Abstract
Objective:
Childhood epilepsy with centrotemporal spikes (CECTS) is a common, focal, transient, developmental epilepsy syndrome characterized by unilateral or bilateral, independent epileptiform spikes in the Rolandic regions of unknown etiology. Given that CECTS presents during a period of dramatic white matter maturation and thatspikes in CECTS are activated during non–rapid eye movement (REM) sleep, we hypothesized that children with CECTS would have aberrant development of white matter connectivity between the thalamus and the Rolandic cortex. We further tested whether Rolandic thalamocortical structural connectivity correlates with spike rate during non-REM sleep.
Methods:
Twenty-three children with CECTS (age = 8-15 years) and 19 controls (age = 7-15 years) underwent 3-T structural and diffusion-weighted magnetic resonance imaging and 72-electrode electroencephalographic recordings. Thalamocortical structural connectivity to Rolandic and non-Rolandic cortices was quantified using probabilistic tractography. Developmental changes in connectivity were compared between groups using bootstrap analyses. Longitudinal analysis was performed in four subjects with 1-year follow-up data. Spike rate was quantified during non-REM sleep using manual and automated techniques and compared to Rolandic connectivity using regression analyses.
Results:
Children with CECTS had aberrant development of thalamocortical connectivity to the Rolandic cortex compared to controls (P = .01), where the expected increase in connectivity with age was not observed in CECTS. There was no difference in the development of thalamocortical connectivity to non-Rolandic regions between CECTS subjects and controls (P = .19). Subjects with CECTS observed longitudinally had reductions in thalamocortical connectivity to the Rolandic cortex over time. No definite relationship was found between Rolandic connectivity and non-REM spike rate (P > .05).
Significance:
These data provide evidence that abnormal maturation of thalamocortical white matter circuits to the Rolandic cortex is a feature of CECTS. Our data further suggest that the abnormalities in these tracts do not recover, but are increasingly dysmature over time, implicating a permanent but potentially compensatory process contributing to disease resolution.
Keywords: BECTS, CECTS, diffusion tensor imaging, DTI, probabilistic tractography
1 ∣. INTRODUCTION
Childhood epilepsy with centrotemporal spikes (CECTS; previously benign epilepsy with centrotemporal spikes or BECTS) is the most common focal childhood epilepsy syndrome, accounting for an estimated 10% of childhood epilepsy cases.1-5 This electroclinical syndrome presents in school-age children and is characterized by a transient period of predominantly nocturnal seizures, subtle cognitive deficits, and abundant sleep-activated spikes in the Rolandic regions on electroencephalography (EEG).6 Clinical seizures classically present as hemifacial twitching, hypersalivation, and focal upper extremity clonus, closely corresponding to Rolandic cortex semiology.7 The etiology of this common epilepsy syndrome remains unknown, and a complex genetic inheritance pattern is presumed.8-12
CECTS exists on a spectrum of sleep-activated epilepsy syndromes, where more severe forms—continuous spike and wave of sleep with encephalopathy (CSWS) and Landau-Kleffner syndrome—are characterized by near-continuous epileptiform spike activity during non–rapid eye movement (REM) sleep.13,14 CECTS and these related sleep-activated epilepsy syndromes present during a dramatic period of brain white matter maturation. During school age, healthy children normally undergo diffuse macrostructural increases in subcortical white matter volume and tract size, as well as strengthening of white matter microstructure, such as increased myelination and fiber bundling.15,16
As the thalamus is a central regulator of cortical sleep oscillations, the stereotyped sleep-activated spikes observed in CECTS could indicate a disruption in the development of the thalamocortical circuit.17,18 Previous work has hinted that the thalamocortical circuit may be disrupted in related developmental epilepsy syndromes characterized by sleep-activated spikes. Children with CSWS tended to have a smaller thalamus compared to controls.19 Children with CSWS are more likely to have an early life thalamic lesion compared to children with a lower spike burden,20 and thalamic lesions in children with CSWS correspond to ipsilateral reductions in white matter volume.21 Furthermore, a prospective study found that half of children with early life thalamic hemorrhage subsequently develop sleep-activated spikes.22
Given that CECTS presents during a period of dramatic white matter maturation and is characterized by stereotyped, focal abnormalities in sleep electrophysiology, we hypothesized that children with CECTS would have focal developmental abnormalities in thalamocortical connectivity specific to the Rolandic cortex compared to controls. We further hypothesized that aberrant thalamocortical connectivity would correlate with non-REM spike burden in these children. Identification of focal thalamocortical white matter abnormalities in CECTS would provide insight into the structural circuit underlying this common neurodevelopmental disease and the developmental changes to this circuit that support disease resolution.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study sample
Children aged 4-15 years who received a clinical diagnosis of CECTS by a child neurologist following International League Against Epilepsy criteria, including history of at least one focal motor or generalized seizure and EEG-confirmed sleep-activated centrotemporal spikes, were eligible for this prospective study.13,23 Control subjects without a history of seizure or known neurological disorder were also recruited. Subjects with a history of autism spectrum disorder, intellectual disability, or other unrelated neurological disease were excluded. Children with attention disorders and mild learning difficulties were included, as these profiles are consistent with known CECTS comorbidities.24 Approval from the Partners Institutional Review Board was received prior to study implementation.
Twenty-seven children with CECTS and 19 control children were enrolled. Three children with CECTS did not complete magnetic resonance imaging (MRI) acquisition. One further child with CECTS was excluded due to excessive motion artifact during MRI. In total, 23 children with CECTS (age = 8-15 years, 18 male) and 19 control subjects (age = 7-15 years, nine male) were included in this study. Duration seizure-free in CECTS subjects at the time of data acquisition, which predicts disease resolution,25 ranged from 0 to 51 months. Clinical characteristics are summarized in Table 1. Four CECTS subjects returned after a minimum of 12 months (range = 1.1-1.8 years) for repeat evaluations. Among these cases, duration seizure-free ranged from 18 to 64 months. Nineteen CECTS subjects (13 male) had non-REM sleep (excluding N1) identified on EEG and were included in the analysis of thalamocortical connectivity and spike burden. Mean duration between MRI and EEG was 4.8 days (range = 0-36).
TABLE 1.
Descriptive characteristics of the study sample
| Characteristic | CECTS, n = 23 | Control, n = 19 | P |
|---|---|---|---|
| Age, mean y (range) | 11.4 (8-15) | 10.7 (7-15) | .09a |
| Gender | 18 M | 9 M | .04b,c |
| Handedness | 20 right, 3 left | ||
| Duration seizure-free, mean mo (range) | 11.8 (0-51) | ||
| Laterality of discharges, n = 19 with NREM EEG | 15 B, 3 R, 0 L, 1 N |
Abbreviations: B, bilateral independent discharges; CECTS, childhood epilepsy with centrotemporal spikes; EEG, electroencephalogram; L, left hemisphere only; M, male; N, neither; NREM, non–rapid eye movement; R, right hemisphere only.
Two-sample t test.
Chi-square test. Both age and gender were tested as predictors of connectivity index (see Statistical Analyses).
Significant at alpha = .05.
2.2 ∣. MRI data acquisition and preprocessing
An overview of MRI preprocessing and analysis is provided in Figure 1. High-resolution structural and diffusion imaging data were acquired on a 3-T Magnetom Prisma MRI scanner (Siemens) using a 64-channel head coil with the following sequences: diffusion tensor imaging (DTI; 64 diffusion-encoding directions, echo time [TE] = 82 milliseconds, repetition time [TR] = 8080 milliseconds, flip angle = 90°, voxel size = 2.0 × 2.0 × 2.0 mm, diffusion sensitivity b = 2000 s/mm2, number of slices = 74, skip 0), multiecho magnetization-prepared rapid acquisition gradient echo (MEMPRAGE; TE = 1.74 milliseconds, TR = 2530 milliseconds, flip angle = 7°, voxel size = 1 × 1 × 1 mm), and multiecho Fast Low Angle Shot (TE = 1.85, 3.85, 5.85, 7.85, 9.85, 11.85, 13.85, 15.85 milliseconds, TR = 2000 milliseconds, flip angle = 5°, voxel size = 1 × 1 × 1 mm). Eddy current distortion, field inhomogeneities, and motion were corrected using FSL-FMRIB.26 DTIFIT was used to compute a voxelwise diffusion tensor model. MEMPRAGE data were coregistered to DTI data using an affine intrasubject transformation matrix generated by FreeSurfer’s bbregister tool. BEDPOSTX was used to generate posterior distributions of possible fiber directions within each voxel, representing local probability density of fiber orientation. Further explanation of this technique, which uses Markov Chain Monte-Carlo sampling, is described by Behrens and colleagues.27
FIGURE 1.
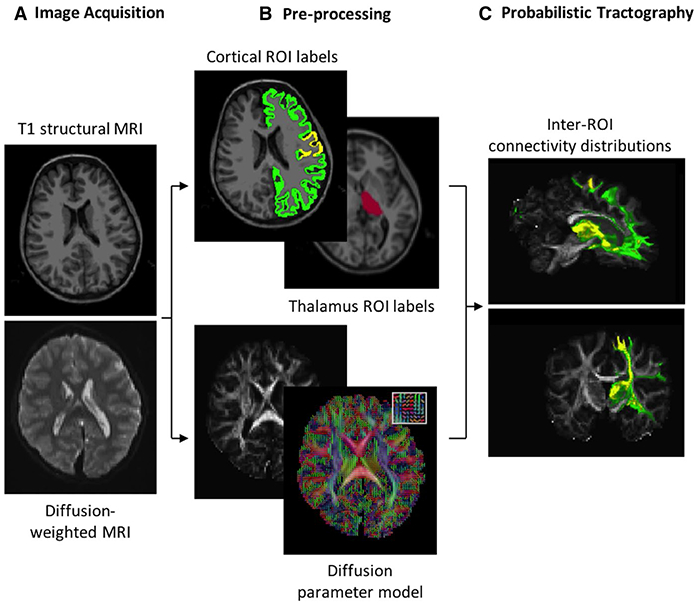
Thalamocortical tractography processing pipeline. A, High-resolution structural and diffusion magnetic resonance images (MRIs) are acquired. B, The Desikan-Killiany atlas is used to label Rolandic (yellow) and non-Rolandic (green) cortex and the thalamus (red) in structural MRIs (top). Diffusion MRIs are used to extract diffusion parameters per voxel from 64 gradient directions (example principal directions of diffusion for 25 voxels shown in inset). C, Distribution of diffusion parameters is repeatedly sampled to infer the probability of white matter tracts between regions of interest (ROIs)
2.3 ∣. Region of interest masks
Diffusion seed and target region of interest (ROI) masks were created using Desikan-Killiany atlas labels fit to the structural MRI of each subject.28 Seed masks included left and right hemisphere thalamus labels. Target masks included left and right hemisphere Rolandic cortex, comprised of pre- and post-central gyri, and left and right non-Rolandic cortex labels. Exclusion masks were created encompassing the hemisphere contralateral to seed and target ROIs, noncortical gray matter including brain stem and cerebellum, and nontarget ipsilateral cortex to ensure anatomically plausible pathways between thalamus and cortical ROIs. All masks were visually inspected to confirm appropriate fitting.
2.4 ∣. Probabilistic tractography
Probabilistic tractography was executed using PROBTRACKX2, with specification of seed and target ROI masks. At each seed voxel, the local posterior distribution generated by BEDPOSTX was sampled, and a 0.5-mm step along the resulting vector direction was taken. The process was repeated until termination of the streamline at the target mask or rejection criteria were met. Streamlines were rejected when reaching 2000 steps without arriving at the target or failing to meet a 0.2 curvature threshold (ie, minimum angle of 80°). Five hundred streamlines were executed per seed voxel. This stepwise procedure was repeated for each target ROI by hemisphere.
To control for variations in volume, the number of streamlines initiated from the seed thalamus successfully terminating in the target cortical ROI was extracted from the resulting tractography matrices, and normalized by the total number of streamlines originating from seed voxels following previous procedures,29 where:
Results per hemisphere were noted to be qualitatively similar and averaged to yield two normalized connectivity indices (CIs) per subject: Rolandic CI and non-Rolandic CI.
2.5 ∣. EEG data acquisition and preprocessing
EEG data were collected using a 70-channel electrode cap and bilateral manually placed temporal electrodes at a sampling rate of 2035 Hz. Data were reviewed by a board-certified clinical neurophysiologist (C.J.C.). Spike rate in the Rolandic cortex was quantified by hemisphere in each subject in three manners: (1) manual detection through visual inspection by a board-certified neurophysiologist (C.J.C.) following standard criteria30; (2) using a validated commercial automated wavelet-based detector, Persyst31,32; and (3) using SpikeNet, a validated automated spike detector developed at our institution.33,34 For each method, all available non-REM sleep epochs were included (mean = 15.2 minutes, range = 4-48 minutes), and a spike rate for each hemisphere was calculated. To minimize artifactual detections, only spikes detected as maximal in the central and temporal channels (consistent with classic CECTS neurophysiology) were included.
2.6 ∣. Statistical analyses
We used linear regression to assess age and gender as predictors of CI in CECTS and control groups. Nonparametric bootstrap analyses were performed to assess differences in CI slope over age between groups. Two groups of equal size to CECTS and control groups were sampled from the union of both groups (N = 42) and used to calculate a mean slope difference. This process was iterated over 10 000 trials to ensure convergence. The resulting distribution of slope differences was evaluated against the empirical slope difference between groups at alpha = .05. To assess the relationship between CI and spike rate, spike rate during non-REM sleep was computed by hemisphere and compared to ipsilateral CI using linear regression with CI as a predictor of spike rate, age as a fixed effect, and subject as a random effect. For these analyses, we applied the inverse hyperbolic sine transformation to the spike rate, as this is similar to the logarithmic transformation, but suitable for observations with zero values.35 We used the Benjamini-Hochberg procedure to control the false discovery rate among approaches (q = 0.05).36
3 ∣. RESULTS
Maturation of Rolandic thalamocortical connectivity is abnormal in CECTS
In controls, we observed the expected increase in thalamocortical connectivity to the Rolandic cortex with age (Rolandic CI range = 0.016-0.057), where each 1-year increase in age was associated with a 0.003-unit increase in CI (P = .01). In CECTS subjects, no relationship between thalamocortical connectivity to the Rolandic cortex and age was observed (P = .69), and visual inspection revealed a more uniform thalamocortical connectivity across all ages (Rolandic CI range = 0.027-0.048). Bootstrap analysis comparing the difference in the trajectories of white matter connectivity over age confirmed a difference (P = .01), in which children with CECTS had a decreased slope compared to controls (Figure 2). Gender was not a predictor of connectivity in CECTS (P = .57) or controls (P = .61).
FIGURE 2.
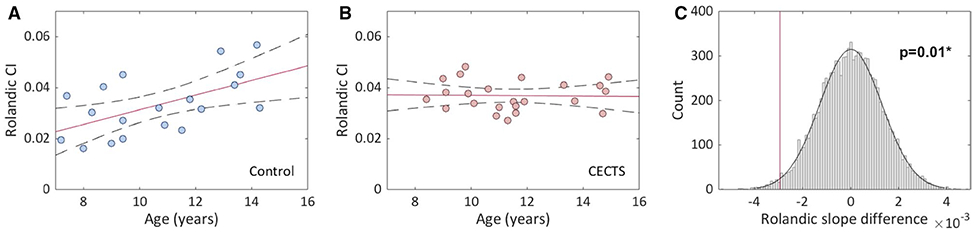
Relationship between thalamocortical connectivity index (CI) to the Rolandic cortex and age in controls and childhood epilepsy with centrotemporal spikes (CECTS). A, Visual analysis reveals a relationship between age and CI among controls. Solid line indicates linear regression model fit; dashed lines indicate 95% confidence intervals. B, Visual analysis reveals no relationship between age and CI among CECTS subjects. C, Bootstrap analysis reveals a difference between the slopes of controls and CECTS subjects (P = .01; *statistically significant)
Maturation of thalamocortical connectivity to regions outside of the Rolandic cortex is normal in CECTS
In both controls and children with CECTS, visual analysis revealed a broad range of CI from thalamus to non-Rolandic regions (control range = 0.119-0.228, CECTS range = 0.133-0.239), and no relationship with age was observed (CECTS, P = .63; control, P = .13). Bootstrap analysis comparing the difference in the trajectories of white matter connectivity over age identified no difference between groups (P = .19, Figure 3). We note that because the non-Rolandic ROI label is much larger than the Rolandic ROI label, higher CI values were observed from thalamus to non-Rolandic cortex in both controls and CECTS.
FIGURE 3.
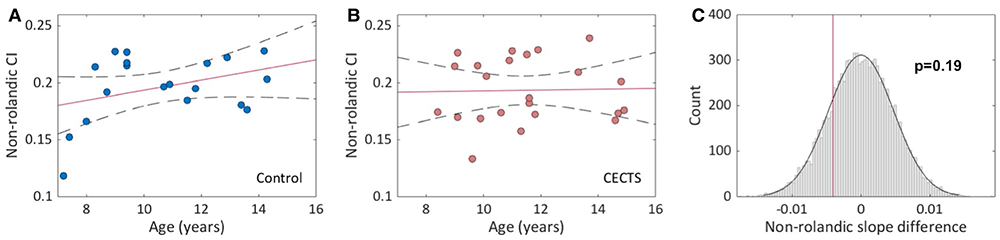
Relationship between thalamocortical connectivity index (CI) to non-Rolandic cortex and age in childhood epilepsy with centrotemporal spikes (CECTS) and healthy controls. A, Visual analysis reveals no relationship between age and CI among (A) controls or (B) CECTS. Solid line indicates linear regression model fit; dashed lines indicate 95% confidence intervals. C, Bootstrap analysis reveals no difference between the slopes of the controls and CECTS subjects (P = .19)
3.1 ∣. Thalamocortical connectivity to the Rolandic cortex decreases over time in CECTS
Four children with CECTS returned after at least 12 months for repeat data collection, allowing for longitudinal analysis (return age = 12.8-15.1, three male). Consistent with cross-sectional data, all longitudinal CECTS subjects showed an abnormal decrease in thalamocortical connectivity to the Rolandic cortex over the follow-up period (Figure 4). Reductions ranged from 0.04% to 11.5%, with a mean 0.002-unit decrease in the CI per year.
FIGURE 4.
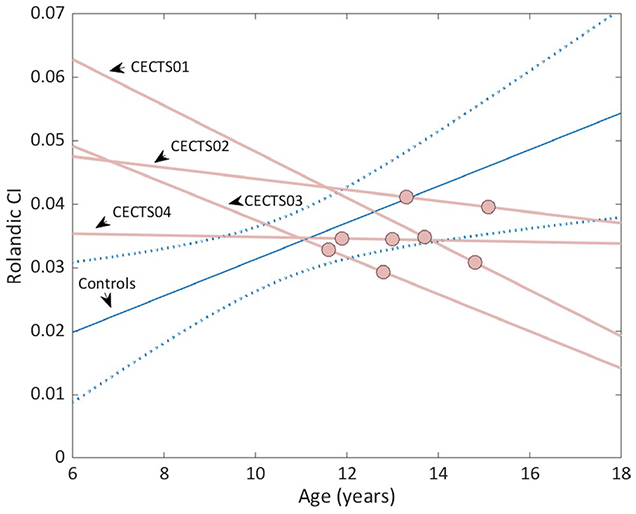
Relationship between thalamocortical connectivity to the Rolandic cortex and age among longitudinal subjects. Rather than increase with age as observed in controls (blue line indicates linear regression model fit and blue dotted lines indicate 95% confidence interval of cross-sectional controls, n = 19), in each longitudinal childhood epilepsy with centrotemporal spikes (CECTS) case available (n = 4), thalamocortical connectivity index (CI) to the Rolandic cortex decreased with age
3.2 ∣. Thalamocortical connectivity in the Rolandic cortex and Rolandic spike rate
Among CECTS subjects with non-REM sleep EEG available (n = 19 CECTS, n = 38 hemispheres), no relationship between thalamocortical connectivity to the Rolandic cortex and Rolandic spike rate was found using manual spike detection (n = 38 hemispheres, P = .61) or either of two independent automated spike detectors (Persyst, n = 32 hemispheres, P = .45; and SpikeNet, n = 38 hemispheres, P = .12; Figure 5). We note that the EEG data of three subjects were too short to include in the Persyst analysis.
FIGURE 5.
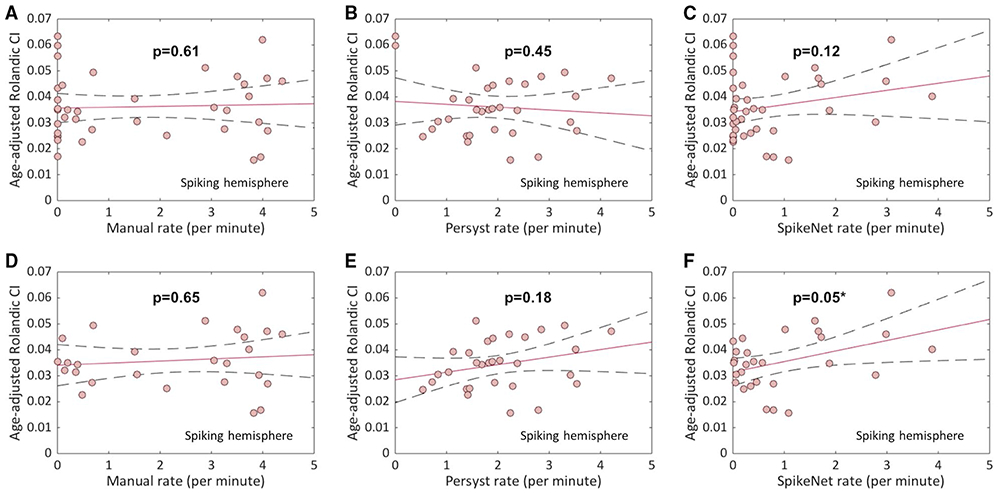
Relationship between Rolandic thalamocortical connectivity and ipsilateral Rolandic spike rate in childhood epilepsy with centrotemporal spikes (CECTS). Among CECTS subjects, there was no relationship found between ipsilateral connectivity index (CI) and each hemisphere’s corresponding spike rate using (A) manual (n = 38 hemispheres, P = .61), (B) Persyst (n = 32 hemispheres, P = .45), and (C) SpikeNet (n = 38 hemispheres, P = .12) techniques. Including only hemispheres with a nonzero spike rate (D-F), a possible relationship was found using SpikeNet (n = 28 hemispheres, P = .05; *statistically significant), but not manual (n = 26 hemispheres, P = .65) or Persyst (n = 30 hemispheres, P = .18) detection. Solid line indicates linear regression model fit; dashed lines indicate 95% confidence intervals
Including only hemispheres with nonzero spike rate, we found a possible relationship between spike rate and CI using the SpikeNet detector (P = .05); however, this finding did not achieve significance after correcting for multiple comparisons. No relationship was observed between hemisphere CI and ipsilateral spike rate when excluding left-handed subjects (P > .05).
4 ∣. DISCUSSION
CECTS is a common, transient, developmental epilepsy characterized by focal epileptiform spikes and seizures in the Rolandic cortex that are most pronounced during non-REM sleep. The underlying cause of CECTS remains unknown. In this study, we show that thalamocortical white matter connectivity to the Rolandic cortex is abnormal in children with CECTS, suggesting that focal disruption of this thalamocortical circuit could provide a common pathophysiological mechanism of this disease.
Consistent with the well-established literature on white matter maturation during childhood and adolescence, we observed a linear increase in structural connectivity between the thalamus and the Rolandic cortex in healthy controls over adolescence and early teenage years.15,16 This maturation is thought to result from increases in myelination, axon diameter, and density of white matter fiber tracts, each resulting in stronger structural connectivity.15,16 In contrast, children with CECTS demonstrate no increase in thalamocortical connectivity to the Rolandic regions over the same age periods. Children with CECTS demonstrate uniform connectivity across ages, resulting in qualitatively increased connectivity at younger ages and qualitatively decreased connectivity at older ages compared to controls. These observations, coupled with our longitudinal findings showing decreased connectivity over time in children with CECTS, suggest that abnormally increased thalamocortical connectivity to the Rolandic regions could contribute to disease onset. Conversely, abnormally decreased connectivity in older children could indicate compensatory mechanisms necessary for disease resolution. Alternatively, the electrophysiological impact of structural abnormalities in the Rolandic thalamocortical circuit may be mitigated by other structural or physiologic changes across development. For example, subcortical association fibers integrating Rolandic cortical regions compete with thalamocortical input, mature during childhood and adolescence, and are also abnormal in children with CECTS.37,38 Overall, these data reveal that children with CECTS do not have a transient disease, but rather develop successful compensatory mechanisms that result in transient symptomatology.
Interestingly, changes in connectivity to the Rolandic cortex were not clearly associated with differences in non-REM spike rate in our cohort. There are a number of potential physiologic explanations for this unexpected finding. First, spike rate is not a reliable biomarker of disease progression in CECTS.38,39 This is further supported by previous findings that centrotemporal spikes are not sufficient for the development of CECTS and are associated with a number of other epilepsy and neurocognitive disorders.40 Other compensatory mechanisms, such as those mentioned above, may contribute to spike rate, requiring a more complex and complete model to identify the relationship between thalamocortical connectivity and sleep-activated spikes.
Alternatively, it is possible we were not able to systematically detect spike activity. Manual detection of epileptiform activity on EEG demonstrates inconsistent interrater agreement; however, review by a neurophysiology-trained specialist, as in this study, may increase reliability.33,41,42 The Persyst algorithm, although validated, has been previously associated with a high rate of false positives.43 In its validation study, the SpikeNet algorithm was directly compared to neurophysiology-trained experts and Persyst, exceeding both in performance.34 Here, we detected a possible relationship between spike rate and CI in hemispheres with active spikes using SpikeNet, although this possible finding requires validation in a second dataset. Finally, although spike rate has been observed to remain stable over periods of sleep and the course of weeks, it is possible that our data sample was underpowered to identify a small effect.44,45
We utilized a popular, advanced probabilistic tractography algorithm to compute thalamocortical connectivity in a well-characterized dataset of children with CECTS. Probabilistic tractography offers a number of advantages over deterministic approaches, including the estimation of the relative strength of connectivity and sensitivity to focal abnormalities along fiber tracts.27,39 This technique has also been previously validated in the reconstruction of thalamocortical radiations.27,46 Despite these advantages, the largest limitation of our study is the small size, limiting the power to identify small effects. The preliminary findings in our small longitudinal cohort must be interpreted cautiously and require future validation. Furthermore, CECTS is now understood to be a mild epileptic encephalopathy and the relationship between thalamocortical connectivity and cognitive function was not evaluated here.
CECTS is common neurodevelopmental disorder characterized by seizures and cognitive dysfunction.3,6 This work contributes to the growing literature supporting that structural abnormalities in the thalamocortical circuit contribute to the pathophysiology of this disorder.14,19-22,47-51 Future work is required to better understand the compensatory mechanisms underlying symptom resolution in CECTS to improve our understanding of the mechanisms responsible for epilepsy and to enable the development of strategies to prevent or accelerate resolution of CECTS and related developmental epilepsies.
Key Points.
Children with CECTS have abnormal development of thalamocortical circuits to the Rolandic cortex compared to controls
Rolandic thalamocortical abnormalities in children with CECTS increase with age
There is not a clear relationship between thalamocortical connectivity to the Rolandic cortex and spike rate in CECTS using available spike detection approaches
ACKNOWLEDGMENTS
This work was supported by National Institute of Neurological Disorders and Stroke K23-NS092923 and National Science Foundation Division of Mathematical Sciences 1451384. The authors would like to thank Wenting Xie and Daniel Song for their assistance in MRI and EEG data collection for this project.
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Number: K23-NS092923 and R01NS115868
Footnotes
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Astradsson A, Olafsson E, Ludvigsson P, Björgvinsson H, Hauser WA. Rolandic epilepsy: an incidence study in Iceland. Epilepsia. 1998;39(8):884–6. [DOI] [PubMed] [Google Scholar]
- 2.Larsson K, Eeg-Olofsson O. A population based study of epilepsy in children from a Swedish county. Eur J Paediatr Neurol. 2006;10(3):107–13. [DOI] [PubMed] [Google Scholar]
- 3.Callenbach PMC, Bouma PAD, Geerts AT, Arts WF, Stroink H, Peeters EA, et al. Long term outcome of benign childhood epilepsy with centrotemporal spikes: Dutch Study of Epilepsy in Childhood. Seizure. 2010;19(8):501–6. [DOI] [PubMed] [Google Scholar]
- 4.Camfield CS, Camfield PR. Rolandic epilepsy has little effect on adult life 30 years later: a population-based study. Neurology. 2014;82(13):1162–6. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Rychlik K. The course of childhood-onset epilepsy over the first two decades: a prospective, longitudinal study. Epilepsia. 2015;56(1):40–8. [DOI] [PubMed] [Google Scholar]
- 6.Bouma PA, Bovenkerk AC, Westendorp RG, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal spikes: a meta-analysis. Neurology. 1997;48(2):430–7. [DOI] [PubMed] [Google Scholar]
- 7.Panayiotopoulos CP, Michael M, Sanders S, Valeta T, Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain. 2008;131(9):2264–86. [DOI] [PubMed] [Google Scholar]
- 8.Vadlamudi L, Harvey AS, Connellan MM, Milne RL, Hopper JL, Scheffer IE, et al. Is benign Rolandic epilepsy genetically determined? Ann Neurol. 2004;56(1):129–32. [DOI] [PubMed] [Google Scholar]
- 9.Vadlamudi L, Kjeldsen MJ, Corey LA, Solaas MH, Friis ML, Pellock JM, et al. Analyzing the etiology of benign Rolandic epilepsy: a multicenter twin collaboration. Epilepsia. 2006;47(3):550–5. [DOI] [PubMed] [Google Scholar]
- 10.Strug LJ, Clarke T, Chiang T, Chien M, Baskurt Z, Li W, et al. Centrotemporal sharp wave EEG trait in Rolandic epilepsy maps to elongator protein complex 4 (ELP4). Eur J Hum Genet. 2009;17(9):1171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with Rolandic spikes. Nat Genet. 2013;45(9):1067–72. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Qian P, Xu X, Liu X, Wu X, Zhang Y, et al. GRIN2A mutations in epilepsy-aphasia spectrum disorders. Brain Dev. 2018;40(3):205–10. [DOI] [PubMed] [Google Scholar]
- 13.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde BW, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez Fernández I, Chapman KE, Peters JM, Harini C, Rotenberg A, Loddenkemper T. Continuous spikes and waves during sleep: electroclinical presentation and suggestions for management. Epilepsy Res Treatment. 2013;2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. [DOI] [PubMed] [Google Scholar]
- 16.Paus T Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–8. [DOI] [PubMed] [Google Scholar]
- 17.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85. [DOI] [PubMed] [Google Scholar]
- 18.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75(2):125–41. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez Fernández I, Peters JM, Akhondi-Asl A, Klehm J, Warfield SK, Loddenkemper T. Reduced thalamic volume in patients with electrical status epilepticus in sleep. Epilepsy Res. 2017;130:74–80. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez Fernández I, Takeoka M, Tas E, Peters JM, Prabhu SP, Stannard KM, et al. Early thalamic lesions in patients with sleep-potentiated epileptiform activity. Neurology. 2012;78(22):1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leal A, Calado E, Vieira JP, Mendonca C, Ferreira JC, Ferreira H, et al. Anatomical and physiological basis of continuous spike-wave of sleep syndrome after early thalamic lesions. Epilepsy Behav. 2018;78:243–55. [DOI] [PubMed] [Google Scholar]
- 22.Kersbergen KJ, de Vries LS, Leijten FSS, Braun KP, Nievelstein RA, Groenendaal F, et al. Neonatal thalamic hemorrhage is strongly associated with electrical status epilepticus in slow wave sleep. Epilepsia. 2013;54(4):733–40. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. [DOI] [PubMed] [Google Scholar]
- 24.Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: a systematic review and meta-analysis. Epilepsia. 2017;58(10):1673–85. [DOI] [PubMed] [Google Scholar]
- 25.Ross EE, Stoyell SM, Kramer MA, Berg AT, Chu CJ. The natural history of seizures and neuropsychiatric symptoms in childhood epilepsy with centrotemporal spikes (CECTS). Epilepsy Behav. 2020;103(Pt A):106437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–86. [DOI] [PubMed] [Google Scholar]
- 27.Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–88. [DOI] [PubMed] [Google Scholar]
- 28.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 29.Chu CJ, Tanaka N, Diaz J, Edlow BL, Wu O, Hamalainen M, et al. EEG functional connectivity is partially predicted by underlying white matter connectivity. Neuroimage. 2015;108:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–31. [PubMed] [Google Scholar]
- 31.Scheuer ML, Bagic A, Wilson SB. Spike detection: inter-reader agreement and a statistical Turing test on a large data set. Clin Neurophysiol. 2017;128(1):243–50. [DOI] [PubMed] [Google Scholar]
- 32.Joshi CN, Chapman KE, Bear JJ, Wilson SB, Walleigh DJ, Scheuer ML. Semiautomated spike detection software Persyst 13 is non-inferior to human readers when calculating the spike-wave index in electrical status epilepticus in sleep. J Clin Neurophysiol. 2018;35(5):370–4. [DOI] [PubMed] [Google Scholar]
- 33.Jing J, Herlopian A, Karakis I, Ng M, Halford JJ, Lam A, et al. Interrater reliability of experts in identifying interictal epileptiform discharges in electroencephalograms. JAMA Neurol. 2020;77(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing J, Sun H, Kim JA, Herlopian A, Karakis I, Ng M, et al. Development of expert-level automated detection of epileptiform discharges during electroencephalogram interpretation. JAMA Neurol. 2020;77(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burbidge JB, Magee L, Robb AL. Alternative transformations to handle extreme values of the dependent variable. J Am Stat Assoc. 1988;83(401):123–7. [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 37.Oyefiade AA, Ameis S, Lerch JP, Rockel C, Szulc KU, Scantlebury N, et al. Development of short-range white matter in healthy children and adolescents. Hum Brain Mapp. 2018;39(1):204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrowski LM, Song DY, Thorn EL, Ross EE, Stoyell SM, Chinappen DM, et al. Dysmature superficial white matter microstructure in developmental focal epilepsy. Brain Commun. 2019;1(1):fcz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie W, Ross EE, Kramer MA, Eden UT, Chu CJ. Timing matters: impact of anticonvulsant drug treatment and spikes on seizure risk in benign epilepsy with centrotemporal spikes. Epilepsia Open. 2018;3(3):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31(49):17821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagheri E, Dauwels J, Dean BC, Waters CG, Westover MB, Halford JJ. Interictal epileptiform discharge characteristics underlying expert interrater agreement. Clin Neurophysiol. 2017;128(10):1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benbadis SR, LaFrance WC, Papandonatos GD, Korabathina K, Lin K, Kraemer HC. Interrater reliability of EEG-video monitoring. Neurology. 2009;73(11):843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halford JJ, Westover MB, LaRoche SM, Macken MP, Kutluay E, Edwards JC, et al. Interictal epileptiform discharge detection in EEG in different practice settings. J Clin Neurophysiol. 2018;35(5):375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2015;45:85–91. [DOI] [PubMed] [Google Scholar]
- 45.Tenney JR, Glauser T, Altaye M, Szaflarski JP, Spencer C, Morita D, et al. Longitudinal stability of interictal spikes in benign epilepsy with centrotemporal spikes. Epilepsia. 2016;57(5): 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aeby A, Liu Y, Tiège XD, Denolin V, David P, Baleriaux D, et al. Maturation of thalamic radiations between 34 and 41 weeks’ gestation: a combined voxel-based study and probabilistic tractography with diffusion tensor imaging. Am J Neuroradiol. 2009;30(9):1780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snead OC. Basic mechanisms of generalized absence seizures. Ann Neurol. 1995;37(2):146–57. [DOI] [PubMed] [Google Scholar]
- 48.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–92. [DOI] [PubMed] [Google Scholar]
- 49.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paz JT, Bryant AS, Peng K, Fenno L, Yizhar O, Frankel WN, et al. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat Neurosci. 2011;14(9):1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maheshwari A, Noebels JL. Monogenic models of absence epilepsy: windows into the complex balance between inhibition and excitation in thalamocortical microcircuits. Prog Brain Res. 2014;213:223–52. [DOI] [PubMed] [Google Scholar]


