Abstract
Objective:
The objective of this review is to discuss the therapeutic use and differential treatment response to Levo-carnitine (L-carnitine) treatment in septic shock, and to demonstrate common lessons learned that are important to the advancement of precision medicine approaches to sepsis. We propose that significant interpatient variability in the metabolic response to L-carnitine and clinical outcomes can be used to elucidate the mechanistic underpinnings that contribute to sepsis heterogeneity.
Methods:
A narrative review was conducted that focused on explaining interpatient variability in L-carnitine treatment response. Relevant biological and patient-level characteristics considered include genetic, metabolic, and morphomic phenotypes; potential drug interactions; and pharmacokinetics.
Main Results:
Despite promising results in a phase I study, a recent phase II clinical trial of L-carnitine treatment in septic shock showed a non-significant reduction in mortality. However, L-carnitine treatment induces significant interpatient variability in L-carnitine and acylcarnitine concentrations over time. In particular, administration of L-carnitine induces a broad, dynamic range of serum concentrations and measured peak concentrations are associated with mortality. Applied systems pharmacology may explain variability in drug responsiveness by using patient characteristics to identify pre-treatment phenotypes most likely to derive benefit from L-carnitine. Moreover, provocation of sepsis metabolism with L-carnitine offers a unique opportunity to identify metabolic response signatures associated with patient outcomes. These approaches can unmask latent metabolic pathways deranged in the sepsis syndrome and offer insight into the pathophysiology, progression, and heterogeneity of the disease.
Conclusion:
The compiled evidence suggests there are several potential explanations for the variability in carnitine concentrations and clinical response to L-carnitine in septic shock. These serve as important confounders that should be considered in interpretation of L-carnitine clinical studies and broadly holds lessons for future clinical trial design in sepsis. Consideration of these factors are needed if precision medicine in sepsis is to be achieved.
Keywords: critical care, septic shock, pharmacometabolomics, systems pharmacology
Epidemiology and Heterogeneity of Sepsis
Sepsis is a life threatening, dysregulated host response to infection, which is characterized by systemic organ dysfunction.1 One in three Americans who die in the hospital have sepsis, and in 2017 there were an estimated 48.9 million cases worldwide.2
The sepsis syndrome is a highly heterogeneous, with patients presenting along a continuum of clinical signs, symptoms, and severity of illness.3 The mechanism and pathophysiology underlying highly variable clinical trajectories in sepsis are complex, and the precise reason(s) some patients exhibit severe dysregulated responses while others recover from their initial infection in an uncomplicated fashion remains poorly understood. Such host-response heterogeneity muddies the interpretation of treatment response and is a major reason why novel pharmacotherapy often fails. Absence of adequate stratification of patients based on their underlying pathophysiology may contribute to this. .4 The need to advance mechanistic understanding of sepsis heterogeneity has led to calls from the National Institute of General Medical Sciences for studies that seek to determine the effect of patient characteristics on differential treatment response (NOT-GM-19-054). Teasing out this variability is necessary to bring about a precision medicine approach to sepsis.
Ample evidence suggests a hypermetabolic component and derangement of host metabolism that is central to sepsis pathophysiology.5 Recently revised consensus guidelines define the most severe manifestation, septic shock, as infection with sustained hypotension despite recommended evidence-based treatment interventions (e.g., fluid resuscitation), and pertinent to this discussion, metabolic dysfunction and/or tissue hypoperfusion as evidenced by an elevated blood lactate concentration.1 Hyperglycemia, protein catabolism, and lipolysis are similarly known to occur in sepsis and contribute to poor patient outcomes.6 While several studies have targeted lactate as a resuscitation goal7–9, these trials have typically utilized fluids, vasopressors, or other agents designed to improve organ perfusion under the assumption that lactate elevations are predominantly explained by ongoing tissue ischemia, which may not necessarily be true.10 Current pharmacotherapy neither targets nor corrects these metabolic perturbations, although restoration of host bioenergetics offers a promising therapeutic target. Moreover, given the prevalence, persistent mortality, and lack of specific treatment paradigms, there is a critical need to advance understanding of the range and extent of the metabolic consequences of sepsis beyond observational studies.
Herein, we discuss clinical trials of L-carnitine, an important regulator of mitochondrial and metabolic homeostasis, for the treatment of septic shock. We consider how patient-level biological variables impact response to treatment and propose that provocation with L-carnitine offers a novel and unique opportunity to improve mechanistic understanding of the heterogeneity and metabolic consequences of sepsis.
Physiological Role of Carnitine and Treatment in Sepsis Patients
Carnitine is an endogenous, polar small-molecule derived from lysine and methionine, which plays a well-established, crucial role in transport of long-chain fatty acids into the mitochondria for β-oxidation. Other key roles during times of metabolic stress include maintenance of coenzyme A homeostasis, metabolic flexibility and promotion of normal TCA cycle function, and further oxidation of fatty acids by peroxisomes.11 A full, in-depth review of carnitine and acylcarnitine homeostasis and biochemistry is outside the scope of this paper, and it has been extensively reviewed elsewhere.11, 12 Briefly, the carnitine shuttle allows for fatty acid entrance to the mitochondria for oxidation and subsequent energy production through transfer of acyl groups and conversion into acylcarnitines (Figure 1).
Figure 1: Overview of carnitine transport and enzymatic conversions in the cell.
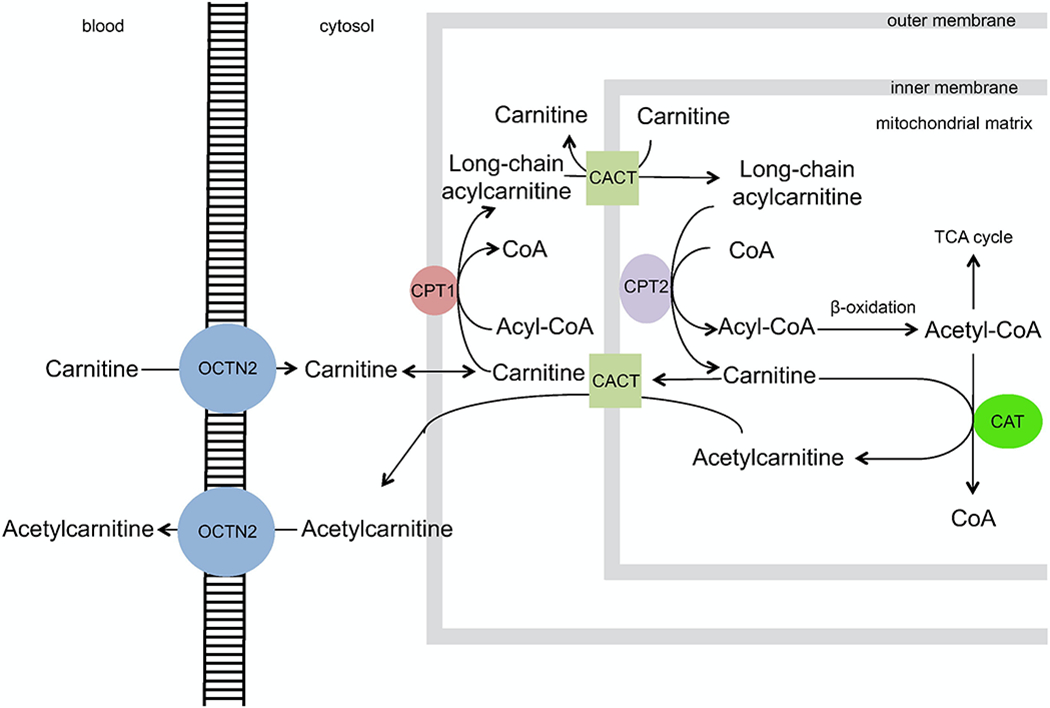
Carnitine enters the cell from the blood through an organic cation transporter (OCTN2), after which carnitine palmitoyl transferase I (CPT-1) facilitates the conversion of carnitine and long chain fatty acid-CoAs to acylcarnitines and coenzyme A (CoA). The transporter carnitine-acylcarnitine translocase (CACT) moves the newly formed long-chain acylcarnitines into the mitochondrial matrix in exchange for free carnitine. Here, long chain acyl groups are transferred back to CoA by carnitine palmitoyl transferase II (CPT-II). The newly regenerated acyl-CoA undergoes β-oxidation into Acetyl-CoA, which feeds into the TCA cycle. Alternatively, carnitine acetyl-transferase (CAT) converts free carnitine and Acetyl-CoA to acetylcarnitine, which can freely diffuse through CACT and OCTN2 back into the bloodstream. This latter process may be enhanced during sepsis and times of metabolic stress, serving as a crucial sink for excess acetyl groups that may be toxic to the cell. The ladder cartoon represents the plasma membrane separating the blood and the cytosol of the cell, while grey boxes represent the outer and inner membranes of the mitochondria. (Open-source through the Creative Commons Attribution, obtained with permission from https://doi.org/10.1016/j.ebiom.2017.01.026).58
In sepsis, mitochondrial dysfunction has been increasingly reported as a critical factor in persistent organ failure and altered peripheral cell mitochondrial function is known to be associated with sepsis mortality.13, 14 Further evidence of mitochondrial dysfunction includes elevations of systemic acylcarnitines, indicating incomplete β-oxidation of fatty acids, and the presence of mitochondrial DNA in plasma.15, 16 Sepsis alterations in mitochondrial function and lipid metabolism are associated with kidney and liver function that are driven in part through inhibition of the pyruvate dehydrogenase complex and decreased activity of carnitine palmitoyltransferase I.17, 18 Prior clinical studies of intravenous (IV) L-carnitine and acetylcarnitine given to patients in cardiogenic and circulatory shock found an overall positive effect on hemodynamic parameters and patient survival.19–21
These principles served as the basis for two recent clinical trials of L-carnitine in septic shock. The first was a phase I, randomized, double-blind clinical trial of L-carnitine (12 g IV) vs. saline placebo conducted in 31 patients with septic shock enrolled within 16 hours of diagnosis.22 Study drug was given as an IV bolus (33% of total dose), followed by a 12-hour infusion that delivered the remaining drug. This study found no difference in the reduction of Sequential Organ Failure Assessment (SOFA) score at 24 hours, but there was an improvement in mortality at 28 days (4/16 vs. 9/15, p=0.048) and 1-year (8/16 vs. 12/15, p = 0.081) in L-carnitine treated patients. Adverse events sometimes attributable to L-carnitine, including gastrointestinal distress, body odor, and an decreased seizure threshold were not observed in the study. In addition, serious adverse events were not significantly different between the L-carnitine and placebo treatment arms. A follow-up phase II multicenter, double-blind, adaptive dose-finding trial randomized 250 patients within 24 hours of identified septic shock to IV L-carnitine (6 g, 12 g, or 18 g) vs. placebo.23 In the primary analysis, the highest dose (18 g) of L-carnitine was not found to be superior to placebo in reducing the total SOFA score at 48 hours, and the predicted probability of success of a subsequent phase III trial in reducing mortality at 28 days did not exceed the a priori threshold of 90%. The 6 g and 12 g L-carnitine doses underperformed in the trial and were adaptively dropped from the randomization scheme as the trial progressed. Three, interim, pre-planned safety and futility analyses were completed by an independent data safety monitoring board.
However, the primary endpoints of both clinical studies do not describe a critical component of drug response to supplemental L-carnitine in patients with septic shock. The pharmacometabolomics data from the Phase I trial reveal substantial interpatient variability in serum carnitine and acetylcarnitine concentrations post-infusion.24, 25 Patients receiving L-carnitine in the phase I study had 24-hour post infusion (T24) serum carnitine levels ranging from 30 μM to over 1600 μM (median = 368 μM). The temporal changes in carnitine and acetylcarnitine for the treatment and placebo arms are shown in Figure 2. Critically, L-carnitine treated non-survivors (based on 1-year mortality) had elevated carnitine and acetylcarnitine (C2), short chain acylcarnitines (C3, C4, and C5), and long chain acylcarnitines (C14 and C16) compared to L-carnitine treated survivors. This suggests the observed variability in measured peak concentrations and metabolic response profiles are associated with clinical outcomes. As such, identification of the patient-level factors associated with peak carnitine/acylcarnitine concentrations may help identify patient most likely to derive a mortality benefit from L-carnitine and inform the design of future clinical studies.
Figure 2: Carnitine treatment induces a metabolic phenotype whereby serum carnitine and acetylcarnitine concentrations are elevated in sepsis non-survivors.
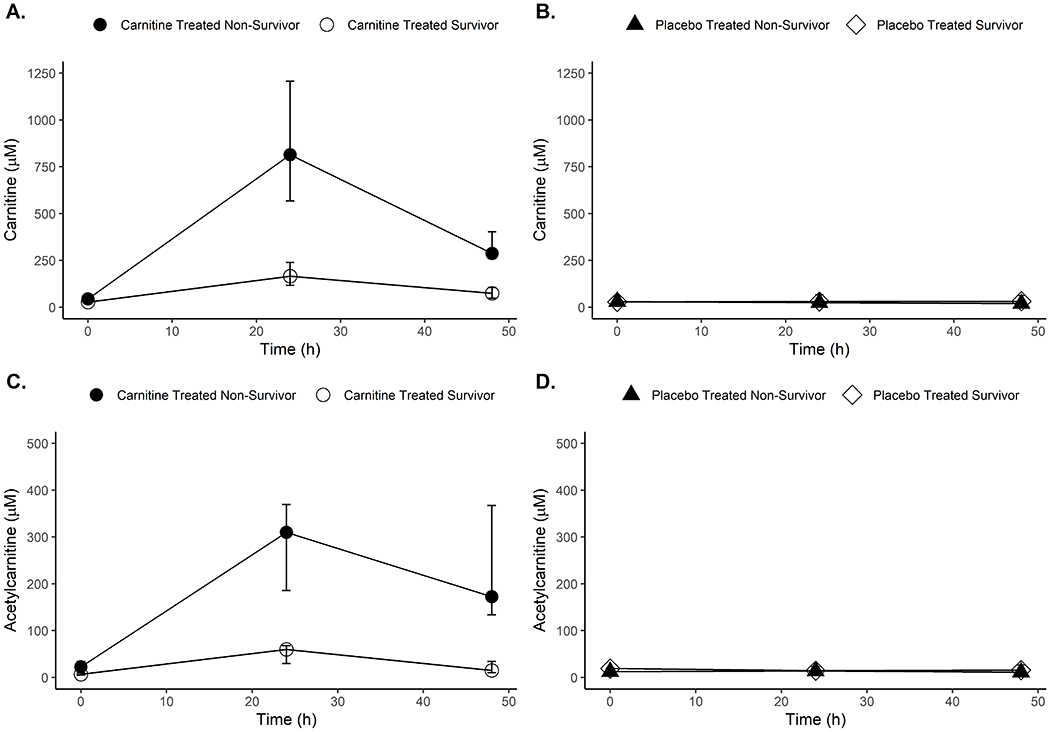
Serum concentrations of carnitine and acetylcarnitine are plotted over time for patients treated with either L-carnitine (panels A and C) or saline placebo (panels B and D). Data plotted are the median, 25th, and 75th percentile of observed serum concentrations, and the Mann-Whitney U test was used to determine significant differences between non-survivors and survivors at each timepoint. All p-values are corrected for multiple comparison using a false discovery rate method according to Storey et. al59 and are reported as q-values. L-carnitine treated non-survivors (N=7–8) at 1-year had significantly higher concentrations of carnitine relative to survivors (N=8) at baseline (BL, q=0.02); 24-hours (T24, q=0.004); and 48-hours (T48, q=0.02) post-treatment. Similar trends were observed for acetylcarnitine (BL, q=0.01; T24, q=0.003; and T48, q=0.02). No significant differences in carnitine or acetylcarntine concentrations were observed between placebo treated non-survivors (N=8–12) and survivors (n=3).
Candidate Mechanisms of Interpatient Variability of Drug Response in Sepsis
Pharmacogenomics:
Pharmacogenomics seeks to explain variability in drug exposure and response based on genetic differences between individuals. Genetic variation in drug metabolizing enzymes, transporters, and targets impacts an individual’s exposure and/or response to a given pharmacologic therapy, which can manifest as distinct drug-response phenotypes. Genetic variability is known to alter patient response to across disease states and in medications commonly used in the intensive care unit (ICU).26 Treatment and dosing paradigms which incorporate patient-specific pharmacogenomic data hold promise in decreasing adverse drug events (ADRs) and improving efficacy.27 Moreover, rationale clinical trial enrollment based on pharmacogenomic phenotypes can foster a more homogenous patient cohort and target patient populations most likely to benefit from therapy (Table 1).
Table 1:
Impact of patient-level variables that could influence the outcome of future clinical trials of sepsis therapeutics.
| Candidate mechanisms of interpatient variability of drug response in sepsis | Impact on L-Carnitine trial design and interpretation | Influence on improving precision medicine in sepsis |
|---|---|---|
| Pharmacogenomics | Genetic variance in the transport receptor of L-Carnitine (OCTN2) may influence drug concentration at site of action | Stratify patients by genotype at the time clinical trial enrollment |
| Drug Interactions | Co-administration of OCTN2 inhibitors, including commonly used antibiotics and vasopressors, may influence drug concentrations | Thorough screening for potential drug interactions by clinical pharmacists at time of trial enrollment and post-hoc |
| Pharmacometabolomics | Baseline and dynamic metabolic signatures are associated with elevated drug concentrations and patient mortality | Target metabolic subgroups for trial enrollment and measure metabolic response signatures post drug administration |
| Morphomics | Patient muscle mass and body composition may influence metabolic adaptability, energetic stores, and drug distribution | Consider variation in body size and composition when testing targeted metabolic therapeutics |
| Renal function and Pharmacokinetics (PK) | Altered renal clearance and reabsorption of drug and acyl-metabolites may influence drug concentrations and patient outcomes | Embedded clinical pharmacology studies to quantify sepsis-pathophysiology induced alterations in drug PK |
Genetic variability in a number of enzymes and transporters could contribute to L-carnitine drug response including those highlighted in the carnitine shuttle (Figure 1). Carnitine acts intracellularly and is highly sequestered in skeletal muscle and other tissues of the body.11 Given the polar structure of carnitine, active sodium-dependent transport by organic cation/carnitine transporters (OCTNs) is required for entry from the blood into the cell and subsequent facilitation of fatty acid β-oxidation. The primary carnitine transporter, OCTN2, thus represents the focus of this section.
The OCTN2 transporter is encoded by the SLC22A5 gene located on chromosome 5q31.1. Spanning 25 kb, the 10 exons of this gene encode the full length 557 amino acid protein. Numerous autosomal recessive mutations in the SLC22A5 gene are responsible for primary carnitine deficiency and results in low serum carnitine levels due to the kidney’s impaired ability to reabsorb the molecule.28 Missense mutations are exceedingly rare, result in severe metabolic and mitochondrial dysfunction, and manifest clinically as a primary carnitine deficiency at a young age. As such, loss of function mutations are unlikely to play a role in explaining variability in L-carnitine concentrations or response in clinical studies of adults with septic shock. Nonetheless, given the vital role of OCTN2 in carnitine uptake into the cell, and considering the large doses administered in these trials, more common genetic polymorphisms in OCTN2 resulting in reduced function and / or expression may improve understanding of the mechanisms that explain the broad dynamic range of carnitine concentrations following supplementation.
Common polymorphisms (i.e., minor allele frequency greater than 1%) in the OCTN2 gene and their impact on carnitine transport outside the context of primary carnitine deficiency are rare.29–31 Three SNPs (Phe17Leu, Tyr449Asp, Val481Asp) were associated with reduced OCTN2 function compared to wild-type (WT), and a SNP in the promoter region of the gene (−207C>G) was associated with increased carnitine transport capacity and trended toward increase mRNA expression in cell lines.29 Out of these, only the promoter region variant (−207C>G, rs2631367) could be considered common according to the National Center for Biotechnology Information database of genetic variation (dbSNP).32 Further studies have observed a tissue-specificity to the −207C>G variant’s effect on mRNA expression levels.30, 31
To supplement the limited literature regarding common polymorphisms effecting OCTN2, we conducted a systematic bioinformatics search for potentially relevant SNPs. We queried the Genotype-Tissue Expression (GTEx) Project (available at https://gtexportal.org/home/), which seeks to explain variability in mRNA expression levels from previously healthy human cadavers with whole genome sequencing.33 The goal of this query was to determine common genetic variants (i.e., SNPs) that significantly alter gene expression of the OCTN2 transporter. Using expression quantitative trait loci (eQTL) analysis, approximately 1500 variants were found to be associated with altered gene expression at the tissue level. Summing across more than 6,000 SNP/tissue pairs, the variant with the largest effect on net OCTN2 gene expression was the promoter region variant (−207C>G, rs2631367).
In previously unpublished data from our group, patients treated with L-carnitine in the phase I trial22 were genotyped for the OCTN2 (−207C>G) SNP. In this preliminary study, fourteen patients had both genomic and serum carnitine concentrations measured at 24 hours (T24). Among these, four patients were wild-type (CC), while ten carried one or two copies of the G allele. Patients with the C/G or G/G genotype trended toward lower T24 plasma levels of L-carnitine (p=0.11), suggesting that genetic variation in the OCTN2 transporter may contribute to variability and persistent elevations in L-carnitine following supplementation during septic shock. More pharmacogenetic studies are needed and are underway in the phase II trial23 to determine if variation in OCTN2 and other carnitine-specific enzymes and / or transporters explain interpatient variability in L-carnitine drug response.
Drug Interactions:
Drug interactions occur when the activity, exposure, or effectiveness of a drug is impacted by the presence of another drug. Co-administered drugs may inhibit or induce expression of important enzymes or transporters, compete at target binding sites, or act in a synergistic or antagonistic fashion. Different combinations of drugs and their interactions introduces variability in the pharmacokinetics (PK) and pharmacodynamic response (PD) to pharmacologic therapy, which may put patients at increased risk of ADRs and either mitigate or enhance therapeutic efficacy. Critically ill patients are at increased risk of drug interactions and subsequent complications given comorbidities and disease complications that are often present (e.g., renal failure) and the requisite complex treatments regimens prescribed.34, 35 In other disease states such as cancer, there is a high prevalence of drug interactions in patients enrolled in clinical trials.36 Drug interactions in critically ill patients may pose a similar threat to trial validity and patient health and should be systematically screened and considered (Table 1).
For L-carnitine, several drugs are reported to inhibit the OCTN2 transporter and therefore could contribute to interpatient variability in exposure. These drugs can also cause secondary carnitine deficiency through inhibition of the OCTN2 transporter in the kidneys leading to decreased efficiency of reabsorption.37 Of particular interest, in the setting of sepsis, are two widely used classes of medications, namely antibiotics and vasopressors. Previous reports have demonstrated that cefepime and levofloxacin inhibit OCTN2 in vitro.38, 39 While the choice of antibiotic therapy in sepsis depends on a number of patient specific factors, cefepime and levofloxacin are two commonly used antibiotics in the United States and are both recommended options in evidence-based best practices. Vasopressors such as norepinephrine and other catecholamines, used to maintain blood pressure support, and other commonly used medications including omeprazole and valproic acid inhibit OCTN2 and could similarly impact L-carnitine drug response.37 In addition to omeprazole, other proton-pump inhibitors, including pantoprazole and lansoprazole, have been shown to inhibit similar organic ion transporters but whether they interfere with the function of OCTN2 and carnitine transport has not been reported.40
Propofol, a short-acting hypnotic and sedative that is widely used in the ICU, may play a critical role in understanding variable drug response to L-carnitine. Propofol is known to inhibit carnitine palmitoyltransferase I and the mitochondrial electron transport chain, which leads to incomplete β-oxidation of fatty acids.41 The induced metabolic disruptions have been linked to propofol infusion syndrome or PRIS, a severe adverse effect of propofol that includes bradycardia, arrhythmias, rhabdomyolysis, metabolic acidosis, hepatomegaly, hyperlipidemia, and organ failure. Moreover, animal and in-vitro experiments have suggested a role for L-carnitine and acetylcarnitine in restoring propofol inhibition of fatty acid metabolism.42, 43
Variable exposure to one or more of these drugs could influence resulting blood concentrations and subsequent metabolic response to supplemental L-carnitine. Other mechanisms are certainly possible such that concomitant medications and variable patient feeding routinely in the treatment of septic shock may further confound the clinical studies discussed above. Presently, the clinical relevance of such interactions and how they should be managed is currently unknown. Further investigation into the use of these drug inhibitors and the effect on L-carnitine concentrations in the phase II study is underway.
Pharmacometabolomics:
Metabolomics seeks to identify and quantify small molecules, the full collection of which define the metabolome, in a given biofluid.44 The metabolome constitutes a read-out of underlying cellular and biochemical events that reflect the genetic makeup of the host, transcriptomic and proteomic influence, as well as variability in the microbiome and environmental exposure. As such, metabolomics represents the culmination of these important regulators on the host. In addition, given that metabolism is dynamic on a practical and physiological time-scale, this sensitivity can inform heterogeneity in disease trajectory and treatment response. Pharmacometabolomics exploits this paradigm and is aimed at understanding and predicting response to drug treatment. In short, clinical application of metabolomics holds great promise in improving the diagnosis and risk stratification of critically ill patients, furthering drug discovery through metabolic signatures of drug response and/or ADRs, and elucidating biochemical pathways involved in the pathophysiology of critical illness (Table 1).
A pharmacometabolomic approach was utilized to understand baseline metabolic differences in patients treated in the Phase I study of L-carnitine.25 Patients treated with L-carnitine who had low baseline levels of the ketone levels,3-hydroxybutyrate, also had lower post-treatment carnitine levels at 24 hours. The L-carnitine treated, low-ketone patients also had better clinical outcomes as evidenced by a timelier reduction in vasopressor requirement and decreased 1-year mortality. A follow-up, an untargeted metabolomics approach was employed in male patients from the Phase I study.45 L-carnitine treated non-survivors were found to have post-treatment elevations in metabolites related to vascular inflammation including histamine, allysine, and fibrinopeptide A. Along with the differential metabolic response of survivors and non-survivors highlighted in Figure 2, these data suggest both baseline metabolic signatures and metabolic profiles over time may be predictive of L-carnitine treatment responsiveness.
Morphomics:
Analytic morphomics is a new and rapidly growing scientific discipline within precision pharmacotherapy that studies how variation in body size, composition and structure are associated with drug and disease response.46 In sepsis, two recent meta-analyses have observed a paradox between body composition and survival, whereby particularly overweight (BMI between 25 kg/m2 and 29.9 kg/m2), and to a lesser extent obese (BMI between 30 kg/m2 and 40 kg/m2), patients tend to have better mortality outcomes compared to normal weight individuals (BMI between 18.5 kg/m2 and 24.9 kg/m2).47, 48 Notably, underweight (BMI less than 18.5 kg/m2) and morbidly obese (BMI greater than 40 kg/m2) patients were found to have similar risk of mortality relative to normal weight individuals. Neither measured peak concentrations of L-carnitine nor mortality were significantly associated with BMI in patients who received study drug in the phase I study. However, the observed “obesity paradox” reinforces the concept of a metabolic and energy-driven component to sepsis pathophysiology and has a number of possible pathophysiological explanations including increased energy stores, anti-inflammatory mediator release from adipose tissue, and lipoprotein binding of bacterial cellular components.49
Another possible explanation is that increased muscle mass offers energetic and metabolic adaptability to patients within a window of the BMI spectrum. Protein catabolism and subsequent myopathy is observed in critically ill patients, and skeletal muscle, an important energetic source to the host, experiences mitochondrial injury over the course of sepsis.50 Indeed, recent studies have found an association between low muscle mass and increased risk of mortality for patients with sepsis. In 74 patients with liver cirrhosis and sepsis, patients with low muscle mass (defined as mid-arm muscle circumference lower than the 5th percentile of the population) had increased mortality compared to patients with normal muscle mass (47% compared to 26%, p=0.06).51 In a separate retrospective review of 627 patients with a diagnosis of sepsis and an available abdominal computed tomography scan of the psoas muscle, muscle mass depletion was associated with 28-day mortality in both univariate and multivariate logistic regression (OR 2.79, p=0.01).52 Given the extent of protein catabolism, the sepsis-obesity paradox, and the known sequestering of carnitine into muscle tissue, morphomics and variability in body composition offers a currently untapped field that could aid in explaining the observed variability in response to supplemental L-carnitine and patient mortality in sepsis broadly (Table 1).
Pharmacokinetics and Renal Function:
Pharmacokinetics (PK) as a science seeks to understand what the body does with and to drugs. More specifically, it is the study of how drugs are absorbed, distributed, metabolized, and eliminated from the body. Previous studies have highlighted that there is profound sepsis-induced variation in drug PK. The reasons for this are likely multifaceted but include altered protein binding, perturbed vascular and tissue permeability, decreased hepatic and renal blood flow, and lower activity of drug metabolizing enzymes.53 High interpatient variability in drug PK in sepsis clinical trials contributes to overall heterogeneity of the patient cohort and may confound trial results unless careful analysis of drug exposure is considered (Table 1).
The PK of L-carnitine has been explored, however no studies have determined the precise PK of L-carnitine in sepsis or at such high intravenous doses. As discussed above, OCTN2 is a critical carnitine transporter that is responsible for carnitine uptake into cells/tissues, however it is also responsible for reabsorption of carnitine in the kidney proximal tubule. As such, kidney function may play a vital role in the interpatient variability in serum carnitine concentrations that result following supplementation. Previous reviews, report an average renal clearance of endogenous carnitine of 1-3 mL/min, indicating that, at physiologically relevant concentrations, up to 99% of carnitine is reabsorbed by the kidney.54 Exogenous carnitine administered to healthy volunteers, increased renal clearance of carnitine and acetylcarnitine, indicating saturation of the OCTN2 transporter and the reabsorption process, which may be relevant for supraphysiologic doses of intravenous carnitine like those given in septic shock trials.54 Unfortunately, urine samples were not collected in these studies, which prevents us from estimating renal clearance of relevant carnitine species in these patients. Both studies reported similar serum creatinine levels among survivors and non-survivors indicating renal function alone does not explain heterogeneity in L-carnitine and acylcarnitine concentrations among patients. However, the reliability of creatinine as a biomarker in the setting of acute kidney injury (AKI), sepsis and other critical illness, and in drug development broadly been called into question.55, 56 New investigations of biomarkers of kidney injury and function are underway, but have yet to be widely adapted or clinically validated. Further investigations of the variability in L-carnitine drug response stratified by the presence of AKI and acute liver injury, and among other measures of organ dysfunction are warranted before precise clinical recommendation can be made in these patient groups. Moreover, modeling the impact of patient-level biological variables such as sex, age, and race is critical to understand the observed heterogeneity in L-carnitine drug response.
Metabolic provocation with supplemental L-carnitine:
While the approaches outlined above offer an opportunity to identify septic patients most likely to respond to L-carnitine, understanding the metabolic response signature of L-carnitine treated patients holds value beyond a potential therapeutic benefit. Outside of sepsis, the concept of provoked metabolic testing is used to uncover latent disease phenotypes. For example, a glucose tolerance test is used to diagnosis a previously undetectable pre-diabetic phenotype in pregnant women. As seen in Figure 2, the metabolic response profiles of the placebo arm did not differentiate patient mortality at one-year, as they did for L-carnitine treated patients. Critically, this finding suggests the possibility that treatment with L-carnitine amplifies or incites a phenotype of sepsis mortality and underlying derangement in carnitine homeostasis. Indeed, elevations in plasma acylcarnitines are understood to be a measure of mitochondrial dysfunction and altered coenzyme A homeostasis in other metabolic diseases, and elevated acetylcarnitine was recently found to be predictive of plasma cytokine levels, blood culture positivity, multi-organ dysfunction, and mortality in patients with sepsis.57 Others have shown that short chain acylcarnitines levels are related to plasma mitochondrial DNA, an indicator of cellular damage, and that acylcarnitines are predictive of mortality in critically ill patients.15, 16 Together, these data suggest derangements of the carnitine/acylcarnitine pool may be indicative of metabolic dysfunction and/or worsening sepsis that is predictive of mortality.
A metabolic test with supplemental L-carnitine can provoke biochemical pathways in sepsis and amplify signals of underlying mitochondrial dysfunction and perturbed energy pathways. A more complete investigation of other metabolite profiles that are disrupted upon treatment may also lead to new insights into underlying disease mechanism and pathophysiology. While there are a number of sepsis metabolomics studies that confirm the substantial metabolic disturbances of the disease, they do not inform distinct sepsis phenotypes in the way that a metabolic provocation test could. The substantial variability in response to L-carnitine exposure and subsequent mortality differences indicate phenotypic differences between groups. In aggregate, this observation introduces the principle that even in the presence of a disease like sepsis, which is known to induce a substantial metabolic perturbation, provocation of metabolism is required to bring the full dynamic range into view.
Conclusion and Future Directions
L-carnitine and acylcarnitine concentrations are highly variable following L-carnitine supplementation in septic shock, and the observed interpatient variability is associated with patient mortality. The heterogeneity of sepsis and drug response complicates the interpretation of a therapeutic value of L-carnitine and other potential sepsis pharmacotherapies. Currently, a careful analysis of the phase II clinical trial to inform the design of, and the results from, a phase III trial are needed before L-carnitine treatment can be recommended for a specific sepsis patient population. However, even though more work needs to be done, a strategy using the patient-level factors and biological variables that impact L-carnitine drug response could be used in the a priori identification of patients who are most likely to derive the greatest benefit from treatment. Well defined phenotypes of drug response could serve as inclusion-exclusion criteria and aid in the design and interpretation of future phase III clinical studies of L-carnitine. Such information will need to be balanced with threats to clinical and external validity, as well as consideration to the ability to recruit a sufficient patient population.
The approach outlined here is applicable to other emerging sepsis therapeutics and could aid in developing a precision medicine approach to sepsis and the design of early-phase clinical trials in critical illness. Moreover, provoking metabolism in septic shock with L-carnitine supplementation offers a unique opportunity to define metabolic signatures of survival and elucidate biochemical pathways deranged in the sepsis syndrome. Such an approach offers a novel mechanism to further the understanding of sepsis pathophysiology and progression, as well as elucidate drug response phenotypes.
Acknowledgements/Sources of support:
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under award number R01GM111400. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIGMS or NIH.
Footnotes
Conflict of interest: The authors claim no conflict of interest.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;8:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 2020;10219:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019;20:2003–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. The Lancet Infectious diseases 2015;5:581–614. [DOI] [PubMed] [Google Scholar]
- 5.Pravda J Metabolic theory of septic shock. World journal of critical care medicine 2014;2:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Triba MN, Amathieu R, et al. Nuclear magnetic resonance-based serum metabolomic analysis reveals different disease evolution profiles between septic shock survivors and non-survivors. Critical Care 2019;1:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 2010;6:752–61. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. Jama 2010;8:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. Jama 2019;7:654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 2005;9462:871–5. [DOI] [PubMed] [Google Scholar]
- 11.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clinical pharmacokinetics 2012;9:553–72. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Black SM. CARNITINE HOMEOSTASIS, MITOCHONDRIAL FUNCTION, AND CARDIOVASCULAR DISEASE. Drug discovery today Disease mechanisms 2009;1–4:e31–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014;1:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Protti A, Fortunato F, Artoni A, et al. Platelet mitochondrial dysfunction in critically ill patients: comparison between sepsis and cardiogenic shock. Critical care (London, England) 2015;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langley RJ, Tsalik EL, van Velkinburgh JC, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 2013;195:195ra95–95ra95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson PI, Nakahira K, Rogers AJ, et al. Plasma mitochondrial DNA and metabolomic alterations in severe critical illness. Crit Care 2018;1:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton S, Fukumoto K, Stefanutti G, Spitz L, Zammit VA, Pierro A. Myocardial carnitine palmitoyltransferase I as a target for oxidative modification in inflammation and sepsis. Biochemical Society transactions 2003;Pt 6:1133–6. [DOI] [PubMed] [Google Scholar]
- 18.Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock (Augusta, Ga) 1996;2:89–94. [DOI] [PubMed] [Google Scholar]
- 19.Gasparetto A, Corbucci GG, De Blasi RA, et al. Influence of acetyl-L-carnitine infusion on haemodynamic parameters and survival of circulatory-shock patients. International journal of clinical pharmacology research 1991;2:83–92. [PubMed] [Google Scholar]
- 20.Corbucci GG, Loche F. L-carnitine in cardiogenic shock therapy: pharmacodynamic aspects and clinical data. International journal of clinical pharmacology research 1993;2:87–91. [PubMed] [Google Scholar]
- 21.Corbucci GG, Lettieri B. Cardiogenic shock and L-carnitine: clinical data and therapeutic perspectives. International journal of clinical pharmacology research 1991;6:283–93. [PubMed] [Google Scholar]
- 22.Puskarich MA, Kline JA, Krabill V, Claremont H, Jones AE. Preliminary safety and efficacy of L-carnitine infusion for the treatment of vasopressor-dependent septic shock: a randomized control trial. JPEN Journal of parenteral and enteral nutrition 2014;6:736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones AE, Puskarich MA, Shapiro NI, et al. Effect of Levocarnitine vs Placebo as an Adjunctive Treatment for Septic Shock: The Rapid Administration of Carnitine in Sepsis (RACE) Randomized Clinical TrialEffect of Levocarnitine vs Placebo as an Adjunctive Treatment for Septic ShockEffect of Levocarnitine vs Placebo as an Adjunctive Treatment for Septic Shock. JAMA Network Open 2018;8:e186076–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puskarich MA, Evans CR, Karnovsky A, Das AK, Jones AE, Stringer KA. Septic Shock Nonsurvivors Have Persistently Elevated Acylcarnitines Following Carnitine Supplementation. Shock 2018;4:412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puskarich MA, Finkel MA, Karnovsky A, et al. Pharmacometabolomics of l-carnitine treatment response phenotypes in patients with septic shock. Annals of the American Thoracic Society 2015;1:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacKenzie M, Hall R. Pharmacogenomics and pharmacogenetics for the intensive care unit: a narrative review. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2017;1:45–64. [DOI] [PubMed] [Google Scholar]
- 27.Vincent J-L. The coming era of precision medicine for intensive care. Critical care (London, England) 2017;Suppl 3:314–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet journal of rare diseases 2012;68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban TJ, Gallagher RC, Brown C, et al. Functional genetic diversity in the high-affinity carnitine transporter OCTN2 (SLC22A5). Molecular pharmacology 2006;5:1602–11. [DOI] [PubMed] [Google Scholar]
- 30.Tahara H, Yee SW, Urban TJ, et al. Functional genetic variation in the basal promoter of the organic cation/carnitine transporters OCTN1 (SLC22A4) and OCTN2 (SLC22A5). The Journal of pharmacology and experimental therapeutics 2009;1:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grube M, Meyer zu Schwabedissen HE, Prager D, et al. Uptake of cardiovascular drugs into the human heart: expression, regulation, and function of the carnitine transporter OCTN2 (SLC22A5). Circulation 2006;8:1114–22. [DOI] [PubMed] [Google Scholar]
- 32.Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic acids research 2001;1:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013;6:580–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanham D, Spinewine A, Hantson P, Wittebole X, Wouters D, Sneyers B. Drug-drug interactions in the intensive care unit: Do they really matter? Journal of critical care 2017;97–103. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulos J, Smithburger PL. Common drug interactions leading to adverse drug events in the intensive care unit: management and pharmacokinetic considerations. Critical care medicine 2010;6 Suppl:S126–35. [DOI] [PubMed] [Google Scholar]
- 36.Marcath LA, Coe TD, Hoylman EK, Redman BG, Hertz DL. Prevalence of drug-drug interactions in oncology patients enrolled on National Clinical Trials Network oncology clinical trials. BMC cancer 2018;1:1155–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pochini L, Scalise M, Galluccio M, Indiveri C. OCTN cation transporters in health and disease: role as drug targets and assay development. Journal of biomolecular screening 2013;8:851–67. [DOI] [PubMed] [Google Scholar]
- 38.Ganapathy ME, Huang W, Rajan DP, et al. β-Lactam Antibiotics as Substrates for OCTN2, an Organic Cation/Carnitine Transporter. Journal of Biological Chemistry 2000;3:1699–707. [DOI] [PubMed] [Google Scholar]
- 39.Hirano T, Yasuda S, Osaka Y, Kobayashi M, Itagaki S, Iseki K. Mechanism of the inhibitory effect of zwitterionic drugs (levofloxacin and grepafloxacin) on carnitine transporter (OCTN2) in Caco-2 cells. Biochimica et biophysica acta 2006;11:1743–50. [DOI] [PubMed] [Google Scholar]
- 40.Nies AT, Hofmann U, Resch C, Schaeffeler E, Rius M, Schwab M. Proton Pump Inhibitors Inhibit Metformin Uptake by Organic Cation Transporters (OCTs). PLOS ONE 2011;7:e22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirrakhimov AE, Voore P, Halytskyy O, Khan M, Ali AM. Propofol infusion syndrome in adults: a clinical update. Crit Care Res Pract 2015;260385–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Rainosek SW, Sadovova N, et al. Protective effect of acetyl-L-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 2014;49–57. [DOI] [PubMed] [Google Scholar]
- 43.Moriyama T, Kiyonaga N, Ushikai M, Kawaguchi H, Horiuchi M, Kanmura Y. Effects of L-Carnitine on Propofol-Induced Inhibition of Free Fatty Acid Metabolism in Fasted Rats and in Vitro. Open Journal of Anesthesiology 2018;11. [Google Scholar]
- 44.Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. American journal of respiratory and critical care medicine 2011;6:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans CR, Karnovsky A, Puskarich MA, Michailidis G, Jones AE, Stringer KA. Untargeted Metabolomics Differentiates l-Carnitine Treated Septic Shock 1-Year Survivors and Nonsurvivors. Journal of Proteome Research 2019;5:2004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chughtai K, Song Y, Zhang P, et al. Analytic morphomics: a novel CT imaging approach to quantify adipose tissue and muscle composition in allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation 2016;3:446–50. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Liu X, Chen Q, Liu C, Huang C, Fang X. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC anesthesiology 2017;1:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, Eichacker PQ. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Critical care (London, England) 2016;1:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng PY, Eikermann M. The obesity conundrum in sepsis. BMC anesthesiology 2017;1:147–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mofarrahi M, Sigala I, Guo Y, et al. Autophagy and skeletal muscles in sepsis. PloS one 2012;10:e47265–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucidi C, Lattanzi B, Di Gregorio V, et al. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver international : official journal of the International Association for the Study of the Liver 2018;5:851–57. [DOI] [PubMed] [Google Scholar]
- 52.Lee Y, Park HK, Kim WY, Kim MC, Jung W, Ko BS. Muscle Mass Depletion Associated with Poor Outcome of Sepsis in the Emergency Department. Annals of nutrition & metabolism 2018;4:336–44. [DOI] [PubMed] [Google Scholar]
- 53.De Paepe P, Belpaire FM, Buylaert WA. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clinical pharmacokinetics 2002;14:1135–51. [DOI] [PubMed] [Google Scholar]
- 54.Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Annals of the New York Academy of Sciences 2004;30–41. [DOI] [PubMed] [Google Scholar]
- 55.Koyner JL. Assessment and diagnosis of renal dysfunction in the ICU. Chest 2012;6:1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crass RL, Pai MP. Estimating Renal Function in Drug Development: Time to Take the Fork in the Road. Journal of clinical pharmacology 2019;2:159–67. [DOI] [PubMed] [Google Scholar]
- 57.Chung KP, Chen GY, Chuang TY, et al. Increased Plasma Acetylcarnitine in Sepsis Is Associated With Multiple Organ Dysfunction and Mortality: A Multicenter Cohort Study. Crit Care Med 2019;2:210–18. [DOI] [PubMed] [Google Scholar]
- 58.Semba RD, Trehan I, Li X, et al. Environmental Enteric Dysfunction is Associated with Carnitine Deficiency and Altered Fatty Acid Oxidation. EBioMedicine 2017;57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences of the United States of America 2003;16:9440–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


