Abstract
Background:
Reirradiation of head and neck cancer is associated with high rates of locoregional failure and potentially severe treatment-related toxicity. We report our institutional experience of reirradiation using modern highly-conformal radiotherapy approaches in patients with prior oropharyngeal radiation.
Methods:
We reviewed patients receiving curative-intent reirradiation with intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT), and proton beam radiotherapy (PBT) at our institution from 1999 – 2019. Disease control, survival, and toxicity rates following reirradiation were determined.
Results:
Sixty-nine patients were evaluated. Local control, progression-free survival, and overall survival at 2-years following reirradiation were 77%, 35%, and 51%, respectively. Grade 3 or greater (G3+) late toxicities occurred in 46% of patients and 41% required feeding tube placement during or after reirradiation.
Conclusions:
In select patients with prior oropharyngeal radiation, highly-conformal reirradiation offers acceptable local control, but G3+ toxicity and out-of-field failure rates remain high. These findings warrant continued evaluation of new multimodality approaches to improve oncologic outcomes.
Keywords: Reirradiation, oropharyngeal carcinoma, highly-conformal radiotherapy
Introduction:
Head and neck cancer patients who develop recurrent disease or a second primary tumor after receiving definitive treatment represent a challenging population for which curative treatments are limited and toxicity risks are significant. Historically, salvage surgery has been used for recurrent head and neck cancer, but for selected patients who are not operative candidates due to patient- and disease-related factors, reirradiation using conformal radiotherapy techniques including intensity-modulated radiation therapy (IMRT), passive-scattering proton therapy (PSPT), intensity-modulated proton therapy (IMPT), and stereotactic body radiation therapy (SBRT) has emerged as an alternative treatment approach.1–4 Several ongoing investigations for reirradiation of head and neck cancer have focused on further reducing treatment-related acute and late toxicities, identifying factors to guide appropriate selection of reirradiation modality, and assessing combinations of reirradiation with targeted therapy and immunotherapy.
Recurrent oropharyngeal squamous cell carcinomas in particular have high rates of relapse and treatment-related toxicity such as severe dysphagia, fibrosis, and soft tissue/bone necrosis following curative-intent reirradiation.4 Factors including tumor volume, reirradiation dose, and receipt of systemic therapy have been associated with rates of relapse and treatment-related toxicity in multiple retrospective studies.5 In general, higher reirradiation doses and use of concurrent systemic therapy have been associated with improved local control rates, but these data are likely influenced by patient selection factors inherent to retrospective studies. While treatment of smaller tumors with SBRT and other conformal techniques may reduce late toxicity1,2,4, the use of higher reirradiation doses and concurrent systemic therapy may contribute to increased toxicity rates.4,6,7
Here, we report our institutional experience with reirradiation in patients who previously received oropharyngeal radiation. We explore local and out-of-field disease control, survival, and toxicity rates following reirradiation with modern conformal radiotherapy techniques including IMRT, SBRT, and PBT. With efforts underway to combine radiotherapy with immunotherapy, modern surgical approaches, and novel technologies such as radiation-enhancing nanoparticles8, these data can serve as a baseline for clinical outcomes using highly-conformal radiotherapy for oropharyngeal reirradiation.
Materials and Methods:
Study Design and Eligibility
In this institutional review board-approved study, we performed a retrospective analysis of patients receiving curative-intent reirradiation treated at The University of Texas MD Anderson Cancer Center from August 16, 1999 through March 19, 2019. Inclusion criteria were patients who previously received a minimum of 45 Gy to the oropharynx and who subsequently developed a recurrent or second primary cancer treated with curative-intent reirradiation using highly conformal radiotherapy (IMRT, PBT, or SBRT). Patients were eligible regardless of whether they received salvage surgery or systemic therapy (induction or concurrent) at the time of recurrence.
Disease failure was defined relative to the distance from the high-dose reirradiation target volume. Reirradiation treatment volume for SBRT was defined as the combined PTV of all prescribed dose levels. Local failures included failures inside (in-field) or within 2 cm (marginal) outside the high-dose target volume. Regional failures included failures in the neck or non-targeted mucosa > 2 cm from the high-dose target volume. Distant failures included those occurring outside the head and neck region. Out-of-field failures included those outside the high-dose target volume, including non-targeted mucosa > 2 cm from high-dose target volume, neck, and distant failures. Toxicity was determined using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Acute adverse events were defined as occurring during and/or within 30 days of reirradiation; late adverse events occurred after 30 days of completing reirradiation. Acute grade 3 (G3) mucositis was defined as confluent mucositis associated with severe pain limiting self-care; G3 dysphagia was defined as severely altered eating or swallowing, feeding tube/TPN requirement, or hospitalization; G3 dermatitis was defined as moist desquamation and minor bleeding not occurring in skin folds.
Radiation Planning
Our departmental practice is to use a GTV-to-CTV expansion of 5–8 mm (respecting normal anatomic borders) for IMRT and PBT, and a PTV expansion of 3–5 mm. For SBRT, we previously utilized 2 mm PTV margins to cover the target volume with 95% of the prescribed dose and did not initially stratify by anatomic subsite (mucosal, skull base, or neck).9 In our current practice, however, we utilize a 3-4 mm PTV margin for oropharyngeal tumors to account for the additional positional soft tissue uncertainty within this subsite.10 Prior to treatment all patients and contours are evaluated at a bi-weekly Head and Neck Radiation Oncology Quality Assurance Planning Conference.
Statistical Methods
Patient, disease, and treatment characteristics were summarized using descriptive statistics. Progression-free survival (PFS), distant metastasis-free survival (DMFS), overall survival (OS), local control (LC), and locoregional control (LRC) were calculated from the time of completion of reirradiation using the Kaplan-Meier method. The log-rank test was used for comparisons of survival between groups. The reverse Kaplan-Meier method was used to calculate follow-up. Statistical analysis was performed using JMP Pro 14.0.0 (SAS Institute Inc., Cary, NC) and GraphPad Prism 8.0.0 (GraphPad Software, Inc., San Diego, CA) software.
Results:
Patient, Disease, and Treatment Characteristics
Sixty-nine patients with a median age of 65 years (range 27 – 84 years) met inclusion eligibility and were analyzed. The majority of patients were men (84%). Fifty-two patients (75%) had Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Tumors were classified as recurrent primary tumors in 40 patients (58%) and second primary tumors in 29 (42%). There were 52 (75%) solitary mucosal and 17 (25%) multisite recurrent or second primary tumors. Recurrent subsites included: the base of tongue (70%), tonsil (13%), soft palate (4%), pharyngeal wall/parapharyngeal space (10%), and carotid space (3%). The most common treatment location was base of tongue for IMRT (63%; n = 25 of 40), SBRT (80%; n = 12 of 15), and PBT (79%; n = 11 of 14).
The median radiation dose of the initial therapy was 68 Gy (range 45 – 75 Gy). Reirradiation was performed with IMRT for 40 patients (58%), SBRT for 15 patients (22%), and PBT for 14 patients (20%). The median interval between initial therapy and reirradiation was 8.6 years (range 0.4 – 21.3 years) for IMRT, 2.8 years (0.6 – 25.3 years) for SBRT, and 3.3 years (1.0 – 20.5 years) for PBT. The median dose and fractionation for reirradiation was 66 Gy (50 – 70 Gy) in 33 fractions (25 – 44 fractions) for IMRT, 45 Gy (40 – 45 Gy) in 5 fractions for SBRT, and 66 Gy(RBE) (34 – 70 Gy(RBE)) in 33 fractions (17 – 35 fractions) for PBT. The median reirradiation target volumes were 72 cm3 (20 – 294 cm3) for IMRT, 67 cm3 (31 – 123 cm3) for SBRT, and 54 cm3 (18 – 191 cm3) for PBT (p=0.46). A representative treatment plan for reirradiation is shown in Figure 1.
Figure 1.
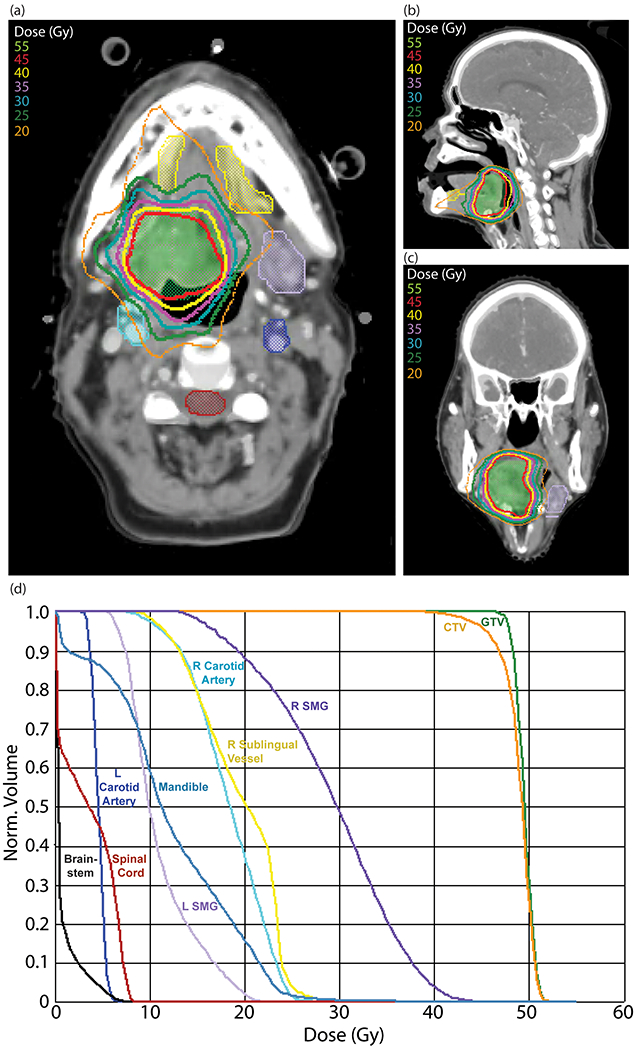
Reirradiation plan for recurrent oropharyngeal carcinoma treated with SBRT (45 Gy/5 fractions) with representative (a) axial, (b) sagittal, and (c) coronal views. Structures shown include: GTV (green); sublingual vessel avoidance structure (yellow); left submandibular gland (light purple); spinal cord (maroon); right carotid artery (teal); left carotid artery (blue); (d) dose-volume histogram for SBRT reirradiation plan. Abbreviations: SBRT, Stereotactic body radiation therapy; GTV, Gross target volume.
Salvage surgery was performed in 13 patients (19%) prior to reirradiation. No patients reirradiated with SBRT underwent salvage surgery. Reirradiation after salvage surgery was indicated for residual disease (38%; n = 5 of 13), positive/close margins (31%; n = 4 of 13), extracapsular extension (15%; n = 2 of 13), or perineural invasion (15%; n = 2 of 13). Tissue flaps were placed at the time of salvage surgery in 9 patients (13%). Prior to reirradiation, carotid stenting was performed in 2 of 69 patients (3%) and carotid endarterectomy was performed in 5 of 69 patients (7%). Fifty-two patients (75%) received chemotherapy (39 patients [57%] with concurrent and 13 patients [19%] with induction and concurrent). Concurrent systemic therapy was platinum-based in 30 patients (44%), cetuximab in 21 patients (30%), and docetaxel in 1 patient (1%). Additional patient, disease, and treatment characteristics are included in Table 1.
Table 1.
Patient, Disease, and Treatment Characteristics
| Characteristica | No. of Patients (%) |
|---|---|
| Age (Median and range) | 65 (27–84 years) |
| Sex | |
| Male | 58 (84) |
| Female | 11 (16) |
| Recurrent disease site | |
| Base of tongue | 48 (70) |
| Tonsil | 9 (13) |
| Soft palate | 3 (4) |
| Pharyngeal wall/Parapharyngeal space | 7 (10) |
| Carotid space | 2 (3) |
| Recurrence type | |
| Recurrent | 40 (58) |
| Second Primary | 29 (42) |
| HPV Status | |
| Positive | 13 (19) |
| Negative | 15 (22) |
| Unknown | 41 (59) |
| ECOG Performance Status | |
| 0 | 12 (17) |
| 1 | 40 (58) |
| 2 | 9 (13) |
| Unknown | 8 (12) |
| Reirradiation modality | |
| IMRT | 40 (58) |
| SBRT | 15 (22) |
| PBT | 14 (20) |
| Previous radiation dose, Gy (Median and range) | |
| IMRT | 66 (45–75) |
| SBRT | 70 (60–72) |
| PBT | 68 (46–72) |
| Reirradiation interval, years (Median and range) | |
| IMRT | 8.6 (0.4–21.3) |
| SBRT | 2.8 (0.6–25.3) |
| PBT | 3.3 (1.0–20.5) |
| Reirradiation dose, Gy (Median and range) | |
| IMRT | 66 (50–70) |
| SBRT | 45 (40–45) |
| PBT | 66 (34–70) |
| Reirradiation fractions (Median and range) | |
| IMRT | 33 (25–44) |
| SBRT | 5 (5–5) |
| PBT | 33 (17–35) |
| Reirradiation target volume, cm3 (Median and range)b | |
| IMRT | 72 (20–294) |
| SBRT | 67 (31–123) |
| PBT | 54 (18–191) |
| Proton reirradiation modality | |
| Active scanning (IMPT) | 11 (79) |
| Passive scatter (PSPT) | 3 (21) |
| Salvage surgery performed | |
| Yes | 13 (19) |
| No | 56 (81) |
| Received tissue flap at time of salvage surgery | |
| Yes | 9 (13) |
| No | 60 (87) |
| Chemotherapy sequence | |
| Induction | 0 (0) |
| Concurrent | 39 (57) |
| Induction + Concurrent | 13 (19) |
| None | 17 (25) |
| Concurrent chemotherapy type | |
| Platinum-based | 30 (44) |
| Cetuximab | 21 (30) |
| Docetaxel | 1 (1) |
| None | 17 (25) |
Abbreviations: HPV, Human papilloma virus; ECOG, Eastern Cooperative Oncology Group; IMRT, Intensity modulated radiation therapy; SBRT, Stereotactic body radiation therapy; PBT, Proton beam radiotherapy; IMPT, Intensity modulated proton therapy; PSPT, Passively scattered proton therapy.
Reirradiation target volume for SBRT defined as sum of volumes for individual dose levels
Disease Control and Survival with Reirradiation
The 1-year and 2-year PFS rates were 47% and 35%, respectively, from the time of completion of reirradiation. DMFS and OS rates at 1-year were 58% and 61%, respectively, and at 2-years were 42% and 51%, respectively. The median PFS was 10 months, and the median OS was 28 months. The median OS rates for patients with recurrent and second primary tumors were 23 months and 34 months, respectively, but no significant differences in PFS or OS were observed based on the type of recurrence (PFS: p=0.85; OS: p=0.20; Supp. Figure 1).
Following completion of reirradiation, the 1-year and 2-year LC rates were 87% and 77%, respectively. LRC rates were 71% and 63% at 1-year and 2-years, respectively. No significant differences were found between the type of recurrence and LC following reirradiation (p=0.70; Supp. Figure 1). There were 12 local failures (32%; n = 12 of 38), 13 regional failures (34%; n = 13 of 38), and 13 distant failures (34%; n = 13 of 38). Twenty-one patients (30%; n = 21 of 69) experienced an out-of-field failure. Two patients failed in non-targeted mucosa (3%; n = 2 of 69), 10 patients failed in the neck (14%; n = 10 of 69), and 1 patient failed in both non-targeted mucosa and neck (1%; n = 1 of 69). Eight patients (12%; n = 8 of 69) developed distant failures only, and five patients (7%; n = 5 of 69) developed both regional and distant failures.
Toxicity Associated with Reirradiation
Thirty-two patients (46%) experienced Grade 3 or greater (G3+) treatment-related late toxicities as detailed in Table 2 and Supplemental Table 1. There were 2 (3%; n = 2 of 69) Grade 5 events and 11 (16%; n = 11 of 69) Grade 4 events. Among G3+ late toxicities, 3 were oropharyngeal hemorrhage associated with the lingual artery requiring embolization. The median time to hemorrhage involving the lingual artery was 8 months (range 2 – 9 months). One patient received IMRT, one patient received PBT, and one patient received SBRT. One patient receiving SBRT developed an oropharyngeal hemorrhage requiring awake tracheostomy approximately 15 months after completing therapy. The proportion of Grade 4 and Grade 5 events by treatment technique were 10 events in 40 patients (25%) treated with IMRT, 2 events in 15 patients (13%) treated with SBRT, and 1 event in 14 patients (7%) treated with PBT. (p=0.28) The median treatment volumes for patients experiencing a Grade 3, Grade 4, or Grade 5 late toxicity were 72 cm3 (n = 19), 60.5 cm3 (n = 11), and 183.9 cm3 (n = 2), respectively, compared to patients without a Grade 3 or greater toxicity (61.4 cm3). (p=0.21) The most common G3+ late toxicities included dysphagia, trismus, and osteonecrosis. Additional toxicities included esophageal stricture, dehydration, oropharyngeal hemorrhage, xerostomia, and an orocutaneous fistula. Feeding tube placement was required for 49 patients (71%) overall. Twenty-one patients (30%) had non-prophylactic feeding tubes placed before undergoing reirradiation, with eighteen of these patients (86%; n = 18 of 21) having a feeding tube at their last follow-up visit. Feeding tubes were placed in 18 additional patients (26%) during or within 90 days of completing therapy and 10 patients (14%) greater than 90 days after completing therapy. (Table 3)
Table 2.
Grade 3 or Greater Late Toxicitiesa
| IMRT | SBRT | PBT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N b | % | Reirradiation volume (cm3)c | N | % | Reirradiation volume (cm3) | N | % | Reirradiation volume (cm3) | |
| All patients | 40 | 58 | 72 (20.2-293.9) | 15 | 22 | 67.3 (31.2-122.5) | 14 | 20 | 54.1 (18.1-191.1) |
| G3+ Late Toxicity | 25 | 78 | 71.3 (27.4-293.9) | 3 | 9 | 67.3 (45.4-95.9) | 4 | 13 | 63 (49.9-184.7) |
| Osteonecrosis | 9 | 82 | 60.5 (27.4 – 141.9) | 1 | 9 | 45.4 | 1 | 9 | 184.7 |
| Fibrosisd | 9 | 69 | 57.7 (38.8 – 293.9) | 1 | 8 | 67.3 | 3 | 23 | 52.9 (49.9 – 73.8) |
| Hemorrhage | 1 | 25 | 183 | 2 | 50 | 70.7 (45.4 – 95.9) | 1 | 25 | 184.7 |
| No G3+ Late Toxicity | 15 | 41 | 87.4 (20.2-270.8) | 12 | 32 | 65.4 (31.2-122.5) | 10 | 27 | 49.6 (18.1-191.1) |
Abbreviations: IMRT, Intensity modulated radiation therapy; SBRT, Stereotactic body radiation therapy; PBT, Proton beam radiotherapy; G3+, Grade 3 or greater.
Number of patients and corresponding row percentages are provided. For categories of G3+ late toxicity (osteonecrosis, fibrosis, or hemorrhage), the number of events and corresponding row percentages are provided.
Median and range (if applicable) of reirradiation target volume reported.
Includes trismus and esophageal strictures/severe dysphagia requiring dilatation
Table 3.
Feeding Tube Placementa
| Feeding Tube Placementb | IMRT | SBRT | PBT | Overall |
|---|---|---|---|---|
| Prior to reirradiation | 11 (27) | 6 (40) | 4 (29) | 21 (30) |
| During or ≤ 90 days after completing reirradiation | 14 (35) | 1 (7) | 3 (21) | 18 (26) |
| > 90 days after completing reirradiation | 4 (10) | 5 (33) | 1 (7) | 10 (14) |
| Overall | 29 (73) | 12 (80) | 8 (57) | 49 (71) |
Abbreviations: IMRT, Intensity modulated radiation therapy; SBRT, Stereotactic body radiation therapy; PBT, Proton beam radiotherapy.
Data indicates number of patients and corresponding percentage in parentheses.
Grade 3 or greater (G3+) acute toxicities occurred in 24 of 40 (60%) patients receiving IMRT, including 13 G3 mucositis, 10 G3 dysphagia, and 1 G4 mucositis with ulceration and intractable pain leading to ICU hospitalization; 6 of 14 (43%) patients receiving PBT, including G3 mucositis (n = 2 events), G3 dysphagia (n = 3) and G3 dermatitis (n = 3); and no acute G3+ toxicities were observed after SBRT (p<0.05 vs. PBT; p<0.001 vs. IMRT). However, 6 of 15 (40%) patients receiving SBRT reported G1–2 mucositis/odynophagia peaking at 2 weeks after treatment and consisting of pain requiring non-opioid analgesics. Two of these patients developed persistent/recurrent thrush.
Discussion:
We report clinical outcomes of patients reirradiated with curative intent using highly-conformal radiotherapy for recurrent or second primary cancers previously treated with high-dose radiotherapy to the oropharynx. Our primary conclusions are first, the majority of patients experienced progressive disease by 1 year after reirradiation. Second, a combination of in-field and out-of-field treatment failures contributed to disease progression, with a promising 2-year local control rate (77%) achieved in the treated areas. Third, Grade 3+ late toxicities including lingual artery hemorrhage, dysphagia, trismus, and osteonecrosis were common (46%), and a significant percentage of patients (41%) required feeding tube placement during or after reirradiation.
Our results regarding disease control accord with previous studies of reirradiation of head and neck cancers using conformal radiotherapy techniques in more heterogeneous populations. For IMRT reirradiation, 2-year locoregional control rates of 48 – 65% have been reported.4,5,7,11 Studies of PBT reirradiation demonstrate 2-year locoregional control rates of 73 – 80%.2,5,12 SBRT reirradiation studies have reported 2-year locoregional control rates of 28 – 64%.5,13–17 Whereas prior studies have combined local and regional failures in reporting disease control outcomes, we report local and regional control rates separately to help delineate patterns of failure of reirradiation with highly-conformal radiotherapy. We observed both in-field failures and out-of-field failures involving regional lymph nodes and uninvolved pharyngeal mucosa. The 2-year local control rate of 77% demonstrates that reirradiation with conformal radiotherapy can be an effective strategy for achieving disease control in the areas treated. Consistent with prior studies, we did not identify an association between specific reirradiation modality including protons and disease control; however, determining an association between specific reirradiation modality and clinical outcomes is best addressed by ongoing prospective, randomized trials in larger populations.
The decision regarding reirradiation modality at our institution is made primarily based on tumor volume. Our department’s ongoing phase II randomized trial (SOAR-HN; ClinicalTrials.gov Identifier: NCT03164460) requires the pre-treatment tumor volume to be < 60 cm3 for a single lesion and < 100 cm3 for all total lesions to be eligible for SBRT reirradiation. Tumor volumes larger than this are dispositioned for conventionally fractionated IMRT or PBT. These volume thresholds were guided by IMRT and PBT reirradiation data, in which CTV > 50 cm3 was a significant predictor of Grade 3 or higher toxicity.2,4 This difference in toxicity based on tumor volume was particularly evident among those reirradiated to the oropharynx/oral cavity and skull base.18 Among those meeting criteria for SBRT reirradiation, preferences of the patient and primary multidisciplinary team also guide treatment decisions. For example, a shorter treatment duration may be preferred in instances of suspected biologic tumor radioresistance such as recurrent tumor within a prior high-dose field or to expedite adjuvant systemic therapy when the risk of regional or distant recurrence risk is deemed to be high. Similarly, the use of PBT versus IMRT is a clinical decision by the managing physician and multidisciplinary team taking into consideration the required tumoricidal dose and adjacent normal tissue constraints. In most cases, a comparison between proton and photon-based plans was performed. In our department, these criteria are generally applied to all patients being considered for SBRT reirradiation and evaluated at our bi-weekly Head and Neck Radiation Oncology Quality Assurance planning conference.19,20
Patients with recurrences in the oropharynx/mucosa, base of skull, or neck are potentially eligible for enrollment on SOAR-HN, and analyses from this trial may shed light on identifying specific anatomic subsites that may be more amenable to SBRT-based reirradiation with the caveat that anatomic subsite is not a pre-specified stratification variable, which in our opinion, is a key factor impacting outcomes and toxicity Emerging treatment approaches such as combining conformal radiotherapy with locally-administered radioenhancers are currently under study to determine potential impacts on disease control for head and neck cancer, soft tissue sarcoma, gastrointestinal cancers, and multiple additional sites.8 Concurrent and adjuvant systemic therapy with SBRT reirradiation including immune checkpoint inhibitors may provide benefits for in-field and out-of-field disease control, and this treatment approach is currently being evaluated in RTOG 3507 (KEYSTROKE; ClinicalTrials.gov Identifier: NCT03546582), a phase II randomized trial of SBRT with or without pembrolizumab for locoregionally recurrent or second primary head and neck cancer. In comparison to SOAR-HN, RTOG 3507 allows a greater variety of fractionation approaches and permits larger tumors, and collectively these studies will help define more tailored treatment approaches and guide selection of patients requiring head and neck reirradiation.
Treatment-related toxicity is a significant concern for patients receiving reirradiation and remains a major consideration in appropriately selecting patients for reirradiation to the head and neck. For IMRT reirradiation, Grade 3 or greater late toxicity rates of 15 – 56%, have been reported.4,5,7,11 PBT reirradiation results in Grade 3 or greater late toxicities between 20 – 25%.2,5,12 SBRT reirradiation studies have reported Grade 3 or greater late toxicity rates of 3 – 32%.5,13–17 Our results generally agree with reported toxicity rates from other institutions. In addition, the combined G4-5 treatment-related toxicity rate (19%) approaches the high rate of treatment-related G4-5 events (>30%) observed in the RTOG 9610 and RTOG 9911 trials, suggesting the highest risk cohorts may represent those reirradiated to the oropharyngeal mucosa and tumors adjacent to the lingual artery and its branches.21,22 While concurrent chemotherapy was not associated with increased acute toxicity in this study, our recent experience suggests that concurrent cetuximab and SBRT or PBT can be associated with mucositis involving the anterior oral cavity outside of the low dose fall off region and folliculitis of the chest and forehead outside of the reirradiation field. Ongoing prospective studies in larger patient populations will provide greater detail on treatment factors associated with both acute and late toxicities.
Although a significant percentage of patients required feeding tube placement during or after reirradiation, it is not currently our department’s routine practice to recommend prophylactic feeding tube placement. Patients receiving care in a multidisciplinary setting who adhere to rigorous swallowing exercises during and after radiation have reduced placement of feeding tubes and less long-term swallowing dysfunction and dependence on enteral nutrition.23 With close multidisciplinary monitoring during and after treatment, many patients can be spared a feeding tube, and we therefore attempt to avoid prophylactic feeding tubes when safely possible. This approach may not be suitable in clinical settings with more limited resources, limited access to speech pathology expertise, or less frequent monitoring, and in these settings prophylactic feeding tube placement may be warranted to mitigate the risk of undernutrition.
While PBT toxicity in this small, selected population was comparable to other modalities, we have previously observed in larger, heterogeneous populations that PBT reirradiation to pharyngeal mucosal sites is associated with 30% rate of acute G3 toxicity and 16.7% rate of chronic G3 toxicity, in addition to several potential Grade 5 acute and sub-acute toxicities (3-5%) related to osteoradionecrosis, hemoptysis, and acute cerebral infarction occurring within 5 months of completing treatment.2 This suggests that the mucosal target may be more susceptible to the dose heterogeneity (also observed with step-and-shoot IMRT), range uncertainty, and RBE uncertainty of PBT, and therefore greater caution may be needed to prevent more severe mucosal toxicity when utilizing PBT.2 Our IMRT cohort consisted of greater than 90% step-and-shoot as the use of VMAT for head and neck radiotherapy/reirradiation was not in common use at our institution until 2016. Of note, a significant proportion of patients in the current study were treated with PSPT techniques; because modern proton therapy centers have transitioned to using more conformal IMPT techniques, it is possible the toxicity rates may be further reduced over time as IMPT becomes more widely adopted.
To reduce the likelihood of severe, potentially fatal toxicities resulting from damage to the carotid artery, lingual artery, or other major vessels, our institution uses specific avoidance structures during treatment planning. We currently aim to keep the lingual vessel avoidance structure maximum dose less than 30 Gy (to 0.3 cm3) if the artery is outside the target volume (< 5 mm from target) or to avoid hot spots if the artery is within the target volume. Additional normal tissue dose constraints for reirradiation used in our department are included in Supplemental Table 2. In the future, improved techniques for radiotherapy delivery, more effective surgical and systemic treatment options, and better patient selection are anticipated to further reduce treatment-related acute and late toxicities of head and neck reirradiation.
This study has several limitations that are inherent to retrospective, single-institutional studies. First, the patterns of care including radiotherapy techniques changed during the long duration of this study such that the reported rates of local control and toxicity may not reflect outcomes with modern standard-of-care therapy. For example, SBRT reirradiation patients are increasingly treated at our institution with adjuvant systemic therapy including pembrolizumab to improve disease control. However, our data do generally accord with results from other institutions obtained over a similar time period. Second, the limited sample size and heterogeneity in our population limits our ability to identify subgroups who may derive greater therapeutic benefit from reirradiation; studies with larger populations ideally in prospective, randomized trial settings are necessary to help clinicians properly select patients for reirradiation. Third, a significant percentage of patients did not have HPV status available which would be expected to influence the observed clinical outcomes. Fourth, because our institution treats a high volume of patients requiring reirradiation and these patients undergo thorough multi-disciplinary evaluations before, during, and after completing treatment, the reported rates of disease control and toxicity may not readily translate to practice settings with lower volumes of head and neck reirradiation cases.
Despite advances in modern highly-conformal radiotherapy and multimodality care, patients with recurrent head and neck cancer receiving reirradiation after prior oropharyngeal radiation experience high rates of treatment failure and significant treatment-related toxicity. Future multimodality approaches to augment the efficacy and reduce toxicity of radiotherapy may improve outcomes for patients with recurrent head and neck cancer.
Supplementary Material
Figure 2.
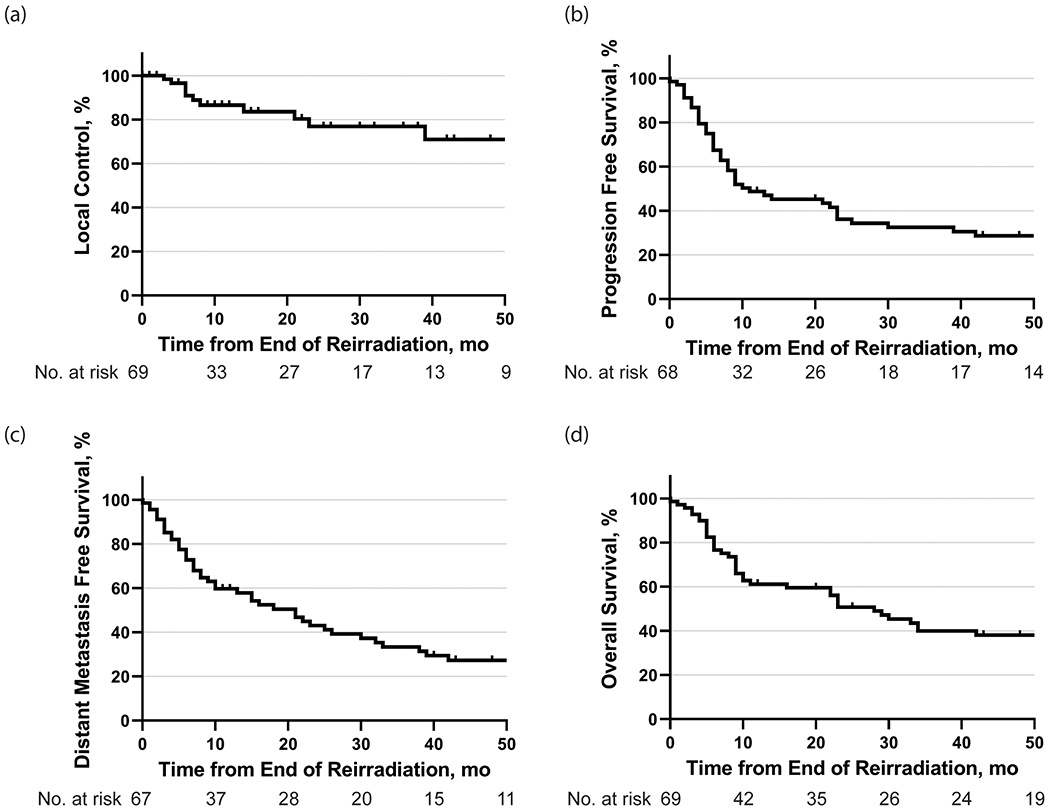
(a) Local control, (b) progression-free survival, (c) distant metastasis-free survival, and (d) overall survival following reirradiation.
Figure 3.
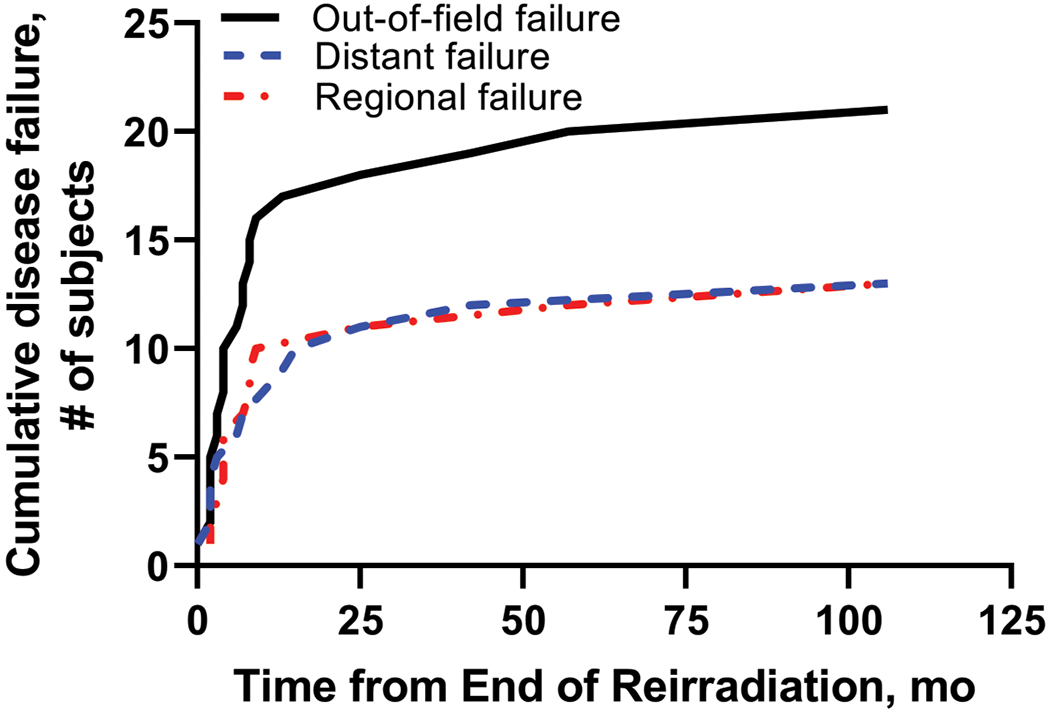
Cumulative out-of-field failures following reirradiation.
Acknowledgements:
This work was supported in part by the NIH/NCI under award number P30CA016672. The authors would like to acknowledge Rong Ye for helpful suggestions on this manuscript.
References:
- 1.Sulman EP, Schwartz DL, Le TT, et al. IMRT Reirradiation of Head and Neck Cancer-Disease Control and Morbidity Outcomes. Int J Radiat Oncol Biol Phys. 2009. doi: 10.1016/j.ijrobp.2008.04.021 [DOI] [PubMed] [Google Scholar]
- 2.Phan J, Sio TT, Nguyen TP, et al. Reirradiation of Head and Neck Cancers With Proton Therapy: Outcomes and Analyses. Int J Radiat Oncol Biol Phys. 2016. doi: 10.1016/j.ijrobp.2016.03.053 [DOI] [PubMed] [Google Scholar]
- 3.Pollard C, Nguyen TP, Ng SP, et al. Clinical outcomes after local field conformal reirradiation of patients with retropharyngeal nodal metastasis. Head Neck. 2017;39(10):2079–2087. doi: 10.1002/hed.24872 [DOI] [PubMed] [Google Scholar]
- 4.Takiar V, Garden AS, Ma D, et al. Reirradiation of Head and Neck Cancers With Intensity Modulated Radiation Therapy: Outcomes and Analyses. Int J Radiat Oncol. 2016;95(4):1117–1131. doi: 10.1016/j.ijrobp.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 5.Ho JC, Phan J. Reirradiation of head and neck cancer using modern highly conformal techniques. Head Neck. 2018;40(9):2078–2093. doi: 10.1002/hed.25180 [DOI] [PubMed] [Google Scholar]
- 6.Vargo JA, Wegner RE, Heron DE, et al. Stereotactic body radiation therapy for locally recurrent, previously irradiated nonsquamous cell cancers of the head and neck. Head Neck. 2012;34(8):1153–1161. doi: 10.1002/hed.21889 [DOI] [PubMed] [Google Scholar]
- 7.Duprez F, Berwouts D, Madani I, et al. High-dose reirradiation with intensity-modulated radiotherapy for recurrent head-and-neck cancer: Disease control, survival and toxicity. Radiother Oncol. 2014;111(3):388–392. doi: 10.1016/j.radonc.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 8.Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. July 2019. doi: 10.1016/S1470-2045(19)30326-2 [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Wang C, Tung S, et al. Improved setup and positioning accuracy using a three-point customized cushion/mask/bite-block immobilization system for stereotactic reirradiation of head and neck cancer. J Appl Clin Med Phys. 2016;17(3):180–189. doi: 10.1120/jacmp.v17i3.6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesko S, Wang H, Tung S, et al. Estimating PTV Margins in Head and Neck Stereotactic Ablative Radiation Therapy (SABR) Through Target Site Analysis of Positioning and Intrafractional Accuracy. Int J Radiat Oncol Biol Phys. 2020. doi: 10.1016/j.ijrobp.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis KK, Ross HJ, Garrett AL, et al. Outcomes of patients with loco-regionally recurrent or new primary squamous cell carcinomas of the head and neck treated with curative intent reirradiation at Mayo Clinic. Radiat Oncol. 2016;11:55. doi: 10.1186/s13014-016-0630-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald MW, Zolali-Meybodi O, Lehnert SJ, et al. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int J Radiat Oncol Biol Phys. 2016;96(4):808–819. doi: 10.1016/j.ijrobp.2016.07.037 [DOI] [PubMed] [Google Scholar]
- 13.Rwigema J-CM, Heron DE, Ferris RL, et al. The Impact of Tumor Volume and Radiotherapy Dose on Outcome in Previously Irradiated Recurrent Squamous Cell Carcinoma of the Head and Neck Treated With Stereotactic Body Radiation Therapy. Am J Clin Oncol. 2011;34(4):372–379. doi: 10.1097/COC.0b013e3181e84dc0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kress MAS, Sen N, Unger KR, et al. Safety and efficacy of hypofractionated stereotactic body reirradiation in head and neck cancer: Long-term follow-up of a large series. Head Neck. 2015;37(10):1403–1409. doi: 10.1002/hed.23763 [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki H, Ogita M, Himei K, et al. Reirradiation using robotic image-guided stereotactic radiotherapy of recurrent head and neck cancer. J Radiat Res. 2016;57(3):288–293. doi: 10.1093/jrr/rrw004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roh KW, Jang JS, Kim MS, et al. Fractionated Stereotactic Radiotherapy as Reirradiation for Locally Recurrent Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2009. doi: 10.1016/j.ijrobp.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui F, Patel M, Khan M, et al. Stereotactic Body Radiation Therapy for Primary, Recurrent, and Metastatic Tumors in the Head-and-Neck Region. Int J Radiat Oncol Biol Phys. 2009;74(4):1047–1053. doi: 10.1016/j.ijrobp.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 18.Ng SP, Wang H, Pollard C, et al. Patient Outcomes after Reirradiation of Small Skull Base Tumors using Stereotactic Body Radiotherapy, Intensity Modulated Radiotherapy, or Proton Therapy. J Neurol Surg Part B Skull Base. July 2019. doi: 10.1055/s-0039-1694052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal DI, Asper JA, Barker JL, et al. Importance of patient examination to clinical quality assurance in head and neck radiation oncology. Head Neck. 2006. doi: 10.1002/hed.20446 [DOI] [PubMed] [Google Scholar]
- 20.Cardenas CE, Mohamed ASR, Tao R, et al. Prospective Qualitative and Quantitative Analysis of Real-Time Peer Review Quality Assurance Rounds Incorporating Direct Physical Examination for Head and Neck Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017. doi: 10.1016/j.ijrobp.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25(30):4800–4805. doi: 10.1200/JCO.2006.07.9194 [DOI] [PubMed] [Google Scholar]
- 22.Spencer SA, Harris J, Wheeler RH, et al. RTOG 96-10: Reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys. 2001. doi: 10.1016/S0360-3016(01)01745-X [DOI] [PubMed] [Google Scholar]
- 23.Bhayani MK, Hutcheson KA, Barringer DA, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: Factors affecting placement and dependence. In: Head and Neck. Vol 35 ; 2013:1634–1640. doi: 10.1002/hed.23200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


