Abstract
Problem
Evaluation of Zika virus (ZIKV)-specific humoral and cellular immune response in pregnant women exposed to ZIKV.
Method of Study
In this observational, prospective cohort study, we recruited pregnant women presenting for prenatal ultrasound for ZIKV exposure at a single academic teaching hospital in Boston, MA from November 2016 to December 2018. We collected blood, urine, and cervicovaginal swabs antepartum, intrapartum, and postpartum; and cord blood and placenta at delivery. We used experimental assays to calculate quantitative viral loads, ZIKV-specific immunoglobulin titers, and ZIKV-specific T cell responses.
Results
We enrolled 22 participants, three of which had serologic-confirmed ZIKV infection. No participants demonstrated sustained ZIKV shedding. ZIKV-specific IgG/IgM antibody were sustained throughout pregnancy and postpartum. ZIKV envelope and capsid-specific T cell responses were also observed, albeit inconsistent. No newborns in this cohort had congenital Zika syndrome. Infant cord blood of infected mothers exhibited ZIKV-specific IgG, but not IgM antibodies.
Conclusions
We detected a robust, prolonged maternal humoral immune response to ZIKV during pregnancy and postpartum. We also demonstrated evidence for efficient transplacental antibody transfer from mother to infant at birth, supporting the importance of neonatal passive immunity to ZIKV. Maternal T cell responses were less consistent amongst pregnant women infected with ZIKV.
Keywords: Congenital infection, maternal immune response, pregnancy, transplacental transfer, Zika virus
GRAPHICAL ABSTRACT
Introduction
Zika virus (ZIKV) has recently become a major concern worldwide due to the causative association with spontaneous abortion and congenital Zika syndrome (CZS).1–3 CZS is characterized by impaired fetal growth, microcephaly, arthrogryposis, developmental delay, hearing and vision impairments, and other birth defects with significant lifelong neurodevelopmental sequelae. Symptoms of ZIKV infection in adults are typically self-limiting and mild (fever, rash, conjunctivitis, joint pain) when compared to other members of the flavivirus family (including dengue, yellow fever, Japanese encephalitis, and West Nile viruses) and rarely causes severe complications like Guillain-Barré syndrome.4,5 ZIKV is unique in its capacity to be transmitted through mosquito bites and sexual contact; and is one of the few known viruses that can be transmitted from mother to child during pregnancy. Prevalence of CZS amongst ZIKV-infected pregnant women varies by region. During the 2016 epidemic and public health emergency in Brazil, as high as 42% of infants born to ZIKV-infected women were reported to have clinical sequelae6; however, these initial reports were likely to be overestimated due to non-specific definitions of microcephaly with 35–87% of infants with reported microcephaly attributable to CZS.7,8 Data from the United States Zika Pregnancy Registry observed that 6% of infants born to women with laboratory evidence of ZIKV infection during pregnancy exhibited CZS, with an 11% prevalence in women infected in the first trimester.9 A similar rate was reported in the French territories in the Americas.10 Moreover, newer reports suggests that 14–22% of infants exposed to ZIKV developed ZIKV-associated birth defects, neurodevelopmental abnormalities associated with ZIKV infection, or both later in life.11 Although the global epidemic has subsided, this mosquito-borne, and sexually transmitted virus is likely to resurface as an important cause of congenital infection because there is no available treatment to prevent CZS.
Sustained ZIKV viremia has been reported in pregnant women,12 and several studies have revealed the importance of various anatomic reservoirs, such as semen, cerebrospinal fluid, and lymph nodes.13,14 This predisposition to sustained viremia in pregnancy suggests that ZIKV has tropism for placenta and fetal brain—tissues which are typically protected from maternal immune attack in order to sustain the allogeneic developing pregnancy. By using the immunologically protected placenta and fetal compartment as a reservoir, persistent ZIKV shedding and destruction during pregnancy may result in the clinical outcomes of miscarriage, impaired fetal growth, and CZS.15–18 Variation in maternal exposure timing, route, and the characteristics of the maternal immune response may be helpful in predicting which pregnancies result in CZS.19 Although the adaptive immune response has been studied in mouse and non-human primate models of ZIKV infection.20,21 little is understood about the human immunologic response to ZIKV during pregnancy. This study evaluated maternal immune responses that were associated with viral convalescence as well as fetal immune responses in infant cord blood.
Materials and Methods
Human subjects
The study was approved by the Beth Israel Deaconess Medical Center (BIDMC) institutional review board (protocol #2016P-000334).
Study design
We conducted a longitudinal prospective cohort study at a single academic teaching hospital in Boston, MA from November 2016 to December 2018. We approached pregnant women age 18 years or older presenting to the BIDMC Department of Obstetrics and Gynecology for ultrasound or Maternal Fetal Medicine consultation for possible exposure to ZIKV. Participants were eligible if they were 18 years or older, pregnant at any gestational age and had possible ZIKV exposure. ZIKV exposure was defined as travel to a region of local ZIKV transmission within 12 weeks of conception through the 3rd trimester or sexual contact with a partner who traveled to an area of ZIKV transmission within 6 months prior to conception. Participants answered a brief questionnaire detailing travel history, symptoms of ZIKV infection (including fever, rash, myalgia, or arthralgia), sexual history, partner travel history and symptoms, vaccine history, and history of infection with other flaviviruses.
Clinical ZIKV testing
At BIDMC, pregnant patients exposed to ZIKV were offered clinical testing in accordance to the recommended Center for Disease Control and Prevention (CDC) guidelines in place at the time of the visit.22 We collected blood samples for serum ZIKV IgM enzyme-linked immunoassay (EIA) and/or serum ZIKV polymerase chain reaction (PCR). We also collected urine samples for ZIKV PCR if the exposure occurred within less than 2 weeks of testing. We submitted all samples to the Massachusetts Department of Public Health (MDPH) for approval and clinical testing. This cohort of ZIKV-exposed participants were categorized using the clinical serology test results (i.e. positive or negative by ZIKV IgM EIA or unknown if they opted out of specimen collection).
Human specimen collection
We screened each participant for fetal growth restriction and microcephaly every 4–6 weeks in congruence with the CDC and the American College of Obstetricians and Gynecologists (ACOG) guidelines for ZIKV exposure management at time of enrollment. We collected maternal blood, urine, and cervicovaginal specimens at time of enrollment, intrapartum (time of delivery) and at the postpartum visit (2–8 weeks following delivery). Umbilical cord blood and placental fragments were also collected from participants at the time of delivery. Placenta fragments were saved in RNALater (Life Technologies, CA, USA) for ZIKV RT-PCR. For any participants undergoing amniocentesis during the enrollment period, we collected discarded amniotic fluid supernatant.
We obtained patient demographics, obstetric history, prenatal ultrasound reports, delivery outcomes, and neonatal clinical information from the electronic medical record. We obtained neonatal blood samples only when there was a clinical reason (i.e. non-research) to do so for up to three months post-delivery.
Human blood processing
Maternal blood and infant umbilical cord blood were collected in tubes containing EDTA and in serum separator tubes. Serum was aspirated and saved for viral load and ELISA antibodies. Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat of EDTA tubes after centrifugation using a Ficoll gradient. Viable PBMCs were counted and stored in 10% DMSO in FBS freezing media at 107 cells/mL in liquid nitrogen for ELISPOT assays.
Study assays
ZIKV RT-PCR
RT-PCR assays were performed on all maternal blood, urine, cervical swabs, placenta, and umbilical cord blood to monitor viral loads, as previously described.23,24 RNA was extracted from blood plasma, urine, cervical swabs, and placenta with QIAcube HT (QIAGEN, Germany) using the Qiacube 96 Cador pathogen HT or the Qiacube 96 RNeasy 96 kit as previously described.14 RNA standards were generated from wild-type ZIKV BeH815744 capsid (C) gene using the AmpliCap-Max T 7 High Yield Message Maker Kit (CellScript, WI, USA) and purified with RNA clean and concentrator kit (Zymo Research, CA, USA). RNA quality and concentration were assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay Viral loads were reported as virus particles (VP) per ml or virus particles per 106 cells with assay sensitivity of >100 copies/ml and >100 ZIKV copies/106 cells.
ZIKV Serum ELISA antibody titers
Human ELISA kits (Alpha Diagnostic International, TX, USA) to ZIKV envelope (E) protein and ZIKV nonstructural protein 1 (NS1) were used to determine endpoint binding antibody titers from maternal and infant cord blood serum using a previously described protocol.23 Both of these kits detect ZIKV-specific IgG. For IgM detection, a ZIKV ELISA using SPH2015 NS1 (Sino Biological, Inc, China) was developed. 96-well plates were coated with 100μg of ZIKV E or NS1 protein at a concentration of 1μg/mL overnight at 4°C, washed with PBS containing 0.05% Tween 20 (PBS-T) and blocked using Blocker™ Casein (Thermo Fisher Scientific, USA) per manufacturer’s guidelines. Serum was added to coated plates in 3-fold serial dilutions from 1:25 to 1:54,675 and incubated for 1 hour at room temperature. After washing with PBS-T, anti-human IgM HRP-conjugate (Thermo Fisher Scientific, USA) was added and incubated at room temperature for 1 hour, followed by washing with PBS-T. Plates were developed in TMB substrate (SeraCare Life Sciences, MA, USA) for 2.5 minutes prior to addition of stop solution (SeraCare Life Sciences, MA, USA), then analyzed at 450nm/550nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices, CA, USA). ELISA endpoint titers, defined as the highest reciprocal serum dilution that yielded an absorbance >2-fold over background, were reported as Log10 endpoint titers.
ZIKV-specific interferon-γ T cell responses by ELISPOT
Cellular immune responses specific to ZIKV were assessed by interferon-γ (IFN-γ) ELISPOT assays using pools of overlapping 15-amino-acid peptides covering the pre-membrane (prM), E, C, and NS1 proteins (JPT, Berlin, Germany), as previously described.23,24 96-well multiscreen plates (Millipore, MA, USA) were pre-treated with 1μg/well of anti-human IFN-γ (BD Biosciences, CA, USA) overnight in endotoxin-free Dulbecco’s PBS (DPBS). After coating for 18–72 hours, plates were washed with DPBS, blocked using RPMI 1640 media containing 10% FBS, and then 2×105 PBMCs were co-incubated with 2μg/mL per well of peptide for 15–20 hours at 37°C. The plates were washed with DPBS-Tween 7 times, incubated with 1μg/mL per well of biotinylated anti-human IFN-γ (BD Biosciences, CA, USA) for 2 to 4 hours at room temperature, followed by 4 washes with DPBS-Tween and 1.33μg/mL per well of alkaline phosphatase-conjugated anti-biotin (Southern Biotechnology Associates, AL, USA) for 2 to 3 hours at room temperature. Plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, IL, USA), stopped by washing with tap water, and read using an ELISPOT reader (KS ELISPOT Reader, Carl Zeiss). The numbers of spot-forming cells (SFC) per 106 cells were calculated subtracted over background (PBMCs incubated with media and DMSO without peptide). Background levels were typically < 15 SFC per 106 cells.
Statistical Analysis
We collected and stored data using REDCap, a HIPAA compliant, web-based data collection tool stored in a restricted-access folder on the BIDMC secure server. Analysis and display of virologic and immunologic data was performed using GraphPad Prism v6.03 (GraphPad Software, CA, USA).
Results
Enrollment
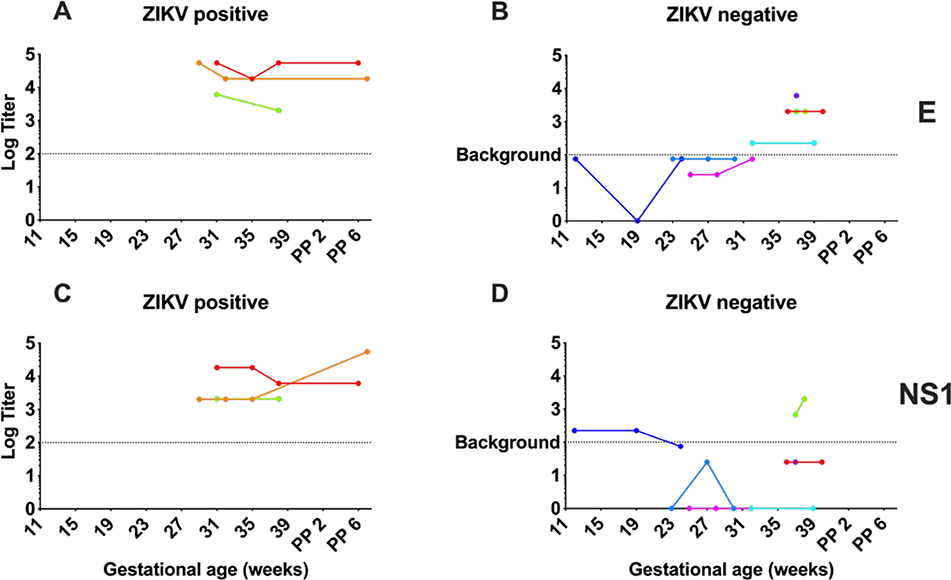
We enrolled 22 participants (Table 1). Three participants had positive MDPH ZIKV serologic testing, 8 had negative testing, and 11 had unknown clinical ZIKV testing status either because they declined testing or were outside of the temporal testing guidelines by MDPH. One of the participants with positive ZIKV serologic testing (displayed in red for Figure 1A, 1C and 2A, 2C, 2E, 2G; patient 1 in Figure 3) had a symptomatic infection during travel to a high-transmission region in the first trimester and also had detectable ZIKV by PCR on clinical testing of the urine. Another participant with ZIKV positive serology (displayed in orange for Figure 1A, 1C and 2A, 2C, 2E, 2G; patient 2 in Figure 3) likely represents a preconception ZIKV infection. She reported symptoms of infection within the 12 weeks preceding conception, had ongoing exposure from living in a high-transmission region until the second trimester, and then underwent clinical ZIKV testing when she presented for care in Boston at 27 weeks. The third participant with ZIKV positive serology (displayed in green for Figure 1A, 1C and 2A, 2C, 2E, 2G) reported no symptoms and was also living in a high-transmission region from preconception until the 2nd trimester; therefore, timing of infection is uncertain. This third participant had a delivery outside of BIDMC; therefore, no infant cord blood was collected.
Table 1.
Characteristics of study participants
| Maternal age (year) | 33 ± 5 |
| Gestational age at delivery (week) | 38 ± 5 |
| Race/Ethnicity | |
| White | 6 (27) |
| Black | 2 (9) |
| Asian | 2 (9) |
| Multi-racial | 1 (5) |
| Unknown/Other | 11 (50) |
| Hispanic | 11 (50) |
| Clinical Zika testing | |
| Negative | 8 (36) |
| Positive | 3 (14) |
| Unknown/declined | 11 (50) |
| Gestational Age at Exposure | |
| Preconception | 13 (59) |
| First trimester | 16 (72) |
| Second trimester | 9 (41) |
| Third trimester | 3 (14) |
| Exposure type | |
| Living in high transmission area | 9 (41) |
| Travel to high transmission area | 14 (64) |
| Sexual exposure | 16 (73) |
| Exposure region | |
| North America | 5 (23) |
| Caribbean | 11 (50) |
| Central America | 1 (5) |
| South America | 6 (27) |
| Africa/Cape Verde | 1 (5) |
| Asia/Pacific Islands | 0 |
| Infection history | |
| Symptoms of Zika infection | 3 (14) |
| Prior non-Zika flavivirus infection | 2 (9) |
| Vaccine history | |
| Yellow fever vaccine | 5 (23) |
All values expressed as mean ± standard deviation or n (%)
Figure 1. Maternal ZIKV antibody levels persist during pregnancy and postpartum.
Maternal serum log10 E-specific ELISA titers (A, B) and NS1-specific ELISA titers (C, D) following ZIKV exposure from 11 weeks gestational age in pregnancy to 6 weeks postpartum (PP). Serial titers in participants with positive clinical ZIKV testing (A, C) compared to antibody titers in participants with negative ZIKV testing (B, D). Assay background displayed on each graph as a dotted line.
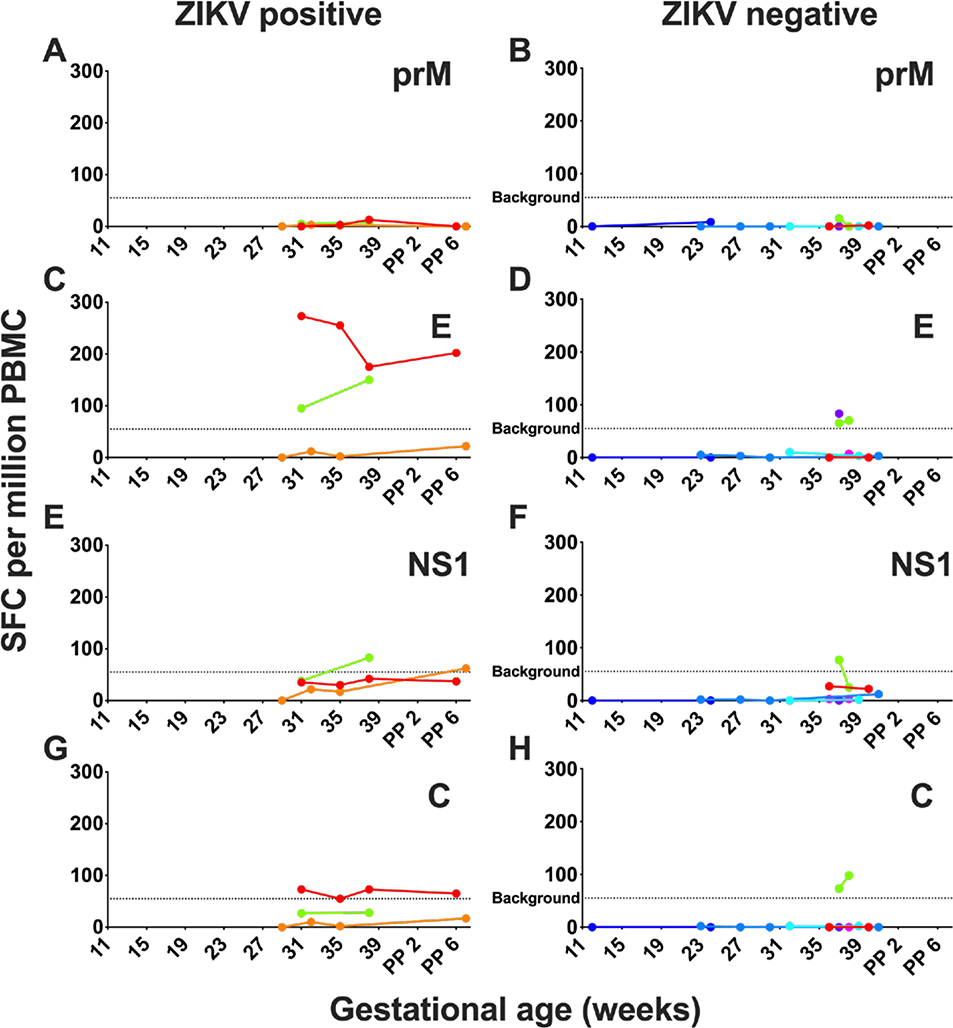
Figure 2. Human maternal cellular immune response following ZIKV exposure.
Maternal cellular immune responses were assessed by interferon-γ ELISPOT assays to prM (A, B), E (C, D), NS1 (E, F), and C (G, H) peptides during pregnancy, delivery, and postpartum in participants with negative clinical ZIKV testing (B, D, F, H) and positive ZIKV testing (A, C, E, G) expressed in units of spot-forming units (SFC) per million peripheral blood mononuclear cells (PBMC). Dotted indicates limit of assay positivity.
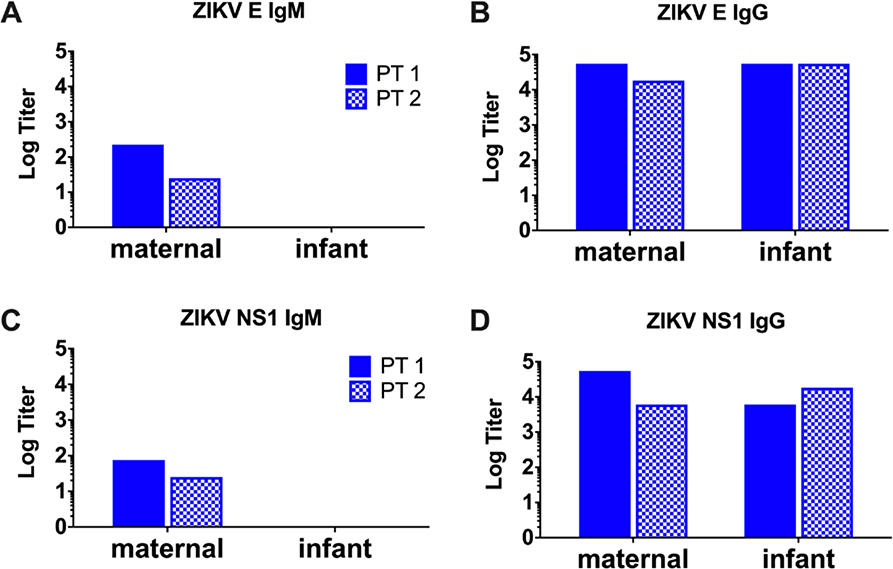
Figure 3. Passive immunity to ZIKV, but not primary fetal immune responses in infant cord blood at delivery.
Serum ELISA for ZIKV E and NS1 IgM (A and C, respectively) antibody levels expressed as log titer in maternal blood (left) and infant cord blood (right) in two patients with positive clinical ZIKV testing. Serum ELISA for ZIKV E and NS1 IgG (B and D, respectively) antibody levels in maternal blood (left) and infant cord blood (right) in the same two patients.
Seventy-three percent of participants reported exposure to ZIKV from sexual contact with a partner living or traveling to a high-transmission area. Sixty-four percent reported exposure to ZIKV from travel to high-transmission area, with the Caribbean being the most common destination. The majority of participants reported exposure to ZIKV during the preconception and/or first trimester of pregnancy. None of the maternal blood, urine, placenta, or infant cord blood samples submitted to MDPH for clinical ZIKV RT-PCR testing at the time of delivery returned as positive. There was no concern for neonatal brain abnormalities or arthrogryposis on prenatal or postnatal imaging for any of the participants and there were no cases of clinically diagnosed CZS.
ZIKV shedding
We monitored ZIKV shedding in maternal blood plasma, urine, and cervicovaginal swabs during pregnancy, delivery, and postpartum. We evaluated for fetal and placental ZIKV infection through infant umbilical cord plasma and placental sampling. All maternal specimens (plasma, urine, cervicovaginal swab, amniotic fluid supernatant) had undetectable ZIKV load by RT-PCR for all time points (data not shown). Our assays did not identify detectable ZIKV viral loads in any of the cord blood or placenta samples. These data show that sustained maternal ZIKV viremia was not observed in our participants, including the 3 with confirmed ZIKV infection during pregnancy.
Adaptive immune responses to maternal ZIKV infection in pregnancy
In the three ZIKV-positive participants, we detected persistent, ZIKV-specific serum antibodies in high titers during pregnancy (Figure 1). For two of the participants who were followed through delivery and postpartum, we continued to detect elevated antibody titers for both ZIKV protein E (Figure 1A) and NS1 (Figure 1C) postpartum. By comparison, in the ZIKV-negative participants, the assays did not detect ZIKV-specific antibody levels consistently above the background (Figure 1B and 1D).
A number of ZIKV-negative participants had low-level ZIKV-specific antibody seropositivity on our experimental assays. This could be explained by participant exposure to dengue and yellow fever viruses (which have overlapping geographic distributions with ZIKV), or prior yellow fever vaccination which may result in detection of cross-reactive dengue and yellow fever antibodies.25,26 Many ZIKV-negative and unknown participants reported prior yellow fever vaccination. Specific ZIKV neutralization assays may be helpful in ruling out serologic cross-reactivity in our cohort.
We evaluated ZIKV-specific cellular immune responses to prM, E, NS1, and C peptides. We did not detect T cell responses to prM peptides above the assay background (Figure 2A, 2B) in any participants. In two participants with ZIKV-positive testing, there was a robust and persistent IFN-γ response to E peptide (Figure 2C). Responses to ZIKV NS1 and C peptides were variable (Figure 2E, 2G). In contrast, we did not detect a sustained cellular response to any of the ZIKV peptides among ZIKV-negative and unknown participants, (Figure 2B, 2D, 2F, 2H).
Adaptive immunity in infants of ZIKV-infected pregnancies
Infant cord blood was collected at the time of delivery and examined for both ZIKV-specific humoral and cellular responses. In two of the cord blood samples from infected mothers, high titers of IgG were observed and closely mirrored the maternal antibody titers at the time of delivery (Figure 3A). These data are consistent with the observations that IgG is transported across the placenta from maternal to fetal circulation via the placental FcRn receptor.27 Transfer of ZIKV-specific IgG appears to be efficient, with no obvious change in concentration from maternal to fetal circulation. In contrast, human pentameric IgM does not get transported transplacentally.28 While NS1-specific IgM was detected in the serum of ZIKV-infected mothers, IgM was not detected in infant serum (Figure 3B). These data suggest that ZIKV-specific antibodies in cord blood reflected passive maternal immunity rather than primary infection of the fetus or contamination with maternal blood. Consistent with these data, no T cell responses to ZIKV antigens by ELISPOT IFN-γ assays were detected in cord blood specimens of ZIKV-infected mothers (data not shown).
Comment
Principal Findings
Our data show adaptive immunity persists throughout pregnancy and postpartum in ZIKV-infected pregnant women.
We also demonstrate maternal to fetal transfer of ZIKV-specific IgG in infant cord blood at the time of delivery. Recently, ZIKV-specific and Dengue virus-specific transplacental IgG transport has been reported in a cohort of mother/infant pairs from Brazil.29 ZIKV-specific IgG has been quantified in infant blood from another case-control study of infants with microcephaly in Brazil.30 Our work complements the findings of these two groups and is the first to report transplacental ZIKV-specific IgG transfer in the United States, in a low-transmission population. Efficient transfer of maternal ZIKV-specific IgG to infant circulation has been observed in the Brazilian population29, which is also corroborated in our study. We speculate that such maternal antibodies may have beneficial effects for the fetus and newborn.
Clinical and Research Implications
Future work needs to confirm these findings in larger numbers of maternal-fetal pairs and explore the possibility that passive transfer of maternal antibodies may help protect a fetus or neonate from CZS. This may be a useful tool in predicting clinical outcomes and CZS. Furthermore, identification of the type of antibodies undergoing efficient transplacental transfer, that are also effective at ZIKV neutralization, may aid in vaccine development.
Strengths and limitations
Strengths of this study include the prospective longitudinal design and rigorous experimental assays used to evaluate cellular T cell and antibody responses to ZIKV. This is one of the few longitudinal studies that evaluate T cell responses during natural infection in human pregnancy and specifically to evaluate ZIKV-specific adaptive immunity in maternal and infant cord blood from ZIKV-infected mothers living in the United States.
The major limitations of this study include the small sample size and absence of infants affected by CZS. This prohibits conclusions about the effect of maternal immune responses on incidence of feto-placental disease or in CZS. Enrollment in this study protocol was less than anticipated due to the rapid decline in the incidence of ZIKV infection in pregnancy during this time period. Larger studies in regions of ongoing transmission would allow further evaluation for maternal immune responses in natural infection in association with the rate of CZS. It is possible that waning IgM levels following infection could lead to falsely negative clinical ZIKV IgM EIA result and misclassification of participants into the ZIKV-negative group. All participants had clinical testing within less than 12 weeks of exposure per CDC guidelines to limit this potential for false negative classification. Furthermore, in participants with ZIKV-positive testing, the ELISA IgM titers were detected throughout pregnancy and postpartum, and demonstrated persistence for over 10 months from initial exposure. This suggests that persistent IgM response after ZIKV infection, and low likelihood for false negative clinical ZIKV testing within this cohort.
In our small cohort, we did not see persistence of viral shedding in blood, urine, or cervicovaginal fluid as has been demonstrated previously.12 These data indicate that persistent viremia is not necessarily a hallmark of ZIKV infection in pregnant women.
Conclusions
Persistent maternal adaptive immune responses to ZIKV infection occur in pregnancy, including ZIKV-specific antibody and cellular immune responses. We also demonstrate transplacental transfer of maternal antibodies to the fetus in natural infection with ZIKV-specific IgG in cord blood at delivery. Maternal IFN-γ T cell responses appear to be less consistent than antibody responses in our cohort. In animal studies, absence of ZIKV-specific T cell response does not appear to significantly impair immunity to ZIKV.24,31 There is a critical need for improved understanding of ZIKV-specific immune responses in maternal-fetal pairs to inform preventative therapies such as vaccines and therapeutic strategies to block vertical transmission during pregnancy. Indeed, vaccine development for ZIKV has progressed at a rapid and promising rate, and purified neutralizing antibodies to ZIKV has become a highly promising candidate currently in Phase I clinical trials.32
Acknowledgements
The authors thank members of the Department of Obstetrics and Gynecology Division of Research including Michele Hacker for assistance in design and implementation of this clinical protocol. The authors thank Barouch Lab members Michael Boyd for assistance with the viral load assays, Katherine McMahan for assistance with ELISA assays, and David Jetton who coordinated the ELISPOT assays for the study. We acknowledge NIH grants (AI124377, AI128751), the Ragon Institute of MGH, MIT, and Harvard. A.Y. Collier received support from the grant K12HD000849, awarded to the Reproductive Scientist Development Program by the Eunice Kennedy Shriver National Institute of Child Health & Human Development and Burroughs Wellcome Fund.
Footnotes
Conflict of Interest/Disclosure Statement
The authors report no conflict of interest. The findings have been presented previously at the Society for Reproductive Investigation Meeting in San Diego, California in March 2018.
References
- 1.Mlakar J, Korva M, Tul N, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374(10):951–958. doi: 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- 2.van der Eijk AA, van Genderen PJ, Verdijk RM, et al. Miscarriage Associated with Zika Virus Infection. N Engl J Med. 2016. doi: 10.1056/NEJMc1605898 [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374(20):1981–1987. doi: 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 4.Cao-Lormeau V-M, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak caused by Zika virus infection in French Polynesia. Lancet. 2016. doi: 10.1017/CBO9781107415324.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dos Santos T, Rodriguez A, Almiron M, et al. Zika Virus and the Guillain-Barré syndrome - Case series from seven countries. N Engl J Med. 2016. doi: 10.1056/NEJMc1609015 [DOI] [PubMed] [Google Scholar]
- 6.Brasil P, Pereira JP Jr., Raja Gabaglia C, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016. doi: 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso D, Ko AI, Baud D. Zika Virus Infection — After the Pandemic. Longo DL, ed. N Engl J Med. 2019;381(15):1444–1457. doi: 10.1056/NEJMra1808246 [DOI] [PubMed] [Google Scholar]
- 8.Krow-Lucal ER, de Andrade MR, Cananéa JNA, et al. Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraíba, Brazil, in 2015–16: a case-control study. Lancet Child Adolesc Heal. 2018. doi: 10.1016/S2352-4642(18)30020-8 [DOI] [PubMed] [Google Scholar]
- 9.Honein MA, Dawson AL, Petersen EE, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA. 2016. doi: 10.1001/jama.2016.19006 [DOI] [PubMed] [Google Scholar]
- 10.Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018. doi: 10.1056/NEJMoa1709481 [DOI] [PubMed] [Google Scholar]
- 11.Rice ME, Galang RR, Roth NM, et al. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital zika virus infection — U.S. Territories and freely associated states, 2018. Morb Mortal Wkly Rep. 2018;67(31):858–867. doi: 10.15585/mmwr.mm6731e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meaney-Delman D, Oduyebo T, Polen KN, et al. Prolonged Detection of Zika Virus RNA in Pregnant Women. Obs Gynecol. 2016;128(4):724–730. doi: 10.1097/AOG.0000000000001625 [DOI] [PubMed] [Google Scholar]
- 13.Mead PS, Duggal NK, Hook SA, et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med. 2018. doi: 10.1056/NEJMoa1711038 [DOI] [PubMed] [Google Scholar]
- 14.Aid M, Abbink P, Larocca RA, et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell. 2017;169(4):610–620 e14. doi: 10.1016/j.cell.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatnagar J, Rabeneck DB, Martines RB, et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg Infect Dis. 2017;23(3). doi: 10.3201/eid2303.161499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Noronha L, Zanluca C, Burger M, et al. Zika virus infection at different pregnancy stages: Anatomopathological findings, target cells and viral persistence in placental tissues. Front Microbiol. 2018. doi: 10.3389/fmicb.2018.02266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reagan-Steiner S, Simeone R, Simon E, et al. Evaluation of placental and fetal tissue specimens for Zika virus infection — 50 states and district of Columbia, January-December, 2016. Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6624a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mysorekar IU, Diamond MS. Modeling Zika Virus Infection in Pregnancy. N Engl J Med. 2016;375(5):481–484. doi: 10.1056/NEJMcibr1605445 [DOI] [PubMed] [Google Scholar]
- 19.J. C, Y. L, P. Y, S. A, S. R, J. S. Outcomes of congenital Zika disease depend on timing of infection and maternal-fetal immunophysiological conditions. J Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JM, Lebratti TJ, Richner JM, et al. Cellular and Humoral Immunity Protect against Vaginal Zika Virus Infection in Mice. J Virol. 2018. doi: 10.1128/jvi.00038-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngono AE, Shresta S. Immune Response to Dengue and Zika. Annu Rev Immunol. 2018. doi: 10.1146/annurev-immunol-042617-053142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: Interim guidance for preconception counseling and prevention of sexual transmission of zika virus for persons with possible zika virus exposure — United States, september 2016. Morb Mortal Wkly Rep. 2016. doi: 10.15585/mmwr.mm6539e1 [DOI] [PubMed] [Google Scholar]
- 23.Abbink P, Larocca RA, De La Barrera RA, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science (80- ). 2016. doi: 10.1126/science.aah6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larocca RA, Abbink P, Peron JP, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536(7617):474–478. doi: 10.1038/nature18952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozza FA, Moreira-Soto A, Rockstroh A, et al. Differential shedding and antibody kinetics of zika and Chikungunya viruses, Brazil. Emerg Infect Dis. 2019. doi: 10.3201/eid2502.180166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe S, Tan NWW, Chan KWK, Vasudevan SG. Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J Infect Dis. 2019. doi: 10.1093/infdis/jiy482 [DOI] [PubMed] [Google Scholar]
- 27.Jennewein MF, Goldfarb I, Dolatshahi S, et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell. 2019;178(1):202–215.e14. doi: 10.1016/j.cell.2019.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams CB, Eisenstein EM, Cole FS. PART IX Immunology and Infections In: Gleason CA, Devaskar SU, eds. Avery’s Diseases of the Newborn. 9th ed. Elsevier/Saunders; 2012:445–467. [Google Scholar]
- 29.Singh T, Lopez CA, Giuberti C, et al. Efficient transplacental IgG transfer in women infected with Zika virus during pregnancy. PLoS Negl Trop Dis. 2019. doi: 10.1371/journal.pntd.0007648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castanha PMS, Souza WV, Braga C, et al. Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil. PLoS Negl Trop Dis. 2018;13(3). doi: 10.1371/journal.pntd.0007246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barouch DH, Thomas SJ, Michael NL. Prospects for a Zika Virus Vaccine. Immunity. 2017. doi: 10.1016/j.immuni.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modjarrad K, Lin L, George SL, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet. 2018. doi: 10.1016/S0140-6736(17)33106-9 [DOI] [PMC free article] [PubMed] [Google Scholar]