Abstract
Background:
In animal models, it is possible to induce different alcohol-related dysmorphic abnormalities based on the timing of prenatal alcohol exposure (PAE). Our objective was to assess whether patterns of PAE differentially predict alcohol-related dysmorphic features in 415 infants.
Methods:
We analyzed a prospective pregnancy cohort in western Ukraine enrolled between 2008–2014. Five distinct trajectories were previously identified to summarize prenatal alcohol exposure: (A) minimal/no PAE (n=253), (B) low/moderate PAE with reduction early in gestation (n=78), (C) low/moderate sustained PAE (n=20), (D) moderate/high PAE with reduction early in gestation (n=45), and (E) high sustained PAE (n=19). A dysmorphology exam of body size, 3 cardinal and 15 non-cardinal dysmorphic features was performed at approximately 6–12 months of age. A modified dysmorphology score was created based on previously published weights. Univariate comparisons were made between each dysmorphic feature and trajectory group. Features that differed by trajectory group were assessed in multivariable analyses. Models were adjusted for maternal age, prenatal vitamin use, socioeconomic status, smoking, and child’s age at dysmorphology exam, with censoring weights for losses to follow-up.
Results:
The three highest trajectories predicted total dysmorphology score, with larger effects in sustained exposure groups. Cardinal features: the three highest trajectories were each associated with a 2–3-fold increased risk of having 2+ cardinal facial features. When assessed individually, there were no consistent associations between the individual trajectories and each cardinal feature. Non-cardinal features: The three highest trajectories were associated with increased risk of hypotelorism. Only the highest trajectory was associated with heart murmur. The highest trajectory predicted <10th centile for sex and age on height, weight and head circumference; and moderate/high with reduction trajectory also predicted height.
Conclusions:
While we did not observe differential results based on specific trajectories of exposure, findings support the wide range of dysmorphic features associated with PAE, particularly at high and sustained levels.
Keywords: prenatal alcohol exposure, dysmorphology, epidemiology
Introduction
Fetal alcohol spectrum disorders (FASD) is a continuum of developmental disorders that is initiated by prenatal alcohol use and results in lifelong consequences for the child. Across diagnostic schema, there is a general consensus regarding the importance of the three cardinal features of thin smooth vermillion border of the upper lip, smooth philtrum and short palpebral fissures (Astley, 2004; Bower et al., 2016; Cook et al., 2015; Hoyme et al., 2016) associated with the diagnosis of fetal alcohol syndrome (FAS). Among children with prenatal alcohol exposure (PAE), other minor structural malformations have been reported, and typically occur in higher frequency among individuals with FAS relative to individuals with other FASD diagnoses on the spectrum (partial FAS, alcohol related neurodevelopmental disorders) or those without PAE (del Campo and Jones, 2017; Feldman et al., 2011; Hoyme et al., 2016, 2005; Jones, 2011; Jones et al., 2010). The cumulative score for dysmorphic features has been found to be predictive of maternal report of alcohol consumption (May et al., 2013, 2006) and the child’s neurodevelopmental and adaptive outcomes (Lynch et al., 2015; May et al., 2006).
In animal models, researchers have successfully produced differential alcohol-related dysmorphic abnormalities based on the timing of PAE. In a series of papers, acute PAE administered on a single gestational day ranging from day 7–10 in mice (equivalent to the 3rd-5th weeks postfertilization in humans) had markedly different signatures with respect to structural brain abnormalities depending on the specific day of exposure (Godin et al., 2010; O’Leary-Moore et al., 2010; Parnell et al., 2013, 2009). To date, only a handful of studies in humans have explored whether timing or dose of PAE is associated with specific dysmorphic features (Feldman et al., 2011) or total dysmorphic score (May et al., 2013, 2006), yet no striking patterns have emerged. There is great interest in further elucidating the association between PAE and dysmorphic outcomes, especially as to whether features differ by timing, duration or quantity of exposure. First, as markers of exposure, the non-cardinal features could add additional elements that aid in identification of children prenatally exposed to alcohol. This is highly relevant to treatment providers, as recognizing individuals affected by PAE in the absence of the cardinal features relies on historical report of prenatal exposure, which is often difficult to obtain. A second motivation for studying the features by timing, quantity and duration of PAE is that the results, like those from animal models, may elucidate mechanisms whereby exposure affects fetal development. Although the insult leading to some features including heart defects (Zhang et al., 2020) and select craniofacial defects (Godin et al., 2010; Sulik, 2005) are known to occur from first trimester PAE, the sensitive periods for many of the other features remain unknown.
From a prospective birth cohort, we previously classified PAE into trajectories, accounting for timing, frequency and quantity of exposure across gestation. We reported that trajectories of PAE were associated with body size at birth and neurodevelopmental deficits in 6–12 month old infants (Bandoli et al., 2019). Notably, although confidence intervals overlapped, we observed low-to-moderate sustained exposure was more strongly associated with most negative infant outcomes than moderate-to-high exposure with early reduction, suggesting that duration of exposure was equally or more impactful than quantity of exposure for some outcomes. The purpose of this study was to determine whether these same trajectories of PAE were differentially associated with cardinal and non-cardinal dysmorphic features in the same birth cohort.
Materials and methods
Data for this analysis are from a prospective cohort study of pregnant women in western Ukraine conducted as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) supported by the National Institute on Alcohol Abuse and Alcoholism (www.CIFASD.org), and carried out by the Omni-Net for Children program in Ukraine . This study has been described elsewhere in detail (Bandoli et al., 2019; Chambers et al., 2014; Coles et al., 2015). Briefly, all pregnant women who presented to one of two centralized prenatal care facilities in Ukraine, the Rivne Regional Medical Diagnostic Center and the Khmelnytsky Perinatal Center, between 2008–2014 were eligible for screening about their alcohol consumption around conception and the most recent month of pregnancy. Women who reported at least weekly binge episodes of 4–5 alcoholic drinks/occasion, at least 5 episodes of 3–4 drinks, or at least 10 episodes of 1–2 drinks in the month around conception and/or in the most recent month of pregnancy were recruited. Following identification of an exposed participant, the next minimally exposed or unexposed woman (<2 drinks per occasion and no more than 2 drinks per week in the month around conception and no alcohol in the most recent month of pregnancy) was recruited for participation. Women were interviewed about demographics, behaviors and pregnancy characteristics using standard questionnaires upon enrollment and again at approximately 32 weeks of gestation.
Exposure trajectories
After enrollment, women who reported ever being drinkers in their lifetime completed a timeline follow-back assessment of day-by-day alcohol consumption by type, quantity and frequency in a typical week around conception and in the most recent two weeks of pregnancy (Sobell and Sobell, 2000). Quantity and frequency of alcohol consumption in responses to these questions was summarized as the average number of drinks per day over the period for which the mother was reporting as a reflection of the overall quantity of alcohol consumed. This information was then converted into absolute ounces of alcohol per day (ozAA/day). At a follow-up pregnancy visit around 32 weeks of gestation, women were asked if their alcohol consumption had changed since the enrollment visit, and if yes, were again asked to recall alcohol consumption for the previous seven days commensurate with the enrollment visit procedures.
From this information, we previously performed k-means longitudinal (Genolini and Falissard, 2016, 2010) analysis to identify similar patterns of individual PAE trajectories on 471 infants with complete exposure information captured at the two prenatal visits. Details of modeling and the results have been described (Bandoli et al., 2019). A 5-trajectory solution was selected based on quality criterion and sample size considerations (Figure 1). Trajectories were best described as minimal to no PAE throughout gestation (trajectory A; n=277), low/moderate exposure with reduction or discontinuation early in gestation (trajectory B; n=96), low/moderate exposure sustained across gestation (trajectory C; n=23), moderate/high exposure with reduction or discontinuation early in gestation (trajectory D; n=51), and high exposure sustained across gestation (trajectory E; n=24).
Figure 1.
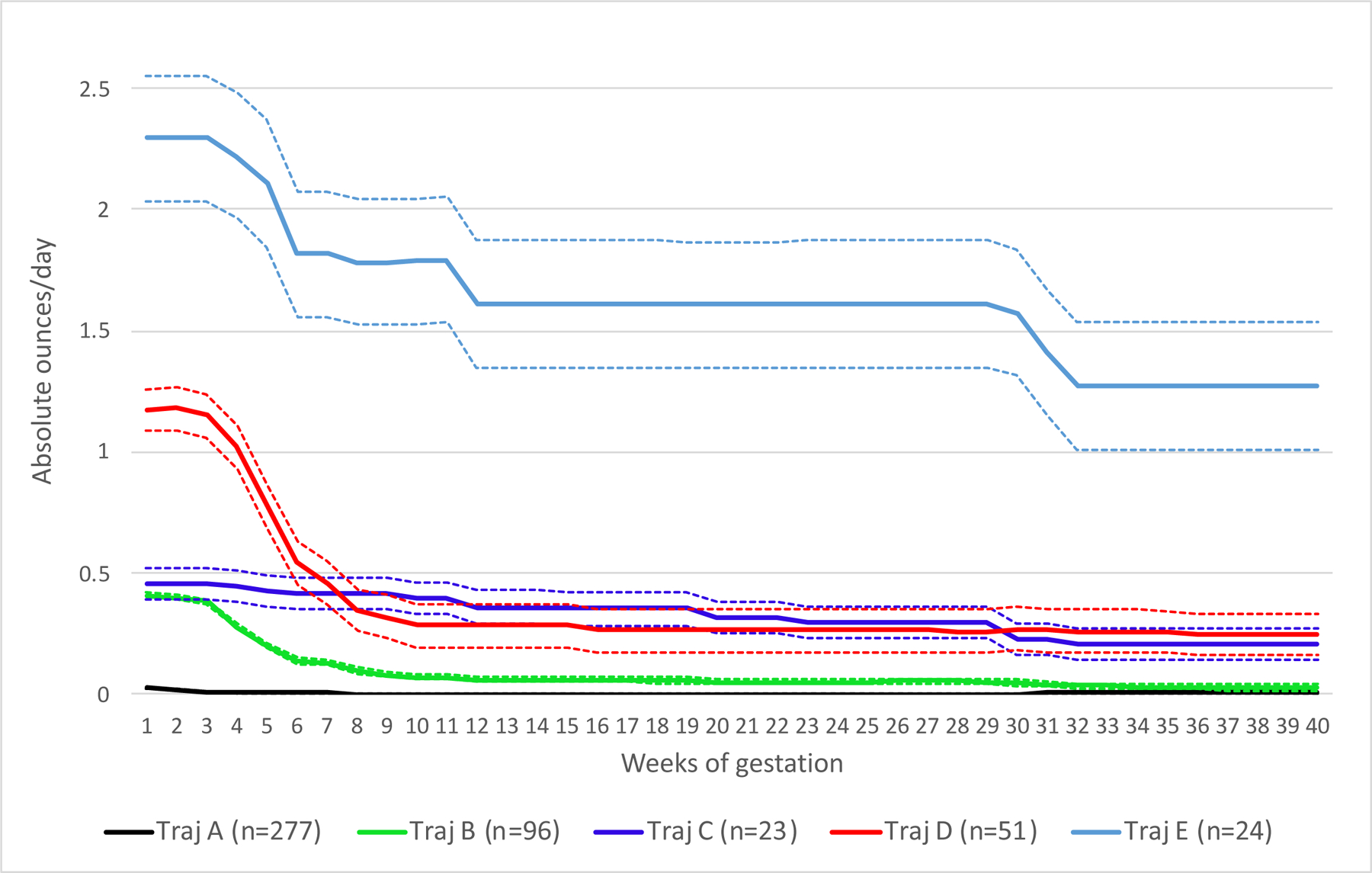
Mean absolute ounces of alcohol per day (solid lines) and 95% confidence intervals (hashed lines) of the five trajectory groups in the full cohort (n=471).
Dysmorphology outcomes
Infant dysmorphology exams were performed by study pediatricians/geneticists at each of the study sites following specialized training, using a standard protocol and an examination checklist. The exams were completed at a median age of 12 months (range 0–52 months, interquartile range 5 months). Some children had more than one exam performed. Therefore, we prioritized exams in the following manner: 1) between 9 and 13 months of age, 2) between 4 and 8 months of age, 3) between 14 and 18 months of age, 4) all other ages, using the first assessment that met this prioritization. Dysmorphology exams included assessment of height, weight, head circumference, the three cardinal features (short palpebral fissure length, thin smooth vermilion border, smooth philtrum), innercanthal distance, midface hypoplasia, epicanthal folds, anteverted nose, long philtrum, clinodactyly, camptodactyly, aberrant creases of the palm, railroad track ears, strabismus, ptosis, decreased pronation or supination of the arms, heart murmur, heart defect, and neurologic problems (hypertonic, hypotonic, seizures). A modified dysmorphology score was created using weights specified by May and Hoyme (Hoyme et al., 2016). From the list of features included in the 2016 May and Hoyme scoring paradigm, flat nasal bridge, hypoplastic fingernails, hypertrichosis and prognathism were not assessed in the Ukraine sample; resulting in a potential dysmorphology scoring range of 0–36.
There were 687 infants with dysmorphology exams, and 471 infants with trajectory exposures available for analysis. Infants without exposure trajectories had mothers that were enrolled later in gestation and did not have the second prenatal visit necessary for creating trajectories. As previously reported (Bandoli et al., 2019), women who completed both pregnancy visits were more likely to have higher socioeconomic status (SES), have attended college, realized they were pregnant approximately one week earlier in gestation (mean 5.6 weeks), and were less likely to have a preterm delivery than women who only completed one pregnancy visit. They did not differ on maternal smoking behaviors, vitamin use, cohabitation status, age, or amount of alcohol consumption reported at the enrollment visit.
Further, from the 471 infants with exposure trajectories, 56 (11.9%) were missing dysmorphology exams, resulting in an analytic sample of 415 infants.
Analyses
We first assessed the univariate associations between the trajectory groups and each dysmorphic feature and total dysmorphology score. Chi square with Fisher’s exact tests were used for categorical outcomes, and ANOVA was used for continuous outcomes. Outcomes that were associated with trajectory groups (p<0.05) were then tested using multivariable log-linear regression (categorical outcomes) or multivariable linear regression (continuous outcomes). For rare outcomes with very few cases per trajectory, we collapsed trajectories into a 3-level variable (A: minimal/none, B/D: decreasing, and C/E: sustained exposure) for analyses. The decision to group the decreasing trajectories (B,D) and sustaining trajectories (C,E) together was done based on similar maternal characteristics in those pairings (Bandoli et al., 2019). Models were adjusted with propensity scores (created by regressing the exposure trajectory variable on potential confounders with multinomial logistic regression) for prenatal vitamin use, maternal age and SES, maternal pregnancy smoking, offspring age at dysmorphology exam. Infants missing dysmorphology exams (n=56) were more likely to be in the B or E exposure trajectories and be born to mothers of lower educational attainment and SES. We created inverse probability censoring weights to re-weight the sample based on these characteristics and applied the weights in all multivariable analyses.
A small subset of participants (n=22) enrolled as minimally/unexposed based on self-report of alcohol use, but were judged to be unreliable by study staff. In a sensitivity analysis, all models were repeated excluding these participants. Longitudinal trajectory analysis was previously performed in R; all subsequent analyses were conducted in SAS 9.4.
Results
There were 415 infants with dysmorphology exams and PAE trajectories available for analysis. From the initial trajectory creation (n=471), 91%, 81%, 87%, 88%, 79% were retained in analysis for trajectories A-E, respectively. Means and standard deviations of absolute ounces of alcohol per day for each trajectory group are shown at multiple gestational time points in Supplemental Table 1. With exception of the minimal/none trajectory (A), all other trajectories had almost universal alcohol exposure at week 1. By week six, 27% of women in the ‘decreasing use’ trajectories (B,D) were no longer reporting alcohol use, compared to 0% in the two sustaining trajectories (C,E). At 12 weeks of gestation, 40% of women in the decreasing trajectories were abstaining from alcohol, compared to 0–5% in the sustaining trajectories.
Univariate frequencies of dysmorphic outcomes often varied by exposure trajectory (Table 1). Infants with sustained alcohol exposure (C and E) had higher frequencies of having higher counts of the three cardinal features, short innercanthal distance (hypotelorism), and heart defects. Infants with the highest sustained exposure were more likely to have weight and head circumference less than the 10th centile for sex and age, aberrant creases of the palm, heart murmur, and a higher overall dysmorphology score. The other dysmorphic features assessed generally did not vary by exposure trajectory.
Table 1.
Dysmorphic features by alcohol trajectory group among 415 infants in Ukraine
| Full sample | A: Minimal/none | B: Low/moderate, decrease | C: Low/moderate, sustained | D: Moderate/high, decrease | E: High, sustained | ||
|---|---|---|---|---|---|---|---|
| n=415 | n=253 | n=78 | n=20 | n=45 | n=19 | p value | |
| <0.0001 | |||||||
| 0 | 295 (71.1) | 200 (79.1) | 48 (61.5) | 9 (45.0) | 26 (57.8) | 12 (63.6) | |
| 1 | 70 (16.9) | 36 (14.2) | 21 (26.9) | 5 (25.0) | 7 (15.6) | 1 (5.3) | |
| 2 | 37 (8.9) | 16 (6.3) | 8 (10.3) | 3 (15.0) | 9 (20.0) | 1 (5.3) | |
| 3 | 13 (3.1) | 1 (0.4) | 1 (1.3) | 3 (15.0) | 3 (6.7) | 5 (26.3) | |
| Palpebral fissure <10th centile (3) | 59 (14.2) | 13 (5.1) | 17 (21.8) | 9 (45.0) | 14 (31.1) | 6 (31.6) | <0.0001 |
| Smooth philtrum (3) | 44 (10.6) | 18 (7.1) | 7 (9.0) | 4 (20.0) | 9 (20.0) | 6 (31.6) | 0.001 |
| Thin vermilion border (3) | 80 (19.3) | 40 (15.8) | 16 (20.5) | 7 (35.0) | 11 (24.4) | 6 (31.6) | 0.08 |
| Body size measures | |||||||
| Height <10th centile (2) | 42 (10.1) | 16 (6.3) | 7 (9.0) | 2 (10.0) | 12 (26.7) | 5 (26.3) | <0.0001 |
| Weight <10th centile (1) | 41 (9.9) | 15 (5.9) | 10 (12.8) | 2 (10.0) | 7 (15.6) | 7 (36.8) | <0.0001 |
| Head circumference <10th centile (3) | 28 (6.8) | 9 (3.5) | 4 (5.1) | 3 (15.0) | 6 (13.3) | 6 (31.6) | <0.0001 |
| Non-cardinal features | |||||||
| Innercanthal distance <25th centile (2) | 54 (13.0) | 18 (7.1) | 11 (14.1) | 6 (30.0) | 12 (26.7) | 7 (36.8) | <0.0001 |
| Midface hypoplasia (2) | 7 (1.7) | 2 (0.8) | 2 (2.6) | 0 (0.0) | 2 (4.4) | 1 (5.3) | 0.11 |
| Epicanthal folds (2) | 241 (58.1) | 144 (57.1) | 51 (65.4) | 10 (50.0) | 25 (55.6) | 11 (57.8) | 0.64 |
| Anteverted nares (2) | 82 (19.8) | 45 (17.7) | 17 (21.8) | 7 (35.0) | 8 (17.8) | 5 (26.3) | 0.32 |
| Long philtrum (2) | 42 (10.1) | 20 (7.9) | 9 (11.5) | 3 (15.0) | 7 (15.9) | 3 (15.8) | 0.23 |
| Clinodactyly (2) | 50 (12.1) | 33 (13.4) | 12 (15.4) | 2 (10.0) | 1 (2.3) | 2 (10.5) | 0.20 |
| Camptodactyly (2) | 7 (1.7) | 4 (1.6) | 1 (1.3) | 0 (0.0) | 1 (2.2) | 1 (5.3) | 0.51 |
| Aberrant palmar crease (2) | 51 (12.3) | 28 (11.0) | 11 (14.3) | 2 (10.0) | 6 (13.3) | 4 (21.1) | 0.64 |
| Railroad track ears (1) | 38 (9.2) | 19 (7.5) | 8 (10.3) | 6 (30.0) | 4 (8.9) | 1 (5.3) | 0.04 |
| Strabismus (1) | 12 (2.9) | 5 (2.0) | 3 (3.9) | 1 (5.0) | 2 (4.4) | 1 (5.3) | 0.33 |
| Ptosis (1) | 4 (1.0) | 2 (0.8) | 0 (0.0) | 1 (5.0) | 1 (2.2) | 0 (0.0) | 0.20 |
| Decreased pronation/supination arms (1) | 6 (1.5) | 1 (0.4) | 1 (1.3) | 0 (0.0) | 3 (6.7) | 1 (5.3) | 0.01 |
| Heart murmur (1) | 50 (12.1) | 19 (7.5) | 9 (11.5) | 4 (20.0) | 9 (20.0) | 9 (47.4) | <0.0001 |
| Heart defect (0) | 18 (4.3) | 5 (2.0) | 2 (2.6) | 3 (15.0) | 4 (8.9) | 4 (22.2) | <0.0001 |
| Neurologic problems (0) | 11 (2.7) | 7 (2.8) | 0 (0.0) | 1 (5.0) | 2 (4.4) | 1 (5.3) | 0.20 |
| Dysmorphology score (mean, sd) | 4.7 (4.5) | 2.5 (2.6) | 3.8 (3.5) | 5.9 (4.8) | 5.1 (5.7) | 7.3 (8.7) | <0.0001 |
Numbers in parenthesis denote score assigned to each feature in dysmorphology score (adapted from Hoyme and May, 2016)
In multivariable linear regression analysis, infants with exposure in the top three trajectories (C-E) had increased total dysmorphology score in dose response fashion (p<0.0001) compared with infants who had minimal to no PAE (Table 2). Point estimates for sustained use (trajectory C) were slightly higher than decreased use (trajectory D), although the confidence intervals fully overlapped.
Table 2.
Beta estimates for dysmorphology score (observed range 0–31)
| Mean, SD | Unadjusted beta | Adjusted beta1 | |
|---|---|---|---|
| A: Minimal/none | 3.6 (3.2) | reference | reference |
| B: Low/moderate, decrease | 5.1 (3.9) | 1.55 (0.46, 2.64) | 0.83 (−0.29, 1.96) |
| C: Low/moderate, sustained | 7.4 (5.4) | 3.71 (1.75, 5.66) | 2.69 (0.74, 4.64) |
| D: Moderate/high, decrease | 6.5 (6.2) | 2.89 (1.53, 4.25) | 2.29 (0.88, 3.71) |
| E: High, sustained | 8.9 (9.7) | 5.25 (3.26, 7.25) | 4.81 (2.89, 6.72) |
Models adjusted with propensity scores for maternal prenatal vitamin use, age, socioeconomic status, pregnancy smoking, and infant age at dysmorphology exam. Inverse probability censoring weights applied for all models to account for loss to follow up.
Infants with the three highest trajectories of exposure were more likely to have two or more of the cardinal features compared to infants in the lowest exposure trajectory (Figure 2). When the cardinal features were individually examined, there was no discernable pattern of associations with exposure groups. Short palpebral fissure length had the strongest associations with the trajectories (3–6 fold increase in risk estimates) and all trajectories were above the null. Conversely, only the high sustained exposure trajectory (E) was associated with a smooth philtrum, and the two sustained use trajectories (C,E) were associated with a 2-fold increase in having a thin smooth vermillion border, although confidence intervals for the highest sustained use trajectory included the null.
Figure 2.
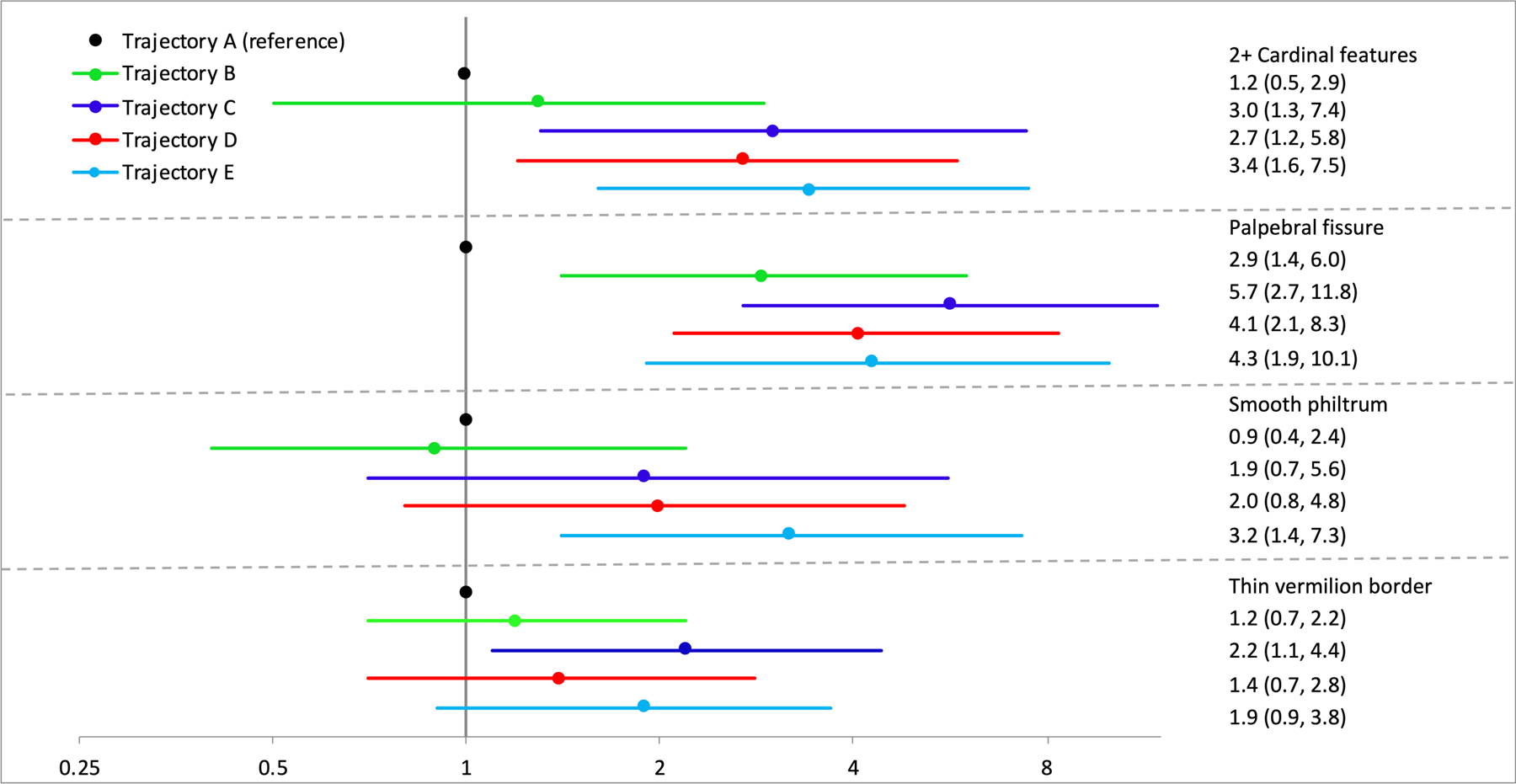
Adjusted risk ratios for cardinal features. Models adjusted with propensity scores for maternal prenatal vitamin use, age, socioeconomic status, pregnancy smoking, and infant age at dysmorphology exam. Inverse probability censoring weights applied for all models to account for losses to follow up.
Four non-cardinal features were tested in multivariable analyses. The top three trajectories of exposure were associated with hypotelorism (Figure 3, top panel). Compared to infants with the lowest trajectory of PAE, only those with the highest sustained exposure were at an increased risk for heart murmur (Figure 3, bottom panel). Due to very low prevalence of railroad track ears and heart defects (Table 3), we collapsed exposure into three groups [minimal/no exposure (A), low or moderate decreasing exposure (B,D) or low to high sustained exposure (C,E)]. Although point estimates for railroad track ears were 2-fold higher in infants with sustained exposure, confidence intervals crossed the null. Those same infants were at a 5-fold increased risk of heart defects compared to the lowest exposure trajectory, however confidence intervals were wide. The decreasing trajectories (B,D) were not associated with railroad track ears or heart defects. Due to the very low prevalence of decreased pronation or supination of the arms, multivariable analyses were not performed.
Figure 3.
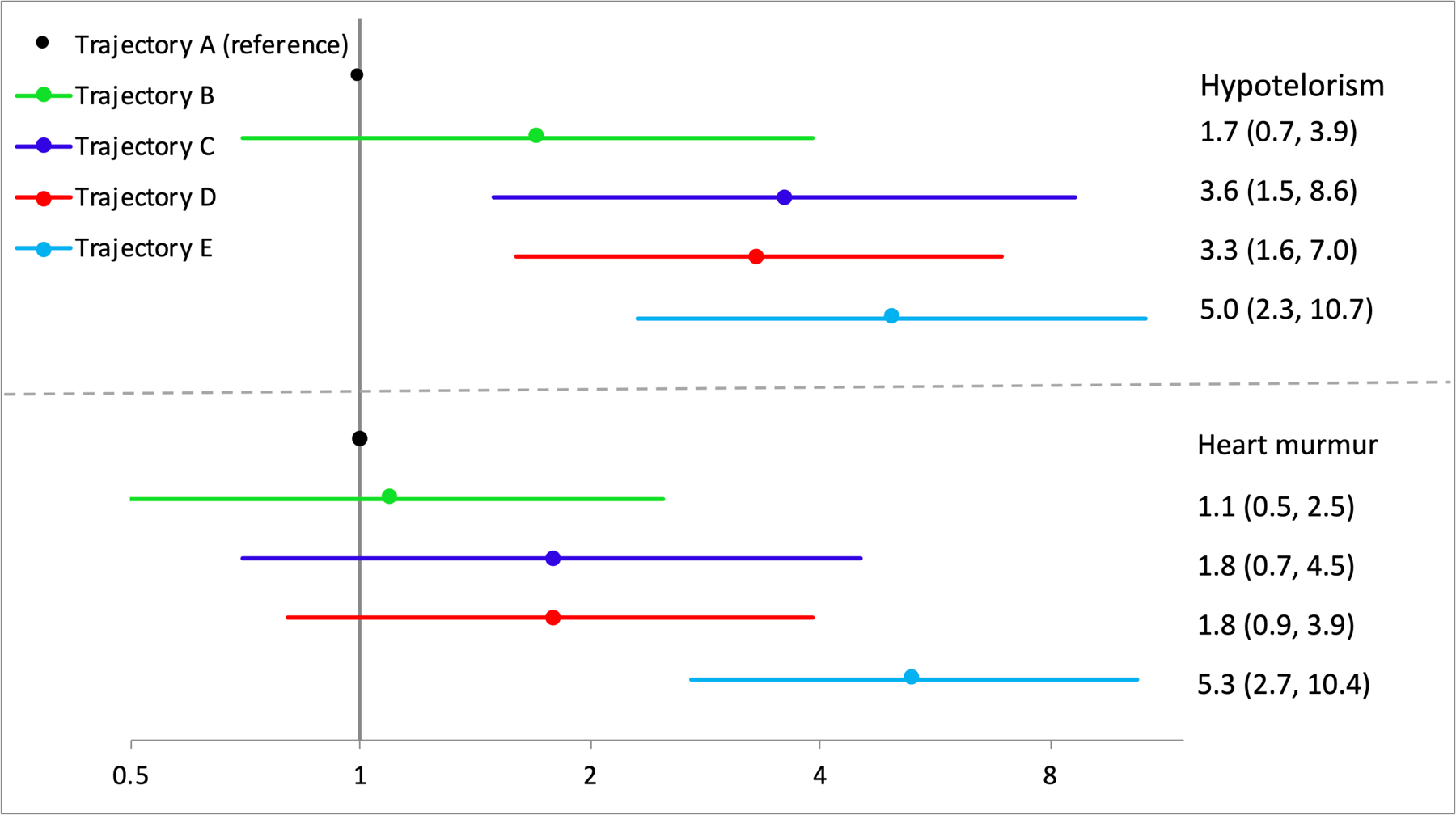
Adjusted risk ratios for non-cardinal features. Models adjusted with propensity scores for maternal prenatal vitamin use, age, socioeconomic status, pregnancy smoking, and infant age at dysmorphology exam. Inverse probability censoring weights applied for all models to account for losses to follow up.
Table 3.
Risk estimates for other non-cardinal features
| Railroad track ears | n, % | Unadjusted RR | Adjusted RR1 |
|---|---|---|---|
| 19 (7.5) | reference | reference | |
| Decrease (B, D) | 12 (9.8) | 1.30 (0.65, 2.60) | 1.18 (0.48, 2.86) |
| Sustained (C, E) | 7 (18.0) | 2.39 (0.18, 5.31) | 2.06 (0.85, 4.96) |
| Heart defect | |||
| None (A) | 5 (1.9) | reference | reference |
| Decrease (B, D) | 6 (4.9) | 2.46 (0.77, 7.93) | 1.22 (0.31, 4.90) |
| Sustained (C, E) | 7 (18.0) | 9.08 (3.03, 27.02) | 5.29 (1.39, 20.06) |
Models adjusted with propensity scores for maternal prenatal vitamin use, age, socioeconomic status, pregnancy smoking, and infant age at dysmorphology exam. Inverse probability censoring weights applied for all models to account for loss to follow up.
When body size measures were analyzed (Figure 4), infants in the highest exposure trajectory were at an increased risk to fall below the 10th centile for sex and age on height, weight and head circumference. Additionally, infants with moderate to high exposure with reduction (D) were also at an increased risk of reduced height percentile. No other exposure groups were associated with any other body size measures.
Figure 4.
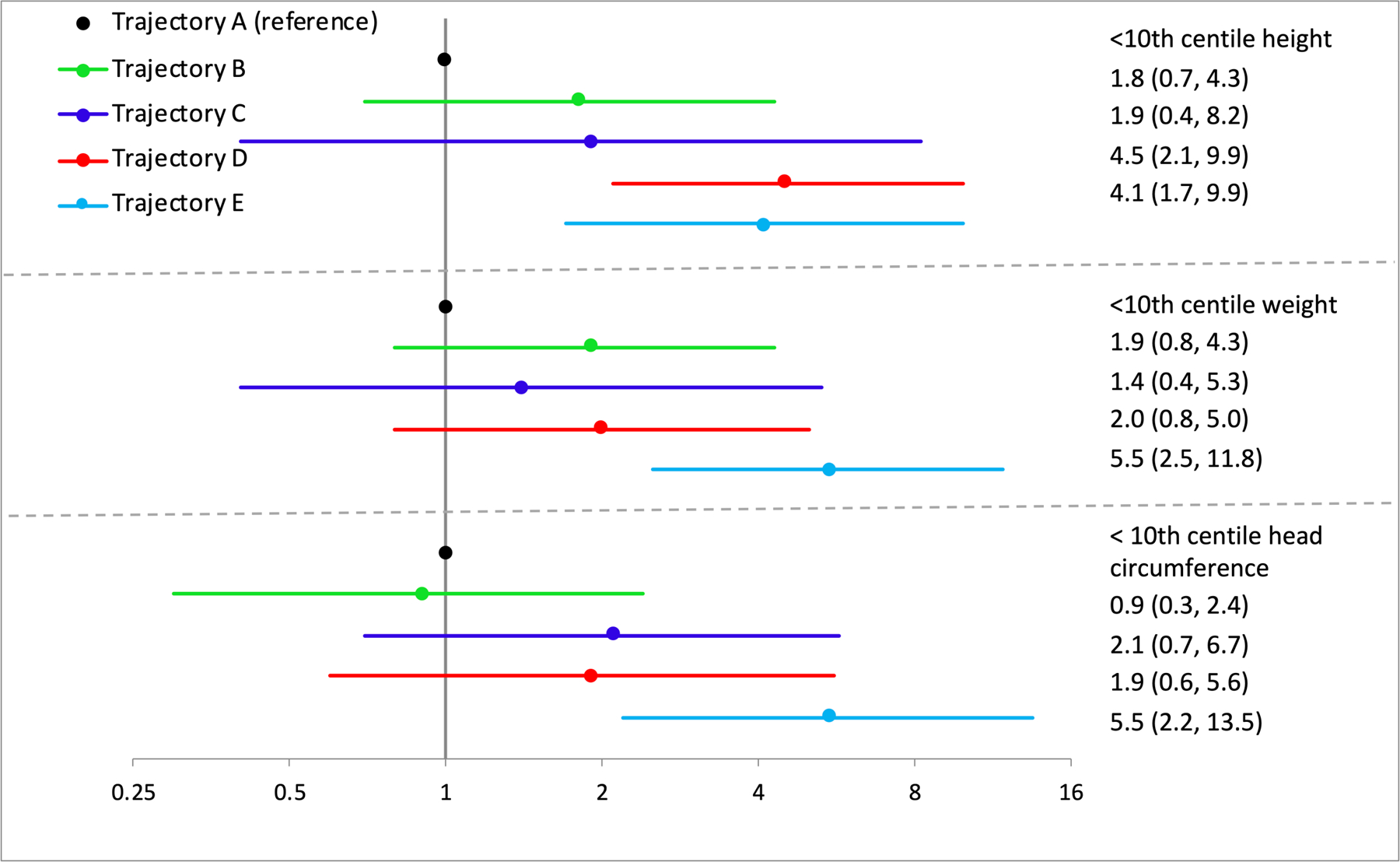
Adjusted risk ratios for body size measures. Models adjusted with propensity scores for maternal prenatal vitamin use, age, socioeconomic status, pregnancy smoking, and infant age at dysmorphology exam. Inverse probability censoring weights applied for all models to account for losses to follow up.
In the sensitivity analysis, 22 participants whose self-reported alcohol use was deemed unreliable were excluded from analyses (trajectories A (n=15) and B (n=7)). Results were unchanged in all models (results not shown).
Discussion
Identification of individuals affected by PAE has been limited by difficulty in confirming exposure and the low prevalence of the cardinal features that are required for the diagnosis of FAS. There is great interest in assessing other non-cardinal features to determine whether they are differentially expressed based on timing, quantity and frequency of PAE. Similar to previous studies, we found a higher overall dysmorphology score associated with exposure, and the score increased as a function of duration and quantity of PAE. Additionally, the three highest trajectories were associated with having 2+ cardinal features; however, there were no consistent associations between the individual trajectories and each cardinal feature alone. For non-cardinal features, the three highest trajectories predicted hypotelorism, but estimates did not differ from one another. Only the highest, sustained exposure trajectory was associated with heart murmur, and when the two sustained use trajectories were collapsed, they also predicted heart defects. Finally, while the highest sustained exposure trajectory was associated with reduced body size for all three measures (height, weight and head circumference), there were no other notable body size findings based on patterns of exposure.
We had anticipated that the associations between select features and the exposure trajectories may differ based on early high (trajectory D) versus lower sustained (trajectory C) exposure. Although there is heterogeneity within the two exposure trajectories leading to overlapping confidence intervals (Figure 1), at the group level, infants in trajectory D have much higher PAE very early in pregnancy, but then cross under trajectory C by the end of the first trimester, and are more likely to have no further PAE after the first trimester. In our previous analysis, low-to-moderate sustained exposure was more strongly associated with deficits in continuous measures of weight and length at birth, and select neurodevelopmental outcomes in infancy than moderate-to-high exposure with early reduction (Bandoli et al., 2019). We anticipated that we may see these same differential effects between those two trajectories in features influenced by exposure after the first trimester, including but not limited to palpebral fissure length, aberrant palmar creases, neurologic problems, camptodactyly, hypotelorism, strabismus and ptosis. Although there was some evidence of this in beta estimates for the total dysmorphology score (C trajectory beta estimate 2.3 vs. D trajectory beta estimate 1.9), as well as in risk ratios for short palpebral fissures (C trajectory aRR 5.7 vs. D trajectory aRR 4.1) and thin smooth vermillion border (C trajectory aRR 2.2 vs D trajectory aRR 1.4), the estimates did not differ from each other statistically, as confidence intervals widely overlapped for the two trajectory estimates for both outcomes. Additionally, given the large numbers of comparisons, these findings could have been due to chance.
Others have also analyzed dysmorphic features by timing or quantity of alcohol. In univariate analyses from a sample of first-graders in Italy, the overall dysmorphology score was associated with maternal recall of drinks per month in the second and third trimesters, although estimates did not differ from each other (May et al., 2006). A second study categorized patterns of alcohol exposure by quantity and frequency measures (e.g., number of drinks on weekends, weekdays, per week, per drinking day, and within each trimester). When analyzed individually as bivariate correlations, most exposure measures significantly predicted total dysmorphology score, and estimates were relatively similar to each other. However, when analyzed after controlling for first trimester exposure, second and third trimester exposure were no longer associated with total dysmorphology score. The authors interpreted this as demonstrating the importance of the first trimester for development of most facial features and many of the other minor anomalies sensitive to alcohol exposure (May et al., 2013). Although we do not have any trajectories with only later exposure to test that hypothesis, we did not see stronger effects in the trajectory with moderate/high exposure with reduction (D) vs. the low/moderate sustained exposure (C), which would have supported that hypothesis. In fact, the total dysmorphology score was higher in trajectory C, likely due to continued exposure after the first trimester.
At least one other study has evaluated individual dysmorphic features with patterns or timing of prenatal alcohol exposure. In a prospective birth cohort, patterns of drinking were characterized by quantity, timing and frequency (drinks/day in each trimester, binge episodes in each trimester), and those measures were assessed with the risk of minor structural malformations that occurred in at least 5% of the sample (Feldman et al., 2011). Specifically, naevus flammeus neonatorum, long philtrum, clinodactyly, altered ear shape, hypoplastic fingernails, anteverted nares, epicanthal folds, and hypertelorism were evaluated. From these, only naevus flammeus neonatorum was associated with PAE, and effect estimates were quite similar based on the gestational timing of exposure. Although we observed different results for the common features evaluated between the two studies, our sample had more pregnancies exposed to alcohol based on the design, with more statistical power to detect differences.
When interpreting the findings, the limitations of the study should be considered. Alcohol use in pregnancy was self-reported, which could have led to misclassification of exposure. Although we would typically expect this to result in bias towards the null based on underreporting, with multiple exposure groups this is not guaranteed. Additionally, trajectories were created from exposure that was assigned based on estimated gestational week. Errors in estimation of last menstrual period would lead to misclassification of exposure, although we would not expect this to be differential by trajectory group assignment or outcome. Finally, the trajectories are constrained by sample size. The sample size in the two sustaining trajectories was small, limiting our ability to differentiate effect estimates for those two groups. Additionally, there is heterogeneity within the resulting trajectories (e.g.- 51% of women in the moderate/high with reduction trajectory abstained from alcohol in the 3rd trimester, but 49% did not), which reduces the precision of effect estimates. Continuing to characterize exposure into longitudinal trajectories from larger datasets will lead to less variance within trajectory groups.
In summary, although exposure trajectories did not differentiate risk for individual dysmorphic features as we had anticipated, there are still interesting findings from this approach. Alcohol trajectories predicted total dysmorphology score in a dose response fashion, reaffirming the message that cessation of alcohol early and for the entirety of gestation is the safest approach. As additional data becomes available, we hope to create more homogenous trajectories to further attempt to illuminate patterns of features based on gestational timing of alcohol exposure.
Supplementary Material
Acknowledgement
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at http://cifasd.org/. Research described in this manuscript was supported by grants U01AA014835 (PI: CD Chambers) and U24AA014815 (PI: KL Jones) funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). We wish to acknowledge the contribution of: OMNI-Net, Ukraine, and the participating families and staff in Rivne and Khmelnytsky, Ukraine. Gretchen Bandoli is funded by the National Institutes on Alcohol Abuse and Alcoholism (1 K01 AA027811-01).
References
- Astley S (2004) Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code, 3rd ed. Seattle, University of Washington Publication Services. [Google Scholar]
- Bandoli G, Coles CD, Kable JA, Wertelecki W, Yevtushok L, Zymak-Zakutnya N, Wells A, Granovska IV, Pashtepa AO, Chambers CD (2019) Patterns of Prenatal Alcohol Use That Predict Infant Growth and Development. Pediatrics e20182399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower C, Elliott E, on behalf of the Steering Group (2016) Report to the Australian Government Department of Health: “Australian Guide to the diagnosis of Fetal Alcohol Spectrum Disorder (FASD).
- Chambers CD, Yevtushok L, Zymak-Zakutnya N, Korzhynskyy Y, Ostapchuk L, Akhmedzhanova D, Chan PH, Xu R, Wertelecki W (2014) Prevalence and Predictors of Maternal Alcohol Consumption in Two Regions of Ukraine. Alcohol Clin Exp Res 38:1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Granovska IV, Pashtepa AO, Chambers CD (2015) Dose and Timing of Prenatal Alcohol Exposure and Maternal Supplements: Developmental Effects on 6-Month-Old Infants. Matern Child Health J 19:2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, Leblanc N, Loock CA, Lutke J, Msw BFM, Mba AAM (2015) Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. C Can Med Assoc J 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo M, Jones KL (2017) A review of the physical features of the fetal alcohol spectrum disorders. Eur J Med Genet 60:55–64. [DOI] [PubMed] [Google Scholar]
- Feldman HS, Jones KL, Lindsay S, Slymen D, Klonoff-Cohen H, Kao K, Rao S, Chambers C (2011) Patterns of prenatal alcohol exposure and associated non-characteristic minor structural malformations: A prospective study. Am J Med Genet Part A 155:2949–2955. [DOI] [PubMed] [Google Scholar]
- Genolini C, Falissard B (2016) Package “kml.” Available at: https://cran.r-project.org/web/packages/kml/kml.pdf Accessed May 7, 2020.
- Genolini C, Falissard B (2010) KmL: K-means for Longitudinal Data. Comput Stat 25:317–328. [Google Scholar]
- Godin EA, O’Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK (2010) Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: Effects of acute insult on gestational day 7. Alcohol Clin Exp Res 34:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138:e20154256–e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 institute of medicine criteria. Pediatrics 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL (2011) The effects of alcohol on fetal development. Birth Defects Res Part C - Embryo Today Rev 93:3–11. [DOI] [PubMed] [Google Scholar]
- Jones KL, Hoyme HE, Robinson LK, del Campo M, Manning MA, Prewitt LM, Chambers CD (2010) Fetal Alcohol Spectrum Disorders: Extending the Range of Structural Defects. Am J Med Genet A 152A:2731–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Kable JA, Coles CD (2015) Prenatal alcohol exposure, adaptive function, and entry into adult roles in a prospective study of young adults. Neurotoxicol Teratol 51:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais A-SS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDHH, Hoyme HE, Tabachnick B, Seedat S (2013) Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug Alcohol Depend 133:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Phillip Gossage J, Kalberg WO, Eugene Hoyme H, Robinson LK, Coriale G, Jones KL, Del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M (2006) Epidemiology of FASD in a Province in Italy: Prevalence and Characteristics of Children in a Random Sample of Schools. Alcohol Clin Exp Res 30:1562–1575. [DOI] [PubMed] [Google Scholar]
- O’Leary-Moore SK, Parnell SE, Godin EA, Dehart DB, Ament JJ, Khan AA, Johnson GA, Styner MA, Sulik KK (2010) Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth Defects Res Part A Clin Mol Teratol 88:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Holloway HT, O’Leary-Moore SK, Dehart DB, Paniaqua B, Oguz I, Budin F, Styner MA, Johnson GA, Sulik KK (2013) Magnetic resonance microscopy-based analyses of the neuroanatomical effects of gestational day 9 ethanol exposure in mice. Neurotoxicol Teratol 39:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK (2009) Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: Effects of acute insult on gestational day 8. Alcohol Clin Exp Res 33:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M (2000) Alcohol timeline follow-back (TLFB) In: Handbook of Psychiatric Measures. , p 477 Washington, DC, American Psychiatric Association. [Google Scholar]
- Sulik KK (2005) Genesis of Alcohol-Induced Craniofacial Dysmorphism. Exp Biol Med 230:366–375. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang L, Yang T, Chen L, Zhao L, Wang T, Chen L, Ye Z, Zheng Z, Qin J (2020) Parental alcohol consumption and the risk of congenital heart diseases in offspring: An updated systematic review and meta-analysis. Eur J Prev Cardiol 27:410–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


