Abstract
Purpose
To demonstrate the feasibility of integrating the MT preparations required for inhomogeneous MT (ihMT) within an MPRAGE style acquisition. Such a sequence allows for reduced power deposition and easy inclusion of other modules.
Methods
An ihMT MPRAGE style sequence (ihMTRAGE) was initially simulated to investigate acquisition of the 3D ihMT data sequentially, or in an interleaved manner. The ihMTRAGE sequence was implemented on a 3T clinical scanner to acquire ihMT data from the brain and spine.
Results
Both simulations and in-vivo data provided an ihMT signal significantly greater using a sequential ihMTRAGE acquisition, compared with an interleaved implementation. Comparison with a steady state ihMT acquisition (defined as having one MT RF pulse between successive acquisition modules) demonstrated how ihMTRAGE allows for a reduction in average power deposition, or greater ihMT signal at equal average power deposition. Inclusion of a prospective motion correction module did not significantly affect the ihMT signal obtained from regions of interest in the brain. The ihMTRAGE acquisition allowed combination with a spatial saturation module to reduce phase wrap artifacts in a cervical spinal cord acquisition.
Conclusions
Use of preparations necessary for ihMT experiments within an MPRAGE style sequence provides a useful alternative for acquiring 3D ihMT data. Compared with our steady state implementation, ihMTRAGE provided reduced power deposition whilst allowing use of the maximum intensity from off-resonance RF pulses. 3D ihMTRAGE allowed combination of other modules with the preparation necessary for ihMT experiments, specifically motion compensation and spatial saturation modules.
Keywords: brain, inhomogeneous magnetization transfer, ihMT, MPRAGE, MT, myelin, spinal cord
Introduction
The inhomogeneous magnetization transfer (ihMT) technique continues to gain traction as a useful MRI tool. This is in part due to its simplicity; ihMT is calculated from the acquisition following two types of off-resonance RF preparations: data acquired following off-resonance RF irradiation applied at two, equal but opposite frequencies, are subtracted from data acquired with single offset frequency RF irradiation (1). As such, data for regular magnetization transfer (MT) is also acquired as part of the ihMT experiment and can be used for comparison. Application in studies of spinal cord, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) shows ihMT: changes with aging and cervical level (2); decreases in ALS more significantly relative to healthy controls in comparison with MT and diffusion measures (3); and, correlates more strongly with MS disability compared to the MT ratio (MTR) (4), respectively. Comparison of ihMT with other MRI techniques related to microstructure, particularly looking at white matter (WM), demonstrates the ability to provide complementary information (5,6). The semi-quantitative ihMT ratio (ihMTR) used as a metric also shows high intra- and inter-scanner reliability and reproducibility (7). All but two of these studies were conducted using single-slice ihMT acquisitions, and all prior to the revelation that a significant increase in ihMT signal is possible with a decrease in the RF duty cycle (for the same time-averaged power) (8,9).
A 3D ihMT sequence that takes advantage of low duty cycle effects, and takes a magnetization preparation approach that permits inclusion of other commonly utilized modules more easily (relative to a steady state sequence), would be a functional and invaluable tool in future MRI studies. Mchinda et al. demonstrates a low duty cycle implementation within a steady state spoiled gradient-echo acquisition for whole brain ihMT (8). To reduce RF deposition, and thus the specific absorption rate (SAR), they propose application of the off-resonance MT pulses only during acquisitions relating to the center portion of k-space. Such a solution may be less ideal when looking at high frequency (resolution) information. More recently, low duty cycle ihMT data were acquired at 3T within clinically acceptable SAR limits using a preparatory module and a single slice EPI acquisition (9). The MPRAGE acquisition provides a modular baseline sequence and was introduced as a 3D acquisition (10). In theory, with no RF pulses applied, the recovery period included as part of the MPRAGE sequence provides an alternative mechanism by which average power deposition (and SAR) can be reduced to compensate for the high B1 off-resonance RF required for increases in ihMT signal (8,9). An ihMT MPRAGE style acquisition (ihMTRAGE), by allowing for recovery of the longitudinal magnetization, and without on-resonance pulses placed between off-resonance RF (as in steady state implementations) to further attenuate the longitudinal magnetization, may provide higher signal-to-noise ratio (SNR) data prior to processing.
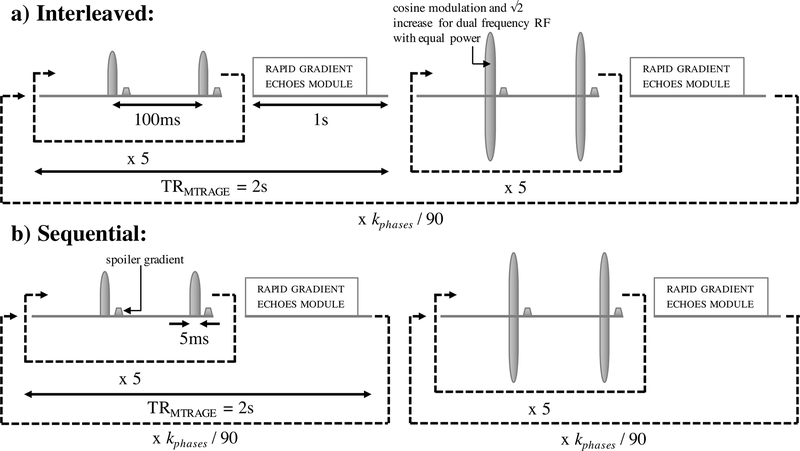
In this work, we combine the magnetization preparations required for the ihMT experiment within an MPRAGE sequence for 3D acquisitions. Off-resonance RF pulses replace the inversion pulse and wait time in the preparatory module associated with common MPRAGE implementations (11). The parameters of the MT preparations for ihMT were based on those that provide the greatest ihMT signal in brain tissue for the single slice EPI acquisition (9). The recovery period (and to a lesser extent the acquisition period) associated with the MPRAGE sequence can be used to reduce the average SAR over the acquisition duration or allow for increases in the power of the MT pulses employed. A radial fan beam (rfb) k-space segmentation scheme was utilized to reduce scan time and provide a Cartesian acquisition strategy originating in the center of k-space and proceeding outward (12). The segmented acquisition of k-space in MPRAGE allows the type of MT preparation to be changed between the single and dual off-resonance frequency experiments required for ihMT (1). By acquiring data in such an interleaved manner, i.e. cycling between single and dual frequency RF irradiation prior to acquisition of a complete 3D k-space (Fig. 1a), the two types of preparation required for ihMT are acquired closer together in time. This interleaved approach reduces the time available for motion to affect subtracted datasets, but any motion would be spread over the time required for acquisition of all ihMT data. Such an interleaved acquisition is similar to the recommendation to achieve accurate label/control subtraction in 3D gradient- and spin-echo readouts for arterial spin-labeled perfusion MRI (13). By comparison a sequential approach, in which segments are acquired to complete 3D k-space sequentially before a change in the preparation type, would reduce the time for motion to affect a single 3D dataset (Fig. 1b). We study the signal resultant from both interleaved and sequential ihMTRAGE acquisitions by simulation and application in vivo.
Figure 1.
Illustration of ihMTRAGE sequence configured for: a) interleaved acquisition of data following single and then dual frequency irradiation, or b) sequential acquisition of complete 3D volume with one type of preparation before moving on to the next. Parameter values relate to the sequences used to acquire ihMT data from the brain of healthy human volunteers.
The prepared magnetization of the ihMTRAGE acquisition provides motivation for this work: The effect of changes to the ihMT preparations can be more easily separated from the effect of on-resonance pulses associated with acquisition; The recovery period reduces the average power deposition over the entire scan; And, the 3D ihMTRAGE sequence can be setup to include other MRI modules associated with changing the magnetization or altering sensitivity to motion. To this latter point, we experimented with inclusion of a spatial saturation module, as well as the prospective motion correction (PROMO) module within the ihMTRAGE sequence (14).
Methods
Simulations
The ihMT signal model was based on the difference between the two pool model often utilized in quantitative MT with and without inclusion of a dipolar order reservoir (15). This equated to the difference between the longitudinal magnetizations of the free, measurable pool following preparation by off-resonance RF irradiation applied at a single offset frequency and preparation at dual offset frequencies centered on-resonance, or for this study ihMTz. The signal from WM and grey matter (GM) regions of the brain were simulated by numerical integration in steps of 0.1 ms (ode45 function in Matlab R2018a, Mathworks, USA). Further details on the ihMT signal model are provided in the Appendix. Parameter values for WM and GM tissues were based on prior literature (9). Specifically, for WM/GM the following values were used: thermal equilibrium longitudinal magnetization of the free pool, A, M0A = 1.0/1.0, and macromolecular pool, B, M0B = 0.100/0.035; longitudinal relaxation rates R1A = 0.92/0.55 s−1 and R1B 1.0/1.0 s−1 of pools A and B respectively; transverse relaxation rates T2A = 69/99 ms and T2B = 9.0/7.6 μs of pools A and B respectively; exchange rate between the two pools R = 60/51 s−1; and, dipolar relaxation time T1D = 6.2/5.9 ms. Multiple dipolar order reservoirs can be included in the ihMT signal model (15,16). However, only a single dipolar order reservoir was considered to match the model from which the aforementioned parameter values were output as a result of fits to data acquired with similar, low-duty cycle preparations (9). Optimized parameters associated with the ihMTRAGE preparation were based on optimization of the ihMT signal in prior literature and hardware constraints at 3T. This included the off-resonance frequency for the MT pulses of ±7 kHz (1), low duty cycle of 5% (9), and a peak B1 of the single frequency off-resonance RF pulses of 15 μT.
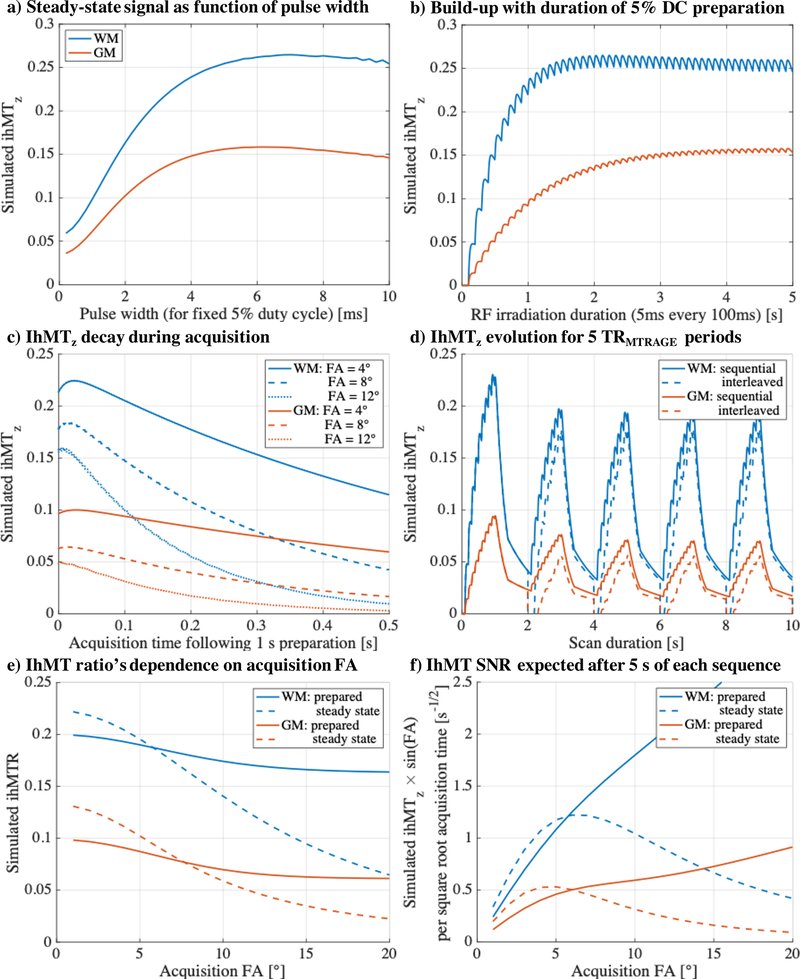
Further optimization of the sequence was guided by simulation of ihMTz. Initial simulation was carried out for long, 5 s off-resonance RF durations to examine ihMTz as a function of pulse width, and then ihMTz was simulated for preparatory periods up to 5 s for a fixed 5 ms pulse width. The effect of on-resonance RF, and their flip angle (FA) for acquisition, on ihMTz was simulated following a 1 s preparatory period with 5 ms MT pulses every 100 ms (as later utilized in experiments). Simulated on-resonance RF pulses (for the readout) were assumed to be instantaneous.
Simulation by numerical integration provided a simple means to follow the progression of ihMTz and guide acquisition, i.e. dummy scans required and expectations in comparison to a steady state ihMT acquisition. Although the ihMTz (based on the differences between longitudinal magnetizations) is not strictly equivalent to the MR signal, it provides a proportional indication of how the ihMT signal develops through each sequence. For the purposes of this study, the steady state ihMT acquisition was defined as having only one off-resonance MT RF pulse between successive acquisition modules, and a recovery period (if present) less than the time between adjacent MT pulses. Such a definition makes the sequence comparable (albeit with more readouts) to previous steady state ihMT implementations (5,7), as well as a recent one that makes use of multiband pulses to achieve simultaneous RF irradiation on- and off-resonance (17). This was in contrast to the prepared, ihMTRAGE sequence in which multiple MT pulses were utilized within the preparation module prior to acquisition. The progression of ihMTz during both interleaved and sequential implementations of ihMTRAGE were also simulated (1s preparation; 400 ms imaging module of 100 phase encodes; FA = 8°; repetition time of the preparatory, plus acquisition, plus recovery module, TRMTRAGE = 2 s). The interleaved signal was simulated by interchange of the final longitudinal magnetization, following a single or dual frequency RF preparation, with the initial magnetization used to simulate the next type of preparation. For comparison with a steady state sequence, the sequential ihMTRAGE implementation was altered slightly: 5ms pulses every 130ms to provide an achievable root mean square B1 of 3 μT for peak B1 = 15μT for the steady state sequence at 3T; 1.04s preparation; TRMTRAGE = 2.08s (twice the preparation duration). The steady state sequence was thus simulated with 5 ms off-resonance RF pulses for the preparation, followed by a 40 ms imaging module of 10 phase encodes, and TRMTRAGE = 130ms. The product of ihMTz immediately following the preparation and the sine of the FA was calculated to provide an indication of the ihMT SNR as a function of the FA for acquisition. Matlab code for all of the aforementioned simulations, including the underlying model used for numerical integration, has been posted at https://github.com/gvarma617/ihMTRAGE-optimize (hash 0310455).
Analysis of the difference in point spread functions (PSFs) between the prepared and steady state ihMT acquisitions was achieved using the results from simulation by numerical integration, combined with details of the rfb k-space view ordering: Beginning with an empty 2D matrix equal to the extent of phase encodes used for acquisition of prepared and steady state ihMT data, the matrix was filled in accordance with the rfb segment view ordering for parameters used in vivo but without parallel imaging. Thus, matrices representing the prepared and steady state strategies were filled with signal values 90 and 10 points at a time respectively. The signal values were the simulated longitudinal magnetizations multiplied by sin(FA = 8°), following 3/10 TRMTRAGE dummy cycles with 8/1 MT pulse preparations for the prepared/steady state acquisition. Matrices representing the signal were expanded with zeros to 8 times the size prior to Fourier transform. This representation of the PSF was convolved with the Fourier transform of a Shepp-Logan phantom, and inverse Fourier transform of the result provided images indicative of the effect of the PSFs from prepared and steady state acquisitions. Data representing the PSFs and their effect were simulated for WM tissue prepared with single and dual frequency off-resonance irradiation independently. The difference between the two was used to provide an indication of the ihMT PSFs in WM and the resultant ihMT images.
Human volunteer experiments
MRI was carried out in accordance with a protocol approved by our institutional review board, and all subjects provided written informed consent. IhMTRAGE was implemented on a 3T scanner (MR 750, GE Healthcare, USA) and data were acquired with a 32 channel receive head coil. Data were acquired using the interleaved, sequential with PROMO, and sequential without PROMO implementations of ihMTRAGE from brains of healthy volunteers (n = 4; age ranges = 26–53 years). Based on the results of simulations and prior literature (9), the preparatory module consisted of Tukey (cosine fraction, r = 0.2) shaped pulses of 5 ms and peak B1s of 15μT (for single offset frequency irradiation) every 100 ms for 1s (Fig. 1). Parameters for the rfb acquisition were: FA = 8 °; FOV = 30.7 × 30.7 × 18.2 cm3; matrix = 128 × 128 × 76; TE/TR = 1.5/4.1 ms; 4x Autocalibrating Reconstruction for Cartesian (ARC) imaging (2x in each phase encode direction); and, 80% phase FOV. TRMTRAGE, was initially 2 s (with 90 readouts per TRMTRAGE), but increased to 2.5 s when the PROMO module was included. Three dummy occurrences of TRMTRAGE preceded acquisition of each 3D volume. The 500ms PROMO module consisted of 5 sets of 3 orthogonal low FA (= 8°), single-shot spiral acquisitions as described in literature (14), and occurred prior to the preparatory module for brain ihMT MRI. Data for a total of five 3D volumes were collected with different types of preparation: a zero power preparation for normalization of the ihMT signal, Szp; a single positive offset frequency preparation, Ssing+; a single negative offset frequency preparation (to minimize effects from MT asymmetry (18)), Ssing-; and, two dual offset frequency preparations, Sdual. Dual frequency RF irradiation was achieved by cosine modulation of the MT pulses to more accurately reflect the ihMT model used for simulations (i.e. negation of dipolar order by simultaneous application at positive and negative values of the offset frequency to give Equation A2). Also, cosine modulated MT pulses are employed in the study that was used to guide the preparation’s structure (Fig. 1), and from which WM/GM tissue parameters used in simulations were obtained (9). Compared with dual frequency RF irradiation by frequency alternation of adjacent MT pulses, cosine modulation does not filter shorter T1D components thereby providing greater sensitivity to dipolar order (19). However, this comes at the expense of reduced specificity to longer T1D components such as those associated with myelinated tissues (20).
The 3D ihMTRAGE acquisition (sequential without PROMO) was modified to allow comparison with the steady state implementation of the ihMT experiments (n = 3; age ranges = 24–36 years). For these experiments the following acquisition parameters were changed: FOV = 25.6 × 25.6 × 16.8 cm3; matrix = 128 × 128 × 84; TE/TR = 1.6/4.3 ms; 1.04 s preparatory period consisting of the 5 ms off-resonance pulses applied every 130ms; and, TRMTRAGE = 2.08 s. To follow our prior definition, the steady state sequence was achieved by reducing: the number of off-resonance pulses in the preparatory period to one and TRMTRAGE to 130ms to match the off-resonance RF duty cycle of the ihMTRAGE preparation, and the readouts per TRMTRAGE from 90 to 10 (TE/TR = 2/5 ms). The FA was maintained at 8°, which based on simulations of this steady state sequence was only 2° above the optimal FA for ihMT SNR in WM (Fig. 2f). This provided a 78% faster acquisition of the data for the ihMT experiment (excluding dummies), allowing for more averaging within the same scan time, albeit for a much greater average SAR (Table 1). However, scanner average SAR limits over 6 minutes prevented acquisition with the same total scan duration with this steady state sequence in all three volunteers, highlighting an advantage of the ihMTRAGE implementation. A maximum average 10 s SAR matched steady state sequence, achieved by lowering the peak B1s of the off-resonance pulses, was also compared with ihMTRAGE acquired in a further three subjects (age ranges = 24–41 years).
Figure 2.
Plots of ihMTz, the difference between independently simulated signals (using numerical integration) with single and dual frequency MT preparations, for parameters relating to WM (in blue) and GM (in orange) tissues. a) Simulated ihMTz as a function of pulse width represent the average value following 4.5 to 5 s of RF irradiation to account for the modulation observed as a result of the low 5% duty cycle. b) IhMTz increases to a plateau with duration of the 5 ms off-resonance RF pulses simulated every 100 ms for the preparatory module of ihMTRAGE. c) Simulated ihMTz decreases with increasing: acquisition time for the imaging module; and, FA of on-resonance RF applied every 4 ms for readout. The imaging module is simulated following two cycles of 2 s TRMTRAGEs that include a 1 s preparation from (b), i.e. 5 s of ihMTRAGE. d) Simulation of ihMTR during five cycles of the ihMTRAGE sequence shows a difference between the sequential (solid line) and interleaved (dashed line) implementation. The interleaved signal becomes negative every TRMTRAGE as interchange of the signals results in Sdual > Ssing. Plots of: e) the ihMTR; and, f) a proxy for ihMT SNR per square root acquisition time (of 100 phase encodes including preparation and recovery periods), following simulation of 5.2 s of prepared ihMTRAGE and steady state sequences, as a function of the on-resonance FA.
Table 1. Results from comparison of prepared and steady state sequences.
Parameters relating to prepared and steady state implementations of the ihMT experiments, including TRMTRAGE that differentiates the two, SAR, and corpus callosum relative SNR (per square root acquisition time in zero-power preparation and ihMT images), MTR, and ihMTR. The average values ± standard errors across healthy volunteers are provided. An asterisk denotes unequal acquisition of Sdual volumes due to average SAR limits imposed by the scanner.
| Prepared | Steady state | ||
|---|---|---|---|
| Peak B1 of single frequency MT pulses [μT] | 15 | 15 | 11 |
| Maximum SAR over 10 s scan period [W/kg] | 2.0 | 3.8 | 2.0 |
| TRMTRAGE [ms] | 2080 | 130 | 130 |
| Dummy TRMTRAGEs | 3 | 10 | 10 |
| Time to acquire one 3D volume, T3Dvol [s] | 56 | 30 | 30 |
| Number of Ssing / Sdual volumes acquired | 2 / 2 | 4 / 3* | 6 / 6 |
| Total acquisition time for ihMT, TihMT [s] | 224 | 210 | 360 |
| Number of subjects from which data acquired | 6 | 3 | 3 |
| Szp relative SNR per square root T3Dvol [s−1/2] | 9.6 ± 0.3 | 8.0 ± 0.7 | 8.0 ± 0.1 |
| ihMT relative SNR per square root TihMT [s−1/2] | 1.72 ± 0.08 | 1.27 ± 0.19 | 0.84 ± 0.02 |
| Corpus callosum MTR | 0.293 ± 0.003 | 0.252 ± 0.025 | 0.205 ± 0.002 |
| Corpus callosum ihMTR | 0.177 ± 0.004 | 0.151 ± 0.012 | 0.110 ± 0.001 |
Cervical spinal cord ihMT MRI was conducted to demonstrate the feasibility of placing a spatial saturation module within the sequential ihMTRAGE acquisition. Data were acquired from two healthy volunteers (female, ages = 23–25 years) using only the posterior elements of a 16 channel receive head-neck-spine (HNS) coil. Limitations imposed by the setup only allowed for peak B1s of 12μT. In order to compensate for the reduction in B1, simulations guided us to the use of longer, 10 ms Tukey shaped pulses every 130 ms for 1.04s in the preparation, and TRMTRAGE = 2.04s, to achieve similar ihMT signal as from the brain. Parameters for the rfb acquisition were maintained, apart from: FOV = 22.4 × 22.4 × 4.5–6.7 cm3; matrix = 160 × 160 × 32–48; TE/TR = 2/6 ms; 70 readouts per TRMTRAGE; 1.5x ARC in slice phase encode direction; and, 40–60% phase FOV. In one of the subjects, an additional 80% phase FOV ihMT dataset (acquired in a longer scan time) was collected without spatial saturation to assess any difference made by its absence. To compensate for the decrease in SNR from the smaller FOV, higher resolution ihMTRAGE acquisition, data relating to Ssing and Sdual were acquired 9 times each for averaging.
Data processing
Data were processed offline using custom scripts developed in Matlab (R2018a, Mathworks, USA) and other tools specified. Maps of MTR and ihMTR were calculated based on established methods: For MTR, division of data following dual offset frequency RF irradiation divided by data acquired with a zero power preparation and subtraction of the result from unity, i.e. MTR = 1 - Sdual / Szp; For ihMTR, subtraction of data following dual offset frequency irradiation from that prepared with single offset frequency RF irradiation applied with the same power, and division of the result by data acquired with a zero power preparation, i.e. ihMTR = (Ssing+ + Ssing- - 2 × Sdual) / Szp. Although this relates to twice the difference between Ssing and Sdual, we maintain the definition used in prior literature and established by acquisition of two separate Ssing datasets to account for MT asymmetry (1,18), Maps of MTR and ihMTR displayed in figures were segmented using the Statistical Parametric Mapping 12 (SPM12) open-source Matlab toolbox (21), and images were resized to twice their resolution using bicubic interpolation.
Analysis of relative sharpness and SNR was conducted on averaged 3D image volumes or calculated ihMT images, where ihMT = Ssing+ + Ssing- - 2 × Sdual, to circumvent the impact from pixel value division by null or negligible values in low signal regions. A measure of sharpness was obtained using the function of the same name found in the k-Wave open source Matlab toolbox (22,23). Specifically, the sharpness metric calculated was based on the Brenner gradient, which computes the sum of the centered finite-difference at each pixel in each Cartesian direction. Results from the 3D volumes were normalized across the three implementations (interleaved, sequential with PROMO, and sequential without PROMO) since they were conducted in the same MRI session using the same hardware and sequence parameters. Thus, differences in sharpness were expected to reflect the type of implementation as well as the contrast. The resultant ihMT data were normalized across the three implementations separately due to the pixel values calculation by addition and subtraction of the 3D volumes. Calculation of relative SNR from data acquired in the same MRI session and with identical acceleration factors (24,25), for comparison between the prepared and steady state implementations were based on regions manually drawn on the ihMT images to encompass the corpus callosum and an area of similar size with no expected signal (i.e. noise) superior to the head. A value of 0.8 of the mean in the noise of Szp images was used to estimate its standard deviation (26).
For region of interest (ROI) analysis in the brain, data from each type of ihMTRAGE acquisition (interleaved and sequential, with and without PROMO) were processed using both SPM12 and FSL (21,27). First data from a FOV matched, T1-weighted (MPRAGE) acquisition were segmented and normalized to MNI152 space. MT-weighted images were aligned to the Szp images using rigid registration with 6 degrees of freedom. The Szp and realigned MT data from the ihMTRAGE acquisition were registered with the T1-weighted data using affine registration prior to calculation of ihMTR maps. The ihMTR maps were normalized to MNI152 space by applying the deformation field calculated for the normalization of the T1-weighted data. We overlaid the JHU ICBM-DTI-81 WM label atlas (28) and the maximum probability tissue labels derived from the MICCAI 2012 Grand Challenge and Workshop on Multi-Atlas Labeling provided by Neuromorphometrics, Inc. (29–31) onto ihMTR maps, from which mean values were extracted for several ROIs. The different implementations of ihMTRAGE (interleaved, sequential with PROMO, and sequential without PROMO) were compared using these mean ROI values and two-tailed, paired Student’s t-tests.
MTR and ihMTR values calculated from spinal cord acquisitions with and without spatial saturation were compared using manually drawn ROIs. Average values were calculated from ROIs in the: cerebellum GM; cerebellum WM; cervical spinal cord; medulla oblongata; and, pons. The linear relationship between ROI averages from data acquired with and without the spatial saturation module was measured by calculation of the Pearson correlation coefficient, r.
Results
Simulations
The results from simulations were used to guide the sequence used for in vivo acquisition and determine the expected ihMT signal. For a constant, low duty cycle of 5%, the average simulated ihMT as a function of pulse width following a 5 s off-resonance RF irradiation preparation shows a peak at 7.5 and 6.2 ms for WM and GM respectively (Fig. 2a). The simulated values of ihMT at a pulse width of 5 ms are within 5% of the maximum. The preparatory module of the ihMTRAGE sequence requires a trade-off between acquisition within an acceptable scan time and maximizing the ihMT signal. Concentrating on WM tissue, simulation of ihMT as a function of the RF irradiation duration (for RF pulse widths of 5ms with a 5% duty cycle) shows after 1 s WM ihMT is within an acceptable 10% of the maximum (Fig. 2b). Modulation of the ihMT signal during the 100 ms off-resonance RF TR is also expected based on simulations. Simulation of the acquisition module following off-resonance RF preparation shows a more rapid decay of ihMTz with increasing FA and increasing acquisition time (Fig. 2c), following an initial peak for lower FAs due to the differing contribution from the more restricted (semi-solid) pool. For a moderate FA of 8°, acquisition time of 0.4 s (excitation every 4 ms), and a TRMTRAGE of 2 s, simulations suggest at least one TRMTRAGE is required to achieve a pseudo steady state (Fig. 2d). A recovery from negative values for the interleaved implementation resulted from the interchange between signals relating to the single and dual off-resonance frequency preparations every TRMTRAGE = 2 s. The ihMT signal from the sequential implementation is expected to be greater than that from the interleaved acquisition. Simulation of the sequential ihMTRAGE sequence showed a decrease in ihMTR with acquisition FA (Fig. 2e). However, this decrease in ihMTR was subtler in comparison to the steady state implementation. The product of the prepared ihMTRAGE signal and the sine of the FA divided by the square root of the time for acquisition of 100 phase encodes (including preparation and recovery periods, i.e. TRMTRAGE = 2.08 s) showed an increase with FA (Fig. 2f). Simulation of the steady state implementation as a function of the FA, also for the acquisition time of 100 phase encodes, i.e. 1.3 s, suggested a humped response, and thus different peak or optimal FA values for WM and GM.
Human volunteer experiments
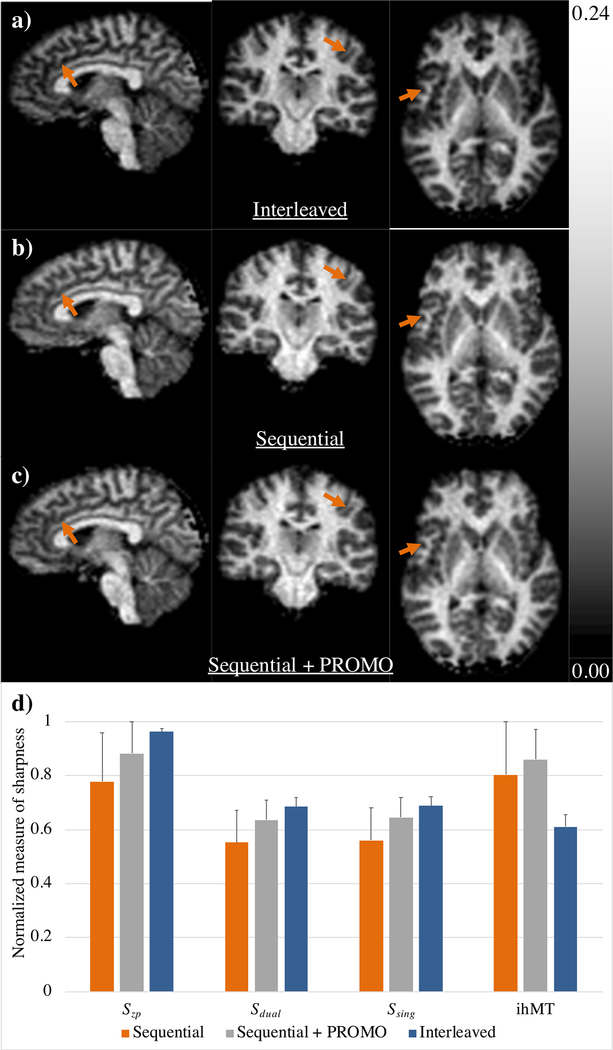
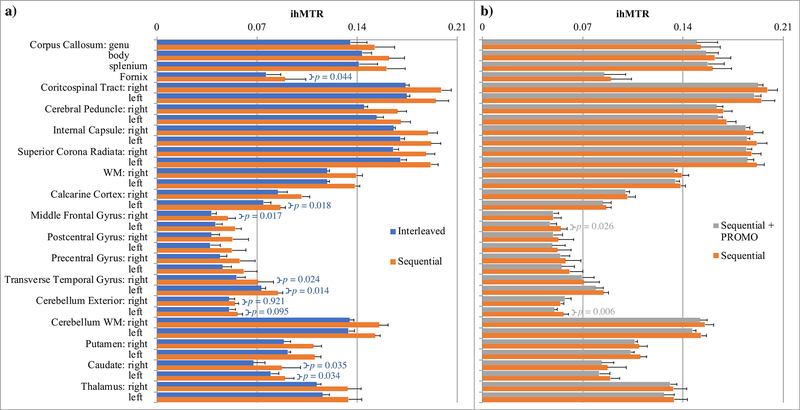
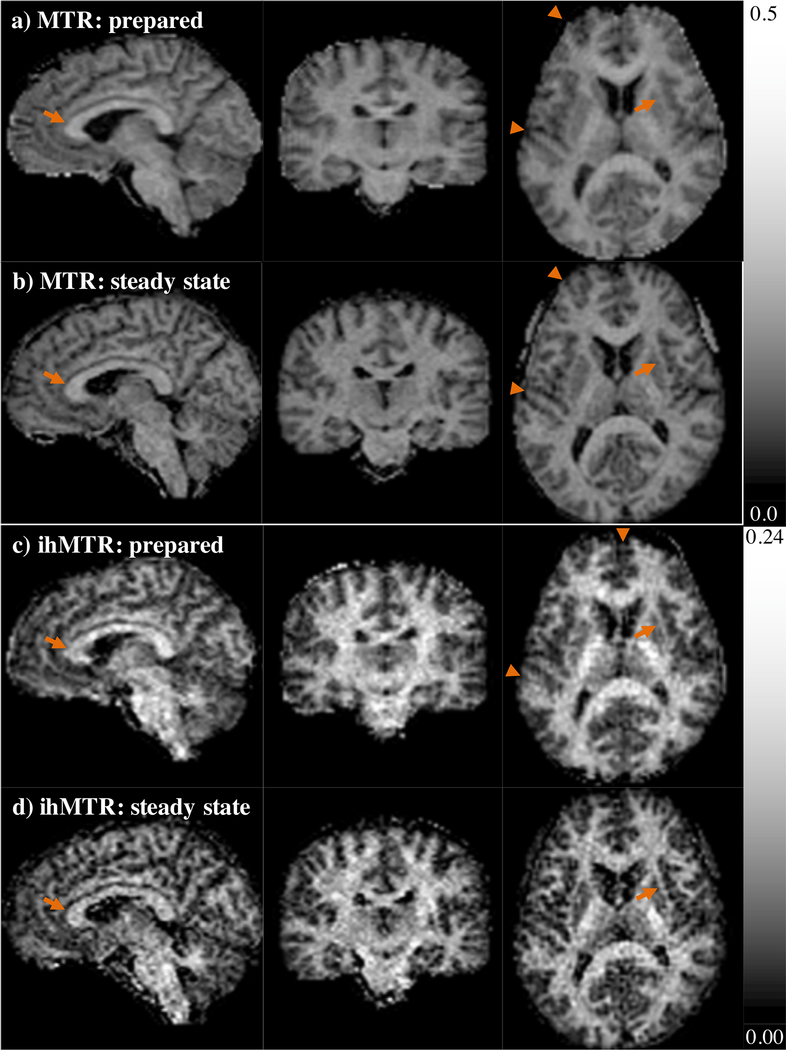
The ihMTRAGE sequence provided 3D maps of ihMTR from the brain in as little as 6 mins. Both interleaved and sequential implementations provided 3D ihMTR maps with no sign of artifacts from reformatting into other planes (Figs. 3a–c). Both sequential with PROMO and interleaved implementations provided sharper acquired volumes relating to Szp, Ssing, and Sdual contrasts than sequential without PROMO (Fig. 3d). Interestingly, the resultant ihMT volumes from the interleaved implementation averaged the lowest in the sharpness metric, in comparison to the other two sequential implementations. As expected from the results of simulations (Fig. 2d), the ihMT signal was lower for the interleaved implementation, with lower average ihMTR values measured across all ROIs in WM and GM (Fig. 4). By comparison, inclusion of the PROMO module in the sequential ihMTRAGE acquisition provided lower, but not significantly different, ihMTR values measured across all but two brain ROIs (Fig. 4). Maps of ihMTR from the sequential ihMTRAGE acquisition with and without PROMO were visually comparable, with no strong indication of motion artifacts observed in either (Figs. 3b–c). These results suggest the additional time within TRMTRAGE to accommodate the PROMO module had a minimal effect on the ihMT signal.
Figure 3.
Representative maps of ihMTR from a healthy volunteer calculated from data collected with a) interleaved, and sequential implementations of ihMTRAGE, b) without and c) with the PROMO module. The column on the left shows the ihMTR maps from data as acquired in the sagittal orientation. Center and rightmost columns are maps of ihMTR reformatted in the coronal and axial planes respectively. Arrows highlight structures that appear more clearly defined in the interleaved and sequential with PROMO acquisitions that can be considered more motion insensitive. d) Bar plot of the normalized measure of sharpness from averaged 3D acquisitions and ihMT image data, averaged over all healthy volunteers (error bars of standard error).
Figure 4.
Bar plots of average ihMTR over all healthy volunteers (error bars of standard error), as measured in ROIs within the brain, comparing the interleaved implementation (blue) with the sequential acquisition without (orange) and with the PROMO module (gray). P-values following two-tailed, paired Student’s t-tests are provided for: a) p > 0.01 in comparison between the interleaved and sequential implementations (blue) since a difference is expected; and b) p < 0.05 in comparison between the sequential acquisitions with and without the PROMO module (gray) since its addition should not affect ihMTRs, based on simulations.
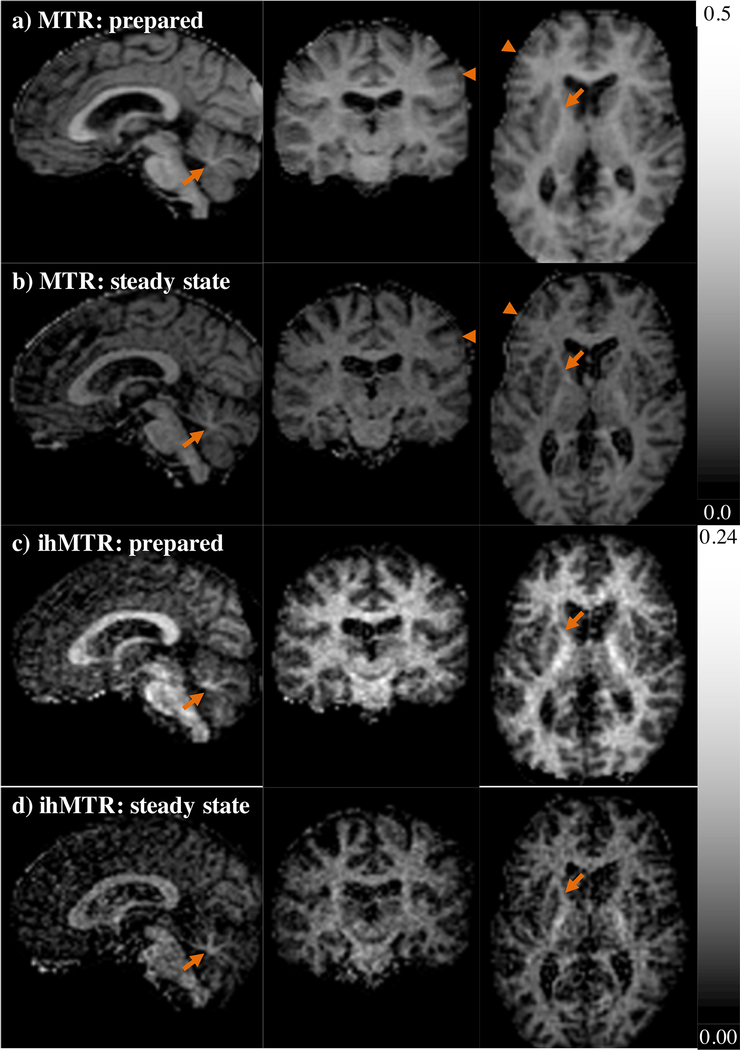
In comparison to the steady state ihMT sequence employed in this study, ihMTRAGE provided greater relative SNR images and ihMT signal for approximately equal total scan times and for lower average SAR values (Table 1, Figs. 5–6). Indeed, for a scanner hardware imposed maximum peak B1 of 15 μT for pulses of the single frequency MT preparation, the steady state ihMT sequence was unable to acquire data for the same total scan duration as the ihMTRAGE sequence in one subject due to average SAR limits. With the same high power off-resonance RF and pulse repetition time, i.e. 5 ms every 130 ms, the prepared ihMTRAGE sequence had a lower SAR due to a TRMTRAGE twice the length of the preparation period that reduced the B1,RMS (neglecting on-resonance pulses) by 29%. The ihMTRAGE sequence provided a means to reduce the SAR, averaging 1.2 W/kg over the entire scan, through use of the recovery period. This period also allowed recovery of the longitudinal magnetization and an increased relative SNR from the sequences applied (Table 1). Smaller average MTR and ihMTR values were measured in the corpus callosum for the steady state ihMT acquisitions. In the case of the steady state ihMT acquisition with maximum average 10 s SAR matched to the ihMTRAGE sequence, a greater reduction in ihMTR (38%) was measured as a result of the lower RF power, i.e. 11 μT peak B1s of the single frequency MT pulses (Figs. 6c–d).
Figure 5.
Comparison of ihMTRAGE with the steady state sequence using the same peak B1s of MT pulses. Maps of a-b) MTR, calculated using Sdual, and c-d) ihMTR, from data acquired with: a, c) the ihMTRAGE sequence in which the MT contrast is prepared before acquisition; and, b, d) a sequence that achieved a steady state with readouts between MT pulses, applied in the same subject. Arrows highlight a less blurred, sharper appearance of fiber bundles in maps from the steady state sequence.
Figure 6.
Comparison of the two types of sequence acquired with the same maximum average 10 s SAR values measured by the scanner, but different peak B1s of MT pulses. Maps from one subject of a-b) MTR, calculated using Sdual, and c-d) ihMTR, from data acquired with: a, c) the ihMTRAGE sequence in which the MT contrast is prepared before acquisition; and, b, d) a sequence that achieved a steady state with readouts between MT pulses. Arrows highlight a less blurred, sharper appearance of fiber bundles in maps from the steady state sequence.
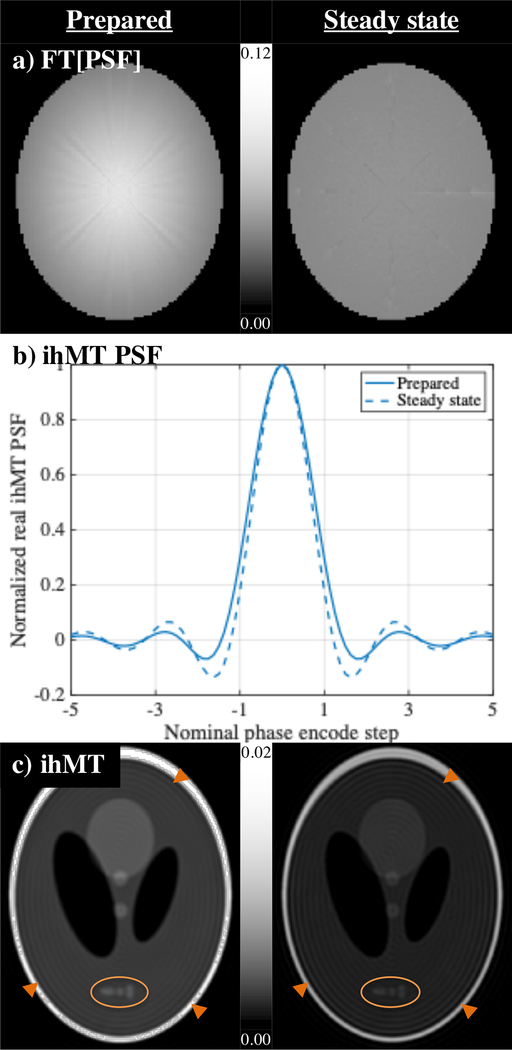
Comparison of the PSFs associated with the prepared and steady state acquisitions suggested the latter was less susceptible to blurring. Combination of the simulated WM Ssing signal with the rfb view ordering showed a more intense central region from the ihMTRAGE acquisition relative to the more uniform distribution predicted for the steady state (Fig. 7a). The ihMT PSF from the prepared acquisition had a slightly wider main lobe (Fig. 7b), which contributed to greater blurring in the resultant ihMT image relative to that from the steady state implementation (ovals in Fig. 7c). Indeed, certain WM structures in both MTR and ihMTR maps did appear more clearly defined for the steady state acquisition, with a less blurred, sharper appearance of fiber bundles (arrows in Figs. 5–6). Most noticeable though was a Gibbs ringing observed in reconstructed images from both ihMTRAGE and steady state simulated signal phantoms (arrowheads in Fig. 7c). Although less prominent for the maps reconstructed using steady state acquisition data, Gibbs ringing was also observed in maps from in vivo data (arrowheads in Figs. 5–6).
Figure 7.
Simulations to illustrate the effect of the PSFs from prepared (left) and steady state (right) implementations. a) The Fourier transform of the PSF is formed by simulation of the transverse signal in WM following single frequency MT for each type of sequence mapped onto the acquisition matrix in the order dictated by the rfb segmentation. b) Plot of the simulated PSF through its center shows a wider main lobe and less prominent sidebands from the prepared, ihMTRAGE sequence. c) Reconstruction of the simulated Shepp-Logan phantom following convolution with the PSF shows its effect on the WM ihMT signal. Arrowheads highlight Gibb’s ringing artifacts in all reconstructed images of the phantom.
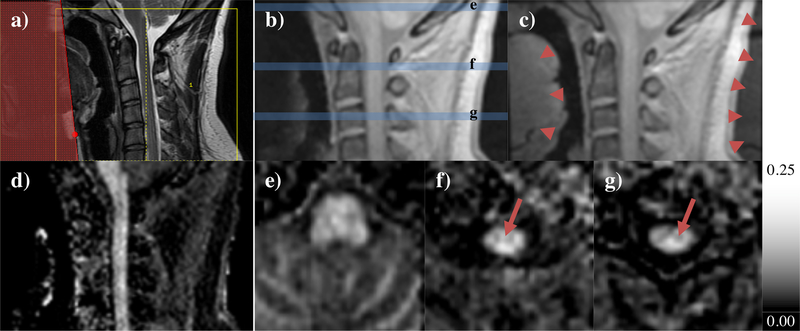
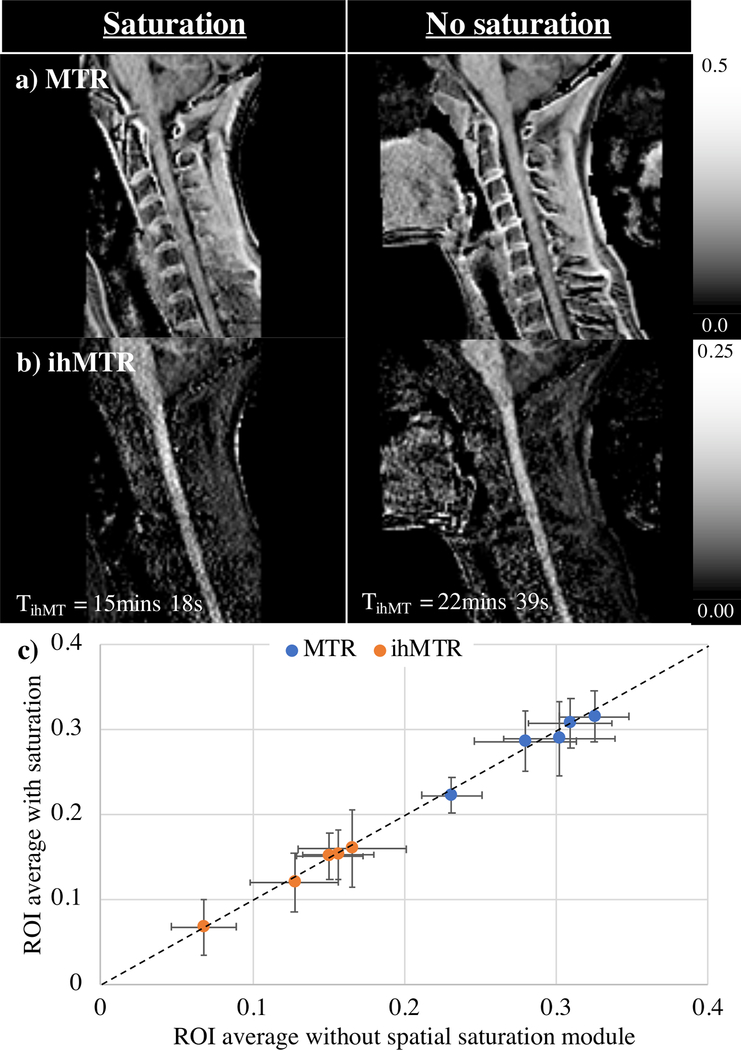
Combination of a spatial saturation module within the ihMTRAGE sequence allowed acquisition of 1.4 mm isotropic resolution ihMT data from the cervical spine. Placement of a spatial saturation band anterior to the ihMTRAGE acquisition volume was used to reduce the phase FOV, and thus the scan time for a 3D volume by attenuating the signal from the jaw (Figs. 8a–c, 9a–b). Maps of ihMTR showed a strong signal from the spinal cord relative to the surrounding tissues, including muscle (Figs. 8d–g). Lower ihMTR values were visible from the center of the spinal cord consistent with the butterfly shape associated with GM (Fig. 8f). MTR and ihMTR maps with and without the spatial saturation module were relatively similar (Fig. 9a–b); Comparison of average MTR and ihMTR ROI values provided a strong linear correlation, r = 0.977 and 0.996 respectively (Fig. 9c). Use of the spatial saturation module allowed for a reduction in the time for acquisition of ihMT data, TihMT by 32% and the phase FOV, from 80 to 40%, but resulted in noisier maps based on larger standard deviations in all ROIs considered.
Figure 8.
a) Sagittal T2-weighted image used for planning of ihMTRAGE acquisition (yellow box) and placement of spatial saturation band (red shaded box). Zero power prepared images (Szp) from ihMTRAGE acquisition b) with, and c) without application of the spatial saturation band. Arrowheads illustrate regions from which signal was attenuated when covered with spatial saturation band, including signal folded over in phase-encode direction. Maps of ihMTR from data combined for an effective thickness of 5.6 mm, d) as acquired in the sagittal orientation, and e-g) reformatted in the axial plane. Sample axial cuts at the levels illustrated in (b) show dominant ihMT signal from e) the cerebellum, and e-g) the spinal cord. Arrows in f-g) highlight the GM contrast visible towards the center of the cord.
Figure 9.
Comparison of cervical spinal cord acquisition with (left) and without (right) use of a spatial saturation module. The phase FOV increased from 40 to 80% upon removal of spatial saturation to minimize fold-over artifacts in maps of a) MTR and b) ihMTR, from data combined for an effective thickness of 5.6 mm. An increased phase FOV resulted in longer acquisition times for ihMT data, TihMT. c) Plot of average MTR and ihMTR from cerebellum WM/GM, cervical spinal cord, medulla oblongata, and pons ROIs (error bars of standard deviation) compares values from data acquired with and without spatial saturation, with dashed identity line for reference.
Discussion
Simulation of ihMTR values provided guidance on what to expect from the ihMTRAGE sequence and were used in consideration of its design. Beginning with a low, 5% duty cycle for the off-resonance pulses used for MT preparation that is found to increase the ihMT signal (8,9), simulations were used to confirm choice of a 5 ms pulse provided close to the maximum ihMTR from WM and GM tissues (Fig. 2a). While the simulated WM ihMTz was found to reach 90% of the maximum after 1 s preparation duration, the simulated GM ihMT value only achieved two thirds of its maximum (Fig. 2b). Although a difference in the ihMT signal intensity based on the type of preparation is found to change the specificity of ihMT to myelin, it has little effect on the sensitivity of ihMT to myelin content (20). As opposed to lengthening the preparation duration, and thus the scan time, this simulation framework might be used to maximize the ihMT signal from other tissues, e.g. GM for myeloarchitectonic mapping of the cerebral cortex (32).
Simulation of the ihMT signal also provided a way to estimate the PSF based on each type of acquisition and its effect on the image data. Combination of the simulated signal with the view ordering of the rfb acquisition showed a more homogeneous distribution across the phase encodes acquired by the steady state sequence (Fig. 7a). An expected result given the 90 readouts per TRMTRAGE employed for the prepared ihMTRAGE sequence versus 10 for the steady state. This resulted in a wider main lobe from the PSF of the ihMTRAGE acquisition (Fig. 7b), which contributed to less well-defined structures in the simulated phantom relative to the steady state acquisition (Fig. 7c). Maps of MTR and ihMTR from the steady state acquisition were also suggestive of sharper WM structures and less blurring (arrows in Figs. 5–6), but a more rigorous and quantitative comparison of SNR matched data is required (33). Although simulation of the signal without parallel imaging (Fig. 7a) corresponds to an increase in scan time, the radial signal decay and thus the PSF should be similar to that in vivo since the rfb view ordering compensates for the extra phase encodes by reducing the angular size of each fan beam (12). Simulations of ihMT as a function of the FA (Fig. 2c), taken as an indirect indication of the signals from the two types of preparation, suggests a difference between the PSFs relating to Ssing and Sdual, and WM and GM tissues. The combined effect from different tissues and the single and dual off-resonance acquisitions add to the complexity of predicting the effect of the PSF. Nonetheless simulations might be used to develop variable FA trains to reduce the effects on the PSF (34). Gibbs ringing was observed in both phantom and in vivo reconstructions (arrowheads in Figs. 5–7). Smaller sidelobes in the ihMTRAGE PSF (relative to the steady state acquisition) appeared to be a consequence of the apodizing effect from greater signal decay during the rfb view ordering (Figs. 7a–b). However, more visible Gibbs ringing from ihMTRAGE data acquired in vivo (Figs. 5–6), may be due to increased signal differences between adjacent structures, relative to the steady state acquisition (Fig. 2f), or a consequence of the discrete sampling of the images.
Both simulated and experimental results showed a decrease in ihMTR using an interleaved approach compared with the sequential ihMTRAGE acquisition (Figs. 1, 2d, 3, 4). Such a difference was dictated by the choice of TRMTRAGE, since lengthening either: the preparation duration to reach the steady state value (Fig. 2b); or, the time between acquisition and preparation to allow complete recovery, would result in the same ihMTR as for the first cycle of the ihMTRAGE sequence (Fig. 2d). However, ihMTRs measured in the corpus callosum with sequential ihMTRAGE were comparable to those using the same preparation before an EPI acquisition (Fig. 4d in (9)), including a higher value in the splenium compared with the genu. For the parameters explored in these ihMT experiments, there was a significant difference in the average ihMTR values between interleaved and sequential (without PROMO) ihMTRAGE from most brain tissue ROIs across subjects (Fig. 4). This would require consideration against any advantage offered by the interleaved acquisition, which allowed the two sets of signal for ihMT (Ssing and Sdual) from the same segment of k-space to be acquired closer together, as for label/control experiments in arterial spin-labeled perfusion MRI (13).
The measure of sharpness provided some indication of the relative quality of images from interleaved, sequential with PROMO, and sequential without PROMO implementations. Although the sequential implementation failed to score the highest sharpness value in any of the acquired 3D or ihMT image volumes, the ihMT sharpness being lowest for the interleaved implementation, in comparison to the other two sequential implementations, seems counterintuitive given the interleaved providing the highest sharpness values of the three implementations from the Ssing and Sdual images (Fig. 3d). Possible reasons include the overall consistency and averaging of the interleaved acquisition; Since the Ssing and Sdual data are collected together, any motion is more likely to be globally distributed along the entire acquisition (as opposed to confined to a single 3D volume) resulting in relatively stable sharpness values. Hence, while certain structures might be more visible in ihMT images since less time elapses between acquisition of the same segment of data (arrows in Figs. 3a–b), a more global blurring might also be present. It is worth noting that none of the differences in sharpness measured between implementations were considered significant, with all p values ≥ 0.15.
Nonetheless, we were able to demonstrate inclusion of a PROMO module for application of ihMTRAGE in the brain, and the use of a spatial saturation module for ihMT of the cervical spine. In contrast to comparison with the interleaved acquisition, sequential ihMTRAGE without and with PROMO showed no significant difference in average ihMTR values from the majority of brain tissue ROIs (Fig. 4). In fact, p values from Student’s t-tests < 0.03 were only found for two ROIs both in GM, from which the low ihMT signal might have contributed. None of the ROIs presented with a significant difference in Szp from sequential ihMTRAGE with and without PROMO. Although the on-resonance pulses associated with PROMO might have serendipitously compensated for the additional recovery afforded by the longer TRMTRAGE of 2.5 s, simulations suggest an overall decrease in TRMTRAGE would increase T1 recovery effects and the difference between images from ihMTRAGE with and without PROMO. Demonstration of the spatial saturation module in ihMTRAGE allowed attenuation of the signal from the anterior portion of the jaw for ihMT of the cervical spine (Figs. 8–9). This in turn allowed the prescribed scan volume and thus scan time to be reduced, while minimizing contamination of the spinal cord ihMT data due to phase wrap. Use of the spatial saturation module had no noticeable effect on MTR or ihMTR values, with average ROI values from data acquired with and without spatial saturation falling close to the line of identity (Fig. 9c). These two demonstrations of the feasibility to add other MRI modules within the ihMTRAGE sequence provide potential for further combination of contrasts and/or optimizations with ihMT. For example, addition of a diffusion-weighting module might help elucidate further the orientation dependence of the ihMT signal (1,5,6), and/or possible ways to mitigate its contribution.
The recovery period that is integral to this prepared style ihMT sequence, i.e. ihMTRAGE, allows for a reduction in the average power deposition over the scan duration in comparison to the steady state approach (Table 1). A reduction in average power deposition allowed the maximum peak B1 to be utilized with lower SAR over the entire scan, in comparison to the steady state acquisition with peak B1 of the single frequency MT pulses of 15 μT (Table 1). The average ihMTR value across the corpus callosum of 0.152 from this steady state acquisition was comparable to that achieved at 1.5T by Mchinda et al. (Fig. 6b in (8)), albeit for approximately half the root mean square B1 over TR but using cosine modulated pulses to achieve dual frequency off-resonance irradiation. Matching the SAR during acquisition of the steady state ihMT sequence to that during the preparation of the ihMTRAGE sequence required a reduction in peak B1 of the single frequency MT pulses to 11 μT. Such a reduction resulted in a decrease in MTR and ihMTR values (Table 1, Fig. 6), as expected from prior literature on which the preparation was based (9). The FA employed for on-resonance pulses was maintained at 8° to allow easier matching with the power deposition and SAR of the ihMTRAGE acquisition. However the ihMTR values obtained from the steady state acquisitions could be increased by reducing the FA for acquisition or the number of readouts per TRMTRAGE (Figs. 2c,e). Simulations support further optimization of both prepared and steady state approaches. In particular, for the steady state implementation, use of the FA that corresponds to a peak in the product of the ihMTz and the sine of the FA, which provided an indication of ihMT SNR (Fig. 2f), as for rapid gradient echo MT MRI (35). Although ihMT relative SNR was found to be lower in the steady state implementation than for the prepared ihMTRAGE sequence (Table 1), simulations suggest use of FA = 8° achieved 95% of the maximum ihMT steady state signal available from WM.
The definition for the steady state used here, i.e. one off-resonance MT RF pulse between successive acquisition modules, and a recovery period (if present) less than the time between adjacent MT pulses, also holds for other instances of simultaneous dual offset RF irradiation (5,7,17). The ihMT acquisitions proposed by Mchinda et al. within a steady state sequence does not fit this definition because it makes use of multiple, closely spaced, <1 ms, MT pulses of alternating frequency for the dual frequency experiments. Although the sequence proposed by Malik et al. constitutes a true steady state with one readout per TR (17), that implementation is relatively SAR intensive whilst not making use of the increased ihMT signal available through a low duty cycle implementation (8,9). Use of multiple readouts, whether in a prepared or steady state ihMT sequence, provides a means for scan time efficiency.
Addition of the recovery time component adds to an already complex optimization problem with respect to the ihMT experiment and the targeted signal and/or its dependency (e.g. T1 sensitivity). This might result in different ihMTRAGE sequences or at least types of preparation being developed to target specific applications, to the detriment of easy comparison of different studies. Whilst this allows for further development of ihMTRAGE, simulations using the framework provided can guide optimization of the sequence, including the effects from combination with other MRI modules.
As well as demonstrating ihMTRAGE, to the best of our knowledge and literature searches this work also provided the first demonstration of MT data acquired with an MPRAGE style sequence. This, along with the low off-resonance RF duty cycle and relatively large offset frequency employed makes a meaningful comparison of MTR values obtained in this study, with prior literature, difficult. Both MTR and ihMTR represent semi-quantitative measurements that are dependent on the sequence implemented (8,36). Extraction of quantitative parameters based on models of the MT and ihMT signals are possible with acquisition of additional data (9,37). As for our simulations (Fig. 2d), such models might easily incorporate details of the interleaved implementation to account for reductions in the ihMTR (Figs. 3–4). The ±7kHz off-resonance RF irradiation utilized is within the optimal range recommended for macromolecular proton fraction mapping with a single MT measurement (38), and thus the MT data from the ihMTRAGE acquisition might also be used for this purpose.
Conclusions
Implementation of the preparations necessary for ihMT (and MT) within an MPRAGE acquisition, i.e. ihMTRAGE, allowed separation of the preparation, acquisition and time for longitudinal magnetization recovery. The latter could be used to reduce overall power deposition and thereby meet SAR limits, without resorting to a reduction in the B1 of the MT pulses used for signal preparation, specifically in comparison to a steady state ihMT acquisition. Both interleaved and sequential acquisitions of the different preparations for ihMT were possible: An interleaved implementation reduced the time between acquisition of the same k-space segment with different preparations. However incomplete recovery of the signal between changes in the preparation resulted in higher ihMTR values from the sequential implementation. The ihMTRAGE implementation also allows for the addition of other MRI modules, with successful inclusion of modules: to decrease motion sensitivity (with PROMO), and for spatially selective saturation of the signal, demonstrated.
Acknowledgements
The research reported in this publication was supported in part by GE Healthcare, and in part by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R21NS114546. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
The ihMT signal was simulated based on a two-pool MT model that considers the interchange of (longitudinal) magnetization, Mz between the free pool A and macromolecular pool B. The macromolecular pool B can include a dipolar order component β that corresponds to the inverse spin temperature of the dipolar order (15,39,40). Inclusion of a single dipolar component was used to describe the change in magnetization during single frequency off-resonance RF irradiation:
| [A1] |
RrfA represents the effect of off-resonance RF irradiation on pool A and was assumed to be equal to (ω12/(2πΔ)2)/T2A based on a Lorentzian lineshape, where ω12 is the power of the RF pulse and Δ is the frequency offset in Hz. RrfB, sometimes referred to by W, was equal to πω12g(2πΔ), where g(2πΔ) was based on a super-Lorentzian lineshape for pool B (41). As a result D2, which represents the local field, was taken to be 1/(15T2B2) (40).
Dual frequency off-resonance RF irradiation achieved by cosine modulated pulses allowed accurate simulation using the two-pool MT system without a dipolar component. The change in magnetization during dual frequency RF irradiation applied symmetrically around on-resonance was described by these two coupled differential equations:
| [A2] |
The effect of on-resonance RF was simulated by instantaneous reduction in the longitudinal magnetization of pool A MzA by multiplication with cos(FA).
Thus, ihMTz was simulated based on the resultant free pool longitudinal magnetization MzA calculated from A2 (the two differential equations relating to RF applied symmetrically around on-resonance) subtracted from MzA calculated from A1 (the set of differential equations that describe single offset frequency RF irradiation).
References
- 1.Varma G, Duhamel G, De Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn. Reson. Med 2015;73:614–622. doi: 10.1002/mrm.25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taso M, Girard OM, Duhamel G, le Troter A, Feiweier T, Guye M, Ranjeva JP, Callot V. Tract-specific and age-related variations of the spinal cord microstructure: A multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed. 2016;29:817–832. doi: 10.1002/nbm.3530. [DOI] [PubMed] [Google Scholar]
- 3.Rasoanandrianina H, Grapperon A-M, Taso M, Girard OM, Duhamel G, Guye M, Ranjeva J-P, Attarian S, Verschueren A, Callot V. Region‐specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed. 2017;30:e3801. doi: 10.1002/nbm.3801. [DOI] [PubMed] [Google Scholar]
- 4.Van Obberghen E, Mchinda S, le Troter A, et al. Evaluation of the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI for multiple sclerosis. Am. J. Neuroradiol 2018;39:634–641. doi: 10.3174/ajnr.A5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geeraert BL, Lebel RM, Mah AC, Deoni SC, Alsop DC, Varma G, Lebel C. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. Neuroimage 2018;182:343–350. doi: 10.1016/j.neuroimage.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Ercan E, Varma G, Mädler B, et al. Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magn. Reson. Med. 2018. doi: 10.1002/mrm.27211. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Chen T, Tian H, Xue H, Ren H, Li L, Fan Q, Wen B, Ren Z. Reproducibility of inhomogeneous magnetization transfer (ihMT): A test-retest, multi-site study. Magn. Reson. Imaging 2019;57:243–249. doi: 10.1016/j.mri.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Mchinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn. Reson. Med 2018;79:2607–2619. doi: 10.1002/mrm.26907. [DOI] [PubMed] [Google Scholar]
- 9.Varma G, Girard OM, Mchinda S, Prevost VH, Grant AK, Duhamel G, Alsop DC. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. J. Magn. Reson 2018;296:60–71. doi: 10.1016/j.jmr.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Mugler JP, Brookeman JR. Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE). Magn. Reson. Med 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 11.Mugler JP, Brookeman JR. Rapid three‐dimensional T1‐weighted MR imaging with the MP‐RAGE sequence. J. Magn. Reson. Imaging 1991;1:561–567. doi: 10.1002/jmri.1880010509. [DOI] [PubMed] [Google Scholar]
- 12.Saranathan M, Tourdias T, Bayram E, Ghanouni P, Rutt BK. Optimization of white-matter-nulled magnetization prepared rapid gradient echo (MP-RAGE) imaging. Magn. Reson. Med 2015;73:1786–1794. doi: 10.1002/mrm.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsop DC, Detre JA, Golay X, et al. Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications : A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. 2015;116:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, Kuperman J, Dale A. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magn. Reson. Med 2010;63:91–105. doi: 10.1002/mrm.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma G, Girard OM, Prevost VH, Grant AK, Duhamel GD, Alsop DC. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J. Magn. Reson 2015;260:67–76. doi: 10.1016/j.jmr.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho VND, Hertanu A, Grélard A, et al. MRI assessment of multiple dipolar relaxation time (T1D) components in biological tissues interpreted with a generalized inhomogeneous magnetization transfer (ihMT) model. J. Magn. Reson [Internet] 2020;311:106668. doi: 10.1016/j.jmr.2019.106668. [DOI] [PubMed] [Google Scholar]
- 17.Malik SJ, Teixeira RPAG, West DJ, Wood TC, Hajnal JV. Steady‐state imaging with inhomogeneous magnetization transfer contrast using multiband radiofrequency pulses. Magn. Reson. Med 2020;83:935–949. doi: 10.1002/mrm.27984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevost VH, Girard OM, Varma G, Alsop DC, Duhamel G. Minimizing the effects of magnetization transfer asymmetry on inhomogeneous magnetization transfer (ihMT) at ultra-high magnetic field (11.75 T). Magn. Reson. Mater. Physics, Biol. Med 2016;29:699–709. doi: 10.1007/s10334-015-0523-2. [DOI] [PubMed] [Google Scholar]
- 19.Prevost VH, Girard OM, Mchinda S, Varma G, Alsop DC, Duhamel G. Optimization of inhomogeneous magnetization transfer (ihMT) MRI contrast for preclinical studies using dipolar relaxation time (T1D) filtering. NMR Biomed. 2017;30:e3706. doi: 10.1002/nbm.3706. [DOI] [PubMed] [Google Scholar]
- 20.Duhamel G, Prevost VH, Cayre M, Hertanu A, Mchinda S, Carvalho VN, Varma G, Durbec P, Alsop DC, Girard OM. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. Neuroimage [Internet] 2019;199:289–303. doi: 10.1016/j.neuroimage.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Treeby BE, Cox BT. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt 2010;15:021314. doi: 10.1117/1.3360308. [DOI] [PubMed] [Google Scholar]
- 23.Treeby BE, Varslot TK, Zhang EZ, Laufer JG, Beard PC. Automatic sound speed selection in photoacoustic image reconstruction using an autofocus approach. J. Biomed. Opt 2011;16:090501. doi: 10.1117/1.3619139. [DOI] [PubMed] [Google Scholar]
- 24.Reeder SB. Measurement of Signal-to-Noise Ratio and Parallel Imaging In: Schoenberg SO, Dietrich O, Reiser MF, editors. Parallel Imaging in Clinical MR Applications. Berlin; New York: Springer; 2007. pp. 49–61. [Google Scholar]
- 25.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of Signal-to-Noise Ratios in MR Images: Influence of Multichannel Coils, Parallel Imaging, and Reconstruction Filters. J. Magn. Reson. Imaging 2007;26:375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 26.Henkelman RM. Measurement of signal intensities in the presence of noise in MR images. Med. Phys 1985;12:232–233. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI atlas of human white matter 1st Editio. Elsevier; 2005. [Google Scholar]
- 29.Friston KJ, Stephan KE, Lund TE, Morcom A, Kiebel S. Mixed-effects and fMRI studies. 2005;24:244–252. doi: 10.1016/j.neuroimage.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Klein A, Andersson J, Ardekani BA, et al. NeuroImage Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage [Internet] 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litvak V, Jha A, Flandin G, Friston K. NeuroImage Convolution models for induced electromagnetic responses. Neuroimage [Internet] 2013;64:388–398. doi: 10.1016/j.neuroimage.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munsch F, Varma G, Taso M, Girard OM, Guidon A, Duhamel G, Alsop DC. Myeloarchitectonic mapping of cortical gray matter with 3D inhomogeneous magnetization transfer (ihMT). In: Proceedings 27th Scientific Meeting, International Society for Magnetic Resonance in Medicine; 2019. p. 1048. [Google Scholar]
- 33.Osadebey ME, Pedersen M, Arnold DL, Wendel-Mitoraj KE. Blind blur assessment of MRI images using parallel multiscale difference of Gaussian filters. Biomed. Eng Online [Internet] 2018;17:1–22. doi: 10.1186/s12938-018-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mugler JP, Epstein FH, Brookeman JR. Shaping the signal response during the approach to steady state in three-dimensional magnetization-prepared rapid gradient-echo imaging using variable flip angles. Magn. Reson. Med 1992;28:165–185. [DOI] [PubMed] [Google Scholar]
- 35.Pike GB. Pulsed magnetization transfer contrast in gradient echo imaging: A two-pool analytic description of signal response. Magn. Reson. Med 1996;36:95–103. doi: 10.1002/mrm.1910360117. [DOI] [PubMed] [Google Scholar]
- 36.Cercignani M, Symms MR, Ron M, Barker GJ. 3D MTR measurement: From 1.5 T to 3.0 T. Neuroimage 2006;31:181–186. doi: 10.1016/j.neuroimage.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Portnoy S, Stanisz GJ. Modeling pulsed magnetization transfer. Magn. Reson. Med 2007;58:144–155. doi: 10.1002/mrm.21244. [DOI] [PubMed] [Google Scholar]
- 38.Yarnykh VL. Fast macromolecular proton fraction mapping from a single off-resonance magnetization transfer measurement. Magn. Reson. Med 2012;68:166–178. doi: 10.1002/mrm.23224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman M Spin temperature and nuclear magnetic resonance in solids. Oxford University Press; 1970. [Google Scholar]
- 40.Morrison C, Stanisz G, Henkelman RM. Modeling Magnetization Transfer for Biological-like Systems Using a Semi-solid Pool with a Super-Lorentzian Lineshape and Dipolar Reservoir. J. Magn. Reson. Ser. B 1995;108:103–113. doi: 10.1006/jmrb.1995.1111. [DOI] [PubMed] [Google Scholar]
- 41.Morrison C, Mark Henkelman R. A Model for Magnetization Transfer in Tissues. Magn. Reson. Med 1995;33:475–482. doi: 10.1002/mrm.1910330404. [DOI] [PubMed] [Google Scholar]