Abstract
Background:
Twenty million Americans suffer from peripheral nerve injury. These patients often develop chronic pain and sensory dysfunctions. In the past decade, neuroimaging studies showed that these changes are associated with altered cortical excitation-inhibition balance and maladaptive plasticity. We tested if neuromodulation of the deprived sensory cortex could restore the cortical balance, and whether it would be effective in alleviating sensory complications.
Objective:
We tested if non-invasive repetitive transcranial magnetic stimulation (rTMS) which induces neuronal excitability, and cell-specific magnetic activation via the Electromagnetic-perceptive gene (EPG) which is a novel gene that was identified and cloned from glass catfish and demonstrated to evoke neural responses when magnetically stimulated, can restore cortical excitability.
Methods:
A rat model of forepaw denervation was used. rTMS was delivered every other day for 30 days, starting at the acute or at the chronic post-injury phase. A minimally-invasive neuromodulation via EPG was performed every day for 30 staring at the chronic phase. A battery of behavioral tests was performed in the days and weeks following limb denervation in EPG-treated rats, and behavioral tests, fMRI and immunochemistry were performed in rTMS-treated rats.
Results:
The results demonstrate that neuromodulation significantly improved long-term mobility, decreased anxiety and enhanced neuroplasticity. The results identify that both acute and delayed rTMS intervention facilitated rehabilitation. Moreover, the results implicate EPG as an effective cell-specific neuromodulation approach.
Conclusion:
Together, these results reinforce the growing amount of evidence from human and animal studies that are establishing neuromodulation as an effective strategy to promote plasticity and rehabilitation.
Graphical Abstract
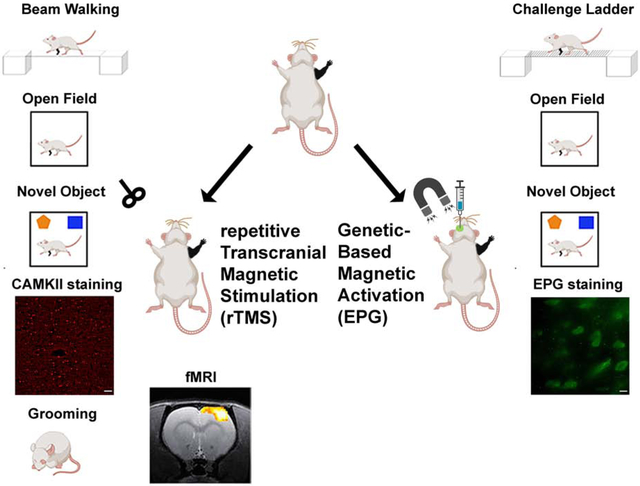
Introduction
Twenty million individuals in the United States are suffering from peripheral nerve injury. Current strategies to facilitate recovery following peripheral nerve injury mainly focus on manipulating the activity of the injured and the non-injured limbs. However, in spite of refined surgical techniques and the available rehabilitation strategies, the clinical outcome in adults is generally poor with persisting sensory dysfunction and pain complications (Lundborg, 2003; Abler et al., 2005).
Over the last 35 years, studies have shown that acute or chronic disturbance to sensory afferents is reflected in distorted cortical representations. These anatomical and functional changes are evident by electrophysiology and fMRI methods and may impact clinical outcome. For example, human studies suggest a strong correlation between abnormal post-injury cortical responses that are often observed with fMRI to the degree of sensory dysfunctions and phantom limb pain (Flor et al., 1995; Dettmers et al., 2001; Flor, 2002). The neural mechanisms implicated in the post-injury cortical changes have been extensively studied in animal models; Studies indicate that peripheral injury evokes cellular mechanisms effecting immediate (Han et al., 2013) and long-term (Pelled et al., 2007; Pelled et al., 2009; Li et al., 2011; Yu et al., 2012; Jouroukhin et al., 2014) function of the primary somatosensory cortex (S1) contralateral and ipsilateral to the injured limb. These mechanisms include alteration in the excitation-inhibition balance (Blom et al., 2014), changes in GABAergic function (Capaday et al., 2000), and increases in the activity of inhibitory interneurons in cortical layer 5 (L5) in the affected (deprived) cortex (Pelled et al., 2009; Li et al., 2011; Han et al., 2013). Therefore, it is conceivable that post-injury cellular changes affect neurorehabilitation and may dictate the degree of recovery.
Evidence from human studies support that modulation of cortical function, and specifically, increasing cortical excitation, have clinical implications. For example, removing the afferents of the “good hand” via tourniquet-induced anesthesia, anesthetic block, and constraint induced therapy lead to improved hand function (Bjorkman et al., 2005; Rosen et al., 2006). Harnessing the brain’s innate plasticity mechanisms through non-invasive methods such as transcranial magnetic stimulation (TMS) has recently gained interest for use in functional and behavioral research as well as rehabilitation research after brain injury (Celnik et al., 2009; Guo et al., 2015; Lu et al., 2015) and neurodegenerative diseases (Shin and Pelled, 2017). Various studies showed promising results using TMS in humans (Sung et al., 2013; Bocci et al., 2016), primates (Mueller et al., 2014), and rodents (Lu et al., 2015; Andreou et al., 2016; Shin et al., 2018; Kloosterboer and Funke, 2019; Krishnan et al., 2019). Importantly, TMS has shown effectiveness in manipulating transcallosal communication in patients suffering from peripheral injury (Karl et al., 2001) and alleviating pain associated with injury (Leo and Latif, 2007). TMS has also been shown to increase neuronal excitability and markers associated with plasticity such as brain-derived neurotrophic factor (BDNF)(Gersner et al., 2011), c-fos (Zhang et al., 2007) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Lu et al., 2015). However, it is still not clear when is the optimal and most effective time for TMS intervention (acute, subacute or chronic phase) to take place after injury. Identifying the most effective stimulation protocols in an animal model could impact moving this treatment intervention forward. Furthermore, evidence suggest that TMS increases the activity of a wide type of neural population (Hoppenrath and Funke, 2013) and currently there is no protocol that enables targeting a specific neural population. Thus, developing neuromodulation strategies to restore normal neural excitability levels with cell-specific precision could lay the groundwork for transforming current clinical practice.
Major advances in molecular and synthetic biology have revolutionized the capability to control cell excitability in living organisms. One of these technologies, magnetic manipulation by the electromagnetic preceptive gene (EPG), allows minimally invasive and cell-specific neuromodulation using external magnetic fields. EPG is a protein that is sensitive to electromagnetic fields which was recently identified in the fish glass catfish (Krishnan et al., 2018; Hunt et al., 2020; Hwang et al., 2020). Recent work had demonstrated that calcium imaging in mammalian cells and cultured neurons expressing EPG activated remotely by magnetic fields led to increases in intracellular calcium concentrations, indicative of cellular excitability. Moreover, wireless magnetic activation of EPG in rat motor cortex induced motor evoked responses of the contralateral forelimb in vivo. Expressing EPG in S1 contralateral to the injury in rats may provide a way to increase excitation by specifically targeting the excitatory cortical neurons and minimizing off-target affects.
Here we capitalized on a battery of behavioral tests, functional MRI (fMRI) and immunohistochemistry to test the effectivity of rTMS intervention, and behavioral tests and immunohistochemistry to test the effectivity of EPG-based neuromodulation in improving short- and long-term sensory, motor, and cognitive outcomes in a rodent model of peripheral nerve injury.
Results
TMS enhances sensorimotor functions
We first tested if non-invasive brain stimulation via rTMS focused on the deprived S1 (contralateral to the denervated forelimb) is an effective strategy to facilitate plasticity and rehabilitation. We performed a battery of behavioral tests to characterize sensorimotor and cognitive functions associated with denervation injury. An illustration depicting the animal model and the different modulation strategies is shown in Fig. 1. A beam walk test with two different width settings was performed every other week to evaluate sensorimotor functions. The results show that denervated rats that received rTMS treatment every other day for 30 days, starting the day after injury (Den-rTMS-Acute, n=6), showed significantly shorter traverse times and enhanced mobility compared to rats that received rTMS treatment starting 3-weeks after injury (Den-rTMS-Delayed, n=6) and injured rats that received no treatment (Den-No rTMS, n=6). This was true for both the 6.3 cm width (Den-rTMS-Acute, 5.64 ±0.4 s; Den-rTMS-Delayed, 6.35 ±0.4 s; Den-No rTMS, 20.56 ±2.0 s; Control, 5.95 ±0.2 s; F (3,12) =24.27, p<0.05; Acute vs. No rTMS: HSD p < 0.05 at each time point; Delayed vs. No rTMS: HSD p < 0.05 at each time point; Acute vs Delayed: NS) and the more challenging, 3.9 cm width beam (Den-rTMS-Acute, 6.29 ±0.4 s; Den-rTMS-Delayed, 7.02 ±0.3 s; Den-No rTMS, 26.4 ±3.4 s; Control, 5.95 ±0.2 s; F (3,11) =13.57, p<0.05) (Fig. 2(A)). The results show a shortening in the traverse time reflecting an improvement in sensorimotor functions and mobility throughout the course of rTMS treatment. The results demonstrate that at the end of the 4-week rTMS treatment, both the Acute and the Delayed group’s traverse times were shortened and similar to the Control group times; The Den-rTMS-Acute group demonstrated an improvement of 61.3% on the 6.3 cm width challenge beam walk, and a 64.8% on the 3.9 cm challenge beam walk after 4 weeks of treatment. The Den-rTMS-Delayed demonstrated an improvement of 54.7% on the 6.3 cm challenge, and a 55.1% on the 3.9 cm challenge, and the Den-No rTMS showed only a 19% and 37.8% improvement on the 6.3 cm and 3.9 cm challenge, respectively, over the same time frame.
Fig. 1.
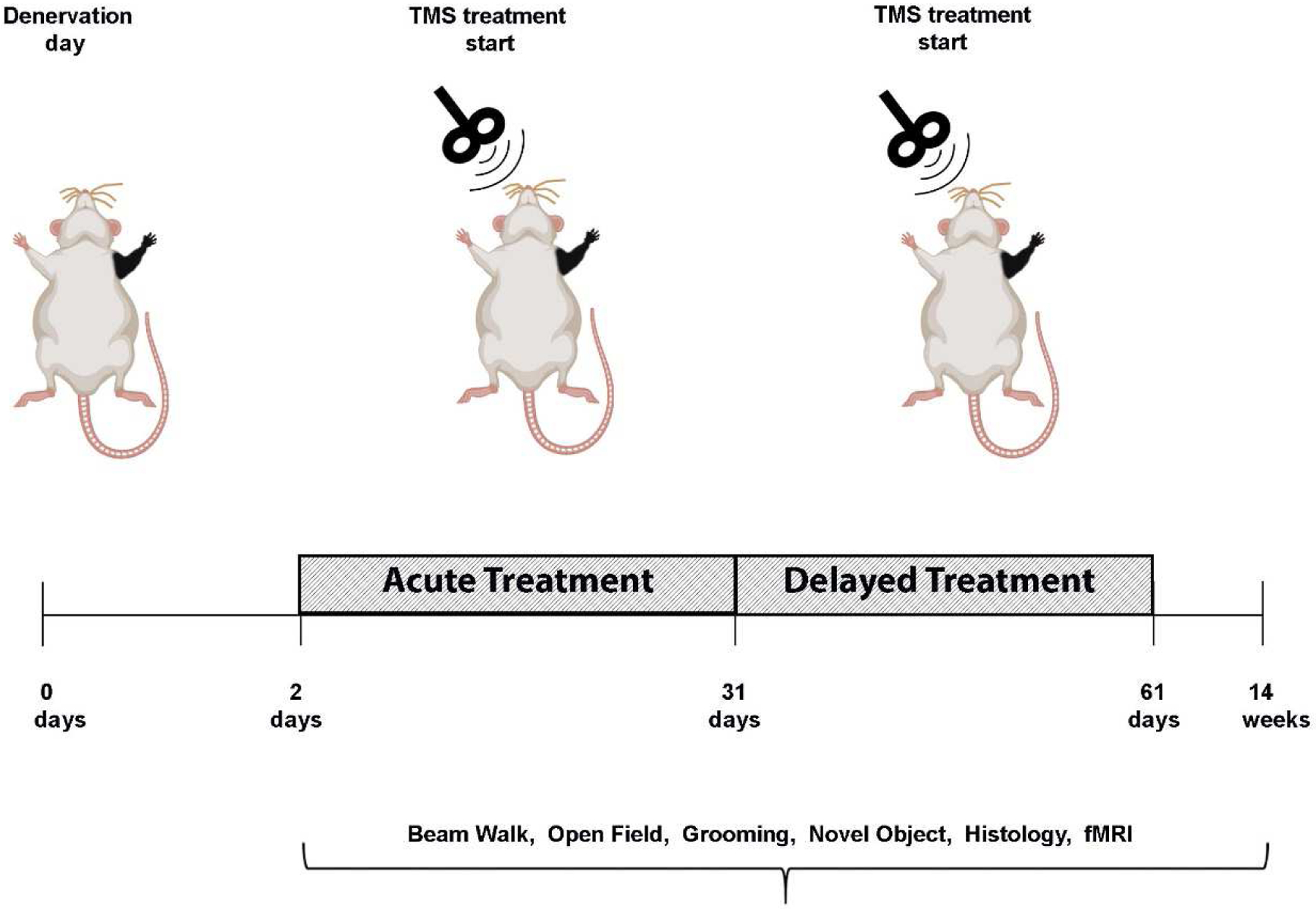
Diagram demonstrating the experimental design of neuromodulation via rTMS. After denervation, rats received rTMS treatment every other day, for 30 days. The intervention began at the acute phase, a day after denervation (Den-rTMS-Acute) or at the sub-acute phase, two weeks following denervation (Den-rTMS-Delayed). The rTMS coil was placed over the left S1, contralateral to the denervated forepaw and delivered 10 min of 10 Hz stimulation. A control group was denervated but did not receive any treatment and an additional control group were not injured and did not receive rTMS treatment.
Fig. 2.
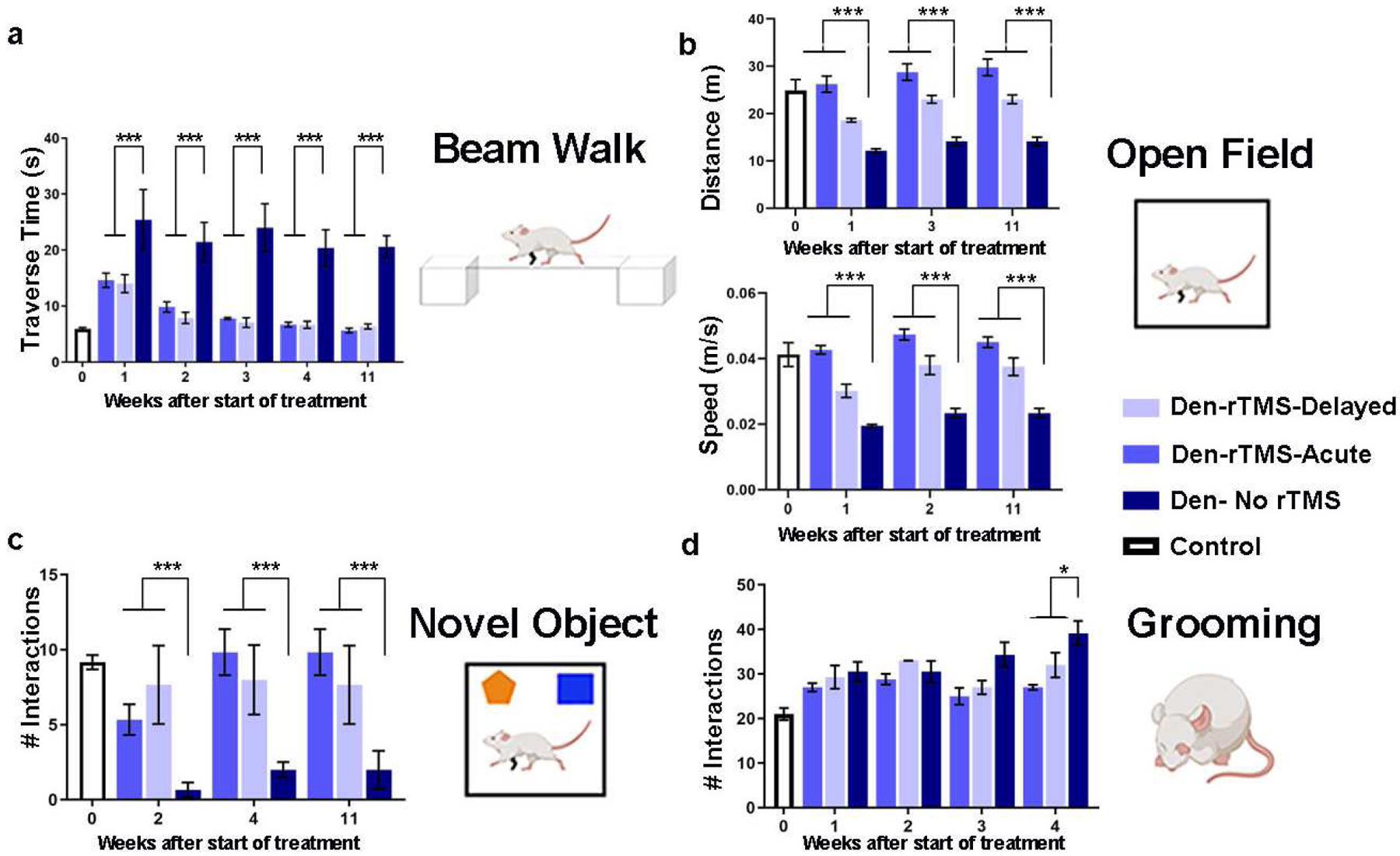
A battery of behavioral tests to assess sensorimotor and cognitive functions was performed before, throughout, and after the rTMS intervention. (A) Sensorimotor functions were evaluated by the traverse time on a 6.3 cm beam walk. (B) Sensorimotor and cognitive functions were evaluated by the time and the velocity of movement in the open field arena. (C) Emotional and cognitive function were evaluated by the time the rats spent exploring new objects in their arena. (D) Sensory dysfunction and pain associated with denervation was monitored by the number of strokes rats made in the self-grooming test. Results show that rTMS intervention leads to improved long-term mobility and decreased anxiety. Furthermore, rats that received rTMS treatment immediately after denervation (Den-rTMS-Acute) exhibited the greatest improvement compared to Den-rTMS-Delayed and Den-No rTMS (*, p<0.05; ***, p<0.001).
An open field test where rats were placed in a 109 cm × 35.56 cm arena and their movement videotaped with a ceiling camera was performed every other week to assess locomotion and anxiety. During the 10 min test, the denervated rats that received rTMS treatment starting the day after injury showed significant increases in the averaged speed (values at week-4: Den-rTMS-Acute, 0.047±0.0016 m/s; Den-rTMS-Delayed, 0.038±0.0028 m/s; Den-No rTMS, 0.023±0.0014 m/s; Control, 0.041 ±0.003 m/s; F (3,6) = 7.028, p<0.05; Acute vs. No rTMS: p < 10−5 for each time point; Delay vs. No rTMS: p < 0.02 at each time point; Acute vs. Delay: NS), and traveled a greater distance compared to the other groups (Den-rTMS-Acute, 28.75±1.75 m; Den-rTMS-Delayed, 23.01±0.8 m; Den-No rTMS, 14.11±0.9 m; Control, 24.88±2.31 m; F (3,6) = 30.64, p<0.05; Acute TMS vs. No rTMS: p < 10−4 for each time point; Delay vs. No rTMS: p < 0.05 at each time point; Acute vs. Delay: NS). The results demonstrate that these improvements lasted throughout the rTMS treatment sessions and weeks following the completion of treatment. After 4 weeks of treatment the Den-rTMS-Acute group increased their speed by 56.9%, while the Den-rTMS-Delayed and the Den-No rTMS increased by only 10.9% and 19.6%, respectively (Fig. 2(B)).
A novel object recognition test was carried out to evaluate the rats’ emotional and cognitive functions reflected by the rats’ interest in new objects placed in the open field arena, as was indicated by the number of times the rats approached the object. This test is known to evaluate anxiety and depression levels which are often increased in patients suffering from chronic pain. The results indicated that denervated rats that received rTMS treatment starting the day after injury spent significantly greater time exploring both the familiar (Den-rTMS-Acute, 7.16±0.7 approaches; Den-rTMS-Delayed, 3±1.5 approaches; Den-No rTMS, 3.8±1.7 approaches; Control, 7.6±1.2 approaches; F (3,9) = 21.07, p<0.05) and the novel objects (Den-rTMS-Acute, 9.8±1.5 approaches; Den-rTMS-Delayed, 7.6±2.6 approaches; Den-No rTMS, 2 ± 0.5 approaches; Control, 9.1±0.4 approaches; F(3,6) = 12.82, p<0.05; Acute vs No rTMS: P < 0.05 at all time points; Delay vs No rTMS: P < 0.05 at time points 1 & 2;). Rats that received the rTMS treatment immediately after injury have shown the greatest gradual increase in the time they spent exploring the novel object over the course of the treatment regime (Den-rTMS-Acute, 84%, Den-rTMS-Delayed, 66.6%, Den-No rTMS, 33.3%) (Fig. 2(C)).
An additional method to assess sensory dysfunctions and pain associated with injury is monitoring the self-grooming behavior. Over-grooming such as compulsive licking, scratching, and biting on the limbs are often observed in animals suffering from nociceptive pain (Berridge and Fentress, 1987; Berridge et al., 1987; Koplovitch et al., 2012; Kalueff et al., 2016). Rats were placed in a clean cage and videotaped for 20 min, and the number of strokes the rats made during that time was counted. The results show that self-grooming had gradually increased over the weeks after denervation in all denervated rats. Analysis of variance showed modest evidence (p<0.05) for variation among groups, but no significance for comparisons of either rTMS group against no rTMS (HSD p>0.05). However, denervated rats that received rTMS treatment showed a marginally significant slower increase in self-grooming compared to denervated rats that did not receive rTMS treatment (P = 0.05 for comparison of slopes of linear regression on time) (Fig. 2(D)).
We then sought to determine if the rTMS treatment induced improvements in sensorimotor and cognitive functions that were observed in the behavioral tests also had physiological correlates. We measured whether rTMS treatment led to long-term plasticity and sensorimotor function. Measurements of Blood-Oxygenation-Level-Dependent (BOLD) fMRI responses evoked by tactile stimulation of the non-injured forelimb were performed 12 weeks after denervation, and 8 weeks after the end of rTMS treatment. Fig. 3 shows BOLD fMRI activation Z maps of individual Denervated-rTMS-Acute and Denervated- No rTMS rats overlaid on high-resolution anatomical MRI images across S1, as well as the statistics for the groups. The number of activated voxels (General Linear Model statistics with a Z score>2.3, corresponding to p<0.05) in S1 induced by tactile stimulation was calculated for Den-rTMS-Acute (n=5) and Den-No rTMS (n=5) groups. The results demonstrate that rTMS treatment led to significant increase in the number of activated voxels across S1 (Den-rTMS-Acute, 150.8±23.3 voxels; Den-No rTMS, 91.6±7.9; p<0.05) suggesting an increase in neuroplasticity in S1 contralateral to the injured forelimb.
Fig 3.
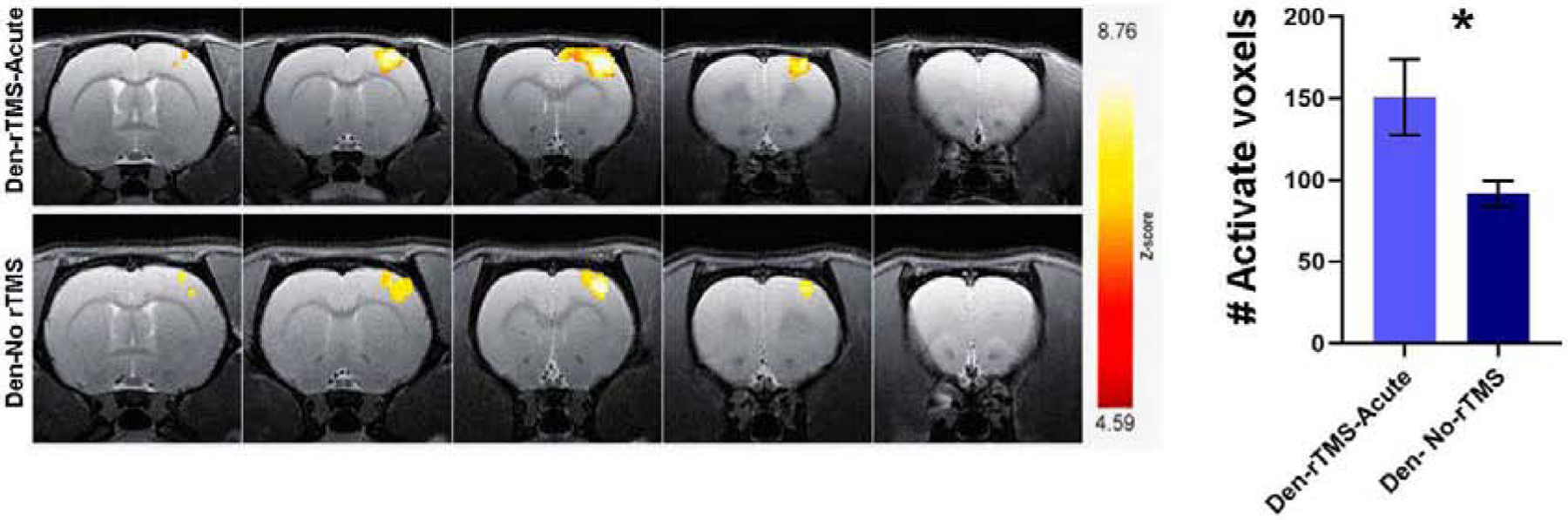
fMRI BOLD responses to intact forepaw stimulation eight weeks after rTMS intervention. (A) Representative BOLD z-score activation maps corresponding to p<0.05, overlaid on high resolution coronal images. (B) The average number of activated voxels in S1. The significantly greater fMRI activation exhibited by the Den-rTMS-Acute compared to Den-No rTMS suggests enhanced neuroplasticity.
Further immunostaining to identify biomarkers associated with neuroplasticity were performed on 25-μm thick brain slices obtained from rats that were sacrificed 16 weeks after the denervation procedure. We calculated the number of cells and the expression levels of CaMKII, a gene known to be involved in long-term potentiation (LTP). The results showed that rats that received rTMS treatment starting the day after injury exhibited a significantly greater fluorescence intensity of CaMKII (Den-rTMS-Acute, 617.5±60 cells), compared to both rats that received delayed rTMS treatment (Den-rTMS-Delayed, 472±13 cells) and denervated rats that did not receive rTMS treatment (Den- No rTMS, 362±23 cells; F (2,3) = 22.61, (p<0.05)). Fig. 4 shows the normalized CaMKII intensity across the deprived S1 (contralateral to denervated forelimb). The non-invasive fMRI and the immunostaining results are consistent with the behavioral tests, and together the results show that rTMS treatment that started at the acute phase after injury led to neuroplasticity and rehabilitation. Furthermore, the results suggest that the rTMS treatment induced long-term neuroplasticity changes that were evident in the behavioral, system, and cellular levels, lasting for months after the treatment has ceased.
Fig. 4.
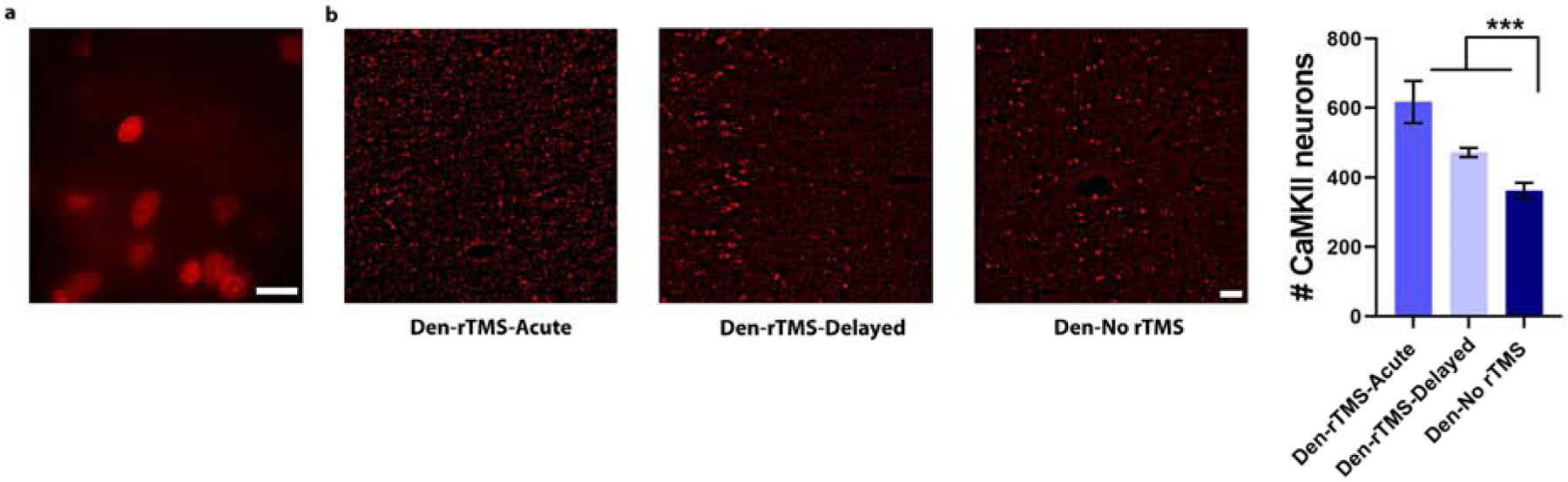
Immunostaining for CaMKII, a marker for neuroplasticity, in S1 contralateral to the denervated forepaw that was subjected to the rTMS intervention. (A) High-magnification image of neurons immunostained for CaMKII (100×, scale bar= 10 μm). (B) Microscopy images demonstrate increased fluorescent in S1 neurons in Den-rTMS-Acute and Den-rTMS-Delayed compared to Den-No rTMS. (C) Quantification of the number of neurons expressing CaMKII (Scale bar = 50 μm) (***, P<0.001).
Cell-specific neuromodulation via EPG
EPG is a protein that is sensitive to magnetic fields that, upon magnetic activation, increases neural excitability. We tested if expression of EPG in excitatory cortical neurons would restore normal excitation-inhibition balance in deprived S1, which could lead to increased plasticity and rehabilitation.
Right forepaw denervation was performed in 11 rats. One week after the denervation procedure, rats were stereotaxiclly injected with a virus encoding for EPG under the CaMKII promotor (AAV-CaMKII:EPG-GFP). Virus was injected into four different locations covering S1 (layers 4 and 5) contralateral to the denervated limb (Den-EPG, n=6). Control rats went through a similar procedure but were injected with virus containing only a GFP marker (Den-Control, n=5). Three weeks after virus injection, and four weeks after denervation, we placed an electromagnet generating a field of 41 mT inside the rat’s skull, directly over S1 expressing EPG, which was contralateral to the denervated limb. A diagram of the experimental paradigm is shown in Fig. 5. The electromagnet consisted of a ferromagnetic core wound with 2,000 turns of magnet wire, and a simulation demonstrating the magnitude of the magnetic fields experienced by the rat based on the dielectric properties of brain tissue and bone(Tang et al., 2016) are shown in Fig. 6(A). The magnetic stimulation was performed for 16 min once a day, for 30 days, while rats were anesthetized with 2% isoflurane. Immunohistochemistry was performed on brain slices obtained from all of the rats in this study to confirm EPG expression in S1 (Fig. 5).
Fig. 5.
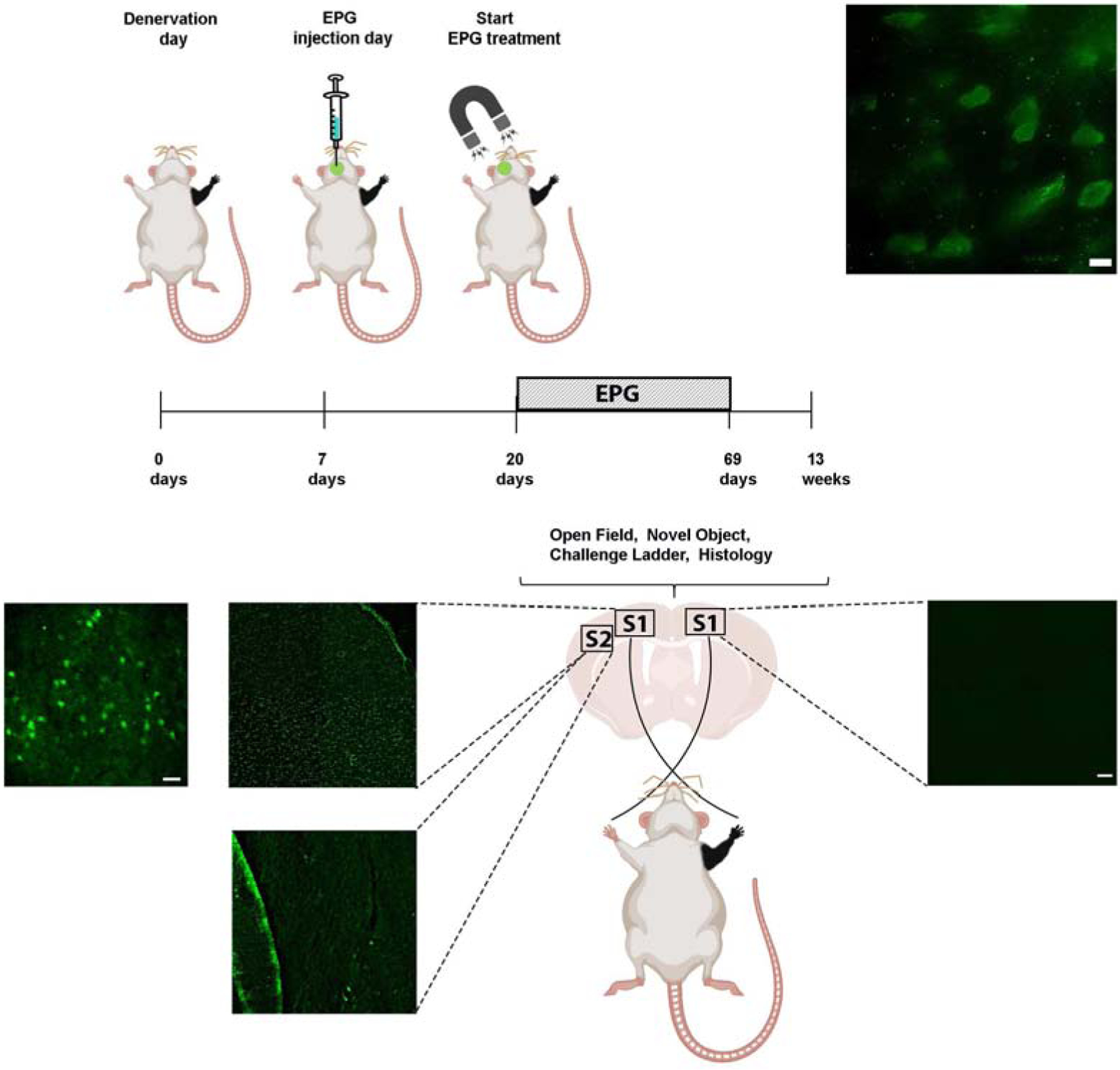
Diagram demonstrating the experimental design of neuromodulation via EPG. Virus encoding to the EPG was stereotaxicly injected into the left S1, contralateral to the denervated forepaw (Den-EPG). Denervated control rats were injected with a virus encoding for a fluorescence protein (Den-Control). An electromagnet was placed over the left S1 starting three weeks following stereotaxic injection. The electromagnet delivered magnetic field stimulation for 16 minutes once a day, for 30 days. Immunostaining images in the primary somatosensory cortex showing EPG expression in fixed brain sections using anti-FLAG antibody in left S1, and right, non-injected S1. On the left S1, EPG can be detected with high-magnification of 100× (upper panel, scale bar= 10 μm), 40× (Scale bar=20 μm) and 4× magnification (Scale bar = 50 μm). No EPG was detected in secondary somatosensory cortex (S2), and in the right, non-injected S1. 4× magnification (Scale bar = 50 μm). No EPG was detected in secondary somatosensory cortex (S2), and in the right, non-injected S1.
Fig 6.
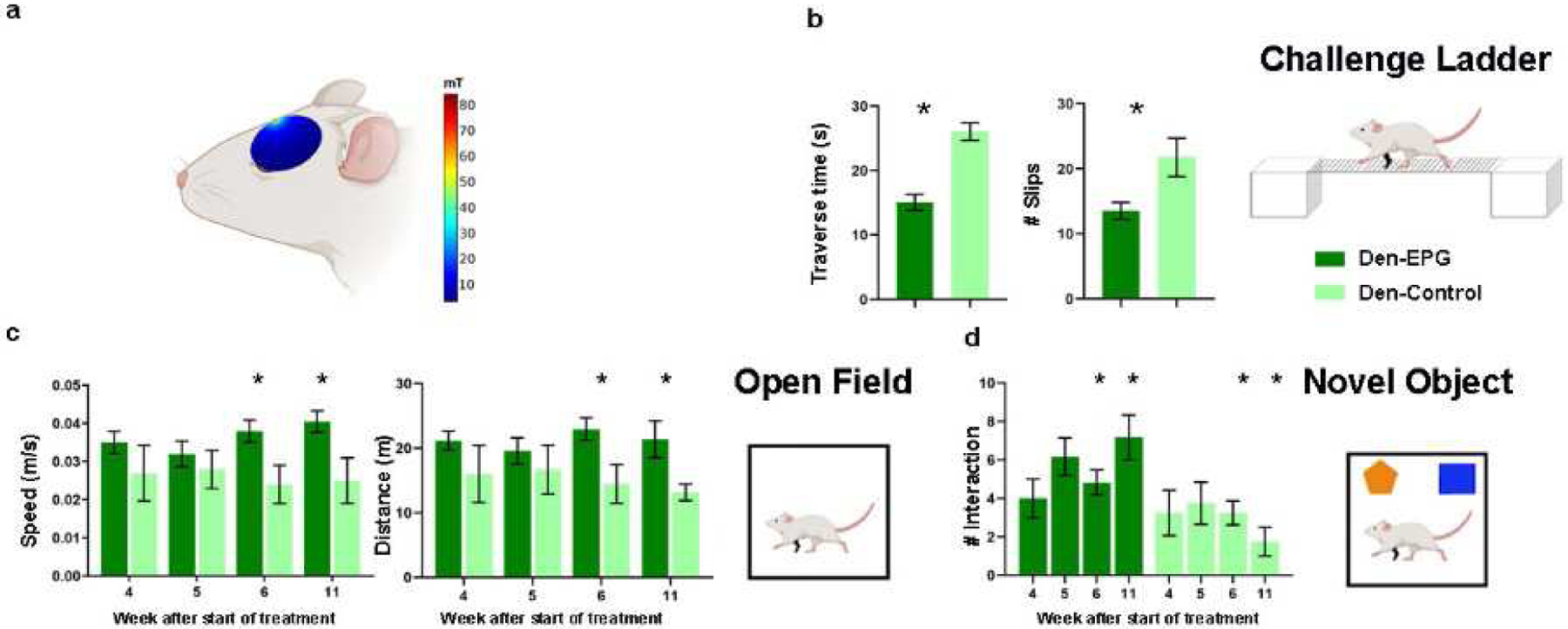
A battery of behavioral tests to assess sensorimotor and cognitive functions was performed throughout and after magnetic activation of EPG. (A) The magnitude of the magnetic field (mT) in a sagittal plane of a simulated ellipsoidal rat brain. The rat skull and brain were modeled using dielectric properties consistent with human bone and brain tissue. (B) Sensorimotor functions were evaluated by the traverse time and number of footfalls on a challenge ladder. (C) Sensorimotor and cognitive functions were evaluated by the time and the velocity of movement in the open field arena. (D) Emotional and cognitive function were evaluated by the time the rats spent exploring new objects in their arena. The results demonstrate that the Den-EPG exhibited significant and long-term improvement in sensorimotor functions compared to the Den-Control group (*, p<0.05).
A battery of behavioral tests to characterize sensorimotor and cognitive function associated with denervation injury and EPG treatment, was performed throughout the course of the stimulation. Long-term improvement in sensorimotor functions and mobility was evaluated using the challenge ladder test, whereas the travers time and the number of slips were determined by laser sensors. This test was performed 12 weeks following denervation, 8 weeks after EPG injection, and 4 weeks after the EPG magnetic stimulation treatment ended. The results show that Den-EPG rats had crossed the ladder in a significantly shorter time (Den-EPG, 15.08±1.2 s; Den-Control, 26.05±1.3 s; p<0.05) and exhibited fewer slips (Den-EPG, 13.5±1.3 s; Den-Control, 21.75±2.9 s; p<0.05) (Fig. 6(B)).
Open field was performed once a week throughout the EPG magnetic treatment. The results demonstrated that within three weeks after starting the magnetic stimulation, the denervated-EPG rats showed significant increases in speed (values at week-4: Den-EPG, 0.04±0.002 m/s; Den-Control, 0.02±0.006 m/s; p<0.05), and traveled a greater distance compared to the control group (Den-EPG, 22.94±1.7 m; Den-Control, 14.45±3 m; p<0.05) (Fig. 6(C)). These improvements lasted for a month after the magnetic stimulation treatments ended, suggesting that the EPG manipulation induced long-term neuroplasticity changes in S1 circuitry.
Den-EPG rats also demonstrated increased interest in new objects during the novel object recognition test. Significant increase of the time they spent exploring the new object, compared to controls, was observed four weeks after the magnetic stimulation treatment ended (Den-EPG, 7.16±1.1 approaches, Den-Control, 1.75±0.7 approaches; p<0.05) (Fig. 6(D)). Overall, the results show that EPG neuromodulation in denervated rats led to substantial improvement in sensorimotor function and rehabilitation.
Discussion
Evidence from human studies suggest that rTMS application over the affected cortex can significantly decrease phantom limb pain, anxiety and depression in amputees (Topper et al., 2003; Malavera et al., 2016). In agreement with these reports, our study demonstrates that neuromodulation in the days and the weeks following peripheral nerve injury leads to short-term and long-term plasticity and neurorehabilitation. This is the first study to test the effectivity of rTMS in a rat model of peripheral nerve injury and test different stimulation protocols. Daily neuromodulation regimes with rTMS have shown to improve sensory, motor, and an overall well-being of the injured rats in a battery of behavioral and imaging tests that were performed up to 8 weeks after the rTMS treatment ended. The results suggest that both an immediate and delay rTMS intervention are effective. This builds on a growing bulk of evidence demonstrating that peripheral nerve injury leads to immediate changes in neural function that may dictate the degree of future rehabilitation (Levy et al., 2002; Werhahn et al., 2002a; Werhahn et al., 2002b; Pelled et al., 2009; Han et al., 2013). Immediate changes in both spontaneous and evoked neural activity have been also demonstrated in models of spinal cord injury (Aguilar et al., 2010). Indeed, and early intervention of rTMS therapy in a rodent model of spinal cord injury has also shown to be more effective compared to later-stage intervention (Krishnan et al., 2019). Nevertheless, delayed rTMS stimulation also led to behavioral improvement compared to rats that did not receive any treatment. Thus, post-injury rTMS treatments may be tailored to benefit patients in the acute, sub-acute, and even chronic phases.
Neuromodulation by rTMS may provide an effective, accessible, relatively inexpensive and completely non-invasive approach to attenuate pain associated with peripheral nerve injury and improve sensorimotor outcomes. Nevertheless, there are ongoing efforts to develop minimally invasive therapeutic strategies that will diminish non-specific activation but will allow temporal precision. Tools such as optogenetics and chemogenetics have the advantages of cell type specificity and superior spatial and temporal resolution compared to prior neuromodulation methods. Indeed, we have previously shown that neuromodulation via optogenetics approaches was successful in restoring cortical excitation-inhibition balance in the weeks following the peripheral nerve injury (Li et al., 2011). Specifically, light activation of halorhodopsin in the healthy cortex combined with forepaw stimulation lead to increase of excitatory neuronal activity in the deprived somatosensory cortex of peripheral nerve injured rats. However, one of the drawbacks of this technology is the requirement to deliver the light directly into the target neural population. Here we tested if neuromodulation via the magnetic sensitive protein EPG, which provides cell and temporal specificity while being activated remotely via non-invasive electromagnetic fields (Krishnan et al., 2018), can be utilized to restore cortical excitability and achieve similar sensorimotor outcomes compared to rTMS. The results demonstrate that daily magnetic activation of EPG improved sensory, motor, and an overall well-being of the injured rats in a battery of behavioral tests that were performed up to 4 weeks after the EPG treatment ended.
Growing amounts of evidence from human and animal studies are establishing neuromodulation as an effective mechanism to strengthen and promote cortical functions (Shin and Pelled, 2017). The behavioral results indicate that both rTMS and EPG treatment have led to considerable improvement in sensorimotor functions. Overall, both acute and sub-acute rTMS treatment alleviated pain as evident by the behavioral assays performed. In addition, neuromodulation via EPG is a new and upcoming technology and efforts are being made towards discovering the molecular structure and the signal transduction basis of this phenomenon. It is also anticipated that utilizing synthetic and molecular biology approaches as well as improving in the hardware will make the EPG function more robust. The EPG technology complements other neuromodulation methods and expands the current toolbox for basic and translational research. Since rTMS intervention is already FDA approved for other conditions, and can be readily translated to clinical practice for patients with peripheral nerve injury and amputation, this study was design to provide a comprehensive characterization of how this intervention affect neural functions the cellular, network and behavior levels. Nonetheless, this is the first application of EPG-intervention in an animal model of injury. Thus, the experiments were design to build the hardware required for magnetic stimulation, validate EPG expression, and test if this approach could also lead to behavioral modifications. Future studies will further characterize EPG-based neuromodulation on cellular and network levels.
Materials and Methods
Animals:
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Michigan State University Animal Care and Use Committee. Thirty-five Sprague-Dawley rats (19 male and 16 female) were provided with food and water ad libitum and housed in a room with a reverse cycle.
Surgeries and Stimulation:
Forepaw denervation was performed on 29 eight weeks old rats weighing 80–90 g. Rats were anesthetized with 2% isoflurane which was delivered through a nose cone. Skin incision was made on the right forepaw, and the radial, median and ulnar nerves were cut, and a 5 mm gap was made in each one. The incision was closed with silk sutures and tissue glue. Tramadol (0.1 mg/300 mg) was administrated orally for 5 days after the injury.
The TMS system was equipped with a figure eight, 25 mm custom rodent coil (Magstim, Rapid2) that was secured by a metal frame over the left hemisphere directly on the head such that the center of the coil was on top of the left S1 (bregma 0). This coil design has been shown to induce focal stimulation in rats (Boonzaier et al., 2020). TMS was delivered once a day, for 31 days with the following settings: 4 s cycles of 10 Hz stimuli, 26 s interval, and 7 cycles (total of 280 pulses per day, 1680 total stimuli). This stimulation frequency has been found to have long-term effects in rats (Lu et al., 2015; Shin et al., 2018; Krishnan et al., 2019; Seewoo et al., 2019). During the stimulation, rats were anesthetized with 2% isoflurane. Denervated control rats that did not receive the TMS treatments were subjected to the same daily anesthesia protocol for the same length of time.
The EPG was identified and cloned from the glass catfish (Krishnan et al., 2018). Until recently, the transparent glass catfish was commonly identified as Kryptopterus bicirrhis but is now known to be Kryptopterus vitreolus. (Ng and Kottelat, 2013). Eleven rats received stereotaxic injection a week following the denervation surgery: 6 of them were injected with virus encoding for EPG under CaMKII promoter (pAAV2-CaMKII::EPG-IRES-hrGFP), and 5 with virus encoding only to GFP (pAAV2-CaMKII::IRES-hrGFP). Rats were anesthetized with 2% isoflurane which was delivered through a nose cone and secured in a stereotaxic frame. The microinjection needle was placed in four locations in the left primary somatosensory cortex (S1) area: AP: +0.2 mm and +0.3, ML:−3.8 mm and −3.2. A volume of 1 μL of virus was injected in each location starting at a depth of 1.2 mm and retracting the needle up to a depth of 0.8 mm. EPG expression was limited to excitatory neurons in layers 4 and 5.
Rats were divided into the following six groups: 1. Denervated rats that started receiving rTMS 48 hours following denervation (Den-rTMS-Acute, n=6). 2. Denervated rats that started receiving TMS 3 weeks following denervation (Den-rTMS-Delayed, n=6). 3. Denervated rats not receiving TMS (Den-No rTMS, n=6). 4. Non-denervated not receiving TMS (Control, n=6). 5. Denervated rats injected with virus containing EPG in S1 contralateral to denervated limb (Den-EPG, n=6). 6. Denervated rats injected with virus containing only GFP in S1 contralateral to denervated limb (Den-Control, n=5). Behavioral Assessments: A comprehensive battery of behavioral tests to assess sensory, motor, and cognitive functions was performed over 30 days since the beginning of TMS therapy. Grooming: Rats were placed separately in a clean cage (43.62cm (L) × 22.86cm (W) × 20.32cm (H)) with food and water. Grooming was recorded for 20 min. The first minute was considered habituation period, and the rest of the 19 min were analyzed. The number of interactions on each part of the chain grooming actions was counted for each individual. This test was performed once a week.
Open Field:
The open field was carried out in an arena with the following dimensions (L) 109 cm × (W) 35.56 cm × 142.24 cm (H) (San Diego Instruments). During the session, the open field was isolated from the observer, and the light intensity was maintained stable. Movements were recorded by a ceiling mounted camera for 10 min. The freezing time, total distance and averaged velocity were analyzed by an automated tracking system (ANY-Maze software, San Diego, USA). After each session the arena was cleaned with 70% ethanol. This test was performed every two weeks (for TMS treated rats), and once a week (EPG rats).
Novel Object Recognition:
Rats were placed in the open field arena. In the first stage, rats were acclimating to the environment (5 min). In the second stage two identical objects were placed in the arena and the rat got familiarized with them (5 min). In the third stage we replaced one of the objects for a new and unfamiliar object (5 min). The time spent exploring the novel object was analyzed by automated tracking (ANY-maze software). This test was performed every two weeks (for TMS treated rats), and once a week (EPG rats).
Beam Walk Test:
TMS-treated rats were placed on one end of 114.3 cm-long suspended, narrow wooden beam. Two different widths were tested: 6.3 cm and 3 cm. The traverse time from one end to the other was measured. Three training sessions were performed for each animal once a week. For the denervated-TMS group, the rats started to walk on the 6.3 cm width beam and then were challenged on the 3 cm wide beam.
Challenge Ladder:
Den-EPG rats crossed a 114 cm-long horizontal suspended ladder with rungs spaced 1.3 cm apart (San Diego Instruments, USA). The traverse time and the number of failures to place the paw correctly on the ladder were observed. Two training sessions were performed for each animal on test days, with this test being performed only once.
Functional MRI:
fMRI activity was assessed in denervated rats that received TMS (TMS acute, n=5) and rats that did not receive the TMS (denervated no-TMS, n=5). Rats were anesthetized with dexmedetomidine (0.1 mg/kg/h, SC) which is known to preserve neurovascular coupling (Pelled, 2011; Jouroukhin et al., 2014; Li et al., 2014). Rats were then placed in a 7 T/16 cm horizontal bore small-animal scanner (Bruker BioSpin, Rheinstetten, Germany). A 72-mm quadrature volume coil and a 15-mm-diameter surface coil were used to transmit and receive magnetic resonance signals, respectively. Respiration rate, heart rate, and partial pressure of oxygen were continuously monitored throughout fMRI measurements (Starr Life Sciences, Pennsylvania, USA). For fMRI, FID-EPI was used with a resolution of 150 × 150 × 1000 μm. Five, 1 mm thick coronal slices covering the primary somatosensory cortex (S1) were acquired (effective echo time (TE), 16 ms; repetition time (TR), 1000 ms; bandwidth, 333 KHz; field of view (FOV), 3.5 × 3.5 cm; matrix size, 128 × 128). A T2-weighted TurboRARE sequence was used to acquire high-resolution anatomical images (TE, 33 ms; TR, 2500 ms; bandwidth, 250 KHz; FOV, 3.5 × 3.5 cm; matrix size, 256 × 256) corresponding to the fMRI measurements. Two needle electrodes were inserted into the left and right forepaws to deliver electrical stimulation. Electrical stimulation was applied in two 40 s trains (3 Hz, 0.4 mA, and 0.4 ms). fMRI analysis was performed using SPM fMRat software (SPM, University College London, UK). Activation maps were obtained using the general linear model. The experimental design was rest <stimulate, T-contrast Thresholded:FWE (Family Wise Error) and P<0.05, Extent threshold k=2, Z-score statistics were used with a threshold of Z>2.3.
Electromagnetic stimulation:
The electromagnet used to deliver the magnetic stimulation to the rats’ brains consisted of a ferromagnetic Iron-Nickel core wound with 2,000 turns of 30 AWG magnet wire. Iron core had dimensions of 16.29 cm in length and a diameter of 1.05 cm. A 45 Degree angle was cut into each end, so that both tips of the core came to a point. During stimulation, 5 V was applied to across the connections of the magnet and a current of about 390 mA flowed through the coil to generate the magnetic field. An external digital signal was used to turn the magnet on and off. Measurements of the magnet at varying distances from the core demonstrated that a magnetic field value of 41 mT was generated just in front of the core.
Finite element analysis was performed using ComSol Multiphysics to simulate the magnetic field stimulation delivered to the rat brain. An electromagnet with 2,000 turns wound around a ferromagnetic Iron-Nickel core with a length of 16.29 cm, a diameter of 1.05 cm, and a relative permeability of 100,000 was used to stimulate the rat brain. A current of 390 mA was passed through the simulated coils and the tip of the core was placed 0.5 mm from the surface of the skull. The rat skull and brain were modeled by concentric ellipses, the larger of which having dimensions 21 mm × 11 mm × 16 mm, with a skull thickness of 0.7 mm used (Tang et al., 2016). Dielectric properties for human bone and brain tissue were used to represent bone and brain tissue in the rat, specifically a relative permittivity of 1.53 × 103 and 6.10 × 104, a relative permeability of 1 and 1, and a conductivity of 2.03 × 10−2 S/m and 1.06 × 10−1 S/m respectively (Tang et al., 2016).
Immunochemistry of Brain slices:
Rats were perfused with 0.1 M phosphate buffer saline solution (PBS) in pH 7.4 followed by ice cold 4% paraformaldehyde solution and the brains were removed. Brains were sliced on a cryostat to obtain 20 μm thick sections. Sections were incubated overnight with primary antibodies to detect CaMKII (anti-CaMKII rabbit, Abcam #ab52476); FLAG (anti-flag mouse antibody, Abcam #ab49763), and GFP (anti-GFP chicken polyclonal antibody, Abcam #ab13970). Sections were incubated for 3 h at room temperature with secondary antibodies, processed with ProLonng Gold antifade reagent with DAPI (Thermo Fischer Scientific 2078923) and then imaged on the DeltaVision microscope. ImageJ was used for analysis.
Statistics:
The number of rats in each group was determined to achieve a power of 0.93 assuming an effect of 1 standard deviation while minimizing the number of rats in each group. All the results and figures show mean ± standard error of mean (SEM). Each analysis compared outcomes across several treatment groups; analysis of variance (ANOVA) was used to flag comparisons with at least one significant difference. Within each ANOVA, there are several comparisons of interest, and to address multiple comparisons issues, we used the studentized range statistic. The studentized range statistic (a.k.a. Tukey’s Honest Significant Difference (HSD)) is an exact test for each of the possible comparisons between two groups embedded in a multi-way ANOVA, where significance is adjusted to control the false positive probability for all pairwise comparisons at the specified level.
Highlights.
We developed state-of-the-art neuromodulation strategies to augment recovery from injury, including repetitive transcranial magnetic stimulation (rTMS) and a novel, neural-specific technology based on a novel magnetic-sensitive gene (EPG). This work impacts basic, translational and clinical research on several levels, including:
Neuromodulation by TMS promoted brain plasticity as was demonstrated by a comprehensive battery of behavioral tests, functional MRI and immunohistochemistry; Genetic-based neuromodulation also promoted brain plasticity as was demonstrated by a comprehensive battery of behavioral tests.
The development of a novel minimally-invasive and wireless technology to control neural firing rate in a cell-specific, region-specific, and temporal-specific manner without the ongoing need for implanted electrodes, optic fibers and stimulation devices.
Both the rTMS and the EPG-based neuromodulation strategies demonstrate that the excitation of the affected cortex facilitates neuroplasticity. These conceptual strategies and rTMS treatment protocols could be readily translated into clinical practice.
Acknowledgments
This work was supported by National Institutes of Health grants R01NS072171, R01NS098231 and R01NS104306.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Walter H, Wunderlich A, Grothe J, Schonfeldt-Lecuona C, Spitzer M, Herwig U (2005) Side effects of transcranial magnetic stimulation biased task performance in a cognitive neuroscience study. Brain Topogr 17:193–196. [DOI] [PubMed] [Google Scholar]
- Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A, Foffani G (2010) Spinal cord injury immediately changes the state of the brain. J Neurosci 30:7528–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ (2016) Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain 139:2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Fentress JC (1987) Deafferentation does not disrupt natural rules of action syntax. Behav Brain Res 23:69–76. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Fentress JC, Parr H (1987) Natural syntax rules control action sequence of rats. Behav Brain Res 23:59–68. [DOI] [PubMed] [Google Scholar]
- Bjorkman A, Rosen B, Lundborg G (2005) Enhanced function in nerve-injured hands after contralateral deafferentation. Neuroreport 16:517–519. [DOI] [PubMed] [Google Scholar]
- Blom SM, Pfister JP, Santello M, Senn W, Nevian T (2014) Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci 34:5754–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci T, Hensghens MJ, Di Rollo A, Parenti L, Barloscio D, Rossi S, Sartucci F (2016) Impaired interhemispheric processing in early Huntington’s Disease: A transcranial magnetic stimulation study. Clin Neurophysiol 127:1750–1752. [DOI] [PubMed] [Google Scholar]
- Boonzaier J, Petrov PI, Otte WM, Smirnov N, Neggers SFW, Dijkhuizen RM (2020) Design and Evaluation of a Rodent-Specific Transcranial Magnetic Stimulation Coil: An In Silico and In Vivo Validation Study. Neuromodulation 23:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Richardson MP, Rothwell JC, Brooks DJ (2000) Long-term changes of GABAergic function in the sensorimotor cortex of amputees. A combined magnetic stimulation and 11C-flumazenil PET study. Exp Brain Res 133:552–556. [DOI] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG (2009) Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke 40:1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmers C, Adler T, Rzanny R, van Schayck R, Gaser C, Weiss T, Miltner WH, Bruckner L, Weiller C (2001) Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neurosci Lett 307:109–112. [DOI] [PubMed] [Google Scholar]
- Flor H (2002) Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol 1:182–189. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E (1995) Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375:482–484. [DOI] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A (2011) Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci 31:7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Li H, Lv Y, Lu H, Niu J, Sun J, Yang GY, Ren C, Tong S (2015) Pulsed Transcranial Ultrasound Stimulation Immediately After The Ischemic Brain Injury is Neuroprotective. IEEE Trans Biomed Eng 62:2352–2357. [DOI] [PubMed] [Google Scholar]
- Han Y, Li N, Zeiler SR, Pelled G (2013) Peripheral nerve injury induces immediate increases in layer v neuronal activity. Neurorehabil Neural Repair 27:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenrath K, Funke K (2013) Time-course of changes in neuronal activity markers following iTBS-TMS of the rat neocortex. Neurosci Lett 536:19–23. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Reimers M, Udpa L, Jimenez GSD, Moore M, Gilad AA, Pelled G (2020) Swimming direction of the Glass Catfish, Kryptopterus bicirrhis, is responsive to magnetic stimulation. bioRxiv 20200813250035. [Google Scholar]
- Hwang J, Choi Y, Lee K, Krishnan V, Pelled G, Gilad AA, Choi J (2020) Regulation of Electromagnetic Perceptive Gene Using Ferromagnetic Particles for the External Control of Calcium Ion Transport. Biomolecules 10(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouroukhin Y, Nonyane BA, Gilad AA, Pelled G (2014) Molecular neuroimaging of post-injury plasticity. J Mol Neurosci 54:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H (2001) Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 21:3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterboer E, Funke K (2019) Repetitive transcranial magnetic stimulation recovers cortical map plasticity induced by sensory deprivation due to deafferentiation. J Physiol 597:4025–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplovitch P, Minert A, Devor M (2012) Spontaneous pain in partial nerve injury models of neuropathy and the role of nociceptive sensory cover. Exp Neurol 236:103–111. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Park SA, Shin SS, Alon L, Tressler CM, Stokes W, Banerjee J, Sorrell ME, Tian Y, Fridman GY (2018) Wireless control of cellular function by activation of a novel protein responsive to electromagnetic fields. Scientific reports 8:8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan VS, Shin SS, Belegu V, Celnik P, Reimers M, Smith KR, Pelled G (2019) Multimodal evaluation of TMS-induced somatosensory plasticity and behavioral recovery in rats with contusion spinal cord injury. Frontiers in neuroscience 13:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo RJ, Latif T (2007) Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. J Pain 8:453–459. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG (2002) Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol 52:755–761. [DOI] [PubMed] [Google Scholar]
- Li N, van Zijl P, Thakor N, Pelled G (2014) Study of the spatial correlation between neuronal activity and BOLD fMRI responses evoked by sensory and channelrhodopsin-2 stimulation in the rat somatosensory cortex. J Mol Neurosci 53:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Downey JE, Bar-Shir A, Gilad AA, Walczak P, Kim H, Joel SE, Pekar JJ, Thakor NV, Pelled G (2011) Optogenetic-guided cortical plasticity after nerve injury. Proc Natl Acad Sci U S A 108:8838–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kobilo T, Robertson C, Tong S, Celnik P, Pelled G (2015) Transcranial magnetic stimulation facilitates neurorehabilitation after pediatric traumatic brain injury. Sci Rep 5:14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G (2003) Richard P. Bunge memorial lecture. Nerve injury and repair--a challenge to the plastic brain. J Peripher Nerv Syst 8:209–226. [DOI] [PubMed] [Google Scholar]
- Malavera A, Silva FA, Fregni F, Carrillo S, Garcia RG (2016) Repetitive Transcranial Magnetic Stimulation for Phantom Limb Pain in Land Mine Victims: A Double-Blinded, Randomized, Sham-Controlled Trial. J Pain 17:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JK, Grigsby EM, Prevosto V, Petraglia FW 3rd, Rao H, Deng ZD, Peterchev AV, Sommer MA, Egner T, Platt ML, Grill WM (2014) Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci 17:1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Kottelat M (2013) After eighty years of misidentification, a name for the glass catfish (Teleostei: Siluridae). Zootaxa 3630:308–316. [DOI] [PubMed] [Google Scholar]
- Pelled G (2011) MRI of neuronal plasticity in rodent models. Methods Mol Biol 711:567–578. [DOI] [PubMed] [Google Scholar]
- Pelled G, Chuang KH, Dodd SJ, Koretsky AP (2007) Functional MRI detection of bilateral cortical reorganization in the rodent brain following peripheral nerve deafferentation. Neuroimage 37:262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled G, Bergstrom DA, Tierney PL, Conroy RS, Chuang KH, Yu D, Leopold DA, Walters JR, Koretsky AP (2009) Ipsilateral cortical fMRI responses after peripheral nerve damage in rats reflect increased interneuron activity. Proc Natl Acad Sci U S A 106:14114–14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B, Bjorkman A, Lundborg G (2006) Improved sensory relearning after nerve repair induced by selective temporary anaesthesia - a new concept in hand rehabilitation. J Hand Surg [Br] 31:126–132. [DOI] [PubMed] [Google Scholar]
- Seewoo BJ, Feindel KW, Etherington SJ, Rodger J (2019) Frequency-specific effects of low-intensity rTMS can persist for up to 2 weeks post-stimulation: A longitudinal rs-fMRI/MRS study in rats. Brain Stimul 12:1526–1536. [DOI] [PubMed] [Google Scholar]
- Shin SS, Pelled G (2017) Novel Neuromodulation Techniques to Assess Interhemispheric Communication in Neural Injury and Neurodegenerative Diseases. Front Neural Circuits 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Krishnan V, Stokes W, Robertson C, Celnik P, Chen Y, Song X, Lu H, Liu P, Pelled G (2018) Transcranial magnetic stimulation and environmental enrichment enhances cortical excitability and functional outcomes after traumatic brain injury. Brain Stimulation 11:1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung WH, Wang CP, Chou CL, Chen YC, Chang YC, Tsai PY (2013) Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke 44:1375–1382. [DOI] [PubMed] [Google Scholar]
- Tang AD, Lowe AS, Garrett AR, Woodward R, Bennett W, Canty AJ, Garry MI, Hinder MR, Summers JJ, Gersner R, Rotenberg A, Thickbroom G, Walton J, Rodger J (2016) Construction and Evaluation of Rodent-Specific rTMS Coils. Front Neural Circuit 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper R, Foltys H, Meister IG, Sparing R, Boroojerdi B (2003) Repetitive transcranial magnetic stimulation of the parietal cortex transiently ameliorates phantom limb pain-like syndrome. Clin Neurophysiol 114:1521–1530. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG (2002a) Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain 125:1402–1413. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG (2002b) Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci 5:936–938. [DOI] [PubMed] [Google Scholar]
- Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP (2012) Thalamocortical inputs show post-critical-period plasticity. Neuron 74:731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mei Y, Liu C, Yu S (2007) Effect of transcranial magnetic stimulation on the expression of c-Fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J Huazhong Univ Sci Technolog Med Sci 27:415–418. [DOI] [PubMed] [Google Scholar]


