Abstract
Objective
Adults with radiographic knee OA (rKOA) are at increased risk of mortality and walking difficulty may modify this relation. Little is known about specific aspects of walking difficulty that increase mortality risk. We investigated the association of walking speed (objective measure of walking difficulty) with mortality and examined the threshold that best discriminated this risk in adults with rKOA.
Methods
Participants with rKOA from the Johnston County Osteoarthritis Project (JoCoOA, longitudinal population-based cohort), Osteoarthritis Initiative and Multicenter Osteoarthritis Study (OAI and MOST, cohorts of individuals with or at high risk of knee OA) were included. Baseline speed was measured via 2.4-meter (m) walk test (short-distance) in JoCoOA and 20-m walk test (standard-distance) in OAI and MOST. To examine the association of walking speed with mortality risk over nine years, hazard ratios (aHR) and 95% confidence intervals (CI) were calculated from Cox regression models adjusted for potential confounders., A Maximal Likelihood Ratio Chi-square Approach was utilized to identify an optimal threshold of walking speed predictive of mortality.
Results
Deaths after 9 years of follow-up occurred in 23.3% (290/1244) of JoCoOA and 5.9% (249/4215) of OAI+MOST. Walking 0.2 meters/second slower during short- and standard-distance walk tests was associated with 23% (aHR[95%CI]; 1.23[1.10, 1.39]) and 25% (1.25[1.09, 1.43]) higher mortality risk, respectively. Walking <0.5 meters/second on short-distance and <1.2 meters/second standard-distance walk tests, best discriminated those with and without mortality risk.
Conclusion
Slower walking speed measured via short- and standard-distance walk tests was associated with increased mortality risk in adults with rKOA.
Keywords: Gait speed, Arthritis, Death, Physical Function, Performance-based measures
Introduction
Knee osteoarthritis (OA) is a significant public health problem in older adults. Over 250 million adults worldwide have OA1, and OA is a leading cause of functional limitation, such as difficulty walking2–5. Further, as age advances, the risk of and the adverse health consequences related to OA increases6. Previous population based studies in United States, United Kingdom and China have shown that adults with knee OA are at increased risk for all-cause mortality7–10. Among adults with knee OA, those who self-report difficulty walking have a 51% higher risk for all-cause mortality compared to those with no difficulty11. Characterizing the severity of difficulty walking is important to predict health outcomes, and can be objectively performed with walking speed (i.e., dividing distance walked by time). Slow walking measured over standard distances, e.g., 20 meters, is associated with an increased risk of all-cause mortality and other markers of health in well-functioning older adults12–15. Consequently, walking speed is a useful clinical vital sign of health14,15, and could be assessed regularly in clinical settings15.
Measuring speed requires walking over a set distance. However, the distance for a walk test is often chosen based on space availability at a clinic as there is no standard distance established to measure walking speed. Shorter distance tests do influence walk speed16. For example, one to two meters are needed for acceleration when starting to walk, which occupies ∼5% to 10% of the total distance traveled during a standard 20-meter walk. However, during a short 4-meter walk test, the acceleration phase occupies roughly 25% to 50% of the distance traveled, which may influence the measured walking speed. Hence, the time it takes to get up to a comfortable walking speed occupies a higher proportion of the short walk test than a 20-meter walk test. At present, it is unclear if walking speed measured using a short-distance walk test is associated with risk for all-cause mortality in adults with radiographic knee OA.
Additionally, it is unclear if there is an optimal threshold of walking speed measured from short-distance or standard-distance walk test that best discriminates those with and without the risk of all-cause mortality among adults with radiographic knee OA. We employed a novel technique called the Maximal Likelihood Ratio Chi-square Approach17 for identifying thresholds that best discriminate the risk of mortality in adults with radiographic knee OA. This approach is comparable to a receiver operating characteristic (ROC) curve, though this approach accounts for time-to-event and censoring, i.e., duration of time to death and dropouts.
The overall aim of this study was to investigate the association of walking speed with the risk of all-cause mortality in adults with radiographic knee OA from three large prospective observational cohort studies. We specifically aimed to investigate the association of walking speed measured from a (i) short-distance test using the data from the Johnston County Osteoarthritis Project (JoCoOA) and (ii) standard-distance test using the data from the Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis Study (MOST). Based on previous literature,14,15 we hypothesized that slower walking speed regardless of whether being measured using short or standard-distance tests will be associated with increased mortality risk. We also calculated optimal thresholds of walking speed specific to each test that discriminated those with and without mortality risk. This investigation is important as it provides valuable information that can be applied clinically for short- and standard-distance tests to identify the risk of mortality among adults with radiographic knee OA.
Methods
Study participants
We used data from three large prospective observational cohort studies, i.e., the Johnston County Osteoarthritis Project (JoCoOA), Osteoarthritis Initiative (OAI), and Multicenter Osteoarthritis Study (MOST). In the JoCoOA, participants were not selected based on disease status, while participants in the OAI and MOST were either at risk for or had knee OA. However, in all three cohort studies, participants were community-dwelling adults who had knee radiographs were taken at baseline. For the purpose of this study, we only included participants with radiographic knee OA defined as Kellgren–Lawrence grade ≥ 2 on x-ray in one or both knees at baseline.
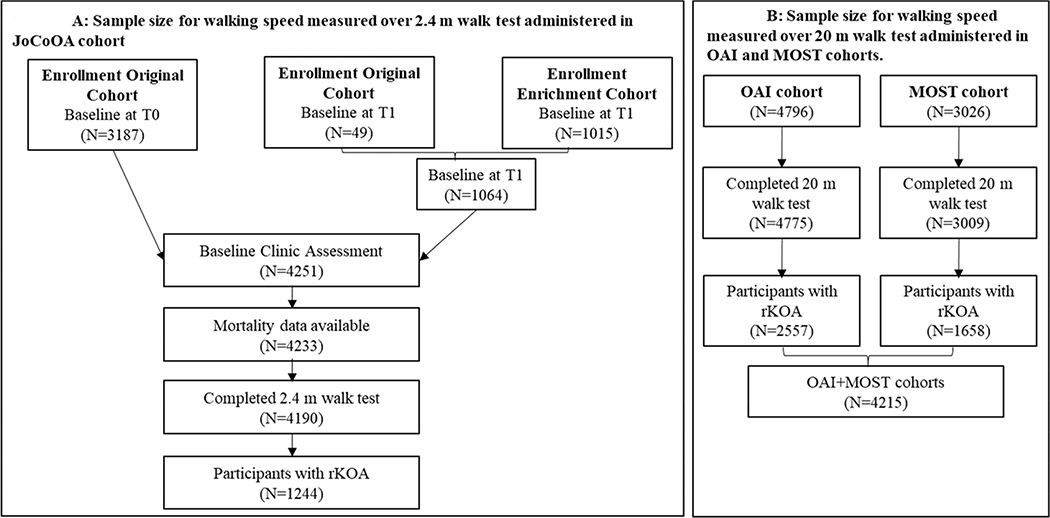
Detailed descriptions of JoCoOA7,18, OAI19, and MOST20 eligibility criteria have been published elsewhere. Briefly, the JoCoOA enrolled residents aged 45 years or older from one of six townships in Johnston County, North Carolina, using a population-based sampling strategy. The OAI recruited adults aged 45–79 years from clinical sites located in Baltimore, Maryland; Pittsburgh, Pennsylvania; Pawtucket, Rhode Island; and Columbus, Ohio. MOST recruited adults aged 50–79 years from Birmingham, Alabama; and Iowa City, Iowa. Study protocols were approved by the relevant institutional review boards. All participants provided written informed consent to participate before enrollment. Figure 1A–B provides a summary of how the final analytic sample was obtained. The current analysis for walking speed measured using a short-distance walk test included data from participants enrolled in the JoCoOA who completed the baseline assessment of walking speed. Specifically, the walking speed assessment was conducted during 1991–1998 for the original enrollment cohort and during 2003–2004 for the enrichment cohort (and for 49 original cohort participants who did not have the assessment at baseline (Figure 1a)). The current analysis for walking speed measured using standard-distance walk test included data from participants who completed the baseline assessment of walking speed conducted between 2004–2006 in the OAI study and 2003–2005 in the MOST study (Figure 1b).
Figure 1.
A–B. Analytic sample size for walking speed measured over a 2.4-m (short-distance) walk test (A) and a 20-m (standard-distance) walk test (B)
rKOA=radiographic knee osteoarthritis
Study Outcome
All-cause mortality
In the JoCoOA, time to all-cause mortality was quantified from the baseline visit (conducted between 1991–1998 or 2003–2004) to the date of death. The date and cause of death were ascertained using the National Death Index (NDI). Additionally, known deaths not found through NDI records, but confirmed through local vital records searches from the Johnston County Register of Deeds office were included. The local vital records search provides information regarding deaths in Johnston County. Follow-up time was calculated from baseline assessment until death, or until administrative censoring, which took place when a participant reached the end of the study period (December 31, 2015). The total follow-up period for the JoCoOA was more than 24 years. However, for the purpose of this analysis, the total follow-up time of the sample was truncated to 9 years to be consistent with the average follow-up time for the OAI and MOST studies.
In the OAI, time to all-cause mortality was quantified from the baseline visit (conducted between 2004–2006) to the date of death. The date of death was confirmed through obituary or death certificates by the OAI study team, when available. Follow-up time was calculated from baseline assessment until death, or until administrative censoring, which took place when a participant reached the end of the study period (March 31, 2017). The average time of follow-up was 10.2 years for the entire OAI sample.
In the MOST, the date of death and assessment visits were not available because the consent of releasing these data were not taken from the study participants. However, the time to all-cause mortality was provided in the requested dataset by the MOST study team, and it was quantified in months from the baseline visit (conducted between 2003–2005) to the date of death. The deaths of study participants were confirmed through obituary or death certificates by the MOST study team, when available. Follow-up time was calculated from baseline assessment until death, or until administrative censoring, which took place when a participant reached the end of the study period (i.e., 84-month clinic visit). The average time of follow-up was 7.3 years for the entire MOST sample.
For this study, data from the OAI and MOST were combined, given the method used to quantify walking speed was the same. A similar approach has been used by other studies where the data from OAI and MOST were combined together, given the same assessment tools were used to measure a construct21. Therefore, the average time of the follow-up for the OAI and MOST sample together was 9.1 years. Further, there was almost no lost-to follow-up because ascertainment of death by the National Death Index (JoCoOA) and by obituary and death certificate (OAI and MOST studies) was complete and accurate.
Study exposure
Short-distance walk test
In the JoCoOA, a 2.4-meter (m) (short-distance) walk test was used to calculate walking speed during the baseline visit for both the original and enrichment enrollment cohorts. During the walk test, the participants were instructed to walk at their usual speed over a marked 2.4 m course in an unobstructed and dedicated room. The participants did not have the room to walk beyond the 2.4 m mark. Walking time was assessed with a digital stopwatch and recorded in seconds to the nearest tenth of second in two trials over a 2.4 m distance. The two trials were averaged, and walking speed in meters/second (m/s) was calculated as the total distance (2.4 m) divided by the total average time to complete the walk test. The 2.4-m walk test has fair to good test-retest reliability (intraclass correlation coefficients > 0.5) for measuring walking speed in older adults22,23.
Standard-distance walk test
In the OAI and MOST, the 20-m (standard-distance) walk test was used to calculate walking speed during the baseline visit. During the walk test, the participants were instructed to walk at their usual speed over a marked 20-m course in an unobstructed and dedicated corridor. Participants were allowed to walk (i.e., could take three more steps) after they crossed the 20-m mark. A digital stopwatch was used to record the time to complete the test. The timing began at the initial movement from standing at the start and stopped when they crossed the 20-m mark. Walking speed in m/s was calculated by dividing the total distance (20 meters) by the total time to complete the walk test (seconds). The 20-m walk test has high test-retest reliability (intraclass correlation coefficients > 0.9 or Spearman correlation co-efficient > 0.8) for measuring walking speed in adults with knee OA24–26.
Potential confounders
We considered the following baseline factors as potential confounders based on their association with walking speed and all-cause mortality13,27–33: age, sex (female versus male), race/ethnicity (white versus non-white), education (less than college graduate versus at least college graduate), body mass index (BMI, kg/m2) computed from weight and height assessment, comorbidity measured using the modified Charlson comorbidity index34, depressive symptoms measured using the Center for Epidemiologic Studies Depression Scale (≥ 16 versus <16)35, and symptomatic knee OA, which was defined as the presence of knee pain, aching or stiffness on most days in the past month during the previous year in either right or left knee and presence of concomitant radiographic knee OA. These factors were ascertained at the study enrollment by interview, questionnaire, and/or direct measurement, as appropriate.
Statistical Analysis
We described the study sample by calculating means and standard deviations for continuous variables and percentages for categorical variables. After testing the proportional hazards assumptions were met using the Supremum Test, we examined the association of walking speed with all-cause mortality over nine years by calculating hazard ratios (HR) and 95% confidence intervals (95%CI) from the Cox regression model, which was adjusted for potential confounders (aHR). The standard error of measurement (SEM) [SEM = Standard deviation × √(1-reliability)] for walking speed measured using short-distance walk test was 0.16 m/s (SEM = 0.22 × √(1–0.5)). To account for SEM for short-distance walk test, we calculated the aHR per 0.2 m/s change in walking speed with mortality risk. We used the same interval for the change in walking speed measured using standard-distance walk test to ensure the consistency.
Sima and Gönen17 considered several techniques modifying ROC-based methods and test-based methods for investigating thresholds predictive of a time to event outcome17. Accounting for time-to-event and for censoring provides critical information about the time at risk, especially when the outcomes are measured at different time points. For example, the walking speed threshold for survival time longer than four years may be different from survival time longer than seven years. Therefore, prior approaches to identify optimal thresholds for uncensored binary outcomes needed modification. Based on their simulation studies, Sima and Gönen17 recommended the use of an approach maximizing the likelihood ratio test for the selection of the optimal threshold. Therefore, we used the maximal Chi-square method to identify the optimal threshold of walking speed that predicted the risk of all-cause mortality17. Specifically, we ran unadjusted Cox models for different thresholds of walking speed. We then identified the model that gave the maximal Chi-Square value. This method maximizes the concordance between walking speed and mortality risk, which is a metric used to evaluate the performance of the thresholds when there are censored endpoints. This method is similar to maximizing the Youden index, a metric employed when using a ROC method. We also calculated the proportion of those at risk of mortality, and the sensitivity, specificity, and positive and negative likelihood ratios of the thresholds for walking speed from short-distance and standard-distance walk tests.
We ran separate analyses for short- and standard-distance walk tests. To investigate the stability of the study findings for the standard-distance walk test, we ran separate analyses using OAI and MOST cohorts either independently or combined into one sample. An individual patient data meta-analysis accounting for clustering by the study of origin was carried out when using data was combined from both cohorts (OAI and MOST). To account for potential differences in baseline hazards for each cohort, a one-step stratified Cox regression model was used to examine the association of walking speed and mortality risk. Additionally, to identify the optimal threshold predictive of mortality risk in this sample, the likelihood ratio was obtained from a one-step stratified Cox regression model for different walking speed thresholds. Consequently, an optimal walking speed threshold corresponded to the one-step stratified Cox regression model that yielded the maximal likelihood ratio. All the analyses were conducted in SAS 9.4 (Statistical Analytical Software, Version 9.4, SAS institute, Cary, North Carolina, USA)
Results
Short-distance walk test
Of the 4251 participants enrolled in the JoCoOA, 1244 participants met study criteria by completing the 2.4-m walk test and had radiographic knee OA at the baseline visit. The average age was 65.2 ± 10.8 years (mean ± sd), BMI 31.9 ± 7.7 kg/m2, over half were women (63%), and were white (63%), and 6.7% were at least a college graduate. 23.4% of the analytic sample (n=290) died over nine years (Table 1).
Table 1:
Characteristics of study participants with radiographic knee OA (rKOA) enrolled in the Johnston County Osteoarthritis Project (JoCoOA), Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis Study (MOST), OAI only and MOST only cohorts.
| JoCoOA | OAI+MOST | OAI | MOST | |
|---|---|---|---|---|
| Total sample N | 1244 | 4215 | 2557 | 1658 |
| Age, years mean ±SD | 65.2±10.8 | 63.1±8.6 | 62.6±9.0 | 64.0±8.0 |
| Women % (n) | 66.8 (831) | 59.0 (2497) | 57.8 (1477) | 61.5 (1020) |
| Race, white % (n) | 63.0 (784) | 79.0 (3328) | 77.3 (1976) | 81.5 (1352) |
| Education, at least college graduate % (n) | 6.7 (83) | 50.6 (2134) | 57.2 (1463) | 40.5 (671) |
| aBMI, kg/m2 mean ±SD | 31.9±7.7 | 30.6±5.6 | 29.6±4.8 | 32.0±6.4 |
| bComorbidities mean ±SD | 1.4±1.4 | 0.5±0.9 | 0.4±0.8 | 0.6±1.0 |
| cPresence of depression % (n) | 13.3 (164) | 11.5 (484) | 9.9 (252) | 14.0 (232) |
| dPresence of symptomatic knee OA | 60.9 (757) | 52.5 (2212) | 54.1 (1384) | 49.9 (828) |
| Baseline speed, meters/second mean ±SD | 0.70±0.25 | 1.24±0.22 | 1.30±0.22 | 1.16±0.21 |
| Number of deaths % (n) | 23.4 (290) | 5.9 (249) | 6.5 (167) | 5.0 (82) |
| Time to deaths, years mean ±SD | 4.9±2.4 | 6.3±2.6 | 6.6±2.5 | 4.8±2.0 |
| Total follow-up time, years mean ±SD | 8.0±2.2 | 9.1±3.0 | 10.2±3.2 | 7.3±1.3 |
Note.
BMI=Body Mass Index
Comorbidities were measured using the modified Charlson comorbidity index
Participants were classified with depression present if the score on the Center for Epidemiologic Studies Depression Scale was ≥ 16
Participants were classified as symptomatic knee OA present if they reported the presence of knee pain, aching or stiffness on most days in the past month during the previous year in either knee and had rKOA in either knee.
Walking 0.2 m/s slower during the short-distance walk test was associated with an 23% (aHR 1.23, 95% CI [1.10, 1.39]) higher risk of mortality over nine years in the adults with radiographic knee OA (Table 2). Walking slower than 0.5 m/s on the short-distance walk test was an optimal threshold to best discriminate those with and without mortality risk in adults with radiographic knee OA since it yielded maximal chi-square value in unadjusted Cox models (Table 3). The optimal threshold, i.e., < 0.5 m/s, on a short-distance walk test yielded 84% specificity, 32% sensitivity and a negative likelihood ratio of 0.80, (95% CI [0.74, 0.87]).
Table 2:
Association of walking speed measured over a 2.4-m walk test in the Johnston County Osteoarthritis (JoCoOA; A) and a 20-m walk test in Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis Study (MOST; B), OAI only (C), and MOST only (D) with the risk of all-cause mortality over nine years among adults with radiographic knee osteoarthritis (rKOA)
| Test | Unadjusted HR [95%CI] | Adjusted HR [95%CI] |
|---|---|---|
| Walking 0.2 meters/second slower on | ||
| 2.4 m (short-distance) walk test | ||
| A) JoCoOA cohort | 1.40 [1.27, 1.55]* | a1.23 [1.10, 1.39]* |
| 20-m (standard-distance) walk test | ||
| B) OAI + MOST cohorts | b1.51 [1.35, 1.69]* | a,b1.25 [1.09, 1.43]* |
| C) OAI cohort only | 1.53 [1.33, .75]* | a1.32 [1.12, 1.55]* |
| D) MOST cohort only | 1.47 [1.22, 1.78]* | a1.11 [0.88, 1.40] |
Note.
Adjusted for sex, race, education, baseline age, body mass index, comorbidities, the presence of depression and symptomatic knee OA.
Cox model stratified by study origin
HR=hazard ratio, CI=confidence interval
Denotes statistical significance
Table 3:
Maximal Likelihood Ratio (LR) Chi-Square (χ2) Approachd to identify the optimal threshold of walking speed measured via a 2.4-m walk test in the Johnston County Osteoarthritis Project (JoCoOA; A) and a 20-m walk test in the Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis Study (MOST;B), OAI only (C) and MOST only (D) cohorts that predicted the risk of excess mortality and diagnostic evaluation of thresholds in adults with radiographic knee osteoarthritis (rKOA).
| Thresholds | Number of deaths/Total people (%) | aHR[95% CI] | bLR χ2 | Diagnostic Evaluation Value[95%CI] |
||||
|---|---|---|---|---|---|---|---|---|
| Walk <threshold | Walk ≥threshold | Sensitivity | Specificity | Negative LR | Positive LR | |||
| (A) 2.4-m (short-distance) walk test administered in the JoCoOA | ||||||||
| 0.4 m/s | 54/132 (40.9) | 236/1112 (21.2) | a1.69[1.23, 2.33] | 24.8 | 18.6[14.3, 23.6] | 91.8[89.9, 93.5] | 0.89[0.84, 0.94] | 2.3[1.7, 3.1] |
| e0.5 m/s | 94/243 (38.4) | 196/1001 (19.6) | a1.60[1.22, 2.11] | e38.0 | 32.4[27.1, 38.1] | 84.4[81.9, 86.6] | 0.80[0.74, 0.87] | 2.1[1.7, 2.6] |
| 0.6 m/s | 141/430 (32.3) | 149/814 (18.3) | a1.44[1.11, 1.88] | 31.9 | 48.6[42.7, 54.5] | 69.7[66.7, 72.6] | 0.74[0.65, 0.83] | 1.6[1.4, 1.9] |
| 0.7 m/s | 191/649 (29.4) | 99/595 (16.6) | a1.50[1.14, 1.97] | 30.0 | 65.9[60.1, 71.3] | 52.0[48.8, 55.2] | 0.66[0.55, 0.78] | 1.4[1.2, 1.5] |
| (B) 20-m (standard-distance) walk test administered in the OAI and MOST | ||||||||
| 1.0 m/s | 53/537 (9.9) | 196/3678 (5.3) | a,c1.42[1.00, 2.02] | 20.7 | 21.3[16.4, 26.9] | 87.8[86.7, 88.8] | 0.90[0.84, 0.96] | 1.7[1.4, 2.3] |
| 1.1 m/s | 101/1035 (9.8) | 148/3180 (4.7) | a,c1.76[1.31, 2.36] | 43.5 | 40.6[34.4, 46.9] | 76.5[75.1, 77.8] | 0.78[0.70, 0.86] | 1.7[1.5, 2.0] |
| e1.2 m/s | 153/1738 (8.8) | 96/2477 (3.9) | a,c1.96[1.47, 2.62] | e57.5 | 61.5[55.1, 67.5] | 60.0[58.5, 61.6] | 0.64[0.55, 0.75] | 1.5[1.4, 1.7] |
| 1.3 m/s | 186/2514 (7.4) | 63/1701 (3.7) | a,c1.61[1.18, 2.21] | 37.6 | 74.7[68.8, 80.0] | 58.7[57.2, 60.2] | 0.43[0.35, 0.53] | 1.8[1.7, 2.0] |
| (C) 20-m (standard-distance) walk test administered in the OAI only | ||||||||
| 1.0 m/s | 27/205 (13.2) | 140/2353 (6.0) | a1.71[1.08, 2.70] | 14.5 | 16.2[10.9, 22.6] | 92.6[91.4, 93.6] | 0.91[0.85, 0.97] | 2.2[1.5, 3.2] |
| 1.1 m/s | 51/438 (11.6) | 116/2119 (5.5) | a1.71[1.18, 2.48] | 22.0 | 30.5[23.7, 38.1] | 83.8[82.3, 85.3] | 0.83[0.75, 0.92] | 1.9[1.5, 2.4] |
| e1.2 m/s | 90/816 (11.0) | 77/1741 (4.4) | a2.05[1.46, 2.89] | e40.3 | 53.9[46.0, 61.6] | 69.6[67.7, 71.5] | 0.66[0.56, 0.78] | 1.8[1.5, 2.1] |
| 1.3 m/s | 116/1264 (9.2) | 51/1293 (3.9) | a1.78[1.25, 2.55] | 32.4 | 69.5[61.9, 76.3] | 52.0[49.9, 54.0] | 0.59[0.47, 0.74] | 1.5[1.3, 1.6] |
| (D) 20-m (standard-distance) walk test administered in the MOST only | ||||||||
| 1.0 m/s | 26/332 (7.8) | 56/1326 (4.2) | a1.09[0.63, 1.90] | 6.8 | 31.7[21.9, 42.9] | 80.6[78.5, 82.5] | 0.85[0.73, 0.98] | 1.6[1.2, 2.3] |
| e1.1 m/s | 50/597 (8.4) | 32/1061 (3.0) | a1.98[1.21, 3.25] | e22.1 | 61.0[49.6, 71.6] | 65.3[62.9, 67.6] | 0.60[0.45, 0.79] | 1.8[1.5, 2.1] |
| 1.2 m/s | 63/922 (6.8) | 19/736 (2.6) | a1.89[1.08, 3.32] | 17.2 | 76.8[66.2, 85.4] | 45.5[43.0, 48.0] | 0.51[0.34, 0.76] | 1.4[1.2, 1.6] |
| 1.3 m/s | 70/1250 (5.6) | 12/408 (2.9) | a1.25[0.65, 2.41] | 5.6 | 85.4[75.8, 92.2] | 25.1[23.0, 27.4] | 0.58[0.34, 0.99] | 1.1[1.0, 1.3] |
Note. m/s=meters/second
Adjusted for baseline age, body mass index, sex, race, education, comorbidities, depression (≤ vs. >16), and symptomatic knee OA (yes or no)
LR χ2 values are obtained from unadjusted Cox models for (A), (C) and (D) and from Cox model stratified by study orgin was used in (B)
Cox model stratified by study oirigin (OAI or MOST) was used when the data from OAI and MOST cohorts were combined into one sample
Approach states that higher chi-square values represent greater concordance between the threshold and mortality
Model that yielded a maximum χ2 value
aHR=adjusted hazard ratio, CI=confidence interval
Standard-distance walk test
Of the 7822 participants recruited for the OAI and MOST studies, 4215 participants met study inclusion criteria by completing the 20-m walk test and had radiographic knee OA at the baseline visit. The average age was 63.1 ± 8.6 years (mean ± sd), BMI 30.6 ± 5.6 kg/m2, over half were women (59%), the majority (79%) were white, and 51% were at least a college graduate. 5.9% of the analytic sample died (n=249) over nine years (Table 1). On average, participants in the OAI study were slightly younger, had a lower BMI, higher walking speed, lower proportion reported depression compared to those in the MOST study (Table 1).
Walking 0.2 m/s slower during the standard-distance walk test was associated with a 25% (aHR 1.25, 95% CI [1.09, 1.43]) higher risk of mortality over nine years in the adults with radiographic knee OA (Table 2). We found similar findings when we investigated this association in the OAI only. However, the association between walking speed and mortality was attenuated and less precise in the MOST only.
Walking slower than 1.2 m/s on a standard-distance walk test was an optimal threshold to best discriminate those with and without mortality risk in adults with radiographic knee OA. This threshold yielded the maximal chi-square value in the unadjusted Cox model stratified by study origin (Table 3). The optimal threshold, i.e., < 1.2 m/s, on a standard-distance walk test yielded 60% specificity and 62% sensitivity, and a negative likelihood ratio of 0.64, (95% CI [0.55, 0.75]). This optimal threshold was similar when examined using the data from the OAI cohort only. However, walking slower than 1.1 m/s on a standard-distance walk test was the optimal threshold that best discriminates those with and without mortality risk in adults with radiographic knee OA using the data from the MOST cohort only (Table 3).
Discussion
We found walking speed, irrespective of whether it was measured using short- or standard-distance walk tests, was associated with increased risk of all-cause mortality over nine years among adults with radiographic knee OA, after adjusting for potential confounders. Specifically, we found walking slower than 0.5 m/s on the short-distance and 1.2 m/s on the standard-distance walk tests were optimal thresholds that discriminated adults with radiographic knee OA with and without mortality risk over nine years.
We found that slow walking speed was strongly associated with all-cause mortality in adults with radiographic knee OA. This finding is consistent with previous studies showing that knee OA increases the risk of mortality by making walking difficult for adults11. Impairments in one or more body systems37,, including vision, lower extremity strength36,37, postural control37 and aerobic capacity38, may reduce walking speed, which in turn may increase the risk for adverse health consequences. Specifically previous studies have reported that slow walking speed was a strong predictor of adverse health outcomes, i.e., mortality and prolonged hospitalization difficulty crossing the streets using timed signals in older adults12–15,39, poor response to rehabilitation in adults after stroke14,40, structural worsenin gi nthe patellofemoral joint in adults after anterior cruciate ligament reconstruction41 and increased risk of incident radiographic and symptomatic knee OA42. Slow walking speed was associated with an increased odds of loss of work due to health status43 and reduced ability to engage in daily walking, i.e., walking fewer steps per day in adults with knee OA44. Inability to participate in daily walking has shown to be associated with increased risk of mortality in older adults45,46. Therefore, slow walking speed probably represents impairments in one or more body systems, which in turn may explain its association with increased mortality risk in adults with knee OA.
We found that the optimal thresholds that best discriminated those with and without mortality risk for a short-distance walk test and a standard-distance walk test were walking slower than 0.5 m/s and 1.2 m/s, respectively. These differences are likely due to the acceleration and deceleration walking phases to achieve a self-selected usual pace47. Najafi et al.47 found that older adults walk faster on a 20-m walk test compared to the 10-m walk test47. Peters et al16 found that walking speed obtained using the 4-m walk test should not be used interchangeably with the 10-m walk test. Based on our study findings and past evidence, we recommend using different thresholds to determine the risk of mortality for short- (i..e, 2.4-m walk test) and standard- (20-m walk test) distance walk tests. However, we strongly caution performing a direct comparison of association and thresholds of walking speed measured using a short- vs. standard distance walk test to mortality risk solely based on the findings of this study because the walk tests were administered in different cohorts. The short-distance walk test was administered in a population-based cohort, while the standard-distance walk test was administered in a cohort that had or was at risk of developing knee OA. We restricted all samples to adults with radiographic knee OA, which was ascertained using a similar methodology and ensured a similar average follow-up time frame. Yet, there were many other differences (including geographic location) across the cohorts besides the differences in participant characteristics (including age, education, sex, and race) and incidence of mortality, which could contribute to unmeasured confounding. The JoCoOA sample was slightly older, there was a higher proportion of females, higher mortality incidence and there was a much lower proportion of participants with at least college education, compared to OAI and MOST samples.
Given our study findings, health care professionals should consider measuring walking speed as part of routine clinical practice. Walking speed is a simple and reliable measure, and recommended as a clinical outcome in adults with knee OA48. Assessing walking speed alone may aid in identifying patients with knee OA who are at risk of poor future health outcomes, and in need for early investigation and management 12,14,15.
The major strength of our study is that we used three large datasets with a long follow-up (9 years) and comprehensively assessed data on adults with or at risk of knee OA, walking speed, and other comorbidities. However, our study had some limitations. First, we caution generalizing the results of our study to all individuals with knee OA, since the majority of the sample who completed the 20-m walk test (OAI and MOST) was white and highly educated. Second, we did not account for intercurrent events such as hospitalization or knee replacement, which may have occurred during follow-up when we investigated the association of walking speed with all-cause mortality. We believe understanding how such events alter the association of walking speed with all-cause mortality is important in future research. Third, in the OAI and MOST, the participants had room to walk beyond the 20-m mark, so the deceleration phase of the walk test after the timing had stopped was not the part of the 20-m course. However, while testing walking speed in JoCoOA, there was little room to decelerate after crossing the 2.4 m mark, and the timing had stopped. Therefore, the deceleration phase may not have been the same between short- and standard-distance walk tests. This may be one reason why a lower threshold was found on short-distance walk test compared to the standard distance walk test. In an ideal study design, both short- and standard-distance walk tests would be administered in the same participants, which could be done in future work. However, in this study, we used the retrospective study design to investigate the research question so we could leverage the previously collected large data. Lastly, the association of walking speed and mortality risk was attenuated and was less precise in the MOST only. We believe the lower incidence of mortality in the MOST sample (5.0%) may have limited precise estimation of the association between walking speed and mortality risk.
Conclusion
Slow walking speed during a short-distance or standard-distance walk test may signify a higher risk of all-cause mortality over nine years in adults with radiographic knee OA. The threshold of walking speed that discriminated mortality risk was walking slower than 0.5 m/s on a short-distance walk test and walking slower than 1.2 m/s on a standard-distance walk test. Health professionals may consider referring patients with radiographic knee OA who walk < 0.5 m/s on a 2.4-m walk or < 1.2 m/s on a 20-m walk test for further examination to manage OA-related impairments and functional limitation.
Acknowledgments
The authors want to thank Irina Tolstykh for assistance with the MOST data.
Role of funding source: The study was supported in part by the University Doctoral fellowship award from Unidel Foundation, National Institutes of Health (R21-AR071079-01A1, K12HD055931-01, K23AR070913, T32-HD007490, F32AR073090, K24-AR070892, U54 GM104941, and P30 AR072520-01).
Data from Johnston County Osteoarthritis Project (JoCoOA), Osteoarthritis Initiative (OAI), and Multicenter Osteoarthritis Study (MOST) have been used for this study.
The support for JoCoOA was provided by the Centers for Disease Control (CDC) S043, S1734, S3486, S3810 and U01DP003206; Multidisciplinary Clinical Research Center (MCRC) of the UNC Thurston Arthritis Research Center, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) P60AR064166; and NIAMS R01AR065937.
The OAI is a public-private partnership composed of five contracts (N01-AR- 2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the NIH.
The MOST is comprised of four cooperative grants (Felson – AG18820; Torner – AG18832, Lewis – AG18947, and Nevitt – AG19069) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by MOST study investigators. This manuscript was prepared using a JoCoOA, OAI, and MOST datasets and does not necessarily reflect the opinions or views of the JoCoOA, OAI, and MOST investigators, the NIH or CDC, or the private funding partners.
Footnotes
Competing interest statement: There are no conflicts of interest. Also, all authors have no disclosures.
Ethics: The study has Institutional Review Board approval from the University of Delaware and all research sites involved in conducting JoCoOA, OAI, and MOST studies. All participants provided written informed consent before enrollment in the JoCoOA, OAI, and MOST studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, et al. UK health performance: findings of the Global Burden of Disease Study 2010. The lancet. 2013;381(9871):997–1020. [DOI] [PubMed] [Google Scholar]
- 3.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. American Journal of Public Health. 1994;84(3):351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubertsson J, Petersson IF, Thorstensson CA, Englund M. Risk of sick leave and disability pension in working-age women and men with knee osteoarthritis. Annals of the Rheumatic Diseases. 2013;72(3):401. [DOI] [PubMed] [Google Scholar]
- 6.Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleveland RJ, Alvarez C, Schwartz T, Losina E, Renner J, Jordan J, et al. The impact of painful knee osteoarthritis on mortality: a community-based cohort study with over 24 years of follow-up. Osteoarthritis and cartilage. 2019;27(4):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. Bmj. 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Niu J, Huang J, Ke Y, Tang X, Wu X, et al. Knee osteoarthritis and all-cause mortality: the Wuchuan Osteoarthritis Study. Osteoarthritis and Cartilage. 2015;23(7):1154–7. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Nguyen UDT, Lane NE, Lu N, Wei J, Lei G, et al. Knee osteoarthritis, potential mediators, and risk of all-cause mortality: data from the Osteoarthritis Initiative. Arthritis care & research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawker GA, Croxford R, Bierman AS, Harvey PJ, Ravi B, Stanaitis I, et al. All-Cause Mortality and Serious Cardiovascular Events in People with Hip and Knee Osteoarthritis: A Population Based Cohort Study. PLOS ONE. 2014;9(3):e91286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. Jama. 2011;305(1):50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic Value of Usual Gait Speed in Well Functioning Older People—Results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53(10):1675–80. [DOI] [PubMed] [Google Scholar]
- 14.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. Journal of geriatric physical therapy. 2009;32(2):2–5. [PubMed] [Google Scholar]
- 15.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. Journal of aging and physical activity. 2015;23(2):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. Journal of geriatric physical therapy 2013;36(1):24–30. [DOI] [PubMed] [Google Scholar]
- 17.Sima CS, Gönen M. Optimal cutpoint estimation with censored data. Journal of Statistical Theory and Practice. 2013;7(2):345–59. [Google Scholar]
- 18.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 19.Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. The Knee. 2009;16(6):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2007;56(9):2986–92. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(6):789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jette AM, Jette DU, Ng J, Plotkin DJ, Bach MA. Are performance-based measures sufficiently reliable for use in multicenter trials? Musculoskeletal Impairment (MSI) Study Group. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(1):M3–M6. [DOI] [PubMed] [Google Scholar]
- 23.Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R, Katzoff M. Reliability and prevalence of physical performance examination assessing mobility and balance in older persons in the US: data from the Third National Health and Nutrition Examination Survey. Journal of the American Geriatrics Society. 2000;48(9):1136–41. [DOI] [PubMed] [Google Scholar]
- 24.Motyl JM, Driban JB, McAdams E, Price LL, McAlindon TE. Test-retest reliability and sensitivity of the 20-meter walk test among patients with knee osteoarthritis. BMC musculoskeletal disorders. 2013;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen M, Crosbie J, Edmonds J. Reliability of gait measurements in people with osteoarthritis of the knee. Physical therapy. 1997;77(9):944–53. [DOI] [PubMed] [Google Scholar]
- 26.Villadsen A, Roos EM, Overgaard S, Holsgaard-Larsen AJ Ajopm, rehabilitation. Agreement and reliability of functional performance and muscle power in patients with advanced osteoarthritis of the hip or knee. 2012;91(5):401–10. [DOI] [PubMed] [Google Scholar]
- 27.Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2009;57(2):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: the role of health and socioeconomic status over the life course. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2012;67(2):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–6. [DOI] [PubMed] [Google Scholar]
- 30.Hannan MT, Anderson JJ, Pincus T, Felson DT. Educational attainment and osteoarthritis: differential associations with radiographic changes and symptom reporting. Journal of clinical epidemiology. 1992;45(2):139–47. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Annals of internal medicine. 2000;133(8):635–46. [DOI] [PubMed] [Google Scholar]
- 32.Samson MM, Crowe A, De Vreede P, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging clinical and experimental research. 2001;13(1):16–21. [DOI] [PubMed] [Google Scholar]
- 33.Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. Lancet. 2012;379(9823):1331–40. [DOI] [PubMed] [Google Scholar]
- 34.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care 1996:73–84. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 36.Bohannon RW. Comfortable and maximum walking speed of adults aged 20—79 years: reference values and determinants. Age and ageing. 1997;26(1):15–9. [DOI] [PubMed] [Google Scholar]
- 37.Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Experimental gerontology. 2013;48(3):358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiser WM, Hays NP, Rogers SC, Kajkenova O, Williams AE, Evans CM, et al. Energetics of walking in elderly people: factors related to gait speed. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65(12):1332–7. [DOI] [PubMed] [Google Scholar]
- 39.Langlois JA, Keyl PM, Guralnik JM, Foley DJ, Marottoli RA, Wallace RB. Characteristics of older pedestrians who have difficulty crossing the street. American journal of public health. 1997;87(3):393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldie PA, Matyas TA, Evans OM. Deficit and change in gait velocity during rehabilitation after stroke. Archives of physical medicine and rehabilitation. 1996;77(10):1074–82. [DOI] [PubMed] [Google Scholar]
- 41.Capin JJ, Williams JR, Neal K, Khandha A, Durkee L, Ito N, et al. Slower Walking Speed Is Related to Early Femoral Trochlear Cartilage Degradation After ACL Reconstruction. Journal of Orthopaedic Research®. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purser JL, Golightly YM, Feng Q, Helmick CG, Renner JB, Jordan JM. Association of slower walking speed with incident knee osteoarthritis-related outcomes. Arthritis care & research. 2012;64(7):1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkness C, Fritz J, Marcus R, Asche C, Callahan L, Ren J. Walking speed is associated with work status in adults with knee osteoarthritis (OA): The osteoarthritis initiative study. Osteoarthritis and Cartilage. 2015;23:A341–A2. [Google Scholar]
- 44.Master H, Thoma LM, Christiansen MB, Polakowski E, Schmitt LA, White DK. Minimum performance on clinical tests of physical function to predict walking 6000 steps/day in knee osteoarthritis: An observational study. Arthritis care & research. 2018;70(7):1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee I-M, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of Step Volume and Intensity With All-Cause Mortality in Older Women. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saint-Maurice PF, Troiano RP, Bassett DR Jr., Graubard BI, Carlson SA, Shiroma EJ, et al. Association of Daily Step Count and Step Intensity With Mortality Among US Adults. JAMA. 2020;323(12):1151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait & posture. 2009;29(2):261–6. [DOI] [PubMed] [Google Scholar]
- 48.Dobson F, Hinman RS, Hall M, Marshall CJ, Sayer T, Anderson C, et al. Reliability and measurement error of the Osteoarthritis Research Society International (OARSI) recommended performance-based tests of physical function in people with hip and knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2017;25(11):1792–6. [DOI] [PubMed] [Google Scholar]