Abstract
The current study examined two possible mechanisms, evocative gene-environment correlation and prenatal factors, in accounting for child effects on parental negativity. Participants included 561 children adopted at birth, and their adoptive parents and birth parents within a prospective longitudinal adoption study. Findings indicated child effects on parental negativity, such that toddlers’ negative reactivity at 18 months was positively associated with adoptive parents’ over-reactive and hostile parenting at 27 months. Furthermore, we found that child effects on parental negativity were partially due to heritable (e.g., birth mother internalizing problems and substance use) and prenatal factors (e.g., birth mother illicit drug use during pregnancy) that influence children’s negative reactivity at 18 months. The current study provides critical evidence for “child on parent” effects.
Keywords: “child on parent” effects, evocative gene-environment correlation, maternal illicit drug use during pregnancy, negative reactivity, parental negativity
Since the publication of Bell’s (1968) article presenting a “child on parent” effects model, there has been an increasing consensus that children play an important role in the way they are parented (e.g., Davidov, Knafo-Noam, Serbin, & Moss, 2015). The literature supports that children are not passive recipients of parenting; rather, they can evoke substantial and systematic reactions from their parents (Davidov et al., 2015; Klahr & Burt, 2014), thereby indirectly influencing their own behavior. One child behavior, in particular – negative reactivity, may figure prominently in child evocative effects on parental behaviors beginning in toddlerhood. This is supported by studies showing that highly reactive children pose more difficulties to their parents and therefore evoke less responsive and more emotionally reactive and hostile parenting behaviors from their parents than do children with low to moderate reactivity (e.g., Bridgett et al., 2009; Popp, Spinrad, & Smith, 2008).
Negative reactivity has been defined as children’s tendency to react with negative emotionality in response to stressors, including anger proneness, fearfulness, difficultness and irritability (Rothbart & Bates, 2006). Specifically, during toddlerhood, as children begin to seek autonomy and have increased physical mobility, coupled with little cognitive appreciation for the consequences of their behavior on themselves and others, their negative emotionality and willful defiance also increases (Shaw & Bell, 1993). In particular, parents often respond to more reactive children with greater control (e.g., limit-setting) and more parental negativity (Shaw & Bell, 1993). For example, previous studies have shown that toddlers with high negative reactivity are more likely to elicit hostile and non-responsive parenting compared to toddlers with low to moderate levels of negative reactivity (Bridgett et al., 2009; Popp et al., 2008). In turn, negative responses from parents may lead to a higher frequency of children’s dysregulated behavior (e.g., oppositional and aggressive behavior), putting children at increased risk for cascading effects that may lead to conduct problems and poor academic achievement at school-age and beyond (Shaw, Gilliom, Ingoldsby, & Nagin, 2003). These studies help to confirm child effects on parental negativity during early childhood. However, the mechanisms underlying child effects on parental negativity are not fully understood, especially in early development.
In the current study, we propose two mechanisms that may help to explain child effects on parental negativity. First, evocative gene-environment correlation (rGE) may be operating. Evocative rGE is present when heritable characteristics of an individual, in this case a child, evoke systematic reactions from their environment, in this case parenting behavior (e.g., Scarr & McCartney, 1983). Second, based on previous findings that prenatal environments have important influences on child behavior (e.g., Alessandri, Sullivan, Imaizumi, & Lewis, 1993), coupled with findings of child evocative effects (e.g., Roben et al., 2015), we propose that child effects on parental negativity may also be influenced by prenatal factors via their influence on child behavior.
The current study aims to systematically examine these two possible mechanisms of child effects on parental negativity via negative reactivity during toddlerhood. Most research examining child effects on parental negativity has studied biologically related families. Such work cannot unambiguously reveal the mechanisms underlying child effects on parental negativity. This is because in biologically related families the associations between child characteristics and parenting could be explained by shared heritable influences between parent and child or by prenatal and postnatal environmental influences or by passive rGE influences (confounding between genes shared between parent and child and the rearing environments) (Ge et al., 1996; Scarr & McCartney, 1983). In addition, associations between prenatal environment and child outcomes in children biologically related to parents may be due in part to confounds between prenatal environment, postnatal environment and heritable influences. The current study addresses gaps in the research literature by using a parent-offspring adoption design to examine child effects from child negative reactivity at 18 months to adoptive parents’ parental negativity at 27 months. The roles of heritable and prenatal influences in explaining child effects will be examined simultaneously, while controlling for postnatal rearing environmental influences. In the parent-offspring adoption design, because the birth parents provide only their genes, and in the case of the birth mother the prenatal environment, links between the adopted child and their birth parent(s) are best explained as heritable and/or prenatal influences. In contrast, the adoptive parents are genetically unrelated to the adopted child but provide the rearing environment. Thus, links between the adopted child and their adoptive parents are best explained as shared environmental. The parent-offspring adoption design allows us to 1) eliminate passive rGE influences, as the adopted child and their adoptive parents are genetically unrelated; and 2) disentangle heritable and prenatal influences from postnatal rearing environmental influences. Through the use of parent-offspring adoption design, which identifies child effects arising from child’s heritable and prenatal factors, we will be able to provide conclusive evidence for child effects.
Evocative rGE in Explaining Child Effects on Parental Negativity
Negative reactivity is partially influenced by heritable factors (Goldsmith, Buss, & Lemery, 1997). Children’s inherited characteristics, such as negative reactivity and difficult temperament, can evoke comparably negatively valenced reactions from parents (e.g., Scarr & McCartney, 1983). Genetically informed studies, such as twin and parent-offspring adoption designs, have found evidence for evocative rGE in explaining child effects on parental negativity (e.g., Boivin et al., 2005; Ge et al., 1996; Marceau, Horwitz, et al., 2013). For example, studies using child-based twin designs show that heritable influences partially account for the association between child characteristics (e.g., child difficult temperament) and parental negativity during early childhood, indicating the existence of gene-environment correlation (e.g., Boivin et al., 2005). Other studies have adopted the novel extended children of twins design, mainly focusing on adolescents, and indicated that evocative rGE explained child effects in the association between adolescent externalizing problems and parental negativity (e.g., Marceau, Horwitz, et al., 2013). Studies using adoption designs have found that adopted adolescents who were at heritable risk for antisocial behaviors received more negative parenting from adoptive parents than adolescents not at heritable risk (Ge et al., 1996). In addition, there is evidence of evocative rGE in young children (e.g., Braungart, Fulker, & Plomin, 1992; Harold et al., 2013). For example, using the same sample as in the current study, birth mothers’ attention deficit hyperactivity symptoms were associated with greater adoptive mothers’ parental negativity at children’s age of 4.5 years, and this association was partially accounted for by child disruptive behavior at 4.5 years (Harold et al., 2013). In another report, birth mothers’ externalizing psychopathology was associated with greater parental negativity from adoptive mothers at children’s age 9 months, but only when adoptive mothers’ marital problems were also present (Fearon et al., 2015). These studies provide consistent evidence for evocative rGE in partially accounting for child effects on parental negativity. However, many previous investigations using different samples have focused on older children (e.g., adolescents) and have not identified specific child characteristics that may mediate associations between heritable factors and parental negativity.
Prenatal Influences in Explaining Child Effects on Parental Negativity
Child effects on parental negativity may also be influenced by prenatal environmental influences (e.g., Knopik, Neiderhiser, de Geus, & Boomsma, 2016). In the present report, we focus on maternal illicit drug use during pregnancy as especially relevant to the development of child negative reactivity, which has been shown to evoke parental negativity. Our focus is on maternal use of one or more illicit drugs (referred to as maternal illicit drug use) rather than maternal extensive use of a single illicit drug during pregnancy because: (1) mothers who use illicit drugs during pregnancy often use multiple drugs (licit or illicit), making it difficult to isolate the effect of a single drug from overlapping poly-drug exposures (Singer, 1999); (2) in the current sample, which was not selected based on the presence of prenatal drug use, we lack the power to estimate the effects of each specific illicit drug used during pregnancy; and (3) importantly, we were interested in the cumulative effects of illicit drugs used during pregnancy. One way to examine the effects of prenatal illicit drug use is to focus on one illicit drug (e.g., marijuana) and control for the effects of other drugs. A complementary method is to examine the cumulative effects of (illicit) drugs used during pregnancy, as done in studies based on the cumulative prenatal stress models, given that prenatal use of one or more drugs could act as a cumulative biological stressor during pregnancy (Conradt et al., 2014; Fisher et al., 2011). Specifically, recent work has indicated that in addition to teratogenic effects, maternal use of one or more drugs during pregnancy serves as a cumulative prenatal stressor, which may expose the fetus to high cumulative levels of maternal cortisol and alter fetus’s physiological reactivity to the rearing environments (e.g., Lester, Marsit, Conradt, Bromer, & Padbury, 2012). Thus, in the current study, we followed the line of reasoning from previous work and examined the effects of maternal use of one or more illicit drugs during pregnancy on child negative reactivity, with the caveat that the biological mechanisms underlying the effects of different illicit drugs on child negative reactivity may be different.
Maternal illicit drug use during pregnancy, including cocaine, marijuana, and amphetamine (the most often studied illicit drugs), has been associated with reduced negative emotional reactivity in infants and toddlers (e.g., Alessandri et al., 1993; LaGasse et al., 2011; Molitor, Mayes, & Ward, 2003; Roumell, Wille, Abramson, & Delaney, 1997). For example, 18-month-old toddlers whose mothers used cocaine with or without marijuana, alcohol, and tobacco during pregnancy exhibited lower levels of negative emotions (e.g., anger, distress) after inoculation (Roumell et al., 1997) and showed less negative reactivity during a separation task (Molitor et al., 2003) compared to unexposed children. However, this finding of less reactivity in children whose mothers used illicit drugs while pregnant has not been consistently replicated across studies. Specifically, toddlers whose mothers used illicit drugs (mainly cocaine, marijuana, and amphetamine) during pregnancy displayed more negative reactivity during emotionally distressful situations (e.g., “still-face” procedure, arm restraint task) and were rated as more negatively reactive (e.g., difficult) by their mothers than toddlers whose mothers did not use drugs during pregnancy (e.g., Kirlic et al., 2013; Metosky & Vondra, 1995). Of note, during early childhood, child negative reactivity in response to stressors is typically adaptive and normative at appropriate levels (Razza, Martin, & Brooks-Gunn, 2012). Both excessive (high) and blunted (flat) reactivity are maladaptive and atypical responses to stressors, both of which have been associated with more problem behaviors compared to children who display low to moderate levels of reactivity (Cole, Zahn-Waxler, Fox, Usher, & Welsh, 1996).
Disentangling Prenatal from Heritable Influences in Explaining Child Effects on Parental Negativity
It is important to clarify if the effects of prenatal illicit drug exposure on child functioning are confounded by heritable or postnatal rearing environmental influences before inferring that prenatal influences are primary (Knopik et al., 2016). Specifically, child emotional and behavioral outcomes that are influenced by pregnancy drug use may also be influenced by heritable factors or influenced by postnatal rearing environments (D’Onofrio et al., 2008; Neiderhiser et al., 2016). Thus, the effects of prenatal drug use on child negative reactivity drawn from studies based on biological families cannot be fully attributed to direct prenatal environmental effects. To date, however, few studies of the effects of prenatal drug use have used genetically informed designs. In addition, few studies have considered in parallel the effects of heritable and prenatal factors on child effects on parental negativity. Previous work using the same parent-offspring adoption sample as the current study indicates prenatal effects (e.g., perinatal internalizing symptoms, prenatal and neonatal complications) on toddlers’ behaviors (Marceau, Hajal, et al., 2013; Neiderhiser et al., 2016; Roben et al., 2015), although these studies have mainly focused on toddlers’ problem behaviors rather than temperamental characteristics. In addition, previous work has shown evocative rGE effects on parenting during early childhood (e.g., Elam et al., 2014; Fearon et al., 2015; Klahr et al., 2017), although many of these previous investigations did not identify specific child characteristics that mediate the association between heritable influences and parenting. The current study aimed to address the gap and extend previous work by simultaneously examining the effects of heritable factors and maternal illicit drug use during pregnancy on child effects on parental negativity via child negative reactivity, while controlling for postnatal rearing environmental influences, using the parent-offspring adoption design. We also controlled for possible confounding by maternal alcohol and tobacco use during pregnancy and other obstetric complications when examining the effects of pregnancy use of illicit drugs.
It should be noted that the parent-offspring adoption design does not perfectly disentangle prenatal influences from heritable influences. However, by carefully measuring birth parents’ psychopathology characteristics and birth mothers’ prenatal environments separately, the adoption design can help to clarify the effects of heritable and prenatal factors on child development (e.g., negative reactivity). In addition, we have included information on birth fathers, which provides additional information to distinguish between heritable and prenatal influences because birth fathers do not provide the prenatal environment, only heritable influences (Loehlin, 2016).
The Present Study
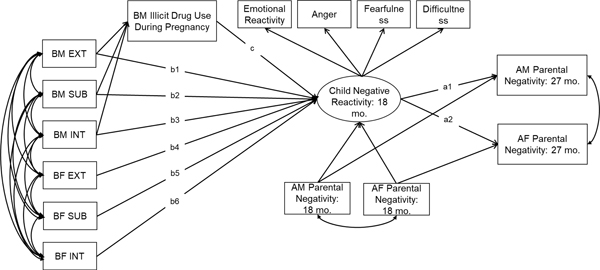
The current study used 561 children adopted at birth, their adoptive parents, and their birth parents to examine (1) child effects on adoptive mothers’ (AM) and adoptive fathers’ (AF) parental negativity at 27 months, and (2) whether heritable influences and birth mother (BM) illicit drug use during pregnancy evoke parental negativity of adoptive parents via child negative reactivity at 18 months. Heritable influences are indicated by birth parents’ psychopathology (e.g., externalizing problems, internalizing problems and substance use). We hypothesized that: (1) higher negative reactivity in children at 18 months would be associated with higher levels of AM and AF’s parental negativity at 27 months, and (2) birth parents’ psychopathology and BM illicit drug use during pregnancy would be indirectly associated with AM and AF’s parental negativity at 27 months and these associations would be mediated by child negative reactivity at 18 months. As shown in Figure 1, hypothesis 1 is tested by paths labeled as a1 and a2. Hypothesis 2 is tested by paths labeled as b1-b6 to a1-a2 and c to a1-a2. We also examined the direct effects of birth parents’ psychopathology and BM illicit drug use during pregnancy on AM and AF’s parental negativity at 27 months. These paths are not shown in Figure 1 to increase interpretability.
Figure 1.
Child effects on adoptive parents’ parental negativity via child negative reactivity: the role of heritable and prenatal factors.
Note. AM = adoptive mother; AF = adoptive father; BM = birth mother; BF = birth father. EXT = externalizing problems; INT = internalizing problems; SUB = substance use. The composites of heritable factors (e.g., EXT, INT, SUB) are shown as observed variables in the figure, but they are factor scores created using principal component analysis. The direct effects of birth parents’ psychopathology and BM prenatal illicit drug use on AM and AF’s parental negativity were not shown in Figure 1 to increase interpretability.
Method
Participants
The sample is from the Early Growth and Development Study (EGDS) Cohorts I and II, which includes 561 adopted children, their adoptive parents and birth parents (Leve et al., 2019). The eligibility criteria for EGDS included: (a) domestic adoption placement, (b) the adoption placement occurred within 3 months postpartum, (c) adoptive family were not biologically related to the child, (d) no known major medical conditions such as extreme prematurity or extensive medical surgeries, and (e) birth and adoptive parents were able to read and understand English at the eighth-grade level. We recruited families from 45 adoption agencies located in 15 states across the United States, between 2003 and 2010. We worked with adoption agencies that favored both open and closed adoptions, including public, private, religious, and secular agencies.
Forty-two percent of the children were female and 56% were Caucasian, 19% were multi-racial, 13% were African American, 11% were Latino and 1% were other or unknown ethnicity or not reported. The median age of the child at the adoption placement was 2 days (SD = 12.45, 0 – 91 days). The adoptive parents typically had college educations and were middle- to upper-class families. The mean age of adoptive mother was 38 (SD = 5.60, 24 – 55 years) and the mean age of adoptive father was 38 (SD = 5.82, 24 – 60 years) at adopted child birth. The ethnicity of adoptive mothers was 92% Caucasian, 4% African American, 2% Hispanic/Latino, 1% multi-racial and 1% other or unknown ethnicity or not reported. The ethnicity of adoptive fathers was 90% Caucasian, 5% African American, 2% Hispanic/ Latino, 1% multi-racial and 2% other or unknown ethnicity or not reported. Birth parents (BP) typically had high school education and had household annual incomes less than $25,000. The mean age of birth mother was 23 (SD = 6.03, 14 – 43 years) and the mean age of birth father (BF) was 24 (SD = 7.78, 15 – 59 years) at adopted child birth. The ethnicity of birth mothers was 70% Caucasian, 13% African American, 7% Hispanic/Latino, 5% multi-racial and 5% other or unknown ethnicity or not reported and the ethnicity of birth fathers was 70% Caucasian, 12% African American, 10% Hispanic/Latino, 5% multi-racial and 3% other or unknown ethnicity or not reported. Data from adopted children, adoptive parents and birth parents were used in the present study. For birth parents, four assessments were used when the adopted child was 5, 18, 27, and 54 months of age. For adoptive parents and adopted children, two assessments were used when the adopted child was 18 and 27 months of age. More recruitment, assessment, and demographic information is available elsewhere (Leve et al., 2019).
It should be noted that there were 41 (7%) same-sex couples in EGDS. For families with two adoptive mothers (N = 23), we averaged across both mothers to create an index score for adoptive mother report and set adoptive father report to missing. Similarly, for families with two adoptive fathers (N = 18), we averaged across fathers to create an index score for adoptive father report and set adoptive mother report to missing.
Measures
Toddler Negative Reactivity.
We used a latent factor representing each child’s negative reactivity as identified using four indicators: Emotional reactivity, Anger, Fearfulness, and Difficultness at 18 months, as illustrated in Figure 1. These indicators collectively provide a valid and comprehensive index of the negative reactivity (Rothbart & Bates, 2006) and were measured as follows.
Emotional Reactivity.
Emotional reactivity was measured when children were 18 months of age using an average of AM and AF’s reports on the 9-item Emotional Reactivity subscale (α = .57 – .58) from the Child Behavior Checklist 1.5 – 5 years (CBCL: Achenbach & Rescorla, 2000). This subscale asks questions about whether the child shows sudden changes in mood, and is worried, upset by new people or situations and disturbed by any change in routine. The CBCL 1.5 – 5 years is scored on a 3-point Likert scale. The scores were averaged across AM and AF to create an index score for emotional reactivity at 18 months (r = .37 between AM and AF reports).
Anger.
Anger was measured using an average of AM and AF’s reports on the 19-item Anger Proneness subscale (α = .89) from the Toddler Behavior Assessment Questionnaire (TBAQ: Goldsmith, 1996) at 18 months. The TBAQ is appropriate for use in children from 16 to 32 months of age (Goldsmith, 1996) and is scored on a 7-point Likert scale. This subscale measures crying, protesting, hitting, pouting, or other signs of anger in situations involving conflict with another child or the caregiver. The scores were averaged across AM and AF to create an index score for anger at 18 months (r = .42 between AM and AF reports).
Fearfulness.
Fearfulness was measured using an average of AM and AF’s reports on the 28-item Social Fearfulness subscale (α = .82 – .84) from the TBAQ (Goldsmith, 1996) at 18 months. This subscale measures inhibition, distress, withdrawal, or other signs of fearfulness in novel situations. The scores were averaged across AM and AF to create an index score for fearfulness at 18 months (r = .63 between AM and AF reports).
Difficultness.
Difficultness was measured using an average of AM and AF’s reports on the 7-item Difficult factor subscale (α = .85 – .86) from the Infant Characteristics Questionnaire (ICQ: Bates, Freeland, & Lounsbury, 1979) at 18 months. The ICQ is appropriate for use in infants and toddlers and is scored on a 7-point Likert scale. This subscale measures parental perception of child negative emotionality (e.g., “changeable mood”, “how easily upset). The scores were averaged across AM and AF to create an index score for difficult temperament at 18 months (r = .59 between AM and AF reports).
Adoptive Parents’ Parental Negativity at 18 and 27 months.
Both AM and AF’s parental negativity were assessed at child ages of 18 and 27 months. At 18 months, AM and AF completed the 10-item Over-reactive subscale (α = .77 – .79) from the Parenting Scale (Arnold, O’leary, Wolff, & Acker, 1993). Adoptive parents reported their own parental discipline “mistakes” such as displays of anger, meanness and irritability on a 7-pont Likert scale.
At 27 months, parental negativity was assessed using AM and AF self-reports of their over-reactive parenting on the 10-item Over-reactive subscale (α = .77 – .79) from the Parenting Scale (Arnold et al., 1993) and hostility towards their child on the 5-item Hostility subscale (α = .70 – .74) from Iowa Family Interaction Rating Scales (Conger & Melby, 2000). Similar to the Parenting Scale, the Iowa Family Interaction Rating Scales is scored on a 7-point Likert scale. For both AM and AF, over-reactive subscale and hostility subscale were significantly correlated (rs = .66 and .64 for AM and AF, respectively). Thus, we were able to create composite scores of over-reactivity and hostility for both AM and AF’s parental negativity at 27 months, respectively. Specifically, we standardized over-reactive subscale scores and hostility subscale scores and then averaged the standardized scores of these two subscales to create the composite scores for AM’s parental negativity and AF’s parental negativity, respectively.
Index of Heritable Risk.
BP characteristics were used to index heritable risks for child negative reactivity and disentangle influences from prenatal illicit drug use from heritable risk. In the current study, we used composites of heritable risk for externalizing problems, internalizing problems and substance use for BM and BF separately. These composites of heritable risk were created using principal component analyses (PCA) based on BP’s: 1) lifetime psychiatric diagnoses through Diagnostic Interview Schedule (DIS: Robins, Helzer, Croughan, & Ratcliff, 1981) and Composite International Diagnostic Interview (CIDI: Kessler & Üstün, 2004); 2) current psychopathology symptoms through DIS and CIDI; 3) age of onset of each disorder assessed via DIS and CIDI, and 4) proportion of first degree relatives (mother, father, and up to three siblings) endorsing the same class of problems. The DIS and CIDI were assessed at the child age of 18 months for both cohorts (N = 561) and then assessed again at the child age of 56 months for Cohort I (N = 361) only. To separate heritable from prenatal influences, we coded the heritable risk indicator, including diagnosis score and symptom count scores, as absent if the onset of symptoms for a given disorder appeared only during pregnancy. The composites of heritable risk have been used previously in Marceau et al. (2019) and detailed rationale, data preparation, missing data analysis, score creation, and scripts information are available elsewhere (Marceau et al., 2019).
Birth Mother Illicit Drug Use during Pregnancy.
BM illicit drug use during pregnancy was derived from self-reports of frequency of illicit drug use during pregnancy using a Pregnancy History Calendar method (modified version of the Life History Calendar method: Caspi et al., 1996; Pickett, Kasza, Biesecker, Wright, & Wakschlag, 2009) collected at 5 months postpartum. The Pregnancy History Calendar method has shown to be reliable and valid for obtaining data on prenatal substance use (Pickett et al., 2009). Specifically, BMs were asked if they used each of the following illicit drugs during pregnancy and the frequency of each illicit drug use during pregnancy on a 4-point Likert scale ranging from rarely, 1 to 6 times during pregnancy to regularly, most days of the week or everyday. There are mainly two strategies that have been used to examine maternal use of one or more drugs during pregnancy. One strategy has been to compare children prenatally exposed to any illicit drugs to children who were not exposed to illicit drugs (yes vs. no) (e.g., Creanga et al., 2012). This approach, however, decreases the power to detect the effects of prenatal illicit drug use on child development especially when the effect is subtle and confounded by other risk factors, such as inherited risks and poor rearing environment. A different strategy has been to compute a summative index for maternal use of each drug during pregnancy (e.g., Conradt et al., 2014). This latter strategy is able to more sensitively estimate the effects of maternal pregnancy use of illicit drugs on child development than is the group comparison approach. In the current study, we computed (and used) a summative frequency for maternal use of every illicit drug during pregnancy, including sedatives, tranquilizers, amphetamines, painkillers, inhalants, marijuana, cocaine, hallucinogens, and heroin, to indicate maternal illicit drug use during pregnancy. Of note, for Cohort I we only asked their substance use behaviors during pregnancy, whereas for Cohort II we asked their substance use behaviors during each trimester of the pregnancy. Because we included data for both cohorts, we cannot examine the data by trimester.
Twenty-five percent of the BMs used illicit drugs during pregnancy, which is well above the population prevalence rates of use (5.5%) (National Institute on Drug Abuse, 1996). The most frequently used illicit drugs were marijuana (16.8%) and amphetamines (6.9%). Of those BMs who used illicit drugs during pregnancy: 38.2% were rare users, 1 to 6 times during pregnancy; 14.6% were infrequent users, about once a month; 18.8% were somewhat regular users, once or twice a week; and 28.5% were regular users, most days of the week or every day. In addition, of those BMs who used illicit drugs during pregnancy, 68% used only one illicit drug, 22% used two illicit drugs, and 10% used more than two illicit drugs during pregnancy.
Control variables.
Additional control variables were included in the analyses to account for possible confounds if they were significantly correlated with child negative reactivity at 18 months and AM and AF’s negative parenting at child age 27 months.
Adoption openness.
Because this study used data from an adoption sample, adoptive parents’ reports of the child and their parenting may be influenced by their perceived knowledge of or contact with the birth parents (Ge et al., 2008). Therefore, we included a composite index of BM’s and adoptive parents’ perceived openness, ranging from very closed to very open, at 5–9 months as a covariate. For birth mothers, 3.8% perceived the adoption as closed to very closed (e.g., no or only general information about the adoptive parents), 27.9% perceived the adoption as semi-open to mediated (e.g., written communication, no face-to-face contact), 68.3% perceived the adoption as open to very open (e.g., visit a couple of time per year and communicate by telephone, mail, or email). For adoptive parents, 4.8 – 6.4% perceived the adoption as closed to very closed (no or only general information about the birth parents), 33.2 – 34.1% perceived the adoption as semi-open to mediated (written communication, no face-to-face contact), 60.4 – 61.1% perceived the adoption as open to very open (visit a couple of time per year and communicate by telephone, mail, or email).The score was standardized before analysis.
Perinatal risk index.
Perinatal risks have been linked with BM illicit drug use during pregnancy and negative reactivity in children (Gutteling et al., 2005). We therefore controlled for obstetric complications using an index derived from BM reports and coded medical records based on an adaptation of the McNeil-Sjöström Scale for Obstetric Complications (see Marceau et al., 2016; McNeil, 1995 for more details). Substance use during pregnancy was excluded from the obstetric complications score for these analyses. The item scores range from 1 (not harmful or relevant) to 6 (very great harm to or deviation in offspring).
Child Sex.
Child sex was coded as 1 (male) or 2 (female).
Birth Mother Tobacco and Alcohol Use during Pregnancy.
BM tobacco and alcohol use during pregnancy were based on self-reports of frequency of tobacco and alcohol use during pregnancy using the modified version of the Life History Calendar method described above. Specifically, for tobacco use during pregnancy, we used the mean number of cigarettes smoked per day, averaged across three trimesters as the index score. For alcohol use during pregnancy, we used the average number of glasses (or cans) of alcohol in a week, averaged across three trimesters as the index score. The percentage of BMs who reported using tobacco and alcohol during pregnancy were 43% and 23%, respectively.
Attrition Analysis
Data collection was attempted for birth parents at child ages of 5, 18, 27, and 54 months and for each child and adoptive parents at child ages of 18 and 27 months. However, some birth and adoptive families declined to participate in the study at a given wave or declined to complete a measure at one or more of the assessments. In addition to attrition, low BF participation (varying from 30% to 36% based on the particular variable) is another major source of missing data. However, BF’s data are important because they account for 50% of the child’s genes and allow prenatal effects to be distinguished from heritable influences (Loehlin, 2016), we included BFs in the model despite the high rate of BF missingness. Of the 561 linked sets of participants, the proportion of missingness is listed below: AM report of child emotional reactivity, anger, fearfulness and difficultness at 18 months of age: 6.8% – 20.0%, AF report of child emotional reactivity, anger, fearfulness and difficultness at 18 months of age: 12.1% – 24.6%, AM report of her own over-reactive and hostile parenting behaviors at child ages of 18 and 27 months: 10.7% – 15.2%, AF report of his own over-reactive and hostile parenting behaviors at child ages of 18 and 27 months: 16.4% – 20.1%, BM report of illicit drug use during pregnancy: 2.1%, indices of heritable risk from BM: 12.7% – 20.9%, and indices of heritable risk from BF: 68.8% – 69.9%. We conducted a targeted missing data analysis to systematically examine missing data mechanisms of the two primary sources of missing data, birth father participation and attrition. Specifically, we examined whether there were appreciable differences in the study variables and demographic variables across groups where (1) birth father did vs. did not participate, and (2) the outcome variables (adoptive parents’ parental negativity at 27 months) were missing vs. available (to index attribution) using one-way analysis of variance (of note, Kruskal-Wallis H test was used for binary study and demographic variables). Study variables include birth mother psychopathology variables, birth mother prenatal substance use frequency variables (illicit drug, alcohol, and tobacco use), child negative reactivity variables (difficult temperament, fear, anger, and emotional reactivity), and adoptive parents’ parental negativity at 18 and 27 months. Demographic variables include child sex, adoption openness, perinatal risk index, adoptive parents demographics (age at child’s birth, household income, education), and birth parents demographics (age at child’s birth, household income, education). For birth father participation, only 1 out of the 26 comparisons was significant (adjusted p value = .002): birth father participation was associated with adoption openness such that birth families where birth father participated had more open adoption, F(1,556) = 15.78, p < .001. For adoptive parents’ parental negativity (attrition), the study and demographic variables were not associated with attrition (adjusted p values = .002 for adoptive mothers’ and adoptive fathers’ parental negativity separately). Large percentage of missing BF data was handled in the PCA analyses when creating the indices of heritable risk. Specifically, within the PCA analyses, data were imputed to generate the complete observations of the indicators and then the PCA analyses on the complete observations were examined which created heritable risk composites. For more details about handling missing data in the PCA analyses, please refer to Marceau et al. (2019). To handle the attrition specific to the current study, we used Bayesian estimation procedures with full information estimation, similar to full-information maximum likelihood method in the frequentist framework, to simultaneously model the missingness and other key hypotheses of interest.
Data Analysis and Bayesian Modeling
Analyses proceeded in two steps. First, descriptive statistics (means, standard deviations and intercorrelations) of primary theoretical variables were provided. Second, Bayesian structural equation model (BSEM) was conducted to simultaneously examine: a) the latent construct negative reactivity based on emotional reactivity, anger, fearfulness and difficult temperament indicators; and b) the evocative effects from child negative reactivity to AM and AF’s parental negativity at 27 months after controlling for AM and AF’s parental negativity at 18 months, respectively; and c) paths from heritable risks for externalizing problems, internalizing problems and substance use for both BM and BF, and BM illicit drug use during pregnancy to AM and AF’s parental negativity at 27 months directly and indirectly via child negative reactivity at 18 months after controlling for AM and AF’s parental negativity at 18 months, BM prenatal alcohol and tobacco use, obstetric complications and adoption openness. All the analyses (including the indirect effects) were performed with Mplus 8 using Bayesian estimation (Muthén & Muthén, 2015). Posterior Predictive Checking (PPC) – Posterior Predictive p-value (PPP) and the 95% credible interval (CI) for the degree of deviation between the observed and the replicated Chi-Square values – were used to indicate the fit of the proposed model (Muthén & Asparouhov, 2012). The model fit is acceptable if the PPP value is larger than 0.05 (lower PPP indicates poorer fit) and the 95% CI for the difference between the observed and the replicated Chi-Square values contains 0 (Muthén & Asparouhov, 2012).
The current study used Bayesian estimation, which provides robust and straightforward quantification of the magnitudes and uncertainty of particular effects, such as indirect effects, when examining mediation models. From a Bayesian perspective, a parameter is considered as a random variable with a probability distribution rather than a true fixed value (from the frequentist perspective). Based on the a priori information and data, Bayesian modeling generates the distribution of the parameter of interest, which is referred to as the posterior distribution. The priori information is a probability distribution, which captures all available knowledge about the parameter of interest, and a likelihood function quantifies the likelihood of the data given a set of parameters (e.g., mean and variance of the parameter). Combining the prior information and the likelihood function via Bayes’ theorem defines a posterior distribution, which summarize the relative probability of parameter values. In Bayesian modeling, Markov chain Monte Carlo (MCMC) methods, in our case Gibbs sampling as implemented in Mplus (Asparouhov & Muthén, 2010), is used to generate the joint posterior distribution of all parameters. Convergence is assessed by running multiple chains for estimation purposes, and evaluating the ratio between the variability between and within chains by means of the potential scale reduction (PSR) factor (Gelman & Rubin, 1992), and several other heuristic measures available in Mplus (Asparouhov & Muthén, 2010). If the posterior distributions yield highly discrepant MCMC samples across chains (i.e., inferential results are inconsistent based on chains with different starting values), this indicates that the posterior distributions as approximated using the MCMC method of choice have not approached the target distributions and more iterations or other model adaptions are needed.
The Bayesian estimation provides posterior distributions and credible intervals based on the percentiles of the posterior, allowing for the possibility of non-normal and asymmetric (e.g., skewed) distributions of parameter estimates (Muthén & Asparouhov, 2012). This is particularly important in examining indirect effects, because the sampling distributions of the indirect effects are usually non-normal and non-symmetric. In addition, credible intervals used in Bayesian estimation are more interpretable compared to confidence intervals used in the frequentist analysis. For example, a 95% credible interval means that there is a 95% probability that the parameter of interest will fall between the upper and lower limits, whereas a 95% confidence interval has a different meaning: out of 100 replications of the same experiments, 95 of them will capture the true population (parameter) value. For more information about the Bayesian modeling in developmental science and additional technical details, please refer to Van de Schoot et al. (2014).
Results
Descriptive Analysis
Means, standard deviations, and inter-correlations among the study variables are presented in Table 1. Child emotional reactivity, anger, fearfulness and difficultness were moderately correlated with each other (rs = .23 – .55, ps < .05), confirming the validity of utilizing a single latent construct negative reactivity derived from these four indicators. Child emotional reactivity, anger and difficultness (but not fearfulness) were positively correlated with AM and AF’s parental negativity at both 18 and 27 months (rs = .08 – .30, ps < .08), suggesting that children with higher negative reactivity, except for fearfulness, were more likely to have adoptive parents who had higher levels of parental negativity at both 18 and 27 months. Interestingly, BM illicit drug use during pregnancy was negatively correlated with child anger (trend) (r = −.08, p < .08), child difficultness (r = −.14, p < .05) and AM’s over-reactive parenting at 18 months (trend) (r = −.09, p < .08), indicating that higher frequency of BM illicit drug use during pregnancy was associated with lower levels of child negative reactivity and lower AM’s over-reactive parenting at 18 months.
Table 1.
Means, Standard Deviations, and Intercorrelations among Study Variables
| M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child Outcomes (18 months) | ||||||||||||||||||
| 1. Emotional Reactivity | 1.60 (1.30) | - | ||||||||||||||||
| 2. Anger | 3.39 (.63) | .33** | - | |||||||||||||||
| 3. Fearfulness | 3.74 (.84) | .23** | .27** | - | ||||||||||||||
| 4. Difficultness | 21.80 (5.43) | .47** | .55** | .24** | - | |||||||||||||
| Rearing Environments (Parenting) | ||||||||||||||||||
| 5. AM Ovrreactivity (18 months) | 1.86 (.59) | .16** | .25** | −.02 | .26** | - | ||||||||||||
| 6. AF Overreactivity (18 months) | 1.89 (.59) | .08† | .24** | .08† | .21** | .34** | - | |||||||||||
| 7. AM Overreactivity (27 months) | 2.06 (.62) | .21* | .27** | .02 | .25** | .72** | .27** | - | ||||||||||
| 8. AF Overreactivity (27 months) | 2.06 (.61) | .12* | .25** | .05 | .24** | .27** | .69** | .27** | - | |||||||||
| 9. AM Hostility (27 months) | 9.04 (2.70) | .19** | .23** | .02 | .29** | .51** | .18** | .66** | .26** | - | ||||||||
| 10. AF Hostility (27 months) | 8.91 (2.60) | .21** | .29** | .02 | .30** | .30** | .51** | .31** | .64** | .33** | - | |||||||
| Indices of Heritable Risk | ||||||||||||||||||
| 11. BM Externalizing Problems | 0 (1) | −.02 | −.02 | −.01 | −.08† | −.05 | −.02 | −.07 | .05 | −.03 | .04 | - | ||||||
| 12. BM Internalizing Problems | 0 (1) | .05 | −.01 | −.06 | .03 | −.06 | −.03 | −.02 | −.04 | −.01 | −.05 | .30** | - | |||||
| 13. BM Substance Use | 0 (1) | −.03 | .01 | −.05 | −.03 | −.06 | −.03 | −.05 | 0 | 0 | −.04 | .55** | .38** | - | ||||
| 14. BF Externalizing Problems | 0 (1) | −.02 | −.07 | −.08 | −.04 | .03 | .02 | .05 | .05 | .02 | .02 | .32** | .13** | .24** | - | |||
| 15. BF Internalizing Problems | 0 (1) | .02 | −.03 | −.03 | −.03 | −.03 | −.02 | 0 | 0 | −.03 | 0 | 0 | .12* | .09* | .21** | - | ||
| 16. BF Substance Use | 0 (1) | −.02 | −.08 | −.07 | −.02 | .03 | .01 | .03 | 0 | 0 | .02 | .07 | .07 | .21** | .61** | .22** | - | |
| BM Prenatal Illicit Drug Use | ||||||||||||||||||
| 17. Summative Frequency of All Illicit Drugs | .09 (.21) | −.07 | −.08† | .03 | −.14** | −.09† | −.04 | −.05 | −.06 | −.04 | −.08 | .25** | .21** | .36** | .15** | .05 | .17* | - |
Note. AM = adoptive mother; AF = adoptive father; BM = birth mother; BF = birth father.
p < .08.
p < .05.
p < .01.
Bayesian Structural Equation Model
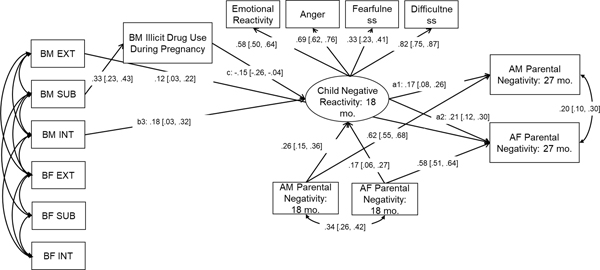
For Bayesian estimation, we used 2 chains and 40,000 fixed number of iterations for each Markov Chain Monte Carlo (MCMC) chain. The first 20,000 iterations were considered as the “burn-in” phase, which were not used to generate the posterior distribution. Only the second 20,000 iterations were used to generate the posterior distribution and assess convergence. The Bayesian structural equation model showed a good fit, PPP = .15 and the 95% CI for the difference between the observed and the replicated Chi-Square values was [−25.23, 87.37]. Model convergence was assessed by examining the potential scale reduction (PSR) factor value (PSR < 1.05), the Kolmogorov-Smirnov test, trace plots, and auto-correlation plots of the parameter estimates (Asparouhov & Muthén, 2010). All the criteria supported model convergence. Detailed information about the model convergence is reported in Appendix S1. Results (standardized solution) are presented in Figure 2.
Figure 2.
Child effects on adoptive parents’ parental negativity via child negative reactivity: the role of heritable and prenatal factors.
Note. Standardized parameter estimates and 95% credible intervals of parameter estimates. AM = adoptive mother; AF = adoptive father; BM = birth mother; BF = birth father. EXT = externalizing problems; INT = internalizing problems; SUB = substance use. The parameter estimates of the covariance among birth parents’ psychopathology scores were omitted due to limited space. Non-significant parameter estimates are not shown in the figure.
Examining Child Effects on AM and AF Parental Negativity.
Child emotional reactivity, anger, fearfulness and difficultness all significantly loaded on the latent construct “negative reactivity” (βs = .33 – .82, 95% CIs all above 0). Child negative reactivity at 18 months was associated with AM’s parental negativity at 27 months (β = .17, 95% CI [.08, .26]) after controlling for AM’s parental negativity at 18 months (β = .62, 95% CI [.55, .68]), such that children who were higher in negative reactivity at 18 months were more likely to elicit higher parental negativity from mothers at the successive assessment. Similarly, a significant positive association between child negative reactivity at 18 months and AF’s parental negativity at 27 months was observed (β = .21, 95% CI [.12, .30]) after controlling for AF’s parental negativity at 18 months (β = .58, 95% CI [.51, .64]). The findings supported a child effects model in which child negative reactivity elicits negative responses from both mothers and fathers.
Examining Evocative rGE in Explaining Child Effects on Parental Negativity.
BM internalizing problems (heritable risk for internalizing problems) were positively associated with child negative reactivity at age 18 months (β = .18, 95% CI [.03, .32]), such that children had higher negative reactivity if they were at heritable risk for internalizing problems from their BM. A significant indirect pathway was also observed from BM internalizing problems via child negative reactivity to AM’s parental negativity (β = .03, 95% CI [.01, .07]) and AF’s parental negativity (β = .04, 95% CI [.01, .09]) at 27 months, providing evidence for evocative rGE in explaining child effects on both AM’s and AF’s parental negativity. However, other heritable risks from BM and all heritable risks from BF were not directly associated with child negative reactivity at 18 months. We did post-hoc analyses with Benjamini-Hochberg procedure to correct for multiple testing of the heritable influences (Benjamini & Hochberg, 1995). Specifically, we calculated a z test statistic using the estimated posterior means and standard errors and examined whether the test statistic passed statistical significance with a false positive threshold of q = .10 (Benjamini & Hochberg, 1995). The results indicated that the heritable influences from birth mother internalizing problems to child negative reactivity were still significant after multiple correction (p = .08).
Interestingly, although BM substance use did not directly predict child negative reactivity, it was indirectly related to child negative reactivity via prenatal illicit drug use (β = −.02, 95% CI [−.04, −.01]), suggesting that heritable effects from BM substance use on child negative reactivity operated through prenatal risks. In addition, BM externalizing problems had a direct impact on AF’s parental negativity (β = .12, 95% CI [.03, .22]), indicating evocative rGE effects on parental negativity. However, this association was not explained by child negative reactivity at 18 months.
Examining BM Illicit Drug Use during Pregnancy in Explaining Child Effects on Parental Negativity.
BM illicit drug use during pregnancy was negatively associated with child negative reactivity at 18 months (β = −.15, 95% CI [−.26, −.04]) after controlling for prenatal alcohol use (β = .09, 95% CI [−.01, .18]) and prenatal tobacco use (β = .05, 95% CI [−.05, .15]). For children whose BM used illicit drugs more frequently during pregnancy, they showed lower levels of negative reactivity at 18 months, compared to children whose BM did not use illicit drugs or who were infrequent users during pregnancy. Of note, we included birth mothers’ and birth fathers’ substance use (heritable risks for substance use) as predictors in the same model, thus controlling for the potential influences from heritable factors. Significant indirect pathways were also observed from BM illicit drug use during pregnancy via child negative reactivity to AM’s parental negativity (β = −.10, 95% CI [−.22, −.02]) and AF’s parental negativity (β = −.13, 95% CI [−.26, −0.03]), providing evidence for prenatal illicit drug exposure in explaining child effects on parental negativity.
For the control variables, child sex was not associated with child negative reactivity nor adoptive parents’ parental negativity (95% CIs contained 0). Adoption openness and perinatal risk were not associated with adoptive parents’ parental negativity (95% CIs contained 0).
In sum, we found evidence for child effects on parental negativity, such that child negative reactivity at 18 months was associated with both AM and AF’s parental negativity at 27 months. In addition, child effects on parental negativity were partially explained by BM internalizing problems and substance use and BM illicit drug use during pregnancy, indicating evocative rGE and prenatal factors in explaining child effects on parenting. We calculated the differences in the summed residual variance of adoptive mothers’ and adoptive fathers’ parental negativity in models with and without indirect effects via the mediator (child negative reactivity) and divided by the summed residual variance of adoptive mothers’ and adoptive fathers’ parental negativity in models without indirect effects via the mediator. In total, child negative reactivity explained 6% of the associations between birth mother internalizing problems, birth mother illicit drug use during pregnancy and adoptive mothers’ and adoptive fathers’ parental negativity.
Secondary Analysis
It is possible that parents have a tendency to portray the child and themselves in a positive way, or respond in a socially desirable way consistently across various assessments, thus showing consistent reporter bias across different constructs. To check the robustness of the current findings, we re-examined the model by using a confirmatory factor analysis (CFA) method grounded within the multi-trait multi-method framework (Hutton & Chow, 2014; Marsh & Bailey, 1991), also referred to as the CFA marker technique (Williams, Edwards, & Vandenberg, 2003), to extract common method variance associated with being a mother vs. a father. Specifically, we created a latent variable that loaded on all mother reported variables (loadings all fixed at 1), and another latent variable that loaded on all father reported variables (loadings all fixed at 1). That way, systematic reporter biases would be captured by the latent variables, and not affect other estimates. The resulting pattern of findings was similar to that of the primary analyses, suggesting that reporter bias effects were minimal. The detailed findings of the model using the CFA marker technique are available from the author upon request.
Discussion
The current study aimed to examine child effects on parental negativity via child negative reactivity and whether heritable influences and birth mother illicit drug use during pregnancy (prenatal factor) account for such child effects on parental negativity. Findings indicated child effects on parental negativity, such that toddlers’ negative reactivity at 18 months was associated with adoptive mothers’ and adoptive fathers’ over-reactive and hostile parenting at 27 months. Furthermore, we found indirect associations between 1) child heritable risks indicated by birth mother internalizing problems and substance use, and 2) birth mother illicit drug use during pregnancy and adoptive parents’ parental negativity at 27 months after controlling for adoptive parents’ parental negativity at 18 months. These associations were mediated by child negative reactivity at 18 months, further supporting the two mechanisms that we proposed as underlying child-driven effects. In addition, birth mother externalizing problems had direct impacts on adoptive fathers’ parental negativity at 27 months, indicating that evocative rGE was operating underlying parental negativity, although the association was not accounted for by child negative reactivity at 18 months.
Consistent with our hypothesis, child negative reactivity at 18 months elicited parental negativity at 27 months, such that adoptive parents of children with higher levels of negative reactivity increased their over-reactivity and hostility in response to that child between ages 18 and 27 months. This was present for both adoptive mothers and adoptive fathers. This pattern of child effects is consistent with earlier findings that young children with high levels of negative reactivity elicit higher levels of parental negativity (Bridgett et al., 2009; Harold et al., 2013). The findings empirically supported Bell’s original theory (Bell, 1968) that children are not passive recipients of their rearing environments; rather, they are active in interacting with their parents and partially modify parenting behaviors toward them. The current finding adds substantially to the growing number of empirical studies examining child effects on parenting in early childhood.
The current study found a significant indirect association between birth mother heritable risk for internalizing problems and adoptive parents’ parental negativity at 27 months via child negative reactivity, indicating heritable factors involved in adult internalizing problems are emergent in early childhood and evoke parental negativity from both mothers and fathers toward the child. The result is consistent with the twin studies that have found maternal hostile-reactive behaviors were moderately associated with their twin infants’ genetically influenced difficultness (Boivin et al., 2005). The current findings yielded evidence of evocative rGE in early child development and contributed to the growing body of literature that underscores the evocative rGE as a critical mechanism underlying child evocative effects on parenting (e.g., Boivin et al., 2005; Harold et al., 2013). Of note, contrary to our expectation, birth father heritable risk for internalizing problems was not associated with child negative reactivity. This finding may be because of low variability (lower prevalence of diagnosis and lower symptoms) in birth father internalizing problems, which may lead to decreased power in detecting birth father heritable influences on child outcomes. The impact of birth father internalizing problems on child negative reactivity may be evident when children get older. It is also possible that the effects of birth mother internalizing problems on child negative reactivity are via prenatal environmental influences, thus the birth father-child associations were not significant, although this was not tested in the current study.
Birth mother substance use (heritable risk) was not directly related to child negative reactivity, but it had an indirect effect on child negative reactivity via prenatal illicit drug use. In other words, heritable influences of substance use were operating partially through prenatal risks of drug use. The finding is consistent with the previous work using the current sample (Marceau, Hajal, et al., 2013; Pemberton et al., 2010). For example, birth mother lifetime substance use was positively associated with their drug use during pregnancy, which in turn influenced child sleep problems (Marceau, Hajal, et al., 2013). However, birth mother lifetime substance use was not directly associated with child sleep problems. Similarly, previous studies have also found that birth mother internalizing problems indirectly influenced child problem behaviors through pregnancy risk factors (e.g., frequency of prenatal care) (Marceau, Hajal, et al., 2013; Pemberton et al., 2010). These findings indicate that because of the genetic overlap between prenatal substance use and substance dependence, women who use drugs during pregnancy are likely to be at heritable risks for substance use disorders (Knopik et al., 2016), although these two phenotypes were not highly correlated in the current study (r = .36, p < .01). Furthermore, the current study found a significant direct association between birth mother externalizing problems and adoptive fathers’ parental negativity, indicating evocative rGE effects on changes in fathers’ parental negativity from 18 to 27 months, which is consistent with the prior work (e.g., Klahr et al., 2017). However, this association was not explained by child negative reactivity at 18 months. Future work is needed to examine whether other child characteristics, such as impulsivity, activity level or aggressive behavior, may explain this relation.
In addition to heritable influences, birth mother illicit drug use during pregnancy was negatively associated with child negative reactivity, such that children whose birth mothers used illicit drugs more frequently during pregnancy showed significantly lower levels of negative reactivity compared to their peers. Our findings are consistent with studies that have found low reactivity of prenatally drug-exposed children (Alessandri et al., 1993; Molitor et al., 2003; Roumell et al., 1997). There are, however, studies that have found high reactivity in children whose mothers used drugs while pregnant (Metosky & Vondra, 1995; Richardson, Goldschmidt, & Willford, 2008) which is not consistent with our findings. During early childhood, it is adaptive for children to display negative reactivity at appropriate levels, which can help them to achieve their goals (Razza et al., 2012). Children with low reactivity are considered atypical compared to children with moderate levels of reactivity and are at risk for developing later problem behaviors, including antisocial problems, depression, and anxiety (Cole et al., 1996). There are several possible explanations for the low reactivity. Firstly, these low reactive children may be emotionally blunted or display emotional flatness such that they are not responsive to their environments regardless of the valence of the environments (Molitor et al., 2003). Secondly, it could also be that these low reactive children are under-aroused or less easily aroused under low to moderate stressful situations (Alessandri et al., 1993). These possible links have been reported in previous studies, although they were not directly examined in the current study and need further examination.
The findings from the current study should be interpreted in light of the following limitations. First, there is a large proportion of missing data for birth father. Although 30%−36% of birth fathers participated in the study, the recruitment rate of birth father is relatively high compared to other studies of birth fathers who completed an adoption plan, because this population is difficult to locate and recruit. Birth fathers are critical, as they provide 50% of genes for adopted children and combining birth father’s heritable influences with birth mother’s heritable and prenatal influences enables us to disentangle prenatal risks from heritable influences and jointly examine both heritable and prenatal influences on child negative reactivity. Second, full-information Bayes estimation procedures in Mplus were performed to handle missing observations in the model. Full-information Bayes estimation procedures assume missing data are missing completely at random or missing at random and may not be adequate in the presence of non-ignorable missingness. Empirical studies often assume that the data under study are missing at random, as was assumed in this study. That is, we assumed that the missingness mechanism depended only on variables that were observed in the study. We did not address the more challenging scenario of non-ignorable missingness, in which missingness depends on variables that ought to be, but were not measured, is difficult – or impossible -- to test comprehensively (Little & Rubin, 2002). In future studies, it would be of interest to explore different ways to handle missing data issues, such as multiple imputation procedures, Bayesian selection models, and pattern mixture models, which is currently not available in Mplus using Bayesian estimation. Third, we were unable to estimate the effects of specific illicit drugs used during pregnancy. In the current community sample, the frequency of use for each illicit drug was relatively low compared to rates in at-risk samples, making it difficult to identify the effects of pregnancy use of a specific illicit drug on child negative reactivity. In addition, women who use one illicit drug during pregnancy often use other drugs, making it difficult to isolate the effect of a specific drug from use of multiple drugs. Excluding women with poly-drug use during pregnancy and including prenatal one-drug users could then result in the most representative group of illicit drug users during pregnancy being excluded (Moe & Slinning, 2002). Thus, in the current study, we examined the effects of maternal use of one or more illicit drugs during pregnancy on child negative reactivity instead of use of specific drugs. Fourth, the current study relied on parent reports of child temperaments and parenting, which may inflate the associations between child negative reactivity and parental negativity due to rater bias, although mother and father reports on child negative reactivity were combined to minimize rater bias. Including data from other informants and contexts, such as observational ratings when relevant, could provide additional information about the generalizability of child on parent effects.
Of note, the parent-offspring adoption design is somewhat limited by the research design, selective placement, and generalizability. Specifically, the parent-offspring adoption design does not perfectly disentangle prenatal influences from heritable influences. Birth parents’ psychopathology could also affect the adopted child via prenatal environmental influences (and through other pathways). Although we controlled for birth mothers’ obstetric complications, there are other possible prenatal factors (e.g., maternal hormone levels or abuse during pregnancy) that may be influenced by birth parents’ psychopathology and may be relevant for the development of child negative reactivity. However, we did not have information on birth mothers’ hormonal levels or abuse during pregnancy, limiting the extent to which we can consider alternative pathways from birth mother psychopathology to the child. Furthermore, the indices of heritable risk (e.g., birth parent psychopathology) were not exhaustive and cannot capture all heritable influences. However, we have included information on birth fathers, which provides additional information to distinguish between heritable and prenatal influences because birth fathers do not provide the prenatal environment, only heritable influences. We also take an approach similar to studies using biologically-related family designs to disentangle prenatal from postnatal influences on child development. Specifically, we have statistically controlled for indices of heritable risk and regressed birth mothers’ illicit drug use during pregnancy on the indices of heritable risk when examining the role of prenatal illicit drug use in child negative reactivity. In addition, to maximize separation of heritable and prenatal influences, we have coded the indices of heritable risk, including diagnosis scores and symptom count scores, as absent if the onset of symptoms for a given disorder appeared only during pregnancy (not before or after pregnancy, or in other words, a pregnancy-limited disorder). Nevertheless, the analysis and the study design may not be fully satisfactory to disentangle prenatal from heritable factors.
In the current study, it is possible that the associations between birth mother internalizing problems and adoptive parents’ parental negativity could be partially explained by selective placement between birth and adoptive parents, as this cannot be empirically disentangled from evocative gene-environment correlation. However, prior reports using this sample have tested for selective placement between birth parents and adoptive parents and found little evidence of selective placement on characteristics such as personality, self-worth, executive function, temperament, financial needs, and intelligence (Leve et al., 2019). In regard to generalizability, the findings may be biased towards families with middle to high socio-economic backgrounds and towards children who are at risk for exposure to prenatal adversity and psychopathology. Specifically, in the current sample, adoptive families were from middle to high socio-economic backgrounds, while birth parents were, on average, from lower socio-economic backgrounds and at higher risk for prenatal substance use and psychopathology. Birth parents did display variability in their socio-economic status, prenatal substance use, and psychopathology, increasing our confidence in the generalizability of the current findings. Nevertheless, the current study provides important and useful information on the mechanisms of child evocative effects on parents and the mechanisms may be universal across various socio-economic backgrounds.
Despite these limitations, the current study indicates child effects on parental negativity via child negative reactivity. The current study supports the proposed mechanisms that child effects on parental negativity are partially due to heritable and prenatal factors that influence children’s behaviors, especially in early development. In this case, children actively interact with their parents and partially modify parenting behaviors toward them based on their characteristics, which is due in part to heritable and prenatal influences. This study advances empirical tests of mechanisms underlying child effects on parental negativity and provides important evidence for “child on parent” effects and implications in early negative reactivity development intervention.
Supplementary Material
Acknowledgments
The Early Growth and Development Study was supported by grant R01 HD042608 from the National Institute of Child Health and Human Development (NICHD), the National Institute on Drug Abuse (NIDA), and the Office of Behavioral and Social Sciences Research (OBSSR), NIH, U.S. PHS (PI Years 1–5: David Reiss, MD; PI Years 6–10: Leslie Leve, PhD), grant R01 DA020585 from NIDA, the National Institute on Mental Health (NIMH), the OBSSR, NIH, U.S. PHS (PI: Jenae Neiderhiser, PhD), grant R01 MH092118 from NIMH (PIs: Jenae Neiderhiser, PhD and Leslie Leve, PhD), grant R01 DA045108 from NIDA (PI: Jenae Neiderhiser), and grant UH3 OD023389 from the Office of the Director, NIH, U.S., PHS (MPIs: Leslie Leve, PhD., Jenae Neiderhiser, Ph.D., & Jody Ganiban, PhD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. We would like to thank the birth and adoptive families who participated in this study and the adoption agencies who helped with the recruitment of study participants.
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms & profiles: An integrated system of multi-informant assessment; Child behavior checklist for ages 1 1/2–5; Language development survey; Caregiver-teacher report form: University of Vermont. [Google Scholar]
- Alessandri SM, Sullivan MW, Imaizumi S, & Lewis M. (1993). Learning and emotional responsivity in cocaine-exposed infants. Developmental psychology, 29(6), 989–997. doi: 10.1037/0012-1649.29.6.989 [DOI] [Google Scholar]
- Arnold DS, O’leary SG, Wolff LS, & Acker MM (1993). The Parenting Scale: a measure of dysfunctional parenting in discipline situations. Psychological assessment, 5(2), 137. [Google Scholar]
- Asparouhov T, & Muthén B. (2010). Bayesian analysis using Mplus: Technical implementation. Manuscript submitted for publication. [Google Scholar]
- Bates JE, Freeland CAB, & Lounsbury ML (1979). Measurement of infant difficultness. Child development, 50(3), 794–803. doi: 10.2307/1128946 [DOI] [PubMed] [Google Scholar]
- Bell RQ (1968). A reinterpretation of the direction of effects in studies of socialization. Psychological review, 75(2), 81–95. doi: 10.1037/h0025583 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B, 57(1), 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Boivin M, Perusse D, Dionne G, Saysset V, Zoccolillo M, Tarabulsy GM, . . . Tremblay RE. (2005). The genetic‐environmental etiology of parents’ perceptions and self‐assessed behaviours toward their 5‐month‐old infants in a large twin and singleton sample. Journal of Child Psychology and Psychiatry, 46(6), 612–630. doi: 10.1111/j.1469-7610.2004.00375.x [DOI] [PubMed] [Google Scholar]
- Braungart JM, Fulker DW, & Plomin R. (1992). Genetic mediation of the home environment during infancy: A sibling adoption study of the HOME. Developmental psychology, 28(6), 1048. [Google Scholar]
- Bridgett DJ, Gartstein MA, Putnam SP, McKay T, Iddins E, Robertson C, . . . Rittmueller A (2009). Maternal and contextual influences and the effect of temperament development during infancy on parenting in toddlerhood. Infant Behavior and Development, 32(1), 103–116. doi: 10.1016/j.infbeh.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, . . . Silva PA. (1996). The life history calendar: a research and clinical assessment method for collecting retrospective event-history data. International journal of methods in psychiatric research, 6, 101–114. doi: [DOI] [Google Scholar]
- Cole PM, Zahn-Waxler C, Fox NA, Usher BA, & Welsh JD (1996). Individual differences in emotion regulation and behavior problems in preschool children. Journal of abnormal psychology, 105(4), 518–529. doi: 10.1037/0021-843X.105.4.518 [DOI] [PubMed] [Google Scholar]
- Conger RD, & Melby JN (2000). The Iowa family interaction rating scales: Instrument summary Family observational coding systems (pp. 49–74): Psychology Press. [Google Scholar]
- Conradt E, Abar B, Sheinkopf S, Lester B, Lagasse L, Seifer R, . . . Whitaker T. (2014). The role of prenatal substance exposure and early adversity on parasympathetic functioning from 3 to 6 years of age. Developmental psychobiology, 56(4), 821–835. doi: 10.1002/dev.21155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanga AA, Sabel JC, Ko JY, Wasserman CR, Shapiro-Mendoza CK, Taylor P, . . . Gynecology. (2012). Maternal drug use and its effect on neonates: a population-based study in Washington State. 119(5), 924–933. doi: 10.1097/AOG.0b013e31824ea276 [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, & Lahey BB (2008). Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Development and Psychopathology, 20(1), 139–164. doi: 10.1017/S0954579408000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidov M, Knafo-Noam A, Serbin LA, & Moss E. (2015). The influential child: How children affect their environment and influence their own risk and resilience. Development and Psychopathology, 27(4pt1), 947–951. doi: 10.1017/S0954579415000619 [DOI] [PubMed] [Google Scholar]
- Elam KK, Harold GT, Neiderhiser JM, Reiss D, Shaw DS, Natsuaki MN, . . . Leve LD. (2014). Adoptive parent hostility and children’s peer behavior problems: Examining the role of genetically informed child attributes on adoptive parent behavior. Developmental psychology, 50(5), 1543–1552. doi: 10.1037/a0035470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon RP, Reiss D, Leve LD, Shaw DS, Scaramella LV, Ganiban JM, & Neiderhiser JM (2015). Child-evoked maternal negativity from 9 to 27 months: Evidence of gene–environment correlation and its moderation by marital distress. Development and Psychopathology, 27(4pt1), 1251–1265. doi: 10.1017/S0954579414000868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Lester BM, DeGarmo DS, Lagasse LL, Lin H, Shankaran S, . . . Whitaker T. (2011). The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Development and Psychopathology, 23(3), 777–788. doi: 10.1017/S0954579411000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates W, Troughton E, & Stewart MA (1996). The developmental interface between nature and nurture: A mutual influence model of child antisocial behavior and parent behaviors. Developmental psychology, 32(4), 574–589. doi: 10.1037/0012-1649.32.4.574 [DOI] [Google Scholar]
- Ge X, Natsuaki MN, Martin DM, Leve LD, Neiderhiser JM, Shaw DS, . . . Reiss D. (2008). Bridging the divide: openness in adoption and postadoption psychosocial adjustment among birth and adoptive parents. Journal of Family Psychology, 22(4), 529–540. doi: 10.1037/a0012817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, & Rubin DB (1992). Inference from iterative simulation using multiple sequences. Statistical science, 7(4), 457–472. [Google Scholar]
- Goldsmith H. (1996). Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child development, 67(1), 218–235. doi: 10.2307/1131697 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Buss KA, & Lemery KS (1997). Toddler and childhood temperament: Expanded content, stronger genetic evidence, new evidence for the importance of environment. Developmental psychology, 33(6), 891–905. doi: 10.1037/0012-1649.33.6.891 [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, & Buitelaar JK (2005). The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European child & adolescent psychiatry, 14(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, . . . Thapar A. (2013). Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. Journal of Child Psychology and Psychiatry, 54(10), 10.1111/jcpp.121001038-121001046. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton RS, & Chow S-M (2014). Longitudinal multi-trait-state-method model using ordinal data. Multivariate behavioral research, 49(3), 269–282. doi: 10.1080/00273171.2014.903832 [DOI] [PubMed] [Google Scholar]
- Kessler RC, & Üstün TB (2004). The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI). International journal of methods in psychiatric research, 13(2), 93–121. doi: 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirlic N, Newman E, LaGasse LL, Derauf C, Shah R, Smith LM, . . . Strauss A (2013). Cortisol reactivity in two-year-old children prenatally exposed to methamphetamine. Journal of studies on alcohol and drugs, 74(3), 447–451. doi: 10.15288/jsad.2013.74.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr AM, & Burt SA (2014). Elucidating the etiology of individual differences in parenting: A meta-analysis of behavioral genetic research. Psychological bulletin, 140(2), 544–586. doi: 10.1037/a0034205 [DOI] [PubMed] [Google Scholar]
- Klahr AM, Burt SA, Leve LD, Shaw DS, Ganiban JM, Reiss D, & Neiderhiser JM (2017). Birth and Adoptive Parent Antisocial Behavior and Parenting: A Study of Evocative Gene–Environment Correlation. Child development, 88(2), 505–513. doi: 10.1111/cdev.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Neiderhiser JM, de Geus E, & Boomsma D. (2016). The Importance of the Prenatal Environment in Behavioral Genetics: Introduction to Special Issue. Behavior genetics, 46(3), 281–285. doi: 10.1007/s10519-016-9790-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, . . . Wilcox T. (2011). Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicology and teratology, 33(1), 166–175. doi: 10.1016/j.ntt.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Marsit CJ, Conradt E, Bromer C, & Padbury J. (2012). Behavioral epigenetics and the developmental origins of child mental health disorders. Journal of developmental origins of health disease, 3(6), 395–408. doi: 10.1017/S2040174412000426 [DOI] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Ganiban JM, Natsuaki MN, Shaw DS, & Reiss D. (2019). The Early Growth and Development Study: A Dual-Family Adoption Study from Birth Through Adolescence. Twin Research and Human Genetics, 1–12. doi: 10.1017/thg.2019.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R, & Rubin D. (2002). Statistical analysis with missing data. Wiley; New York. doi: 10.1002/9781119013563 [DOI] [Google Scholar]
- Loehlin JC (2016). What can an adoption study tell us about the effect of prenatal environment on a trait? Behavior genetics, 46(3), 329–333. doi: 10.1007/s10519-015-9730-x [DOI] [PubMed] [Google Scholar]
- Marceau K, De Araujo-Greecher M, Miller ES, Massey SH, Mayes LC, Ganiban JM, . . . Neiderhiser JM (2016). The perinatal risk index: early risks experienced by domestic adoptees in the United States. PloS one, 11(3), e0150486. doi: 10.1371/journal.pone.0150486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Hajal N, Leve LD, Reiss D, Shaw DS, Ganiban JM, . . . Neiderhiser JM (2013). Measurement and associations of pregnancy risk factors with genetic influences, postnatal environmental influences, and toddler behavior. International Journal of Behavioral Development, 37(4), 366–375. doi: 10.1177/0165025413489378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Horwitz BN, Narusyte J, Ganiban JM, Spotts EL, Reiss D, & Neiderhiser JM (2013). Gene–environment correlation underlying the association between parental negativity and adolescent externalizing problems. Child development, 84(6), 2031–2046. doi: 10.1111/cdev.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Rolan E, Leve LD, Ganiban JM, Reiss D, Shaw DS, . . . Neiderhiser JM (2019). Parenting and prenatal risk as moderators of genetic influences on conduct problems during middle childhood. Developmental psychology, 55(6), 1164–1181. doi: 10.1037/dev0000701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, & Bailey M. (1991). Confirmatory factor analyses of multitrait-multimethod data: A comparison of alternative models. Applied psychological measurement, 15(1), 47–70. doi: 10.1177/014662169101500106 [DOI] [Google Scholar]
- McNeil T. (1995). McNeil-Sjöström scale for obstetric complications. Lund University Department of Psychiatry, Malmö University Hospital, Malmö, Sweden. [Google Scholar]
- Metosky P, & Vondra J. (1995). Prenatal drug exposure and play and coping in toddlers: a comparison study. Infant Behavior and Development, 18(1), 15–25. doi: 10.1016/0163-6383(95)90004-7 [DOI] [Google Scholar]
- Moe V, & Slinning K. (2002). Prenatal drug exposure and the conceptualization of long-term effects. Scandinavian Journal of Psychology, 43(1), 41–47. doi: doi: 10.1111/1467-9450.00267 [DOI] [PubMed] [Google Scholar]
- Molitor A, Mayes LC, & Ward A. (2003). Emotion regulation behavior during a separation procedure in 18-month-old children of mothers using cocaine and other drugs. Development and Psychopathology, 15(1), 39–54. doi: 10.1017/S0954579403000038 [DOI] [PubMed] [Google Scholar]
- Muthén B, & Asparouhov T. (2012). Bayesian structural equation modeling: a more flexible representation of substantive theory. Psychological methods, 17(3), 313. doi: 10.1037/a0026802 [DOI] [PubMed] [Google Scholar]
- Muthén L, & Muthén B. (2015). Mplus. The comprehensive modelling program for applied researchers: user’s guide, 5. [Google Scholar]
- National Institute on Drug Abuse (NIDA). (1996). National Pregnancy & Health Survey: Drug Use Among Women Delivering Livebirths, 1992. Rockville, Md: National Institute on Drug Abuse, Division of Epidemiology and Prevention Research; US Department of Health and Human Services, National Institutes of Health. [Google Scholar]
- Neiderhiser JM, Marceau K, De Araujo-Greecher M, Ganiban JM, Mayes LC, Shaw DS, . . . Leve LD (2016). Estimating the Roles of Genetic Risk, Perinatal Risk, and Marital Hostility on Early Childhood Adjustment: Medical Records and Self-Reports. Behavior genetics, 46(3), 334–352. doi: 10.1007/s10519-016-9788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton CK, Neiderhiser JM, Leve LD, Natsuaki MN, Shaw DS, Reiss D, & Ge X. (2010). Influence of parental depressive symptoms on adopted toddler behaviors: An emerging developmental cascade of genetic and environmental effects. Development and Psychopathology, 22(4), 803–818. doi: 10.1017/S0954579410000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Kasza K, Biesecker G, Wright RJ, & Wakschlag LS (2009). Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine & Tobacco Research, 11(10), 1166–1174. doi: 10.1093/ntr/ntp117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp TK, Spinrad TL, & Smith CL (2008). The relation of cumulative demographic risk to mothers’ responsivity and control: Examining the role of toddler temperament. Infancy, 13(5), 496–518. doi: 10.1080/15250000802329446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razza RA, Martin A, & Brooks-Gunn J. (2012). Anger and children’s socioemotional development: Can parenting elicit a positive side to a negative emotion? Journal of Child and Family Studies, 21(5), 845–856. doi: 10.1007/s10826-011-9545-1 [DOI] [Google Scholar]
- Richardson GA, Goldschmidt L, & Willford J. (2008). The effects of prenatal cocaine use on infant development. Neurotoxicology and teratology, 30(2), 96–106. doi: 10.1016/j.ntt.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roben CK, Moore GA, Cole PM, Molenaar P, Leve LD, Shaw DS, . . . Neiderhiser JM (2015). Transactional patterns of maternal depressive symptoms and mother–child mutual negativity in an adoption sample. Infant and Child Development, 24(3), 322–342. doi: 10.1002/icd.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, & Ratcliff KS (1981). National Institute of Mental Health diagnostic interview schedule: Its history, characteristics, and validity. Archives of general psychiatry, 38(4), 381–389. doi: 10.1001/archpsyc.1981.01780290015001 [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament. Handbook of child psychology. [Google Scholar]
- Roumell N, Wille D, Abramson L, & Delaney V. (1997). Facial expressivity to acute pain in cocaine‐exposed toddlers. Infant Mental Health Journal, 18(3), 274–281. doi: [DOI] [Google Scholar]
- Scarr S, & McCartney K. (1983). How people make their own environments: A theory of genotype→ environment effects. Child development, 424–435. [DOI] [PubMed] [Google Scholar]
- Shaw DS, & Bell RQ (1993). Developmental theories of parental contributors to antisocial behavior. Journal of abnormal child psychology, 21(5), 493–518. doi: 10.1007/BF00916316 [DOI] [PubMed] [Google Scholar]
- Shaw DS, Gilliom M, Ingoldsby EM, & Nagin DS (2003). Trajectories leading to school-age conduct problems. Developmental psychology, 39(2), 189–200. [DOI] [PubMed] [Google Scholar]
- Singer LT (1999). Advances and redirections in understanding effects of fetal drug exposure. Journal of Drug Issues, 29(2), 253–262. doi: 10.1177/002204269902900207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Schoot R, Kaplan D, Denissen J, Asendorpf JB, Neyer FJ, & Van Aken MA (2014). A gentle introduction to Bayesian analysis: Applications to developmental research. Child development, 85(3), 842–860. doi: 10.1111/cdev.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Edwards JR, & Vandenberg RJ (2003). Recent advances in causal modeling methods for organizational and management research. Journal of management, 29(6), 903–936. doi: 10.1016/S0149-2063_03_00084-9 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.