following injury and thus, E2 deficiency in females would theoretically further exacerbate loss of strength via this mechanism. In support of this theory, muscles from Ovx mice have been shown to be weaker following various injuries (e.g., freeze injury, eccentric contraction-induced injury) compared to control or Ovx+E2 mice (Schneider et al., 2004; Kosir et al., 2015; Le et al., 2018; Collins et al., 2019). Estradiol may influence recovery of strength by regulating various pathways of skeletal muscle degeneration and regeneration. These include, but are not limited to, immune cells (Tiidus et al., 2001; Le et al., 2018) and muscle stem cells (i.e., satellite cells) (Tiidus et al., 2005). For instance, following a freeze-induced injury, a moderate dose of E2 given to Ovx mice increased neutrophil recruitment and recovery of muscle strength over that of a placebo treatment (Le et al., 2018). While acute inflammation including neutrophils and macrophages dominate the initial degenerative phase following injury (Arnold et al., 2007), the capacity for muscle to regenerate is largely due to the satellite cell population (Rathbone et al., 2003; Lepper et al., 2011). Satellite cells are small, mitotically quiescent stem cells that reside under the basal lamina of the muscle fiber (Mauro, 1961) and express the transcription factor Pax7 (Seale et al., 2000; Bosnakovski et al., 2008). Depleting satellite cells prevents skeletal muscle regeneration following injury (Lepper et al., 2011; Fry et al., 2015). Thus, the regenerative capacity of skeletal muscle requires maintenance of the satellite cell population. We recently reported that loss of E2 or estrogen receptor-α (ERα) in satellite cells reduces satellite cell number in skeletal muscles of female mice through a process involving apoptosis (Collins et al., 2019). These data indicate E2 regulates satellite cell maintenance in females under steady-state conditions, and likely muscle regeneration and recovery of strength following a single, isolated injury from an insult such as cardiotoxin or barium chloride (BaCl2).
In a physiological setting, skeletal muscle does not typically sustain a single injury, but rather numerous injuries over a lifetime. Repeated injuries have previously been used to assess skeletal muscle stress resistance, resilience, and adaptability in healthy and diseased mouse models, such as muscular dystrophy and malignant hypothermia (Corona et al., 2008; Baumann et al., 2020). However, is it unclear how repeated injuries to E2 deficient skeletal muscle impacts recovery, satellite cell number, and subsequent strength gains. Therefore, the purpose of this study was to (1) determine the extent to which E2 deficiency affects strength recovery when muscle is challenged by multiple injuries and, (2) assess how satellite cell number is influenced by the combination of E2 deficiency and repetitive skeletal muscle injuries. Considering that skeletal muscle regeneration following injury requires satellite cells and E2 maintains satellite cell number, in this study we hypothesized that recovery of strength following repeated injuries is impaired in mice without E2.
METHODS
Ethical approval
All animal procedures were approved by the Institutional Animal Care and Use Committees at the University of Minnesota (A3456). For all in vivo procedures (Ovx surgery and torque measurements), mice were initially anesthetized in an induction chamber using isoflurane, and then maintained by inhalation of isoflurane via a nose cone (1.25%, 125 mL O2 per min). Mice were euthanized with an overdose of sodium pentobarbital (IP injection at 200 mg/kg) at the completion of the study. Investigators understand the ethical principles and ensure that the work complies with the animal ethics checklist of the journal.
Experimental animals and design
Female wildtype (C57Bl/6) mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) at 12 wk of age, and aged to 16 wk prior to the initiation of the study. Mice were housed in groups of 4-5 and had access to phytoestrogen-free rodent chow (Harlan-Teklad #2019; Indianapolis, IN, USA) and water ad libitum. The housing room was maintained on a 14:10 h light:dark cycle with controlled temperature and humidity.
Mice were randomly assigned to one of two treatment groups: Placebo (n=24) or E2 (n=21). Four hours prior to the surgery, mice were given a subcutaneous injection of slow-release buprenorphine. Immediately following the Ovx surgery, mice received a placebo or slow-release E2 pellet. Three weeks following Ovx surgery, mice were tested for in vivo maximal isometric torque of the anterior crural muscles [tibialis anterior (TA), extensor digitorum longus, extensor hallucis muscles] (Pre; Figure 1) and retested weekly four times in a repeated measures design (i.e., pre-injury, and 7, 14 and 21 d post-injury). Immediately after recording in vivo maximal isometric torque during the pre-injury and each of the 21-d assessments, TA muscles were injured with intramuscular injections of BaCl2. Thus, over a 10-wk period TA muscles were injured three times with BaCl2 and assessed for maximal isometric torque production on 10 separate occasions (Figure 1). Mice were monitored daily for 3 d following all surgical procedure (ovariectomy or BaCl2 injections). TA muscles that were injured once or three times were given 63 or 21 d to recover, respectively. A subset of mice within each group served as uninjured controls. At the end point, uteri and TA muscles were dissected and weighed. Uterine mass <30 mg was used as an inclusion parameter to indicate successful Ovx surgery (Wood et al., 2007).
Figure 1. Experimental timeline for treatment, assessment of in vivo isometric torque, injury induction and tissue collection.
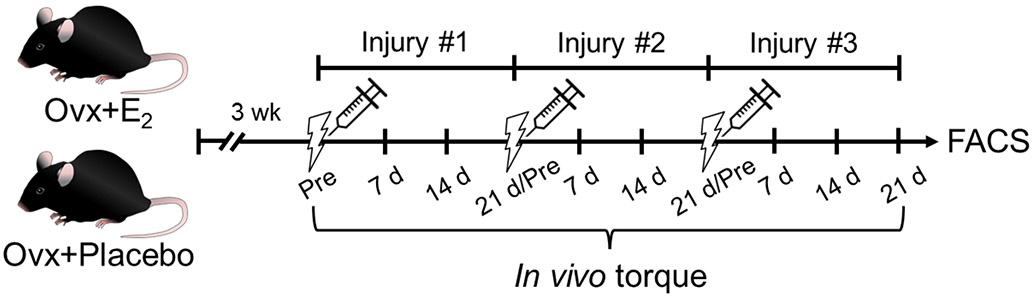
Mice were randomly assigned to one of two treatment groups prior to an ovariectomy (Ovx) surgery: Placebo or 17β-estradiol (E2). In vivo isometric torque of the anterior crural muscles was then measured before (Pre), and 7, 14 and 21 d following repeated BaCl2-induced injuries. After recovery from the first or third injuries, tissues were collected and satellite cell number of injured and uninjured tibialis anterior (TA) muscles was measured using FACS.
The total number of satellite cells in TA muscles was measured by FACS, with muscles designated as uninjured, injured once or injured three times. To determine adaptive strength gains, defined as the gain in torque from the initial pre-injury torque (Pre; Figure 1) to 21 d post-injury for each injury, a percent change was calculated as: ((21 d Post-Injury Torque − Pre-Injury Torque of Injury #1) / Pre-Injury Torque of Injury #1) * 100. Torque data expressed as fractional change following the first and second injuries for a subset of mice were previously reported (Collins et al., 2019).
Experimental methodology
Ovx surgery and treatment pellets
Under aseptic conditions, bilateral Ovx was performed through two small dorsal incisions between the iliac crest and the lower ribs (Moran et al., 2007). Immediately after Ovx, mice were implanted with pellets containing placebo or 0.18 mg E2 released over a 60-d period (Innovative Research of America, Sarasota, FL). This dose of E2 has been shown to mimic physiological levels in female mice (Greising et al., 2011; Le et al., 2018).
In vivo analysis of muscle torque
As previously described (Baumann et al., 2014), anesthetized mice were placed on a temperature-controlled platform to maintain core body temperature. The left knee was clamped and the left foot was secured to an aluminum “shoe” that is attached to the shaft of a 300B servomotor (Aurora Scientific, ON, Canada). Sterilized platinum needle electrodes were inserted through the skin for stimulation of the left common peroneal nerve. Stimulation voltage and needle electrode placement were optimized with isometric tetanic contractions (200 ms train of 0.1 ms pulses at 200 Hz). Contractile function of the anterior crural muscles was then assessed by measuring isometric torque as a function of stimulation frequency, with the highest recorded torque defined as maximal isometric torque.
BaCl2-induced injury
To induce skeletal muscle injury several methods have been used previously, including natural toxins (cardiotoxin, notexin), chemical reagents (BaCl2), physical injury (freeze or crush injury), medications (bupivacaine), and eccentric-contraction induced injury (Tierney & Sacco, 2016). Here, we use local exposure of BaCl2 (1.2% in sterile demineralized water; ~57mM) (Ricca Chemical Company, Arlington, TX) to induce muscle injury because, unlike freeze injury, BaCl2 causes myofiber necrosis through calcium-induced proteolysis, which does not affect the surrounding mononuclear population (i.e., satellite cells and fibroblasts) (Hansen et al., 1984; Caldwell et al., 1990; Cornelison et al., 2004; Murphy et al., 2011; Hardy et al., 2016; Morton et al., 2019). BaCl2 is a widely used method of injury due to its ability to cause consistent, repeatable muscle injury, as well as the absence of regulatory restrictions that accompany the purchase and use of natural toxins. Briefly, to induce injury, a small incision was made to expose the TA muscle, the Hamilton syringe needle was inserted at a 30° angle into the distal end of the TA muscle, pulled parallel to the tibia and 25 μL of BaCl2 was slowly injected. The syringe was held in place for 30 s following the injection to prevent fluid loss and the skin incision was closed with a single suture. To determine the volume of 1.2% BaCl2 to completely injure the TA muscle, we performed preliminary experiments with 25 μL or 50 μL injections via insulin or Hamilton syringe. We found that > 90% of the TA myofibers were affected histologically after injecting 25 μL 1.2% BaCl2 and preferred delivery with Hamilton syringe.
Isolation, staining, and FACS analyses of satellite cells
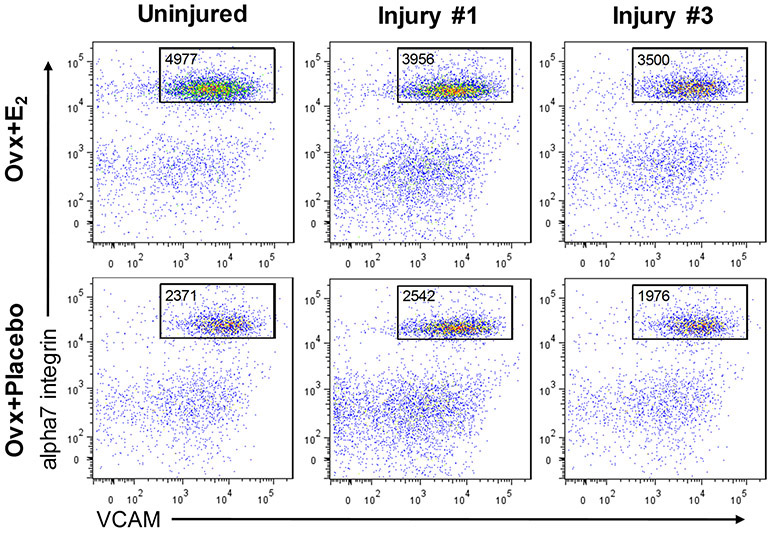
Isolation of satellite cells from TA muscles was performed as described in detail previously (Collins et al., 2019). Briefly, TA muscles were digested with collagenase type II and dispase (17101-015 and 17105-041, respectively; Gibco, Grand Island, NY). Mononuclear cells were stained using an antibody mixture of PE-Cy7 rat anti-mouse CD31 (clone 390), PE-Cy7 rat anti-mouse CD45 (clone 30-F11), Biotin rat anti-mouse CD106 (clone 429(MVCAM.A)) and PE Streptavidin from BD Biosciences (San Diego, CA); and alpha7 integrin 647 (clone R2F2) from AbLab (Vancouver, B.C., Canada). Samples were incubated with antibody cocktail, washed, and resuspended with FACS staining medium containing propidium iodide for analysis on a FACSAriaII SORP (BD Biosciences, San Diego, CA). Total satellite cells (lineage negative; VCAM, alpha7 double positive cells) were analyzed while draining the entire sample from each TA muscle sample (Figure 2).
Figure 2. Representative FACS plots of cells isolated from TA muscles of Ovx mice with and without 17β-estradiol treatment.
FACS plots show total satellite cells in TA muscles from Ovx+E2 and Ovx+Placebo mice that were quantified by lineage negative; VCAM, alpha7 integrin double positive cells. All gated events from individual TA muscles are shown.
Statistical analyses
To analyze the effect of treatment (Placebo vs. E2) on torque (Pre, 7 d, 14 d, 21 d for Injuries #1-#3) or adaptive strength gains (Pre and 21 d following Injuries #1-#3) across time, a repeated measures two-way analysis of variance (ANOVA) was utilized (with time as the repeated measure). A two-way ANOVA was used to assess the effect of treatment across time (Uninjured, Post-Injury #1 and Post-Injury #3) for satellite cell number and TA muscle mass. Bonferroni post hoc tests were performed in the event of a significant interaction or main effect of time. T-tests were used to detect difference in body mass and uterine mass between treatment or across time (study start and end times). An α level of <0.05 was used for all analyses. Data are presented as mean±SD. All statistical testing was performed using SigmaPlot version 12.5 (Systat Software, San Jose, CA).
RESULTS
Mouse body and uterine masses
Body mass did not differ between mice designated to Placebo or E2 groups (20.2±0.9 vs. 20.3±1.5 g; p=0.780) prior to the Ovx surgery. Both groups gained body mass 12 weeks following the surgery (p<0.001), however the Ovx+Placebo mice weighed 20% more than the Ovx+E2 mice (30.3±4.4 vs. 25.1±2.3 g; p<0.001). Uterine mass was ~9-fold less in Ovx+Placebo than Ovx+E2 mice (16.1±4.9 vs. 141.3±40.3 mg; p<0.001) with all uteri being < 25 mg in Ovx+Placebo mice.
Maximal isometric torque
To assess recovery of strength after injuries, in vivo isometric torque was measured in Ovx+Placebo and Ovx+E2 mice at several time points. An interaction between treatment and time was observed for maximal isometric torque (p<0.001). Isometric torque did not differ between groups prior to the first injury (Pre; 2.09±0.27 vs. 1.91±0.13 mN·m, p=0.146) and was greater in Ovx+Placebo than Ovx+E2 mice at day 7 following injury #1 (p=0.046; Figure 3A & B). In contrast, at 7, 14 and 21 days after the second injury, Ovx+Placebo mice produced 13-22% less isometric torque than Ovx+E2 mice (p≤0.021). Isometric torque following the third injury tended to be lower in Ovx+Placebo mice at day 14 (p=0.070), and was 11% less than Ovx+E2 mice by day 21 (2.67±0.23 vs. 3.01±0.40 mN·m, p=0.029; Figure 3A & B).
Figure 3. Mice that lack E2 are weaker following repeated injuries.
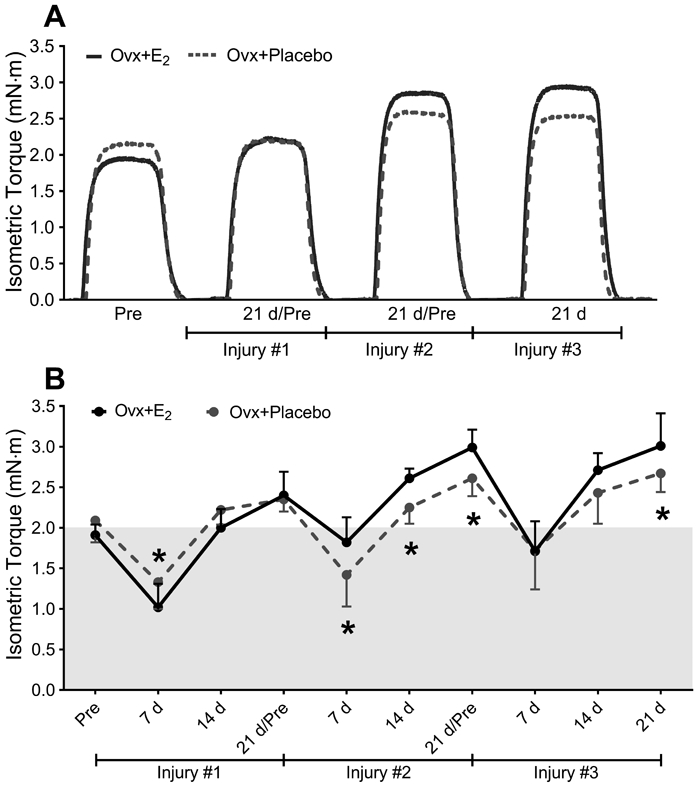
(A) Representative tracings of maximal isometric torque by anterior crural muscles before (Pre) and 21 d following one, two or three BaCl2-induced injures. (B) Repeated in vivo isometric torque measurements were made on Ovx+E2 and Ovx+Placebo mice before injury, and at 7-d intervals after one, two and three BaCl2–induced injuries. Data points above shaded area represent adaptive strength gains. Ovx; ovariectomized, E2; 17β-estradiol. Sample size per group, n=7 mice. Values are presented as mean±SD. Post hoc results following a significant interaction between treatment and time are shown in B (p<0.05). *Significantly different from Ovx+E2 at given time point.
Because in vivo maximal isometric torque appeared to increase above the initial pre-injury torque during the latter recovery periods (unshaded area, Figure 3B), we calculated adaptive strength gains at 21 d after each injury. When expressed as a percent change relative to Pre, both groups experienced an increase in isometric torque by the end of the study (p=0.005). These adaptive strength gains were not significantly different between the Ovx+Placebo and Ovx+E2 mice following first injury (14 vs. 26%; p=0.194). However, after the second and third injuries, adaptive strength gains at the 21 d post-injuries were less in Ovx+Placebo mice, with strength only increasing by 26-29% vs. 57-58% in Ovx+E2 mice relative to their respective Pre torque values (p≤0.002).
TA muscle mass and satellite cell numbers
To determine if the differences observed in maximal isometric torque were due to differences in muscle hypertrophy, we assessed mass of the uninjured and recovered TA muscles following the single and triple injury. An interaction between treatment and time was detected for TA muscle mass (p=0.006). Following the third injury, TA muscles of the Ovx+Placebo mice weighed 20% less than that of the Ovx+E2 mice (p<0.001; Figure 4A). Within the Ovx+E2 group, TA muscle mass was greater following injuries #1 and #3 compared to uninjured muscles indicating hypertrophy at the 21-d recovery times (p≤0.012; Figure 4A).
Figure 4. E2 improves the maintenance of satellite cell number before and after injuries.
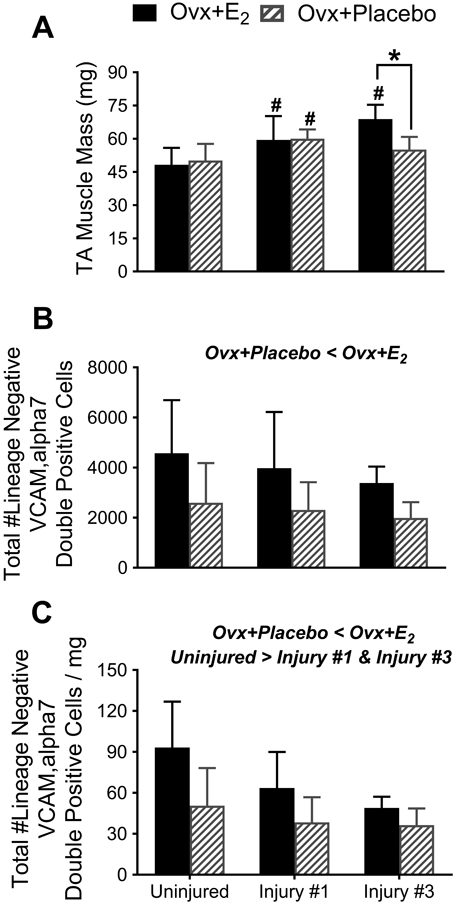
(A) Differences in TA muscle masses due to estradiol treatment depend on the number of injuries (interaction effect; p<0.006). (B) The total number of satellite cells in TA muscles, quantified by FACS as lineage negative;VCAM, alpha7 double-positive cells, is greater in ovariectomized mice treated with estradiol (main effect of treatment; p<0.001). (C) The total number of satellite cells normalized to muscle mass is greater in ovariectomized mice treated with estradiol (main effect of treatment; p,0.001) and lower with injury (main effect of time; p=0.002). Data was derived from the TA muscles of Ovx+E2 and Ovx+Placebo mice that were not injured, or from TA muscles that had recovered from one or three BaCl2-induced injuries. Sample size per group, n=7-16 mice. Ovx; ovariectomized, E2; 17β-estradiol. Values are presented as mean±SD. For A, following the significant interaction pairwise post-hoc results are indicated by: *Significantly different from Ovx+E2 at given time point; #Significantly different from Uninjured within treatment group. Main effects of two-way ANOVAs are indicated above each set of bars in B and C.
To quantify satellite cells, we used flow cytometry to count the number of lineage negative; VCAM, alpha7 integrin positive cells in entire TA muscles. The number of satellite cells in TA muscles was approximately 42% lower in Ovx+Placebo than Ovx+E2 mice (treatment effect; p<0.001; Figure 4B). The number of times that the TA muscle was injured did not alter the absolute number of satellite cells (time effect; p=0.230; Figure 4B). No treatment by time interaction was detected (p=0.849).
As with absolute satellite cell number (Figure 4B), satellite cell number per mg of TA muscle mass was lower in Ovx+Placebo than Ovx+E2 mice (treatment effect; p<0.001; Figure 4C). In contrast to absolute numbers, when accounting for hypertrophy as measured by muscle mass, there was a main effect of time (p=0.002). Satellite cell number per muscle mass was 26-40% less after injury #1 and #3 compared to that of uninjured muscles. No treatment by time interaction was detected (p=0.174).
DISCUSSION
The purpose of this study was to determine the extent to which E2 deficiency affects recovery of skeletal muscle strength and satellite cell number when challenged by multiple injuries. This report is the first to measure recovery of strength at several time points following three injuries in ovariectomized mice with and without estradiol treatment (Ovx+E2 and Ovx+Placebo, respectively). It is also one of few studies to measure total muscle satellite cell number by FACS using a repetitive injury model. The major findings of this study are: 1) after repeated injuries, muscles from Ovx+Placebo mice exhibit a blunted ability to adapt as indicated by smaller and weaker muscles compared to those from Ovx+E2 mice, and 2) satellite cell number is lower in Ovx+Placebo mice compared to Ovx+E2 mice regardless of injury status (uninjured or injured) or the number of injuries (1, 2 or 3). Taken together our data support the notion that the loss of E2 blunts adaptive strength gains and that the number of satellite cells likely contributes to the impairment. However, considering that recovery of strength occurred in Ovx mice, we are unable to support the hypothesis that recovery of strength would be impaired in mice without E2.
One of the more salient mechanisms that regulates skeletal muscle regeneration is the homeostatic maintenance of the satellite cell pool (Shefer et al., 2006), with loss of satellite cells being detrimental to regeneration. Decrements in satellite cell number can arise from changes in the muscle environment (Dumont et al., 2015), such as loss of E2-ERα signaling (Collins et al., 2019). In the present study, BaCl2-induced injuries were used to evaluate skeletal muscle regeneration with parallel analyses of strength recovery and adaptability. Hardy et al. reported that satellite cell number decreased 53% 18 h after a BaCl2–induced injury followed by an increase in proliferation resulting in a 4-fold increase in satellite cell number (Hardy et al., 2016). By three months post-injury, satellite cell number returned to baseline (Hardy et al., 2016). We quantified satellite cell number in TA muscles that were uninjured or recovered following a single or triple injury. The results presented here show that absolute satellite cell number remains low in Ovx+Placebo mice after a single injury or three repeated injuries, while the absolute satellite cell number in Ovx+E2 muscle remains consistently higher (Figure 4B). Since further decrements in absolute satellite cell number with E2 deficiency were not observed with repeated injuries, our data suggest that the remaining population of satellite cells in the Ovx+Placebo muscle are resilient to pro-apoptotic signals and maintain their ability to self-renew.
Several studies have investigated factors that influence satellite cell number and muscle regeneration, yet the link between satellite cells and recovery of strength has been minimally studied. Combining satellite cell ablation and hindlimb casting to model muscle contractures, Dayanidhi et al. (2020) showed that a reduced number of satellite cells impaired muscle’s ability to add sarcomeres in series and recover from the immobilization-induced contracture. That study suggested that there is a relationship between satellite cell number and muscle growth. Using an eccentric contraction-induced injury model and irradiation to eliminate satellite cells, Rathbone et al. (2003) reported that recovery of in vivo maximal isometric torque 35 d after a single injury was 25% less when satellite cells were eliminated. We are aware of no studies that determined the effect of reduced satellite cell number on strength recovery following multiple injuries. The results presented here show that regardless of BaCl2 injury number, maximal isometric torque of Ovx+Placebo mice fully recover and increase 21 d post-injury compared to that of their pre-injury torque. However, when E2 was present and satellite cell number was maintained, Ovx+E2 mice were able to produce more torque as well as greater strength gains following repeated injures compared to Ovx+Placebo mice (Figure 3). We suggest that satellite cells that survived in the E2 deficient muscle were resilient and capable of regenerating injured muscle to support recovery of strength, yet were insufficient to induce adaptive strength gains (i.e., hypertrophy) as measured in TA muscle of Ovx+E2 mice (Figure 4A). These data are in agreement with Egner et al., (Egner et al., 2016) who showed that overload hypertrophy was prevented in satellite cell-deficient mice indicating that satellite cells are necessary for effective muscle hypertrophy. Overall, our data suggest that E2 deficiency impairs the ability of muscle to adapt after repeated injuries and that mechanistically satellite cell number contributes to this functional deficit.
In summary, we report that E2 deficiency impairs the adaptive potential of skeletal muscle following repeated BaCl2-induced injuries, as measured by skeletal muscle mass and strength. The blunted ability of E2 deficient muscle to recover and adapt to repeated injuries is likely multifactorial, however our data suggest one mechanism is reduction in the number of satellite cells. We highlight the role of E2 in maintaining muscle strength, an important consideration in the context of aging because repeated injuries accumulate over a lifetime and can contribute to age-related strength loss (Brooks & Faulkner, 1994). Thus, our findings have implications for aging, hormone replacement and regenerative medicine in regards to maintaining satellite cell number and ultimately the preservation of skeletal muscle’s adaptive potential.
NEW FINDINGS.
What is the central question of this study?
Estradiol (E2) plays an important role in regulating skeletal muscle strength in females. Here, we asked to what extent E2 deficiency affects recovery of strength and satellite cell number when muscle is challenged by multiple injuries.
What is the main finding and its importance?
E2 deficiency impairs the adaptive potential of skeletal muscle following repeated injuries, as measured by muscle mass and strength. The impairment is likely multifactorial with our data indicating that one mechanism is reduction in satellite cell number. Our findings have implications for aging, hormone replacement and regenerative medicine in regards to maintaining satellite cell number and ultimately the preservation of skeletal muscle’s adaptive potential.
Acknowledgments
FUNDING
This study was supported by NIH grants R01-AG031743 (DAL), R01-AG062899 (DAL and MK), T32-AG029796 (AAL), and T32-AR007612 (CWB).
ABBREVIATIONS
- FACS
fluorescent-activated cell sorting
- E2
17β-estradiol
- Ovx
ovariectomy
- ERα
estrogen receptor-alpha
- BaCl2
barium chloride
- TA
tibialis anterior
Footnotes
COMPETING INTERESTS
None declared.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK & Chazaud B (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204, 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CW, Rogers RG, Gahlot N & Ingalls CP (2014). Eccentric contractions disrupt FKBP 12 content in mouse skeletal muscle. Physiol Rep 2, e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CW, Warren GL & Lowe DA (2020). Plasmalemma Function Is Rapidly Restored in Mdx Muscle after Eccentric Contractions. Med Sci Sports Exerc 52, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC & Kyba M (2008). Prospective isolation of skeletal muscle stem cells with a pax7 reporter. Stem Cells 26, 3194–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV & Faulkner JA (1994). Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc 26, 432–439. [PubMed] [Google Scholar]
- Caldwell C, Mattey D & Weller R (1990). Role of the basement membrane in the regeneration of skeletal muscle. Neuropathology and applied neurobiology 16, 225–238. [DOI] [PubMed] [Google Scholar]
- Collins BC, Arpke RW, Larson AA, Baumann CW, Xie N, Cabelka CA, Nash NL, Juppi HK, Laakkonen EK, Sipilä S, Kovanen V, Spangenburg EE, Kyba M & Lowe DA (2019). Estrogen regulates the satellite cell compartment in females. Cell Rep 28, 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BC, Mader TL, Cabelka CA, Iñigo MR, Spangenburg EE & Lowe DA (2018). Deletion of estrogen receptor α in skeletal muscle results in impaired contractility in female mice. J Appl Physiol 124, 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison D, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC & Olwin BB (2004). Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes & development 18, 2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona BT, Rouviere C, Hamilton SL & Ingalls CP (2008). Eccentric contractions do not induce rhabdomyolysis in malignant hyperthermia susceptible mice. J Appl Physiol 105, 1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanidhi S, Kinney MC, Dykstra PB & Lieber RL (2020). Does a reduced number of muscle stem cells impair the addition of sarcomeres and recovery from a skeletal muscle contracture? a transgenic mouse model. A Publication of The Association of Bone and Joint Surgeons®∣ CORR® 478, 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont NA, Wang YX & Rudnicki MA (2015). Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142, 1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner IM, Bruusgaard JC & Gundersen K (2016). Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 143, 2898–2906. [DOI] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ & Peterson CA (2015). Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL & Lowe DA (2011). Estradiol’s beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol 110, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Lowe DA & Warren GL (2009). Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci 64, 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TR, Dineen DX & Petrak R (1984). Mechanism of action of barium ion on rat aortic smooth muscle. American Journal of Physiology-Cell Physiology 246, C235–C241. [DOI] [PubMed] [Google Scholar]
- Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM, Tajbakhsh S, Rocheteau P & Chrétien F (2016). Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11, e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanga-Sollo E, Thornton K, White ME & Dayton WR (2017). Role of G protein-coupled estrogen receptor-1 in estradiol 17β-induced alterations in protein synthesis and protein degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol 58, 90–96. [DOI] [PubMed] [Google Scholar]
- Kosir AM, Mader TL, Greising AG, Novotny SA, Baltgalvis KA & Lowe DA (2015). Influence of ovarian hormones on strength loss in healthy and dystrophic female mice. Med Sci Sports Exerc 47, 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Colla A, Vasconsuelo A, Milanesi L & Pronsato L (2017). 17β-Estradiol Protects Skeletal Myoblasts From Apoptosis Through p53, Bcl-2, and FoxO Families. J Cell Biol 118, 104–115. [DOI] [PubMed] [Google Scholar]
- Laakkonen EK, Soliymani R, Karvinen S, Kaprio J, Kujala UM, Baumann M, Sipilä S, Kovanen V & Lalowski M (2017). Estrogenic regulation of skeletal muscle proteome: a study of premenopausal women and postmenopausal MZ cotwins discordant for hormonal therapy. Aging Cell 16, 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Collins BC, Colson BA, Kararigas G & Lowe DA (2016). Estradiol modulates myosin regulatory light chain phosphorylation and contractility in skeletal muscle of female mice. Am J Physiol Endocrinol Metab 310, E724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le G, Novotny SA, Mader TL, Greising SM, Chan SSK, Kyba M, Lowe DA & Warren GL (2018). A moderate oestradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated. J Physiol 596, 4665–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA & Fan C-M (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A (1961). Satellite cell of skeletal muscle fibers. The Journal of biophysical and biochemical cytology 9, 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AL, Nelson SA, Landisch RM, Warren GL & Lowe DA (2007). Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol 102, 1387–1393. [DOI] [PubMed] [Google Scholar]
- Morton AB, Norton CE, Jacobsen NL, Fernando CA, Cornelison D & Segal SS (2019). Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels. Skeletal muscle 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA & Kardon G (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SK, Rook KM, Siddle NC, Bruce SA & Woledge RC (1993). Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 84, 95–98. [DOI] [PubMed] [Google Scholar]
- Qaisar R, Renaud G, Hedstrom Y, Pollanen E, Ronkainen P, Kaprio J, Alen M, Sipila S, Artemenko K, Bergquist J, Kovanen V & Larsson L (2013). Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J Physiol 591, 2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone CR, Wenke JC, Warren GL & Armstrong RB (2003). Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 285, R1490–1495. [DOI] [PubMed] [Google Scholar]
- Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS & Hevener AL (2016). Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8, 334ra354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BSP, Fine JP, Nadolski T & Tiidus PM (2004). The effects of estradiol and progesterone on plantarflexor muscle fatigue in ovariectomized mice. Biol Res Nurs 5, 265–275. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P & Rudnicki MA (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB & Yablonka-Reuveni Z (2006). Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294, 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MT & Sacco A (2016). Inducing and evaluating skeletal muscle injury by notexin and barium chloride In Skeletal Muscle Regeneration in the Mouse, pp. 53–60. Springer. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Deller M & Liu XL (2005). Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand 184, 67–72. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Holden D, Bombardier E, Zajchowski S, Enns D & Belcastro A (2001). Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol 79, 400–406. [PubMed] [Google Scholar]
- Valencia AP, Schappal AE, Morris EM, Thyfault JP, Lowe DA & Spangenburg EE (2016). The presence of the ovary prevents hepatic mitochondrial oxidative stress in young and aged female mice through glutathione peroxidase 1. Exp Gerontol 73, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KLM & Khokha R (2007). Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 133, 1035–1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.