Abstract
Background
The orbitofrontal cortex (OFC) encodes internal representations of outcomes and subjective value to facilitate flexible reward-seeking. OFC activation is associated with drug seeking in both human subjects and animal models. OFC plays a role in alcohol use, but studies in animal models have produced conflicting results with some showing decreased seeking after OFC inactivation but others showing increased seeking or no changes. In part this may be due to the different measures of alcohol seeking used (e.g., homecage drinking vs. operant seeking).
Methods
We characterized the impact of transient inactivation of OFC (primarily lateral and, to a lesser extent, ventral subregions) using inhibitory hM4Di designer receptors exclusively activated by designer drugs (DREADDs). OFC neurons were transiently inhibited during 10% and 20% alcohol (ethanol) and sucrose homecage consumption, fixed ratio (FR1) operant self-administration, and cue-induced reinstatement of either 10% ethanol or sucrose in male and female rats.
Results
OFC inactivation did not affect sucrose or ethanol consumption in the homecage, nor did it influence seeking or consumption under FR1 operant conditions. In contrast, OFC inactivation suppressed cued-induced reinstatement for both ethanol and sucrose in both male and female rats.
Conclusions
Our results are aligned with previous work indicating a selective suppressive effect of OFC inactivation on reinstatement for alcohol and other drugs of abuse. They extend these findings to demonstrate no effect on homecage consumption or FR1 seeking as well as showing an impact of sucrose reinstatement. These data indicate that OFC plays a uniquely important role when reward seeking is driven by associations between external stimuli and internal representations of reward value, both for natural and drug rewards. They further implicate the OFC as a key structure driving relapse-associated seeking and potentially contributing to alcohol use disorder and other diseases of compulsive reward seeking.
Keywords: prefrontal, alcohol, DREADD, relapse, sex differences
INTRODUCTION
The orbitofrontal cortex (OFC) regulates flexible decision-making in human and non-human animals, permitting acquisition of positive, and avoidance of negative, reinforcers. (Stalnaker et al., 2015, Rudebeck and Murray, 2014, Wallis, 2011, Balleine et al., 2011). Accumulating evidence indicates substantial OFC involvement in motivation to acquire drugs of abuse, as well as disruption of OFC after chronic drug use (Volkow and Fowler, 2000, London et al., 2000, Schoenbaum and Shaham, 2008, Dom et al., 2005). There has been a growing interest in the contributions of OFC to alcohol seeking and use (Moorman, 2018). In humans, OFC is activated during alcohol craving induced by cues and alcohol itself (Wrase et al., 2002, Tapert et al., 2003, Myrick et al., 2004). Chronic alcohol disrupts OFC basal function (Nicolas et al., 1993, Kuruoglu et al., 1996, Volkow et al., 1997) and reduces OFC volume (Beck et al., 2012, Durazzo et al., 2011, Rando et al., 2011). In non-human animals, there is an increasing number of observations that OFC is associated with alcohol use. OFC neurons are activated during alcohol seeking (Jupp et al., 2011, Barak et al., 2013, Laguesse et al., 2016, Bianchi et al., 2018). Inhibition of OFC reduces cue- and context-induced reinstatement of alcohol seeking (Bianchi et al., 2018, Arinze and Moorman, 2020), and disruption of mTORC1 signaling in OFC decreased alcohol seeking during extinction (Morisot et al., 2019). In other studies, however, lesions or DREADD (designer receptor exclusively activated by designer drugs; (Armbruster et al., 2007)) inhibition of OFC increased drinking in rats (Ray et al., 2018) and mice treated with chronic ethanol (den Hartog et al., 2016). Despite some conflicting results, these data indicate a clear involvement of OFC in alcohol seeking.
Thus there are clear indicators of significant OFC involvement in alcohol use, but our understanding of which aspects of alcohol seeking are controlled by OFC are not well established, and there are some inconsistencies in previous findings. To address this issue, we used DREADDs to transiently suppress the activity of the same OFC neurons during three types of alcohol and sucrose seeking: homecage consumption, FR1 self-administration, and cue-induced reinstatement. Studies were performed in both males and females to assess potential sex differences in OFC contributions to alcohol seeking. The results of our study indicate a selective role for OFC in reinstatement, but not homecage consumption or FR1 alcohol or sucrose seeking. Reinstatement of seeking of both ethanol (Experiment 1) and sucrose (Experiment 2) was affected, indicating a general role for OFC in cue-driven reward seeking that encompasses both natural and drug rewards. Our data suggest that OFC may be a particularly relevant structure in the context of relapse in humans, both for alcohol and other drugs of abuse, as well as for natural rewards.
MATERIALS AND METHODS
Experimental design
Male (n = 24) and female (n = 24) Wistar rats (~200–300 g upon arrival; Charles River, Wilmington MA Laboratories) were used in these studies. Rats were divided into three experimental cohorts: Experiment 1a (DREADD tests of ethanol (EtOH) and sucrose seeking/consumption), Experiment 1b (GFP control tests of EtOH and sucrose seeking/consumption), and Experiment 2 (DREADD tests of sucrose seeking and consumption). Experiment 2 was conducted to assess the impact of DREADD manipulation on natural reward seeking and consumption in the absence of any history of EtOH use. In Experiment 1 (DREADD – 1a, and GFP – 1b), male and female Wistar rats (n = 36, 18 males, 18 females) were trained to drink 20% ethanol (EtOH; Fisher Scientific, Pittsburgh, PA) in their homecages using the intermittent access to EtOH paradigm (Wise, 1973). EtOH was given in homecages with ad lib access to food and water. Of these rats, 16 (8 male and 8 female) were ultimately tested with DREADD manipulations (Experiment 1a) and 12 (6 male and 6 female) were tested as GFP controls (Experiment 1b). The remaining rats were not included in the study based on suboptimal DREADD expression (see below). Rats used in Experiment 2 (DREADD and sucrose; n = 12, 6 males and 6 females) received 15% sucrose in their homecages for approximately 1 week and no EtOH. Following homecage access, rats received surgery followed by operant training and testing (Fig. 1A). Within each experiment, all rats were tested during homecage drinking, self-administration, and reinstatement. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst and were designed and conducted in compliance with the National Institutes of Health Guide for the Care and Use of Animals.
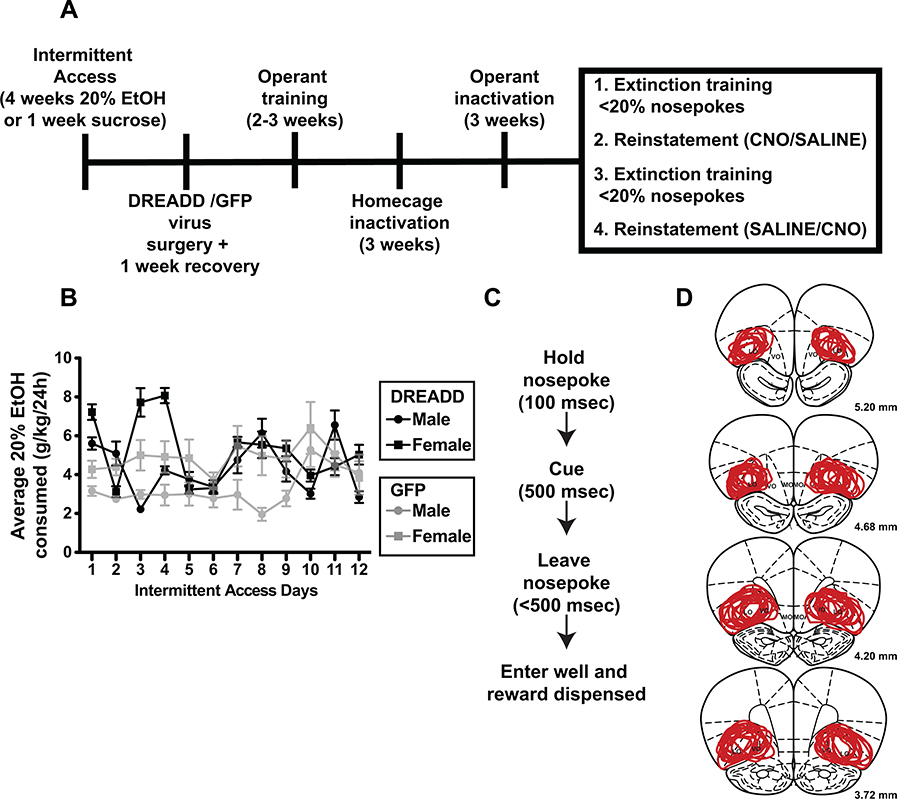
Figure 1.
A) Experimental timeline. See Materials and Methods for details of training and testing order in each Experiment. B) Daily intake of 20% ethanol in male and female rats over the course of 4 weeks of intermittent access in Experiment 1. Data are separated into rat cohorts infused with DREADD or GFP viral vectors. C) FR1 operant task timeline. D) Expression of hM4Di-mCherry in all rats. Overlapping lines indicate regions of expression in all rats included for analysis. Note the main overlapping site is lateral OFC, with some spread into ventral OFC.
Surgery
Under isoflurane anesthesia (1.5%−2.5%), rats in Experiments 1a and 2 received 0.6 μL AAV8-hSyn-hM4D(Gi)-mCherry (Addgene: Watertown, MA) delivered bilaterally to lateral and, to a lesser extent, ventral OFC (A/P 3.6 – 4.6, M/L 2.6 to 3.0, D/V −5.0 to −5.2 mm from bregma) using silica tubing connected to 10μl Hamilton syringes (Hamilton Company, Reno, NV) mounted on microinfusion pumps (UMP3/Micro 4, World Precision Instruments, Sarasota, FL). Extent of expression across rats is shown in Fig. 1D. Rats in Experiment 1b received 0.6 μL AAV8-hSyn-EGFP, (Addgene). Rats received antibiotic (0.1 ml cefazolin, 330 mg/ml, i.m.) and analgesic (meloxicam, 1 mg/kg, s.c.).
Behavioral training
One week after surgery, rats were trained to self-administer 10% EtOH, 20% EtOH, and 15% sucrose (Experiment 1) or 15% sucrose (Experiment 2) in an operant chamber (Med Associates, Fairfax, Vermont) on an FR1 schedule (Fig. 1C). Different rewards were provided on separate days such that only one reward was available on each self-administration day. Self-administration training was conducted during 2 hr sessions during rats’ dark cycles. Following houselight off, FR1 nosepokes produced an auditory cue for 500 msec that predicted the availability of one of three outcomes in a spigot located below the nosepoke: 0.1 ml of either 15% sucrose (5 kHz cue), 10% EtOH (1 kHz cue) or 20% EtOH (10 kHz cue). Different tones were used so that rats could more easily differentiate expected outcome within training and test sessions and to permit selective reinstatement associated with a particular outcome (see below).
Rats were initially trained to respond for 10% EtOH. Once rats reached a criterion of 80% successful trials (i.e., retrieving 10% EtOH within 2 seconds post-cue onset), rats were then trained on FR1 sucrose sessions followed by 20% EtOH sessions. Once rats reliably performed at 80% success in sucrose sessions and then 20% EtOH sessions, homecage inactivation studies commenced, followed by operant inactivation studies (Fig. 1A). During both training and testing, reward wells were checked at the end of each session to verify that delivered sucrose or ethanol was consumed and we confirmed that this was the case by the time that animals reached successful training stages. Training took approximately 2–3 weeks, resulting in an approximate 3–4 week virus incubation period prior to the start of inactivation testing, in line with previous reports (Harper et al., 2019, Purohit et al., 2018, Reimsnider et al., 2007, Smith et al., 2016, Meyer and Bucci, 2016).
Inactivation
Homecage
On separate weeks, rats received 40 mL sucrose, 10% EtOH or 20% EtOH in a sipper tube along with ad libitum water and food daily for 2 hrs. On Mondays, Tuesdays, and Thursdays, rats received solutions without any injection. On Wednesdays and Fridays, 30 min prior to sipper tube placement, rats received an injection of CNO (3 mg/kg in 0.5% DMSO and saline, i.p.) or vehicle (0.5% DMSO in saline, i.p.), in a counterbalanced fashion. Effects of inactivation were tested on a separate reward on each of three consecutive weeks (Experiment 1: 10% EtOH on week 1, 20% EtOH on week 2, and sucrose on week 3) or only for sucrose during 1 week (Experiment 2).
FR1 Operant
The week after homecage inactivation, rats were tested on the effects of OFC inactivation on EtOH and sucrose seeking and consumption in an operant environment. On Wednesdays and Fridays rats were either injected with CNO or vehicle in a counterbalanced fashion 30 min before operant testing. On Mondays, Tuesdays, and Thursdays, rats self-administered one reward in the absence of any treatment. Tests were conducted for a separate reward on each of three consecutive weeks (Experiment 1) or only for sucrose during 1 week (Experiment 2). Reward-week order for operant testing was the same as homecage testing (10% EtOH on week 1, 20% EtOH on week 2, and sucrose on week 3).
Extinction and cued-induced reinstatement
After operant inactivations, rats underwent daily 1 hr extinction sessions. During extinction sessions nosepokes resulted in no cues or rewards, but nosepokes and well entries were recorded. Rats were extinguished for at least 2 weeks until emitting < 20 nosepokes in a 2 hr session for 2 consecutive sessions. Upon meeting criterion, rats received CNO or vehicle prior to cue-induced reinstatement. In reinstatement sessions, a nosepoke, resulted in delivery of a 10% EtOH (Experiment 1) or sucrose (Experiment 2) reward-predicting cue, but no reward was delivered from the spigot. Only one cue/outcome pairing was reinstated per Experiment (10% EtOH in Experiment 1 and sucrose in Experiment 2) to avoid confusion regarding extinguished and reinstated vs. non-reinstated cues. All nosepokes and well entries were recorded. The first nosepoke producing a cue was counted as an initiated trial. Both initiated trials as well as all nosepokes (which included initiated trials as well as other pokes such as multiple pokes for trial initiation, pokes during cue presentation, etc.) were subject to analysis. Similarly, well entries immediately following cue presentation were counted and analyzed, as were all well-entries (including those not associated with cue presentation). After the first reinstatement session, rats were again extinguished to criteria and were re-tested on reinstatement using the counterbalanced injection treatment so that each animal received two reinstatement sessions (CNO and vehicle) for the same cue.
Histology
After the final reinstatement test, rats were perfused with 0.9% NaCl solution followed by 4% paraformaldehyde. Brains were post-fixed overnight with 4% paraformaldehyde and cryoprotected in 20% sucrose/0.1% sodium azide. Forty μm sections were collected. To stain for mCherry, sections were blocked in 3% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) in PBST and incubated overnight with primary antibodies (rabbit anti-DsRed, 1:500; Takara Bio, Kusatsu, Japan). Tissue was incubated with secondary antibodies (594 anti-rabbit, 1:250; Jackson ImmunoResearch) for 2 hrs followed by mounting onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) using CitiFluor (Electron Microscopy Sciences, Hatfield, PA). Brains expressing GFP were blocked similarly with overnight incubation of primary antibodies (chicken anti-GFP (1:2000; Abcam, Cambridge, MA). The next day tissue was incubated for 2 hr in secondary antibodies (488 anti-Chicken, 1:500; Jackson ImmunoResearch) following mounting and coverslipping. Rats were removed from data analysis if significant DREADD expression was observed outside OFC (e.g., in piriform cortex) or if no DREADD expression was observed.
Data analysis
Operant behaviors analyzed included number of initiated trials (the first nosepoke producing a cue/outcome during self-administration or a cue during reinstatement), total number of nosepokes (including those during the intertrial interval, additional pokes after trial initiation, etc.), number of rewards received, number of total well entries (including non-rewarded entries such as those during the intertrial interval, etc.), latency to initiate trials, and latency to enter the reward well after nosepoke/cue delivery. Statistical tests were performed using Prism (GraphPad, San Diego, CA). Two factor mixed model ANOVAs (referred to in Results as “two factor ANOVA”) were conducted to identify effect of treatment (CNO vs. vehicle), sex (male vs. female) and interaction. When data sets contained missing values, Geisser-Greenhouse-corrected mixed effects models (referred to in Results as “mixed effects model”) were used instead of two factor ANOVAs.
RESULTS
Male and female rats consumed 20% ethanol during intermittent access
On average, rats from Experiments 1a (DREADD) and 1b (GFP) consumed around 4 g/kg/24h 20% EtOH across all days of intermittent access (IA) (Fig 1B). Drinking varied across days with an overall increase over IA sessions (F(4.47, 97.59) = 5.36, p = 0.0004; mixed effects model). Male and female rats exhibited similar patterns of drinking during IA, with females drinking more EtOH than males but not significantly (female mean/SEM: 5.22 +/− 0.50 g/kg; male: mean/SEM: 4.31 +/− 0.39 g/kg; effect of sex: F(1, 22) = 2.07, p = 0.16). There was no sex x day interaction (F(11, 240) = 1.07, p= 0.39), although the degree of escalation appeared greater in males than females, which started at a higher drinking level. Similar overall levels of drinking were observed in GFP-expressing rats though in this group there were significant sex differences in consumption (female mean/SEM: 4.83 +/− 0.08 g/kg; male: mean/SEM: 3.29 +/− 0.30 g/kg; effect of sex: F(1, 10) = 24.31, p =0.0006), but no main effect of IA drinking day (F(2.961, 29.61) = 1.79, p =0.17) or interaction (F(11, 110) = 0.76, p = 0.68; two factor ANOVA).
OFC inactivation did not influence consumption of ethanol or sucrose during homecage drinking tests
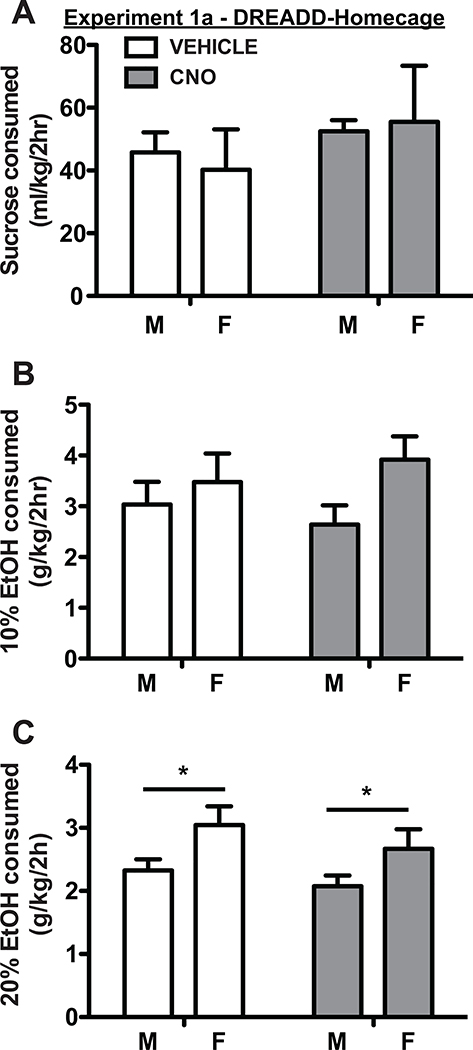
We used AAV to express inhibitory hM4Di DREADDs in lateral and ventral OFC (Fig. 1D). Based on histological verification of DREADD expression, we analyzed results from 16 rats in Experiment 1a (hM4Di-mCherry; 8 male, 8 female), and from 12 rats in Experiment 1b (GFP control; 6 male, 6 female). Following DREADD expression, OFC was inactivated in EtOH and sucrose homecage consumption tests. Rats were allowed free-access to 40 mL of 15% sucrose, 10% EtOH or 20% EtOH for 2 hrs beginning 30 mins after CNO or vehicle injection. One reward type was tested per week (see Materials and Methods). As shown in Fig. 2, OFC inactivation did not change consumption of 15% sucrose (F(1, 12) = 1.81, p = 0.20, two factor ANOVA), 10% EtOH (F(1, 28) = 0.002, p = 0.96, mixed effects model), or 20% EtOH (F(1, 14) = 2.11, p = 0.17; two factor ANOVA). Females consumed the same relative amount of 15% sucrose per body weight as males F(1,14) = 0.007, p = 0.93., slightly more 10% EtOH than males (F(1,14) = 4.18, p = 0.06) and significantly more 20% EtOH than males (F(1,14) = 5.71, p = 0.03). There were no significant interaction effects (all p > 0.05). In GFP-only control rats there were also no main effects of treatment during 15% sucrose (Table 1; F(1, 9) = 0.83, p = 0.39; mixed effects model), 10% EtOH (F(1, 10) = 1.48, p = 0.25; two factor ANOVA), or 20% EtOH (F(1, 10) = 1.03, p = 0.33) sessions. There were no sex differences in sucrose consumption (F(1, 10) = 0.02, p = 0.88), but females drank significantly more 10% EtOH (F(1, 10) = 12.63, p = 0.005) and 20% EtOH (F(1, 10) = 7.24, p = 0.02) than males. There were no interaction effects (all p > 0.05).
Figure 2.
OFC inactivation had no effect on homecage consumption of 15% sucrose (A), 10% ethanol (B), and 20% ethanol (C) in either male or female rats. Female rats consumed more 10% ethanol and significantly more 20% ethanol than male rats, in line with previous findings. In this and following figures, bars show mean and SEM across all rats for each single treatment test session (CNO or vehicle).
Table 1–
Effects of CNO and vehicle treatment in GFP-expressing rats
| Test | Reward Type | M-CNO | M-Veh | F-CNO | F-Veh |
|---|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | ||
| Homecage | 15% sucrose (ml/2hr) | 69.76 (4.94) | 66.58 (7.56) | 81.27 (16.75) | 81.07 (16.48) |
| 10% EtOH (g/kg/2hr) | 2.63 (0.38) | 2.42 (0.29) | 3.01 (0.25) | 2.55 (0.33) | |
| 20% EtOH (g/kg/2hr) | 1.54 (0.17) | 1.43 (0.11) | 2.10 (0.15) | 1.94 (0.22) | |
| FR1 Operant | 15% sucrose (trials initiated) | 127.50 (27.47) | 137.00 (27.15) | 124.00 (15.42) | 134.17 (10.05) |
| 10% EtOH (trials initiated) | 48.00 (9.25) | 33.17 (11.07) | 33.83 (6.15) | 49.00 (9.02) | |
| 20% EtOH (trials initiated) | 35.17 (6.12) | 28.33 (6.82) | 27.83 (2.83) | 31.00 (6.69) | |
| 15% sucrose (rewards collected) | 124.83 (17.45) | 133.33 (27.6) | 114.33 (27.58) | 128.83 (10.81) | |
| 10% EtOH (rewards collected) | 41.50 (11.70) | 28.33 (6.49) | 22.83 (9.3) | 34.17 (10.23) | |
| 20% EtOH (rewards collected) | 34.00 (5.93) | 27.00 (7.14) | 21.50 (3.25) | 23.00 (6.92) | |
| Cued-Reinstatement | 10% EtOH (total nosepokes) | 13.17 (2.77) | 19.00 (1.69) | 19.17 (4.84) | 16.83 (4.19) |
| 10% EtOH (trials initiated) | 10.83 (1.21) | 14.17 (2.77) | 16.17 (4.05) | 19.17 (2.77) | |
| 10% EtOH (latency to initiate trial) | 569.95 (132.41) | 540.77 (181.77) | 558.88 (131.68) | 559.85 (148.23) | |
| 10% EtOH (total well entries) | 154.00 (34.25) | 166.00 (44.23) | 169.83 (43.89) | 172.50 (38.61) | |
| 10% EtOH (well entries after cue) | 5.83 (2.56) | 8.33 (0.88) | 7.00 (1.16) | 10.67 (4.06) | |
| 10% EtOH (latency to enter well) | 1.41 (0.16) | 1.26 (0.17) | 1.37 (0.17) | 1.26 (0.15) | |
Mean and SEM of results of CNO and vehicle treatment for each behavioral measure in male and female rats expressing virally-transduced GFP as a control. The same data are presented here as are presented in graphical format for DREADD-expressing rats. All comparisons of CNO vs. vehicle treatment were non-significant except one: 10% EtOH (trials initiated). The details of these results are described in the text.
OFC inactivation did not influence seeking or consumption of sucrose or ethanol during operant FR1 self-administration
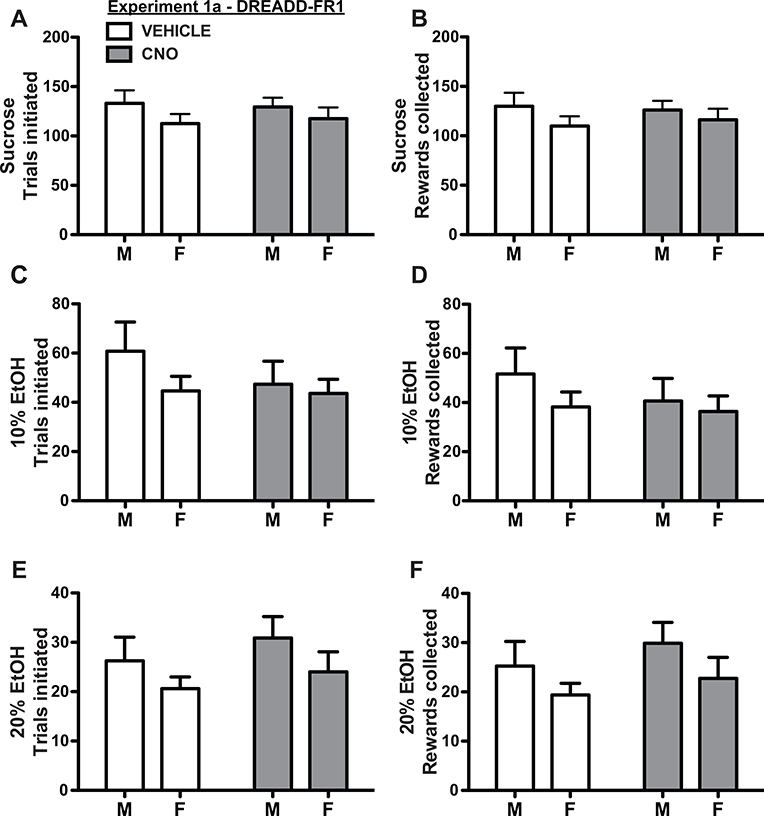
During FR1 operant testing, OFC inactivation did not affect seeking or consumption of sucrose, 10% EtOH, or 20% EtOH as measured by total number of initiated trials or rewards collected (Fig. 3, main effect of treatment, treatment × sex interaction: all p > 0.05; two factor ANOVA) and there were no differences across sex (main effect of sex: all p > 0.05). There were also no significant differences based on either treatment or sex, and no treatment × sex interaction, in total number of nosepokes, total number of well-entries, latency to initiate trials or latency to acquire rewards (all p > 0.05; two factor ANOVA), except for one main effect of sex in latency to consume sucrose rewards (female mean/SEM: 0.65 +/− 0.03 sec; male mean/SEM: 0.88 +/− 0.004 sec; F(1, 14) = 4.77, p = 0.05), but no main effect of treatment (F(1, 14) = 0.83, p = 0.38) or treatment x sex interaction (F(1, 14) = 0.53, p = 0.48). We did observe an influence of reward type on number of trials initiated (F(1.93, 28.88) = 177.50, p < 0.0001, Geisser-Greenhouse-corrected RM two factor ANOVA, all post-hoc comparisons p<0.05) and latency to initiate responses (F(1.81,27.14) = 18.40, p < 0.0001, CNO: all post-hoc comparisons p<0.05, vehicle: sucrose vs. 20% p <0.0001), but no significant influence on latency to consume reward (F(1.44, 21.57) = 3.67, p = 0.06). These results indicate that rats used session information (which reward was available on a specific day) to guide behavior and that when the reward acquisition was initiated, it was relatively automatic, as demonstrated by the lack of significant difference in consumption latencies across reward.
Figure 3.
OFC inactivation had no effect on FR1 seeking (trials initiated - A, C, E) or taking (rewards collected - B, D, F) of sucrose (A-B), 10% ethanol (C-D), or 20% ethanol (E-F) in either male or female rats. Male and female rats exhibited similar levels of seeking and consumption of all reinforcers. Note that most initiated trials resulted in rewards collected, and that rats exhibited high levels of motivation for all reinforcers (though seeking/consumption was greatest for sucrose, followed by 10% ethanol, then 20% ethanol).
In GFP-expressing rats (Table 1), there were no significant main effects of CNO vs. vehicle treatment across all animals (all p > 0.05). We did observe significant sex x treatment interactions in number of 10% EtOH initiated trials (F(1, 10) = 5.39, p = 0.04), total trials (F(1, 10) = 4.97, p = 0.05), and initiation latency (F(1, 9) = 16.22, p = 0.003). However, no posthoc comparisons were significant (Sidak’s MCT, all p > 0.05) except that CNO significantly decreased trial initiation latency in males, but not females (t = 4.01, p= 0006). The overall lack of consistent effects, combined with a lack of effect on any other self-administration or homecage consumption behavior, including in DREADD-expressing rats, leads us to place little emphasis on this result, as considered more in Discussion.
OFC inactivation significantly decreased cue-induced reinstatement of 10% ethanol
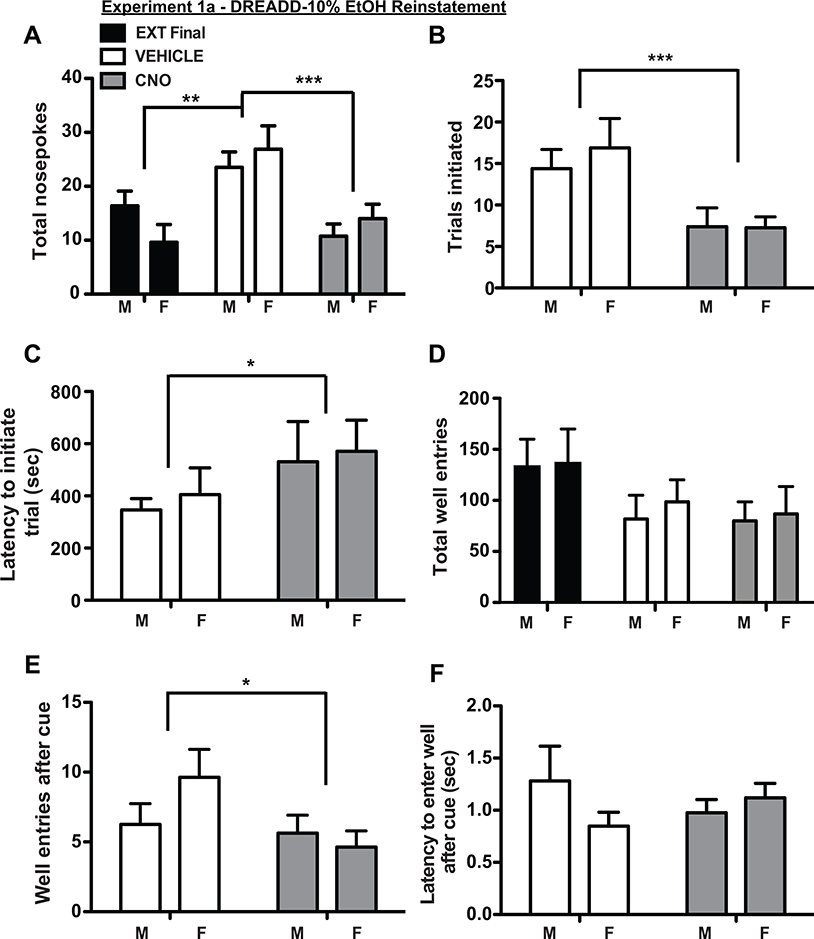
After meeting extinction criteria of <20 nosepokes for 2 consecutive sessions, rats received cue-induced reinstatement sessions in which nosepokes delivered 10% EtOH cues but no EtOH reward. When treated with vehicle, rats exhibited significant cue-induced reinstatement as measured by increased total nosepokes compared to the last day of extinction (Fig 4A, main effect of treatment F(1.92, 26.82) = 12.15, p = 0.0002, post hoc Tukey test extinction vs. vehicle p <0.001, two factor ANOVA). CNO treatment significantly decreased reinstatement relative to vehicle (Fig. 4A, post hoc Tukey test CNO vs. vehicle p <0.005), resulting in nosepokes at the same level as the last extinction session (post hoc Tukey test CNO vs. extinction p >0.05). There were no significant sex differences (main effect of sex F(1, 14) = 0.0002, p = 0.99), and no treatment x sex interaction effect (F(2, 28) = 1.97, p = 0.16). Male and female rats also initiated fewer trials following CNO treatment (Fig 4B; main effect of treatment: F(1, 14) = 14.15, p = 0.002; no main effect of sex: F(1, 14) = 0.18, p = 0.67; no interaction effect: F(1, 14) = 0.35, p = 0.56; two factor ANOVA), and exhibited a longer latency to initiate trials (Fig. 4C, main effect of treatment: F(1, 13) = 5.19, p = 0.04; no main effect of sex F(1, 14) = 0.15, p = 0.71; and no interaction effect F(1, 13) = 0.01, p = 0.92; mixed effects analysis; latency data missing for one male rat in a CNO-treatment session). Total numbers of well-entries were not significantly reduced by OFC inactivation (Fig 4D), but OFC inactivation significantly decreased numbers of well entries after nosepoke-elicited cues (Fig 4E; main effect of treatment F(1, 14) = 4.66, p = 0.049; no main effect of sex F(1, 14) = 0.48, p = 0.50; and no interaction effect F(1, 14) = 2.82, p = 0.12; two factor ANOVA). There were no significant differences in well-entry latency following CNO or vehicle treatment in males or females (Fig. 4F; all p > 0.05; two factor ANOVA). In GFP-expressing rats (Table 1), we observed a significant main effect of session (F(2, 22) = 6.71, p = 0.005; RM one-way ANOVA), which was driven by significant differences between CNO-reinstatement vs. extinction (p < 0.05; Tukey) and vehicle-reinstatement vs. extinction (p < 0.01) but no significant difference between CNO- and vehicle-reinstatement sessions (p > 0.05), indicating no impact of CNO treatment on reinstatement in controls.
Figure 4.
OFC inactivation suppressed reinstatement of 10% ethanol seeking in both male and female rats. (A) Saline treated rats (white bars) exhibited significant reinstatement relative to the final day of extinction (black bars). CNO treatment (gray bars) suppressed total nosepokes relative to saline treatment, and decreased responding to extinction levels. This was true for male and female rats. OFC inactivation also decreased total number of trials initiated (B), increased latency to initiate trials (C), and marginally (but significantly) decreased number of well entries following conditioned cues (D). Total well entries (D) and latency to enter the well (F) were not impacted.
OFC inactivation did not influence homecage or operant sucrose seeking or consumption in ethanol naïve rats
A third cohort of rats received hM4Di delivered to OFC neurons but were not exposed to EtOH intermittent access. Based on histological verification of DREADD expression, we analyzed results from 9 rats in cohort 3 (hM4Di-mCherry; 4 male, 5 female). These rats received homecage sucrose and were trained to self-administer sucrose on an FR1 schedule. Neither OFC inactivation nor sex influenced homecage sucrose consumption (no main effects of treatment or sex, no interaction effect, all p > 0.05, two factor ANOVA). No behavioral parameter measured during FR1 sucrose seeking or consumption was significantly different across treatment or sex (no main effects of treatment or sex, no interaction effect, all p > 0.05, two factor ANOVA). This included total numbers of nosepokes, total number of trials initiated, latency to initiate trial, total numbers of well-entries, number of rewarded well entries, or latency to enter reward port after cue presentation.
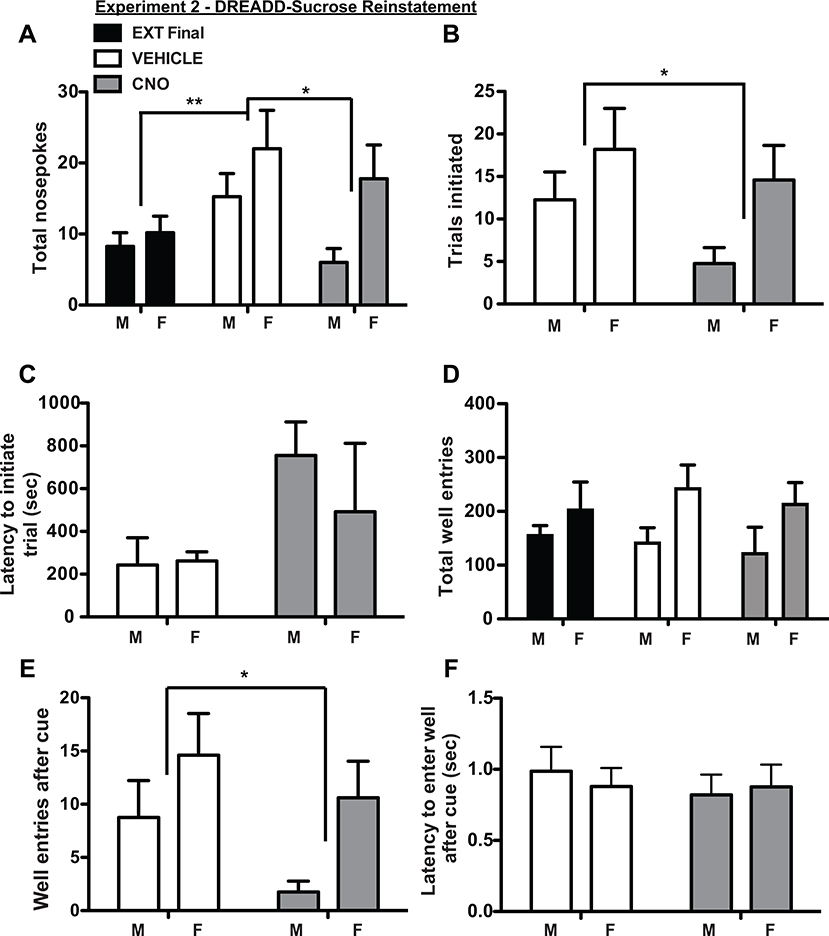
OFC inactivation suppressed 15% sucrose reinstatement
After meeting extinction criteria of <20 nosepokes for 2 consecutive sessions, rats received cue-induced reinstatement trials in which nosepokes delivered sucrose cues but no reward. When treated with vehicle, rats exhibited significant cue-induced reinstatement as measured by increased total nosepokes compared to the last day of extinction (Fig 5A; main effect of session F(1.45, 10.16) = 8.57, p = 0.01, two factor ANOVA; extinction vs. vehicle: p <0.05; Tukey). CNO treatment significantly decreased reinstatement relative to vehicle (Fig. 5A, CNO vs. vehicle: p<0.05), resulting in nosepokes at the same level as the last extinction session (CNO vs. extinction: p > 0.05). There were no significant main effects of sex (F(1, 7) = 2.14, p = 0.19) and no interaction effects (F(2, 14) = 2.22, p = 0.15). CNO significantly decreased numbers of initiated trials (Fig. 5B; F(1, 7) = 9.67, p = 0.02, two factor ANOVA), but there were no sex differences (F(1, 7) = 2.25, p =0.18) and no interaction effects (F(1, 7) = 1.19, p = 0.31). There were no significant differences in latency to initiate trials based on treatment or sex (Fig. 5C; no main effects of treatment or sex, no interaction effect, all p > 0.05, two factor ANOVA). There were no significant differences in total well entries (Fig. 5D), but CNO significantly reduced well-entries following nosepoke-elicited sucrose cues (Fig. 5E; main effect of treatment: F(1, 7) = 6.54, p = 0.04 two factor ANOVA) independent of sex (no main effect of sex: F(1, 7) = 3.01, p = 0.13; no interaction effect: F(1, 7) = 0.49, p = 0.51). There were no treatment or sex influences on latency to enter the reward port after nosepoke-elicited cues (Fig. 5F; no main effects of treatment or sex, no interaction effect, all p > 0.05, two factor ANOVA).
Figure 5.
OFC inactivation suppressed reinstatement of sucrose seeking in both male and female rats. As with 10% ethanol, total number of nosepokes (A), number of initiated trials (B), and well entries after cue (E) were decreased after OFC inactivation, and total well entries (D) and latency to enter the well (F) were not affected. Latency to initiate trials was increased (C), but not significantly. Although differences were observed between male and female rats, particularly after OFC inactivation (e.g., A, B, and E), there were no significant effects of sex and no significant interaction effects between sex and treatment.
DISCUSSION
Understanding how reward seeking is driven by OFC activity is critical, both for understanding OFC contributions to behavior as well as to identify potential correlates of problematic drug or alcohol use such as is seen during relapse. Here we describe a specific role for OFC in reinstatement, but not free-access (in the homecage) or FR1 operant reward seeking of 10% or 20% ethanol or sucrose. During reinstatement, OFC inhibition decreased nosepokes as well as reward well port entry after nosepoke/cue presentation. Latency to initiate trials was also increased. Importantly, total numbers of well-entries, including those not associated with a reinstatement nosepoke/cue, were not decreased. Together these results indicate that OFC inactivation did not nonselectively decrease activity or even reward-seeking activity during reinstatement, but instead specifically reduced the association between the cue and outcome, thereby diminishing the operant seeking response.
In this report we have expanded upon previously-described roles for OFC in ethanol reinstatement (Bianchi et al., 2018, Arinze and Moorman, 2020) in a number of ways. First, we showed that inactivation of (primarily lateral) OFC disrupts cue-induced reinstatement for both alcohol and sucrose, indicating a broad role for OFC in reinstatement of reward seeking. Second, we included measurement of the impact of OFC inactivation on non-operant homecage free access, showing no effect on consumption of 10% or 20% ethanol or sucrose. Third, we extended previous findings to show that OFC inactivation had no impact on 10% ethanol or sucrose self-administration, and that cue-induced reinstatement of 10% ethanol and sucrose was decreased, expanding upon previous findings using 20% ethanol (Arinze and Moorman, 2020). Fourth, we demonstrated no significant sex differences in the impact of OFC inactivation on reward seeking and consumption, which emphasizes the broad role of OFC in reinstatement across sex. An additional novel element of the current study was afforded by the use of DREADDs in that we were able to make these comparisons of OFC inactivation in homecage, self-administration, and reinstatement tests in the same DREADD-expressing neural ensembles in the same individuals. Performing this many repeated comparisons within subjects is challenging if not impossible using techniques such as local pharmacological manipulation (Arinze and Moorman, 2020, Bianchi et al., 2018) given potential uneven dispersal of pharmacological agents across neuron populations during different infusions as well as the potential cumulative influence of tissue damage on neural signaling in the targeted region (Caballero et al., 2019). Combined with previous studies, our data indicate that OFC plays a limited role in driving reward seeking or consumption when reward is freely available. In contrast, OFC plays a more important role when reward seeking is driven by learned associations, such as reward-associated cues, in the absence of primary reinforcers. Intriguingly, this appears to be a fundamental function across individuals and outcomes as we observed consistent results across sex and across sucrose and ethanol reinforcers, in line with previous studies the role of OFC in reinstatement of psychostimulant seeking (see below for discussion).
There were no significant sex differences in the impact of OFC inhibition on any aspect of ethanol or sucrose seeking or consumption tested here. We did observe sex differences in behavior. Females consumed more 20% ethanol and, to a lesser extent, 10% ethanol than males during homecage consumption. In contrast, there were no significant sex differences in operant seeking, either during FR1 self-administration or during reinstatement of ethanol seeking. Females appeared to show greater reinstatement to sucrose than males, and some resistance to OFC inhibition, though this effect was not significant (Fig. 5). The fact that females consumed more ethanol than males during free access but showed no differences in operant behavior indicates that different alcohol-associated behaviors (drinking vs. self-administration vs. reinstatement) are differentially modulated in a sexually-dimorphic fashion, supporting previous findings (Juarez and Barrios de Tomasi, 1999, Priddy et al., 2017, Bertholomey et al., 2016, Bianchi et al., 2018, Randall et al., 2017), though there is some variability with respect to previous findings of sex-differences in reinstatement. Our results are also aligned with previous studies in which OFC regulation of ethanol reinstatement was equivalent across sex, influencing reinstatement approximately equally in both males and females (Bianchi et al., 2018). We do note that relatively small numbers of male and female rats may have reduced our ability to detect subtle statistical differences, particularly in Experiment 2 (sucrose only) and note that there is still a need to explore sex differences in OFC-guided behavior in future studies.
The present results are also aligned with previous findings showing a general role for OFC in regulating reinstatement. This includes reinstatement of ethanol seeking (Bianchi et al., 2018, Arinze and Moorman, 2020), responding during extinction of ethanol seeking (Morisot et al., 2019), and reinstatement of cocaine seeking (Fuchs et al., 2004, Lasseter et al., 2009, Capriles et al., 2003, Kantak et al., 2009). Together, these data indicate an overarching role for OFC in reinstatement of most, if not all, reinforcers. This is supported by the fact that OFC inactivation also decreased reinstatement of sucrose seeking in our study. A previous study showed no effect of OFC lesions on food-primed reinstatement of food seeking (Grakalic et al., 2010), underscoring the specific role of the OFC in reinstatement driven by a learned association such as cues or context.
Our target region for inactivation was lateral, as opposed to medial, OFC as previous studies comparing functional roles of rodent OFC subregions have shown an important role for lateral OFC in value representation and reward seeking (Arguello et al., 2017, Fuchs et al., 2004, Lasseter et al., 2009, Lasseter et al., 2011, Lopatina et al., 2016, Lopatina et al., 2015, Lopatina et al., 2017, Burton et al., 2014) including reinstatement of ethanol seeking (Arinze and Moorman, 2020). Unlike (Arinze and Moorman, 2020), we did not dissociate separable contributions of OFC subregions, so we have referred throughout the text to OFC broadly. However, based on prior work we believe that the main anatomical substrate driving the effects here is lateral OFC (Arinze and Moorman, 2020) (but see cannula placements in (Bianchi et al., 2018)). We do note that there is likely additional heterogeneity in other dimensions (such as anterior-posterior location), and that further work is needed refine our understanding of the specific circuits underlying OFC contributions to reinstatement.
Also of relevance to anatomical specificity, a number of recent studies have indicated that the rodent anterior insula (AI), located immediately caudal to the lateral OFC, plays an important role in reward-related behaviors, including those directed to alcohol. As with OFC, contributions of AI to alcohol seeking are complex and depend on a number of factors such as whether alcohol is presented in conjunction with a paired aversive outcome (Seif et al., 2013) or punishment-imposed abstinence (Campbell et al., 2019). Intriguingly, recent work has shown that chemogenetic inhibition of AI increases alcohol operant self-administration (Jaramillo et al., 2018a) and that chemogenetic activation of AI decreases alcohol (and sucrose) intake (Haaranen et al., 2020). Together these results show that, like OFC, AI is strongly involved in the use of alcohol and other reinforcers. However, despite it close location, AI seems to perform qualitatively different functions: AI inhibition increases drinking and self-administration, whereas OFC inhibition decreases reinstatement, but not drinking or self-administration. It is also worth noting that specific circuits within these regions may differentially contribute to seeking and use of alcohol and other reinforcers as, for example, inactivation of AI projections to nucleus accumbens actually decreased alcohol self-administration (Jaramillo et al., 2018b, Jaramillo et al., 2018a, Seif et al., 2013). In general, addressing both regional and circuit-selective differences in contributions to behavior is a key strategy going forward.
We were surprised to observe a significant decrease in trial initiation latency in male, but not female, GFP-expressing control rats during ethanol self-administration. CNO should have no effect in animals expressing GFP but not DREADDS (Armbruster et al., 2007). Although CNO has been shown to convert to clozapine in vivo (Gomez et al., 2017), nonselective effects of CNO are rarely seen in DREADD non-expressing animals (Mahler and Aston-Jones, 2018). Furthermore, a significant impact of clozapine on behavior would result in decreased locomotor activity (Coward, 1992, Gomez et al., 2017), which is the opposite of what was observed with treatment. The fact that CNO sped responses in control rats suggests that we did not observe an off-target effect of CNO or conversion to clozapine. Further, the fact that this effect was not seen in DREADD-expressing rats during any phase of ethanol or sucrose testing (drinking, self-administration, reinstatement) and the fact that this effect was not seen in control rats during sucrose or 20% ethanol self-administration, nor during reinstatement, suggests that this was a very limited effect. There is no mechanism that we can identify which would explain how CNO could speed responses in male, but not female, control animals in one behavior but not others, and have no effect in DREADD-expressing animals. It is theoretically possible that faster task initiation resulted from an interaction between 10% ethanol and CNO in male rats that was protected against by OFC inactivation. Rather than speculate such extreme explanations, our conservative conclusion is that the effect was driven by small numbers of animals, chance outcomes, or experimenter error on test day. The finding does not undermine the consistent, selective, and strong influence of DREADD inactivation of OFC on both ethanol and sucrose reinstatement in both male and female rats. It does, however, warrant careful experimental control in future studies, however, to assess whether this phenomenon is legitimate or not. We also note a related set of limitations to our studies related to validation of the specific effects of CNO on expressed DREADDs in the OFC. Due to technical issues we were unable to confirm physiological inhibitory effects of CNO on DREADDs in our lab using, for example, electrophysiology or c-Fos. So, although numerous studies have confirmed that CNO does inhibit neurons through the hM4Di receptor, it is possible that the effects observed could be due to other factors besides DREADD inactivation. Related, because we chose to use a synapsin promoter to target the entire OFC, we were unable to specify whether effects of DREADD inactivation were due to pyramidal neuron or GABAergic interneuron inactivation as both populations of neurons presumably expressed DREADDs during testing. Our tentative conclusion is that the combined outcome of inactivating all of OFC was to reduce the activity of projections out of OFC to regulate reinstatement behavior, but of course, these predictions should be tested using cell-type and circuit specific neuronal manipulation to be certain.
Outside of reinstatement, at least two other studies have found an impact of OFC manipulation on motivation for ethanol. In these studies, however, lesions or DREADD inhibition of OFC increased drinking in rats (Ray et al, 2018) and mice treated with chronic ethanol (den Hartog et al, 2016). In the study by den Hartog and colleagues, mice with OFC lesions increased ethanol consumption after, but not before, chronic intermittent ethanol (CIE) vapor treatment. Similar findings were observed with DREADD inactivation. In the manuscript by Ray and colleagues, rats underwent fear conditioning post-OFC lesion followed by extinction and ethanol drinking tests. OFC-lesioned rats consumed more ethanol across all eight drinking sessions. In the current study, DREADD inactivation of OFC had no impact on homecage ethanol consumption or operant self-administration. Given the differences in experimental designs (CIE vs. fear conditioning, vs. no prior treatment), it is somewhat hard to find a unifying role for OFC across contexts, particularly given differential contributions of OFC subregions to motivated behavior as described above. However, one possibility is that both CIE and fear conditioning shift animals away from value-driven, or goal-directed ethanol consumption and towards habitual or craving-associated ethanol use. Given the broad role of OFC in regulating value decisions and goal-directed behavior, OFC inactivation may sever the final link between controlled consumption and enhance drive by other systems, potentially those associated with stress, craving, or habitual behavior such as central amygdala, bed nucleus of the stria terminalis, lateral hypothalamus, noradrenergic nuclei, dorsal striatum, or other structures implicated in ethanol use (Koob, 2014, Corbit and Janak, 2016, Hopf, 2020, Centanni et al., 2019). Though broadly supported by previous work (Baltz et al., 2018, Gremel and Costa, 2013) this hypothesis is still speculative and necessitates further experimental testing. It does, however, line up with our conceptualization of OFC as using maintained associative value representation to guide behavior, discussed below. In the absence of value/goal associated behavioral control, other drives may elevate ethanol use to counteract increased stress or craving associated with the treatments such as fear or CIE used in previous studies.
In combination with previous work, our results demonstrate that OFC is not globally responsible for driving reward seeking. Instead, OFC may be critically involved when representation of value and/or associations between learned cues and representations of reward value must be maintained online in order to direct behavior. In addition to explaining selective impact of OFC inactivation on reinstatement, this framework is also well aligned with the demonstrated role of OFC in behavioral flexibility as seen in reversal learning (Izquierdo et al., 2017, McAlonan and Brown, 2003, Ghods-Sharifi et al., 2008, Groman et al., 2013), set shifting (Sleezer et al., 2016, Chase et al., 2012), and altering responding after outcome devaluation, in which online representations of outcome must be updated to change behavior (Gottfried et al., 2003, Gremel and Costa, 2013, Gourley et al., 2013, West et al., 2011). In general, these observations are also concordant with the concept of OFC neuron ensembles encoding specific aspects of a cognitive “map” (Schuck et al., 2016, Wilson et al., 2014, Lopatina et al., 2017) that permits model-based (as opposed to habitual, or automatic) decision-making behavior (McDannald et al., 2011, Jones et al., 2012, Gremel and Costa, 2013). In the case of reinstatement, OFC networks may map the association between reward outcomes and the cues associated with these outcomes, thus emphasizing the role of OFC in the learning-related elements of reinstatement. During reward seeking in the presence of an available outcome, this learned association is not required and OFC inactivation has no effect on seeking behavior. This conceptualization emphasizes the cognitive aspects of drug seeking, and relapse in particular. Relapse to alcohol and other drugs of abuse is dependent on a maintained representation of the value of the drug reward such that reward-associated cues evoke this representation and drive drug seeking. One potential strategy for reducing relapse therefore might be disrupting the maintained value representation of the outcome via cognitive behavioral strategies or perhaps through modulation of OFC function.
Acknowledgments
This work was supported by NIH research grants AA024571, AA025481, DA041674, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (DEM) and NIH research grant GM099649 (JSH). The authors declare no competing financial interests.
REFERENCES
- ARGUELLO AA, RICHARDSON BD, HALL JL, WANG R, HODGES MA, MITCHELL MP, STUBER GD, ROSSI DJ & FUCHS RA 2017. Role of a Lateral Orbital Frontal Cortex-Basolateral Amygdala Circuit in Cue-Induced Cocaine-Seeking Behavior. Neuropsychopharmacology, 42, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARINZE I & MOORMAN DE 2020. Selective impact of lateral orbitofrontal cortex inactivation on reinstatement of alcohol seeking in male Long-Evans rats. Neuropharmacology, 168, 108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMBRUSTER BN, LI X, PAUSCH MH, HERLITZE S & ROTH BL 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A, 104, 5163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLEINE BW, LEUNG BK & OSTLUND SB 2011. The orbitofrontal cortex, predicted value, and choice. Ann N Y Acad Sci, 1239, 43–50. [DOI] [PubMed] [Google Scholar]
- BALTZ ET, YALCINBAS EA, RENTERIA R & GREMEL CM 2018. Orbital frontal cortex updates state-induced value change for decision-making. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARAK S, LIU F, BEN HAMIDA S, YOWELL QV, NEASTA J, KHARAZIA V, JANAK PH & RON D 2013. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci, 16, 1111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK A, WUSTENBERG T, GENAUCK A, WRASE J, SCHLAGENHAUF F, SMOLKA MN, MANN K & HEINZ A 2012. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry, 69, 842–52. [DOI] [PubMed] [Google Scholar]
- BERTHOLOMEY ML, NAGARAJAN V & TORREGROSSA MM 2016. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl), 233, 2277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHI PC, CARNEIRO DE OLIVEIRA PE, PALOMBO P, LEAO RM, COGO-MOREIRA H, PLANETA CDS & CRUZ FC 2018. Functional inactivation of the orbitofrontal cortex disrupts context-induced reinstatement of alcohol seeking in rats. Drug Alcohol Depend, 186, 102–112. [DOI] [PubMed] [Google Scholar]
- BURTON AC, KASHTELYAN V, BRYDEN DW & ROESCH MR 2014. Increased firing to cues that predict low-value reward in the medial orbitofrontal cortex. Cereb Cortex, 24, 3310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABALLERO JP, SCARPA GB, REMAGE-HEALEY L & MOORMAN DE 2019. Differential Effects of Dorsal and Ventral Medial Prefrontal Cortex Inactivation during Natural Reward Seeking, Extinction, and Cue-Induced Reinstatement. eNeuro, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL EJ, FLANAGAN JPM, WALKER LC, HILL M, MARCHANT NJ & LAWRENCE AJ 2019. Anterior Insular Cortex is Critical for the Propensity to Relapse Following Punishment-Imposed Abstinence of Alcohol Seeking. J Neurosci, 39, 1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPRILES N, RODAROS D, SORGE RE & STEWART J 2003. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl), 168, 66–74. [DOI] [PubMed] [Google Scholar]
- CENTANNI SW, BEDSE G, PATEL S & WINDER DG 2019. Driving the Downward Spiral: Alcohol-Induced Dysregulation of Extended Amygdala Circuits and Negative Affect. Alcohol Clin Exp Res, 43, 2000–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHASE EA, TAIT DS & BROWN VJ 2012. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci, 36, 2368–75. [DOI] [PubMed] [Google Scholar]
- CORBIT LH & JANAK PH 2016. Habitual Alcohol Seeking: Neural Bases and Possible Relations to Alcohol Use Disorders. Alcohol Clin Exp Res, 40, 1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWARD DM 1992. General pharmacology of clozapine. Br J Psychiatry Suppl, 5–11. [PubMed] [Google Scholar]
- DEN HARTOG C, ZAMUDIO-BULCOCK P, NIMITVILAI S, GILSTRAP M, EATON B, FEDAROVICH H, MOTTS A & WOODWARD JJ 2016. Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology, 107, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOM G, SABBE B, HULSTIJN W & VAN DEN BRINK W 2005. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry, 187, 209–20. [DOI] [PubMed] [Google Scholar]
- DURAZZO TC, TOSUN D, BUCKLEY S, GAZDZINSKI S, MON A, FRYER SL & MEYERHOFF DJ 2011. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res, 35, 1187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUCHS RA, EVANS KA, PARKER MP & SEE RE 2004. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci, 24, 6600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHODS-SHARIFI S, HALUK DM & FLORESCO SB 2008. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem, 89, 567–73. [DOI] [PubMed] [Google Scholar]
- GOMEZ JL, BONAVENTURA J, LESNIAK W, MATHEWS WB, SYSA-SHAH P, RODRIGUEZ LA, ELLIS RJ, RICHIE CT, HARVEY BK, DANNALS RF, POMPER MG, BONCI A & MICHAELIDES M 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTFRIED JA, O’DOHERTY J & DOLAN RJ 2003. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301, 1104–7. [DOI] [PubMed] [Google Scholar]
- GOURLEY SL, OLEVSKA A, ZIMMERMANN KS, RESSLER KJ, DILEONE RJ & TAYLOR JR 2013. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci, 38, 2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAKALIC I, PANLILIO LV, QUIROZ C & SCHINDLER CW 2010. Effects of orbitofrontal cortex lesions on cocaine self-administration. Neuroscience, 165, 313–24. [DOI] [PubMed] [Google Scholar]
- GREMEL CM & COSTA RM 2013. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun, 4, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN SM, JAMES AS, SEU E, CRAWFORD MA, HARPSTER SN & JENTSCH JD 2013. Monoamine levels within the orbitofrontal cortex and putamen interact to predict reversal learning performance. Biol Psychiatry, 73, 756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAARANEN M, SCUPPA G, TAMBALO S, JARVI V, BERTOZZI SM, ARMIROTTI A, SOMMER WH, BIFONE A & HYYTIA P 2020. Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl Psychiatry, 10, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARPER KM, KNAPP DJ, BUTLER RK, COOK CA, CRISWELL HE, STUBER GD & BREESE GR 2019. Amygdala Arginine Vasopressin Modulates Chronic Ethanol Withdrawal Anxiety-Like Behavior in the Social Interaction Task. Alcohol Clin Exp Res, 43, 2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPF FW 2020. Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology, 168, 108013. [DOI] [PubMed] [Google Scholar]
- IZQUIERDO A, BRIGMAN JL, RADKE AK, RUDEBECK PH & HOLMES A 2017. The neural basis of reversal learning: An updated perspective. Neuroscience, 345, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAMILLO AA, RANDALL PA, STEWART S, FORTINO B, VAN VOORHIES K & BESHEER J 2018a. Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology, 130, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAMILLO AA, VAN VOORHIES K, RANDALL PA & BESHEER J 2018b. Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav Brain Res, 348, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES JL, ESBER GR, MCDANNALD MA, GRUBER AJ, HERNANDEZ A, MIRENZI A & SCHOENBAUM G 2012. Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science, 338, 953–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUAREZ J & BARRIOS DE TOMASI E 1999. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol, 19, 15–22. [DOI] [PubMed] [Google Scholar]
- JUPP B, KRSTEW E, DEZSI G & LAWRENCE AJ 2011. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol, 162, 880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTAK KM, MASHHOON Y, SILVERMAN DN, JANES AC & GOODRICH CM 2009. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res, 201, 128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF 2014. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol, 125, 33–54. [DOI] [PubMed] [Google Scholar]
- KURUOGLU AC, ARIKAN Z, VURAL G, KARATAS M, ARAC M & ISIK E 1996. Single photon emission computerised tomography in chronic alcoholism. Antisocial personality disorder may be associated with decreased frontal perfusion. Br J Psychiatry, 169, 348–54. [DOI] [PubMed] [Google Scholar]
- LAGUESSE S, MORISOT N, PHAMLUONG K & RON D 2016. Region specific activation of the AKT and mTORC1 pathway in response to excessive alcohol intake in rodents. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSETER HC, RAMIREZ DR, XIE X & FUCHS RA 2009. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci, 30, 1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSETER HC, WELLS AM, XIE X & FUCHS RA 2011. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology, 36, 711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONDON ED, ERNST M, GRANT S, BONSON K & WEINSTEIN A 2000. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex, 10, 334–42. [DOI] [PubMed] [Google Scholar]
- LOPATINA N, MCDANNALD MA, STYER CV, PETERSON JF, SADACCA BF, CHEER JF & SCHOENBAUM G 2016. Medial Orbitofrontal Neurons Preferentially Signal Cues Predicting Changes in Reward during Unblocking. J Neurosci, 36, 8416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPATINA N, MCDANNALD MA, STYER CV, SADACCA BF, CHEER JF & SCHOENBAUM G 2015. Lateral orbitofrontal neurons acquire responses to upshifted, downshifted, or blocked cues during unblocking. Elife, 4, e11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPATINA N, SADACCA BF, MCDANNALD MA, STYER CV, PETERSON JF, CHEER JF & SCHOENBAUM G 2017. Ensembles in medial and lateral orbitofrontal cortex construct cognitive maps emphasizing different features of the behavioral landscape. Behav Neurosci, 131, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER SV & ASTON-JONES G 2018. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology, 43, 934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCALONAN K & BROWN VJ 2003. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res, 146, 97–103. [DOI] [PubMed] [Google Scholar]
- MCDANNALD MA, LUCANTONIO F, BURKE KA, NIV Y & SCHOENBAUM G 2011. Ventral striatum and orbitofrontal cortex are both required for model-based, but not model-free, reinforcement learning. J Neurosci, 31, 2700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER HC & BUCCI DJ 2016. Imbalanced Activity in the Orbitofrontal Cortex and Nucleus Accumbens Impairs Behavioral Inhibition. Curr Biol, 26, 2834–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORMAN DE 2018. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog Neuropsychopharmacol Biol Psychiatry, 87, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORISOT N, PHAMLUONG K, EHINGER Y, BERGER AL, MOFFAT JJ & RON D 2019. mTORC1 in the orbitofrontal cortex promotes habitual alcohol seeking. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRICK H, ANTON RF, LI X, HENDERSON S, DROBES D, VORONIN K & GEORGE MS 2004. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology, 29, 393–402. [DOI] [PubMed] [Google Scholar]
- NICOLAS JM, CATAFAU AM, ESTRUCH R, LOMENA FJ, SALAMERO M, HERRANZ R, MONFORTE R, CARDENAL C & URBANO-MARQUEZ A 1993. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med, 34, 1452–9. [PubMed] [Google Scholar]
- PRIDDY BM, CARMACK SA, THOMAS LC, VENDRUSCOLO JC, KOOB GF & VENDRUSCOLO LF 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav, 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUROHIT K, PAREKH PK, KERN J, LOGAN RW, LIU Z, HUANG Y, MCCLUNG CA, CRABBE JC & OZBURN AR 2018. Pharmacogenetic Manipulation of the Nucleus Accumbens Alters Binge-Like Alcohol Drinking in Mice. Alcohol Clin Exp Res, 42, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL PA, STEWART RT & BESHEER J 2017. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol Biochem Behav, 156, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDO K, HONG KI, BHAGWAGAR Z, LI CS, BERGQUIST K, GUARNACCIA J & SINHA R 2011. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry, 168, 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY MH, HANLON E & MCDANNALD MA 2018. Lateral orbitofrontal cortex partitions mechanisms for fear regulation and alcohol consumption. PLoS One, 13, e0198043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIMSNIDER S, MANFREDSSON FP, MUZYCZKA N & MANDEL RJ 2007. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther, 15, 1504–11. [DOI] [PubMed] [Google Scholar]
- RUDEBECK PH & MURRAY EA 2014. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron, 84, 1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOENBAUM G & SHAHAM Y 2008. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry, 63, 256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUCK NW, CAI MB, WILSON RC & NIV Y 2016. Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron, 91, 1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIF T, CHANG SJ, SIMMS JA, GIBB SL, DADGAR J, CHEN BT, HARVEY BK, RON D, MESSING RO, BONCI A & HOPF FW 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci, 16, 1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEEZER BJ, CASTAGNO MD & HAYDEN BY 2016. Rule Encoding in Orbitofrontal Cortex and Striatum Guides Selection. J Neurosci, 36, 11223–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH KS, BUCCI DJ, LUIKART BW & MAHLER SV 2016. DREADDS: Use and application in behavioral neuroscience. Behav Neurosci, 130, 137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STALNAKER TA, COOCH NK & SCHOENBAUM G 2015. What the orbitofrontal cortex does not do. Nat Neurosci, 18, 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPERT SF, CHEUNG EH, BROWN GG, FRANK LR, PAULUS MP, SCHWEINSBURG AD, MELOY MJ & BROWN SA 2003. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry, 60, 727–35. [DOI] [PubMed] [Google Scholar]
- VOLKOW ND & FOWLER JS 2000. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex, 10, 318–25. [DOI] [PubMed] [Google Scholar]
- VOLKOW ND, WANG GJ, OVERALL JE, HITZEMANN R, FOWLER JS, PAPPAS N, FRECSKA E & PISCANI K 1997. Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin Exp Res, 21, 1278–84. [PubMed] [Google Scholar]
- WALLIS JD 2011. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci, 15, 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEST EA, DESJARDIN JT, GALE K & MALKOVA L 2011. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. J Neurosci, 31, 15128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON RC, TAKAHASHI YK, SCHOENBAUM G & NIV Y 2014. Orbitofrontal cortex as a cognitive map of task space. Neuron, 81, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISE RA 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia, 29, 203–10. [DOI] [PubMed] [Google Scholar]
- WRASE J, GRUSSER SM, KLEIN S, DIENER C, HERMANN D, FLOR H, MANN K, BRAUS DF & HEINZ A 2002. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry, 17, 287–91. [DOI] [PubMed] [Google Scholar]