Abstract
Introduction:
During the anhepatic phase of liver transplantation (LT), fibrinolytic activity increases, since the liver clears tissue plasminogen activator (tPA). We hypothesize that patients who fail to reduce fibrinolytic activity following graft reperfusion will have an increased rate of early allograft dysfunction (EAD).
Methods:
Assessment of fibrinolysis in liver transplant recipients was quantified with thrombelastography (TEG) LY30. Changes in LY30 were assessed after graft reperfusion. The 30-minute post-reperfusion LY30 was subtracted from the anhepatic LY30 quantifying fibrinolytic changes (delta-LY30).
Results:
Seventy-three primary LT patients were included in the analysis. Receiver operating characteristic curve (ROC) analysis identified an inflection point of delta-LY30 −5.3% as a risk factor for EAD. EAD occurred in 44% of these patients compared to 5% in high delta-LY30 (p=0.002).
Conclusion:
LT recipients that develop hyperfibrinolysis who fail to reduce fibrinolytic activity 30 minutes after graft reperfusion had an EAD rate 8-fold higher than patients who had a large reduction in LY30 following reperfusion.
Keywords: Liver transplantation, fibrinolysis, early allograft dysfunction, tissue plasminogen activator
Introduction
Liver transplantation (LT) is the only effective treatment for chronic hepatic failure and end-stage liver disease (ESLD). The waitlist for liver transplant exceeds 17,000 patients in the United States1 resulting in thousands of preventable deaths per year due to lack of available donor organs2. ESLD is estimated to increase by 168% over the next 15 years further exacerbating the donor shortage 3. The deceased donor pool (general population) is estimated to become older and have more comorbidities resulting in lower quality organs that are at risk factor for early graft failure after transplantation 4. The prospect of performing LT with lower quality organs exacerbates the challenges to be faced by the transplant community. Therefore, interventions to improve graft function are essential for the sustainability of LT in the United States.
Early allograft dysfunction (EAD) occurs in roughly 25% of recipients5,6 and is associated with up to a 7-fold increased risk of early graft loss and 10-fold risk of mortality after transplant7. EAD has been proposed as an appealing target to improve graft outcomes8 yet specific mechanisms driving this process remains unclear8,9. Clinically, slow graft function can be appreciated soon after reperfusion of the liver, while the time frame for diagnosis of EAD with objective laboratory data is commonly calculated 7 days after liver transplantation7. An alternative approach to detect EAD would include a functional assay at the time of liver reperfusion, which can augment clinical judgment. It has been well appreciated since the origins of liver transplantation that recipients often develop a hyperfibrinolytic state when the native liver is removed10. However, following reperfusion the majority of patients correct their fibrinolytic state11. This is likely due to the rapid clearance of t-PA by the liver, that has a half-life of 5 minutes in humans due to multiple hepatic endothelial receptors12. Therefore, the measurement of reduction of fibrinolytic activity following graft reperfusion could represent an early marker for graft function in liver transplantation, and provide an opportunity for intraoperative detection of EAD to aid in patient management including future therapeutic interventions.
Recently there have been efforts to standardized the nomenclature of the different fibrinolytic changes following severe injury13. The activation of fibrinolysis followed by inhibition, a term called fibrinolysis shutdown, has been researched in trauma for the past 50 years14-16. Recently, fibrinolysis shutdown in transplant surgery has been associated with adverse outcomes17 and previously been documented to occur using viscoelastic testing11. We therefore, had an interest in evaluating the timing of fibrinolysis shutdown during liver transplantation, and if the timing and magnitude of fibrinolysis shutdown had an impact on early graft function. We hypothesize that liver transplant recipients that fail to reduce hyperfibrinolysis during early graft reperfusion will have a high rate of EAD.
Methods
Patient population
Liver transplant recipients were pre-operatively enrolled in a Colorado Multi-Institutional Review Board study to prospectively collect blood samples for the first 24 hours following surgery. All patients received a LT at the University of Colorado Hospital; which averages – 130 liver transplants a year. Enrollment criteria were adults (>18 years) and deceased donor liver transplant recipients. Patients that received a living liver graft were excluded from the analysis because they only received half of a liver and with less liver parenchyma would have an anticipated difference in response to reducing fibrinolysis due to organ volume rather than function during reperfusion. Donation after cardiac death donors were also excluded as our protocol for this patient population includes the use of tPA during early reperfusion to breakdown presumed microthrombi18. Patient demographics were recorded; including age, sex, co-morbidities, and model for end-stage liver disease (MELD) calculated on laboratory values the day of surgery.
Blood Samples for Viscoelastic Testing
Blood was collected and stored in a 3.5-mL tubes containing 3.2% citrate, and immediately transferred for analysis via a trained professional research assistant. All viscoelastic assays were completed within 2 hours of blood draw. Serial blood samples were obtained before the surgical incision (pre-op), during the native hepatectomy (after hepatic artery ligation), during the anhepatic phase of surgery (15 minutes after removal of native liver from recipient), 30 minutes after reperfusion (determined as the time from unclamping the portal vein), 2 hours after reperfusion and on postoperative day 1 (POD1). These TEG samples were all assayed in the research laboratory and results were blinded to the attending anesthesiologists and transplant surgeons.
Thrombelastography
Blood samples were assayed with the TEG 5000 Hemostatic Analyzer (Haemonetics, Braintree, MA) according to manufacturer’s recommendations. The following measurements were recorded: R time (minutes), angle α, degrees), maximum amplitude (MA, mm), and lysis 30 minutes after MA (LY30, %). Samples were run native, without any activator (n-TEG). Hyperfibrinolysis was defined as an LY30 of > 3% based on the existing definition in the literature19.
Outcomes
The primary outcome of interest was early allograft dysfunction. This was determined using the previously validated definition7; transaminases greater than 2,000 post-operative day 1-7, and INR greater than 1.6 on post-operative day 7, and a bilirubin greater than 10 on post-operative day 7. Secondary outcomes of interest included blood product utilization during the perioperative period, primary non-function of the liver, and overall mortality since follow up.
Statistical Analysis
Statistical analysis was performed using SPSS 23 software (Microsoft, Armonk, NY). Normally distributed data were described as mean and standard deviation and non-normally distributed data were described as the median value with the 25th to 75th percentile values. The 30 minutes reperfusion LY30 was subtracted from the anhepatic LY30 quantifying fibrinolytic changes (delta-LY30). A receiver operating characteristic curve (ROC) was used to define the threshold of delta LY30 for predicting EAD using a Youden index. Patients with a low delta-LY30 based on this cut point were contrasted to patients with a delta LY30 higher than this point and stratified development of hyperfibrinolysis during surgery. Outcomes were contrasted between patient cohorts with a chi square test for categorical outcomes and Mann Whitney U test for continuous variables.
Results
Demographics
Eighty-five patients were enrolled during this study period; for this paper we excluded 8 liver transplant recipients that received organs from living donors and 4 recipients that received organs from donors after cardiac death. The remaining Seventy-three brain dead liver transplant recipients were included in the analysis. The median lab MELD on the day of LT was 22 (14-31). The most common indications for transplantation were cirrhosis secondary to viral hepatitis (32%) followed by alcohol (29%) and nonalcoholic steatohepatitis (11%). Hepatocellular carcinoma (HCC) was present in the final pathology of the native liver in 23% of LT recipients. EAD occurred in 26% of patients, and hyperfibrinolysis during the anhepatic phase of surgery was prevalent in 56% of patients.
Delta Fibrinolysis Following Reperfusion
Of the patients that developed hyperfibrinolysis, the delta-LY30 ROC area under to curve for predicting EAD was 0.749 (95%CI 0.599-0.899 p=0.011). The Youden index was identified to be a delta-LY30 of −5.5%. In the overall patient cohort, 44% of patients failed to develop hyperfibrinolysis during the anhepatic phase of surgery, 31% of patients had a high negative delta-LY30 following reperfusion, and 25% had a low negative delta-LY30. The cohort grouping is depicted in figure 1.
Figure 1:
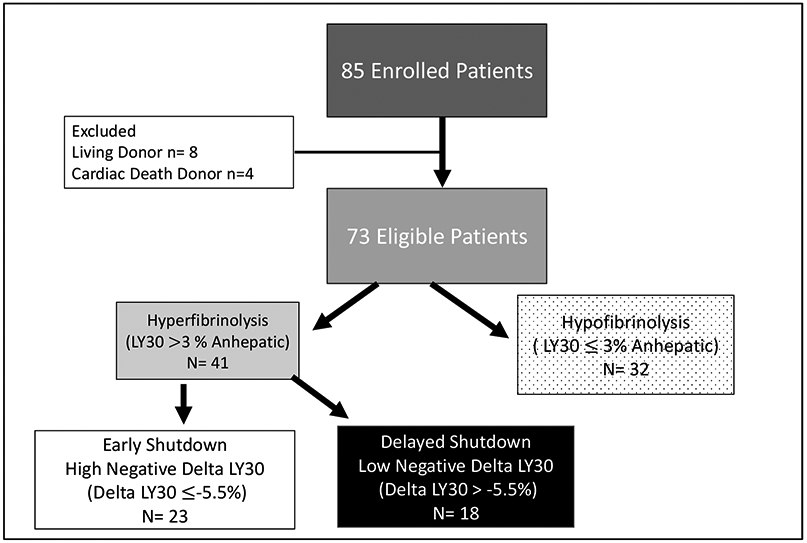
Strengthening the Reporting of Observational studies in Epidemiology (STROBE) Diagram
The temporal trends of LY30 are depicted in Figure 1, which demonstrate differences in LY30 during the anhepatic phase of surgery. The high negative delta-LY30 group having the highest LY30 (p<0.001) during the anhepatic phase or surgery, whereas during the 30-minute reperfusion the low negative delta LY30 had the highest LY30 (p<0.001). The nonhyperfibrinolytic transplant group had sustained low fibrinolytic activity throughout surgery with only one patient receiving an antifibrinolytic. All patients sustained low fibrinolytic activity by 120 minutes of reperfusion which persisted through POD-1. Bases on a standardized definition of fibrinolytic phenotypes20 the patient cohorts were reclassified. The non hyperfibrinolytic group failed to generate a fibrinolytic response and is more appropriately termed hypofibrinolysis. Both of the hyperfibrinolytic cohorts eventually suppressed fibrinolytic activity at 120 minutes of reperfusion which represents fibrinolysis shutdown. The high delta LY30 suppressed fibrinolysis at 30 minutes reperfusion and were renamed early fibrinolysis shutdown (e-SD) and low delta LY30 took an additional 90 minutes following reperfusion to suppress fibrinolytic activity, representing delayed fibrinolysis shutdown (d-SD). The demographics of each cohort are displayed in Table 1 including ischemia times and donor demographics. The only variable found to different between groups was longer warm ischemia time in the low negative delta-LY30 group (p=0.004).
Table 1:
Patient and Donor Demographics
| e-SD | d-SD | Hypofibrinolysis | P Value | |
|---|---|---|---|---|
| Age (Years) | 60 (54-63) | 55 (50-65) | 52 (44-63) | 0.221 |
| Female | %55 | 45% | 41% | 0.665 |
| HCC | 13% | 22% | 39% | 0.181 |
| BMI | 26 (23-30) | 26 (24-29) | 27 (25-32) | 0.637 |
| Pre-op INR | 2.2 (1.5-3.0) | 2.0 (1.5-2.9) | 2.0 (1.4-2.5) | 0.651 |
| Pre-op Plt (100,000) | 54 (39-70) | 63 (36-96) | 65 (40-133) | 0.200 |
| Pre-op LY30 (%) | 0 (0-0.1) | 0 (0-0.5) | 0 (0-0.2) | 0.811 |
| MELD | 25 (18-33) | 23 (16-36) | 20 (13-31) | 0.423 |
| Warm Ischemia Time (Minutes) | 32 (30-36) | 39 (34-44) | 34 (30-40) | 0.004 |
| Cold Ischemia Time (Minutes) | 336 (284-370) | 361 (284-420) | 430 (319-542) | 0.130 |
| Donor Age (Years) | 38 (21-44) | 40 (31-46) | 32 (21-49) | 0.403 |
| Donor Female | 32% | 39% | 28% | 0.811 |
| Donor BMI | 25 (23-31) | 30 (25-33) | 24 (22-28) | 0.078 |
e-SD = Early Shutdown, d-SD= Delayed Shutdown, HCC= Hepatocellular Carcinoma, BMI = Body Mass Index, PLT = Platelet Count, MELD = Model for End Stage Liver Disease, P Value represents Kruskal Wallis test across three groups or Chi Square for categorical variables.
Outcomes
Within groups the overall rate of EAD was the highest in patients with d-SD followed by hypofibrinolysis, and the lowest rate was in patients with high e-SD (Figure 2). The perioperative blood product utilization was highest in the high negative delta-LY30 group during the anhepatic phase of surgery (Figure 3), and remained similar for groups throughout the other time points during surgery. However, the total 24 hours red blood utilization was similar between cohorts, in addition to plasma, platelet and cryoprecipitate transfusions (Table 2). While underpowered to show statistical differences, the d-SD cohort had a 10% rate of primary non-function compared to 0% in the other groups (p=0.058) and a 18% mortality rate (vs 6% hypofibrinolysis, and 0% e-SD p=0.107). TEG indices were not different between groups on post-operative day 1 (Table 3) and not statistically differ in patients with EAD versus non EAD on post-operative day 1 (Table 3).
Figure 2: Temporal Changes in Fibrinolysis During Surgery Between Patient Cohorts.
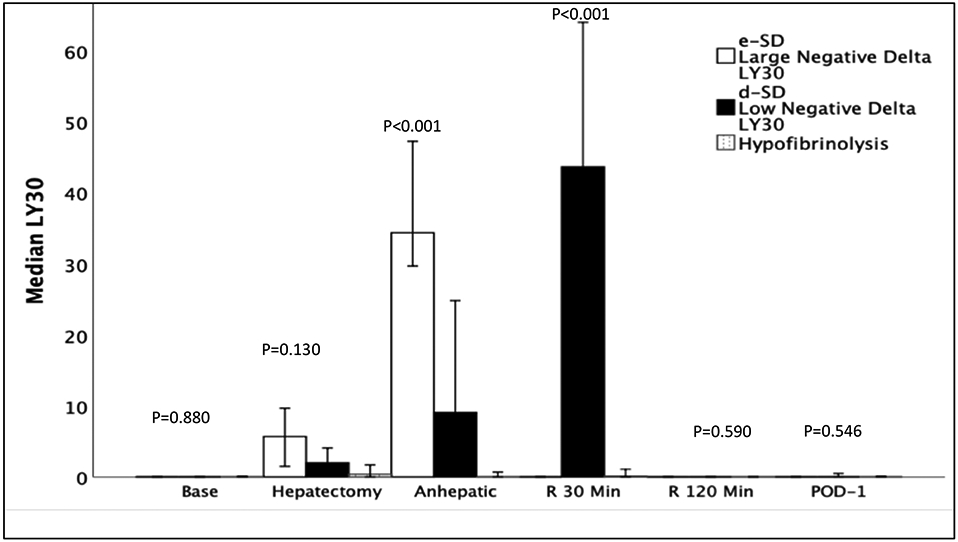
e-SD = Early Shutdown, d-SD= Delayed Shutdown, Base = Baseline Blood Draw, R = reperfusion, Min = Minutes, POD = Post-Operative Day, LY30 = Lysis at 30 Minutes P Value represents Kruskal Wallis test across three groups
Y-axis represents the median LY30 and X axis represents time. The no hyperfibrinolysis group (purple) did not generate a fibrinolytic response to surgery and represent hypofibrinolysis. Conversely, the large negative delta LY30 and low negative delta LY30 generated a fibrinolytic response to surgery that was subsequently inhibited and more appropriately termed fibrinolysis shutdown. The Large negative delta LY30 group suppressed fibrinolysis during early reperfusion and were termed early shutdown (e-SD), whereas the low negative LY30 group had increasing fibrinolysis during early reperfusion and were not inhibited until 120 minutes reperfusion and were termed delayed fibrinolysis shutdown (d-SD).
Figure 3: Rates of Early Allograft Dysfunction Between Recipient Cohorts.
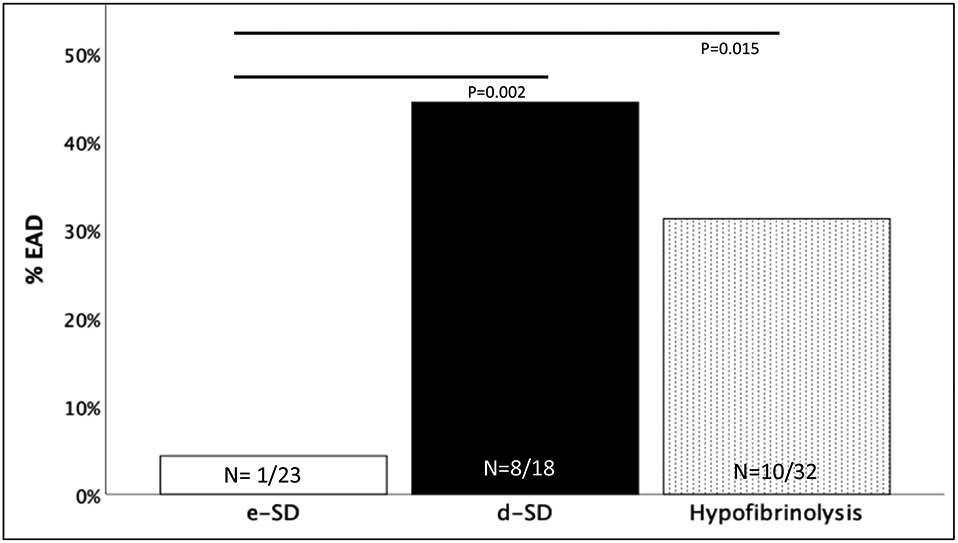
e-SD = Early Shutdown, d-SD= Delayed Shutdown, EAD= Early Allograft Dysfunction, P Values represent Fisher’s Exact test between e-SD and d-SD or Hypofibrinolysis.
Y-axis represents the percent of patients with EAD within the three cohorts along the x-axis
Table 2:
Transfusion Requirements
| e-SD | d-SD | Hypofibrinolysis | P Value | |
|---|---|---|---|---|
| # RBC | 12 | 12 | 9 | 0.766 |
| Transfusions | (4-27) | (5-18) | (4-18) | |
| # Plasma | 17 | 13 | 10 | 0.342 |
| Transfusions | (5-32) | (6-18) | (3-18) | |
| # Plt | 3 | 2 | 1 | 0.360 |
| Transfusion | (1-5) | (1-4) | (0-4) | |
| Coprecipitate | 1 | 1 | 0 | 0.351 |
| Transfusion | (0-2) | (0-1) | (0-2) | |
| TXA | 0 | 0 | 2.6 % | 0.999 |
e-SD = Early Shutdown, d-SD= Delayed Shutdown, RBC = Red Blood Cell Units, Plt = Pooled Platelet Units, TXA = Tranexamic Acid P Value represents Kruskal Wallis test across three groups or Chi Square for categorical variables.
Table 3:
Coagulation Measurements Post-Operative Day 1.
| e-SD | d-SD | Hypo fibrinolysis |
P Value | EAD | No EAD | P Value |
|
|---|---|---|---|---|---|---|---|
| R Time (Minutes) | 10.3 (7.7-13) | 9.9 (7.1-13) | 9.1 (7.6-12) | 0.501 | 10 (8.8-15) | 9.2 (7-12) | 0.180 |
| Angle (Degrees) | 49 (41-55) | 52 (35-55) | 51 (38-59) | 0.706 | 49 (33-53) | 50 (42-58) | 0.542 |
| MA (mm) | 47 (44-52) | 49 (44-55) | 49 (41-58) | 0.709 | 50 (40-55) | 48 (43-54) | 0.867 |
| LY30 % | 0 (0-0) | 0 (0-1.1) | 0 (0-1.3) | 0.546 | 0 (0-1.0) | 0 (0-0.2) | 0.277 |
e-SD = Early Shutdown, d-SD= Delayed Shutdown, MA = Maximum Amplitude, LY30 = Lysis at 30 Minutes. P Value represents Kruskal Wallis test across three groups
Discussion
Liver transplant recipients have three unique patterns of fibrinolytic changes during the perioperative period. While the majority of transplant recipients have low fibrinolytic activity at baseline, over half of patients develop a hyperfibrinolytic state during the anhepatic phase of surgery. Those patients with rapid reversal of hyperfibrinolysis 30 minutes following graft reperfusion (e-SD) had an EAD rate of 4%. This was 10-fold lower (44%) liver transplant recipients who had limited reduction (or an actual increase in fibrinolysis) following reperfusion. This group elevated fibrinolytic activity at 120 minutes following reperfusion (d-SD). In patients that failed to generate a fibrinolytic response (hypofibrinolysis), EAD occurred in roughly 1 in 3 patients (31%). The d-SD group had the highest rate of primary non-function and the highest post-operative mortality rate of the three cohorts.
Predicting adverse outcomes in liver transplantation based on fibrinolytic changes dates back to the 1980’s from the Starzl group in Pittsbugh21. Elevated fibrinolytic activity following graft reperfusion was associated with increased red blood cell and plasma administration in this study. In contrast, a more recent group evaluating living liver donor recipients identified fibrinolysis before the anhepatic phase of surgery in the recipients of adult living donor grafts as a predictor of early graft loss, but hyperfibrinolysis after the anhepatic phase having no adverse associations22. Our study was inconsistent with both of these studies as increased fibrinolytic activity during the anhepatic of surgery was associated with increased blood utilization (Figure 3) but better graft outcomes. However, all of these studies21,22 share a similar finding that patients eventually inhibited fibrinolysis after activation, which is consistent with the term fibrinolysis shutdown. This physiologic event of fibrinolytic activation with subsequent shutdown was first described in trauma14, and subsequently identified in other patients populations that underwent physiologic stress23. Unlike trauma patients that demonstrate poor outcomes with early fibrinolysis shutdown19, this rapid drop in fibrinolysis at 30 minutes following reperfusion in liver transplantation appears to be a biomarker for good graft function.
There are distinct biological factors that drive fibrinolysis in trauma versus transplant. As previously mentioned, the liver is the primarily responsible for clearance of tPA12 with a halflife in the order of minutes24. By removing the liver there is a loss of tPA clearance and an anticipated activation of the fibrinolytic system due to excessive plasminogen activators. This is likely prolonged when a margin graft has been implanted as it has have been demonstrated in a rodent liver transplant models, that transplanted organs with induced poor graft function from prolonged cold ischemia time shed their endothelium25. This loss of endothelium would be associated with the loss of tPA receptors. Therefore, graft implantation of organs with sloughed endothelium would have a delay in correction of fibrinolysis, i.e. d-SD, as appreciated in our study. This is further supported by the warm ischemia time in this group being significantly longer than the hypofibrinolytic and e-SD cohorts. While the d-SD warm ischemia time was only 39 minutes (vs 32 and 34 min) concerns for liver injury occur with greater than 30 minutes of warm ischemia26 and the extra 7-4 minutes could be contributory to more graft ischemia reperfusion injury during transplantation. In trauma patients the liver is intact and the exact mechanisms that drive this process remains unclear. Hemorrhagic shock in animal models27,28 and low systolic blood pressure in trauma patients19 have been associated with hyperfibrinolysis, with concurrent increases in tPA29 and depletion of plasmin inhibitors30. Trauma patients have some factor related to hemorrhagic shock driving tPA release with concurrent depletion of inhibitors, which is not the same as lack of tPA clearance in liver transplantation. The mechanism of early fibrinolysis shutdown in trauma remain unclear as these patients also can have depletion of their fibrinolytic inhibitors30. A limitation in trauma is knowing the exact timing of fibrinolysis activation and their physiology prior to hospital arrival. In addition, it is unclear if all trauma patients that present to the hospital with low fibrinolytic activity have prior activation of their fibrinolytic system20 and could be misclassified as fibrinolysis shutdown. Trauma patients that have the best outcomes present to the hospital with a balanced level of fibrinolysis termed physiologic31. Transplant patients in this study did not retain physiologic fibrinolysis for the duration of the surgery, and at baseline are chronically ill patients.
The Starzl study from the 1980’s21 also identified a cohort of patients failed to develop a fibrinolytic response during liver transplantation, which is consistent with hypofibrinolysis. Hypofibrinolysis is defined as a failure to generate a fibrinolytic response when anticipated. A fibrinolytic response can be generated in healthy individuals by applying a tourniquet to the arm promoting the release of tPA and this results in increased local fibrinolytic activity in the ischemic arm32. Hypofibrinolysis, defined by this measurement has been associated with thrombotic complications in multiple clinical settings32-34. Liver transplant serves another model for identifying hypofibrinolysis, as removal of the liver results in lack of clearance of tPA, and therefore it would be expected that all liver transplant patients would experience hyperfibrinolysis during the anhepatic phase of surgery. Recent studies evaluating fibrinolysis had identified hyperfibrinolysis to occur in 30-71% of liver transplant patients during the peri anhepatic phase of surgery22,35-37 while the remainder fail to generate a fibrinolytic response. In our study a large portion of liver transplant recipients demonstrated a hypofibrinolystic response during surgery (Figure 2) as the median LY30 of the cohort was less than 3% for the duration of surgery. This hypofibrinolytic group represented the majority of EAD (10/19 patients 52%). A recent review of the literature on fibrinolysis phenotypes in trauma20 demonstrates the importance of appropriately differentiating hypofibrinolytic (for failing to generate a fibrinolytic response) to fibrinolytic shutdown (activation and then impairment of fibrinolysis). We see the same importance in differentiating these phenotypes in this study, as d-SD and hypofibrinolysis are both associated with increased rates of EAD, whereas e-SD has favorable outcomes.
The clinical significance of identifying these intraoperative fibrinolytic phenotypes includes risk stratification of patients for appropriate therapeutic interventions following the transplant. The d-SD group in our study only had a high rate of EAD and also had a 11% rate of primary non-function. Patients with primary non-function require re-listing for transplantation as the mortality rate can be as high as 50% even after re-transplantation38. Early identification of d-SD with concerns for PNF could improve this mortality rate as the patients would have less time waiting for a new liver. A group from Spain used intra-operative arterial flows measured with doppler as a predictor for EAD39. Which, similar to our study provides an intra-operative assessment for risk of EAD. This group performed a multivariate regression analysis to identify factors associated with EAD using portal and arterial flows using cut offs to predict EAD and 30-day mortality. Unfortunately, the authors used cut off values for blood flow based on “clinically relevant” points but fail to demonstrate a specific reference to their significance. Furthermore, they report that contrasting EAD to non-EAD for hepatic artery blood flow showed no difference between groups39. Our study uses a prior definition of hyperfibrinolysis19,40 and a receiver operating characteristic curve with a Youden index41 to define a new threshold for pathologic changes in fibrinolysis during surgery, which demonstrates a high performance for accurately predicting EAD. At this time the treatment for d-SD would appear to be supportive (due to lack of reversal of hyperfibrinolysis due to an intrinsic liver problem) and early re listing for transplant if the patient clinically appears to have PND. Future work is warranted to identify if there are donor factors that could be contributory, as we may be underpowered at this time to see significant differences.
There may be a clinical therapy to treat hypofibrinolytic patients. The beneficial effects of fibrinolysis to clear microthrombi in the organs of animals recovering from hemorrhagic shock was demonstrated in animals a half century ago42. This same principle has been adopted in deceased liver donors at high risk of poor graft function, where tPA is administered in the donor at the time of organ recovery to break down presumed small clots in the organs43. A meta-analysis of tPA use in the high risk donor cohort has been demonstrated to improve 1 year graft survival following LT18. However, the routine utilization of tPA in all deceased organ donors is not advocated as this medication has been associated with massive bleeding, particularly when the transplanted liver has marginal functional43. These results begin to support a potential therapeutic role of tPA in the hypofibrinolytic cohort, as those patients who generated a large fibrinolytic response with early recovery had the best outcomes.
Our study has limitations to clinical translation as TEG samples were obtained for research purposes, and not to guide clinical care. Recommended timing of viscoelastic assays during liver transplantation remains ambiguous37 but historically TEG samples obtained during the anhepatic phase of surgery were ignored due perceived risk of over treating transient coagulopathy resulting in a theoretical increased risk of thrombotic complications44. However, two randomized control trials in liver transplantation using viscoelastic testing during regimented time frames have demonstrated a reduction in blood product utilization45,46. Both studies collected the anhepatic and 30-minute post-reperfusion time point45,46. These studies utilized antifibrinolytics as an adjunct to treat hyperfibrinolysis. In the first clinical trial only, plasma transfusions were reduced46 but had no impact on overall survival. In the more recent trial total blood product transfusion, tranexamic acid, and plasma was utilized less in the viscoelastic cohort45, but long term outcomes were not measured. We appreciated that the hyperfibrinolytic group did utilize more blood products during the anhepatic time frame (Figure 3), but was reduced following reperfusion and remained low during the post-operative period. Overall, this cohort had similar blood product utilization to the other cohorts, despite having the highest pre-operative MELD. Only one patient in our study received TXA in our study during the native hepatectomy portion of their surgery which resulted in a hypofibrinolytic phenotype. An additional limitation of this study utilizing an LY30 of 3% as an inflection point for hyperfibrinolysis based trauma patients19. There may be a more specific cut off for an appropriate fibrinolytic response during the anhepatic phase of surgery, as evident with the evolving definitions in trauma which are also viscoelastic testing dependent47. Regardless, it is important to begin to differentiate the different cohorts of fibrinolytic changes during LT.
In conclusion, the utilization of an interval TEGs between the anhepatic phase of LT and 30 minutes following graft reperfusion helps define three specific patient coagulation phenotypes in liver transplantation, and their association with early graft function. Those with early fibrinolysis shutdown (high negative delta-LY30) have the lowest rate of EAD, while patients with delayed fibrinolysis shutdown (low negative delta-LY30) had nine-fold rate of EAD. The hypofibrinolysis (no hyperfibrinolysis) group also appears to be a clinically unique group, that has an elevated risk of EAD compared to e-SD. These data support the routine use of viscoelastic assessment during the anhepatic phase of surgery and 30 minutes after reperfusion to risk stratify patients for EAD in programs that do not routinely utilize antifibrinolytic therapy. In addition, early identification of hypofibrinolysis with TEG may provide an opportunity to reduce the rate of EAD by increasing fibrinolytic activity during surgery.
Figure 4: Red Blood Cell Transfusions During Surgery.
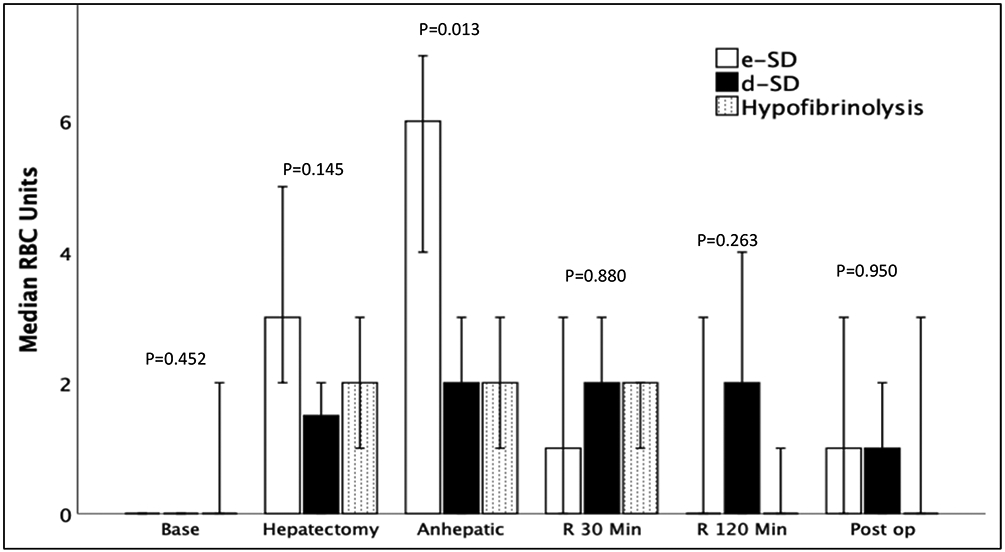
e-SD = Early Shutdown, d-SD= Delayed Shutdown, Baseline = Baseline Blood Draw, R = reperfusion, Min = Minutes, POD = Post-Operative Day, RBC = Red Blood Cells, P Value represents Kruskal Wallis test across three groups.
The Y axis represents the median number of red blood cells transfused from TEG lab draw to subsequent TEG lab draw. The X Axis represents the different blood draw times. The e-SD (green) group had a significant increase in blood product utilization during the anhepatic phase of surgery with a rapid decrease in blood product utilization for the duration of the blood draws. The other phenotypes did not have significantly different blood product utilization but the total red blood cell transfusions between all three phenotypes was the similar.
Highlights.
Thrombelastography identifies three unique fibrinolytic phenotypes during liver transplantation [Hypofibrinolysis, Early Fibrinolysis Shutdown (e-SD) and Delayed Fibrinolysis Shutdown (d-SD)]
Liver transplant patients who have a delay in correction of hyperfibrinolysis (d-SD) hold an 8-fold risk of early allograft function compared to those with early correction of hyperfibrinolysis (e-SD).
Hypofibrinolysis (lack of generating a fibrinolytic response) is also associated with an increased rate of early allograft dysfunction (EAD)
Acknowledgments
This study was supported in part by National Heart Lung and Blood Institute: K99HL151887 and American Society of Transplant Surgeons Veloxis Fellowship Award, and University of Colorado Academic Enrichment Fund
Footnotes
Planned to be Presented at the Southwestern Surgical Congress in Ojai, California, September 2020, But Was Cancelled Due to COVID-19
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19 Suppl 2:184–283. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi J. Liver and Lung Transplant Advances. JAMA : the journal of the American Medical Association. 2019;321(10):930. [DOI] [PubMed] [Google Scholar]
- 3.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16(7):874–884. [DOI] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12 Suppl 1:1–156. [DOI] [PubMed] [Google Scholar]
- 6.Deschenes M Early allograft dysfunction: causes, recognition, and management. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19 Suppl 2:S6–8. [DOI] [PubMed] [Google Scholar]
- 7.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16(8):943–949. [DOI] [PubMed] [Google Scholar]
- 8.Schroppel B, Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney international. 2014;86(2):251–258. [DOI] [PubMed] [Google Scholar]
- 9.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(11):2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Archives of surgery. 1969;98(1):31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bababekov YJ, Nydam TL, Pomposelli JJ, Moore HB. Goal-directed Management of Coagulation: The Right Treatment, the Right Patient, the Right Time. Transplantation. 2018;102(6):e303–e304. [DOI] [PubMed] [Google Scholar]
- 12.Otter M, Kuiper J, van Berkel TJ, Rijken DC. Mechanisms of tissue-type plasminogen activator (tPA) clearance by the liver. Annals of the New York Academy of Sciences. 1992;667:431–442. [DOI] [PubMed] [Google Scholar]
- 13.Moore HB, Moore EE, Neal MD, et al. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesthesia and analgesia. 2019;129(3):762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innes D, Sevitt S. Coagulation and Fibrinolysis in Injured Patients. Journal of clinical pathology. 1964;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HB ME, Gonzalez E, Huebner BJ, Sheppard F, Banerjee A, Sauaia A, Silliman CC. Reperfusion Shutdown: Delayed Onset of Fibrinolysis Resistance after Resuscitation from Hemorrhagic Shock Is Associated with Increased Circulating Levels of Plasminogen Activator Inhibitor-1 and Postinjury Complications. Blood. 2016;128:206. [Google Scholar]
- 16.Roberts DJ, Kalkwarf KJ, Moore HB, et al. Time course and outcomes associated with transient versus persistent fibrinolytic phenotypes after injury: A nested, prospective, multicenter cohort study. The journal of trauma and acute care surgery. 2019;86(2):206–213. [DOI] [PubMed] [Google Scholar]
- 17.Nicolau-Raducu R, Beduschi T, Vianna R, et al. Fibrinolysis Shutdown Is Associated With Thrombotic and Hemorrhagic Complications and Poorer Outcomes After Liver Transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2019;25(3):380–387. [DOI] [PubMed] [Google Scholar]
- 18.Jayant K, Reccia I, Virdis F, Shapiro AMJ. Systematic Review and Meta-Analysis on the Impact of Thrombolytic Therapy in Liver Transplantation Following Donation after Circulatory Death. J Clin Med. 2018;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. The journal of trauma and acute care surgery. 2014;77(6):811–817; discussion 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore HB, Moore EE, Neal MD, et al. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesthesia and analgesia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation. 1989;47(6):978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimauchi T, Yamaura K, Higashi M, Abe K, Yoshizumi T, Hoka S. Fibrinolysis in Living Donor Liver Transplantation Recipients Evaluated Using Thromboelastometry: Impact on Mortality. Transplantation proceedings. 2017;49(9):2117–2121. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti R, Hocking ED, Fearnley GR. Reaction pattern to three stresses--electroplexy, surgery, and myocardial infarction--of fibrinolysis and plasma fibrinogen. Journal of clinical pathology. 1969;22(6):659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korninger C, Stassen JM, Collen D. Turnover of human extrinsic (tissue-type) plasminogen activator in rabbits. Thrombosis and haemostasis. 1981;46(3):658–661. [PubMed] [Google Scholar]
- 25.Stolz DB, Ross MA, Ikeda A, et al. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatology. 2007;46(5):1464–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozmen MM, Oruc MT, Besler HT, et al. Comparison of the effects of continuous and intermittent portal triad occlusion (PTO) in rats. Hepato-gastroenterology. 2003;50(54):2127–2132. [PubMed] [Google Scholar]
- 27.Macko AR, Moore HB, Cap AP, Meledeo MA, Moore EE, Sheppard FR. Tissue injury suppresses fibrinolysis after hemorrhagic shock in nonhuman primates (rhesus macaque). The journal of trauma and acute care surgery. 2017;82(4):750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158(2):386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. The journal of trauma and acute care surgery. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore HB, Moore EE, Chapman MP, et al. Does Tranexamic Acid Improve Clot Strength in Severely Injured Patients Who Have Elevated Fibrin Degradation Products and Low Fibrinolytic Activity, Measured by Thrombelastography? Journal of the American College of Surgeons. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore HB, Moore EE, Liras IN, et al. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. Journal of the American College of Surgeons. 2016;222(4):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen G, Horellou MH, Kruithof EK, Conard J, Samama MM. Residual plasminogen activator inhibitor activity after venous stasis as a criterion for hypofibrinolysis: a study in 83 patients with confirmed deep vein thrombosis. Blood. 1988;72(2):601–605. [PubMed] [Google Scholar]
- 33.Bombardier C, Villalobos-Menuey E, Ruegg K, Hathaway WE, Manco-Johnson MJ, Goldenberg NA. Monitoring hypercoagulability and hypofibrinolysis following acute venous Thromboembolism in children: application of the CloFAL assay in a prospective inception cohort study. Thrombosis research. 2012;130(3):343–349. [DOI] [PubMed] [Google Scholar]
- 34.Garcia Frade LJ, de la Calle H, Torrado MC, Lara JI, Cuellar L, Garcia Avello A. Hypofibrinolysis associated with vasculopathy in non insulin dependent diabetes mellitus. Thrombosis research. 1990;59(1):51–59. [DOI] [PubMed] [Google Scholar]
- 35.Kim EH, Ko JS, Gwak MS, Lee SK, Kim GS. Incidence and clinical significance of hyperfibrinolysis during living donor liver transplantation. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2018;29(3):322–326. [DOI] [PubMed] [Google Scholar]
- 36.Abuelkasem E, Lu S, Tanaka K, Planinsic R, Sakai T. Comparison between thrombelastography and thromboelastometry in hyperfibrinolysis detection during adult liver transplantation. British journal of anaesthesia. 2016;116(4):507–512. [DOI] [PubMed] [Google Scholar]
- 37.Gorlinger K [Coagulation management during liver transplantation]. Hamostaseologie. 2006;26(3 Suppl 1):S64–76. [PubMed] [Google Scholar]
- 38.Kulik U, Lehner F, Klempnauer J, Borlak J. Primary non-function is frequently associated with fatty liver allografts and high mortality after re-transplantation. Liver Int. 2017;37(8):1219–1228. [DOI] [PubMed] [Google Scholar]
- 39.Lominchar PL, Orue-Echebarria MI, Martin L, et al. Hepatic flow is an intraoperative predictor of early allograft dysfunction in whole-graft deceased donor liver transplantation: An observational cohort study. World J Hepatol. 2019;11(9):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pommerening MJ, Goodman MD, Farley DL, et al. Early diagnosis of clinically significant hyperfibrinolysis using thrombelastography velocity curves. Journal of the American College of Surgeons. 2014;219(6):1157–1166. [DOI] [PubMed] [Google Scholar]
- 41.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81. [DOI] [PubMed] [Google Scholar]
- 42.Hardaway RM, Drake DC. Prevention of "irreversible" hemorrhagic shock with fibrinolysin. Annals of surgery. 1963;157:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto K, Eghtesad B, Gunasekaran G, et al. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(12):2665–2672. [DOI] [PubMed] [Google Scholar]
- 44.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesthesia and analgesia. 1985;64(9):888–896. [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnet A, Gilquin N, Steer N, et al. The use of a thromboelastometry-based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: A randomised controlled study. European journal of anaesthesiology. 2019;36(11):825–833. [DOI] [PubMed] [Google Scholar]
- 46.Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplantation proceedings. 2010;42(7):2590–2593. [DOI] [PubMed] [Google Scholar]
- 47.Stettler GR, Moore EE, Moore HB, et al. Redefining Post Injury Fibrinolysis Phenotypes Using Two Viscoelastic Assays. The journal of trauma and acute care surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


