Abstract
Objective
Ultra-high-field 7-Tesla (7T) MRI offers increased signal-to-noise and contrast-to-noise ratios which may improve visualization of cortical malformations. We aim to assess the clinical value of in vivo structural 7T MRI and its post-processing for the noninvasive identification of epileptic brain lesions in patients with pharmacoresistant epilepsy and nonlesional 3T MRI undergoing presurgical evaluation.
Methods
Sixty-seven patients were included who had nonlesional 3T MRI by official radiology report. Epilepsy protocols were used for the 3T and 7T acquisitions. Post-processing of the 7T T1-weighted MP2RAGE sequence was performed using the morphometric analysis program (MAP) with comparison to a normal database consisting of 50 healthy controls. Review of 7T was performed by an experienced board-certified neuroradiologist and at the multimodal patient management conference. The clinical significance of 7T findings was assessed based on intracranial EEG (ICEEG) ictal onset, surgery, post-operative seizure outcomes and histopathology.
Results
Unaided visual review of 7T detected previously unappreciated subtle lesions in 22% (15/67). When aided by 7T MAP, the total yield increased to 43% (29/67). The location of the 7T-identified lesion was identical to or contained within the ICEEG ictal onset in 13/16 (81%). Complete resection of the 7T-identified lesion was associated with seizure freedom (p=0.03). Histopathology of the 7T-identified lesions encountered mainly FCD. 7T MAP yielded 25% more lesions (6/24) than 3T MAP, and showed improved conspicuity in 46% (11/24).
Significance
Our data suggests a major benefit of 7T with post-processing for detecting subtle FCD lesions for patients with pharmacoresistant epilepsy and nonlesional 3T MRI.
Keywords: MRI, 7T, ultra-high field, presurgical evaluation, nonlesional, focal cortical dysplasia
Introduction
Resective surgery is the most effective treatment for pharmacoresistant focal epilepsy, especially in patients with a visible focal lesion identified on magnetic resonance imaging (MRI).1 The ability to identify focal lesions on MRI has markedly improved over the last decade with increased MRI scanner field strengths.2 It was shown that identification of a focal epileptogenic lesion with 3T MRI was approximately 2.5 times as likely as with 1.5T MRI.3 It is not unreasonable to speculate that a similar trend might be seen when moving from 3T to 7T. A few previous studies addressed the usefulness of in vivo structural 7T MRI in patients with pharmacoresistant focal epilepsy undergoing presurgical evaluation.4-12 These studies provided initial evidence that the improved signal-to-noise ratio from 7T MRI can lead to better detection/depiction of some epileptic lesions, particularly focal cortical dysplasia (FCD), even in those cases with a negative 3T MRI.8,9,11 A major limitation of these reports is the lack of histopathological validation or seizure outcome of the identified lesions in a large number of patients.
Another strategy to improve the diagnostic yield of MRI for epilepsy presurgical evaluation is to employ computer-assisted quantitative assessment of the acquired images. Post-processing techniques on 1.5T and 3T MRI have shown promising detection yields for patients with nonlesional MRI by visual analyses.13-17 However, MRI post-processing using 7T data has not yet been attempted in 3T-nonlesional epilepsies. In previous studies, the diagnostic gain of 7T was mainly obtained visually using T2*-weighted gradient echo (GRE), fluid-attenuated inversion recovery (FLAIR) or susceptibility weighted imaging (SWI) sequences.7,9-11 By applying post-processing analysis to the 7T images, an increased diagnostic yield is likely but has yet to be evaluated.
In this study, we aimed to assess the clinical value of in vivo structural 7T MRI and its post-processing in 67 pharmacoresistant epilepsy patients undergoing presurgical evaluation who had a nonlesional 3T MRI. We hypothesized that the combination of 7T visual and post-processing analyses will help visualize previously unidentified subtle FCD lesions for patients with negative 3T MRI, and we further hypothesize that these lesions are concordant with ICEEG ictal onset and need to be included in surgical resection to achieve seizure freedom.
Materials and Methods
Study Design
This is a prospective, single-center study on patients evaluated for epilepsy surgery at Cleveland Clinic Epilepsy Center from 2014 to 2019. Although the Siemens Magnetom Terra 7T MRI scanner was FDA approved for clinical diagnostic use in October 2017, we used the scanner model prior to the FDA approval (Siemens Magnetom) due to the start of the study in 2014; therefore, the scans were performed as part of a research study approved by the Cleveland Clinic institutional review board (IRB). For each patient enrolled, a 7T MRI was performed in addition to the standard presurgical evaluation. The 7T MRI was then reviewed by a board-certified neuroradiologist with 12 years of expertise in epilepsy imaging (SEJ). Additional information generated by the 7T MRI was re-evaluated on the 3T MRI, and positive findings (by visual analysis or MAP) were communicated with the patient’s epileptologist. The strategies for intracranial EEG (ICEEG) electrode implantation and surgical resection in all the patients were routinely discussed based on scalp-EEG video, 3T MRI, FDG-PET, subtraction ictal SPECT co-registered to MRI (SISCOM) and magnetic source imaging (MSI) during a patient management conference (PMC), where 7T MRI results were presented and reviewed again at PMC.
Patient Selection
Patients were included if they had negative 3T MRI by official radiology report and their electroclinical profile (history and scalp video-EEG) suggested focal epilepsy. Patients were excluded if they: (1) could not lay still in the scanner, which included claustrophobia, psychiatric conditions, low body weight (<30 kg) or young age (<10 years); (2) had metal objects in their body/head incompatible with 7T MRI; and (3) would/could not provide informed consent or assent to study. Occasionally, subtle abnormalities from initially negative 3T MRI could be raised when the epileptologist or neurosurgeon reviewed multimodal presurgical evaluation data; these patients were still included in order to compare these findings to 7T results.
Normal Control Subjects
Fifty normal control subjects (23 females/27 males, mean age=30, median=27, range=15-58 years) were included for the purpose of evaluating incidental findings and calculating a normal database for post-processing. All normal subjects were scanned using the same 7T MRI protocol as the one used for patients with epilepsy.
MRI Acquisition Parameters
We used a standard epilepsy protocol on a 7T MRI scanner (Magnetom, Siemens, Erlangen, Germany) with a head-only circularly polarized transmit and 32-channel phased array receive coil (Nova Medical, Wilmington, MA), consistent with our previous study6. The epilepsy-protocol MRI scans obtained as standard care for the epilepsy patients were performed on a 3T scanner (Skyra, Siemens, Erlangen, Germany) using a 32-channel phased array receive coil (Nova Medical, Wilmington, MA) with similar physical characteristics as the one used for 7T. Detailed protocols for both 7T and 3T are included in Appendix 1.
MRI Post-processing
MAP07 was carried out in SPM12 (Wellcome Department of Cognitive Neurology, London, UK) using MATLAB 2015a (MathWorks, Natick, Massachusetts) following previously established methods.14,18 MAP was performed on the UNIDEN images from the MP2RAGE output, which were 3D T1-weighted images that minimized B1-induced inhomogeneities.19 We used a scanner-specific normal average database made from the aforementioned 50 normal controls. Each patient’s data was co-registered to the control group’s data and compared on a voxel-by-voxel basis, yielding three volumetric statistical maps, the junction, extension and thickness maps. The junction file is sensitive to blurring of the gray-white matter junction; the extension file is sensitive to abnormal gyration and extension of gray matter into white matter; the thickness file is sensitive to abnormal cortical thickness. The junction file was processed at a resolution of 0.5 mm3.
The entire brain was searched for candidate MAP abnormalities by an experience reviewer (ZIW), based on visual inspection alone without using automated detection algorithms. Candidate MAP abnormalities were those with significant deviation above a z-score threshold of 6 on the junction file, which was shown to be the most useful map by previous studies20-23. The reviewer also examined whether there was an accompanying region on the extension file (z-score > 6) and the thickness file (z-score > 4). The locations of the candidate-MAP abnormalities were then reviewed on the original MRI by an experienced board-certified neuroradiologist (SEJ), in order to judge if the candidate-MAP abnormality correlated with a native MRI lesion. A consistent 5-point scale to rate the abnormality in each patient: 1, nothing; 2, unlikely; 3, ambiguous; 4, possible; 5, most likely. Only abnormalities with ratings>=3 were regarded as MAP-positive. This process minimizes false positives due to high-z-score areas caused by signal inhomogeneities, artifacts and nonspecific white matter lesions. 3T MAP was performed in a similar manner to that of 7T MAP, except for using a 3T normal databases (detailed in our previous publication21), junction z-score threshold of 4, and with junction file processed at a resolution of 1 mm3. This procedure of performing the MAP methodology was consistent with previous studies from our group and others.14,18,20-22,24,25 The MAP reviewer and the neuroradiologist were blinded to patients’ clinical data and other non-invasive data. Review of 3T and 7T MAP were performed in a randomized order at separate times, so that results from one would not bias the other.
Concordance between 7T and ICEEG
Three image volumes were coregistered using Curry 7 (Compumedics Neuroscan, Hamburg, Germany): the 7T images, the CT obtained immediately after ICEEG implantation and the postoperative MRI (1.5T or 3T). The ICEEG ictal onset location was obtained from a review of the clinical report finalized based on PMC consensus. Once the three image volumes were overlaid, the spatial relationships between the 7T abnormality, clinically defined ICEEG ictal onset and the resection cavity can be visualized, and the complete or incomplete resection of 7T abnormality can be assessed. Considering post-operative movement of tissue around the resection cavity, geometric distortion in MRI and image coregistration error, the lesions were considered as inside the resection if the borders were completely within or <5 mm outside of the resection margin.
Concordance between the 7T findings and ICCEG ictal onset was assessed using a previously published scheme.26 If the location of the 7T-detected lesion matched the ICEEG ictal onset zone, they were considered concordant (7T=ICEEG). If the 7T-detected lesion included the ICEEG ictal onset zone but occupied a bigger brain area, they were considered concordant-plus (7T>ICEEG). Conversely, if the ICEEG ictal onset zone included the 7T-detected lesion but occupied a bigger brain area, they were considered concordant-minus (7T<ICEEG). If there was no overlap between the 7T-detected lesion and ICEEG ictal onset zone, they were considered discordant. When the 7T-detected lesion was not sampled by ICEEG (commonly due to surgical constraints such as nearby vasculature), the concordance was considered indeterminate.
Pathology, Surgery and Seizure Outcomes
Surgical specimens were formalin-fixed and paraffin-embedded, retrieved from the archive and microscopically reviewed by 2 experienced neuropathologists (IB, RC). A selected panel of immunohistochemical stainings were reviewed, as previously recommended for the neuropathology work-up of epilepsy surgery specimens.27,28 FCD lesions were classified according to 2011 ILAE classification guidelines, with considerations of the critical update in 2018.29,30 Post-operative seizure outcomes were classified using ILAE classifications31 and dichotomized as seizure-free (without aura) or not seizure-free at 1-year postoperatively.
Statistical Analyses
Statistical analyses were performed using SAS software (version 9.4; Cary, NC). We used Fisher’s exact test to assess the relationship between parameters and seizure outcomes, as the cell size was less than 5. “N−1” Chi-squared test was used to compare percentages. All analyses were two-sided, at a significant level of 0.05.
Results
Cohort Overview
A total of 67 patients were included (43 males/24 females, 57 right-handed/9 left-handed/1 ambidextrous). The average age was 27.5 years (median=26, range=10 to 55) which is not significantly different from the normal controls (average age=30, median age=27, range=15 to 58). The average epilepsy duration was 14.2 years (median=13, range=0.5 to 45). The average onset age was 13.3 years (median=10, range=0 to 50). A total of 13 patients had temporal lobe epilepsy (TLE) and 54 patients had extra-temporal lobe epilepsy (ETLE). A total of 31 patients underwent ICEEG (28 SEEG and 3 subdural grids with depth electrodes). A total of 25 patients underwent subsequent surgery (21 resections, 4 laser ablations). Complete seizure freedom (no auras) was achieved in 15 patients at 1-year follow-up (15/25, 60%). Detailed demographics and clinical data are in Appendix 2.
Yield of 7T
As detailed in Table 1, unaided visual review of 7T detected previously unappreciated subtle lesions in 22% (15/67). When aided by 7T MAP, the total yield increased to 43% (29/67, examples shown in Figures 1-3). The new findings included the radiological diagnoses of 23 with FCD, 2 with FCD and polymicrogyria (PMG), 1 with FCD and encephalocele, 2 with amygdala enlargement and T1 signal increase, and 1 with vascular malformation. Of the 24 abnormalities detected during MAP-guided review (based on MAP junction file), 42% (10/24) of them had associated changes on the extension file and 8% (2/24) on the thickness file.
Table 1.
Yields of unaided visual review and MAP-guided review on the 7T images, relationship between 7T findings and ICEEG ictal onset, and relationship between 7T findings and resection, 1-year postoperative seizure outcome and pathology.
| 7T Yield (N=67) | ||||
| MAP-guided review + | MAP-guided review − | |||
| Visual review + | 10 | 5 | ||
| Visual review − | 14 | 38 | ||
| 7T Findings and ICEEG Ictal Onset (N=31) | ||||
| 7T+ | 16 | 7 concordant | ||
| 6 concordant-minus | ||||
| 2 discordant | ||||
| 1 indeterminate | ||||
| 7T− | 15 | NA | ||
| 7T Findings, Resection, Seizure Outcome and Pathology (N=25) | ||||
| 7T+ | 17 | 12 complete resection | 10 SF (pathology: 3 IIb, 2 IIa, 1 MOGHE, 2 mMCD II, 2 NA) 2 NSF (pathology: 1 IIb, 1 NA) |
|
| 4 partial resection | 1 SF (pathology: MOGHE) 3 NSF (pathology: 1 MOGHE, 1 NA, 1 encephalocele) |
|||
| 1 no resection | 1 NSF (pathology: NA) | |||
| 7T− | 8 | NA | 4 SF (pathology: 1 IIb, 1 encephalocele, 1 mMCD II, 1 NA) | |
| 4 NSF (pathology: 3 mMCD II, 1 NA) | ||||
1-year postoperative seizure outcome and pathology.
SF=seizure-free; NSF=not seizure-free; NA=not available due to no tissue sent or not applicable
Figure 1.

Illustration of concordant ICEEG ictal onset and 7T finding (detected by MAP-guided review) in a patient with a subtle FCD IIb lesion in the right central sulcus/subcentral gyrus characterized by gray-white blurring (highlighted by red arrows and crosshairs). A: 3T T1-weighted MPRAGE (sagittal); B: 7T T1-weighted MP2RAGE (sagittal); C: 7T MAP junction file (sagittal); D: 7T T2*-weighted GRE (sagittal, zoomed in); E: ictal onset in relation to the subtle lesion detected on 7T (onset contact marked with red; other contacts marked with green); F: postoperative MRI showing complete removal of lesion. Bottom EEG panel: a typical seizure captured during SEEG monitoring; seizure onset at R5-8 is marked by the red arrow; dashed arrows denote the quick spread to Q7-10. The location of ictal onset was exactly in the location of the subtle abnormality detected by 7T MAP. The patient has been seizure-free for 3.75 years following complete removal of the abnormality.
Figure 3.
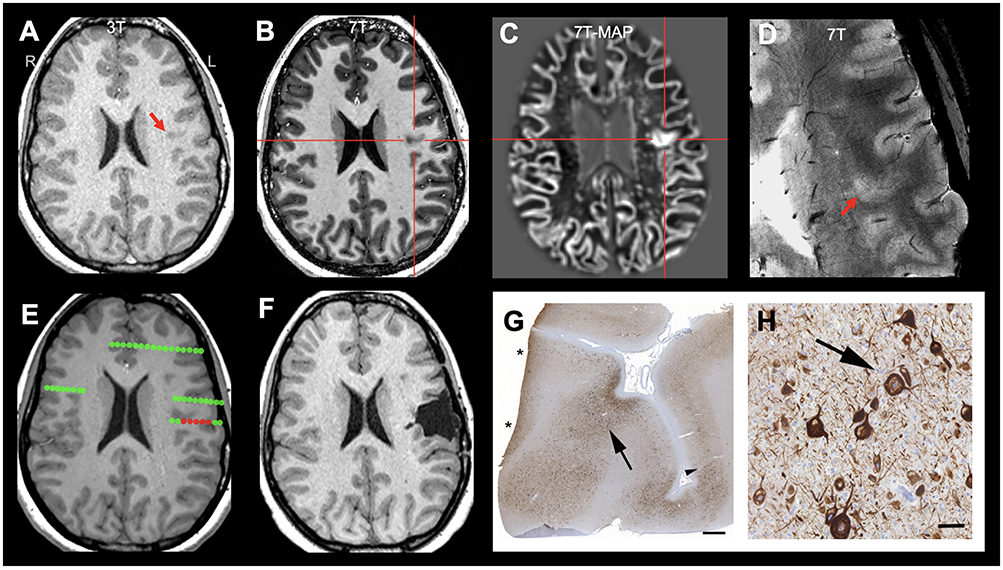
Illustration of concordant ICEEG onset and 7T finding (detected by MAP-guided review) in a patient with a subtle FCD IIa lesion in the left frontal operculum (highlighted by red arrows and crosshairs). A: 3T T1-weighted MPRAGE (axial); B: 7T T1-weighted MP2RAGE (axial); C: 7T MAP junction file (axial); D: 7T T2*-weighted GRE (axial, zoomed in); E: ictal onset in relation to the subtle lesion detected by 7T MAP (onset contact marked with red; other contacts marked with green); F: postoperative MRI showing complete removal of lesion. The patient has been seizure-free for 5 years postoperatively. Pathology panel G: whole slide imaging of immunohistochemical staining using antibodies directed against non-phosphorylated neurofilaments (SMI32). Black arrow indicates the area of highest density of dysmorphic neurons as recognized by intraneuronal neurofilament accumulation. Black arrowhead indicates approximate border of FCD IIa on this plane of section. Note that the section border (asterisks) on the left was not free of dysmorphic neurons. Scale bar = 1 mm. H: zoom-in view of G highlighting dysmorphic neurons; scale bar = 50 μm. There was no evidence of balloon cells.
Upon side-by-side 3T-7T comparison of the 15 cases where the 7T lesion was visually identified, the lesion was seen with less conspicuity on the 3T in the majority of the cases (12 of 15, 80%); less frequently (in 3/15, 20%), the lesion was not appreciated on the 3T because of its small size and its location in between imaging slices (and therefore not optimally captured).
In the 24 cases where the 7T MAP yielded positive results, 6 (25%, 6/24) showed lesions that were not picked up on 3T MAP (due to low z-scores or artifacts); in 11 (46%, 11/24) , the 7T-MAP-delineated abnormality showed improved conspicuity such as clearer connection to cortex or larger extent of abnormal cortical regions (examples shown in Figure 4); in the remaining 7 (29%, 7/24), 7T MAP and 3T MAP showed similar results.
Figure 4:

Comparison of 3T-MAP and 7T-MAP in an example patient who had a subtle FCD IIb lesion at the crown of gyrus in the left superior parietal lobule. 7T-MAP gray-white junction file showed improved conspicuity and delineation of lesion extent. A: 7T T1-weighted MP2RAGE (axial, zoomed); B: 7T MAP junction file (axial, zoomed); C: 3T T1-weighted MPRAGE (axial, zoomed); D: 3T MAP junction file (axial, zoomed).
Concordance with ICEEG Ictal Onset
In the 31 patients who underwent ICEEG in the 3T-nonlesional group, 16 (52%, 16/31) had positive 7T findings (visual or MAP-guided). The 7T findings were identical or contained within the ICEEG ictal onset in 81% (13/16, 7 concordant and 6 concordant-minus); discordant finding was seen in 2 and indeterminate in 1 (details in Table 1, examples shown in Figures 1-3).
Surgery and Postoperative Seizure Outcome
Out of the 29 7T-positive patients, 17 (59%, 17/29) proceeded with surgery and 11 (38%, 11/29) became seizure-free; out of the 38 7T-negative patients, 8 (21%) underwent surgery and 4 (11%, 4/38) became seizure-free; the percentage of patients undergoing surgery and the percentage of patients with postoperative seizure-freedom were both significantly higher in the 7T-positive group (p=0.002, p=0.01, respectively).
When resection extent is taken into account, a total of 12 patients had complete resection of the 7T-detected lesion and 10 (10/12, 83%) became seizure-free at 1 year; 5 patients had partial or no resection of the 7T finding (commonly due to overlap with eloquent cortex) and only one was seizure-free (1/5, 20%, follow up=3 years), with the rest having recurrence at an average of 8 month postoperatively (range=4-12 months). Complete resection of the 7T-detected lesion was significantly associated with seizure-freedom (p=0.03).
Abnormalities Suspected on 3T
In 19 patients, suspicious findings were identified during re-review of the 3T MRI in conjunction with other noninvasive data (EEG, PET, SPECT, MEG). 7T provided additional anatomical details to confirm/re-demonstrate the 3T-suspected lesion in 6 patients (32%, 6/19). In another 13 patients (68%, 13/19), the 3T-suspected lesion was not re-demonstrated by the 7T scan. The majority (77%, 10/13) of these lesions were originally suspected as FCD with gray-white junction blurring on 3T, which was judged to be due to partial volume effects after reviewing the higher-resolution 7T images with more slices. Notably, 5 of these “refuted” lesions were indeed proven to be not epileptogenic by ICEEG and/or surgery.
Temporal Lobe Epilepsy (TLE) vs. Extra-temporal Lobe Epilepsy (ETLE)
A total of 54 patients had ETLE, and 21 of them had 7T-postive findings; 13 patients had TLE and 8 had 7T-postive findings. There is no significant difference in the percentage of 7T-poisitive findings in the TLE vs. ETLE subgroups.
Histopathological Correlates
In the 16 patients who underwent either a complete or a partial resection of the 7T-detected lesions, 12 had tissue available for detailed histopathological examination. All samples showed various types of cortical malformations, including 4 FCD type IIb (Figure 2), 2 FCD IIa (Figure 3), 3 MOGHE (Figure 5), 2 mMCD II and 1 encephalocele.
Figure 2.
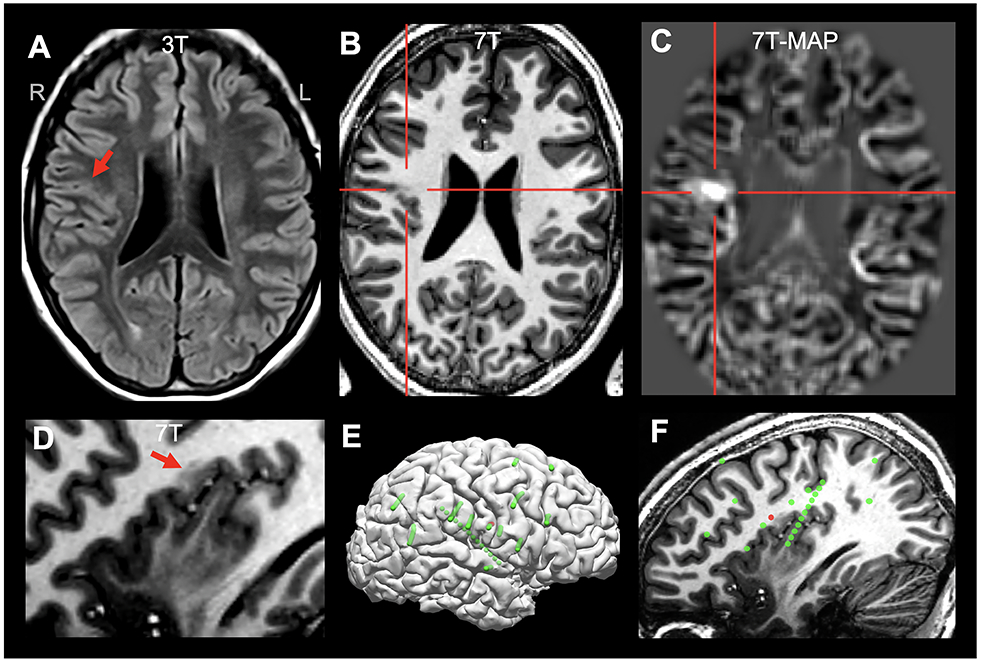
Illustration of concordant ICEEG onset and 7T finding (detected by MAP-guided review) in a patient with a subtle FCD in the right parietal operculum (highlighted by red arrows or crosshairs), characterized by gray-white blurring and T1-weighted signal abnormality. A: 3T FLAIR (axial) on which the lesion was difficult to appreciate; B: 7T T1-weighted MP2RAGE (axial); C: 7T MAP junction file (axial); D: 7T MP2RAGE (sagittal, zoomed in); E: all implanted SEEG electrodes (green spheres: all implanted electrodes; red spheres: ictal onset); F: sagittal view with 7T finding and SEEG electrode location; the 7T-MAP-detected FCD lesion was concordant with ictal onset shown by the SEEG. The patient was seizure-free at 1 year follow up, following complete resection of the lesion. Histopathology was not available due to fragmented tissue.
Figure 5.
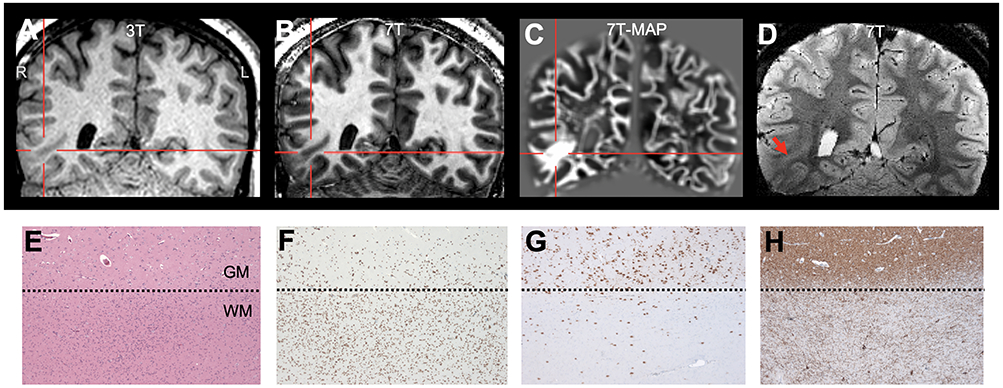
Illustration of 7T findings in a patient with mild malformation of cortical development with oligodendroglial hyperplasia (MOGHE) in the right basal tempo-occipital region. A: 3T T1-weighted MPRAGE (coronal); B: 7T T1-weighted MP2RAGE (coronal); C: 7T MAP junction file (coronal); D: 7T T2*-weighted GRE (coronal). In this patient, the lesion was detected by MAP-guided 7T review. The patient had seizure recurrence (although at a reduced seizure frequency) at 1 year following partial resection of the abnormality. Pathology panel E: increased cell densities at the gray-white-matter junction, with patchy increased cellularity (H&E staining). F: Olig2 immunohistochemistry of the same area as A proving the oligodendroglial origin of cells. In addition to the oligodendroglial hyperplasia, NeuN (G) and Map2 (H) immunohistochemical stainings demonstrated blurred gray-white-matter boundaries with increased density of heterotopic neurons in the subjacent white matter. Dotted lines in E-H: gray-white-matter junction. Scale bar in E = 200μm, which also applies to F-H.
Incidental Findings
In the 50 normal controls, review of 7T showed 3 developmental venous anomalies, 1 pineal cyst, 3 arachnoid cysts, 1 choroidal fissure cyst, and 1 upper spinal cord lesion suggestive of demyelinating disease.
Adverse Events
No adverse events occurred. Approximately half the subjects reported transient disorientation which did not affect the scan.
Discussion
Our study on the largest-to-date cohort of 67 pharmacoresistant epilepsy patients undergoing presurgical evaluation demonstrates a major benefit of 7T for the detection of subtle FCD lesions, in almost half the patients with negative 3T MRI. Our data also showed that guidance from MRI post-processing on 7T significantly increases the yield for FCD detection. An important detail to point out is that, patients with a positive 7T MRI were significantly more likely to undergo surgery and become seizure-free after surgery. These results suggest that the presence of lesion on 7T could perhaps be a positive predictor of the presurgical evaluation success, which is of particular importance when 3T MRI is negative. Our current study significantly contributes to the literature4-12,32 because: (1) it included a larger number of patients to address the relationship between 7T MRI findings and ICEEG ictal onset, histopathology as well as post-operative seizure outcomes; (2) the usage of 7T MRI post-processing to guide 7T visual review was investigated for the first time.
7T in the Setting of Nonlesional 3T
Our data suggest the major added value of 7T is in the subgroup of patients with extratemporal epilepsies due to FCD. The identification of these subtle lesions may significantly improve postoperative seizure outcome, as supported by our data showing the complete resection of the 7T-detected lesion was significantly associated with seizure-free outcome. Our findings were consistent with a few previous reports. Veersema et al. reported a 7T visual analysis yield of 23% in a 3T-nonlesional cohort (9/40); 6 of the 9 patients underwent surgery and FCD was histopathologically confirmed.8 De Ciantis et al. reported that in a cohort of 21 patients with negative 1.5T or 3T MRI, 7T visual analysis detected additional lesions in 6 patients (29%); 4 of the 6 patients underwent surgery and FCD was histopathologically confirmed.9 Feldman et al studied 37 patients with negative 1.5T or 3T MRI, showing that up to 67% had 7T-detected abnormalities with epileptogenic potential; however, only 5 were confirmed by post-operative seizure freedom.11 Due to the small numbers of surgical cases included in these prior studies,8,9,11 no statistical inference could be made. Because of 7T’s higher sensitivity to blood products (particularly with T2*-weighted GRE and SWI sequences), vascular malformations were shown to be better imaged.7,11 In our study, this advantage of 7T led to a previously unseen vascular malformation (P6, details in Appendix 2).
The definition of “nonlesional” or “negative” 3T MRI has long been the debate in the literature, which largely depends on the experience of the MRI reviewer and the information provided for the review. In order to apply a practical standard of patient inclusion, we included all patients with an initially negative official radiology report (issued by a board-certified neuroradiologist), consistent with previous studies20,21. Interestingly, our data showed that in the group of patients whose 3T MRI was thought to be subtly lesional after taking into account of other noninvasive test results, 7T was helpful in not only confirming, but also ruling out some of the “FCD-appearing” normal cortex due to partial volume effects at 3T. It is worth pointing out that refuting suspicious findings does carry clinical significance, as a false-positive MRI may misguide the next steps of clinical management, such as surgical candidacy, need for ICEEG and extent of the surgical resection.
Importance of Post-processing
Our results suggest that using higher magnetic field strength does not diminish the role of MRI post-processing, but rather, a combination of the two methods optimizes the overall gain. In fact, with the smaller voxel size and inter-slice gap, 7T imaging generates more data than 3T, making the importance of computer-assisted review even more pertinent. This was supported by our data showing that unaided visual review alone could generate 22% of yield, while adding MAP to guide the visual analysis led to a markedly higher yield of 43%. This finding is consistent with previous studies on MRI post-processing using 3T/1.5T MRI.14,20,22,24,33,34 Based on recent studies showing more detailed morphological features of FCD being revealed on 7T,9,12,35 using post-processing techniques to target these new features may bring additional yield.
Five patients (P11-P15, Appendix 2) had positive 7T findings by visual review, but had negative MAP. This can be explained by the fact that MAP is not designed to detect mesial temporal structural changes (2/5 patients had amygdala enlargement and signal abnormality) or encephaloceles. In three patients, the FCD detected by visual review of 7T were mainly noted on the T2-weighted, T2*-weighted or FLAIR sequences; since the MAP processing applied here was based on T1-weighted sequence, it generated negative results. This finding calls for incorporating a multi-contrast scheme into MRI post-processing to further increase its yield36,37.
TLE vs. ETLE
A disadvantage of 7T in our current setup, consistent with previous reports,8,32 lies within the artifact induced by B1 field inhomogeneity in the basal temporal area due to head coil geometry, despite the use of dielectric pads. Our data showed no significant differences in the percentage of 7T-positive lesions in the TLE vs. ETLE group; although this should be taken into account with the fact that our cohort has way more ETLE than TLE patients, i.e. there was a selection bias with the 3T-nonlesional nature of the cohort. Nevertheless, the B1 field inhomogeneity artifacts can hamper the recognition of structural changes associated with temporal lobe epilepsy, such as the collateral sulcus and blurring of the temporal pole. One future direction would be to use parallel transmit system with dedicated coil to address the technical issues of the B1 field inhomogeneity.38
Imaging-pathology Correlations
Detailed histological examination revealed FCD IIb and IIa (typically with small size and depth-of-sulcus location), as well as oligodendroglial hyperplasia, which has been recently described as a new histopathologic entity of MCD, i.e. MOGHE39,40 and is typically difficult to see on 1.5T or 3T MRI. Our data suggests the efficacy of 7T MRI with post-processing in elucidating these types of lesions. Particularly, the increased cellular density at the gray-white boundary seen in MOGHE (as exemplified in Figure 5) could explain the efficacy of 7T-MAP gray-white junction file in detecting these lesions.
mMCD II is defined as a mild malformation of cortical development which cannot be put into the category of type I or type II FCD, but neurons exhibited excessive accumulation in the white matter when viewed under the microscope.29 Our data, particularly the more frequent occurrence of mMCD II in the surgical pathology of 7T-negative cases, showed that even with increased signal-to-noise ratio at 7T, neither the existence nor the boundary of this MCD variant can be well recognized.
3T Re-review and 3T MAP
It is interesting to note that after the lesions were pointed out on the 7T, correlates on 3T could be found in the majority of the cases, suggesting that 7T makes it easier for the human eye to detect the subtle lesions by increasing their conspicuity and contrast comparing to the adjacent normal cortex. Similarly, post-processing based on 7T as input makes the features of the subtle lesions stand out more when comparing to those of the adjacent normal cortex, thereby improving conspicuity and delineation of lesion extent (46% of cases), as well as increasing detection (25% of cases). Taken together, our data suggests that conspicuity is the key for subtle lesion detection. When 7T is not accessible, conspicuity of subtle abnormalities may be strengthened by focused re-review together with other noninvasive modalities such as MEG41 and PET42, or by MRI post-processing that enhances the features of FCD on the 3T MRI13,20,23-25,33. In fact, our data show that in the 24 cases where the 7T MAP yielded positive results, 18 were already picked up on 3T MAP, suggesting that even only adding the MAP post-processing on the 3T could already have substantial yield.
Implications for ICEEG and Surgery
The excellent concordance between the 7T-identified lesions and ICEEG-identified ictal onset zone is worth noting. We have previously shown that for those FCD lesions that are of small size and situated at the depth of sulcus, a priori detection of the lesion may lead to a successful surgical resection without the need for ICEEG.43 Given results revealed by the current study, we hypothesize that ICEEG could perhaps be omitted in select patients with 7T-identified, small depth-of-sulcus lesions in the future.
Of note, in some patients, ICEEG ictal onset may be broad, diffuse or simultaneously involving multiple brain regions even with a MRI-positive lesion, reflecting the complexity of managing these cases. This was seen in the 6 cases with a concordant-minus relationship (lesion extent < ICEEG ictal onset). Discrepancy between 7T and ICEEG was encountered in 2 patients (P26, P29, Appendix 2). In both patients, the ICEEG ictal onset showed diffuse pattern, but the 7T-identified abnormality seemed to be uninvolved, or silent. The discrepancy could be consistent with previous studies showing in situ epileptogenicity outside the MRI identified lesion44, or the existence of structurally abnormal but electrically silent lesions21,33,46.
Limitations
Our study, although including the largest-to-date cohort of 67 patients with nonlesional 3T MRI, is still limited by only a portion of the patients having surgery and pathological confirmation.
There was only one neuroradiologist who performed the prospective review, although the 7T findings were re-reviewed and discussed at the PMC which included another neuroradiologist. We are in the process of performing another study with multiple neuroradiologists’ agreement statistics on assessing 7T imaging of epileptic lesions.
The choice of z-score threshold of 6 on 7T MAP was based on empirical experience to reduce false positive results. The choice of threshold could depend on the normal control database used for each institution. The optimal z-score threshold requires further studies.
Our control scans were reviewed for incidental findings and constructing a normal database for MAP. When reviewed, they were known to be controls. We are in the process of performing another study with multiple neuroradiologists assessing 7T imaging of epileptic lesions where control scans are mixed and reviewed in a blinded fashion.
Subtotal sampling of some specimens could result in an underestimation of the epileptic pathologies. Additionally, laser ablation precluded further pathological examination in some cases.
Conclusions
We present the largest-to-date cohort of 67 patients with pharmacoresistant focal epilepsy evaluated with 7T MRI who had a nonlesional 3T MRI. 7T may detect previously unseen subtle lesions, particularly FCD, in almost 50% of patients with negative 3T. MRI post-processing based on 7T significantly increases the yield for FCD detection. When concordant with the patient’s electro-clinical profile, the additional 7T findings should be targeted in the devising of ICEEG implantation and resective/ablative surgical plans.
Supplementary Material
Key Points:
We assessed the clinical value of structural 7T MRI and post-processing in 67 patients with pharmacoresistant epilepsy and nonlesional 3T MRI.
Unaided visual review of 7T detected previously unappreciated subtle lesions in 22%; when aided by MRI-postprocessing, the yield increased to 43%.
The location of the 7T-identified lesion was identical to or contained within the ICEEG ictal onset in 81%.
Complete resection of the 7T-identified lesion was associated with seizure freedom.
7T MRI-postprocessing yielded 25% more lesions than 3T MRI-postprocessing, and showed improved conspicuity in 46%.
Acknowledgement
Supported by NIH R01 NS109439 (ZIW, IB, IN, SEJ) and JoshProvides Epilepsy Assistance Foundation Research Grant (ZIW). The authors would like to acknowledge the generous technical support provided by Dr. Ken Sakaie for the acquisition of 7T studies, and the gracious guidance from Dr. Hans-Jürgen Huppertz on 7T MAP.
Footnotes
Disclosure
Imad Najm is on the Speakers’ bureau of Eisai. Jorge Gonzalez-Martinez received educational grant from Zimmer Biomed. Stephen Jones received travel and speaker fees from SIEMENS Healthineers. All other authors report no disclosures.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Engel JJ. Principle of epilepsy surgery In: Shorvon SD, Dreifuss F, Fish DR, editors. The treatment of Epilepsy. 2nd ed. Oxford, England: Blackwell; 1996. p. 519–29. [Google Scholar]
- 2.Knake S, Triantafyllou C, Wald LL, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005/10/12 2005; 65(7):1026–31. [DOI] [PubMed] [Google Scholar]
- 3.Phal PM, Usmanov A, Nesbit GM, et al. Qualitative comparison of 3-T and 1.5-T MRI in the evaluation of epilepsy. AJR Am J Roentgenol. 2008/08/22 2008; 191(3):890–5. [DOI] [PubMed] [Google Scholar]
- 4.Feldman RE, Rutland JW, Fields MC, et al. Quantification of perivascular spaces at 7 T: A potential MRI biomarker for epilepsy. Seizure. 2018; 54:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer E, Dymerska B, Cardoso PL, et al. Comparison of Routine Brain Imaging at 3 T and 7 T. Invest Radiol. 2016/02/11 2016; 51(8):469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obusez EC, Lowe M, Oh SH, et al. 7T MR of intracranial pathology: Preliminary observations and comparisons to 3T and 1.5T. Neuroimage. 2016/12/05 2017; 168(November 2016):pii: S1053–8119(16), 30648–6. [DOI] [PubMed] [Google Scholar]
- 7.Veersema TJ, van Eijsden P, Gosselaar PH, et al. 7 tesla T2*-weighted MRI as a tool to improve detection of focal cortical dysplasia. Epileptic Disord. 2016/07/21 2016; 18(3):315–23. [DOI] [PubMed] [Google Scholar]
- 8.Veersema TJ, Ferrier CH, Van Eijsden P, et al. Seven tesla MRI improves detection of focal cortical dysplasia in patients with refractory focal epilepsy. Epilepsia Open. 2017; 10(2(2)):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ciantis A, Barba C, Tassi L, et al. 7T MRI in focal epilepsy with unrevealing conventional field strength imaging. Epilepsia. 2016/01/19 2016; 3(57):445–54. [DOI] [PubMed] [Google Scholar]
- 10.Colon AJ, van Osch MJP, Buijs M, et al. Detection superiority of 7 T MRI protocol in patients with epilepsy and suspected focal cortical dysplasia. Acta Neurol Belg. 2016/07/09 2016; 116(3):259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman RE, Delman BN, Pawha PS, et al. 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. PLoS One. 2019; 14(3):e0213642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittau F, Baud MO, Jorge J, et al. MP2RAGE and Susceptibility-Weighted Imaging in Lesional Epilepsy at 7T. J Neuroimaging. 2018; 28(4):365–9. [DOI] [PubMed] [Google Scholar]
- 13.Bernasconi A, Antel SB, Collins DL, et al. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Ann Neurol. 2001/06/21 2001; 49(6):770–5. [PubMed] [Google Scholar]
- 14.Huppertz HJ, Wellmer J, Staack AM, et al. Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia. 2008; 49(5):772–85. [DOI] [PubMed] [Google Scholar]
- 15.Sisodiya SM, Free SL, Stevens JM, et al. Widespread cerebral structural changes in patients with cortical dysgenesis and epilepsy. Brain. 1995/08/01 1995; 118 (Pt 4):1039–50. [DOI] [PubMed] [Google Scholar]
- 16.Bonilha L, Rorden C, Appenzeller S, et al. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage. 2006/07/29 2006; 32(3):1070–9. [DOI] [PubMed] [Google Scholar]
- 17.Colliot O, Antel SB, Naessens VB, et al. In vivo profiling of focal cortical dysplasia on high-resolution MRI with computational models. Epilepsia. 2006; 47(1):134–42. [DOI] [PubMed] [Google Scholar]
- 18.Huppertz H-JJ, Grimm C, Fauser S, et al. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 2005; 67(1–2):35–50. [DOI] [PubMed] [Google Scholar]
- 19.Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2009/10/13 2010; 49(2):1271–81. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZI, Jones SE, Jaisani Z, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. 2015/03/27 2015; 77(6):1060–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ZI, Alexopoulos AV., Jones SE, et al. Linking MRI postprocessing with magnetic source imaging in MRI-negative epilepsy. Ann Neurol. 2014/04/30 2014; 75(5):759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Lin Y, Wang S, et al. Voxel-Based Morphometric MRI Postprocessing in Nonlesional Pediatric Epilepsy Patients Using Pediatric Normal Databases. Eur J Neurol. 2019; :1–11. [DOI] [PubMed] [Google Scholar]
- 23.El Tahry R, Santos SF, Vrielynck P, et al. Additional clinical value of voxel-based morphometric MRI post-processing for MRI-negative epilepsies : a prospective study. Epileptic Disord. 2020; 22(2):1–9. [DOI] [PubMed] [Google Scholar]
- 24.Wagner J, Weber B, Urbach H, et al. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011; 134(10):2844–54. [DOI] [PubMed] [Google Scholar]
- 25.Wong-Kisiel LC, Tovar Quiroga DF, Kenney-Jung DL, et al. Morphometric analysis on T1-weighted MRI complements visual MRI review in focal cortical dysplasia. Epilepsy Res. 2018; 140:184–91. [DOI] [PubMed] [Google Scholar]
- 26.Murakami H, Wang ZI, Marashly A, et al. Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain. 2016/08/28. 2016; 139:2935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumcke I, Spreafico R, Haaker G, et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N Engl J Med. 2017; 377(17):1648–56. [DOI] [PubMed] [Google Scholar]
- 28.Aronica E, Miyata H, Sarnat HB, et al. International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue : A consensus Task Force report from the ILAE Commission on Diagnostic Methods. 2016; :348–58. [DOI] [PubMed] [Google Scholar]
- 29.Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011; 52(1):158–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najm IM, Sarnat HB, Blümcke I. Review: The international consensus classification of Focal Cortical Dysplasia – a critical update 2018. Neuropathol Appl Neurobiol. 2018; 44(1):18–31. [DOI] [PubMed] [Google Scholar]
- 31.Wieser HG, Blume WT, Fish D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001; 42(2):282–6. [PubMed] [Google Scholar]
- 32.Guye M, Bartolomei F, Ranjeva JP. Malformations of cortical development: The role of 7-Tesla magnetic resonance imaging in diagnosis. Rev Neurol (Paris). 2019; :8–13. [DOI] [PubMed] [Google Scholar]
- 33.Hong SJ, Kim H, Schrader D, et al. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014/06/06 2014; 83(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin B, Krishnan B, Adler S, et al. Automated detection of focal cortical dysplasia type II with surface-based magnetic resonance imaging postprocessing and machine learning. Epilepsia. 2018; :982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zucca I, Milesi G, Medici V, et al. Type II FCD: ex vivo 7 Tesla MRI abnormalities and histopathological comparisons. Ann Neurol. 2015/10/09 2015; 79(1):42–58. [DOI] [PubMed] [Google Scholar]
- 36.House PM, Lanz M, Holst B, et al. Comparison of morphometric analysis based on T1- and T2-weighted MRI data for visualization of focal cortical dysplasia. Epilepsy Res. 2013/07/31 2013; 106(3):403–9. [DOI] [PubMed] [Google Scholar]
- 37.Bernasconi A, Bernasconi N, Bernhardt BC, et al. Advances in MRI for “cryptogenic” epilepsies. Nat Rev Neurol. 2011; 7(10):99–108. [DOI] [PubMed] [Google Scholar]
- 38.Gras V, Boland M, Vignaud A, et al. Homogeneous non-selective and slice-selective parallel-transmit excitations at 7 Tesla with universal pulses: A validation study on two commercial RF coils. PLoS One. 2017; 12(8):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schurr J, Coras R, Rossler K, et al. Mild Malformation of Cortical Development with Oligodendroglial Hyperplasia in Frontal Lobe Epilepsy: A New Clinico-Pathological Entity. Brain Pathol. 2016/01/11 2017; 27(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartlieb T, Winkler P, Coras R, et al. Age-related MR characteristics in mild malformation of cortical development with oligodendroglial hyperplasia and epilepsy (MOGHE). Epilepsy Behav. 2018; 91:68–74. [DOI] [PubMed] [Google Scholar]
- 41.Funke ME, Moore K, Orrison WW Jr., et al. The role of magnetoencephalography in “nonlesional” epilepsy. Epilepsia. 2011/07/16 2011; 52 Suppl 4:10–4. [DOI] [PubMed] [Google Scholar]
- 42.Chassoux F, Rodrigo S, Semah F, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010; . [DOI] [PubMed] [Google Scholar]
- 43.Ying Z, Wang I, Blümcke I, et al. A comprehensive clinico-pathological and genetic evaluation of bottom-of-sulcus focal cortical dysplasia in patients with difficult-to-localize focal epilepsy. Epileptic Disord. 2019; 21(1):65–77. [DOI] [PubMed] [Google Scholar]
- 44.Marusic P, Najm IM, Ying Z, et al. Focal cortical dysplasias in eloquent cortex: functional characteristics and correlation with MRI and histopathologic changes. Epilepsia. 2002/03/07 2002; 43(1):27–32. [DOI] [PubMed] [Google Scholar]
- 45.Sarkis RA, Jehi L, Bingaman W, et al. Seizure worsening and its predictors after epilepsy surgery. Epilepsia. 2012; 53(10):1731–8. [DOI] [PubMed] [Google Scholar]
- 46.Bonilha L, Montenegro MA, Rorden C, et al. Voxel-based morphometry reveals excess gray matter concentration in patients with focal cortical dysplasia. Epilepsia. 2006; 47(5):908–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


