Abstract
The hypoxia-inducible factor 1α (HIF-1α) is critically involved in tissue regeneration. Hence, the pharmacological prevention of HIF-1α degradation by prolyl hydroxylase (PHD) under normoxic conditions is emerging as a promising option in regenerative medicine. Using a mouse model of ligature-induced periodontitis and resolution, we tested the ability of an injectable hydrogel-formulated PHD inhibitor, 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid (1,4-DPCA/hydrogel), to promote regeneration of alveolar bone lost owing to experimental periodontitis. Mice injected subcutaneously with 1,4-DPCA/hydrogel at the onset of periodontitis resolution displayed significantly increased gingival HIF-1α protein levels and bone regeneration, as compared to mice treated with vehicle control. The 1,4-DPCA/hydrogel-induced increase in bone regeneration was associated with elevated expression of osteogenic genes, decreased expression of pro-inflammatory cytokine genes and increased abundance of FOXP3+ T regulatory (Treg) cells in the periodontal tissue. The enhancing effect of 1,4-DPCA/hydrogel on Treg cell accumulation and bone regeneration was reversed by AMD3100, an antagonist of the chemokine receptor CXCR4 that mediates Treg cell recruitment. In conclusion, the administration of 1,4-DPCA/hydrogel at the onset of periodontitis resolution promotes CXCR4-dependent accumulation of Treg cells and alveolar bone regeneration, suggesting a novel approach for regaining bone lost due to periodontitis.
Keywords: Hypoxia-inducible factor 1α; 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid; osteogenesis; AMD3100; T regulatory cells
INTRODUCTION
Periodontitis is a chronic inflammatory disease leading to progressive destruction of the tissues that surround and support the teeth, i.e., the gingiva, periodontal ligament, and alveolar bone, collectively known as the periodontium (1, 2). The inflammatory destruction of the periodontium is driven by a dysregulated host response against dysbiotic microbial communities (‘dental plaque biofilm’) that colonize subgingival tooth sites within the so-called periodontal pockets (3). Almost 50% of adults are afflicted by some form of periodontal disease (ranging from mild to severe) with nearly 10% of the global adult population presenting severe periodontitis, which is associated with increased risk of certain systemic disorders (1, 4–6). If not properly treated, periodontitis can lead to tooth loss and thus impaired mastication and esthetics, thereby affecting the quality of life (7, 8).
Currently, the standard-of-care therapy of periodontitis patients involves subgingival mechanical debridement (‘scaling and root planing’) (9), which aims to control the pathogenic dental plaque biofilm and enable inflammation resolution (10). However, mechanical debridement is only partially effective for the majority of periodontitis patients (hence, a high rate of recurrence), whereas a sizeable fraction of patients does not respond favorably to standard treatment (‘refractory periodontitis patients’) (11, 12). Thus, periodontitis remains a serious public health and economic burden (8, 13, 14) and, importantly, alveolar bone lost due to periodontitis shows limited or unpredictable capacity for regeneration even in patients treated with scaling and root planing and conventional surgical periodontal therapy (15–17). With regard to specific approaches to regenerate bone lost owing to periodontitis, progress has been achieved through the surgical implantation of allogeneic or autologous bone material, the use of scaffolds, stem cells, and soluble molecules, such as, bone morphogenetic proteins and various growth factors. However, there is as yet insufficient evidence on the efficacy of emerging periodontal regenerative approaches to warrant recommendation for routine clinical application (18–22). There is, therefore, an unmet need for affordable and effective adjunctive regenerative therapies.
Drug-induced regeneration is a promising alternative approach that uses small-molecule pharmacological molecules that activate pro-regenerative host biological processes (23, 24). An approach based on the transcription factor hypoxia-inducible factor (HIF)-1 was developed based on clues from unique regenerative traits of the Murphy Roths Large (MRL) mice (24, 25). HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits; whereas HIF-1β is constitutively expressed, HIF-1α is regulated by oxygen and accumulates in cells under hypoxic conditions (26). The levels of HIF-1α are regulated by prolyl-4-hydroxylase (PHD) which, under normoxic conditions, hydroxylates proline residues on HIF-1α, thereby targeting it for degradation by the ubiquitin ligase-proteasome system (27). Unlike most laboratory strains of mice, the MRL mouse exhibits an extraordinary capacity to regenerate tissues upon wounding, which has been attributed to their inherent ability to maintain high levels of HIF-1α in response to injury (24, 25). HIF-1α exerts pro-regenerative effects by transcriptional regulation of various genes involved in metabolism, angiogenesis, cell migration, and survival (25, 28–30). Pharmacological inhibition of PHD activity, as by the use of 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid (1,4-DPCA), blocks the degradation of HIF-1α and thus increases its protein levels (24, 31). Importantly, systemic delivery of 1,4-DPCA via an injectable hydrogel carrier (1,4-DPCA/hydrogel) in mice without spontaneous regenerative capacity, promoted tissue regeneration upon ear hole punch injury; this regenerative response was HIF-1α-dependent and reminiscent of that seen in MRL mice (24).
The aim of this study was to evaluate the capacity of 1,4-DPCA/hydrogel to promote re-growth of bone lost due to periodontitis. Specifically, using the ligature-induced periodontitis model in C57BL/6 mice, which do not share the spontaneous regenerative capacity of MRL mice, we tested the ability and underlying mechanisms of 1,4-DPCA/hydrogel to promote alveolar bone regeneration during the resolution phase of periodontitis.
MATERIALS AND METHODS
Mice
C57BL/6 female and male mice were purchased from the Jackson Laboratory. Sex- and age-matched mice (8- to 10-weeks old) were used in experiments. As there were no significant differences in the results obtained with female and male mice, the respective data were pooled per treatment group. Mice were maintained in individually ventilated cages under specific pathogen-free conditions on a standard 12-h light/dark cycle. Food and water were provided ad libitum. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and were performed in compliance with institutional, state, and federal policies.
Cell culture and ELISA
Human gingival epithelial cells (HGEC) were isolated and cultured as previously described (32). Human periodontal ligament (HPDL) cells were purchased from Lonza (cat# CC-7049; Walkersville, MD, USA). The murine osteoblastic progenitor cell line MC3T3-E1 Subclone 4 (ATCC cat# CRL-2593; Manassas, VA, USA) was maintained as previously described (33). HGEC and HPDL cells (1.0 × 104 cells/0.2 ml) were seeded on 96-well plates and MC3T3-E1 cells (5.0 × 105 cells/0.5 ml) were seeded on 12-well plates. The cells were stimulated with 1,4-DPCA (50 μg/ml final concentration; cat# 71220; Cayman Chemical, Ann Arbor, MI, USA) or vehicle control (DMSO; 0.17% final concentration; cat# D5879; Sigma-Aldrich, St. Louis, MO, USA). After 24 h, culture supernatants were collected and the levels of vascular endothelial growth factor (VEGF) and C-X-C motif chemokine ligand 12 (CXCL12) were determined using ELISA kits (cat# DY293B and DY350 for human assays and cat# DY460 and DY493 for mouse assays; R&D Systems, Minneapolis, MN, USA).
Resolution of ligature-induced periodontitis and bone regeneration
Groups of mice were subjected to experimental periodontitis by tying a 5–0 silk ligature around the maxillary left second molar for 10 days. The contralateral tooth was kept unligated as baseline control. To enable periodontitis resolution, the ligatures were removed at day 10 and the mice were sacrificed 5 days later (day 15). Periodontal bone loss was assessed morphometrically in defleshed maxillae using a dissecting microscope (40x) fitted with a video image measurement system (Nikon Instruments, Melville, New York, USA). Specifically, the distance from cement-enamel junction (CEJ) to alveolar bone crest (ABC) was measured on six predetermined points on the ligated second molar and the affected adjacent regions, as we described previously (34). Bone loss was calculated by substracting the six-site total CEJ-ABC distance of the ligated side of each mouse from the six-site total CEJ-ABC distance on the contralateral unligated side. The data were further transformed to indicate bone gain (or loss; negative value) relative to the bone levels of mice that were sacrificed at day 10, as we previously described (33).
Experimental interventions
The 1,4-DPCA/hydrogel used in this study was previously described (24). Briefly, the compound 1,4-DPCA, which is a potent inhibitor of PHDs and stabilizes HIF-1α protein (35), was formulated as 1,4-DPCA drug crystals trapped in a polymer hydrogel comprising a cross-linked network of polyethylene glycol molecules. This hydrogel is ideal as a delivery vehicle as it is biocompatible, displays rapid gelation in situ from a liquid precursor, and releases the drug compound over several days (24). To study the effect of 1,4-DPCA/hydrogel in bone regeneration during resolution, upon removal of the ligatures on day 10, mice were subcutaneously injected (at the base of the neck) with vehicle control (i.e., hydrogel alone) or 1,4-DPCA/hydrogel (50 μg in 25 μl volume), prepared as previously described (24). To study the role of CXCR4 in bone regeneration during resolution, the CXCR4 antagonist AMD3100 (cat# 239825; Merck Millipore, Darmstadt, Germany) was injected (100 μg in 50 μl PBS) intraperitoneally once daily for 5 days after ligature removal on day 10. PBS was used as control.
Flow cytometry and antibodies
Antibodies to the following mouse molecules were purchased from Biolegend (San Diego, CA, USA): CD45 (clone 30-F11), CD3 (clone 145–2C11), CD4 (clone GK1.5) and CD31 (clone 390), IL-10 (clone JES5–16E3), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; clone UC10–4B9), inducible T cell costimulator (ICOS; clone 7E.17G9), CD25 (clone 3C7). Anti-mouse Forkhead box protein P3 (FOXP3) (clone FJK-16s) was from eBioscience and Live/Dead fixable violet dead cell stain kit was from Thermo-Fisher Scientific (Waltham, MA, USA). Mouse gingival tissue was dissected around the area of ligature placement and digested for 50 min at 37°C with Collagenase IV (3.2 mg/ml; cat# 17104019; Thermo-Fisher Scientific) and DNase I (0.15 μg/ml; cat# 10104159001; Sigma-Aldrich) as previously described (36). Single-cell suspensions were obtained by mashing the tissue against a strainer (70 μm size) using plungers and filtered for staining and flow cytometry analysis. Total cell number was counted with a hemocytometer after staining with trypan blue. The cell suspensions were incubated with anti-CD16/CD32 (clone 93, cat# 101320; Biolegend) and fluorochrome-conjugated antibodies against surface markers (as indicated in the figures) in PBS with 2.5% fetal bovine serum (cat# 16141079; Thermo-Fisher Scientific), for 40 min at 4°C degrees in the dark, and then washed. Dead cells were excluded with Live/Dead fixable dye. For intracellular staining of FOXP3, alone or together with IL-10, the cells were fixed and permeabilized with FOXP3/transcription factor staining buffer set (cat# 00-5521-00; eBioscience) and stained for 40 min at 4°C with fluorochrome-conjugated antibody against FOXP3, alone or together with IL-10. Cell acquisition was performed on a NovoCyte flow cytometer (ACEA Biosciences, San Diego, CA, USA). Data were analyzed with NovoExpress software (ACEA Biosciences).
Immunoblotting
Excised gingival tissue was used to extract total protein using NucleoSpin RNA/Protein (cat# 740933.50; Macherey-Nagel, Düren, Germany). The concentration of protein was quantified using Pierce BCA Protein Assay Kit (cat# 23225; Thermo-Fisher Scientific). Samples with equal protein content were separated by SDS-PAGE on acrylamide gels (Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene difluoride membrane (cat# 1620177; Bio-Rad) by electroblotting. The membranes were incubated in blocking buffer (cat# 1706404; Bio-Rad) followed by probing with rabbit anti-mouse HIF-1α (polyclonal, cat# 10006421; Cayman Chemical) and visualization with horseradish peroxidase-conjugated secondary antibody and chemiluminescence using the Amersham Biosciences ECL system (cat# 12644055; GE Healthcare, Chicago, IL, USA). The immunoblots were stripped and reprobed with anti-β actin antibody (13E5; Cell Signaling, Danvers, MA, USA) to control for sample loading. Images were captured using a FluorChem M imaging system (ProteinSimple, San Jose, CA, USA). The density of bands was analyzed using Image Studio Lite (LI-COR Bioscience, Lincoln, NE, USA).
Histology and immunofluorescence histochemistry
Mouse maxillae with surrounding tissue were fixed in 4% paraformaldehyde, decalcified in Decalcifying Solution (Thermo-Fisher Scientific), and then embedded in paraffin. Tissue samples were sectioned at 5-μm thickness in the coronal direction along the long axis of the teeth. The sections were stained with hematoxylin and eosin. For immunofluorescence histochemistry, the sections were stained with antibodies to HIF-1α, CXCR4 (polyclonal, cat# PA3–305; Invitrogen, Carlsbad, CA, USA), CD31 (polyclonal, cat# AF3628; R&D Systems), FOXP3 (clone FJK-16s; Invitrogen), or Ki67 (clone SolA15; Invitrogen), followed by appropriate secondary antibodies (AlexaFluor488-, AlexaFluor594-, or AlexaFluor647-conjugated donkey IgG; Invitrogen). Slides were mounted with cover slips using Fluoroshield mounting medium with DAPI (Abcam, Cambridge, UK). The specificity of staining was confirmed using appropriate isotype controls. Stained images were visualized and captured using a Nikon Eclipse Ni-E automated fluorescent microscope and NIS-Elements software (Nikon Instruments).
RNA in situ hybridization
RNA in situ hybridization was performed with an RNAscope 2.0 (Red) kit, according to the manufacturer’s protocol (Advanced Cell Diagnostics, Newark, CA, USA). Briefly, the tissues were pretreated with H2O2 for 10 min, boiled for 30 min in retrieval buffer, followed by 30 min of protease digestion (RNAscope Protease Plus). The tissues were then incubated with Vegfa and Cxcl12 probes (Advanced Cell Diagnostics) for 2 hours at 40°C. DapB (a bacterial gene coding for dihydrodipicolinate reductase) probe was used as negative control (Advanced Cell Diagnostics). The signal was amplified with sequential hybridization of amplifiers and label probes, then detected by Fast Red. Vegfa and Cxcl12 mRNA were visualized by fluorescence microscopy.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from gingiva tissue using NucleoSpin RNA/Protein and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific) and real-time PCR with cDNA was performed using the Applied Biosystems QuantStudio 3 Real-Time PCR System (Thermo-Fischer Scientific) according to the manufacturer’s protocol. TaqMan probes and gene-specific primers (Supplemental Table S1) for detection and quantification of murine genes investigated in this study were purchased from Thermo-Fisher Scientific. Data were analyzed using the comparative (ΔΔCt) method.
Statistical analysis
For multiple-group comparisons, data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. A two-tailed Student’s t-test was used for two-group comparisons. P values <0.05 were considered to be statistically significant. All statistical analyses were performed using GraphPad Prism software (version 8.2.1).
RESULTS
1,4-DPCA drug/hydrogel increases gingival HIF-1α protein levels and alveolar bone regeneration during the resolution of periodontitis
The ligature-induced periodontitis (LIP) model simulates human periodontitis as it leads to the generation of a local dysbiotic microbiome that drives IL-17-dependent inflammation and alveolar bone loss (34, 36–38). Ligature removal abrogates the dysbiotic microbial challenge leading to inflammation resolution and bone regeneration (33, 39, 40). Two groups of C57BL/6 mice were subjected to ligature-induced periodontitis for 10 days followed by ligature removal for 5 days to enable resolution (‘10dL + 5dR’ treatment). At day 10, the mice were administered 1,4-DPCA/hydrogel (50 μg in 25 μL volume) or vehicle control (hydrogel without drug) by subcutaneous injection at the back of the neck. The effect of 1,4-DPCA/hydrogel on HIF-1α protein expression at day 15 was evaluated by immunoblot analysis (Fig. 1A) and immunofluorescence (Fig. 1B). In the gingival tissue of control mice, HIF-1α protein levels were higher in the ligated side than in the contralateral unligated side (Fig. 1A), consistent with induction of hypoxia in periodontitis (41). However, 1,4-DPCA/hydrogel injection resulted in even higher HIF-1α protein levels than those in vehicle control-injected mice, both in the ligated and the unligated sides (Fig. 1A). Consistently, immunofluorescence revealed that, in 1,4-DPCA/hydrogel-treated mice, HIF-1α was upregulated in the gingival epithelium and the periodontal ligament (PDL) of ligated sides, as compared to the corresponding areas in vehicle control-treated mice (Fig. 1B).
Figure 1: 1,4-DPCA/hydrogel promotes HIF-1α protein levels in the periodontium and alveolar bone regeneration.
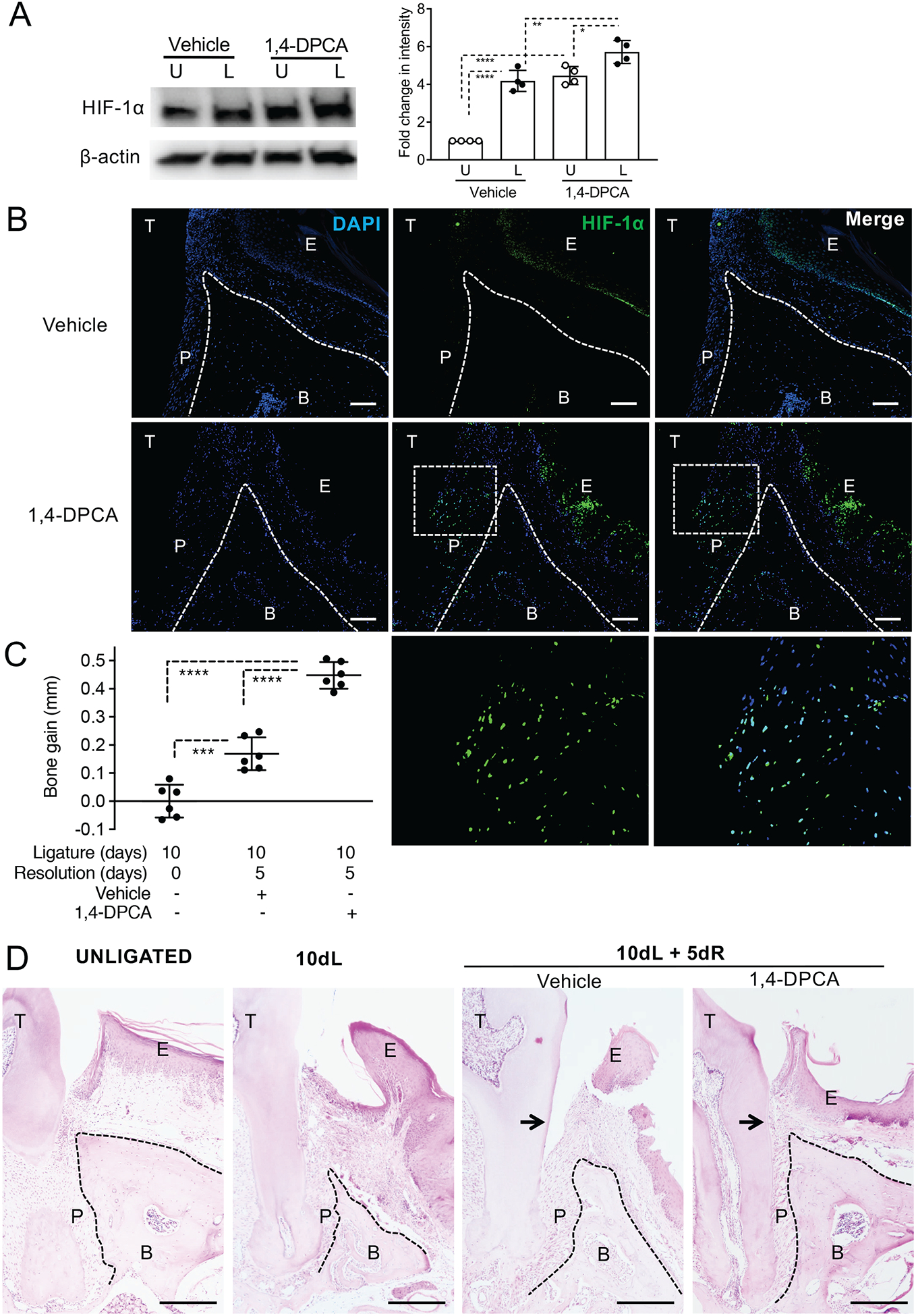
Mice were subjected to LIP for 10 days followed by 5 days without ligatures to enable resolution. At the onset of resolution (day 10), the mice were subcutaneously injected with 1,4-DPCA/hydrogel, or vehicle alone, and were euthanized at day 15 for analysis. (A) The expression of HIF-1α in gingival tissue lysates was analyzed by immunoblotting (left panel). The relative intensity of immunoblotting signals of HIF-1α was quantified by densitometry (right panel). U, unligated; L, ligated. (B) Periodontal tissue sections were stained for HIF-1α protein (green) and DAPI (blue). Scale bars, 100 μm. Bottom row images are magnifications of the rectangle-demarcated areas of the images above. (C) Bone heights (CEJ-ABC distance) were measured and CEJ-ABC data were transformed to indicate bone gain, relative to the bone heights at day 10 (baseline). (D) Periodontal tissue sections were stained with hematoxylin and eosin. Scale bars, 500 μm. Black arrows indicate increased attachment gain in 1,4-DPCA/hydrogel group as compared to vehicle control. NL, not ligated; 10dL, 10 days ligated; 10dL + 5dR, 10 days ligated and 5 days with ligatures removed (resolution). B, Bone; E, Epithelium; P, Periodontal ligament; T, Tooth. Data are shown as means ± SD (A, n=4 mice/per group; B, n=6 mice/per group from two independent experiments, each performed in triplicates). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (ANOVA with Turkey’s multiple-comparisons test).
We have recently shown that ligature removal for 5 days leads to bone regeneration in C57BL/6 mice previously subjected to LIP for 10 days (‘10dL + 5dR’ group), as compared to LIP-subjected mice without ligature removal (‘10dL’ group) (33). Importantly, the treatment with 1,4-DPCA/hydrogel promoted further bone regeneration during LIP resolution as compared to vehicle control (Fig. 1C; P< 0.0001). Histological examination confirmed the enhancing effect of 1,4-DPCA/hydrogel in bone regeneration as compared to the vehicle control, which in turn exhibited bone gain, as compared to the 10dL baseline group (Fig. 1D). Consistent with the upregulation of HIF-1α in the PDL of 1,4-DPCA/hydrogel-treated mice (Fig. 1B, second row and magnified insets), 1,4-DPCA/hydrogel promoted re-growth of the PDL and increased attachment gain, as compared to the vehicle control (Fig. 1D). In fact, the histological appearance of the 1,4-DPCA/hydrogel-treated ligated side was comparable to that of the unligated control (Fig. 1D). Together, these data show that, during resolution of experimental periodontitis, 1,4-DPCA/hydrogel elevates HIF-1α protein levels and leads to improved regeneration of the PDL and bone as compared to vehicle control treatment, resulting in restoration of periodontal tissue integrity.
1,4-DPCA/hydrogel downregulates pro-inflammatory cytokine gene expression and upregulates osteogenic gene expression during periodontitis resolution
We have previously shown that ligature removal from LIP-subjected mice leads to inflammation resolution featuring downregulation of pro-inflammatory cytokine gene expression (40). Similarly in this study, quantitative real-time PCR (qPCR) analysis of the gingival tissue showed that LIP-induced proinflammatory cytokine expression (Il6, Il17a, and Tnf) was significantly higher at day 10 just prior to the removal of ligatures, as compared to 5 days later in the absence of ligatures (Fig. 2, top). Importantly, moreover, this analysis of the gingival tissue revealed that, during the resolving phase of experimental periodontitis, 1,4-DPCA/hydrogel caused further significant decrease of pro-inflammatory cytokine (Il6, Il17a, and Tnf) expression relative to vehicle control treatment (Fig. 2, top). Conversely, mRNA expression of osteogenic genes, the master osteogenic transcription factor Runx2 (Runt-related transcription factor 2), Alpl (alkaline phosphatase), and Bglap (osteocalcin) was significantly decreased at day 10 (relative to their baseline levels prior to LIP). However, the expression of all three osteogenic markers was significantly upregulated after the removal of ligatures (day 15) (Fig. 2, bottom). Importantly, moreover, 1,4-DPCA/hydrogel further significantly promoted the expression of Runx2, Alpl, and Bglap, as compared to vehicle control (Fig. 2, bottom). These data suggest that 1,4-DPCA/hydrogel not only contributes to enhanced resolution of inflammation but augments the expression of osteogenic genes, consistent with its promotional effect on bone regeneration (Fig. 1 C,D).
Figure 2: 1,4-DPCA/hydrogel downregulates pro-inflammatory cytokine gene expression and upregulates osteogenic gene expression during the resolution of periodontitis.
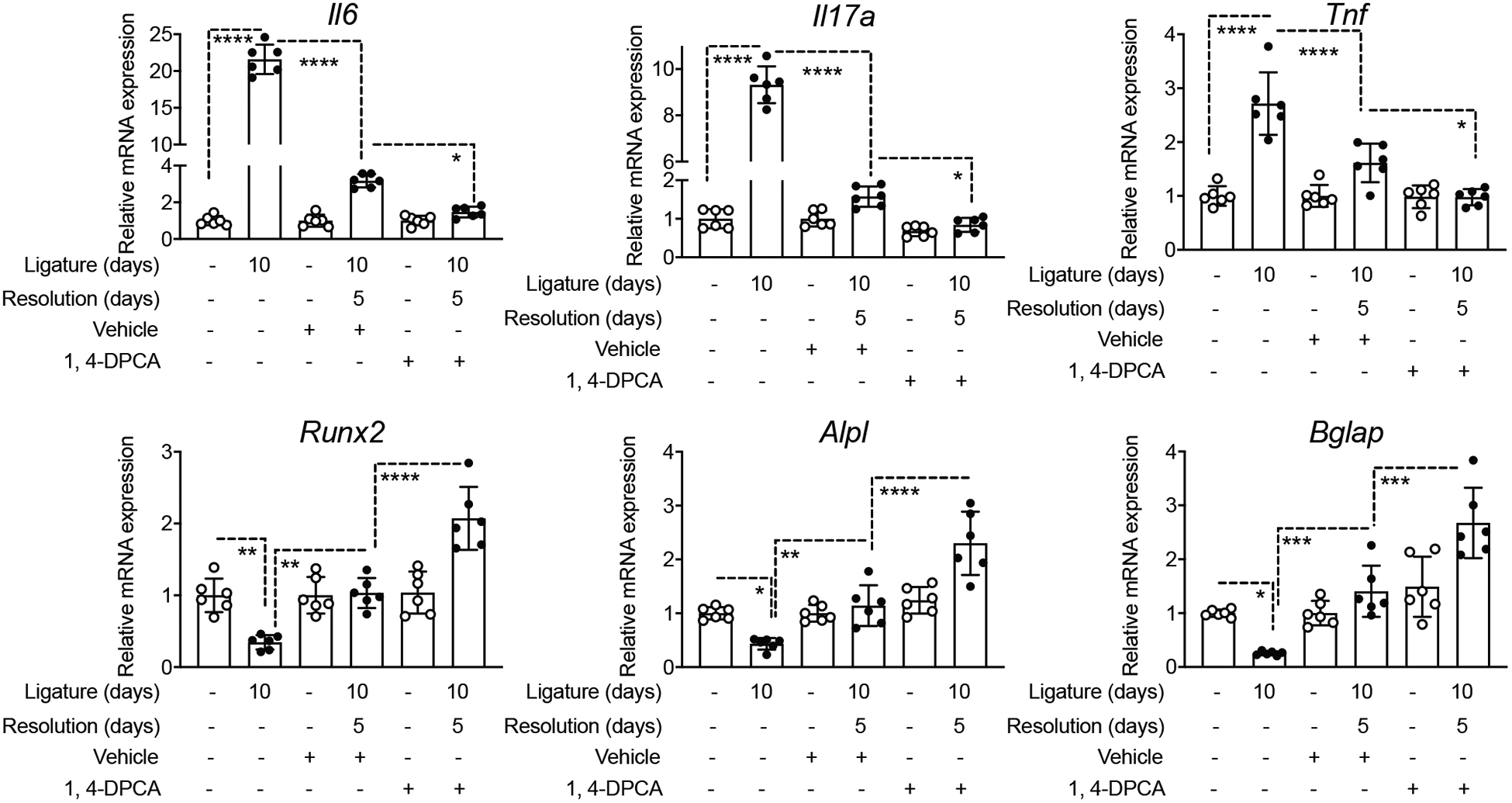
Groups of mice were subjected to LIP for 10 days. One group was euthanized at day 10 for dissecting gingiva for cytokine expression analysis. In two other groups subjected to LIP, the ligatures were removed on day 10 for another 5 days to enable resolution. At the onset of resolution (day 10), these mice were subcutaneously injected once with 1,4-DPCA/hydrogel, or vehicle alone, and were euthanized at day 15. Gingival tissues were analyzed for gene expression by quantitative real-time PCR. The mRNA expression of the indicated molecules was normalized to that of Hprt; results are presented as fold change relative to the mRNA levels of the unligated contralateral sites of the vehicle control-treated mice, assigned an average value of 1. Data are shown as means ± SD (n=6 mice/per group from two independent experiments, each performed in triplicates). **P < 0.01, ***P < 0.001, ****P < 0.0001 (ANOVA with Turkey’s multiple-comparisons test).
1,4-DPCA/hydrogel administered at the onset of resolution leads to increased accumulation of Treg cells in the periodontal tissue
HIF-1α contributes to different stages of wound healing through regulatory effects on cell migration, cell proliferation and survival, release of growth factors, angiogenesis and extracellular matrix synthesis (42). Moreover, HIF-1α was shown to enhance the abundance and function of FOXP3+ T regulatory (Treg) cells (43), which, besides their immune suppressive activities, can also promote tissue repair and regeneration (44–47). We thus investigated whether 1,4-DPCA/hydrogel promotes Treg cell abundance in the periodontal tissue during resolution and whether this activity contributes to the ability of the drug to promote alveolar bone regeneration. To this end, qPCR analysis of the gingival tissue of mice administered 1,4-DPCA/hydrogel, or vehicle control, showed that 1,4-DPCA/hydrogel augmented the expression of Treg-related molecules, namely Foxp3, Tgfb1, and Il10, during the resolution phase (Fig. 3A). Conclusive evidence for the ability of 1,4-DPCA/hydrogel to increase the abundance of Tregs during periodontitis resolution was obtained from a new, independent experiment. Indeed, flow cytometric analysis of the gingival tissue and cervical lymph nodes (cLNs) of mice subjected to ‘10dL + 5dR’ treatment showed that 1,4-DPCA/hydrogel caused an increase in both the Treg cell frequencies (% FOXP3+ cells among CD4+ T cells) and their absolute numbers (Fig. 3B,C,D). Moreover, during periodontitis resolution, 1,4-DPCA/hydrogel treatment increased the relative abundance of Treg cells expressing markers associated with their suppressive function (CD25, IL-10, CTLA-4, ICOS), as compared to vehicle control treatment, in both gingival tissues and draining cLNs (Fig. 3E).
Figure 3: Treatment with 1,4-DPCA/hydrogel leads to increased number of Tregs during the resolution of periodontitis.
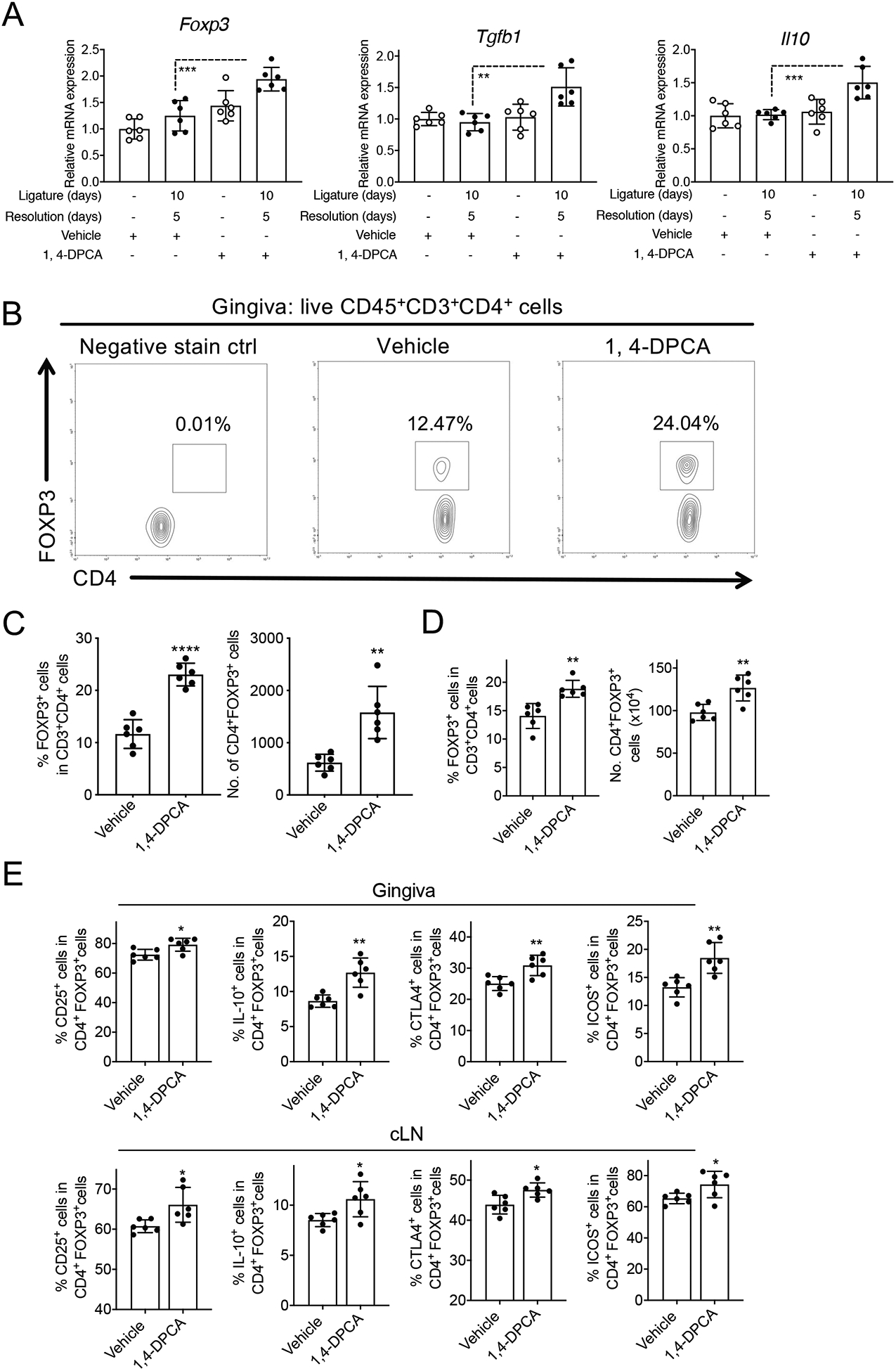
Mice were subjected to LIP for 10 days followed by 5 days without ligatures to enable resolution. At the onset of resolution (day 10), the mice were subcutaneously injected once with 1,4-DPCA/hydrogel, or vehicle alone, and were euthanized on day 15 for analysis. (A) Gene expression in the gingival tissue was analyzed by quantitative real-time PCR. The mRNA expression of the indicated molecules was normalized to that of Hprt; results are presented as fold change relative to the mRNA levels of the unligated contralateral sites of the vehicle control-treated mice, assigned an average value of 1. (B) Representative FACS plots of Treg cells and (C) bar graphs showing percentage of Treg cells in CD4+ T cells (left) and their absolute numbers (right), in gingival tissues of vehicle control-treated and 1,4-DPCA/hydrogel-treated mice on day 15. (D) Bar graphs showing percentage of Treg cells in CD4+ T cells (left) and their absolute numbers (right), in cervical lymph nodes (cLNs) of vehicle control-treated and 1,4-DPCA/hydrogel-treated mice on day 15. (E) Frequency of CD25+, IL-10+, CTLA-4+ and ICOS+ in CD4+FOXP3+Treg cells in gingiva (top) and cLNs (bottom) of vehicle control-treated and 1,4-DPCA/hydrogel-treated mice on day 15. Data are shown as means ± SD (n=6 mice/per group from two independent experiments, each performed in triplicates). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (Student’s t-test).
HIF-1α regulates the expression of multiple target genes, including genes important for angiogenesis (Vegfa) and cell migration (Cxcl12 and Cxcr4; acting respectively as chemokine and chemotactic receptor on several recruited immune cells), which are activities that promote tissue healing and regeneration (48). Regarding cell migration, hypoxia induces high expression of C-X-C motif chemokine receptor 4 (CXCR4) in various cell types in a HIF-1α-dependent manner. This hypoxia–HIF-1α–CXCR4 pathway may regulate cell trafficking and localization in hypoxic tissue microenvironments (49). By means of qPCR analysis of the gingival tissue of the ‘10dL + 5dR’ groups administered 1,4-DPCA/hydrogel or vehicle control, we showed that 1,4-DPCA/hydrogel upregulated the mRNA expression of Vegfa, Cxcl12 and Cxcr4 (Fig. 4A). The ability of this drug to enhance the gingival mRNA expression of Vegfa and Cxcl12 during periodontitis resolution was also confirmed by an RNAscope in situ hybridization (Supplemental fig. S1A). Moreover, 1,4-DPCA/hydrogel enhanced the production of VEGF-A and CXCL12 protein in human gingival epithelial cells and human PDL cells in vitro, whereas it could upregulate the production of VEGF-A but not of CXCL12 in MC3T3-E1 osteoblastic cells (Supplemental fig. S1B). Consistent with the known role of HIF-1α in promoting angiogenesis, 1,4-DPCA/hydrogel increased the numbers of CD31+CD45− endothelial cells in the gingival tissue (Fig. 4B). Immunofluorescence analysis of the periodontal tissue showed enhanced staining for CXCR4 and FOXP3 in the PDL region and gingival connective tissue of mice treated with 1,4-DPCA/hydrogel as compared to vehicle control-treated mice (Fig. 4C). Interestingly, CXCR4, FOXP3, and CD31 appeared to colocalize in the PDL (Fig. 4C, right panel & supplemental fig. S2), suggesting the presence in or around vessels of Treg cells that might express CXCR4. Staining for Ki67 revealed that this proliferation marker was associated with the epithelium rather than with FOXP3+ cells (Fig. 4D); this finding suggests that the enhancing effect of 1,4-DPCA/hydrogel on the numbers of Treg cells is unlikely to involve increased Treg cell proliferation in the gingival tissue. Together, these findings suggest that 1,4-DPCA/hydrogel, administered at the onset of resolution, induces enhanced angiogenesis and Treg cell accumulation, rather than proliferation, in the periodontal tissue.
Figure 4: 1,4-DPCA/hydrogel promotes angiogenesis and the emergence of Treg cells in and around vessels of the periodontium during the resolution of periodontitis.
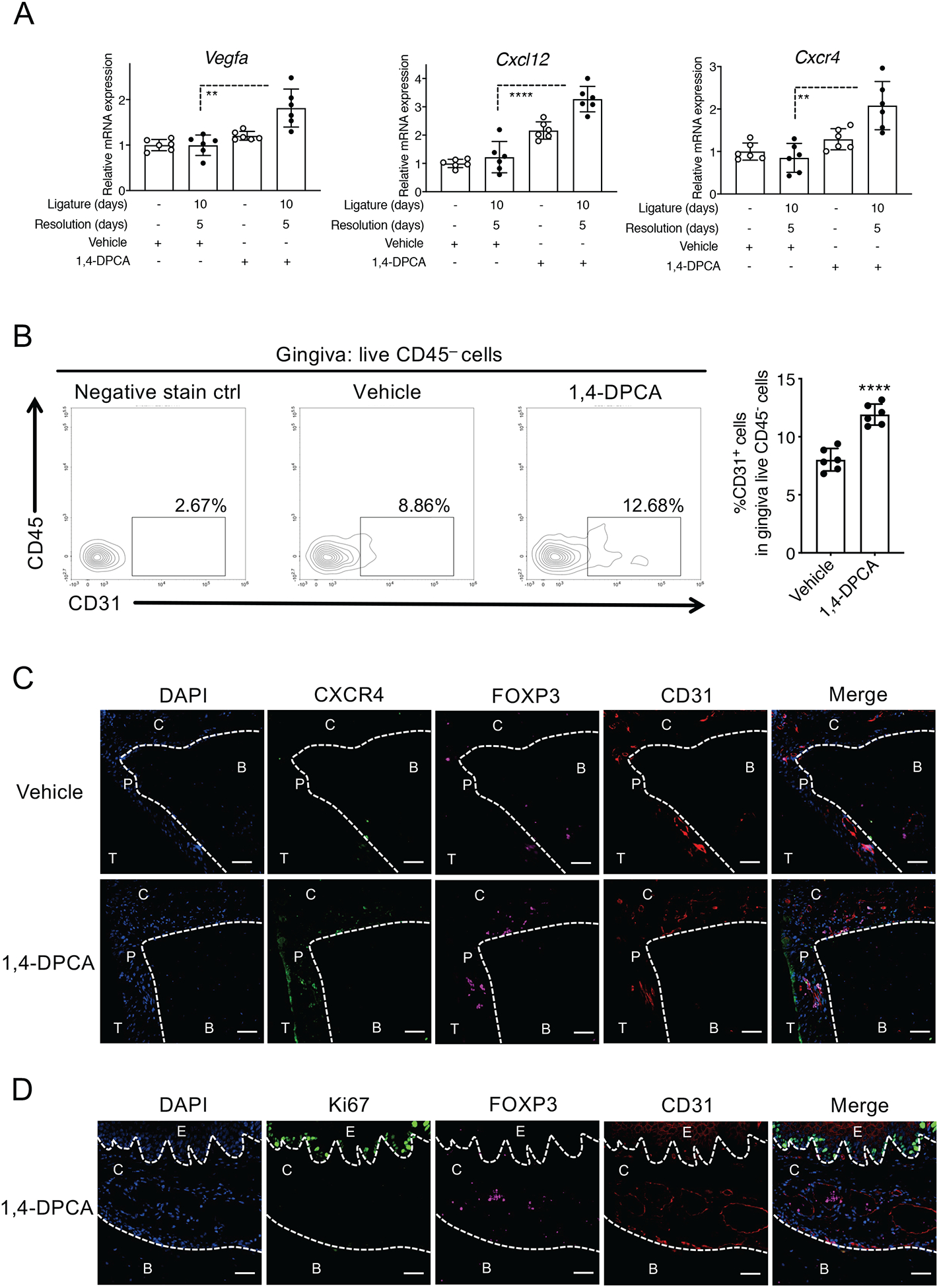
Mice were subjected to LIP for 10 days followed by 5 days without ligatures to enable resolution. At the onset of resolution (day 10), the mice were subcutaneously injected once with 1,4-DPCA/hydrogel or vehicle alone and were euthanized on day 15. (A) Gingival tissues were analyzed for gene expression by quantitative real-time PCR. The mRNA expression of the indicated molecules was normalized to that of Hprt and presented as fold change relative to the mRNA levels of the unligated contralateral sites of the vehicle control-treated mice, assigned an average value of 1. (B) Gingival tissues were harvested on day 15 and processed for flow cytometric analysis. The cells obtained were stained and gated for live CD45−CD31+ cells; shown are the percentage of CD31+ cells in CD45− cells. (C) Periodontal tissue sections were stained for CXCR4 (green), FOXP3 (purple), CD31 (red), and DAPI (blue). Scale bars, 100 μm. (D) Periodontal tissue sections were stained for Ki67 (green), FOXP3 (purple), CD31 (red), and DAPI (blue). Scale bars, 20 μm. B, Bone; C, Connective tissue; E, Epithelium; P, Periodontal ligament; T, Tooth. Data are shown as means ± SD (n=6 mice/per group from two independent experiments, each performed in triplicates). **P < 0.01, ****P < 0.0001 (Student’s t-test).
CXCR4 antagonism inhibits 1,4-DPCA/hydrogel-induced Treg cell accumulation and alveolar bone regeneration
Our data so far suggested that 1,4-DPCA/hydrogel causes accumulation in the periodontal tissue of FOXP3+ Treg cells which do not appear to proliferate locally, thus suggesting that their enhanced numbers might result from increased recruitment. Moreover, Treg cells accumulating in or around vessels colocalize with CXCR4, suggesting that they might express this chemokine receptor. We therefore assessed next if CXCR4 blockade could reduce the numbers of Treg cells in the periodontal tissue. Moreover, if the enhancing effect of 1,4-DPCA/hydrogel on bone regeneration depends on its ability to increase the numbers of Treg cells in the periodontal tissue, then CXCR4 blockade should inhibit bone regeneration.
To address these hypotheses, we intraperitoneally administered mice with a specific and potent antagonist of CXCR4, the bicyclam drug AMD3100 (50), or PBS control, starting on day 10 (when ligatures were removed and 1,4-DPCA/hydrogel was injected) and continuing daily until the day before the end of the experiment on day 15 (5 doses total). Flow cytometric analysis of the gingival tissue of mice euthanized on day 15 revealed that AMD3100 caused a significant reduction in the frequency and absolute numbers of Treg cells, thus reversing the enhancing effect of 1,4-DPCA/hydrogel on Treg cell accumulation (Fig. 5A & supplemental fig. S3). Moreover, mice treated with 1,4-DPCA/hydrogel and AMD3100 displayed significantly less bone regeneration on day 15 than mice treated with 1,4-DPCA/hydrogel and PBS control (Fig. 5B). Consistent with its ability to reverse bone regeneration, AMD3100 also counteracted 1,4-DPCA/hydrogel-induced downregulation of Il6, Il17, and Tnf mRNA expression (Fig. 5C top panels) as well as 1,4-DPCA/hydrogel-induced up-regulation of Runx2, Alpl, and Bglap mRNA expression (Fig. 5C middle panels). Moreover, the 1,4-DPCA/hydrogel-induced expression of Treg-related genes, such as Foxp3, was also inhibited by AMD3100 (Fig. 5C bottom panels), in line with the reduced numbers of Treg cells in the gingival tissue of AMD3100-treated mice (Fig. 5A). Together, these data indicate that the ability of 1,4-DPCA/hydrogel to cause alveolar bone regeneration, accompanied by modulation of pro- and anti-inflammatory genes, is, in large part, dependent upon chemokine receptor CXCR4-dependent Treg cell accumulation.
Figure 5. AMD3100 (CXCR4 antagonist) suppresses Tregs accumulation and bone regeneration induced by 1,4-DPCA/hydrogel during the resolution of periodontitis.
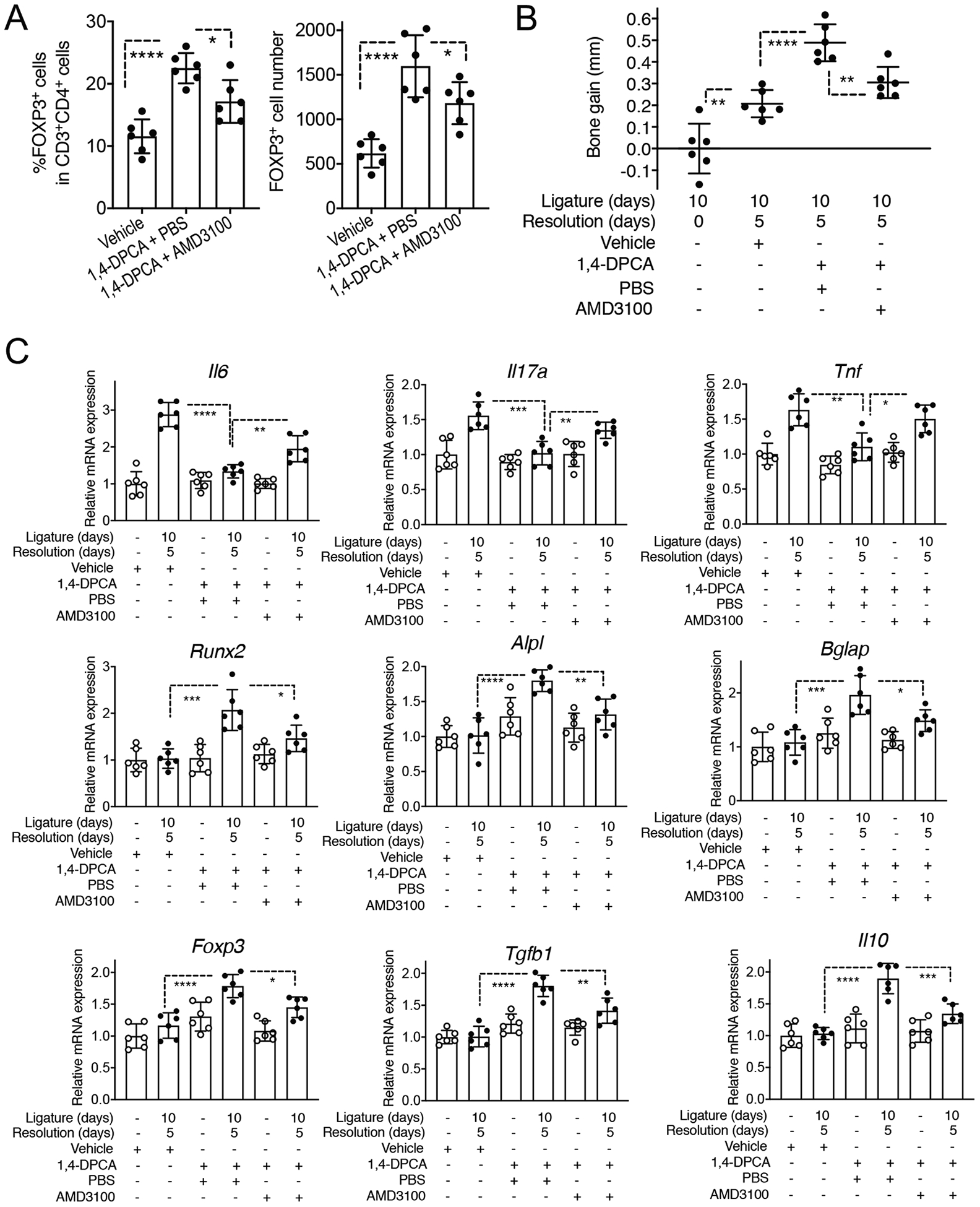
Groups of mice were subjected to LIP for 10 days followed (or not) by 5 days without ligatures to enable resolution. At the onset of resolution (day 10), groups of mice were subcutaneously injected once with vehicle alone (one group) or 1,4-DPCA/hydrogel (two groups). The 1,4-DPCA/hydrogel-treated groups were additionally intraperitoneally injected with AMD3100 or PBS control daily for 5 days. (A) Gingival tissues were harvested on day 15 and processed for flow cytometric analysis to identify FOXP3+ Treg cells. Shown are the percentage of Treg cells in CD4+ T cells (left) and their absolute numbers (right). (B) Bone heights (CEJ-ABC distance) were measured and CEJ-ABC data were transformed to indicate bone gain in 1,4-DPCA/hydrogel-treated mice with or without AMD3100 intervention, relative to the bone heights of untreated mice that were sacrificed at day 10 (baseline). (C) Relative mRNA expression of the indicated molecules in the gingival tissue harvested on day 15. Results were normalized to those of Hprt and presented as fold change relative to the mRNA levels of the corresponding genes in the unligated contralateral sites of vehicle control-treated mice, assigned an average value of 1. Data are shown as means ± SD (n=6 mice/per group from two independent experiments, each performed in triplicates). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (ANOVA with Turkey’s multiple-comparisons test).
DISCUSSION
The ability of 1,4-DPCA/hydrogel drug to stabilize HIF-1α was previously shown to promote tissue regeneration in an ear hole punch injury model in mice (24). Here, using an established mouse model of bone regeneration during periodontitis resolution (33), we showed that 1,4-DPCA/hydrogel significantly promotes new bone formation. The capacity of 1,4-DPCA/hydrogel to increase the gingival tissue levels of HIF-1α suggests that, at least in principle, this drug can mediate an array of HIF-1α-dependent pro-regenerative effects, e.g., growth factor release, matrix synthesis, and cell survival under hypoxia (42, 48, 51). However, mechanistic evidence from this study indicates that the ability of 1,4-DPCA/hydrogel to increase the abundance of periodontal Treg cells expressing markers associated with their regulatory function (CD25, IL-10, CTLA-4, ICOS) is critical for enhancing bone regeneration.
It was previously shown that whole-body hypoxia or pharmacologic inhibition of PHD (by means of the drug AKB-4924) leads to induction of FOXP3 expression and increased abundance of Treg cells in systemic tissues (43). Thus, we cannot exclude the possibility that 1,4-DPCA/hydrogel might have promoted de novo generation of Treg cells in lymph nodes or other lymphoid organs. Nevertheless, a more likely, albeit not mutually exclusive, mechanism is that 1,4-DPCA/hydrogel promoted the accumulation of Treg cells, likely from the circulation. First, Treg cells did not appear to proliferate locally in the periodontium of 1,4-DPCA/hydrogel-treated mice. Importantly, moreover, Treg cells were observed in and around vessels and treatment with an antagonist of the chemokine receptor CXCR4 (AMD3100) resulted in decreased abundance of Treg cells in the periodontal tissue. This finding is in line with reports that hypoxia induces upregulation of CXCR4 in Treg cells and that the interaction of CXCR4 on Tregs with the chemokine CXCL12 enhances Treg cell recruitment (52, 53).
The AMD3100-induced reduction of Treg cell numbers in the periodontal tissue was associated with decreased alveolar bone regeneration, thus implicating Treg cells as important effectors of the pro-regenerative action of the 1,4-DPCA/hydrogel drug. Through their well-established ability to inhibit effector immune responses and promote inflammation resolution (54–57), Treg cells can foster an immune environment that is conducive for tissue repair and regeneration (46, 58). However, Treg cells may additionally have a more direct role in tissue repair and regeneration by regulating the expression of pro-reparative or anabolic molecules (44, 45, 59). Whether and how Treg cells interact with osteoblasts or their mesenchymal stromal cell (MSC) progenitors to promote osteogenesis, as recently speculated (44), is currently uncertain. However, it is interesting that Treg cells were localized in the vicinity of vessels in the PDL, in other words, the perivascular region of the MSC niche, where progenitor cells can differentiate into osteoblasts (60–62).
In addition to HIF-1α, which enhances the abundance and function of FOXP3+ Treg cells (43), HIF-2α was also shown to regulate the suppressive function of Treg cells (63). However, 1,4-DPCA/hydrogel was conclusively shown earlier to not affect the levels of HIF-2α either in vivo or in vitro, although the drug enhanced the levels of HIF-1α in parallel experiments (24), as also observed in the present study. Moreover, the earlier study by Zhang et al (24) demonstrated that the pro-regenerative activities of 1,4-DPCA are dependent on HIF-1α. Indeed, Hif1a siRNA transfection in vivo blocked 1,4-DPCA/hydrogel-induced tissue regeneration in mice after wounding (24). Furthermore, the ability of this drug to upregulate the expression of Vegf and other target genes in fibroblast-like cell cultures was blocked by Hif1a siRNA (24). Therefore, although both HIF isoforms can potentially modulate Treg cells (43, 63), HIF-2α is unlikely to mediate the effects of 1,4-DPCA/hydrogel on Treg cell regulation in our experiments.
HIF-1α and VEGF-A, which were both upregulated by 1,4-DPCA/hydrogel, exhibit activities that couple angiogenesis to osteogenesis (48, 64, 65). HIF-1α induces angiogenesis, which indirectly elevates the supply of oxygen, nutrients and osteogenic growth factors that can promote osteogenesis. Increased VEGF-A-dependent vascularization might lead to a higher input of MSCs/osteoblast progenitors needed for enhanced osteogenesis. In this regard, it should be noted that the reported increased vascularity and bone regeneration (in response to distraction osteogenesis) in mice with constitutive HIF-1α activation in osteoblasts is dependent on VEGF (66). Interestingly, VEGF might enhance osteogenesis also by directly interacting with the VEGF receptor on osteoblasts (64). Thus, the 1,4-DPCA/hydrogel-induced increased abundance of Treg cells in the periodontal tissue, although critically important as demonstrated in our study, may not be the only mechanism contributing to the observed enhanced bone regeneration during the resolution of experimental periodontitis.
In regenerative medicine, many interventional approaches entail implantation of materials (e.g., scaffolds, growth factors, stem cells and combinations thereof) directly into a diseased or injured tissue defect to promote tissue healing and regeneration (67). In the present study, we used a drug (1,4-DPCA/hydrogel) which is injected subcutaneously and can orchestrate tissue regeneration at a distal diseased site, thus representing a more convenient and practical therapeutic strategy. Our results suggest that treatment with 1,4-DPCA/hydrogel should be initiated upon the onset of the resolution of periodontitis, e.g., after mechanical debridement of the periodontal pockets by scaling and root planing. However, it is currently uncertain whether 1,4-DPCA/hydrogel could be beneficial also if administered during active periodontitis, which merits future investigation. Drug-induced regeneration of periodontal bone has also been successfully demonstrated using proresolving lipid mediators. In this regard, the use of the stable lipoxin analog benzo-lipoxin A4 incorporated into microparticles, termed nano-proresolving medicines, promoted regeneration of periodontal bone lost due to periodontitis in the Hanford miniature pig model (68).
In conclusion, using a mouse model of alveolar bone regeneration, we have successfully applied a pro-regenerative intervention involving a simple and affordable drug that obviates the need for surgical implantation of expensive biologics (e.g., growth factors or cell-based drugs) directly into the tissue defect. Whether a locally injectable hydrogel containing 1,4-DPCA that up-regulates HIF-1α protein levels can be used to treat human periodontitis is not known at present but merits future investigation. The fact that PHD inhibitors are currently approved for use in the clinic (25) should facilitate translation from mouse studies to similar regenerative therapies in human periodontitis and perhaps other bone loss disorders.
Supplementary Material
Acknowledgements
Supported by U.S. Public Health Service grants from the National Institutes of Health (DE021104 to EHK, PBM, and GH; DE026152 and DE028561 to GH and TC) and by the Deutsche Forschungsgemeinschaft (SFB1181, project C7 to TC). We thank Dr. Manjunatha Benakanakere (University of Pennsylvania) for providing human gingival epithelial cells and Dr. Martina Rauner (Technical University Dresden) for providing MC3T3-E1 cells.
ABBREVIATIONS
- ABC
alveolar bone crest
- CEJ
cement-enamel junction
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CXCL12
C-X-C motif chemokine ligand 12
- CXCR4
C-X-C motif chemokine receptor 4
- 1,4-DPCA
1,4-dihydrophenonthrolin-4-one-3-carboxylic acid
- FOXP3
Forkhead box protein P3
- HIF-1
hypoxia-inducible factor 1
- HGEC
human gingival epithelial cells
- HPDL
human periodontal ligament
- ICOS
inducible T cell costimulator
- MRL
Murphy Roths Large
- PHD
prolyl-4-hydroxylase
- Runx2
Runt-related transcription factor 2
- Treg
T regulatory cell
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
Phillip B. Messersmith and Ellen Heber-Katz are coinventors on US Patents 9,675,607 and 10,307,415 relating to the 1,4-DPCA/hydrogel drug delivery system. The rest of the authors state that they have no conflicts of interest.
References
- 1.Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15, 30–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinane DF, Stathopoulou PG, and Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 3, article No.17038 [DOI] [PubMed] [Google Scholar]
- 3.Lamont RJ, Koo H, and Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16, 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, and Genco RJ (2015) Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol 86, 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demmer RT, and Papapanou PN (2010) Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000 53, 28–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, and Dietrich T (2017) Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol 44 Suppl 18, S94–S105 [DOI] [PubMed] [Google Scholar]
- 7.Brauchle F, Noack M, and Reich E (2013) Impact of periodontal disease and periodontal therapy on oral health-related quality of life. Int Dent J 63, 306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapple IL (2014) Time to take periodontitis seriously. BMJ 348, g2645. [DOI] [PubMed] [Google Scholar]
- 9.Lang NP, Salvi GE, and Sculean A (2019) Nonsurgical therapy for teeth and implants—When and why? Periodontology 2000 79, 15–21 [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Chiang N, and Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonetti MS, Chapple IL, and Working Group 3 of Seventh European Workshop on, P. (2011) Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38 Suppl 11, 114–118 [DOI] [PubMed] [Google Scholar]
- 12.Colombo AP, Bennet S, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst FE, and Paster BJ (2012) Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol 83, 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, and Marcenes W (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 93, 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beikler T, and Flemmig TF (2011) Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol 2000 55, 87–103 [DOI] [PubMed] [Google Scholar]
- 15.Murakami S (2011) Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000 56, 188–208 [DOI] [PubMed] [Google Scholar]
- 16.Somerman MJ, Ouyang HJ, Berry JE, Saygin NE, Strayhorn CL, D’Errico JA, Hullinger T, and Giannobile WV (1999) Evolution of periodontal regeneration: from the roots’ point of view. J Periodontal Res 34, 420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sculean A, Nikolidakis D, and Schwarz F (2008) Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials – biological foundation and preclinical evidence: A systematic review. J Clin Periodontol 35, 106–116 [DOI] [PubMed] [Google Scholar]
- 18.Lin Z, Rios HF, and Cochran DL (2015) Emerging Regenerative Approaches for Periodontal Reconstruction: A Systematic Review From the AAP Regeneration Workshop. J Periodontol 86, S134–S152 [DOI] [PubMed] [Google Scholar]
- 19.Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple ILC, and Stavropoulos A (2015) Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontology 2000 68, 182–216 [DOI] [PubMed] [Google Scholar]
- 20.Foster BL, and Somerman MJ (2005) Regenerating the periodontium: is there a magic formula? Orthod Craniofac Res 8, 285–291 [DOI] [PubMed] [Google Scholar]
- 21.Murakami S (2017) Emerging Regenerative Approaches for Periodontal Regeneration: The Future Perspective of Cytokine Therapy and Stem Cell Therapy. pp. 135–145, Springer Singapore, Singapore [Google Scholar]
- 22.Cochran DL, Cobb CM, Bashutski JD, Chun Y-HP, Lin Z, Mandelaris GA, McAllister BS, Murakami S, and Rios HF (2015) Emerging Regenerative Approaches for Periodontal Reconstruction: A Consensus Report From the AAP Regeneration Workshop. J Periodontol 86, S153–S156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan F, He Z, Kong L-L, Chen Q, Yuan Q, Zhang S, Ye J, Liu H, Sun X, Geng J, Yuan L, Hong L, Xiao C, Zhang W, Sun X, Li Y, Wang P, Huang L, Wu X, Ji Z, Wu Q, Xia N-S, Gray NS, Chen L, Yun C-H, Deng X, and Zhou D (2016) Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med 8, 352ra108–352ra108 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Strehin I, Bedelbaeva K, Gourevitch D, Clark L, Leferovich J, Messersmith PB, and Heber-Katz E (2015) Drug-induced regeneration in adult mice. Sci Transl Med 7, 290ra292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heber-Katz E (2017) Oxygen, Metabolism, and Regeneration: Lessons from Mice. Trends Mol Med 23, 1024–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang LE, Arany Z, Livingston DM, and Bunn HF (1996) Activation of Hypoxia-inducible Transcription Factor Depends Primarily upon Redox-sensitive Stabilization of Its α Subunit. J Biol Chem 271, 32253–32259 [DOI] [PubMed] [Google Scholar]
- 27.Rahimi N (2012) The Ubiquitin-Proteasome System Meets Angiogenesis. Molecular Cancer Therapeutics 11, 538–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, and Sang N (2016) Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J Cell Biochem 117, 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhry H, and Harris AL (2018) Advances in Hypoxia-Inducible Factor Biology. Cell Metab 27, 281–298 [DOI] [PubMed] [Google Scholar]
- 30.Koivunen P, and Kietzmann T (2018) Hypoxia-Inducible Factor Prolyl 4-Hydroxylases and Metabolism. Trends Mol Med 24, 1021–1035 [DOI] [PubMed] [Google Scholar]
- 31.Love RJ, and Jones KS (2013) Transient inhibition of connective tissue infiltration and collagen deposition into porous poly(lactic-co-glycolic acid) discs. Journal of Biomedical Materials Research Part A 101, 3599–3606 [DOI] [PubMed] [Google Scholar]
- 32.Benakanakere MR, Zhao J, Finoti L, Schattner R, Odabas-Yigit M, and Kinane DF (2019) MicroRNA-663 antagonizes apoptosis antagonizing transcription factor to induce apoptosis in epithelial cells. Apoptosis 24, 108–118 [DOI] [PubMed] [Google Scholar]
- 33.Yuh DY, Maekawa T, Li X, Kajikawa T, Bdeir K, Chavakis T, and Hajishengallis G (2020) The secreted protein DEL-1 activates a β3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J Biol Chem 295 7261–7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe T, and Hajishengallis G (2013) Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods 394, 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerji B, Conejo-Garcia A, McNeill LA, McDonough MA, Buck MR, Hewitson KS, Oldham NJ, and Schofield CJ (2005) The inhibition of factor inhibiting hypoxia-inducible factor (FIH) by beta-oxocarboxylic acids. Chem Commun (Camb), 5438–5440 [DOI] [PubMed] [Google Scholar]
- 36.Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, Morell RJ, Freeman AF, Lazarevic V, Trinchieri G, Diaz PI, Holland SM, Belkaid Y, Hajishengallis G, and Moutsopoulos NM (2018) A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med 10, eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin J, Maekawa T, Abe T, Hajishengallis E, Hosur K, Pyaram K, Mitroulis I, Chavakis T, and Hajishengallis G (2015) DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci Transl Med 7, 307ra155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, and Hajishengallis G (2015) Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun 6, 8272 10.1038/ncomms9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, and Graves DT (2012) Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J 26, 1423–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kourtzelis I, Li X, Mitroulis I, Grosser D, Kajikawa T, Wang B, Grzybek M, von Renesse J, Czogalla A, Troullinaki M, Ferreira A, Doreth C, Ruppova K, Chen LS, Hosur K, Lim JH, Chung KJ, Grossklaus S, Tausche AK, Joosten LAB, Moutsopoulos NM, Wielockx B, Castrillo A, Korostoff JM, Coskun U, Hajishengallis G, and Chavakis T (2019) DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol 20, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng K-T, Li J-P, Ng KM, Tipoe GL, Leung WK, and Fung M-L (2011) Expression of Hypoxia-Inducible Factor-1α in Human Periodontal Tissue. Journal of Periodontology 82, 136–141 [DOI] [PubMed] [Google Scholar]
- 42.Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, Paik KJ, Duscher D, Gurtner GC, Lorenz HP, and Longaker MT (2014) The Role of Hypoxia-Inducible Factor in Wound Healing. Adv Wound Care (New Rochelle) 3, 390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, and Eltzschig HK (2012) Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A 109, E2784–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei H, Schmidt-Bleek K, Dienelt A, Reinke P, and Volk H-D (2015) Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Frontiers in Pharmacology 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, and Rudensky AY (2015) A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 162, 1078–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Tan J, Martino MM, and Lui KO (2018) Regulatory T-Cells: Potential Regulator of Tissue Repair and Regeneration. Front Immunol 9, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delacher M, Imbusch CD, Weichenhan D, Breiling A, Hotz-Wagenblatt A, Träger U, Hofer A-C, Kägebein D, Wang Q, Frauhammer F, Mallm J-P, Bauer K, Herrmann C, Lang PA, Brors B, Plass C, and Feuerer M (2017) Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat Immunol 18, 1160–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin W, Xu L, Zwingenberger S, Gibon E, Goodman SB, and Li G (2017) Mesenchymal stem cells homing to improve bone healing. J Orthop Translat 9, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, and Sica A (2003) Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198, 1391–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, and Moore JP (1998) AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med 4, 72–77 [DOI] [PubMed] [Google Scholar]
- 51.Heber-Katz E, and Messersmith P (2018) Drug delivery and epimorphic salamander-type mouse regeneration: A full parts and labor plan. Adv Drug Deliv Rev 129, 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan M, Jene N, Byrne D, Millar EKA, O’Toole SA, McNeil CM, Bates GJ, Harris AL, Banham AH, Sutherland RL, and Fox SB (2011) Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Research 13, R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou L, Barnett B, Safah H, LaRussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, and Zou W (2004) Bone Marrow Is a Reservoir for CD4+CD25+ Regulatory T Cells that Traffic through CXCL12/CXCR4 Signals. Cancer Research 64, 8451–8455 [DOI] [PubMed] [Google Scholar]
- 54.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, and King LS (2009) CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119, 2898–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi S, Yamaguchi T, Nomura T, and Ono M (2008) Regulatory T cells and immune tolerance. Cell 133, 775–787 [DOI] [PubMed] [Google Scholar]
- 56.Campbell DJ, and Koch MA (2011) Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 11, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, and Ayala A (2014) Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. J Leukoc Biol 96, 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, and Shi S (2011) Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med 17, 1594–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM, and Pacifici R (2018) The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, and Kalajzic I (2013) In vivo identification of periodontal progenitor cells. J Dent Res 92, 709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.San Miguel SM, Fatahi MR, Li H, Igwe JC, Aguila HL, and Kalajzic I (2010) Defining a visual marker of osteoprogenitor cells within the periodontium. Journal of Periodontal Research 45, 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen SC, Marino V, Gronthos S, and Bartold PM (2006) Location of putative stem cells in human periodontal ligament. J Periodontal Res 41, 547–553 [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto A, Hester J, Macklin PS, Kawai K, Uchiyama M, Biggs D, Bishop T, Bull K, Cheng X, Cawthorne E, Coleman ML, Crockford TL, Davies B, Dow LE, Goldin R, Kranc K, Kudo H, Lawson H, McAuliffe J, Milward K, Scudamore CL, Soilleux E, Issa F, Ratcliffe PJ, and Pugh CW (2019) Systemic silencing of PHD2 causes reversible immune regulatory dysfunction. J Clin Invest 129, 3640–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schipani E, Maes C, Carmeliet G, and Semenza GL (2009) Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. Journal of Bone and Mineral Research 24, 1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, and Chavakis T (2007) Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem 282, 26746–26753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, and Clemens TL (2008) Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A 105, 686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, Pillay M, and Motaung KSCM (2018) Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells International 2018, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, and Serhan CN (2015) Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res 94, 148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


