Abstract
Objectives:
To document the need for additional FDA approved medications for the treatment of juvenile idiopathic arthritis (JIA).
Methods:
The electronic medical record of JIA patients treated at Cincinnati Children’s Hospital Medical Center (CCHMC) and data from JIA patients enrolled in the Childhood Arthritis & Rheumatology Research Alliance (CARRA) Registry were included in this study. Unmet medication need was measured in two ways: (A) presence of chronically uncontrolled JIA defined as a physician global-assessment of JIA activity (0–10; 0=inactive) ≥3 OR ≥3 active joints OR a patient global-assessment of well-being (0–10; 0=very well) ≥3, despite sequential use of ≥2 biologic disease-modifying anti-rheumatic drugs (bDMARDs); and (B) use of ≥1 bDMARD not approved for any JIA category.
Results:
At CCHMC, 829 of 1,599 JIA patients (52%) were treated with ≥1 bDMARD and 19% (304/1,599) had been exposed to ≥1 unapproved bDMARD. In the CARRA Registry, 4,766 of 7,379 (65%) children had received ≥1 bDMARD and 1,122 (15%) had been prescribed ≥1 unapproved bDMARD; Of those children treated with ≥1 DMARD, 52% (225/487) at CCHMC and 45% (527/1159) of patients in the CARRA Registry with complete data had chronically uncontrolled JIA despite use of ≥2 bDMARDs.
Conclusion:
Despite the bDMARDs that are currently approved for JIA, there is persistent need for additional therapies to control JIA signs and symptoms. Since FDA approval is critical to insure bDMARD access, the study and licensing of new medications is critical to address the unmet medication need and to further improve JIA outcomes.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) is a chronic pediatric rheumatic disease of unknown etiology that presents in children by the age of 16 years. The Childhood Arthritis Rheumatology Research Alliance (CARRA) is a research network in North America that collects longitudinal information about children with JIA and other pediatric rheumatic diseases in a robust registry (CARRA Registry) that began enrollment in 2015. Cincinnati Children’s Hospital Medical Center (CCHMC) is a free-standing pediatric hospital with an extensive regional referral base.
Given the absence of a cure, the goal of JIA therapy remains the achievement of inactive disease and clinical remission to avoid joint damage and limit the negative impact of JIA on patient growth and development (1). Besides non-steroidal anti-inflammatory medications, corticosteroid joint injections, and synthetic disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate, biologic DMARDs (bDMARDs) are used to control active arthritis. Current JIA treatment guidelines recommend the use of bDMARDs as part of the early treatment for patients with polyarticular and systemic JIA (2, 3).
In the United States (U.S.), the Food and Drug Administration (FDA) is the federal agency charged with overseeing drug manufacturing, labelling, advertisement, and safety of medications and biological products. The Best Pharmaceuticals for Children Act (BPCA) (4) and the Pediatric Research Equity Act (PREA) (5) govern medication approval for JIA in the U.S. While the BPCA encourages drug companies to test their products in children, PREA necessitates the study of new drugs and bDMARDs in children, if there is a pediatric disease similar to the non-orphan adult disease, and if it is likely that the new agent will be used in children (6). The first bDMARD approved in the U.S. for use in JIA was etanercept in 1999 (7). Since then, several additional bDMARDs have been approved for the treatment of JIA. Despite FDA approved drugs for polyarticular JIA and systemic JIA (sJIA), clinical experience suggests that a sizable proportion of children with JIA fail to achieve clinical remission. This raises the possibility of an ongoing need for additional therapeutic options for JIA.
The objective of this research was to investigate the need for additional FDA approved medications to treat signs and symptoms of JIA.
METHODS
Two databases with information about children with JIA and their treatment were used for this study: the JIA Registry integrated in the electronic medical record (EMR) at CCHMC; and the CARRA Registry which prospectively collects data at 71 U.S. and Canadian sites (8). The CCHMC IRB approved the EMR study. The Duke University IRB approved the CARRA Registry study, and all participants were consented for data collection.
Data elements used for the analysis
The focus of the CARRA Registry is to evaluate the safety of therapeutic agents in children with JIA. Secondary objectives include the evaluation of disease outcomes and their associations with medication use. Since 2015, data are collected every 6 months and include clinical assessments, laboratory results, former and current medication use, patient-reported outcomes, and safety events. Due to the initial enrollment strategy (8), the CARRA Registry is enriched for participants who are likely to receive treatment with bDMARDs. Included in this analysis were all CARRA Registry participants with JIA and complete enrollment data prior to May 2019.
In 2007, CCHMC implemented an EMR which features an integrated registry function. Standardized templates capture longitudinal patient-reported outcomes, results of laboratory and radiologic testing, and all clinical data, including medication information from JIA patients are available. Children with JIA are seen approximately every 3 months (range: 1–12 months), depending on disease activity and the impact of JIA on patient functioning. The CCHMC JIA Registry includes children that had ≥2 visits with a JIA diagnosis as per a CCHMC pediatric rheumatologist. Data collected from such patients between February 2008 and March 2019 were included in this analysis.
Data extracted from both registries included: (a) JIA category according to the Revised International League against Rheumatism (ILAR) Classification for JIA (9); (b) date of JIA diagnosis; (c) measured on a 21-circle visual analog scale (21c-VAS), the physician global-assessment of disease activity (MD-global; range: 0–10; 0=no activity, 10=very severe disease activity); (d) also measured on a 21c-VAS, the patient rating of overall well-being (Pat-global; range: 0–10; 0=very well, 10=very poor); and (e) the number of joints with active arthritis (AJC). The MD-global and AJC are considered central for evaluating the success of JIA therapy, hence are included in the criteria to measure clinical remission of JIA (10). The Pat-global captures, among other, patient perceived benefits and side effects of JIA treatments.
Both registries provide discrete longitudinal data on JIA relevant medication usage, including start and stop dates. For the purpose of this study, JIA medication use was limited to bDMARDs and targeted small molecule therapeutics, specifically Janus kinase (JAK) inhibitors, which were grouped under the bDMARD category.
Approaches to assess unmet medication need
Given the absence of generally accepted criteria, we newly defined the presence of “unmet medication need” in two ways: (A) presence of chronically uncontrolled JIA (CU-JIA), i.e. a MD-global ≥3 OR an AJC ≥3 OR a Pat-global ≥3 despite sequential use of ≥2 different bDMARDs; and (B) exposure to one or more bDMARDs not approved for any JIA category. Currently approved for at least one JIA category are the following bDMARDs: intravenously (IV) and/or subcutaneously (SC) administered abatacept, adalimumab, etanercept, tocilizumab, and canakinumab.
Statistical Analysis
Descriptive analysis was done with categorical data summarized as frequencies and percentages, and numerical data as means and standard deviations (SD), respectively. Assessment of CU-JIA despite ≥2 bDMARDs was only calculated in patients with complete information on MD-global, Pat-global and AJC. For both data sources, current and prior use of unapproved bDMARDs was assessed.
CARRA Registry.
The presence of CU-JIA was evaluated at the most recent Registry visit. Therefore, the cross-sectional analysis of CU-JIA need was restricted to participants with a history of use of ≥2 bDMARDs.
CCHMC data.
Since longitudinal data are available for up to 10 years, all patients with CU-JIA were assessed for this outcome at their most recent clinical visit. Additionally, for patients initiating a third or subsequent bDMARD, the most recent visit prior to each bDMARD switch was assessed to confirm CU-JIA.
RESULTS
Patients
There were 1,599 children with JIA included from the CCHMC Registry and 7,379 children with JIA from the CARRA Registry, respectively. The frequencies of JIA patients belonging to specific JIA categories were similar across both cohorts (Table 1). However, the proportion of patients with a disease duration of <3 years and the frequency of patients prescribed ≥1 bDMARD were both higher among CARRA Registry participants compared to patients followed at CCHMC. Nevertheless, in both cohorts, the majority of patients used bDMARDs. In the CCHMC registry, 829 of 1,599 patients (53%) and in the CARRA Registry 4,766 of 7,379 patients (65%) were exposed to one or more bDMARDs in sequence.
Table 1.
Description of cohorts†.
| CCHMC (N=1,599) | CARRA (N=7,379) | |
|---|---|---|
| Age in years; mean (SD) | 16.3 (6.76)* | 12.6 (4.9) ** |
| Disease duration | ||
| < 3 years | 485 (30%) | 3144 (43%) |
| ≥ 3 and < 6 years | 525 (33%) | 1813 (25%) |
| ≥ 6 years | 589 (37%) | 2422 (33%) |
| JIA category | ||
| Persistent oligoarthritis | 302 (19%) | 1864 (25%) |
| Extended oligoarthritis | 217 (14%) | 545 (7%) |
| Polyarticular arthritis; Rheumatoid Factor negative | 538 (34%) | 2327 (32%) |
| Polyarticular arthritis; Rheumatoid Factor positive | 75 (5%) | 505 (7%) |
| Enthesitis-related arthritis or juvenile psoriatic arthritis | 279 (17%) | 1351 (18%) |
| Systemic | 138 (9%) | 623 (8%) |
| Undifferentiated | 50 (3%) | 164 (2%) |
| Treatment without bDMARDs | ||
| Never received DMARD | 435 (27%) | 870 (11%) |
| Conventional DMARD only‡ | 335 (20%) | 1743 (24%) |
| Treatment using bDMARDs¶ | ||
| 1 bDMARD | 342 (21%) | 2992 (41%) |
| 2 bDMARDs | 239 (15%) | 1129 (15%) |
| 3 bDMARDs | 174 (11%) | 428 (6%) |
| 4 bDMARDs | 49 (3%) | 130 (1.8%) |
| 5 or more bDMARDs | 25 (1.6%) | 87 (1.2%) |
| Any bDMARD | 829 (53%) | 4766 (65%) |
Values are N (%) unless stated otherwise;
Age when contributing final cohort information;
Age at most recent CARRA Registry visit;
Methotrexate, sulfasalazine, leflunomide; RF rheumatoid factor
bDMARDs were given alone or combined with a conventional DMARD and included: abatacept IV/SC, adalimumab, anakinra, canakinumab, certolizumab, etanercept, golimumab IV/SC, infliximab, rilonacept, rituximab, secukinumab, tofacitinib; tocilizumab IV/SC, ustekinumab;
Chronically uncontrolled JIA despite use of at least 2 bDMARDs
There were 487 patients from CCHMC and 1,774 from the CARRA Registry who had been treated with ≥2 bDMARDs in sequence. Missing data for MD-global, Pat-global and AJC in the most recent registry visits limited the assessment of CU-JIA to 1,159 of the 1,774 (65%) patients in the CARRA Registry. Table 2 summarizes the results on the presence of CU-JIA despite exposure to ≥2 bDMARDs. Overall, 255 of 487 (52%) of CCHMC patients and 527 of 1,159 (45%) patients in the CARRA Registry fulfilled Definition A of unmet medication need. There were no clear trends between the frequency of unmet medication need and patient disease duration (<3 years, 3 to <6 years; ≥ 6 years). As shown in Table 2, CU-JIA was present in both cohorts (39–66%), despite the use of up to 5 or more DMARDs. Supplemental Table 1 shows the proportion of JIA patients with AJC≥3 OR MD-global≥3, despite exposure to ≥2 bDMARDs for both cohorts.
Table 2:
Patients with chronically uncontrolled JIA (CU-JIA) by number of bDMARDs and disease duration*
| CCHMC JIA Registry – assessed over 3 visits (N = 487) | CARRA Registry – assessed at most recent visit (n=1,159) | |||||
|---|---|---|---|---|---|---|
| n | n/N = % | Number of patients with CU-JIA / n =% | n | n/N = % | Number of patients with CU-JIA / n =% † | |
| Total Number of bDMARDs | ||||||
| 2 bDMARDs used | 239 | 239 /487 = 48% | 113 /239 = 47% | 731 | 731 /1159 = 63% | 283/731 = 39% |
| 3 bDMARDs used | 174 | 174/487 = 37% | 97/174 = 56% | 282 | 282 /1159 = 24% | 149 /282 = 53% |
| 4 bDMARDs used | 49 | 49/487 = 10% | 31/49 = 63% | 87 | 87 /1159 = 8% | 56 /87 = 64% |
| ≥ 5 bDMARDs used | 25 | 25 /487 = 5% | 14 /25= 56% | 59 | 59 /1159 = 5% | 39 / 59 = 66% |
| Disease duration | ||||||
| < 3 years disease duration | 104 | 104 / 487 = 22% | 43 / 104 = 41% | 335 | 335 /1159 = 29% | 164 /335 = 49% |
| ≥ 3 and < 6 years disease duration | 143 | 143 / 487 = 29% | 84 / 143 = 59% | 315 | 315 /1159 = 27% | 131 /315 = 42% |
| ≥ 6 years disease duration | 240 | 240/ 487 = 49% | 128 / 240 = 53% | 509 | 509 /1159 = 44% | 232 /509 = 46% |
| All CU-JIA despite exposure to ≤2 bDMARDs | 255 /487 = 52% | 527/ 1159 = 45% | ||||
Only patients with complete data who were exposed to 2 or more bDMARDs are shown
Despite sequential use of ≥ 2 different bDMARDs, MD-global ≥3 OR AJC > 3 OR Pat-global ≥3 at the last CARRA registry visit or last CCHMC clinic visit or visit in given disease duration interval (< 3years; 3–6 years,> 6 years)
Use of unapproved bDMARDs
Considering Definition B of unmet medication need, we evaluated the exposure to unapproved bDMARDs. Advanced therapies marketed in the U.S. for adults with inflammatory arthritis but not labelled for use in JIA included tofacitinib, anakinra, infliximab, IV and SC golimumab, certolizumab, rilonacept, secukinumab, and ustekinumab. We found that off-label use of bDMARDs was common in both cohorts (CCHMC: 304 patients; CARRA: 1,122 registry participants), ranging from 15–19% of the cohorts’ population (Figure 1).
Figure 1.
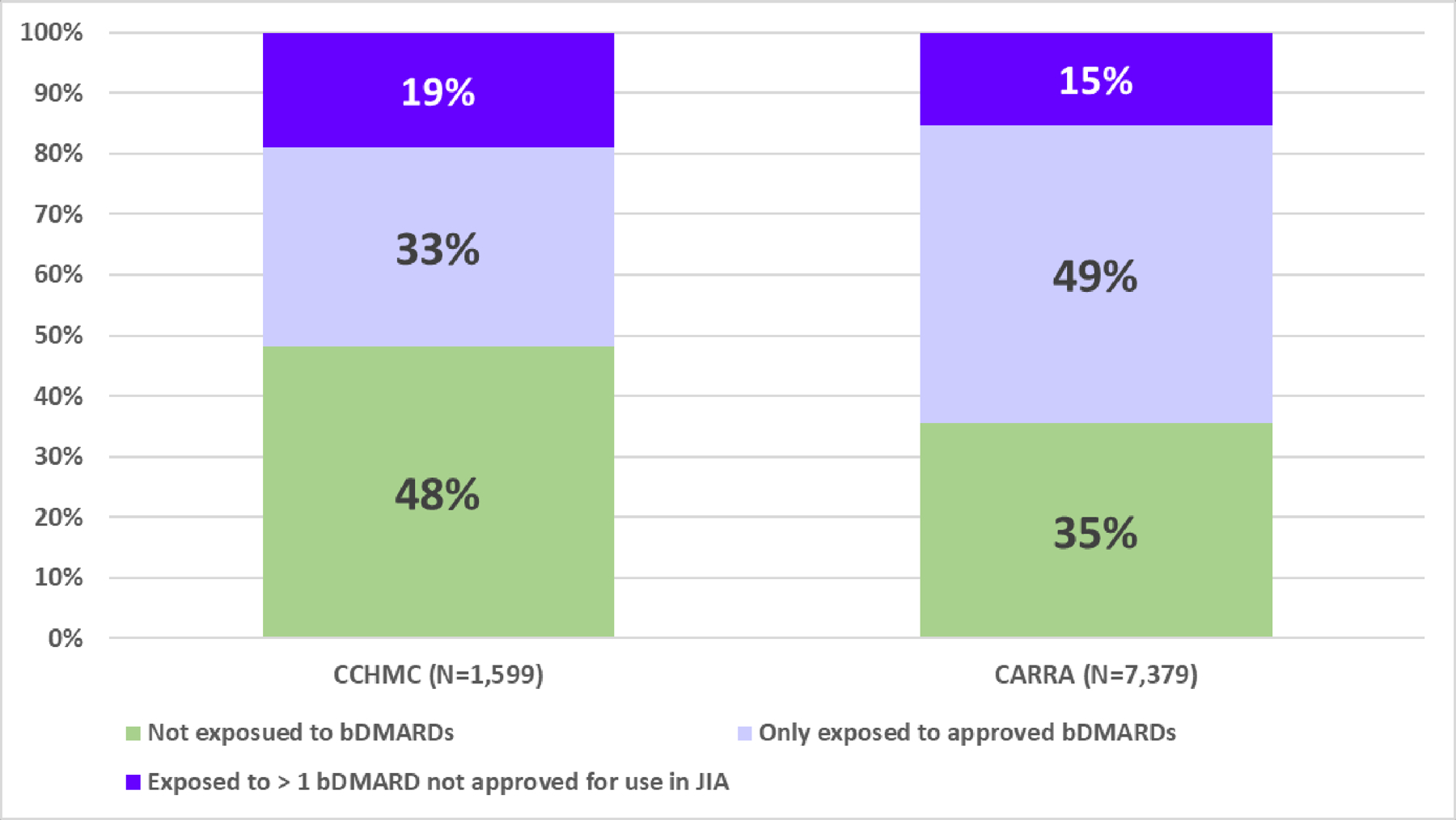
† Unapproved are: anakinra, infliximab, IV/SC golimumab, certolizumab, rilonacept, rituximab, secukinumab, ustekinumab, tofacitinib; approved are: abatacept (IV, SC), adalimumab, etanercept, tocilizumab (IV, SC), canakinumab, tocilizumab (IV, SC); SC administered by subcutaneous injection; IV for intravenous administration
Among JIA patients with more severe disease course that require bDMARDs therapy 37% (304/ 829) of the CCHMC patients and 24% (1,122/4,766) of the children participating in the CARRA Registry participants were exposed to at least 1 unapproved bDMARD.
Systemic JIA
Of the 138 sJIA patients from CCHMC, 85% (117/138) were ever exposed to a bDMARD, and 10 patients (10/117 = 9.1%) continued to have CU-JIA despite exposure to ≥2 bDMARDs. In the CARRA Registry, 88% (546/623) of all sJIA patients were ever treated with a bDMARD, and 35% of the children (55/155 with complete data) had CU-JIA despite exposure to ≥2 bDMARDs.
Enthesitis-related arthritis and juvenile psoriatic arthritis
In the CCHMC cohort, 79% (220/279) of the patients with ERA or jPSA were ever treated with a bDMARD for JIA. There were 31% (27/86) children with ERA or jPSA who continued to experience CU-JIA despite exposure to ≥2 bDMARDs. Similarly, 72% (546/1,351) of children with ERA or jPSA in the CARRA Registry were ever treated with a bDMARD. Among children with ERA or jPSA who were enrolled in the CARRA Registry, 55% (140/253 with complete data) had CU-JIA despite exposure to ≥2 bDMARDs at the most recent registry visit.
DISCUSSION
JIA is the most common pediatric rheumatic disease and often has a chronically active course. Despite overall improved outcomes since the availability of bDMARDs, the results of the current analyses suggest a continued need for additional approved medications. Indeed, as many as 1 in 2 JIA patients treated with ≥2 bDMARDs continued to have CU-JIA in both registries. Taken together, the results of this study underscore the ongoing need for additional medications to treat JIA.
The principal goal of JIA therapy is achieving inactive disease and maintaining clinical remission (3, 11). Among others, children who have inactive disease or are in clinical remission of JIA, lack active arthritis (AJC= 0) and must have the best possible physician global assessment (MD-global= 0). To demonstrate the need for additional medications for the treatment of JIA, this study used a new definition, based on clinical parameters (MD-global, Pat-global, AJC) and the sequential use of ≥2 bDMARDs. The definition was developed a priori by two authors (HBR, DJL) and provides a conservative estimate of medication need because the current target of JIA treatment is clearly not met (2, 3). This is because patients whose well-being is moderately impaired due to JIA (Pat-global ≥ 3) or with an MD-global ≥ 3 and/or who have an AJC of ≥ 3, clearly have not achieved inactive disease status (2, 3). However, even if we had excluded the consideration of the Pat-global from the definition of CU-JIA, then still over 24% of children could be considered to have a need for additional medications.
We demonstrated that off-label use of medication is common in JIA, despite laws that incentivize and require pediatric drug development (4, 5). Presumably, unapproved medications are used because approved therapies have failed to control JIA activity. Off-label use of bDMARDs puts children with JIA at risk of suboptimal outcomes due to potentially inappropriate dosing of bDMARDs. Infliximab, a medication widely used off-label for JIA, provides an informative example. Infliximab is approved for adults with rheumatoid arthritis at dosages as low as 3mg/kg/dose. However, when this weight-adjusted dose was studied in children with JIA, it yielded comparatively lower drug exposure than what was found to be effective in adults, it resulted in higher rates of adverse events and lower responses to therapy compared to what was observed in adults with rheumatoid arthritis (12).
The existing U.S. regulation (PREA) requires the study of bDMARDs proven effective in rheumatoid arthritis only in polyarticular forms of JIA (i.e., rheumatoid factor [RF] positive and RF negative polyarticular JIA, extended oligoarticular JIA, and sJIA with polyarticular course but inactive systemic disease). Other forms of JIA are not currently covered under PREA for medications that have been approved for rheumatoid arthritis. This has the potential for frequent off-label use of bDMARDs in children with certain JIA categories that should be avoided.
The lack of an FDA approved indication is a major barrier to access bDMARDs through both private and public insurance companies. There are currently no bDMARDs that have been labelled for the treatment of ERA or jPSA, although both JIA categories are quite common. As such, the ERA or jPSA groups constituted about 17% of the JIA populations in each of the two cohorts evaluated. Hence, there are sufficient numbers of children with ERA or jPSA that could be enrolled in clinical trials of bDMARDs found to be effective in adults with psoriatic arthritis or spondyloarthritis. Such clinical trials could then support the labelling of these bDMARDs for use in jPSA and ERA.
Systemic JIA is more common than adult onset Still’s disease, the adult correlate of sJIA. Despite the availability of FDA approved medications for sJIA, namely tocilizumab and canakinumab, ongoing disease activity and off-label use of bDMARDs was common among children with sJIA in both cohorts. Anakinra is preferred by many providers for sJIA treatment due to its short half-life and rapid onset of action. However, anakinra has not been FDA approved for use in sJIA, and optimal dosing of anakinra, especially when used for the treatment of very young children in sJIA (13), has not been established.
Our study is not without limitations. Initially, the CARRA Registry preferentially enrolled patients with pJIA and sJIA who were likely to receive treatment with a bDMARD, and there were also missing data that were needed to define CU-JIA (8). These issues may affect the generalizability of the results to the overall JIA population. However, the frequency of patients classified as belonging a given JIA category were similar across registries. Regional or site-specific characteristics in JIA care may impact CCHMC registry results but are not an issue in the CARRA Registry with its broad recruitment from greater than 60 North American sites. However, the results of medication usage were quite similar for both registries, supporting the external validity of our study findings. Reasons for bDMARD discontinuation were not consistently recorded. Thus, we were unable to report the reason for CU-JIA in each patient. Conceivably, reasons also included safety concerns by physicians and patients/families, non-adherence, pain amplification, as well as patient drug intolerance, besides uncontrolled joint inflammation and ongoing disease activity. Arguably, safety concerns and drug intolerance are also reflective of an unmet medication need.
In summary, the results of study of two contemporary cohorts of JIA patients in North America support a profound ongoing need for additional FDA approved drugs for all categories of JIA.
Supplementary Material
ACKNLOWLEDGMENTS OF FUNDING SOURCES
This work could not have been accomplished without the aid of the following organizations: The NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) & the Arthritis Foundation. We would also like to thank all participants and hospital sites that recruited patients for the CARRA Registry.
The study was supported by the PRCSG Coordinating Center and NIAMS P30 AR076316
OTHER ACKNOWLEDGEMENTS:
We thank Drs. Laura Lewandowski and Sangeeta Sule for critical reading of the manuscript. We thank Cathy Patty-Resk and Guy Eakin for their input during the development of this research project.
We would like to thank the faculty and staff who contributed to the collection of the data from CCHMC
Sheila Angeles Han, Alexei Grom, Michael Henrickson, Jennifer Huggins, Rina Mina, Grant Schulert, Janalee Taylor, Tracy Ting, and Patty Vega-Fernandez.
We would also like to thank all participants and hospital sites that recruited patients for the CARRA Registry. The authors thank the following CARRA Registry site principal investigators, sub- investigators and research coordinators:
N. Abel, K. Abulaban, A. Adams, M. Adams, R. Agbayani, S. Akoghlanian, O. Al Ahmed, E. Allenspach, R. Alperin, M. Alpizar, G. Amarilyo, D. Anastasopoulos, E. Anderson, M. Andrew, K. Ardalan, S. Ardoin, E. Baker, I. Balboni, S. Balevic, L. Ballenger, S. Ballinger, N. Balmuri, F. Barbar-Smiley, L. Barillas-Arias, M. Basiaga, K. Baszis, H. Bell-Brunson, E. Beltz, H. Benham, S. Benseler, W. Bernal, , T. Bigley, B. Binstadt, J. Birmingham, C. Black, M. Blakley, J. Bohnsack, J. Boland, A. Boneparth, S. Bowman, E. Brooks, A. Brown, , M. Buckley, M. Buckley, H. Bukulmez, A. Bullington, D. Bullock, B. Cameron, S. Canna, L. Cannon, S. Carpenter, P. Carper, V. Cartwright, E. Cassidy, L. Cerracchio, E. Chalom, J. Chang, A. Chang-Hoftman, V. Chauhan, P. Chira, Y. Chiu, K. Chundru, H. Clairman, D. Co, K. Collins, A. Confair, H. Conlon, R. Connor, A. Cooper, J. Cooper, S. Cooper, C. Correll, R. Corvalan, T. Cospito, D. Costanzo, R. Cron, M. Curry, A. Dalrymple, A. Davis, C. Davis, C. Davis, T. Davis, D. De Ranieri, J. Dean, F. Dedeoglu, M. DeGuzman, N. Delnay, V. Dempsey, E. DeSantis, T. Dickson, J. Dingle, M. Dionizovik-Dimanovski, B. Donaldson, E. Dorsey, S. Dover, J. Dowling, J. Drew, K. Driest, K. Drummond, Q. Du, K. Duarte, D. Durkee, E. Duverger, J. Dvergsten, A. Eberhard, M. Eckert, K. Ede, C. Edens, C. Edens, Y. Edgerly, M. Elder, S. Fadrhonc, C. Failing, D. Fair, M. Falcon, L. Favier, B. Feldman, I. Ferguson, P. Ferguson, B. Ferreira, R. Ferrucho, K. Fields, T. Finkel, M. Fitzgerald, D. Fleck, C. Fleming, O. Flynn, L. Fogel, E. Fox, M. Fox, L. Franco, M. Freeman, S. Froese, R. Fuhlbrigge, J. Fuller, N. George, T. Gergely, K. Gerhold, D. Gerstbacher, M. Gilbert, M. Gillispie-Taylor, E. Giverc, I. Goh, T. Goldberg, D. Goldsmith, E. Gotschlich, A. Gotte, B. Gottlieb, C. Gracia, T. Graham, S. Grevich, T. Griffin, J. Griswold, M. Guevara, P. Guittar, R. Gurion, M. Guzman, T. Hahn, O. Halyabar, E. Hammelev, M. Hance, E. Hansman, A. Hanson, L. Harel, S. Haro, J. Harris, E. Hartigan, J. Hausmann, A. Hay, K. Hayward, J. Heiart, K. Hekl, L. Henderson, M. Henrickson, A. Hersh, K. Hickey, S. Hillyer, L. Hiraki, M. Hiskey, P. Hobday, C. Hoffart, M. Holland, M. Hollander, S. Hong, M. Horwitz, J. Hsu, A. Huber, J. Huggins, R. Hughes, J. Hui-Yuen, C. Hung, J. Huntington, A. Huttenlocher, M. Ibarra, L. Imundo, C. Inman, S. Iqbal, A. Jackson, S. Jackson, K. James, G. Janow, J. Jaquith, S. Jared, N. Johnson, J. Jones, J. Jones, J. Jones, K. Jones, S. Jones, S. Joshi, L. Jung, C. Justice, A. Justiniano, P. Kahn, N. Karan, K. Kaufman, A. Kemp, E. Kessler, M. Khaleel, U. Khalsa, B. Kienzle, S. Kim, Y. Kimura, M. Kitcharoensakkul, T. Klausmeier, K. Klein, M. Klein-Gitelman, A. Kosikowski, L. Kovalick, J. Kracker, S. Kramer, C. Kremer, J. Lai, B. Lang, S. Lapidus, A. Lasky, D. Latham, E. Lawson, R. Laxer, P. Lee, P. Lee, T. Lee, L. Lentini, M. Lerman, D. Levy, S. Li, S. Lieberman, L. Lim, C. Lin, N. Ling, M. Lingis, M. Lo, , N. Luca, S. Lvovich, M. Ma, C. Mackey, C. Madison, J. Madison, B. Malla, J. Maller, M. Malloy, M. Mannion, C. Manos, L.. Marques, A. Martyniuk, T. Mason, S. Mathus, L. McAllister, B. McCallum, K. McCarthy, K. McConnell, D. McCurdy, P. McCurdy Stokes, S. McGuire, I. McHale, A. McHugh, K. McKibben, A. McMonagle, C. McMullen-Jackson, E. Meidan, E. Mellins, E. Mendoza, R. Mercado, A. Merritt, L. Michalowski, P. Miettunen, M. Miller, E. Mirizio, E. Misajon, M. Mitchell, R. Modica, S. Mohan, K. Moore, L. Moorthy, S. Morgan, E. Morgan Dewitt, S. Morris, C. Moss, T. Moussa, V. Mruk, E. Mulvhihill, E. Muscal, B. Nahal, K. Nanda, L. Nassi, S. Nativ, M. Natter, J. Neely, B. Nelson, L. Newhall, L. Ng, E. Nguyen, J. Nicholas, P. Nigrovic, J. Nocton, E. Oberle, B. Obispo, B. O’Brien, T. O’Brien, M. O’Connor, M. Oliver, J. Olson, K. O’Neil, M. Orlando, R. Oz, E. Pagano, A. Paller, N. Pan, S. Panupattanapong, J. Paredes, A. Parsons, J. Patel, K. Pentakota, P. Pepmueller, T. Pfeiffer, K. Phillippi, K. Phillippi, L. Ponder, R. Pooni, S. Prahalad, S. Pratt, S. Protopapas, M. Punaro, B. Puplava, J. Quach, M. Quinlan-Waters, A. Quintero, C. Rabinovich, S. Radhakrishna, J. Rafko, J. Raisian, A. Rakestraw, E. Ramsay, S. Ramsey, A. Reed, A. Reed, A. Reed, H. Reid, A. Reyes, A. Richmond, M. Riebschleger, S. Ringold, M. Riordan, M. Riskalla, M. Ritter, R. Rivas-Chacon, S. Roberson, A. Robinson, E. Rodela, M. Rodriquez, K. Rojas, T. Ronis, M. Rosenkranz, N. Rosenwasser Raines, B. Rosolowski, H. Rothermel, D. Rothman, E. Roth-Wojcicki, K. Rouster - Stevens, T. Rubinstein, N. Ruth, N. Saad, M. Sabatino, S. Sabbagh, R. Sadun, C. Sandborg, A. Sanni, A. Sarkissian, S. Savani, L. Scalzi, S. Scharnhorst, K. Schikler, H. Schmeling, K. Schmidt, E. Schmitt, K. Schollaert-Fitch, G. Schulert, T. Seay, C. Seper, J. Shalen, R. Sheets, A. Shelly, B. Shen, S. Shenoi, K. Shergill, N. Shiff, J. Shirley, M. Shishov, E. Silverman, N. Singer, V. Sivaraman, J. Sletten, A. Smith, C. Smith, J. Smith, J. Smith, E. Smitherman, C. Snider, J. Soep, M. Son, A. Soybilgic, S. Spence, L. Spiegel, J. Spitznagle, R. Sran, H. Srinivasalu, H. Stapp, J. Stasek, K. Steigerwald, Y. Sterba Rakovchik, S. Stern, A. Stevens, B. Stevens, R. Stevenson, K. Stewart, C. Stingl, J. Stokes, M. Stoll, S. Stoops, J. Strelow, E. Stringer, S. Sule, J. Sumner, R. Sundel, A. Sura, M. Sutter, R. Syed, S. Taber, R. Tal, A. Tambralli, A. Taneja, T. Tanner, S. Tapani, G. Tarshish, S. Tarvin, L. Tate, A. Taxter, J. Taylor, M. Terry, M. Tesher, A. Thatayatikom, B. Thomas, T. Ting, A. Tipp, D. Toib, K. Torok, C. Toruner, H. Tory, M. Toth, E. Treemarcki, S. Tse, V. Tubwell, M. Twilt, S. Uriguen, T. Valcarcel, H. Van Mater, L. Vannoy, C. Varghese, N. Vasquez, K. Vazzana, P. Vega-Fernandes, R. Vehe, K. Veiga, J. Velez, J. Verbsky, N. Volpe, S. Vora, J. Wagner, L. Wagner-Weiner, D. Wahezi, H. Waite, J. Walker, H. Walters, T. Wampler Muskardin, C. Wang, L. Waqar, M. Waterfield, M. Watson, A. Watts, B. Waugaman, P. Weiser, J. Weiss, P. Weiss, E. Wershba, A. White, C. Williams, A. Wise, J. Woo, L. Woolnough, T. Wright, E. Wu, A. Yalcindag, M. Yee, E. Yen, R. Yeung, K. Yomogida, Q. Yu, R. Zapata, A. Zartoshti, A. Zeft, R. Zeft, L. Zemel, Y. Zhang, Y. Zhao, A. Zhu, C. Zic
REFERENCES
- 1.Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum 2013;65(10):2499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Care Res (Hoboken) 2019;71(6):703–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Rheumatol 2019;71(6):846–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best pharmaceuticals for children act of 2007. Food and Drug Administration Amendments Act (FDAAA), Title V [Google Scholar]
- 5.Pediatric Research Equity Act. 2003:Pub L No. 108–55
- 6.Field MJ, Boat TF, Institute of Medicine (U.S.), National Research Council (U.S.). Safe and effective medicines for children : pediatric studies conducted under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act Washington, D.C.: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 7.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763–9. [DOI] [PubMed] [Google Scholar]
- 8.Beukelman T, Kimura Y, Ilowite NT, Mieszkalski K, Natter MD, Burrell G, et al. The new Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry: design, rationale, and characteristics of patients enrolled in the first 12 months. Pediatr Rheumatol Online J 2017;15(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31(2):390–2. [PubMed] [Google Scholar]
- 10.Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Clinically inactive disease in a cohort of children with new-onset polyarticular juvenile idiopathic arthritis treated with early aggressive therapy: time to achievement, total duration, and predictors. J Rheumatol. 2014;41(6):1163–70. [DOI] [PubMed] [Google Scholar]
- 11.Ringold S, Weiss PF, Beukelman T, Dewitt EM, Ilowite NT, Kimura Y, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Care Res (Hoboken) 2013;65(10):1551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2007;56(9):3096–106. [DOI] [PubMed] [Google Scholar]
- 13.Urien S, Bardin C, Bader-Meunier B, Mouy R, Compeyrot-Lacassagne S, Foissac F, et al. Anakinra pharmacokinetics in children and adolescents with systemic-onset juvenile idiopathic arthritis and autoinflammatory syndromes. BMC Pharmacol Toxicol 2013;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


