Abstract
The role of stem cells in augmenting reparative processes in the heart after ischemic injury has been successfully demonstrated in small and large animal models. However, the outcomes of cell therapy in clinical trials have been somewhat variable, with overall effects of autologous stem cell therapies demonstrating a modest improvement in cardiac structure and function. How stem cells repair the heart after cardiac injury is still not well understood. Most recent studies suggest that adult derived stem cells act primarily through paracrine signaling to exert beneficial effects, including modulation of immune response, stimulation of new blood vessel formation or by inducing mature myocytes to transiently reenter the cell cycle, rather than robust direct differentiation of the transplanted cells into myocytes. Additionally, data from multiple labs confirmed clearance of stem cells themselves within a few days still leading to functional benefits further confirming the role of paracrine signaling in augmenting cardiac reparative processes rather than direct differentiation of cells. These findings rapidly evolved the field of extracellular vesicles specifically microvesicles as they are active hubs of autocrine, paracrine and endocrine signaling targeting different biological processes. The beneficial effects seen after stem cell transplantation could be linked to the cardioprotective factors packaged in the microvesicles secreted from stem cells. Therefore, stem cell microvesicles provide a new avenue for the treatment of cardiovascular disease through a multitude of mechanisms including cellular communication within the stem cell niches, delivery of genetic information, regulation of the immune system in the heart, and stimulation of angiogenesis which will be discussed in this review.
Keywords: Stem Cell Microvesicles, Heart, Cardiovascular Diseases
Introduction
Cardiovascular Diseases (CVDs) are the leading cause of death worldwide, with about 422 million cases per year.3 CVDs are defined as a group of disorders of the heart and circulatory system.1 The growing number of individuals that are affected by CVDs indicate a necessity for improvements, preventions, and treatments related to the disease. Over the past decades, tremendous progress has been made in the ability to reopen blocked coronary arteries.5 However, reperfusion of the artery cannot rescue the cardiac tissue in the core of the infarct leading to compromised heart function.6
The field of stem cells has created excitement as there is a possibility of targeting reparative processes in organs with limited regeneration capabilities including the heart.7 Stem cells were first thought to be able to replace the scar by repopulating the scarred tissue after injury; however, so far, the evidence for neomyogeneisis has been very limited.9 Nevertheless, there is no doubt that stem cell treatments in animal models and in clinical trials have shown functional improvement that are independent of new myocyte formation because of their ability to target multiple pathways via paracrine factor release.7 It is documented by several labs that stem cells are cleared quickly within a day or two after transplantation; however, this early clearance of stem cells did not affect the functional benefits after injury suggestive of the paracrine role of stem cells being the major contributor in observed functional outcomes. These paracrine factors and other secretory molecules, proteins, miRNA can be loaded in extra-cellular vesicles that bud off the cells as a normal physiological process.
More recently, extracellular vesicles (EVs) have gained importance as they are hubs of signaling and have been suggested as a beneficial and novel therapeutics for the treatment of diseases,14,15,16 including CVDs17. Extracellular vesicles are secreted by all cells including stem cells with increasing evidence in literature emphasizing their role in augmenting repair processes after injury. They have been suggested as a novel target and possible mechanism to help improve CVD prognosis and prevention18. Extracellular vesicles are not only secreted by stem cells but are produced by all cells in the body, therefore comparing the cargo of EV’s under normal and pathological conditions can serve as useful biomarkers to predict early abnormal changes in the body related to diseases. These biomarkers can provide early diagnosis of the cardiac disease, leading to a better outcome in patients with heart problems as well as a potentially new therapeutic strategy for treatment.19,20,21 This review will specifically address the role of the stem cells derived microvesicles and their potential role for the treatment of CVDs.
What are microvesicles?
The knowledge on extracellular vesicles (EVs) is quite broad; however, less is known about microvesicles (MVs). MVs are considered to be 100 −1000nm in size and derived from plasma membrane shedding. MVs can be released by all cells, including stem cells23, circulating cells (such as platelets and T cells)24, vascular cells (such as endothelial cells)25, and tumor cells26 as a part of the normal physiological process. Because of the variety of cellular sources and, therefore, functions, MVs serve an integral part of cellular communication.27
Extracellular vesicles are comprised of microvesicles, exosomes and apoptotic bodies.28 The nomenclature for these different subsets is defined majorly by the size of these subfractions. MVs are about 100–1000nm compared to exosomes that are smaller in size ranging from 50–100nm versus apoptotic bodies that are larger including vesicles that are 1–5μM in size.29 MVs are unique vesicles that bud from the plasma membrane and can be dispersed and found in biologic fluid, such as blood or urine.28 These MVs can deliver various signals between cells for vital cellular communication as well as propagation of multi-signaling processes.28,30 Additionally, MVs shed from the plasma membrane of healthy cells and can be referred to as ectosomes, shedding vesicles, or microparticles28. When MVs are shed, they often keep the surface molecules from the parent cell and retain their cytosolic content, which includes proteins, RNA, DNA, lipids, and miRNAs.31 Alternatively, apoptotic bodies are created when stimuli paired with the extrinsic and intrinsic apoptosis pathways initiate apoptosis. The pathways integrate and form these apoptotic bodies, which are engulfed and recycled.32 Conversely, exosomes are defined as vesicles released during reticulocyte differentiation because of multivesicular endosomes (MVE) fusion with a plasma membrane28. Moreover, the composition of MVs compared to exosomes vary. Exosomes have specific protein contents that differentiate them from MVs.28 MV composition resembles the plasma membrane; however, the main difference is the lack of asymmetric phosphatidylserine (PS) and phosphatidylethanolamine (PE).22,33 Exosomes have increased PS, as well as glycosphingolipids and cholesterol.22,34 However, separating MVs from exosomes is not always straightforward as they are found in similar fluids and share similar origins.22 The differences between the three types of extracellular vesicles have been highlighted in Table 1.
Table 1:
Summary of the characteristics of extracellular vesicles
| Extracellular Vesicle | Size | Origin | Function |
|---|---|---|---|
| Exosomes | 50–100nm | Multivesicular endosome fusion with the plasma membrane as a result of reticulocyte differentiation | Carry and deliver signaling molecules to target cells |
| Microvesicles | 100–1000nm | Shedding from the plasma membrane of parent cells into extracellular space | Cellular communication by cargo delivery, immune response, angiogenesis |
| Apoptotic Bodies | 1–5μM | Extrinsic and intrinsic apoptotic pathways initiation of apoptosis | Engulf and recycle cells |
To date several types of stem cells have been used for treating cardiovascular diseases with modest improvement. Every stem cell type has pros and cons (Table 2). Simultaneously, several different routes have been tested for the delivery of stem, cells for a better outcome (Table 3). In this review, we will highlight the role of microvesicles specifically from stem cells and their contribution in the regulation of different processes/pathways in the heart, as well as the therapeutic approaches and limitations associated with CVD.
Table 2.
Stem cell populations with potential uses in cardiac repair processes
| Stem Cell Type | Purpose | Advantage | Disadvantage |
|---|---|---|---|
| Adipose Derived Stem Cells80, 81 | Differentiate into various cell lineages, regeneration/repair of tissue and organs after injury | Adipose tissue is abundant and less invasive for gathering samples, association with skin cells helps with healing | Susceptible to host environment rejection/immune response, efficiency problems |
| Bone Marrow Derived Stem Cells (including MSCs and HSCs)10, 84 | Tissue and organ regeneration/repair | Many preclinical and clinical studies, easy availability | Modest benefit from clinical trials |
| Cardiac Derived Stem Cells103 | Regulation of cardiac cell turnover to increase regeneration | Promote tissue regeneration, reduce adverse cardiac remodeling, improved LV function, reduced infarct size | Lack of differentiation, modest improvement in cardiac function |
| Embryonic Stem Cells10 | Cardiac regeneration, differentiation into cardiomyocytes | Unlimited self-renewal, can differentiate into any cell type | Often form teratomas and malignant tumors, arrythmias in heart after transplantation |
| Induced Pluripotent Stem Cells10 | Differentiation into cardiomyocytes with full functional capacity of healthy cardiac cells | Use of transcription factors to aid differentiation | Transcription factors are known oncogenes associated with teratoma formation, low and varying efficiency |
| Progenitor Cells (Embryonic and Cardiac)84 | Tissue specific, endogenous differentiation | Tissue specific, provides localized and easily traced differentiation and regeneration | Limited translational application |
| Skeletal Derived Myoblasts10 | Differentiate into skeletal muscle to repair the myocardium | Increased plasticity, derive autologous cells which removes necessity for immunosuppression | Cannot become cardiomyocytes, problems with electric junction formation, risk of ventricular tachycardia |
Table 3.
Possible stem cell delivery models.10
| Delivery Model | Purpose | Advantage | Disadvantage |
|---|---|---|---|
| Intracoronary delivery10,85 | Transvascular approach into coronary artery during occlusion using catheter | Even distribution, simple and well known technique | Low retention, possible microvascular occlusion |
| Intravenous infusion10,86 | Transvascular approach to deliver cells following myocardial infarction | Delivery of different stem cells types including EPCs and MSCs showed improved cardiac function in animal model | Limited clinical application |
| Mobilization of stem cells10,87 | Transvascular approach to draw circulating stem cells to injury site | Noninvasive approach | Increased immune response |
| Direct injection into ventricular wall10, 88 | Deliver cells into ventricular wall after occluded coronary artery | Favored method, works with larger cell types, well studied | Can have poor cell survival, challenging in patients with acute myocardial infarction |
| Transepicardial Injection10,89 | Used with coronary artery bypass graft (CABG) | Direct visualization of myocardium | Difficult to assess efficiency because of CABG |
| Transendocardial injection10,89 | Deliver cells inside the LV with a catheter across the aortic valve | Can use electromechanical mapping for better visualization, cell delivered even during complete occlusion | Can disrupt cardiac tissue architecture, can cause lack of blood supply |
| Transcoronary vein injection10,90 | Uses catheter with ultrasound to delivery cells to myocardium | Deliver cells parallel to ventricular wall and into injured myocardium | Deep injection |
Stem Cell derived Microvesicles Vasculature in Cardiac Disease:
In order to understand how stem cells and their MVs function and communicate with other cells within the body, it is essential to understand the microenvironment in which the stem cells reside. Stem cells co-localize with other cells that dwell within specific tissues. Ultimately the microenvironment in which these stem cells reside yield a specific structural and functional characteristics commonly referred to as the stem cell niche.35 In general, this stem cell niche is anchored in an heterogeneous extracellular matrix that is composed of organ-specific cell types and resident stem cells, which allows for cell to cell signaling.36 The mechanism of tissue or organ specificity of stem cell niches is still not fully understood; however, it is known that the spatial organization and the interaction within the niche are vital for the stem cell specification and the maintenance of existing cells. This guarantees that the stem cells retain their characteristics such as self-renewal and the prevention of differentiaion.37 The overall role of the niche is to provide a location where cell signaling molecules, including MVs, can interact and influence stem cell behavior and function38 as well as tissue specific cells’ behavior and function39. For example, the hematopoietic stem cell (HSC) can be found in the perivascular niche, which helps provide blood cells derived from HSCs when the body is under hematological stress.35
The stem cell uniqueness to the niche environment in the heart and vasculature is vital for the microenvironment interactions of MVs. This is an essential feature of stem cells in the treatment of CVD, as there are specialized stem cell niches in the heart and vasculature for specific signaling and MV function.40 Within those stem cell niches, the stem cell MVs are critical for the cell to cell communication, in both physiological and pathological settings in the heart.41 The stem cell MVs are strong cellular communicators by activating and inhibiting many biological processes within the stem cell niches which will be addressed. The stem cell MVs contain bioactive material that can help regulate, activate, and inhibit a variety of processes associated with stem cells described throughout this review.27 Additionally, there are many forms of cellular communication of stem cell MVs related to CVD. This review will address how stem cell MVs enhance cellular communication in order to lessen the damage caused by CVDs, specifically through the transfer of genetic information, the use of miRNAs, cargo delivery, immunomodulation, and angiogenesis.
Delivery of Genetic Information and Signaling Molecules by MV’s:
Cargo delivery is characteristic of all EVs. Specifically, MV cargo can consist of genetic information, transcription factors, cytokines, growth factors, and cellular receptors.19 The focus of stem cell cargo delivery has been on genetic information such as mRNA, microRNA (miRNA), and DNA.19 Stem cell derived MVs are shown to create a regulatory system that can modulate mRNA delivery and transcription in the target cells as well.42 The MV’s ability to transfer genetic information has been a hallmark and the main feature of this form of cellular communication (Figure 1). Additionally, the transfer of genetic information and bioactive material from stem cell MVs have been shown to alter regulatory networks of endocrine activity, which helped identify ischemia/reperfusion-induced acute injury.43 This genetic transfer has also been associated with stem cell plasticity, and phenotypic regulation.44 For example, stem cell derived MVs can transfer RNA to hematopoietic cells, which cause reprogramming of the cells and hematopoietic cell differentiation.45,46 Furthermore, MVs released from embryonic cells have stem cell specific molecules that help with self-renewal and proliferation of adult stem cells. The MVs from embryonic stem cells (ESCs) also increased the survival of hematopoietic progenitor cells because of the mRNA in the MVs contained increased amounts of pluripotent transcription factors.46 There are reports demonstrating microvesicles derived from mesenchymal stem cells (MSCs) to have a wide range of genetic material transfer that leads to paracrine secretion and increased cardiac differentiation to help enhance cardiomyocyte function after injury.47
Figure 1:
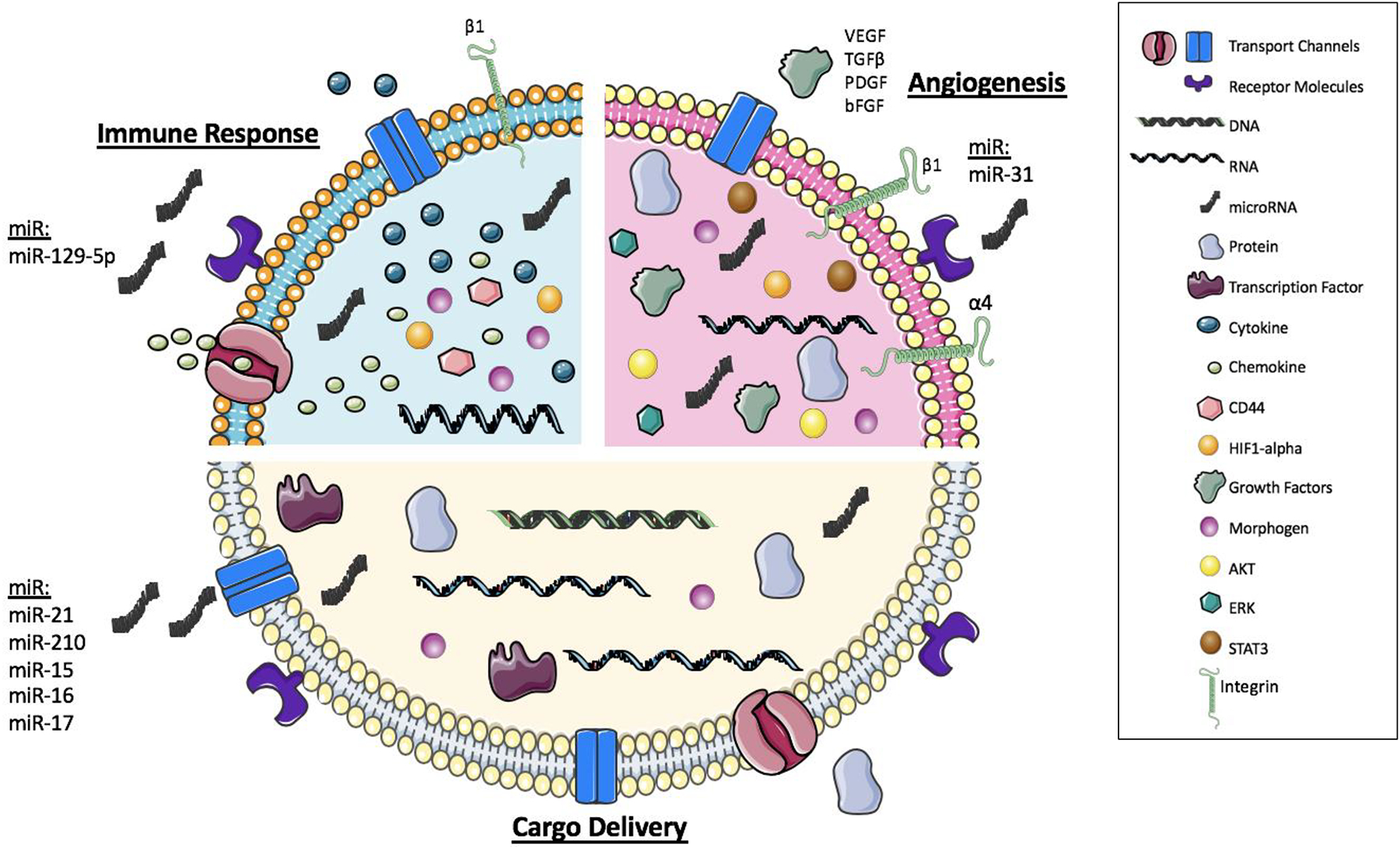
Composition of Stem Cells derived Microvesicles: Schematic illustration of main functions of microvesicles including cargo delivery, angiogenesis, and the immune response Microvesicles can pack a variety of molecules/miRNA / factors that help drive the signaling and function to target cells.
Another type of genetic information delivery that has propelled the field of stem cell MVs is the delivery and use of miRNAs by these microvesicles. MVs from adult human bone marrow and tissue specific MSCs help regulate intracellular communication through select miRNAs.48 Specifically, miR-31 targets HIF-1α to help induce angiogenesis. Similarly, adipose derived stem cells (ASCs) preconditioned with endothelial differentiation medium release this miR-31 and increased angiogenesis.49 miR-129–5p has been shown to be conversely regulated as a response to oxidative stress. The inducible pluripotent stem cells (iPSCs) derived MVs also had cardioprotective miRNAs that help protect against myocardial ischemia and reperfusion, specifically miR-21 and miR-210, which is regulated by HIF-1α.50 In general, MSC derived MVs have a higher amount of miR-15, miR-16, miR-17, miR-31, miR-126, miR-145, miR-221, miR-222, miR-320a, and miR-424, which were profiled from HIF-1α overexpressing MSCs. This study also emphasized that miR-31 has the most extensive role in migration and tube formation.47.
The transfer of genetic information as cargo delivery is vital; however, there are other types of cargo delivery by stem cell MVs. MVs can deliver signaling molecules, such as growth factors and immune regulators (Figure 1), to help combat hypoxic stress in ischemic tissue.51 Delivery of bioactive materials, such as mRNA but also proteins from iPSCs derived MVs to human cardiac MSCs, display a protective effect by altering the transcriptome and proteome profile of the MSCs in the heart.52 Additionally, MVs from the iPSCs contain pluripotent transcription factors that are vital for maintaining the stem cell pluripotency.53,54 Stem cell derived MVs are also capable of transferring morphogens, such as the hedgehog proteins, to help regulate plasma membrane remodeling and differentiation.55
Microvesicles have been demonstrated to be a key therapeutic target, however, they can also be used to assess the extent of cardiac injury.56 Increased MV expression post MI correlates with increased injury severity.57 The molecular profile and delivery of the MVs are increased in myocardial infarctions and stroke indicating their function in the prevention of the disease.51 Because of the uniqueness of the miRNA composition within stem cell MVs, this genetic information could be a beneficial therapy for the prognosis and detection of CVDs. For example, embryonic stem cell derived cardiomyocytes MVs were downregulated when exposed to oxidative stress. This miR-129–5p is inversely related to the level of heart failure, and therefore, the MV levels are a suggested sensitive biomarker of the disease.58
Immune modulation by stem cells derived MV’s during heart injury:
Stem cell MVs have been suggested to play a significant role in the paracrine hypothesis (Figure 1). The paracrine hypothesis proposes that stem cells can release beneficial molecules and initiate signaling that can improve reparative response and function of the injured myocardium.59,60 MVs secreted by cardiac fibroblast derived iPSCs are particularly effective at delivering cytoprotective signals to cardiomyocytes under myocardial ischemia and reperfusion.50 The iPSCs MVs have shown cytoprotective properties that help induce cardiac repair in vitro as well, resulting in increased left ventricular function and vascularization.61 Therefore, EV delivery has been suggested as a potential therapy during ischemic injury resulting from a myocardial infarction.50 However, the mechanism of MV delivery, function, and off-target effects are still not fully understood and need further investigation. MVs derived from embryonic stem cells (ESCs), have been shown to transfer cardioprotective immune signaling to decrease inflammation and increase angiogenesis in turn increasing myocardium viability; this study provides insight on how stem cell mediated MV therapy can be used to target the inflammatory response post-MI.62 Additionally, MSCs have MVs that secrete paracrine factors as well as RNA, which suggested that MSCs could be mediating the immune response through intercellular communication and the secretion of MVs. Extensive analysis of MVs derived from MSC proteome has shown their potential therapeutic effects and ability to help with tissue repair and regeneration as well.63 More specifically, through microarray analysis, it was discovered that these MVs have specific cellular mRNAs, CD44, and β1-integrins that help control proliferation and immunoregulation that can assist with the survival of tubular cells after injury.64 These MSC derived MVs are also capable of immunomodulation through the production of cytokines, which improves the function of the target cells through paracrine signaling.65
The role of stem cell MVs promoting angiogenesis:
Angiogenesis is vital for maintaining and restoring blood supply, nutrient uptake, and oxygen transfer that is often lost following ischemic injury or disease.66 The role of MVs in promoting and inducing angiogenesis has also been widely reported because of cellular communication and enhanced signal transduction (Figure 1).66 Specifically, endothelial progenitor cell (EPC) derived MVs improved neoangiogenesis after ischemia. Using SCID mice, these MVs promoted new vascularization and regeneration in hindlimb ischemia conditions.67 Specifically, EPCs secrete MVs that are capable of inducing angiogenesis in endothelial cells through interaction with the α4 and β1 integrins.68 It has also been suggested that MVs derived by EPCs can activate angiogenesis in endothelial cells resulting in an angiogenic reprogramming.69 The MSC derived MVs have also shown increased angiogenic signaling through their genetic transfer as mentioned previously. However, the MVs derived from MSCs are even beneficial in increasing blood flow recovery and reducing infarct size after ischemic injury due to their ability to promote blood vessel formation.70 Additionally, bone marrow MSC derived MVs have been shown to induce angiogenesis through signaling cascades involving AKT, STAT3, and ERK molecules. It was hypothesized that these signaling molecules increased the transcription of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and transforming growth factor beta (TGFβ) which all contribute to an increase in angiogenesis.66 Another proposed mechanism is through the adipose derived stem cell (ASC) MVs. These MVs increased tube formation of human umbilical vein endothelial cells. Preconditioning these stem cells in endothelial differentiation medium increased the release of MVs and angiogenesis once administered.49 ASC derived MVs have been shown to help with angiogenesis both in vitro and in vivo because of the increased amount of the proliferation cyclin molecules such as cyclin D1 and cyclin A1 and growth factors such as VEGF, FGF, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF).71 These MVs also activated the AKT and ERK pathways of target cells. Like MSCs derived MVs, the ASCs have MVs that promote angiogenesis through the delivery of RNA and miRNA, as mentioned previously.49 The injection of these ASC MVs increased wound healing and emphasized a potential therapeutic of the MVs in angiogenesis.71
Potential Therapies involving Stem Cell MVs in CVD:
Because of the characteristics and functions of stem cell MVs, the use of MVs has been suggested as a key therapeutic in the mediation of CVDs, specifically myocardial infarctions. More recently, the role of these MVs has been shown to increase beneficial cardiac properties and functions to help combat the symptoms of CVDs.18 Some of the leading therapeutic uses of stem cell MVs include the prognosis and prevention of CVDs through improved stem cell therapies, proteomic analyses, and the use of MVs as biomarkers for the disease.
Stem cell therapy to treat CVDs, specifically myocardial infarction, has been limited. Clinical trials with stem cell therapies have not produced beneficial results despite the enormous potential of stem cells. There is still a need to understand the mechanism behind stem cells and cardiac remodeling, particularly with MVs. Nanoshuttles have also been suggested as a therapeutic strategy for stem cell MVs. This method uses the nanoshuttles to relay information from a microenvironment to signal cells for aid in stressed states. Nanoshuttling was originally only used for waste removal in cells.72 Now, nanoshuttling the cargo of stem cell MVs to various target cells could be utilized for cardiac repair and halting cardiac fibrosis.72
Experimental studies also examined the MVs derived from MSCs, ESCs, and cardiac progenitor cells (CPCs) to help drive differentiation of stem cells in the heart. This strategy optimizes the capacity of stem cells relative to their paracrine signaling effects. The stem cells are injected into the heart, and the MVs from these stem cells contain bioactive molecules that assist in paracrine signaling and cell differentiation that would help the stem cells sustain and survive.17,73 The use of MVs to enhance engraftment of stem cells has also been suggested. Similarly, MVs derived from HSCs were shown to improve the transplantation efficiency of HSCs after engraftment. The MVs were proposed to have increased nitric oxide and AKT signaling that improved the engraftment of the stem cells.74 Another therapeutic strategy is to load these stem cell MVs with drugs to help reach the target cells for additional improvements.17
The proteome of various stem cells has been studied, but recently the proteome of MSC MVs was analyzed for their beneficial effect in the treatment of disease. The proteome of the MVs plays a role in a variety of potential therapeutic targets, such as signaling molecules, surface receptors, and cell adhesion, as mentioned earlier. This provides an understanding of MSC MVs in the disease versus healthy state, which could help offer novel therapeutic approaches.28 More comprehensive proteomic studies could give great insight into the potential of stem cell MVs specifically in the treatment of CVDs. Specifically, the technology involving iPSCs and RNA Drug Delivery System (RNA-DDS) has been suggested as a base for the miRNA libraries. The iPSCs are easily amplified in the immune cells, such as monocytes and macrophages, of patients, and therefore the cells and their MVs are potential targets for understanding individual RNA medicine.75
Another characteristic of MVs is their ability to carry cell surface proteins and receptors from their parent cells. They also can contain and maintain molecules from within parent cells such as miRNAs, RNAs, proteins, and lipids, as mentioned previously. All of these molecules could be specific biomarkers for CVDs to help identify the cellular origin.76 Because of their overall composition, MVs have been described as “fingerprints” of diseases. Using MVs from stem cells could provide novel insight and potential predication and prevention of CVDs.17 Specifically, MVs have been associated as biomarkers of a variety of cardiometabolic diseases, such as diabetes, which are easily detectable and attainable through body fluids.76 Most of this work has been done in platelet derived MVs, so there is still unknown potential and work to be done around stem cell MVs as biomarkers of CVD.
Limitations of stem cell MVs:
There are numerous possibilities of stem cell MVs; however, more work is still needed to have a universal and comprehensive understanding of their function and mechanism in the heart. With substantial research being dedicated to microvesicle applications, it is vital to understand the persisting challenges. The current limitations include a lack of detailed mechanism of action of stem cell MVs, standard isolation protocols, large scale production of MVs, and clinical trials utilizing stem cell MVs.
Although microvesicle analyses have impressively evolved in the last decades, the exact mechanisms of biogenesis are still unknown. MV’s are produced by all cell types, the cargo of the MV’s varies depending on the cell condition and densities, therefore there is still a need for a consistent and standard protocol for stem cell MV isolation.17 Another hindering step is the large scale production and purification of MVs that is needed in a clinical setting.17 Because of the signaling potential of stem cell MVs, the off-target effect is still not completely understood, which could contribute to issues in future studies and therapeutics. Stem cells have been considered as a treatment for a variety of CVD because of their plasticity and ability to assist in regeneration.77 So far, research and clinical trials have been inconclusive,78,79 but the emerging stem cell MVs have a new potential still not completely understood. The use of stem cell MVs as biomarkers of CVD is promising; however, there is still a need for large randomized clinical trials. There are currently trials ongoing for human beta cells MVs relative to type I diabetes, but there is a lack of trials corresponding to stem cells MVs in CVDs.17
Conclusions:
Stem cell derived microvesicles signaling mechanism plays a significant role in cellular communication and homeostasis of the stem cell niches in the heart and vasculature. The regulation of these MVs could be a potentially beneficial strategy in identifying and treating a variety of CVDs; however, the mechanism, off-target effects, and isolation protocol for stem cell MVs are still not fully elucidated. Attaining an enhanced and comprehensive understanding of the function of these stem cell derived MVs could significantly augment the stem cell field in the treatment of CVDs in the future. Moreover, with more available literature on microvesicles biogenesis and functions, there are endless opportunities to manipulate their contents to further advance their therapeutic application. The potential of using microvesicles as a therapeutic platform is clearly demonstrated and the possibilities are infinite.
Acknowledgments:
Figures were created using Servier Medical Art templates. Image colors were altered during figure preparation. All templates are licensed under the Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
Sources of Funding: SM is supported by funding from NIH (R56-HL137850, RO1-HL137850) and (SDG-15SDG25550038) from American Heart Association.
Abbreviations
- CVD
Cardiovascular Disease
- EV
Extracellular Vesicles
- MV
Microvesicles
- MVE
multivesicular endosomes
- PS
Phosphatidylserine
- PE
phosphatidylethanolamine
- HSC
hematopoietic stem cell
- MSC
mesenchymal stem cell
- iPSC
induced pluripotent stem cell
- EPC
endothelial progenitor cell
- CPC
cardiac progenitor cell
- ESC
embryonic stem cell
- ASC
adipose derived stem cell
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
- EGF
epidermal growth factor
- PDGF
platelet-derived growth factor
Footnotes
Statistical Analysis of Data
Not applicable
Conflict of Interest
There is no conflict of interest.
Financial Disclosure: None
References
- (1).The Use of Stem Cells for Treatment of Diseases https://kids.frontiersin.org/article/10.3389/frym.2017.00009 (accessed Apr 3, 2020).
- (2).Cardiovascular diseases (CVDs) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed Feb 17, 2020).
- (3).Roth GA; Johnson C; Abajobir A; Abd-Allah F; Abera SF; Abyu G; Ahmed M; Aksut B; Alam T; Alam K; Alla F; Alvis-Guzman N; Amrock S; Ansari H; Ärnlöv J; Asayesh H; Atey TM; Avila-Burgos L; Awasthi A; Banerjee A; Barac A; Bärnighausen T; Barregard L; Bedi N; Belay Ketema E; Bennett D; Berhe G; Bhutta Z; Bitew S; Carapetis J; Carrero JJ; Malta DC; Castañeda-Orjuela CA; Castillo-Rivas J; Catalá-López F; Choi J-Y; Christensen H; Cirillo M; Cooper L; Criqui M; Cundiff D; Damasceno A; Dandona L; Dandona R; Davletov K; Dharmaratne S; Dorairaj P; Dubey M; Ehrenkranz R; El Sayed Zaki M; Faraon EJA; Esteghamati A; Farid T; Farvid M; Feigin V; Ding EL; Fowkes G; Gebrehiwot T; Gillum R; Gold A; Gona P; Gupta R; Habtewold TD; Hafezi-Nejad N; Hailu T; Hailu GB; Hankey G; Hassen HY; Abate KH; Havmoeller R; Hay SI; Horino M; Hotez PJ; Jacobsen K; James S; Javanbakht M; Jeemon P; John D; Jonas J; Kalkonde Y; Karimkhani C; Kasaeian A; Khader Y; Khan A; Khang Y-H; Khera S; Khoja AT; Khubchandani J; Kim D; Kolte D; Kosen S; Krohn KJ; Kumar GA; Kwan GF; Lal DK; Larsson A; Linn S; Lopez A; Lotufo PA; El Razek HMA; Malekzadeh R; Mazidi M; Meier T; Meles KG; Mensah G; Meretoja A; Mezgebe H; Miller T; Mirrakhimov E; Mohammed S; Moran AE; Musa KI; Narula J; Neal B; Ngalesoni F; Nguyen G; Obermeyer CM; Owolabi M; Patton G; Pedro J; Qato D; Qorbani M; Rahimi K; Rai RK; Rawaf S; Ribeiro A; Safiri S; Salomon JA; Santos I; Santric Milicevic M; Sartorius B; Schutte A; Sepanlou S; Shaikh MA; Shin M-J; Shishehbor M; Shore H; Silva DAS; Sobngwi E; Stranges S; Swaminathan S; Tabarés-Seisdedos R; Tadele Atnafu N; Tesfay F; Thakur JS; Thrift A; Topor-Madry R; Truelsen T; Tyrovolas S; Ukwaja KN; Uthman O; Vasankari T; Vlassov V; Vollset SE; Wakayo T; Watkins D; Weintraub R; Werdecker A; Westerman R; Wiysonge CS; Wolfe C; Workicho A; Xu G; Yano Y; Yip P; Yonemoto N; Younis M; Yu C; Vos T; Naghavi M; Murray C Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol 2017, 70 (1), 1–25. 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mensah GA; Roth GA; Fuster V The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol 2019, 74 (20), 2529–2532. 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- (5).Borhani S; Hassanajili S; Ahmadi Tafti SH; Rabbani S Cardiovascular Stents: Overview, Evolution, and next Generation. Prog. Biomater 2018, 7, 175–205. 10.1007/s40204-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Celle TD; Cleutjens JP; Blankesteijn WM; Debets JJ; Smits JF; Janssen BJ Long-Term Structural and Functional Consequences of Cardiac Ischaemia-Reperfusion Injury in Vivo in Mice: Cardiac Ischaemia-Reperfusion Injury. Exp. Physiol 2004, 89 (5), 605–615. 10.1113/expphysiol.2004.027649. [DOI] [PubMed] [Google Scholar]
- (7).Leite CF; Almeida TR; Lopes CS; Dias da Silva VJ Multipotent Stem Cells of the Heart—Do They Have Therapeutic Promise? Front. Physiol 2015, 6 10.3389/fphys.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Laflamme MA; Murry CE Heart Regeneration. Nature 2011, 473 (7347), 326–335. 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ludke A; Li R-K; Weisel RD The Rejuvenation of Aged Stem Cells for Cardiac Repair. Can. J. Cardiol 2014, 30 (11), 1299–1306. 10.1016/j.cjca.2014.03.021. [DOI] [PubMed] [Google Scholar]
- (10).Sun R; Li X; Liu M; Zeng Y; Chen S; Zhang P Advances in Stem Cell Therapy for Cardiovascular Disease (Review). Int. J. Mol. Med 2016, 38 (1), 23–29. 10.3892/ijmm.2016.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mattsson J; Ringdén O; Storb R Graft Failure after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant 2008, 14 (1), 165–170. 10.1016/j.bbmt.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Barker RA; Carpenter MK; Forbes S; Goldman SA; Jamieson C; Murry CE; Takahashi J; Weir G The Challenges of First-in-Human Stem Cell Clinical Trials: What Does This Mean for Ethics and Institutional Review Boards? Stem Cell Rep. 2018, 10 (5), 1429–1431. 10.1016/j.stemcr.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Grivennikov IA Embryonic Stem Cells and the Problem of Directed Differentiation. Biochem. Biokhimiia 2008, 73 (13), 1438–1452. 10.1134/s0006297908130051. [DOI] [PubMed] [Google Scholar]
- (14).Fuhrmann G; Neuer AL; Herrmann IK Extracellular Vesicles – A Promising Avenue for the Detection and Treatment of Infectious Diseases? Eur. J. Pharm. Biopharm 2017, 118, 56–61. 10.1016/j.ejpb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- (15).Galieva LR; James V; Mukhamedshina YO; Rizvanov AA Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front. Neurosci 2019, 13 10.3389/fnins.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Tian J; Casella G; Zhang Y; Rostami A; Li X Potential Roles of Extracellular Vesicles in the Pathophysiology, Diagnosis, and Treatment of Autoimmune Diseases. Int. J. Biol. Sci 2020, 16 (4), 620–632. 10.7150/ijbs.39629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bei Y; Das S; Rodosthenous RS; Holvoet P; Vanhaverbeke M; Monteiro MC; Monteiro VVS; Radosinska J; Bartekova M; Jansen F; Li Q; Rajasingh J; Xiao J Extracellular Vesicles in Cardiovascular Theranostics. Theranostics 2017, 7 (17), 4168–4182. 10.7150/thno.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Amosse J; Martinez MC; Le Lay S Extracellular Vesicles and Cardiovascular Disease Therapy. Stem Cell Investig. 2017, 4 10.21037/sci.2017.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lawson C; Vicencio JM; Yellon DM; Davidson SM Microvesicles and Exosomes: New Players in Metabolic and Cardiovascular Disease. J. Endocrinol 2016, 228 (2), R57–R71. 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- (20).Zaldivia MTK; McFadyen JD; Lim B; Wang X; Peter K Platelet-Derived Microvesicles in Cardiovascular Diseases. Front. Cardiovasc. Med 2017, 4 10.3389/fcvm.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Martinez MC; Tual-Chalot S; Leonetti D; Andriantsitohaina R Microparticles: Targets and Tools in Cardiovascular Disease. Trends Pharmacol. Sci 2011, 32 (11), 659–665. 10.1016/j.tips.2011.06.005. [DOI] [PubMed] [Google Scholar]
- (22).Chong SY; Lee CK; Huang C; Ou YH; Charles CJ; Richards AM; Neupane YR; Pavon MV; Zharkova O; Pastorin G; Wang J-W Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci 2019, 20 (13), 3272 10.3390/ijms20133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Tricarico C; Clancy J; D’Souza-Schorey C Biology and Biogenesis of Shed Microvesicles. Small GTPases 2017, 8 (4), 220–232. 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Vajen T; Mause SF; Koenen RR Microvesicles from Platelets: Novel Drivers of Vascular Inflammation. Thromb. Haemost 2015, 114 (8), 228–236. 10.1160/TH14-11-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Badimon L; Suades R; Arderiu G; Peña E; Chiva-Blanch G; Padró T Microvesicles in Atherosclerosis and Angiogenesis: From Bench to Bedside and Reverse. Front. Cardiovasc. Med 2017, 4 10.3389/fcvm.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Tual-Chalot S; Leonetti D; Andriantsitohaina R; Martínez MC Microvesicles: Intercellular Vectors of Biological Messages. Mol. Interv 2011, 11 (2), 88–94. 10.1124/mi.11.2.5. [DOI] [PubMed] [Google Scholar]
- (27).Camussi G; Deregibus MC; Bruno S; Cantaluppi V; Biancone L Exosomes/Microvesicles as a Mechanism of Cell-to-Cell Communication. Kidney Int. 2010, 78 (9), 838–848. 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- (28).Raposo G; Stoorvogel W Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol 2013, 200 (4), 373–383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).György B; Szabó TG; Pásztói M; Pál Z; Misják P; Aradi B; László V; Pállinger É; Pap E; Kittel Á; Nagy G; Falus A; Buzás EI Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci 2011, 68 (16), 2667–2688. 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).EL Andaloussi S; Mäger I; Breakefield XO; Wood MJA Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov 2013, 12 (5), 347–357. 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- (31).Loyer Xavier; Vion Anne-Clémence; Tedgui Alain; Boulanger Chantal M. Microvesicles as Cell–Cell Messengers in Cardiovascular Diseases. Circ. Res 2014, 114 (2), 345–353. 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- (32).Xu X; Lai Y; Hua Z-C Apoptosis and Apoptotic Body: Disease Message and Therapeutic Target Potentials. Biosci. Rep 2019, 39 (1), BSR20180992 10.1042/BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fadeel B; Xue D The Ins and Outs of Phospholipid Asymmetry in the Plasma Membrane: Roles in Health and Disease. Crit. Rev. Biochem. Mol. Biol 2009, 44 (5), 264–277. 10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sabin K; Kikyo N Microvesicles as Mediators of Tissue Regeneration. Transl. Res 2014, 163 (4), 286–295. 10.1016/j.trsl.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Deregibus MC; Tetta C; Camussi G The Dynamic Stem Cell Microenvironment Is Orchestrated by Microvesicle-Mediated Transfer of Genetic Information. Histol. Histopathol 2010, 25 (3), 397–404. 10.14670/HH-25.397. [DOI] [PubMed] [Google Scholar]
- (36).Ferraro F; Celso CL; Scadden D Adult Stem Cels and Their Niches. In The Cell Biology of Stem Cells; Meshorer E, Plath K, Eds.; Back N, Cohen IR, Lajtha A, Lambris JD, Paoletti R, Series Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, 2010; Vol. 695, pp 155–168. 10.1007/978-1-4419-7037-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pennings S; Liu KJ; Qian H The Stem Cell Niche: Interactions between Stem Cells and Their Environment. Stem Cells Int. 2018, 2018, 1–3. 10.1155/2018/4879379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Ratajczak J; Miekus K; Kucia M; Zhang J; Reca R; Dvorak P; Ratajczak MZ Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20 (5), 847–856. 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- (39).Kishore R; Khan M More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ. Res 2016, 118 (2), 330–343. 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Jadczyk T; Faulkner A; Madeddu P Stem Cell Therapy for Cardiovascular Disease: The Demise of Alchemy and Rise of Pharmacology: Cardiovascular Cell Therapy. Br. J. Pharmacol 2013, 169 (2), 247–268. 10.1111/j.1476-5381.2012.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Laurenzana I; Lamorte D; Trino S; De Luca L; Ambrosino C; Zoppoli P; Ruggieri V; Del Vecchio L; Musto P; Caivano A; Falco G Extracellular Vesicles: A New Prospective in Crosstalk between Microenvironment and Stem Cells in Hematological Malignancies https://www.hindawi.com/journals/sci/2018/9863194/ (accessed Apr 9, 2020). 10.1155/2018/9863194. [DOI] [PMC free article] [PubMed]
- (42).Aliotta JM; Pereira M; Johnson KW; de Paz N; Dooner MS; Puente N; Ayala C; Brilliant K; Berz D; Lee D Microvesicle Entry into Marrow Cells Mediates Tissue-Specific Changes in MRNA by Direct Delivery of MRNA and Induction of Transcription. Exp. Hematol 2010, 38 (3), 233–245. 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zhao L; Hu C; Zhang P; Jiang H; Chen J Genetic Communication by Extracellular Vesicles Is an Important Mechanism Underlying Stem Cell-Based Therapy-Mediated Protection against Acute Kidney Injury. Stem Cell Res. Ther 2019, 10 (1), 119 10.1186/s13287-019-1227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Lee Y; EL Andaloussi S; Wood MJA Exosomes and Microvesicles: Extracellular Vesicles for Genetic Information Transfer and Gene Therapy. Hum. Mol. Genet 2012, 21 (R1), R125–R134. 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- (45).Théry C; Ostrowski M; Segura E Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol 2009, 9 (8), 581–593. 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- (46).Ratajczak J; Miekus K; Kucia M; Zhang J; Reca R; Dvorak P; Ratajczak MZ Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20 (5), 847–856. 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- (47).Haider KH; Aramini B Mircrining the Injured Heart with Stem Cell-Derived Exosomes: An Emerging Strategy of Cell-Free Therapy. Stem Cell Res. Ther 2020, 11 (1), 23 10.1186/s13287-019-1548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Collino F; Deregibus MC; Bruno S; Sterpone L; Aghemo G; Viltono L; Tetta C; Camussi G Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of MiRNAs. PloS One 2010, 5 (7), e11803 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kang T; Jones TM; Naddell C; Bacanamwo M; Calvert JW; Thompson WE; Bond VC; Chen YE; Liu D Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of MiRNA-31. STEM CELLS Transl. Med 2016, 5 (4), 440–450. 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wang Y; Zhang L; Li Y; Chen L; Wang X; Guo W; Zhang X; Qin G; He S; Zimmerman A; Liu Y; Kim I; Weintraub NL; Tang Y Exosomes/Microvesicles from Induced Pluripotent Stem Cells Deliver Cardioprotective MiRNAs and Prevent Cardiomyocyte Apoptosis in the Ischemic Myocardium. Int. J. Cardiol 2015, 192, 61–69. 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Belting M; Christianson HC Role of Exosomes and Microvesicles in Hypoxia-Associated Tumour Development and Cardiovascular Disease. J. Intern. Med 2015, 278 (3), 251–263. 10.1111/joim.12393. [DOI] [PubMed] [Google Scholar]
- (52).Bobis-Wozowicz S; Kmiotek K; Sekula M; Kedracka-Krok S; Kamycka E; Adamiak M; Jankowska U; Madetko-Talowska A; Sarna M; Bik-Multanowski M; Kolcz J; Boruczkowski D; Madeja Z; Dawn B; Zuba-Surma EK Human Induced Pluripotent Stem Cell-Derived Microvesicles Transmit RNAs and Proteins to Recipient Mature Heart Cells Modulating Cell Fate and Behavior. STEM CELLS 2015, 33 (9), 2748–2761. 10.1002/stem.2078. [DOI] [PubMed] [Google Scholar]
- (53).Jung J-H; Fu X; Yang PC Exosomes Generated from IPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases. Circ. Res 2017, 120 (2), 407–417. 10.1161/CIRCRESAHA.116.309307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Zhou J; Ghoroghi S; Benito-Martin A; Wu H; Unachukwu UJ; Einbond LS; Guariglia S; Peinado H; Redenti S Characterization of Induced Pluripotent Stem Cell Microvesicle Genesis, Morphology and Pluripotent Content. Sci. Rep 2016, 6 (1), 19743 10.1038/srep19743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Martínez MC; Larbret F; Zobairi F; Coulombe J; Debili N; Vainchenker W; Ruat M; Freyssinet J-M Transfer of Differentiation Signal by Membrane Microvesicles Harboring Hedgehog Morphogens. Blood 2006, 108 (9), 3012–3020. 10.1182/blood-2006-04-019109. [DOI] [PubMed] [Google Scholar]
- (56).Danielson KM; Das S Extracellular Vesicles in Heart Disease: Excitement for the Future? Exosomes Microvesicles 2014, 2 (1). 10.5772/58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lazar E; Benedek T; Korodi S; Rat N; Lo J; Benedek I Stem Cell-Derived Exosomes - an Emerging Tool for Myocardial Regeneration. World J. Stem Cells 2018, 10 (8), 106–115. 10.4252/wjsc.v10.i8.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ramachandran S; Lowenthal A; Ritner C; Lowenthal S; Bernstein HS Plasma Microvesicle Analysis Identifies MicroRNA 129–5p as a Biomarker of Heart Failure in Univentricular Heart Disease. PLOS ONE 2017, 12 (8), e0183624 10.1371/journal.pone.0183624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Glembotski Christopher C Expanding the Paracrine Hypothesis of Stem Cell–Mediated Repair in the Heart. Circ. Res 2017, 120 (5), 772–774. 10.1161/CIRCRESAHA.116.310298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wagner MJ; Khan M; Mohsin S Healing the Broken Heart; The Immunomodulatory Effects of Stem Cell Therapy. Front. Immunol 2020, 11 10.3389/fimmu.2020.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Adamiak Marta; Cheng Guangming; Bobis-Wozowicz Sylwia; Zhao Lin; Kedracka-Krok Sylwia; Samanta Anweshan; Karnas Elzbieta; Xuan Yu-Ting; Skupien-Rabian Bozena; Chen Xing; Jankowska Urszula; Girgis Magdy; Sekula Malgorzata; Davani Arash; Lasota Slawomir; Vincent Robert J.; Sarna Michal; Newell Kathy L.; Wang Ou-Li; Dudley Nathaniel; Madeja Zbigniew; Dawn Buddhadeb; Zuba-Surma Ewa K Induced Pluripotent Stem Cell (IPSC)–Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than IPSCs. Circ. Res 2018, 122 (2), 296–309. 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yuan Y; Du W; Liu J; Ma W; Zhang L; Du Z; Cai B Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front. Pharmacol 2018, 9, 547 10.3389/fphar.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Kim H-S; Choi D-Y; Yun SJ; Choi S-M; Kang JW; Jung JW; Hwang D; Kim KP; Kim D-W Proteomic Analysis of Microvesicles Derived from Human Mesenchymal Stem Cells. J. Proteome Res 2012, 11 (2), 839–849. 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- (64).Bruno S; Grange C; Deregibus MC; Calogero RA; Saviozzi S; Collino F; Morando L; Busca A; Falda M; Bussolati B; Tetta C; Camussi G Mesenchymal Stem Cell-Derived Microvesicles Protect Against Acute Tubular Injury. J. Am. Soc. Nephrol 2009, 20 (5), 1053–1067. 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Cha JM; Shin EK; Sung JH; Moon GJ; Kim EH; Cho YH; Park HD; Bae H; Kim J; Bang OY Efficient Scalable Production of Therapeutic Microvesicles Derived from Human Mesenchymal Stem Cells. Sci. Rep 2018, 8 (1), 1–16. 10.1038/s41598-018-19211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Bian X; Ma K; Zhang C; Fu X Therapeutic Angiogenesis Using Stem Cell-Derived Extracellular Vesicles: An Emerging Approach for Treatment of Ischemic Diseases. Stem Cell Res. Ther 2019, 10 (1), 158 10.1186/s13287-019-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ranghino A; Cantaluppi V; Grange C; Vitillo L; Fop F; Biancone L; Deregibus MC; Tetta C; Segoloni GP; Camussi G Endothelial Progenitor Cell-Derived Microvesicles Improve Neovascularization in a Murine Model of Hindlimb Ischemia. Int. J. Immunopathol. Pharmacol 2012, 25 (1), 75–85. 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- (68).Deregibus MC; Cantaluppi V; Calogero R; Lo Iacono M; Tetta C; Biancone L; Bruno S; Bussolati B; Camussi G Endothelial Progenitor Cell–Derived Microvesicles Activate an Angiogenic Program in Endothelial Cells by a Horizontal Transfer of MRNA. Blood 2007, 110 (7), 2440–2448. 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- (69).Deregibus MC; Cantaluppi V; Calogero R; Lo Iacono M; Tetta C; Biancone L; Bruno S; Bussolati B; Camussi G Endothelial Progenitor Cell–Derived Microvesicles Activate an Angiogenic Program in Endothelial Cells by a Horizontal Transfer of MRNA. Blood 2007, 110 (7), 2440–2448. 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- (70).Bian S; Zhang L; Duan L; Wang X; Min Y; Yu H Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J. Mol. Med 2014, 92 (4), 387–397. 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- (71).Ren S; Chen J; Duscher D; Liu Y; Guo G; Kang Y; Xiong H; Zhan P; Wang Y; Wang C; Machens H-G; Chen Z Microvesicles from Human Adipose Stem Cells Promote Wound Healing by Optimizing Cellular Functions via AKT and ERK Signaling Pathways. Stem Cell Res. Ther 2019, 10 (1), 47 10.1186/s13287-019-1152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Dougherty JA; Mergaye M; Kumar N; Chen C-A; Angelos MG; Khan M Potential Role of Exosomes in Mending a Broken Heart: Nanoshuttles Propelling Future Clinical Therapeutics Forward https://www.hindawi.com/journals/sci/2017/5785436/ (accessed Mar 31, 2020). 10.1155/2017/5785436. [DOI] [PMC free article] [PubMed]
- (73).Gnecchi M; He H; Noiseux N; Liang OD; Zhang L; Morello F; Mu H; Melo LG; Pratt RE; Ingwall JS; Dzau VJ Evidence Supporting Paracrine Hypothesis for Akt-Modified Mesenchymal Stem Cell-Mediated Cardiac Protection and Functional Improvement. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 2006, 20 (6), 661–669. 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- (74).Jalnapurkar S; Moirangthem RD; Singh S; Limaye L; Kale V Microvesicles Secreted by Nitric Oxide-Primed Mesenchymal Stromal Cells Boost the Engraftment Potential of Hematopoietic Stem Cells. Stem Cells Dayt. Ohio 2019, 37 (1), 128–138. 10.1002/stem.2912. [DOI] [PubMed] [Google Scholar]
- (75).Akao Y; Iio A; Itoh T; Noguchi S; Itoh Y; Ohtsuki Y; Naoe T Microvesicle-Mediated RNA Molecule Delivery System Using Monocytes/Macrophages. Mol. Ther 2011, 19 (2), 395–399. 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Chen Y; Li G; Liu M-L Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics Proteomics Bioinformatics 2018, 16 (1), 50–62. 10.1016/j.gpb.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Abbott JD; Giordano FJ Stem Cells and Cardiovascular Disease. J. Nucl. Cardiol 2003, 10 (4), 403–412. 10.1016/S1071-3581(03)00580-4. [DOI] [PubMed] [Google Scholar]
- (78).LaPar DJ; Kron IL; Yang Z Stem Cell Therapy for Ischemic Heart Disease: Where Are We? Curr. Opin. Organ Transplant 2009, 14 (1), 79–84. 10.1097/MOT.0b013e328320d2e2. [DOI] [PubMed] [Google Scholar]
- (79).Mathur A; Martin J Stem Cells and Repair of the Heart. The Lancet 2004, 364 (9429), 183–192. 10.1016/S0140-6736(04)16632-4. [DOI] [PubMed] [Google Scholar]
- (80).Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6(3):312–321. doi: 10.4252/wjsc.v6.i3.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.() Shukla L, Yuan Y, Shayan R, Greening DW, Karnezis T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front Pharmacol. 2020;11. doi: 10.3389/fphar.2020.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Kim HJ, Park J-S. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev Reprod. 2017;21(1):1–10. doi: 10.12717/DR.2017.21.1.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Barreto S, Hamel L, Schiatti T, Yang Y, George V. Cardiac Progenitor Cells from Stem Cells: Learning from Genetics and Biomaterials. Cells. 2019;8(12). doi: 10.3390/cells8121536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Bui QT, Gertz ZM, Wilensky RL. Intracoronary delivery of bone-marrow-derived stem cells. Stem Cell Res Ther. 2010;1(4):29. doi: 10.1186/scrt29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA [DOI] [PubMed] [Google Scholar]
- (87).Norol F, Merlet P, Isnard R, et al. Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood. 2003;102(13):4361–4368. doi: 10.1182/blood-2003-03-0685 [DOI] [PubMed] [Google Scholar]
- (88).Archundia A, Aceves JL, López-Hernández M, et al. Direct cardiac injection of G-CSF mobilized bone-marrow stem-cells improves ventricular function in old myocardial infarction. Life Sci. 2005;78(3):279–283. doi: 10.1016/j.lfs.2005.04.080 [DOI] [PubMed] [Google Scholar]
- (89).Rj L, M P, M R, et al. Transendocardial and transepicardial intramyocardial fibroblast growth factor-2 administration: myocardial and tissue distribution. Drug Metab Dispos Biol Fate Chem. 2005;33(8):1101–1107. doi: 10.1124/dmd.104.002774 [DOI] [PubMed] [Google Scholar]
- (90).Retrograde delivery of stem cells: promising delivery strategy for myocardial regenerative therapy - Wu - 2011 - Clinical Transplantation - Wiley Online Library. Accessed August 28, 2020 https://onlinelibrary.wiley.com/doi/full/10.1111/j.1399-0012.2011.01508.x [DOI] [PubMed]


