Abstract
Cardiovascular disease is now a leading cause of mortality in people with HIV (PWH). High blood pressure is the major driver of cardiovascular disease. Despite this, little is known about blood pressure in PWH during the early years of antiretroviral therapy (ART). In this prospective cohort study in Tanzania, the authors conducted unobserved blood pressure measurements at enrollment, 3, 6, 12, 18, and 24 months in 500 PWH initiating ART and 504 HIV‐uninfected adults. The authors excluded measurements taken on antihypertensive medications. Although PWH had a significantly lower blood pressure before ART initiation, they had a significantly greater increase in blood pressure during the first 2 years of ART compared to HIV‐uninfected controls. Blood pressure correlates in PWH differed from HIV‐uninfected controls. In PWH, lower baseline CD4+ T‐cell counts were associated with lower blood pressure, and greater increases in CD4+ T‐cell counts on ART were associated with greater increases in blood pressure, both on average and within individuals. In addition, PWH with a systolic blood pressure (SBP) <90 mm Hg at the time of ART initiation had ~30% mortality in the following 3 months due to occult infections. These patients require careful investigation for occult infections, and those with tuberculosis may benefit from corticosteroids.
Keywords: blood pressure, CD4+ T cell, global health, HIV
1. INTRODUCTION
People with HIV (PWH) are at a higher risk of mortality due to cardiovascular disease when compared to the general population. 1 Hypertension is a main driver of cardiovascular disease. 2 Cross‐sectional studies examining the relationship between HIV and hypertension have reported inconsistent results. 3 , 4 Prospective studies have reported a high incidence of hypertension in PWH, 5 , 6 , 7 but have lacked HIV‐uninfected control groups for comparison. The authors of the largest of these cohort studies recently concluded, “an HIV‐negative comparison population is needed to assess the association of the HIV itself with hypertension”. 5 This is especially true for the early ART period when immune reconstitution is occurring in PWH.
In a cross‐sectional study, we previously conducted in Tanzania, PWH were found to have lower blood pressure than HIV‐uninfected adults prior to ART initiation but higher blood pressure several years after initiation of ART. 8 We postulated that immune reconstitution may have played a role in this reversal. 7 This hypothesis is based on evidence from animal models, suggesting that changes in T‐cell immunity contribute to the pathophysiology of hypertension. 9
In this paper, we report an analysis of pre‐ and early ART data from our cohort investigating blood pressure in PWH and HIV‐uninfected controls in Tanzania. Our objectives were as follows: (a) to investigate the pattern of blood pressure change in the first 24 months of ART, (b) to determine factors associated with blood pressure, and (c) to describe the relationship between pre‐ART blood pressure and mortality in PWH.
2. METHODS
2.1. Study design
We conducted a prospective cohort study of PWH and HIV‐uninfected adults. PWH were enrolled from public HIV clinics in the city of Mwanza, Tanzania, along with their HIV‐uninfected “treatment supporters.” According to Tanzanian guidelines, PWH must designate a family member or neighbor as a “treatment supporter” at the time of ART initiation who provides social and medical support. We have previously shown that treatment supporters and PWH have similar socioeconomic characteristics. 8
2.2. Inclusion and exclusion criteria
Inclusion criteria for HIV‐uninfected adults were age 18‐65 years, able and willing to be contacted by cell phone, Tanzanian citizenship, residing in Mwanza City, and able and willing to provide written informed consent. Additional inclusion criteria for PWH were no prior exposure to ART and willing to initiate ART within 1 month. Exclusion criteria for both groups were previous history of cardiovascular events, medical condition with a prognosis of <12 months, and plans to move away from Mwanza.
2.3. Study location
All of the study participants included in this analysis were enrolled from three public outpatient HIV clinics in Mwanza, Tanzania: Bugando Medical Centre, Igoma Health Center, and Nyamagana District Hospital. The prevalence of HIV in the catchment area for these three HIV clinics is approximately 6%, which is similar to the national average. At the time of the study, these HIV clinics were providing outpatient care to over 15 000 PWH.
2.4. Study procedures
All study participants were seen at enrollment, 3 months, 6 months, and then every 6 months thereafter. At every study visit, the study staff administered a standardized questionnaire based on the World Health Organization's STEPwise Surveillance (STEPS) 10 to evaluate risk factors for hypertension. The STEPS questionnaire has been translated into the local language (Kiswahili), has been validated in East Africa, 11 and includes questions about socioeconomic status, diet, and exercise and protocols for anthropomorphic measurements. Additional questions regarding HIV diagnosis and treatment were included. After completing the questionnaire, the study staff conducted a standardized physical examination (including weight, height, and waist circumference) according to the STEPS protocol. BMI was calculated using this height and weight.
2.5. Blood pressure measurement
In order to minimize measurement bias, blood pressure was measured at every study visit using an OMRON HBP‐1300 professional blood pressure monitor following National Health and Nutrition Examination Survey (NHANES) blood pressure procedure. 12 Participants rested for 5 minutes, sitting with back supported, legs uncrossed, and feet flat on floor. Three successive, unobserved blood pressure measurements were taken on the right arm with a 60‐second interval between measurements. Average SBP/DBP was calculated using the second and third measurements. We excluded blood pressure measurements taken while participants were receiving blood pressure–lowering medications.
2.6. Laboratory procedures
Routine point‐of‐care laboratory tests were performed and recorded at all study visits. CD4+ T‐cell counts, total lymphocyte counts, and hemoglobin levels were measured using an automated BD FACSPresto™ System (BD Biosciences). Point‐of‐care random blood glucose levels were measured from a sterile fingerpick (OneTouch Select, LifeScan). For any participant with a random blood glucose level ≥7 mmol/L, a fasting blood glucose measurement was obtained to confirm the diagnosis of diabetes mellitus in accordance with the guidelines of the International Diabetes Federation. 13 Anemia was categorized according to the World Health Organization definition. 14
Additionally, blood and urine were collected at baseline. The blood samples were transported in a cooler to the laboratory of the Tanzanian National Institute for Medical Research (NIMR). Serum creatinine level was measured in the NIMR laboratory using the A25 Analyzer (Biosystems), calibrated by the creatinine Jaffé 2 method. An estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation (without ethnic factor), which has been shown to be the most accurate eGFR calculation for African adults. 15 Urine albumin and creatinine were measured with the Siemens DCA Vantage Analyzer (Siemens Healthcare).
2.7. Data analysis
Data were double‐entered into OpenClinica (OpenClinica LLC) and analyzed using Stata 15 (StataCorp LLC). Outcomes variables included blood pressure and mortality, and the main confounders were age, sex, and adiposity. Continuous variables were summarized by median and interquartile range [IQR]. Categorical variables were summarized by frequency and percentage. We performed simple and multiple linear regression models to determine factors independently associated with blood pressure. We used backward selection, until we arrived at the final model with all remaining factors significant with P‐value of <.05. Forward selection, Akaike information criterion (AIC), and Bayesian information criterion (BIC) were also used for robustness of the final model selection. Probability of survival was estimated using the standard Kaplan‐Meier (KM) method, and the multiple KM curves were compared using the log‐rank test. Factors associated with mortality were assessed using Cox proportional hazard models while adjusting for other covariates. Longitudinal trend of blood pressures was analyzed using linear mixed‐effects models with time and HIV fixed effects and patient‐specific random effects. Tests of HIV group differences in blood pressure trends over time were assessed using the asymptotic chi‐square test. All available data were included in the analysis.
2.8. Ethics
The study was carried out in accordance with Good Clinical Practice. The study and consent forms were approved by the IRBs of Weill Cornell Medicine (Protocol 1506016328), the Tanzanian National Institute of Medical Research (Protocol NIMR/HQ/R.8c/Vol.1/1399), and Bugando Medical Centre (Protocol CREC/074/2015). All participants provided written informed consent. All PWH were provided free treatment according to the Tanzanian national guidelines. 16 For participants with hypertension, we assisted them to purchase medications for hypertension using money from a private foundation. 17
3. RESULTS
3.1. Study population
Between June 2016 and August 2019, we screened 1427 people. Reasons for non‐enrollment were as follows: known cardiovascular disease (125), living outside study area (79), unable/unwilling to consent (76), prior ART use (71), no phone (63), outside age range (7), and prognosis <1 year (6).
We enrolled 1000 participants (496 PWH and 504 HIV‐uninfected). Four of the HIV‐uninfected participants have now been infected with HIV. Since these four participants contribute observation time in both the PWH and HIV‐uninfected groups, we have included these 4 in both groups, making the total number of observations 500 PWH and 504 HIV‐uninfected. We analyzed data up to 2 years of follow‐up for each participant. The mean follow‐up time of participants was 17.3 months. At the time of analysis, 904 participants were in follow‐up, 35 had died, and 61 were lost to follow‐up.
3.2. Baseline characteristics
The baseline characteristics for the 1004 PWH and HIV‐uninfected participants are displayed in Table 1. The average age was similar between PWH and HIV‐uninfected participants (36 [29‐43] vs 35 [26.5‐43] years). Two‐thirds of study participants were female (68.6% vs 66.5%). The median body mass index (BMI) was 22 kg/m2 in both groups. The median CD4+ count of PWH was 413 [164‐594]. Albuminuria (ACR ≥ 30 mg/g) was more common in PWH (14.2% vs 6.8%), though renal function (eGFR < 60 mL/min/1.73 m2) was similar in both groups (10.4% vs 10.7%). Most had never had their blood pressure measured before the day of enrollment (57% vs 61%).
TABLE 1.
Baseline characteristics for study population
| People with HIV (n = 500 a ) | HIV‐uninfected (n = 504 a ) | |
|---|---|---|
| Number (%)/Median [IQR] | Number (%)/Median [IQR] | |
| Age (y) | 36 [29‐43] | 35 [26.5‐43] |
| Female | 343 (68.6%) | 335 (66.5%) |
| Education | ||
| Primary school or less | 408 (81.6%) | 371 (73.6%) |
| Secondary school (form 1‐4) | 75 (15.0%) | 113 (22.4%) |
| University/college | 17 (3.4%) | 20 (4.0%) |
| Monthly income (TSh) | 90 000 [33 000‐181 000] | 80 000 [40 000‐150 000] |
| Mode of transport to clinic | ||
| Private vehicle | 75 (15.0%) | 43 (8.5%) |
| Public transport | 207 (41.4%) | 200 (39.7%) |
| Walk or Bicycle | 218 (43.6%) | 261 (51.8%) |
| Manual labor | 138 (27.6%) | 136 (27.0%) |
| Last blood pressure (BP) measurement | ||
| Within the last year | 172 (34.4%) | 113 (22.4%) |
| More than 1 y ago | 45 (9.0%) | 84 (16.7%) |
| Never | 283 (56.6%) | 307 (60.9%) |
| Antiretroviral therapy (ART) initiated immediately after enrollment | ||
| Tenofovir, lamivudine, efavirenz | 403 (80%) | N/A |
| Tenofovir, lamivudine, dolutegravir | 31 (6.2%) | N/A |
| Other ART regimens | 24 (4.8%) | N/A |
| Delayed ART initiations | 42 (8.4%) | N/A |
| Ever used tobacco | 82 (16.4%) | 52 (10.3%) |
| Currently using tobacco | 26 (5.2%) | 28 (5.6%) |
| Ever used alcohol | 352 (70.4%) | 268 (53.2%) |
| Used alcohol in last year | 182 (36.4%) | 150 (29.8%) |
| Fruit servings/wk | 2 [0.5‐7] | 2 [0‐4] |
| Vegetable servings/wk | 5 [2‐7] | 5 [2‐7] |
| Add salt to cooking | 259 (51.8%) | 238 (47.2%) |
| Soda(s)/d | 1 [1‐2] | 1 [1‐2] |
| Waist circumference (cm) | 78.0 [72.4‐85.5] | 80.5 [73.1‐90.1] |
| Body mass index (kg/m2) | 21.6 [19.2‐24.8] | 22.4 [19.8‐26.7] |
| SBP (mm Hg) | 113.5 [104.0‐124.5] | 123 [112.0‐134.0] |
| Low SBP at baseline (SBP < 90) | 14 (2.8%) | 3 (0.6%) |
| DBP (mm Hg) | 74.5 [68.0‐82.0] | 78 [71.5‐85.0] |
| Diabetes | 12 (2.4%) | 10 (2.0%) |
| Hemoglobin (g/dL) | 12.2 [10.5‐13.8] | 13.6 [12.6‐14.8] |
| Normal | 253 (50.6%) | 427 (84.7%) |
| Mild anemia | 88 (17.6%) | 30 (6.0%) |
| Moderate anemia | 134 (26.8%) | 42 (8.3%) |
| Severe anemia | 25 (5.0%) | 5 (1.0%) |
| eGFR < 60 mL/min/1.73 m2 | 52 (10.4%) | 54 (10.7%) |
| Albumin‐creatinine ratio (mg/g) | 6 [3‐13.5] | 4 [0‐7] |
| Normal (<30 mg/g) | 429 (85.8%) | 470 (93.3%) |
| High (≥30 and ≤ 300 mg/g) | 53 (10.6%) | 28 (5.6%) |
| Very high (≥300 mg/g) | 18 (3.6%) | 6 (1.2%) |
| CD4+ T‐cell count (cells/mm3) | 412.5 [163.5‐594] | 883 [715.5‐1068.5] |
| Total lymphocyte count (cells/mm3) | 1903 [1395‐2404] | 2123 [1763‐2541] |
Four participants were HIV‐uninfected at baseline but seroconverted during follow‐up. These four participants contribute observation time to both study groups and are counted in both groups.
3.3. Baseline blood pressures
At the time of enrollment, both SBP and DBP were significantly lower in the PWH compared to HIV‐uninfected participants. The median SBP was 113.5 [104.0‐124.5] mm Hg in PWH vs 123 [112.0‐134.0] mm Hg in HIV‐uninfected controls (P < .001). The median diastolic blood pressure (DBP) was 74.5 [68.0‐82.0] vs 78 [71.5‐85.0] mm Hg (P = .005).
3.4. Factors associated with enrollment blood pressure
We examined the associations between all exposure variables in Table 1 with SBP at enrollment using univariable and multivariable linear regression models. All exposures significantly associated with higher baseline SBP are displayed in Table 2. After adjusting for confounding in multivariable linear regression models, the factors most strongly associated with higher SBP at baseline were HIV‐uninfected status, higher BMI, male sex, older age, higher hemoglobin, and lower fruit consumption. After adjustment, baseline SBP remained 6.3 [4.3‐8.3] mm Hg lower in PWH.
TABLE 2.
Statistically significant associations between characteristics from Table 1 and baseline systolic blood pressure (SBP) in 1000 people with HIV and HIV‐uninfected participants in Mwanza City, Tanzania
| Univariate analysis |
Interaction a P‐value |
Multivariate analysis | |||
|---|---|---|---|---|---|
| Coefficient [95% CI] SBP in mm Hg | P‐value | Coefficient [95% CI] SBP in mm Hg | P‐value | ||
| HIV infection | −8.66 [−10.72,−6.59] | <.001 | N/A | −6.29 [−8.29,−4.28] | N/A |
| Age (y) | 0.54 [0.44,0.64] | <.001 | .059 | 0.48 [0.38,0.57] | <.001 |
| Sex (male) | 5.35 [3.11,7.61] | <.001 | .420 | 5.54 [3.23,7.85] | <.001 |
| Fruit servings/wk | −0.49 [−0.82,−0.15] | .004 | .854 | −0.37 [−0.67,−0.08] | .014 |
| Waist circumference (cm) | 0.47 [0.38,0.56] | <.001 | .318 | ||
| Body mass index (kg/m2) | 0.85 [0.64,1.05] | <.001 | .459 | 0.67 [0.46,0.87] | <.001 |
| Hemoglobin (g/dL) | 2.09 [1.64,2.53] | <.001 | .046 | 1.06 [0.57,1.54] | <.001 |
| eGFR < 60 mL/min/1.73 m2 | 6.91 [3.46,10.35] | <.001 | .008 | ||
| CD4+ T‐cell count (cells/mm3) | 0.008 [0.005,0.011] | <.001 | .005 | ||
| Manual labor | 3.88 [1.50,6.26] | .001 | .899 | ||
| Time of home smoke exposure (y) | 0.11 [0.03,0.19] | .006 | .203 | ||
Interaction effect with HIV status.
Table S1 displays the factors from Table 1 that were significantly associated with baseline DBP. After adjustment, baseline DBP remained 2.1 [0.8‐4.3] mm Hg lower in PWH.
3.5. Effect modification by HIV status
We analyzed whether exposures associated with SBP and DBP differed by HIV status using a P‐value for interaction (Table 2 and Table S1). HIV status was a significant modifier in the relationship between CD4+ T‐cell count and SBP (see Figure 1A). By stratified linear regression, a statistically significant positive association was seen between CD4+ T‐cell count and SBP in the PWH but not in the HIV‐uninfected group.
FIGURE 1.
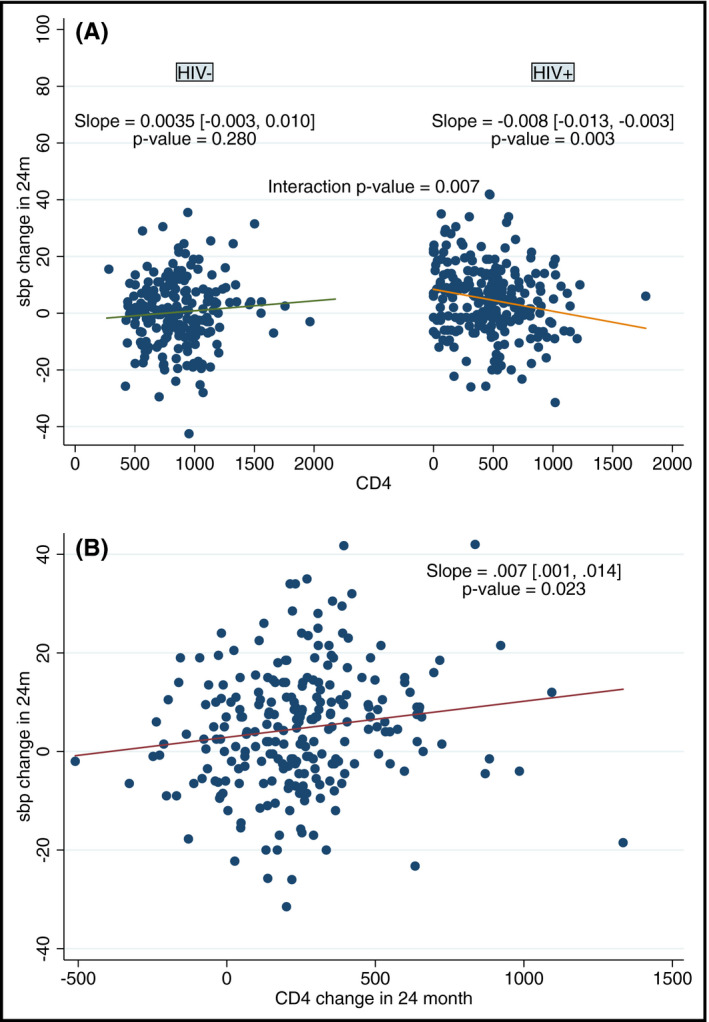
CD4+ T‐cell counts were significantly associated with systolic blood pressure (SBP), but only in people With HIV (PWH). Panel A. Association between baseline CD4+ count and change in SBP from baseline to 24 mo in PWH (right) and HIV‐uninfected adults (left). Panel B. Association between increase in CD4+ T‐cell count and increase in SBP during the first 2 y of ART in PWH
HIV status was also a significant moderator in the relationships between estimated glomerular filtration rate (eGFR) and hemoglobin with SBP (Figure S1A). A statistically significant positive association was seen between eGFR and SBP at baseline in the HIV‐uninfected group but not in PWH . A similar trend was seen for albuminuria with a statistically significant positive association seen between albumin‐creatinine ratio and SBP at baseline in the HIV‐uninfected group but not in PWH (Figure S1B). In contrast, a stronger positive association was seen between hemoglobin and SBP in PWH than in HIV‐uninfected adults (Figure S1C).
3.6. Blood pressure changes over time
All 1000 participants were studied for blood pressure trends. Of 4155 blood pressure measurements included in this analysis, 84 were censored because participants were receiving blood pressure–lowering medications. Figure 2Adepicts the change in mean SBP from baseline over the first 2 years of study follow‐up. For PWH, the average change in SBP from baseline to 24 months was 4.7 mm Hg (95% CI: 3.1‐6.3 mm Hg) vs 0.4 mm Hg (−1.3 to 2.1) in controls. PWH with a CD4+ T‐cell count of <300 at baseline experienced a significantly greater increase in SBP from baseline than those with a higher CD4+ count (Figure 2B). The trends in mean SBP and DBP from baseline to 24 months are displayed in Figures S2 and S3.
FIGURE 2.
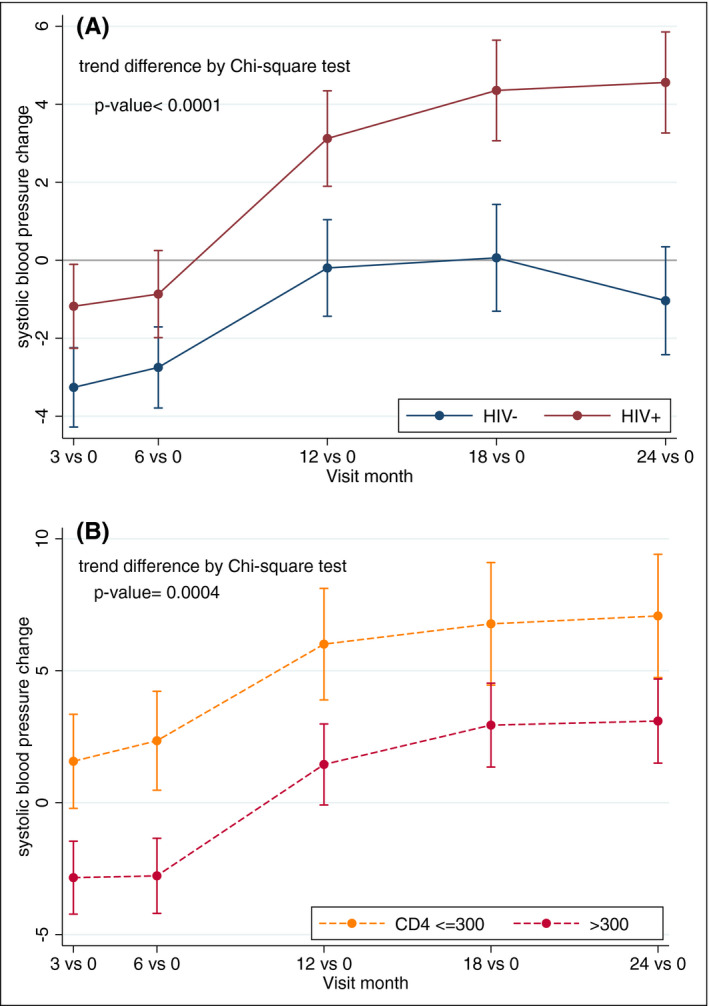
Systolic blood pressure (SBP) increased more rapidly in people with HIV (PWH) compared to controls with an even greater increase observed in PWH with low CD4+ T‐cell counts. Panel A. Changes in SBP from baseline during the first 2 y of antiretroviral therapy in PWH (red line) and HIV‐uninfected controls (blue line). Panel B. Changes in SBP from baseline during the first 2 y of antiretroviral therapy in PWH with CD4+ ≤300 (yellow line) and CD4+ >300 (red line)
We investigated the association between all exposures in Table 1 with change in SBP from baseline to 24 months using linear regression models. Factors significantly associated with greater increases in SBP from baseline to 24 months were HIV infection, lower baseline CD4+, and lower hemoglobin. In this analysis, significant effect modification was observed for baseline CD4+ count (P = .007 for interaction), which was only significant in PWH. Neither total lymphocyte counts nor ART regimen nor delay in ART initiation were significantly associated with change in SBP from baseline to 24 months. In addition, a greater increase in CD4+ count (coefficient: 0.007, P = .023, Figure 1B), hemoglobin (1.37, P < .001), waist circumference (0.52, P < .001), and BMI (1.71, P < .001) during the first 2 years of ART was significantly associated with greater increases in SBP from baseline to 24 months. In multivariate models including changes in CD4+ count, hemoglobin, and waist circumference, we found clear and consistent differences in factors associated with change in SBP over 2 years according to baseline blood pressure. For PWH with SBP < 120 mm Hg at baseline, in a multivariate model including CD4+ count, hemoglobin, and waist circumference, change in CD4+ count remains significantly associated with change in SBP (0.007, P = .042) together with change in hemoglobin (1.00, P = .003) and waist circumference (0.34, P = .004). For PWH with SBP ≥120 mm Hg at baseline, in a multivariate model including the same variables, the only factor significantly associated with increase in SBP was increased waist circumference (0.83, 0.001). BMI was excluded from these models due to co‐linearity with waist circumference.
From the first blood pressure measurement at enrollment through the next 2 study visits (0, 3, and 6 months), blood pressure declined in both PWH and HIV‐uninfected participants. In total, 846 study participants attended all three of these visits. On the first measurement at the 1st visit, 211/846 (24.9%) had a blood pressure ≥140/90 mm Hg. In contrast, using the average of measurements at the 1st visit, only 125 (14.8%) had a blood pressure ≥140/90 mm Hg. By the 2nd and 3rd study visits, only 73 (8.6%) and 52 (6.1%) had a blood pressure that was persistently ≥140/90 mm Hg.
3.7. Baseline SBP and mortality in PWH
Thirty‐four out of 500 PWH (6.8%) died during the 2 years of follow‐up. One HIV‐uninfected control died. Low baseline blood pressure was significantly associated with mortality during the first 2 years of follow‐up in PWH. Figure 3displays the Kaplan‐Meier survival analysis of survival in PWH grouped by baseline blood pressure into three groups: SBP < 90, SBP 90‐139, and SBP ≥ 140 mm Hg. Five out of 17 (29.4%) PWH with baseline blood pressure <90 mm Hg died within 2 years compared to none of the PWH with baseline blood pressure of ≥140 (P < .0001 by log‐rank test). After adjusting for age, sex, and CD4+ count, SBP at baseline < 90 mm Hg remained strongly associated with mortality (aHR = 7.0 [2.6‐19.2], P‐value < .001). The details for these five participants are provided in Table S2. In all 5, the cause of death was tuberculosis with immune reconstitution inflammatory syndrome (IRIS) within 3 months of enrollment.
FIGURE 3.
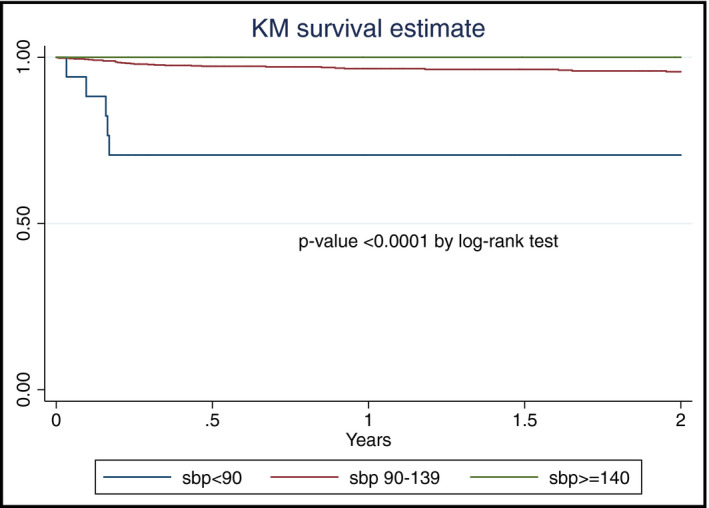
Kaplan‐Meier curve for survival of people with HIV during the first 2 y of follow‐up according to the baseline systolic blood pressure (SBP)
4. DISCUSSION
In our study of 1000 young and middle‐aged Tanzanian adults, PWH experienced a 4 mm Hg greater increase in SBP during the first 2 years of antiretroviral therapy (ART) than similar HIV‐uninfected control participants. This result is both statistically and clinically significant. Meta‐analysis in 61 prospective observational studies demonstrated that even a 2 mm Hg higher SBP in early adulthood or middle age is associated with a 10% higher lifetime risk of death from stroke and a 7% higher risk of death from ischemic heart disease. 18 Meta‐analysis of more clinical trials of antihypertensive treatment in this age‐group has confirmed that a 5 mm Hg reduction in SBP prevents cardiovascular events and reduces all‐cause mortality. 19 These results suggest that the early ART period may represent a critical window of opportunity for interventions to reduce long‐term cardiovascular risk in PWH.
Before ART initiation, PWH had a significantly lower blood pressure than HIV‐uninfected adults. This combination of lower blood pressure before ART initiation and a more rapid increase in blood pressure after ART initiation leads us to an important question—why are the dynamics of blood pressure different in PWH in the early ART period? The answer to this question likely involves a complex interplay between traditional and HIV‐specific risk factors such as immune reconstitution. 20 Several recent cross‐sectional studies have shown that PWH on established ART have the same or less hypertension than HIV‐uninfected controls. 21 , 22 , 23 Other studies, however, have shown higher rates of hypertension in PWH. 24 Our results indicate that further research should focus on the early ART period.
Among PWH, lower baseline CD4+ T‐cell counts were significantly associated with lower baseline blood pressure, and greater increases in CD4+ T‐cell counts during the first 2 years of ART were associated with greater increases in blood pressure in participants with SBP < 120 mm Hg at baseline, even after adjusting for potential confounders. Total lymphocyte counts, on the other hand, were not associated with blood pressure. Of note, a return to good health marked by rising hemoglobin and weight appears to explain some, but not all of the association that we observed between changes in CD4+ T‐cell count and increases in blood pressure. These results imply that increases in blood pressure in the early ART period might be driven by rapid changes in T‐cell immunity during this period (Figure 4). Mice lacking T cells exhibit reduced blood pressure increases in response to salt and angiotensin II infusion. 25 In humanized mice given activated human T cells, by contrast, the CD4+ T cells invade aorta and kidney and produce cytokines IL‐17A and IFN‐gamma, which contribute to hypertension. 26 Similarly in mice with hypertension, CD4+ T cells produce IL‐17A in the kidney and aorta and blocking IL‐17A reduces the blood pressure. 27 Therefore, ART‐naïve PWH may have lower blood pressure due to depletion of CD4+ T cells. After ART initiation and immune reconstitution, abnormally activated CD4+ T cells may invade the aorta and the kidney, leading to a rapid rise in blood pressure. Alternatively, rapid changes in CD4+ T‐cell immunity could induce changes in CD8+ T cells or monocytes that are also known to be involved in the pathophysiology of hypertension.
FIGURE 4.
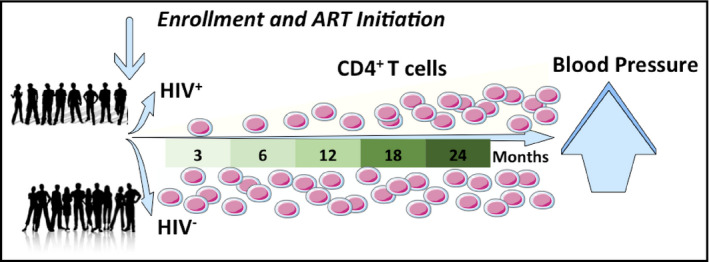
Graphical abstract. In people with HIV who were enrolled before initiation of antiretroviral therapy (ART) and followed for 24 mo, blood pressure increased significantly more than in HIV‐uninfected control participants of similar age and gender. This increase in blood pressure was significantly associated with increases in CD4+ T cells, both on average and within individuals
Several determinants of blood pressure differed significantly by HIV status including hemoglobin, reduced eGFR, and albuminuria. Hemoglobin, for example, was strongly associated with SBP. The regression coefficient for the relationship between hemoglobin and SBP and PWH was twice as great in PWH compared to HIV‐uninfected adults. Among PWH in our study, >30% had moderate or severe anemia, and a reduction of hemoglobin of 5 g/dL was associated with a 10 mm Hg lower SBP. Studies from the general population have reported a small but statistically significant association between hemoglobin and blood pressure. 28 , 29 Higher hemoglobin may directly promote arterial stiffness or may reduce vascular nitric oxide levels leading to vasoconstriction, 30 , 31 and HIV may promote similar changes. 32 This finding deserves further investigation.
We found that both SBP and DBP dropped dramatically between the first, second, and third visits at the HIV clinic (Tables S2 and S3). Furthermore, only 25% of participants whose first blood pressure measurement was elevated had persistent hypertension on three consecutive visits. This could be due to anxiety in a population where blood pressure measurement is rarely performed (nearly 60% of our participants had never had their blood pressure measured). 33 Accurate measurement of blood pressure is a cornerstone of hypertension management. Therefore, as HIV programs in Africa begin to integrate hypertension care into clinical practice, these programs must ensure that standard procedures for measuring multiple blood pressures on different days will be followed.
PWH with SBP < 90 mm Hg at the time of ART initiation should receive special medical attention. Our findings confirm a recent publication from a cohort study of 816 PWH in Haiti in which SBP < 90 mm Hg before ART initiation was associated with a twofold increased odds of mortality. 7 Low blood pressure before ART initiation may be secondary to an undiagnosed infection, which could progress to septic shock and/or severe IRIS after ART initiation. 34 PWH with SBP < 90 mm Hg in before ART initiation should undergo a thorough diagnostic workup for occult infections with close follow‐up. Corticosteroid treatment should be considered in those with tuberculosis to prevent death due to immune reconstitution inflammatory syndrome (IRIS). 35
Our study has limitations. HIV viral loads are not measured at the time of ART initiation in Tanzania and, therefore, were not available as part of the baseline data. Of note, HIV viral load has not been strongly associated with blood pressure in prior studies. 3 , 4 In addition, although we did not observe a significant association between ART exposure and blood pressure, a longer duration of follow‐up may be needed to observe such effects. The results of our study are likely generalizable to other populations of PWH in sub‐Saharan Africa but may not be generalizable to PWH living in high‐income countries where early diagnosis and treatment of HIV are more common and severe immunosuppression is rare.
5. CONCLUSIONS
Our analysis of blood pressure in the pre‐ART and early ART period in PWH and HIV‐uninfected adults has revealed several exciting new findings. First, immune‐suppressed PWH had a significantly lower blood pressure before ART initiation but a significantly greater increase in blood pressure during the first 2 years of ART. Second, lower blood pressure before ART initiation and greater increases in blood pressure were strongly associated with CD4+ T‐cell counts. Third, PWH with an SBP of <90 mm Hg at the time of ART initiation had a 30% risk of death in the first 3 months of ART. Further research is needed to determine the mechanisms underlying the association of blood pressure and T cells in the early ART period. In addition, blood pressure measurement must be regularly and carefully measured in HIV clinics and PWH with low SBP should receive careful investigation and close monitoring.
CONFLICT OF INTEREST
No conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
KR contributed to study design, data collection, data analysis, data interpretation, and manuscript preparation, and revised the manuscript for important intellectual content. BD contributed to study conception and study design, and revised the manuscript for important intellectual content. JK contributed to study conception, study design, data collection, and manuscript preparation, and revised the manuscript for important intellectual content. AK contributed to data analysis, data interpretation, and manuscript preparation, and revised the manuscript for important intellectual content. AM contributed to study conception and study design, and revised the manuscript for important intellectual content. CM contributed to data analysis, data interpretation, and manuscript preparation, and revised manuscript for important intellectual content. ML contributed to study design, data analysis, data interpretation, and manuscript preparation, and revised the manuscript for important intellectual content. SK contributed to study conception, study design, data interpretation, and manuscript preparation, and revised the manuscript for important intellectual content. RP contributed to study conception, study design, data collection, data analysis, data interpretation, and manuscript preparation, and revised the manuscript for important intellectual content. All authors approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge all of our participants, without whom this work would be impossible, as well as our colleagues at Bugando Medical Center who have lent their support to this research.
Reis KG, Desderius B, Kingery J, et al. Blood pressure, T cells, and mortality in people with HIV in Tanzania during the first 2 years of antiretroviral therapy. J Clin Hypertens. 2020;22:1554–1562. 10.1111/jch.13975
Funding information
The study was supported by Dr Peck's NIH K01TW010281 grant, Dr Fitzgerald's NIH K24AI098627 grant, and the Weill Cornell Medicine UL1TR000457 grant.
REFERENCES
- 1. Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep. 2013;10:199‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low‐, middle‐, and high‐income countries. N Engl J Med. 2014;371:818‐827. [DOI] [PubMed] [Google Scholar]
- 3. Dimala CA, Blencowe H, Choukem SP. The association between antiretroviral therapy and selected cardiovascular disease risk factors in sub‐Saharan Africa: a systematic review and meta‐analysis. PLoS One. 2018;13:e0201404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanidas E, Papadopoulos DP, Velliou M, Tsioufis K, Barbetseas J, Papademetriou V. Human immunodeficiency virus infection and hypertension. Is there a connection? Am J Hypertens. 2018;31:389‐393. [DOI] [PubMed] [Google Scholar]
- 5. Brennan AT, Jamieson L, Crowther NJ, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS One. 2018;13:e0204020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez‐Arboli E, Mwamelo K, Kalinjuma AV, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania ‐ a prospective cohort study. PLoS One. 2017;12:e0172089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batavia AS, Severe P, Lee MH, et al. Blood pressure and mortality in a prospective cohort of HIV‐infected adults in Port‐au‐Prince, Haiti. J Hypertens. 2018;36:1533‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross‐sectional study. BMC Med. 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . WHO Steps Instrument. 2012. https://www.who.int/ncds/surveillance/steps/en/. Accessed June 01, 2020.
- 11. Kavishe B, Biraro S, Baisley K, et al. High prevalence of hypertension and of risk factors for non‐communicable diseases (NCDs): a population based cross‐sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med. 2015;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Center for Disease Control . National Health and Nutrition Examination Survey (NHANES): Health Tech/Blood Pressure Procedures Manual. 2009.
- 13. International Diabetes Federation . Global Guideline for Type 2 Diabetes. Brussels, Belgium: International Diabetes Federation; 2012. [Google Scholar]
- 14. World Health Organization . Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 15. Wyatt CM, Schwartz GJ, Owino Ong'or W, et al. Estimating kidney function in HIV‐infected adults in Kenya: comparison to a direct measure of glomerular filtration rate by iohexol clearance. PLoS One. 2013;8:e69601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanzania Ministry of Health . Standard Treatment Guidelines & National Essential Medications List: Tanzania Mainland. 2017.
- 17. Mzombwe M, Desderius B, Kapiga S, Smart L, Peck R. The ethical imperative to treat NCDs during research in Africa. Lancet Glob Heal. 2019;7:e406‐e407. [DOI] [PubMed] [Google Scholar]
- 18. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 19. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta‐analysis. JAMA Cardiol. 2017;2:775‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV‐infected adults. Hypertension. 2018;72:44‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okello S, Ueda P, Kanyesigye M, et al. Association between HIV and blood pressure in adults and role of body weight as a mediator: cross‐sectional study in Uganda. J Clin Hypertens. 2017;19:1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manne‐Goehler J, Siedner MJ, Montana L, et al. Hypertension and diabetes control along the HIV care cascade in rural South Africa. J Int AIDS Soc. 2019;22:e25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feinstein MJ, Kim J‐H, Bibangambah P, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV‐infected and uninfected Ugandans. AIDS Res Hum Retroviruses. 2017;33:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schouten J, Wit FW, Stolte IG, et al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between HIV‐infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787‐1797. [DOI] [PubMed] [Google Scholar]
- 25. Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449‐2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itani HA, McMaster WG, Saleh MA, et al. Activation of human T cells in hypertension: Studies of humanized mice and hypertensive humans. Hypertension. 2016;68:123‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saleh MA, Norlander AE, Madhur MS. Inhibition of interleukin‐17A, but not interleukin‐17F, signaling lowers blood pressure, and reduces end‐organ inflammation in angiotensin II–induced hypertension. JACC Basic to Transl Sci. 2016;1:606‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasmussen JB, Mwaniki DL, Kaduka LU, et al. Hemoglobin levels and blood pressure are associated in rural black Africans. Am J Hum Biol. 2016;28:145‐148. [DOI] [PubMed] [Google Scholar]
- 29. Atsma F, Veldhuizen I, de Kort W, van Kraaij M, Pasker‐de Jong P, Deinum J. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertens. 2012;60:936‐941. [DOI] [PubMed] [Google Scholar]
- 30. Cabrales P, Han G, Nacharaju P, Friedman AJ, Friedman JM. Reversal of hemoglobin‐induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. 2011;300:H49‐H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawamoto R, Tabara Y, Kohara K, et al. A slightly low hemoglobin level is beneficially associated with arterial stiffness in Japanese community‐dwelling women. Clin Exp Hypertens. 2012;34:92‐98. [DOI] [PubMed] [Google Scholar]
- 32. Hansen L, Parker I, Sutliff RL, Platt MO, Gleason RL. Endothelial dysfunction, arterial stiffening, and intima‐media thickening in large arteries from HIV‐1 transgenic mice. Ann Biomed Eng. 2013;41:682‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Etyang AO, Warne B, Kapesa S, et al. Clinical and epidemiological implications of 24‐hour ambulatory blood pressure monitoring for the diagnosis of hypertension in Kenyan adults: a Population‐Based Study. J Am Heart Assoc. 2016;5:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bloomfield GS, Hogan JW, Keter A, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meintjes G, Stek C, Blumenthal L, et al. Prednisone for the prevention of paradoxical tuberculosis‐associated IRIS. N Engl J Med. 2018;379:1915‐1925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


