Abstract
Background:
Sinonasal mucosal melanoma (SNMM) is an aggressive cancer with high mortality. Identifying patients at risk of distant metastasis assists with management and prognostication. We aimed to define the relationship between volume, survival, and risk of distant metastases.
Methods:
A retrospective review of all patients with SNMM treated at a single institution over a 21-year period was conducted. Tumor volume was calculated using cross-sectional imaging and survival analysis was performed.
Results:
Sixty-one patients were included. Tumor volume was predictive of local progression free survival (LPFS) (p=0.032), distant metastases free survival (DMFS) (p=0.002), and overall survival (OS) (p=0.022). It was a better predictor than AJCC stage and T-classification. Tumor volume equal to or greater than 5cm3 was associated with a significantly worse DMFS and OS (p=0.022 and 0.009, respectively).
Conclusion:
Calculation of tumor volume assists in quantifying the risk of distant metastases and death in SNMM.
Keywords: mucosal melanoma, sinonasal, staging systems, tumor volume, survival
INTRODUCTION
Sinonasal mucosal melanoma (SNMM) is a rare and aggressive cancer that accounts for fewer than 4% of sinonasal tumors and 0.5% of all tumors of the head and neck.1-5 Patients with SNMM have a poor prognosis, with a 5-year overall survival of 20–40%,6-10 which is attributed to the significant proportion of patients who develop distant metastases (between 39 to 68%).11-15 The current management paradigm of SNMM includes surgical resection (when feasible) followed by adjuvant radiotherapy.16-19 In recent years, immune checkpoint inhibition has been introduced as treatment for patients with locally advanced or distant disease, but there is limited data to guide the selection of patients most likely to benefit from immunotherapy.20-22
There are several described methods of risk stratification for patients with SNMM. The Ballantyne staging system, which was first proposed in 1970 and subsequently modified by Prasad in 2004, classifies tumors based on histological and anatomical disease extent.23,24 Although useful as a descriptive tool, it offers limited prognostic information for patients.25 Similarly, the American Joint Committee on Cancer (AJCC) initially used a non-specific sinonasal carcinoma staging system for patients with SNMM that offered little in the way of prognostic information for SNMM. In 2009, the AJCC released its first staging system specifically for head and neck mucosal melanomas (HNMM), which stratifies patients based on prognosis and remains the most commonly used system today. None of these staging systems offer information regarding the risk of distant metastases.24-27
The prognostic value of primary tumor volume (TV) in predicting disease progression and survival has been reported in other head and neck cancers,28 but not in SNMM. Our objective was to define the relationship between TV and the risk of distant metastases and overall survival in patients with SNMM.
MATERIALS / METHODS
Patient Selection
Following Institutional Review Board approval, a retrospective review was conducted of all patients with SNMM who presented to a high-volume academic cancer center from January 1997 to December 2018. Cases were identified using diagnostic codes contained within a prospectively maintained skull base database. Patients were included in our study if they had a newly diagnosed and pathologically confirmed diagnosis of melanoma arising from the sinonasal region and had pre-treatment structural imaging available for review. Patients were excluded if radiological imaging was not available for review or if the patient had undergone extensive biopsies or debulking prior to imaging (as per operation report). Patient demographics, tumor factors, treatment details, and outcomes data were collected. Patients were staged retrospectively using AJCC 8th edition staging for head and neck mucosal melanoma (HNMM). Because the AJCC 8th edition does not include prognostic stage groups,29 the 7th edition was used for this purpose.30 Follow-up imaging studies were reviewed to assess for the presence of recurrent locoregional and distant disease.
Calculation of Tumor Volume
Pre-treatment structural imaging (either computed tomography [CT] or magnetic resonance imaging [MRI] of the sinuses with contrast) was reviewed by a neuroradiologist who was blinded to patient outcomes. Patients were assigned a T-classification in accordance with the AJCC 8th edition system for HNMM29, which allowed staging according to the 7th edition as outlined above30. Tri-planar imaging was used to measure tumor diameter in three axes. Ellipsoid primary tumor volume (TV) was calculated using the method described by Dejaco et al, in which tumor volume is equal to (xyz)π/6 where x, y and z are three perpendicular tumor diameter measurements in centimeters.31
To assess the potential effect of interobserver variability, we compared a random sample of ten sets of measurements obtained by the blinded study neuroradiologist with those recorded in pre-operative imaging reports. We then compared these values in order to calculate the Interobserver Correlation Coefficient (ICC).
Treatment
The treatment regimen was variable due to the heterogeneity of the patient group and disease status, but generally involved surgery followed by postoperative radiotherapy, except in those with unresectable disease. Surgery was performed with the goal of gross total resection of the primary tumor either via an open or endoscopic approach. Neck dissection was reserved for those patients with node-positive disease. Six patients were additionally given systemic platinum-based chemotherapy for distant metastases. In the more recent cases, immune checkpoint inhibition (either PD-1 or CTLA-4 checkpoint inhibitor) was incorporated into the treatment regimen.
Outcomes
The primary outcome was overall survival (OS). Secondary outcomes were disease-specific survival (DSS), local progression free survival (LPFS), and distant metastases free survival (DMFS). Outcomes were defined as the time from the diagnosis to the occurrence of an event (recurrence or death) or to the last follow-up.
Statistical Analysis of Outcomes
LPFS, DMFS, OS, and DSS were calculated using the Kaplan Meier method. Univariate analysis was performed using the log rank test. Those factors that were found to be significant on univariate analysis were then assessed by multivariate analysis using a Cox regression analysis and log rank test. Descriptive analyses were performed using chi-square test. A TV cutoff was determined according to OS on a receiver operating characteristics (ROC) curve and selected to achieve the best sensitivity and specificity combination. All analyses were performed using SPSS Statistics v.25 (Windows) software (IBM, Armonk, NY). Reported p-values were two-tailed when available, and for all tests a value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 102 patients who were treated for SNMM at our institution during the study period. Thirty patients were excluded because there was no pre-treatment imaging to review (either because the hard copy had not been uploaded to the electronic system, or because the imaging was performed at an outside institution and the images were not available). Eleven patients were excluded because they had undergone extensive biopsies prior to obtaining structural imaging. This left 61 patients eligible for inclusion in the study. There were 29 males and 32 females, with a mean age at diagnosis of 69 years (Table 1). Median duration of follow-up was 27 months (range, 3 – 161 months).
Table 1.
Patient characteristics at presentation with sinonasal mucosal melanoma
| Patient Characteristics | |
|---|---|
| Total No. of patients | 61 |
| Sex (%) | |
| Female | 32 (52.5%) |
| Male | 29 (47.5%) |
| Age | |
| Mean (range) | 69 (46 – 85) |
| Treatment (%) | |
| Surgery | 51 (83.6%) |
| Radiotherapy | 50 (82.0%) |
| Chemotherapy | 6 (9.8%) |
| Immunotherapya | 20 (32.9%) |
| T classificationb at presentation (%) | |
| 3 | 30 (49.2%) |
| 4a | 22 (36.1%) |
| 4b | 9 (14.8%) |
| N classificationb at presentation (%) | |
| N0 | 57 (93.4%) |
| N1 | 4 (6.6%) |
| M classificationb at presentation (%) | |
| M0 | 54 (88.5%) |
| M1 | 7 (11.5%) |
| Overall stageb | |
| III | 30 (49.2%) |
| IVa | 17 (27.9%) |
| IVb | 7 (11.5%) |
| IVc | 7 (11.5%) |
| Tumor volume | |
| <5 cm3 | 22 (36.1%) |
| ≥5 cm3 | 39 (63.9%) |
Immune checkpoint inhibition with PD-1 or CTLA-4, either alone or in combination
American Joint Committee on Cancer (AJCC) 7th edition staging system
Staging
A combination of imaging modalities was used to stage patients. For local staging, CT sinuses with contrast was used in 29 patients (47.5%), predominately early in the study period, and MRI sinuses in the remaining 32 (52.5%). For distant staging, PET scan was used in 53 patients (86.9%) and CT chest / abdomen / pelvis in 4 patients (6.6%), one of whom also had a bone scan. The remaining three patients, all of whom were treated early in the study period, had a chest radiograph alone as the only means of distant staging (4.9%).
Treatment
Surgery was performed in 50 patients (82.0%); this entailed endoscopic resection in 13, open transfacial resection in 28, and craniofacial resection in 9 patients. Surgery was performed with a goal of gross total resection, this was achieved in 48 patients per the operative report. Due to the variability in quality of historical pathology and operative reports, as well as the piecemeal nature of endoscopic resections, it was not always possible to retrospectively confirm complete microscopic clearance of disease. Surgery was therefore subdivided into “gross total resection” (n=48) and “subtotal resection” (n=2). Surgery was followed by adjuvant radiotherapy in 41 patients (82.0%), and adjuvant chemotherapy in 5 patients (10.0%).
The reasons for not offering surgery to patients were unresectable local disease (n=8), widespread distant metastases (n=2), and patient refusal (n=1). Of the 11 patients treated non-surgically, the remainder all received definitive radiotherapy (n=10), often in combination with immunotherapy (n=7), with the exception of one patient who received palliative chemotherapy and immunotherapy alone.
Outcomes
The median OS was 37 months (range 3 – 161). The 1-, 3-, and 5-year OS estimates were 82.0%, 53.0% and 33.4%, respectively, with corresponding DSS estimates of 83.4%, 55.8%, and 39.0%, respectively.
Local recurrence was observed in 27 patients (44.3%) and regional recurrence in 14 (30.0%). In patients who developed local recurrence, the median time to recurrence was 17 months (range 2 – 115 months). The 1-, 3-, and 5-year LPFS estimates were 81.0%, 61.3%, and 45.4%, respectively.
Seven patients had distant metastases at presentation (11.4%) and a further 31 developed them during the period of observation, resulting in a total of 38 patients with distant metastases (62.3%) The median time to development of distant metastases was 12 months (range 0 – 115 months). The 1-, 3-, and 5-year DMFS estimates were 62.9%, 42.6%, and 29.4%. Twenty-one patients developed both local recurrence / progression and distant metastases (34.4%), and there was no association between LP and DM (p=0.380).
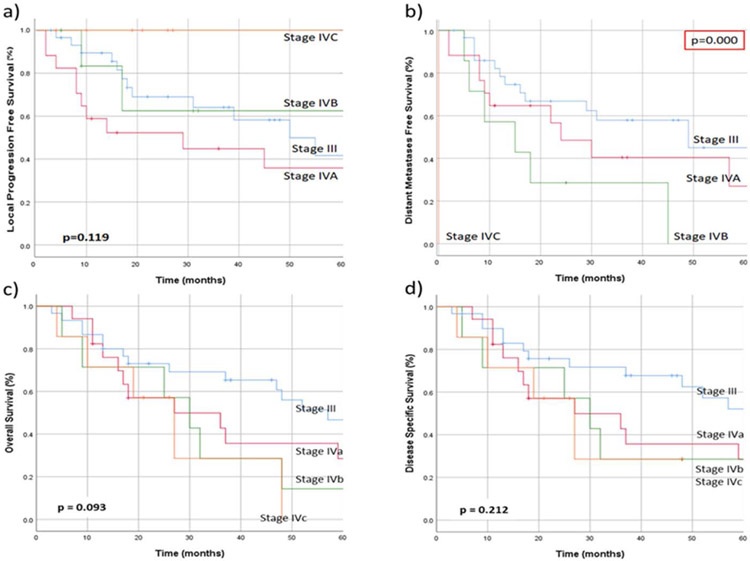
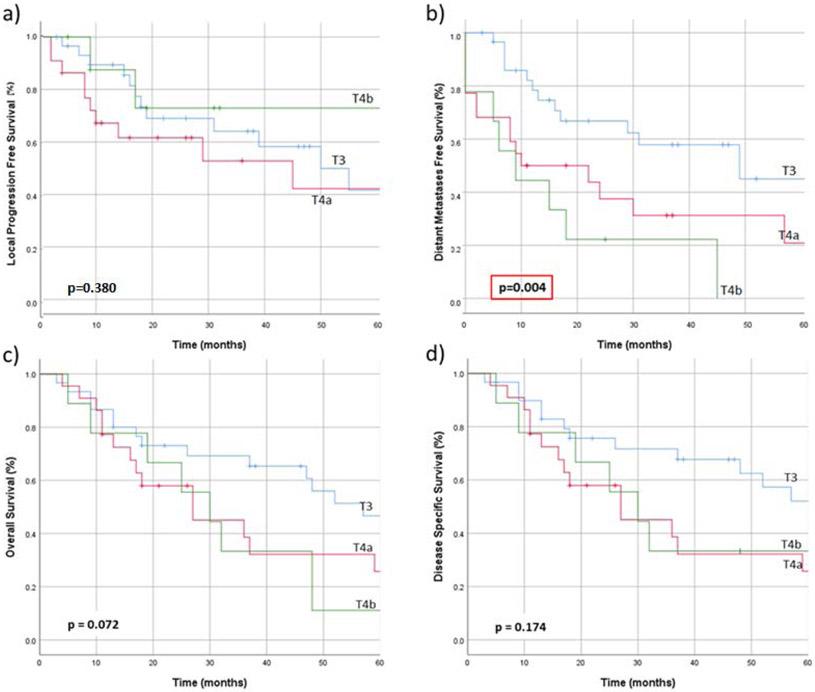
AJCC 7th edition stage grouping was not predictive of LPFS (p=0.119), OS (p=0.093), or DSS (p=0.212). Overall stage did predict DMFS (p<0.001), but this was expected due to the incorporation of distant metastases into the staging system (Figure 2). T-classification predicted DMFS (o=0.004) but not LPFS(p=0.380), OS (p=0.072) or DSS (p=0.174) (Figure 3).
Figure 2.
a) Local progression free survival, b) distant metastases free survival, c) overall survival, and d) disease-specific survival in patients with sinonasal mucosal melanoma, when stratified according to AJCC 7th edition stage grouping.29,30
Figure 3.
a) Local progression free survival, b) distant metastases free survival, c) overall survival, and d) disease-specific survival in patients with sinonasal mucosal melanoma, when stratified according to AJCC 8th edition T-classification.29
Other patient and treatment factors including age, sex, surgical resection, type of resection, and radiotherapy were not predictive of LPFS, DMFS, OS, or DSS. Receiving chemotherapy was predictive of worse OS and DSS (p=0.039, p=0.026, respectively) and this retained significance in a multivariate model incorporating AJCC staging (hazard ratio [HR] 2.454; 91% confidence interval [CI] 1.002 – 6.007; p=0.049).
Tumor Volume
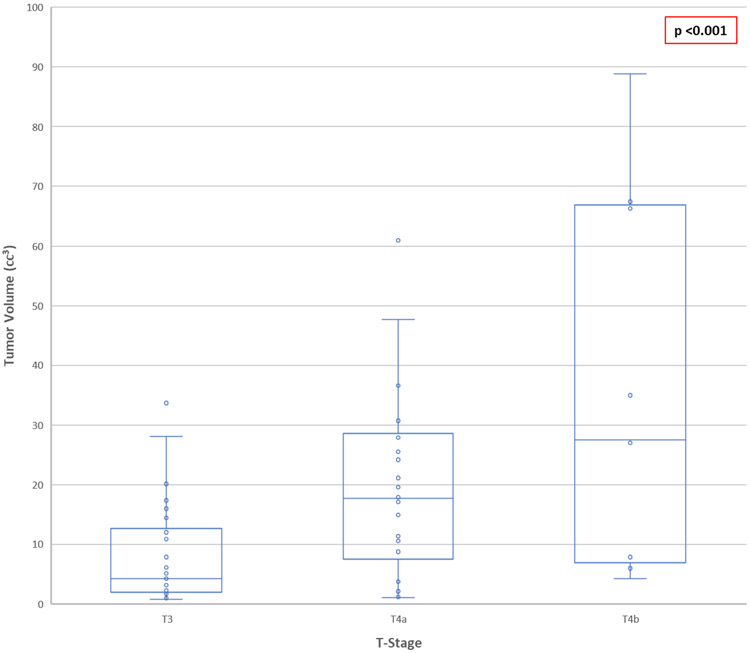
Mean TV was 17.79 cm3 (median, 10.85 cm3; range, 0.75 – 101.79 cm3). TV increased with increasing tumor stage (Figure 1). The mean TV in those patients who went on to develop distant metastases was 21.08 cm3 compared to 11.53 cm3 in patients who remained free of distant metastases (p=0.049). There was no significant different in mean TV in patients who progressed locally compared to those who did not (p=0.261). However, when analyzed as a continuous variable, TV was found to be predictive of LPFS (p=0.032), DMFS (p=0.002), OS (p=0.022), and DSS (p=0.036). On multivariate analysis incorporating AJCC stage, T-classification, and TV, it remained significance only for LPFS (HR 1.031; 95% CI 1.007 – 1.056; p=0.010).
Figure 1.
Tumor volume in patients with sinonasal mucosal melanoma, stratified according to American Joint Committee on Cancer (AJCC) 8th edition T- classification, compared using analysis of variance (ANOVA).
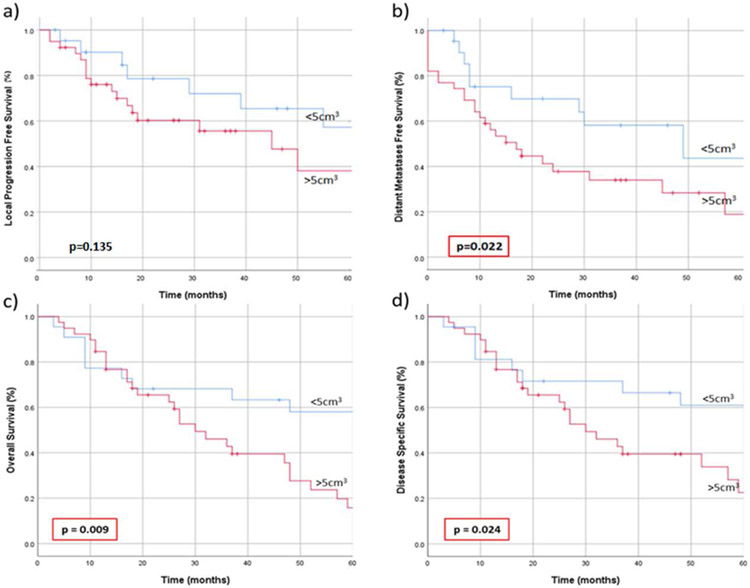
Using a ROC curve to determine the optimal volume cutoff to differentiate between OS outcomes, tumors were divided into “small” (<5 cm3) and “large” (≥5 cm3). The calculations were also repeated for DMFS outcomes with a similar cut off found. Patients with TV ≥5cm3 had worse DMFS (p=0.022), OS (p=0.009), and DSS (p=0.024) (Figure 4). The proportion of patients with small tumors who developed distant metastases within 5 years was 45.5%, compared with 66.7% of patients with large tumors (p =0.290). The 5-year OS was 59.1% for small and 30.8% for large tumors, respectively, with a median survival of 80 months compared to 30 months (p<0.001).
Figure 4.
a) Local progression free survival, b) distant metastases free survival, c) overall survival, and d) disease-specific survival in patients with sinonasal mucosal melanoma, when stratified according to tumor volume into those with “small” tumors (<5 cm3) and “large” tumors (≥5 cm3).
Comparison of a sample of TV measurements obtained by the study neuroradiologist with those recorded in the pre-operative imaging produced an ICC of 0.757.
DISCUSSION
SNMN is an aggressive malignancy, and treatment options are limited by its location adjacent to critical structures within the head and neck. Current staging systems do not attempt to predict the risk of locoregional and distant failure. The prognostic stage grouping has been removed from the AJCC 8th edition staging system which limits the ability of this staging system to be used for prognostication.29 Identifying factors that can predict patient outcomes will result in more accurate prognostication and thus have the potential to guide treatment decisions.
Our results show that TV is predictive of LPFS, DMFS, OS, and DSS among patients with SNMM. This is the first time that tumor volume has been used as a prognostic indicator in this setting.
TV has been described as a predictor of response to radiotherapy in the head and neck radiation oncology literature.32,33 Chen et al found TV < 30 cm3 was predictive of improved cause-specific survival,34 and <40 cm3 was predictive of local-relapse free survival35 in patients with hypopharyngeal cancer treated with primary chemoradiotherapy. Rutkowski found TV was predictive of local control and overall survival in patients with laryngeal cancer.36 Dziegielewski identified a cut off of <2.5 cm3 as being predictive of worse tumor control rates in patients with T3 glottic cancer. Knegjens studied 360 patients undergoing primary chemoradiotherapy for head and neck cancer and found a 14% increased hazard ratio for local recurrence per 10 cm3 increase in tumor volume.37 Dejaco et al measured tumor volume in 125 patients undergoing surgical resection for head and neck cancer, and found that volume was predictive of worse overall survival28
The challenge in using a continuous variable such as TV to predict risk is that it does not enable stratification of patients. For this reason, we divided TV into two groups based on whether the tumor was less than or greater than 5 cm3 in volume. Our results demonstrate tumors ≥5 cm3 had worse DMFS, OS and DSS. There was no significant difference in rates of LPFS (despite the fact that TV was predictive of LPFS when analyzed as a continuous variable), and we hypothesize that this may have been because the 5 cm3 cutoff was not optimized for this outcome. It is important to acknowledge that this is an arbitrary dichotomization, and it is possible that an alternative cutoff volume (or volumes) may be more appropriate. Nevertheless, we believe it is helpful to group patients in this way as it can be used to guide therapeutic decisions. Further research should focus on whether volume can be subdivided into additional risk categories.
To evaluate the usefulness of dichotomized TV as a predictive variable, we compared it to other staging systems. Among our patient cohort, we found the AJCC 7th edition stage grouping for HNMM to be less accurate for predicting OS and DSS. These results are similar to those obtained by Michel et al and Houette et al, who also found the AJCC HNMM stages did not stratify into appropriate survival curves, except among those patients with distant metastases.25,27 This is in contrast to the work of Moya-Plana et al, who found the staging system performed well for OS, PFS, and DMFS.26
One explanation for the limited performance of AJCC staging may be that patients with distant metastases are automatically classified as having the highest stage of disease. Although the presence of metastatic disease has been considered a pre-terminal event, this is now changing in the era of effective systemic therapy. We therefore chose to examine the AJCC T-classification independently.
SNMM is unique in that, due to the poor prognosis and propensity for local recurrence, T-classification begins with T3 disease. This reduces the range of T-classifications that can be assigned to a tumor to T3, T4a, and Tb4. The reduction in range of classifications is arguably beneficial in terms of risk stratification, because it results in fewer comparison groups and therefore an increased likelihood of significant differences. Other authors have shown T-classification to be predictive of OS.7,13,26 We found T-classification did not predict OS but did predict DMFS. This finding should be considered in the context of how T-classification relates to tumor size. The T-classification reflects the anatomical extent of disease, and our results show that it also correlates with the TV. It is therefore unclear whether the association between T-classification and DM is reflective of tumor size or extent. Of note, all patients with tumors <5 cm3 in volume were classified as T3, thus providing further evidence for the crossover between anatomical and volume stratification. It is possible that both factors contribute to the risk of metastatic disease, and further work should focus on determining the relative contributions of each of these factors.
We chose to calculate TV using a validated method of calculating the ellipsoid volume based on maximum orthogonal diameters in three planes. This is in contrast to the methods used in other series, in which the tumor was outlined slice-by-slice and a computer algorithm calculated the total volume.34-39 The computerized method is dependent on the presence of digitalized data and is therefore logistically challenging if only hardcopy films are available, as was the case in some of our earlier cases. The method we used was described31 and validated28 by Dejaco et al, who demonstrated its use over a wide range of tumor sizes. They reported an average 8% underestimation when compared to the gold standard technique. Alternative methods of manually calculating tumor volume, such as a single measurement of tumor diameter or cuboid tumor volume estimates, have been shown to be less accurate.31 However, we recognize that digitalized measurement has advantages over our method, particularly in the case of irregularly shaped tumors, and this is a limitation in our study.
Tumor volume assessment is impaired in patients who have had extensive diagnostic biopsies prior to imaging, thus reducing the true volume of the remaining tumor. It is standard practice at our institution to obtain pre-biopsy imaging in all patients with suspected sinonasal malignancies prior to surgical biopsy. Following this practice will avoid the potential difficulties encountered with measurement of post-biopsy tumors.
We faced technical challenges in the measurement of TV in some instances. For those patients who underwent CT scan with contrast, tumor extent can be difficult to delineate due to trapped secretions. This issue was resolved by the more widespread use of MRI in later years of the study, however it is possible that it may have affected results from patients earlier during the study period. Due to this consideration, as well as the superior soft tissue detail offered by MRI, we suggest that those planning to use tumor volume assessment in their own practice obtain measurements from MRI in preference to CT if possible.
In patients with sessile or multifocal lesions, the full extent of the tumor is sometimes indistinct on imaging. This could potentially lead to underestimation of the TV. Unfortunately, due to the retrospective design of our study, we were not able to identify how many patients had clinically detectable satellite or multifocal lesions at the time of diagnosis. However, be believe the addition of this information may be helpful to further risk-stratify lesions and we encourage future researchers to consider how satellite lesions affect the usefulness of TV as a predictive value.
An important consideration in tumor volume calculation lies in the potential for interobserver variability between reporting neuroradiologists.33 This is particularly true of tumors with an irregular shape. We assessed the potential impact of this variability by calculating the ICC based on the pre-operative imaging reports, with a result of 0.757. According to the guidelines proposed by Koo and Li, this represents good interobserver reliability.40 Nevertheless, the lack of a second independent radiologist in our study for measurement comparison is a limitation in our study design.
We had difficulty establishing true margin status in some patients due to lack of clarity in operative and pathology reports, as well as the piecemeal nature of some endoscopic resections. We recently published a paper from our institution showing no difference in outcomes between patients with SNMM who had traditional wide-margin surgery compared to endoscopic gross total resection19. We therefore classified patients as having undergone “gross total resection (GRT)” or “subtotal resection (STR)”. This classification is based on the surgeon’s subjective assessment and thus is a potential source of bias. Thus, the subjective assessment of GRT leading to uncertainty in determination of true margin status is a limitation of this study.
These results should be interpreted in the context of the observed heterogeneity in treatment strategies. Management protocols have advanced over the study period; immunotherapy, seldom used prior to 5 years ago, has now emerged as a pillar of our current treatment regimen. Whereas the National Comprehensive Cancer Network guidelines provide a suggested management paradigm, a number of our patients were treated as part of clinical trials and therefore deviated from this framework. Given our small sample size, it was not possible to reach any conclusions regarding the effect of these variations on our results.
Although this dataset was prospectively maintained, some data required retrospective collection. Therefore, this study is limited by the usual challenges in including selection bias, variable treatment modalities, and incomplete data. The limited number of patients precluded identification of additional volume cutoffs that would have helped to further stratify risk. While we acknowledge that the rarity of SNMM renders prospective data collection logistically challenging, further research should aim to calculate pre-treatment TV. Evaluation of TV in a larger cohort will result in a better understanding of how TV and other variables interact to predict prognosis.
CONCLUSION
Primary tumor volume is predictive of LPFS, DMFS, OS and DSS on univariate analysis, but only LPFS on multivariate analysis. Consideration should be given to how this measure can be incorporated into future staging systems to improve prognostication. Patients with large tumor volume should be considered as high risk for presenting with or developing distant metastases and having poor survival outcomes. Clinical trials using these parameters are indicated to show the impact of adjuvant therapies such as immunotherapy in these high-risk patients.
Funding Acknowledgment:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest/Disclosure Statement: The authors declare no conflict of interest.
REFERENCES
- 1.Moreno MA, Roberts DB, Kupferman ME, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010;116(9):2215–2223. [DOI] [PubMed] [Google Scholar]
- 2.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24(3):247–257. [DOI] [PubMed] [Google Scholar]
- 3.Bridger AG, Smee D, Baldwin MA, Kwok B, Bridger GP. Experience with mucosal melanoma of the nose and paranasal sinuses. ANZ J Surg. 2005;75(4):192–197. [DOI] [PubMed] [Google Scholar]
- 4.Prasad ML, Busam KJ, Patel SG, Hoshaw-Woodard S, Shah JP, Huvos AG. Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch Pathol Lab Med. 2003;127(8):997–1002. [DOI] [PubMed] [Google Scholar]
- 5.Rinaldo A, Shaha AR, Patel SG, Ferlito A. Primary mucosal melanoma of the nasal cavity and paranasal sinuses. Acta Otolaryngol. 2001;121(8):979–982. [PubMed] [Google Scholar]
- 6.Amit M, Na’ara S, Hanna EY. Contemporary Treatment Approaches to Sinonasal Mucosal Melanoma. Curr Oncol Rep. 2018;20(2):10. [DOI] [PubMed] [Google Scholar]
- 7.Koivunen P, Back L, Pukkila M, et al. Accuracy of the current TNM classification in predicting survival in patients with sinonasal mucosal melanoma. Laryngoscope. 2012;122(8):1734–1738. [DOI] [PubMed] [Google Scholar]
- 8.Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R. Mucosal melanoma of the head and neck: a systematic review of the literature. Int J Radiat Oncol Biol Phys. 2014;90(5):1108–1118. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi D, Bottazzoli M, Turri-Zanoni M, et al. Sinonasal mucosal melanoma: A 12-year experience of 58 cases. Head Neck. 2016;38 Suppl 1:E1737–1745. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YF, Lai CC, Ho CY, Shu CH, Lin CZ. Toward a better understanding of sinonasal mucosal melanoma: clinical review of 23 cases. J Chin Med Assoc. 2007;70(1):24–29. [DOI] [PubMed] [Google Scholar]
- 11.Amit M, Tam S, Abdelmeguid AS, et al. Patterns of Treatment Failure in Patients with Sinonasal Mucosal Melanoma. Ann Surg Oncol. 2018;25(6):1723–1729. [DOI] [PubMed] [Google Scholar]
- 12.Sun CZ, Li QL, Hu ZD, Jiang YE, Song M, Yang AK. Treatment and prognosis in sinonasal mucosal melanoma: A retrospective analysis of 65 patients from a single cancer center. Head Neck. 2014;36(5):675–681. [DOI] [PubMed] [Google Scholar]
- 13.Samstein RM, Carvajal RD, Postow MA, et al. Localized sinonasal mucosal melanoma: Outcomes and associations with stage, radiotherapy, and positron emission tomography response. Head Neck. 2016;38(9):1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56(5):828–834. [DOI] [PubMed] [Google Scholar]
- 15.Sun S, Huang X, Gao L, et al. Long-term treatment outcomes and prognosis of mucosal melanoma of the head and neck: 161 cases from a single institution. Oral Oncol. 2017;74:115–122. [DOI] [PubMed] [Google Scholar]
- 16.Gilain L, Houette A, Montalban A, Mom T, Saroul N. Mucosal melanoma of the nasal cavity and paranasal sinuses. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(6):365–369. [DOI] [PubMed] [Google Scholar]
- 17.Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(8):917–923. [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 19.Sayed Z, Migliacci JC, Cracchiolo JR, et al. Association of Surgical Approach and Margin Status With Oncologic Outcomes Following Gross Total Resection for Sinonasal Melanoma. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganti A, Raman A, Shay A, et al. Treatment modalities in sinonasal mucosal melanoma: A national cancer database analysis. Laryngoscope. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68(7):1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troussier I, Baglin AC, Marcy PY, et al. [Mucosal melanomas of the head and neck: State of the art and current controversies]. Bull Cancer. 2015;102(6):559–567. [DOI] [PubMed] [Google Scholar]
- 23.Ballantyne AJ. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am J Surg. 1970;120(4):425–431. [DOI] [PubMed] [Google Scholar]
- 24.Prasad ML, Patel SG, Huvos AG, Shah JP, Busam KJ. Primary mucosal melanoma of the head and neck: a proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer. 2004;100(8):1657–1664. [DOI] [PubMed] [Google Scholar]
- 25.Michel J, Perret-Court A, Fakhry N, et al. Sinonasal mucosal melanomas: the prognostic value of tumor classifications. Head Neck. 2014;36(3):311–316. [DOI] [PubMed] [Google Scholar]
- 26.Moya-Plana A, Mangin D, Dercle L, et al. Risk-based stratification in head and neck mucosal melanoma. Oral Oncol. 2019;97:44–49. [DOI] [PubMed] [Google Scholar]
- 27.Houette A, Gilain L, Mulliez A, Mom T, Saroul N. Prognostic value of two tumour staging classifications in patients with sinonasal mucosal melanoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(5):313–317. [DOI] [PubMed] [Google Scholar]
- 28.Dejaco D, Steinbichler T, Schartinger VH, et al. Prognostic value of tumor volume in patients with head and neck squamous cell carcinoma treated with primary surgery. Head Neck. 2018;40(4):728–739. [DOI] [PubMed] [Google Scholar]
- 29.Amin MB ES, Greene F, et al. (Eds.). AJCC Cancer Staging Manual, 8th edition. Springer International Publishing; 2017. [Google Scholar]
- 30.Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A. (Eds.). AJCC Cancer Staging Manual, 7th edition. Springer International Publishing; 2010. [Google Scholar]
- 31.Dejaco D, Url C, Schartinger VH, et al. Approximation of head and neck cancer volumes in contrast enhanced CT. Cancer Imaging. 2015;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutkowski T The role of tumor volume in radiotherapy of patients with head and neck cancer. Radiat Oncol. 2014;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendenhall WM, Mancuso AA, Strojan P, et al. Impact of primary tumor volume on local control after definitive radiotherapy for head and neck cancer. Head Neck. 2014;36(9):1363–1367. [DOI] [PubMed] [Google Scholar]
- 34.Chen SW, Yang SN, Liang JA, Lin FJ, Tsai MH. Prognostic impact of tumor volume in patients with stage III-IVA hypopharyngeal cancer without bulky lymph nodes treated with definitive concurrent chemoradiotherapy. Head Neck. 2009;31(6):709–716. [DOI] [PubMed] [Google Scholar]
- 35.Chen SW, Yang SN, Liang JA, Tsai MH, Shiau AC, Lin FJ. Value of computed tomography-based tumor volume as a predictor of outcomes in hypopharyngeal cancer after treatment with definitive radiotherapy. Laryngoscope. 2006;116(11):2012–2017. [DOI] [PubMed] [Google Scholar]
- 36.Rutkowski T, Wygoda A, Skladowski K, et al. Prognostic role of tumor volume for radiotherapy outcome in patient with T2 laryngeal cancer. Strahlenther Onkol. 2013;189(10):861–866. [DOI] [PubMed] [Google Scholar]
- 37.Knegjens JL, Hauptmann M, Pameijer FA, et al. Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck. 2011;33(3):375–382. [DOI] [PubMed] [Google Scholar]
- 38.Dziegielewski PT, Reschly WJ, Morris CG, et al. Tumor volume as a predictor of survival in T3 glottic carcinoma: A novel approach to patient selection. Oral Oncol. 2018;79:47–54. [DOI] [PubMed] [Google Scholar]
- 39.Rutkowski T Impact of initial tumor volume on radiotherapy outcome in patients with T2 glottic cancer. Strahlenther Onkol. 2014;190(5):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]