Abstract
Objective:
The release of neutrophil extracellular traps (NETs) by hyperactive neutrophils has recently been recognized to play an important role in antiphospholipid syndrome (APS). Here, we aimed to evaluate autoantibodies targeting NETs in patients with primary APS, and to determine their potential functions and clinical associations.
Method:
We measured global anti-NET activity in 76 patients with primary APS, 23 patients with systemic lupus erythematosus without antiphospholipid antibodies (aPL), 11 patients with history of unprovoked venous thrombosis without aPL, and 44 healthy controls. The ability of APS sera to degrade NETs was also assessed.
Result:
We found markedly elevated levels of anti-NET IgG and IgM in patients with primary APS as compared with healthy controls (IgG mean optical density 0.55 ± 0.34 vs. 0.33 ± 0.17; IgM mean optical density 0.76 ± 0.51 vs. 0.26 ± 0.23). This anti-NET activity did not correlate with levels of traditional aPL and was relatively stable over time. Mechanistically, anti-NET antibodies (especially of the IgG isotype) impaired the ability of patient sera to degrade NETs (r=0.4, p=0.003). Levels of anti-NET IgM inversely correlated with complement C4 (r=0.4, p=0.019). Clinically, anti-NET antibodies associated with certain APS clinical manifestations, and in particular recurrent venous thrombosis (OR=4.3, p=0.002). Interestingly, anti-NET antibodies also appeared to associate with unprovoked venous thrombosis in the general population (IgM mean optical density 0.67 ± 0.34 vs. 0.26 ± 0.23).
Conclusion:
These data reveal high levels of anti-NET antibodies in patients with primary APS, which may impair NET clearance and activate the complement cascade. These findings may ultimately enable more effective risk stratification.
INTRODUCTION
Antiphospholipid syndrome (APS) is an autoimmune thromboinflammatory disease manifested by arterial, venous, and/or microvascular thrombosis, as well as pregnancy loss(1). Current classification criteria require positive testing for one or more traditional antiphospholipid antibodies (aPL) including anticardiolipin (aCL), anti-beta-2 glycoprotein-I (β2GPI), and lupus anticoagulant, in the setting of a thrombotic event or pregnancy loss. While the pathophysiology of APS remains incompletely understood, aPL-mediated mechanisms that may contribute include activation of endothelial cells, monocytes, platelets, coagulation factors, and complement proteins (1).
Activated neutrophils—and, in particular, neutrophil extracellular trap (NET) formation—have recently received increased scrutiny as drivers of arterial, venous, and microvascular thrombosis (2). NETs are a meshwork of DNA, histones, and microbicidal proteins released from activated neutrophils via a program called NETosis. Neutrophils presumably deploy NETs to trap and kill pathogens (3); however, NETs may also be key players in the pathophysiology of thrombophilic disease states such as cancer, APS, heparin-induced thrombocytopenia, and COVID-19 (2, 4, 5). In APS, aPL engage the neutrophil surface, circumvent normal homeostatic mechanisms, and directly trigger NET release (5). Indeed, even when assessed between thrombotic events, patients with APS have higher levels of circulating NETs as compared with healthy controls, and APS neutrophils have a reduced threshold for spontaneous NETosis when cultured ex vivo (5). Furthermore, neutrophils from patients with APS appear to have increased adhesive potential, dependent upon the activated form of integrin Mac-1, which may potentiate NET release (6). In mouse models of aPL-mediated large-vein thrombosis, depletion of neutrophils, disruption of neutrophil-endothelium interactions, and digestion of NETs are all protective (7).
Intriguingly, one small study has suggested that patients with primary APS have circulating IgG species that bind to NETs (8). However, quantitation, function, and prognostic significance of this anti-NET activity has not been thoroughly investigated. Here, we sought to evaluate anti-NET IgG and IgM antibodies in patients with primary APS, and to determine potential functions and clinical associations.
METHODS
See Supplementary Materials for detailed methodology.
RESULTS AND DISCUSSION
Global anti-NET activity in primary APS.
Utilizing an ELISA platform, we measured anti-NET IgG and IgM antibodies in 76 patients with primary APS, 23 patients with systemic lupus erythematosus (SLE) without aPL, 11 patients with history of unprovoked venous thrombosis without aPL, and 44 healthy controls. The clinical characteristics of these patients are described in Supplementary Table 1. Markedly elevated levels of anti-NET IgG and IgM were detected in patients with primary APS as compared with healthy controls (Figure 1A–B). High levels were also seen in patients with SLE but negative aPL (Figure 1A–B). While anti-NET IgG was not significantly elevated in patients with unprovoked venous thrombosis without aPL (Figure 1A), those patients did have higher levels of anti-NET IgM (Figure 1B). We next considered whether anti-NET activity might correlate with levels of traditional aPL; however, no such correlation was appreciated with either anti-β2GPI IgG/IgM (Figure 1C and Supplementary Figure 1) or lupus anticoagulant (data not shown). To determine the stability of anti-NET antibodies over time, we identified six patients with primary APS who had serial plasma banked over two to four years of follow-up in our clinic. Anti-NET IgG, in particular, showed stability over time (Figure 1D and Supplementary Figure 2). No significant differences were observed in levels of anti-NET IgG or IgM between APS patients who were receiving hydroxychloroquine as compared with those who were not (Supplementary Figure 3). In summary, elevated levels of anti-NET IgG and IgM antibodies are present in patients with primary APS. These antibodies do not correlate with traditional aPL testing, and appear to be quite stable over time in some patients.
Figure 1: High levels of anti-NET antibodies among patients with primary APS.
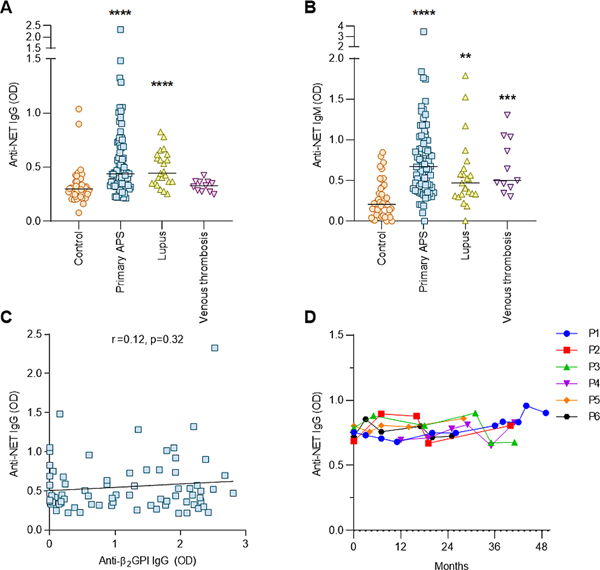
A-B, Anti-NET IgG and IgM were measured in individuals with primary APS, SLE without antiphospholipid antibodies, unprovoked venous thrombosis without antiphospholipid antibodies, or no known disease (healthy controls). Levels of anti-NET IgG and IgM at 450-nm optical density (OD) were compared by Kruskal–Wallis test **p<0.01, ***p<0.001, and ****p<0.0001 as compared with the control group. C, The relationship between anti-NET IgG and anti-β2GPI IgG was assessed by Spearman’s correlation test and linear regression. D, Anti-NET IgG levels were measured in six patients with primary APS who had two to four years of follow-up in our clinic.
Anti-NET antibodies decorate NETs generated by different stimuli.
Anti-NET activity was assessed by immunofluorescence microscopy. When NETs were incubated with plasma from patients with high levels of anti-NET IgG, antibodies robustly decorated NET strands (Figure 2). It has been suggested that NETs generated by different stimuli may have different protein composition (9). For example, phorbol 12-myristate 13-acetate (PMA) induces NETosis through a NADPH oxidase-dependent pathway, and may have lower content of citrullinated histones as compared with calcium ionophore-induced NETs (10). We therefore asked whether there was a difference in anti-NET activity in the context of these different stimuli. Notably, we found a strong correlation between APS samples tested in a PMA-NET ELISA and an ionophore-NET ELISA (r=0.72, p<0.0001 for anti-NET IgG; r=0.71, p<0.0001 for anti-NET IgM; Supplementary Figure 4). In summary, detection of anti-NET activity appears to be relatively independent of the stimulus used to trigger NETosis.
Figure 2: Anti-NET antibodies decorate NETs.
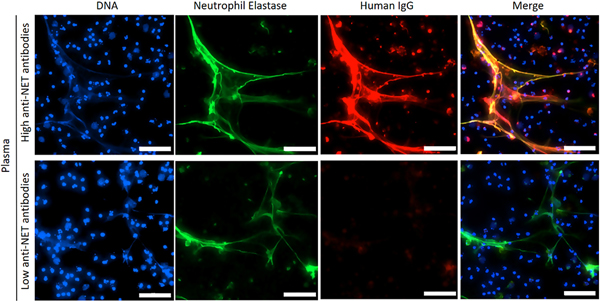
Control neutrophils were stimulated with PMA to generate NETs, which were then incubated with plasma from patients with high (top panels) or low (bottom panels) anti-NET IgG; scale bars=200 microns.
Relationship of anti-NET activity to NET degradation and complement levels.
Given that impaired NET degradation had been appreciated in some patients with SLE (11), we next asked whether anti-NET antibodies might impact the ability of APS sera to degrade NETs. Indeed, sera from patients with both primary APS and secondary APS (Supplementary Table 2) showed impaired NET degradation as compared with healthy controls (Figure 3A). Importantly, high levels of anti-NET IgG correlated with decreased NET degradation (Figure 3B), while depletion of IgG partially restored degradation (Figure 3C). As NETs and associated antibodies are potential activators of the complement system (12), we were interested in whether patients with high anti-NET activity might have evidence of smoldering complement activation. We found a correlation between high levels of anti-NET IgM and depressed complement C4 (Figure 3D), whereas no significant correlation was appreciated for anti-NET IgG (Supplementary Figure 5). In summary, high levels of anti-NET IgG associate with an impaired ability to degrade NETs, while anti-NET IgM may contribute to complement consumption.
Figure 3: Anti-NET antibodies correlate with impaired NET degradation.
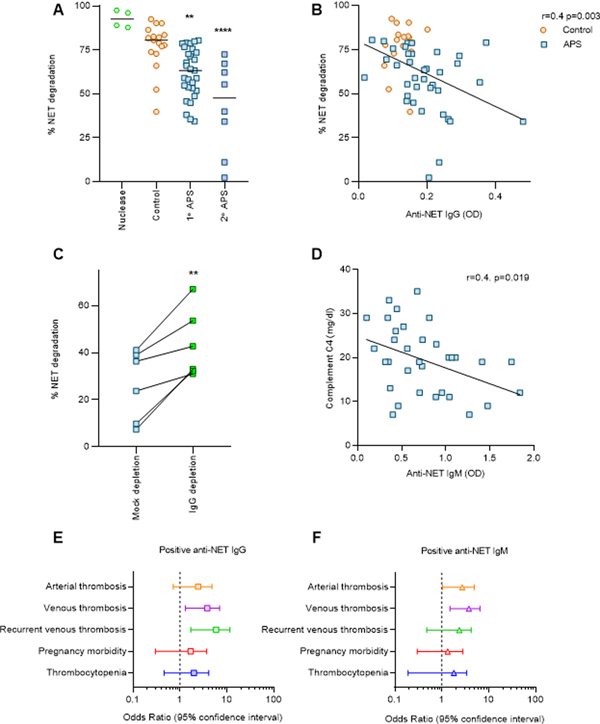
A, NET release was triggered from control neutrophils by PMA. Fresh NETs were then incubated with sera from patients with primary or secondary APS, or from healthy controls. Some samples were treated with Micrococcal nuclease as a positive control. Percentage of NET degradation was compared to the control group by one-way ANOVA; **p<0.01 and ****p<0.0001. B, Correlation between NET degradation and anti-NET IgG was assessed by Spearman’s correlation and linear regression. C, APS serum samples (six unique patients) were depleted of total IgG, and compared with mock-depleted samples. Paired t test was used to assess the difference in NET degradation before and after depletion; **p<0.01. D, Correlation between anti-NET IgM and complement C4 was assessed by Spearman’s correlation and linear regression. E-F, Univariate logistic regression was performed to assess the association between positive anti-NET antibodies and various clinical manifestations in 154 individuals with primary APS, SLE without antiphospholipid antibodies, unprovoked venous thrombosis without antiphospholipid antibodies, or no known disease. Odds ratio and 95% confidence intervals are presented.
Association of anti-NET activity with clinical manifestations.
Finally, we were interested in whether anti-NET antibodies might associate with APS-associated clinical manifestations such as thrombosis and pregnancy loss. After setting a positive cutoff two standard deviations above the mean for healthy controls, univariate logistic regression was performed to evaluate clinical associations among individuals with primary APS (n=76), SLE (n=23), or unprovoked venous thrombosis (n=11); 44 healthy controls were also included in the analysis (Figure 3E and 3F). Twenty patients with primary APS (26.3%), four patients with SLE (17.4%), and two healthy controls (4.5%) had positive anti-NET IgG. For anti-NET IgM, 36 patients with primary APS (47.4%), five patients with SLE (21.7%), four patients with venous thrombosis (36.4%), and three healthy controls (6.8%) tested positive. We found that both anti-NET IgG and IgM were significantly associated with venous thrombosis (IgG OR=3.1, p=0.008; IgM OR=3.2, p=0.001). Positive anti-NET IgG was also significantly associated with recurrent venous thrombosis (OR=4.3, p=0.002), while anti-NET IgM was significantly associated with arterial thrombosis (OR=2.2, p=0.046). When we further limited the analysis to only patients with primary APS, we interestingly found that those patients with recurrent venous thrombosis had significantly higher levels of anti-NET IgG as compared with all other patients with primary APS (Supplementary Figure 6). In summary, anti-NET IgG and IgM are associated with APS clinical manifestations, and anti-NET IgG may especially predict patients with the severe phenotype of recurrent venous thrombosis.
NETs are being increasingly recognized for their role in the pathogenesis of various thromboinflammatory conditions. For example, it has been suggested that increased NET formation, presence of anti-NET antibodies, and impaired NET clearance associate with SLE disease activity and organ damage (13). To date, anti-NET antibodies have been little studied in primary APS. Here, we found that high levels of anti-NET IgG not only predict an impaired ability of APS sera to clear NETs, but also strongly associate with venous thrombosis (especially patients with the severe phenotype of recurrent venous thrombosis). Whether their impact on NET degradation is by shielding NETs from DNase or direct antagonism of DNase requires further investigation. Notably, we also found elevated levels of anti-NET antibodies in SLE patients, which is interesting given that patients with active SLE are at high risk for thrombotic events (14).
The intersection of NETs, complement, and coagulation is an area of increasing study (15). NETs can directly activate complement (15), which in turn promotes coagulation via effects on tissue factor and platelets (15). At the same time, activated complement can promote NET formation, and NETs themselves then serve as a scaffold for thrombosis formation (15). It has been reported that sera from patients with active SLE that do not degrade NETs normally have low levels of complement C3 and C4, indicative of complement system activation (12). Here, we found that high anti-NET IgM significantly correlated with depressed complement C4. It is certainly possible that anti-NET antibodies are important orchestrators of the potentially complex relationship between NETs, complement, and coagulation in thromboinflammatory disease. Future studies seem warranted to elucidate the mechanistic relationship between anti-NET activity and complement activation.
Given that aPL are regularly detected in individuals who never develop clinical manifestations of APS, it is challenging to make decisions regarding primary thrombosis prophylaxis without a reliable strategy for risk stratification. Clinically-relevant biomarkers that predict thrombotic risk and allow sub-phenotyping of asymptomatic aPL carriers and patients with APS are desperately needed. The data presented here suggest that anti-NET antibodies have potential as a new class of clinically-relevant biomarkers that will more effectively risk stratify and sub-phenotype aPL-positive individuals.
Supplementary Material
ACKNOWLEDGEMENTS
YZ was supported by career development grants from the Rheumatology Research Foundation and APS ACTION. JAM was partially supported by the VA Healthcare System. JSK was supported by grants from the NIH (R01HL115138), Lupus Research Alliance, and Burroughs Wellcome Fund. Support for acquisition of patient samples was partially through the Taubman Institute Innovative Program for medical research (to JMK and JEG).
Footnotes
CONFLICTS OF INTEREST: JEG has served on advisory boards for Almirall, Bristol Myers Squibb (BMS), Celgene, AbbVie, and Novartis. JEG has received grant support from SunPharma, Almirall, and both JEG and JMK have received Grant support from Celgene/BMS. JMK has served on advisory boards for AstraZeneca, Eli Lilly, Boehringer Ingleheim, Avion Pharma, and BMS. None of the other authors has any financial conflict of interest to disclose.
REFERENCES
- 1.de Groot PG, de Laat B. Mechanisms of thrombosis in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract Res Clin Rheumatol. 2017;31(3):334–41. [DOI] [PubMed] [Google Scholar]
- 2.Bonaventura A, Liberale L, Carbone F, Vecchie A, Diaz-Canestro C, Camici GG, et al. The Pathophysiological Role of Neutrophil Extracellular Traps in Inflammatory Diseases. Thromb Haemost. 2018;118(1):6–27. [DOI] [PubMed] [Google Scholar]
- 3.Grayson PC, Kaplan MJ. At the Bench: Neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukoc Biol. 2016;99(2):253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67(11):2990–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sule G, Kelley WJ, Gockman K, Yalavarthi S, Vreede AP, Banka AL, et al. Increased Adhesive Potential of Antiphospholipid Syndrome Neutrophils Mediated by beta2 Integrin Mac-1. Arthritis Rheumatol. 2020;72(1):114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight JS, Meng H, Coit P, Yalavarthi S, Sule G, Gandhi AA, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight. 2017;2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leffler J, Stojanovich L, Shoenfeld Y, Bogdanovic G, Hesselstrand R, Blom AM. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin Exp Rheumatol. 2014;32(1):66–70. [PubMed] [Google Scholar]
- 9.Petretto A, Bruschi M, Pratesi F, Croia C, Candiano G, Ghiggeri G, et al. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS One. 2019;14(7):e0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes CL, Shim D, Kernien J, Johnson CJ, Nett JE, Shelef MA. Insight into Neutrophil Extracellular Traps through Systematic Evaluation of Citrullination and Peptidylarginine Deiminases. J Immunol Res. 2019;2019:2160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522–31. [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun Rev. 2017;16(11):1160–73. [DOI] [PubMed] [Google Scholar]
- 14.Thrombosis Petri M. and systemic lupus erythematosus: the Hopkins Lupus Cohort perspective. Scand J Rheumatol. 1996;25(4):191–3. [DOI] [PubMed] [Google Scholar]
- 15.de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol. 2019;16(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


