Abstract
Background
With expansion of antiretroviral therapy (ART) programs, transmission rates are low but new infant infections still occur. We investigated predictors of pre-ART viral load (VL) and CD4+ T-cell counts and percentages in infants diagnosed with HIV at birth in a setting with high coverage of maternal ART and infant prophylaxis.
Methods
As part of an early treatment study, 97 infants with confirmed HIV-infection were identified at a hospital in Johannesburg, South Africa. Infant VL and CD4+ T-cell parameters were measured before ART initiation. Data were collected on maternal characteristics, including VL, CD4+ T-cell counts and ART, and infant characteristics, including sex, birth weight, and mode of delivery.
Results
Pre-ART, median infant VL was 28,405 copies/ml (IQR: 2,515 – 218,150), CD4+ T-cell count 1914 cells/mm3 (IQR: 1474 – 2639) and percentage 40.8% (IQR: 32.2 – 51.2). Most (80.4%) infants were born to mothers who received ART during pregnancy and 97.9% of infants received daily nevirapine prophylaxis until ART initiation at median of 2 days of age (IQR: 1 − 7). Infant pre-ART VL was more likely to be ≥1000 copies/ml when their mothers had VL ≥1000 copies/ml (Odds Ratio [OR] 6.88; 95% CI:2.32, 20.41) and was higher in boys than girls (OR 3.29; 95% CI: 1.07, 9.95). Lower maternal CD4+ T-cell count (<350 cells/mm3) was associated with lower infant CD4+ T-cell count (<1500 cells/mm3) (OR 3.59; 95% CI: 1.24, 10.43).
Conclusions
Pre-ART VL and CD4+ T-cell parameters of intrauterine-infected infants were associated with VL and CD4+ T-cell counts of their mothers. Maternal ART during pregnancy may begin treatment of intrauterine infection and/or may mask the severity of disease in infected infants identified in the current era with high maternal ART coverage.
Keywords: HIV, Maternal, Pediatric, Viral load, Antiretroviral therapy
Introduction
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV, regardless of their CD4+ T-cell count or disease stage, to optimize maternal health and reduce the risk of vertical transmission.1 A major goal of ART is to reduce maternal HIV-1 RNA viral load in the blood to undetectable levels, which in turn reduces the risk of perinatal transmission.2 ART should be initiated as early in pregnancy as possible, or preferably before, and should be continued through pregnancy, breastfeeding and beyond. Expansion of programs to improve access to timely and lifelong ART has dramatically reduced rates of vertical transmission globally but new infant HIV infections continue to occur. In the Southern and Eastern regions of Africa, new infant infections are due to a range of factors, including incident infections in pregnant women, failure to initiate or maintain maternal ART, and lack of retention in care through pregnancy and breastfeeding.3
While it is clear that maternal ART reduces transmission of HIV, less is known about the effect of maternal ART on early viral load dynamics in infants who acquire HIV in utero. Here we describe the pre-ART profile of viral load and CD4+ T-cell parameters in a cohort of intrauterine-infected infants identified as part of a birth testing program in Johannesburg, South Africa. South Africa has been able to achieve excellent coverage (87%) of pregnancies with maternal ART leading to low rates of transmission (<5%).4,5 In this context, we investigated associations between markers of maternal disease severity and maternal ART and pre-ART disease severity in infants. We hypothesized that maternal ART during pregnancy may begin treatment of infant HIV infection in utero resulting in a more favorable pre-ART profile among those infants who acquire HIV infection.
Methods
Study population
Through a birth testing program that included both laboratory-based and point-of-care diagnostic tests for HIV at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa, we identified 108 intrauterine-infected infants between June 2014 and August 2018 and enrolled them into an early treatment study.6 The protocols were approved by the Institutional Review Boards of Columbia University in New York, NY, USA and the University of the Witwatersrand in Johannesburg, South Africa. Mothers or legal guardians signed written informed consent for their own and their infant’s participation.
Measurements
Prior to initiation of ART, usually on the same day that ART was initiated, a quantitative HIV-1 RNA (viral load) was measured using the COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 test, version 2.0 (Roche Molecular Systems, Inc., Branchburg, NJ). Also measured were neonatal pre-ART CD4+ T-cell counts and percentages (TruCount Method, BD Biosciences, Germany). Maternal HIV-1 RNA viral loads and CD4+ T-cell counts were measured during late pregnancy, or soon after delivery. Information on maternal demographics and ART during pregnancy was collected, as well as additional characteristics pertaining to the infants (sex, birth weight, gestational age, mode of delivery, feeding methods and antiretroviral prophylaxis).
Statistical analysis
We included in this analysis 97 mother-infant pairs who had both a maternal HIV-1 RNA viral load result and an infant pre-ART HIV-1 RNA viral load result collected in the first 28 days of life. Descriptive statistics, including means and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables or proportions for categorical variables, were used to describe the profile of the mother-infant pairs. Continuous variables of interest were dichotomized at clinically-relevant values (e.g. 350 cells/mm3 for maternal CD4+ T-cell count and 1000 copies/ml for viral load). Histograms and kernel density estimates were used to graphically depict the distribution of neonatal pre-treatment viral load (on the log10 scale) and CD4+ T-cell percentage and count. Logistic regression models were used to evaluate associations between maternal and infant characteristics (e.g. infant sex, mode of delivery, preterm birth, birthweight, maternal viral load, maternal CD4+ T-cell count, maternal ART) and neonatal pre-ART outcomes including viral load (≥1000 copies/mL vs. <1000 copies/mL), CD4+ T-cell percentage (<30% vs. ≥30%), and CD4+ T-cell count (<1500 vs. ≥1500 cells/mm3). Associations between two continuous variables were graphically depicted using scatterplots. Spearman correlation coefficients were used to assess correlations on a continuous scale. Differences in neonatal pre-ART viral load and CD4+ T-cell values between groups were illustrated by box and whisker plots and significance determined by Wilcoxon rank-sum tests. All analyses were conducted using SAS software version 9.4 (Cary, North Carolina, USA).
Results
Characteristics of the 97 mother-infant pairs are presented in Supplemental Digital Content 1 (Table). Mean maternal age was 27.9 years. Overall, 15.5% of mothers started ART prior to pregnancy, 64.9% initiated ART while pregnant (36.1% <12 weeks, 27.8% ≥12 weeks and 1% at an unknown time), and 19.6% received no ART up until delivery. Of those on ART, 74 (94.9%) were on an efavirenz-based regimen, 3 (3.8%) on a ritonavir-boosted lopinavir-based regimen, and 1 (1.3%) on an unknown regimen. Of the 97 infants, 51.5% were female, mean birthweight was 2840 ± 547 grams (range: 905 – 4150) and 86.6% were born term (≥37 weeks of gestation). Three-quarters of infants (75.3%) were born by vaginal delivery and 78.4% initiated any breastfeeding.
Maternal viral load was measured at a median infant age of 1 day (IQR: 1 – 5) with a median of 38,459 copies/ml (IQR: 1,002 – 121,000) and 24.7% of mothers had a viral load <1,000 copies/ml (5.2% <50 copies/ml). Maternal CD4+ T-cell count was measured at a median infant age of 1 day (IQR: 1 – 4) with a median of 329 cells/mm3 (IQR: 207 ─ 569) and 53.6% had a CD4+ T-cell count <350 cells/mm3 (22.7% <200 cells/mm3). Pre-ART infant viral load was measured at a median infant age of 1 day (IQR: 1 – 6). Most newborns (97.9%, n=74) received nevirapine prophylaxis from birth, prior to the pre-ART measurement, until transition to ART. ART was started in this cohort at a median age of 2 days (IQR: 1 −7, max 104 days).
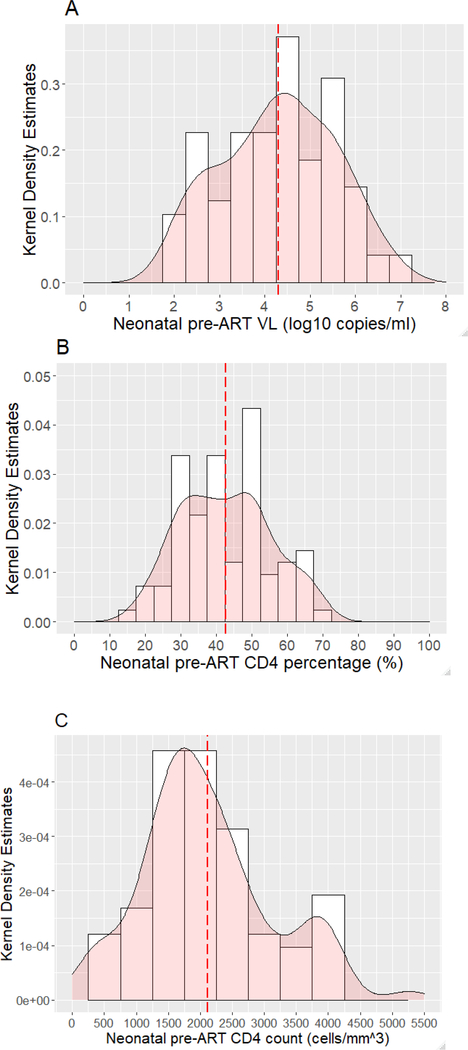
The distribution of neonatal pre-ART viral load (log10 copies/ml) is shown in Figure 1A. The median neonatal pre-ART viral load was 28,405 copies/ml (IQR: 2,515 – 218,150). Of the 97 infants, 19.6% had a pre-ART viral load <1,000 copies/ml (none were below the detection threshold of the assay), 19.6% 1,000–9,999 copies/ml, 28.9% 10,000–99,999 copies/ml, 22.7% 100,000–999,999 copies/ml, and 9.3% >1,000,000 copies/ml. The median neonatal pre-ART CD4+ T-cell percentage was 40.8% (IQR: 32.2 – 51.2) (Figure 1B) and CD4+ T-cell count was 1914 cells/mm3 (IQR: 1474 – 2639) (Figure 1C). Of 83 infants who had a pre-ART CD4+ T-cell available, 7.2% had a CD4+ T-cell % <25, 10.8% 25–29.9, 14.5% 30–34.9 and 67.5% ≥35; 26.5% had a CD4+ T-cell count <1500 cells/mm3.
Figure 1: Distribution neonatal pre-antiretroviral therapy (ART) viral load (VL) (log10 copies/ml), CD4+ T-cell percentage, and CD4+ T-cell count.
Kernel density estimates are shown on the Y-axis and neonatal pre-ART VL (A), CD4+ T-cell percentage (B) and CD4+ T-cell count (C) on the x-axis. Dashed line indicates the mean.
We evaluated predictors of neonatal pre-ART viral load ≥1000 copies/ml (Table 1). In univariable models, boys had a higher odds of having a neonatal pre-ART viral load ≥1000 copies/ml than girls (OR: 3.27, 95% CI: 1.07 – 9.95), infants whose mothers had a viral load ≥1000 copies/ml were more likely to have viral load above this level themselves (OR: 6.88, 95% CI: 2.32 – 20.41) and breastfed infants had higher odds of pre-ART viral load ≥1000 copies/ml (OR: 3.64 (1.23 − 10.80). In multivariable models, only male sex and maternal viral load ≥1000 copies/ml remained significantly associated with a higher odds of a neonatal pre-ART viral load ≥1000 copies/ml (Table 1). The initial univariable association between breastfeeding and neonatal pre-ART viral load ≥1000 copies/ml appeared to be due to confounding. Maternal viral load was higher among breastfeeding mothers with 81.6% having viral load >1000 copies/ml compared to 52.4% among mothers who did not breastfeed (p=0.0097). All mothers who avoided all breastfeeding had received ART compared to 75% of mothers who initiated some breastfeeding (p=0.002). Maternal viral load differences between breastfeeding and non-breastfeeding mothers may explain the association, and, after adjustment, no significant association between neonatal pre-ART viral load and breastfeeding was observed.
Table 1:
Odds ratios of neonatal pre-treatment viral load ≥1000 copies/ml vs. <1000 copies/ml associated with infant and maternal characteristics
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Characteristic | Categories | OR (95% CI) | P | OR (95% CI) | P |
| Sex | Boys vs. Girls | 3.27 (1.07 − 9.95) | 0.037 | 2.96 (0.91 − 9.66) | 0.07 |
| Mode of delivery | Vaginal vs. Cesarean | 2.09 (0.71 − 6.14) | 0.18 | ||
| Preterm status | Preterm vs. Not preterm | 1.40 (0.28 − 6.90) | 0.68 | ||
| Low birthweight | <2500 vs. ≥2500 grams | 1.27 (0.33 − 4.93) | 0.73 | ||
| Maternal viral load | ≥1000 vs. <1000 copies/ml | 6.88 (2.32 −20.41) | 0.0005 | 6.46 (2.12 − 19.69) | 0.001 |
| Maternal ART | None vs. Any | 0.89 (0.26 − 3.08) | 0.86 | ||
| Maternal CD4+ T-cell count | <350 vs. ≥350 cells/mm3 | 0.81 (0.29 − 2.22) | 0.68 | ||
| Breastfeeding | Ever vs. Never | 3.64 (1.23 − 10.80) | 0.02 | ||
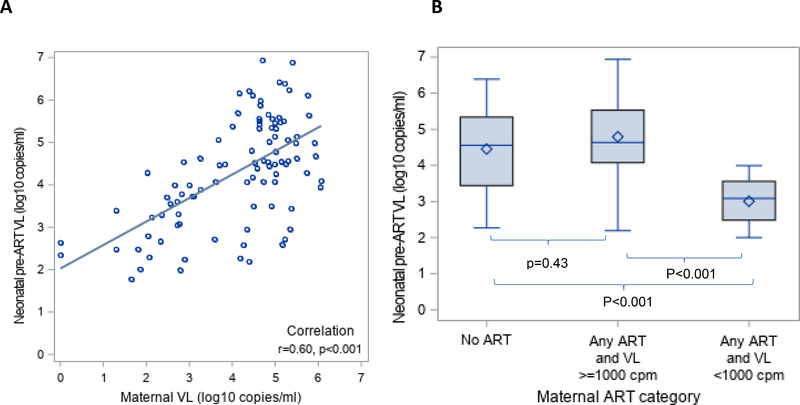
Results were similar if we examined neonatal pre-ART viral load on a continuous scale. Median neonatal pre-ART viral load was higher (36,456 copies/ml) in boys compared to girls (10,335 copies/ml (p=0.0098). Median neonatal pre-ART viral load was 43,300 copies/ml in those whose mothers had viral load ≥1000 copies/mL compared to 1,172 copies/ml in those with a maternal viral load <1000 copies/mL (p<0.001). Results were also similar if maternal viral load was included as a continuous variable. As shown in Figure 2A, maternal viral load was positively and significantly correlated with neonatal pre-ART viral load (r=0.60, p<0.01).
Figure 2: Relationship between maternal viral load (VL) and antiretroviral (ART) category with neonatal pre-ART VL.
(A) Scatterplot of maternal VL (log10 copies/ml) and neonatal pre-ART VL (log10 copies/ml). (B) Box and whisker plot of neonatal pre-ART VL by maternal ART/VL category (No ART, any ART and VL ≥1000 copies/ml, any ART and VL <1000 copies/ml).
Maternal viral load was strongly correlated with whether or not the mother was on ART. In mothers not on ART, the median viral load was 67,800 copies/ml (IQR: 15,100 – 167,102 with 89.5% ≥1000 copies/ml) compared to 30,365 copies/ml (IQR: 608 – 101,765, with 71.8% ≥1000 copies/ml) in mothers on ART. Therefore, we created a categorical variable combining maternal ART and maternal viral load into three categories: 1) No ART, 2) Any maternal ART and maternal viral load ≥1000 copies/ml, and 3) Any maternal ART and maternal viral load <1000 copies/ml. As shown in Figure 2B, neonatal pre-ART viral load was significantly lower (median 875 copies/ml, IQR: 220 − 2,515) among those whose mothers received any ART and had a viral load <1,000 copies/mL compared to those whose mothers received any ART and had a viral load ≥1,000 copies/mL (median 43,225 copies/mL, IQR: 11,941 – 340,276) as well as compared to those whose mothers received no ART (median 36,180 copies/ml, IQR: 2,765 – 218,150), both p<0.001.
As we did with pre-ART viral load, we evaluated whether maternal and infant characteristics were associated with a neonatal pre-ART CD4+ T-cell count <1500 cells/mm3 or neonatal pre-ART CD4+ T-cell percentage <30%. The odds of having a neonatal pre-ART CD4+ T-cell count <1500 cells/mm3 was higher in mothers who had a CD4+ T-cell count <350 cells/mm3 vs. those with a CD4+ T-cell count ≥350 cells/mm3 (see table, Supplemental Digital Content 2). Median neonatal pre-ART CD4+ T-cell count was higher in mothers who had a CD4+ T-cell count ≥350 cells/mm3 than mothers who had a CD4+ T-cell count <350 cells/mm3 (2194 vs. 1827 cells/mm3, p=0.055). Associations with pre-ART CD4+ T-cell percentage were in the same direction but not significant. For example, median neonatal pre-ART CD4+ T-cell percentage was also higher but not significant (47.9 vs. 39.2%, p=0.12) in mothers with high vs low CD4+ T-cell counts respectively. On a continuous scale, maternal CD4+ T-cell count was weakly positively correlated with neonatal pre-ART CD4+ T-cell percentage and CD4+ T-cell count (see figure, Supplemental Digital Content 3A and 3B).
In a comparison of neonatal pre-ART CD4+ T-cell percentage to neonatal pre-ART viral load, a negative linear correlation was observed, with viral load decreasing with increasing CD4+ T-cell percentage and count (see figure, Supplemental Digital Content 4). This relationship was observed in all three maternal ART and viral load groups.
Discussion
We observed strong associations between markers of maternal health and infant pre-ART prognostic markers (viral load and CD4+ T-cell parameters) in a cohort of intrauterine infected infants in South Africa. Mothers with lower viral loads close to delivery were more likely to deliver infants with lower pre-ART viral loads; and mothers with higher CD4+ T-cell counts were more likely to deliver infants with higher CD4+ T-cell counts. The associations we observed between markers of maternal health and neonatal pre-ART viral load and immune status are consistent with studies which have shown associations between maternal health status and disease progression in children living with HIV.7–9 In a large multi-center study in the US prior to the widespread introduction of effective ART, HIV-infected children born to mothers with more advanced disease (low CD4+ T-cell counts and high viral load) progressed more rapidly to AIDS or death than children born to mothers with less advanced disease.7 Similarly, maternal viral load was found to correlate with peak viral load in untreated perinatally-infected infants in Kenya.8 An international meta-analysis combining information from eight multicenter studies found that maternal HIV-1 RNA levels at or close to the time of delivery correlated with the early levels of viremia attained in the infant after the first month of life, and infant disease progression (stage C disease or death).9 Our findings suggest a possible mechanism explaining the link between maternal and child HIV disease progression.
Neonatal pre-ART viral loads were reasonably low in our cohort and few had evidence of severe immunosuppression based on their CD4+ T-cell parameters. Prior studies of the natural history of pediatric HIV infection have described very high pre-ART viral load measurements in infants.10,11 In the same setting over the past decade, we have observed pre-ART viral loads several log higher than we observed here in cohorts of infants with HIV presenting for care.12,13 Interestingly, this profile of unexpectedly low pre-ART viral load has also been reported in two recent cohorts of HIV-infected infants i.e. infants under the age of 28 days. The median pre-ART viral load in a cohort of 44 infants from the UK, Spain, Italy, and Thailand was 17,000 copies/ml (IQR: 962 − 174,882).14 The median pre-ART viral load in a cohort of 40 infants from Botswana was 11,220 copies/ml (IQR: 617 − 72,444) and median CD4+ T-cell percentage of 50 (IQR: 38 − 56).15 Importantly, South Africa’s early infant diagnosis program has also revealed a reduction in the median baseline viral load among HIV-infected infants under a year of age from a high of 6.1 log (n=30) in 2010 to 4.3 log (n=221) in 2016.16
The primary driver of maternal health status is access to ART. The likely explanation for the comparatively lower viral loads and higher CD4+ T-cell counts and percentages in infants observed in our cohort, as well as among the other recent cohorts of early-treated infants, is the expansion of programs that now provide universal ART. We did not have sufficient variability in the use of infant prophylaxis to examine the separate role of prophylaxis in contributing to the improved pre-ART parameters that we observed. Since almost all received prophylaxis, the associations we observed between maternal health and neonatal parameters are in the context of near universal prophylaxis.
In this cohort of infants with intrauterine infection, boys had a higher pre-treatment viral load compared to girls. This is consistent with early results from the European Collaborative Study that reported in 118 perinatally-infected children after 4 years the RNA load was consistently 0.25–0.5 log10 lower for girls than boys.17 Higher CD4+ T-cell counts and percentages in HIV-infected girls than boys have also been reported18 as well as a strong over-representation of females among elite controllers.19 Some have reported higher rates of intrauterine transmission in girls than in boys20 but we observed a roughly equal sex ratio in our cohort of intrauterine infected infants.
This study has several limitations. We report epidemiologic associations and confounders may explain the observed associations. Although it is encouraging that the newborn pre-ART parameters are comparatively better than expected, we and others have observed that response to ART in early treated infants is quite variable.6,15,21 Although prior studies have shown strong correlations between pre-ART viral load and CD4+ T-cell parameters,8,22 it does not necessarily follow, in the context of birth diagnosis and high rates of maternal ART, that these parameters have the same clinical significance. We speculate that maternal ART may mask HIV-related effects in infants with intrauterine infection rendering the usual markers of prognosis less informative. Other limitations include the small sample size from a single site, lack of exact coincidence in time of the measurement of maternal and infant parameters and inability to confirm maternal adherence with ART. Furthermore, our cohort which was derived from a birth testing program and may not be comparable to earlier cohorts that generally over-represented sicker children diagnosed at older ages.
In conclusion, we found low pre-ART viral loads in infants with intrauterine HIV infection with only a small proportion having severe immunosuppression prior to starting ART. We hypothesize that in the current era where mothers receive ART during pregnancy, leading to lower viral loads and better immune parameters, an improved pre-ART profile of infected newborns is observed. We speculate that effective maternal ART in pregnancy may begin treatment of infants with in utero HIV infection. Whether the maintenance of low levels of maternal viremia may provide an added beneficial effect on infant disease progression is still unknown but efforts to ensure maternal virologic suppression to protect maternal health and reduce vertical transmission should remain a priority.
Supplementary Material
Supplemental Digital Content 1: Characteristics of 97 intrauterine-infected infants and their mothers enrolled at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa
Supplemental Digital Content 2: Odds ratios of neonatal pre-treatment CD4+ T-cell count <1500 vs. ≥1500 cells/mm3 associated with infant and maternal characteristics
Supplemental Digital Content 3: Relationship between maternal CD4+ T-cell count and neonatal pre-ART CD4+ T-cell counts and percentages. (A) Scatterplot of maternal CD4+ T-cell count and neonatal pre-ART CD4+ T-cell percentage. (B) Scatter plot of maternal CD4+ T-cell count and neonatal pre-ART CD4+ T-cell count.
Supplemental Digital Content 4: Relationship between neonatal pre-ART CD4+ T-cell percentage and count and neonatal pre-ART viral load (VL; log10 copies/ml), stratified by maternal ART/VL category. (A) Scatterplots of neonatal pre-ART CD4+ T-cell percentage (%) and neonatal pre-ART VL (copies/ml), by maternal ART/VL category (No ART, any ART and VL ≥1000 copies/ml, any ART and VL <1000 copies/ml). (B) Scatterplots of neonatal pre-ART CD4+ T-cell count (cells/mm3) and neonatal pre-ART VL (copies/ml), by maternal ART/VL category (No ART, any ART and VL ≥1000 copies/ml, any ART and VL <1000 copies/ml).
Acknowledgements
We acknowledge the infants and families who participated in the study as well as the study team. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health (U01HD080441), USAID/PEPFAR, the South African National HIV Programme and South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection 2016. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/. [PubMed]
- 2.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. The New England journal of medicine 1999;341:385–93. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF UNAIDS and WHO. Key considerations for programming and prioritization. Going the ‘Last Mile’ to EMTCT: A road map for ending the HIV epidemic in children. 2020;http://www.childrenandaids.org/sites/default/files/2020-02/Last-Mile-To-EMTCT_WhitePaper_UNICEF2020.pdf (accessed 9 May 2020).
- 4.https://www.unaids.org/en/regionscountries/countries/southafrica. (accessed 2 March 2020).
- 5.UNAIDS: AIDSInfo. at https://aidsinfo.unaids.org/.)
- 6.Kuhn L, Strehlau R, Shiau S, et al. Early antiretroviral treatment of infants to attain HIV remission. EClinicalMedicine 2020;18:100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams EJ, Wiener J, Carter R, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. AIDS (London, England) 2003;17:867–77. [DOI] [PubMed] [Google Scholar]
- 8.Obimbo EM, Wamalwa D, Richardson B, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. Journal of acquired immune deficiency syndromes (1999) 2009;51:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannidis JP, Tatsioni A, Abrams EJ, et al. Maternal viral load and rate of disease progression among vertically HIV-1-infected children: an international meta-analysis. AIDS (London, England) 2004;18:99–108. [DOI] [PubMed] [Google Scholar]
- 10.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunological reviews 2013;254:143–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. The New England journal of medicine 1997;336:1337–42. [DOI] [PubMed] [Google Scholar]
- 12.Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. The Journal of infectious diseases 2010;201:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrams EJ, Woldesenbet S, Soares Silva J, et al. Despite Access to Antiretrovirals for Prevention and Treatment, High Rates of Mortality Persist Among HIV-infected Infants and Young Children. The Pediatric infectious disease journal 2017;36:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez-Rodriguez S, Tagarro A, Palma P, et al. Reduced Time to Suppression Among Neonates With HIV Initiating Antiretroviral Therapy Within 7 Days After Birth. Journal of acquired immune deficiency syndromes (1999) 2019;82:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maswabi K, Ajibola G, Bennett K, et al. Safety and Efficacy of Starting Antiretroviral Therapy in the First Week of Life. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazanderani AH, Moyo F, Kufa T, Sherman GG. Brief Report: Declining Baseline Viremia and Escalating Discordant HIV-1 Confirmatory Results Within South Africa’s Early Infant Diagnosis Program, 2010–2016. Journal of acquired immune deficiency syndromes (1999) 2018;77:212–6. [DOI] [PubMed] [Google Scholar]
- 17.European Collaborative Study. Level and pattern of HIV-1-RNA viral load over age: differences between girls and boys? AIDS (London, England) 2002;16:97–104. [DOI] [PubMed] [Google Scholar]
- 18.Mori M, Adland E, Paioni P, et al. Sex Differences in Antiretroviral Therapy Initiation in Pediatric HIV Infection. PloS one 2015;10:e0131591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieira VA, Zuidewind P, Muenchhoff M, et al. Strong sex bias in elite control of paediatric HIV infection. AIDS (London, England) 2019;33:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taha TE, Nour S, Kumwenda NI, et al. Gender differences in perinatal HIV acquisition among African infants. Pediatrics 2005;115:e167–72. [DOI] [PubMed] [Google Scholar]
- 21.Frigati L, Wynberg E, Maritz J, Holgate S, Cotton MF, Rabie H. Antiretroviral Treatment Initiated in the First Month of Life. The Pediatric infectious disease journal 2017;36:584–7. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. Jama 1998;279:756–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Characteristics of 97 intrauterine-infected infants and their mothers enrolled at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa
Supplemental Digital Content 2: Odds ratios of neonatal pre-treatment CD4+ T-cell count <1500 vs. ≥1500 cells/mm3 associated with infant and maternal characteristics
Supplemental Digital Content 3: Relationship between maternal CD4+ T-cell count and neonatal pre-ART CD4+ T-cell counts and percentages. (A) Scatterplot of maternal CD4+ T-cell count and neonatal pre-ART CD4+ T-cell percentage. (B) Scatter plot of maternal CD4+ T-cell count and neonatal pre-ART CD4+ T-cell count.
Supplemental Digital Content 4: Relationship between neonatal pre-ART CD4+ T-cell percentage and count and neonatal pre-ART viral load (VL; log10 copies/ml), stratified by maternal ART/VL category. (A) Scatterplots of neonatal pre-ART CD4+ T-cell percentage (%) and neonatal pre-ART VL (copies/ml), by maternal ART/VL category (No ART, any ART and VL ≥1000 copies/ml, any ART and VL <1000 copies/ml). (B) Scatterplots of neonatal pre-ART CD4+ T-cell count (cells/mm3) and neonatal pre-ART VL (copies/ml), by maternal ART/VL category (No ART, any ART and VL ≥1000 copies/ml, any ART and VL <1000 copies/ml).