Abstract
BACKGROUND/OBJECTIVES:
Over the past decade, feeding tube use in nursing home residents with advanced dementia has declined by 50% among white and black patients. Little is known about whether a similar reduction has occurred in other invasive interventions, such as mechanical ventilation.
DESIGN:
Retrospective cohort study.
SETTING:
Acute-care hospitals in the United States.
PARTICIPANTS:
Medicare beneficiaries with advanced dementia who previously resided in a nursing home and were hospitalized between 2001 and 2014 with pneumonia and/or septicemia and of either black or white race.
MEASUREMENT:
Invasive mechanical ventilation (IMV), as identified by International Classification of Diseases (ICD) procedure codes. Two multivariable logistic regression models examined the association between race and the likelihood of receiving IMV, adjusting for patients’ demographics, physical function, and comorbidities. A hospital fixed-effects model examined the association of race within a hospital, whereas a random-effects logistic model was used to estimate the between-hospital variation in the probability of receiving IMV and examine the overall association of race and use of IMV.
RESULTS:
Between 2001 and 2014, 289,017 patients with advanced dementia were hospitalized for pneumonia or septicemia. Use of IMV increased from 3.7% to 12.1% in white patients and from 8.6% to 21.8% in blacks. Among those ventilated, 1-year mortality rates remained high, at 82.7% for whites and 84.2% for blacks dying in 2013. Compared with whites, blacks had a higher odds of receiving IMV in the fixed-effects (within-hospital) model (adjusted odds ratio (AOR) = 1.34; 95% confidence interval (CI) = 1.29–1.39) and in the random-effects (between-hospital) model (AOR = 1.46; 95% CI = 1.40–1.51).
CONCLUSION:
IMV use in patients with advanced dementia has increased substantially, with black patients having a larger increase than whites, based, in part, on the hospitals where black patients receive care.
Keywords: invasive mechanical ventilation, race, secular trends
INTRODUCTION
Studies suggest that the prevalence of dementia, which affects approximately 5.8 million people in the United States,1 is higher in older blacks than in older whites.2–4 Racial/ethnic differences in the intensity of end-of-life care are well documented, with blacks being more likely than whites to be admitted to an intensive care unit (ICU) at the end of life5 and to die in an acute-care hospital,5,6 and less likely to stop dialysis7 or use hospice services.8 Among those residing in nursing homes, blacks are also more likely than whites to be admitted to the hospital at the end of life and less likely to enroll in hospice.8,9 These observed differences have been partly attributed to geographic variation in health care, particularly as racial/ethnic minorities live disproportionately in parts of the United States that have lower quality hospital care.8,10–12 Few longitudinal studies have documented whether racial/ethnic differences in end-of-life care utilization for patients with advanced dementia have changed with efforts to improve communication, advance care planning, and access to hospice and palliative care services over the last decade. To this end, Mitchell and colleagues evaluated racial/ethnic differences in rates of feeding tube insertion, finding that, although rates still remained higher in black patients with advanced dementia than in whites, they had declined from 37.5% in 2000 to 17.5% in 2014.13
The cognitive and functional decline that occurs with progression of dementia places people at risk for respiratory complications. Among hospitalized patients with advanced dementia, pneumonia and/or sepsis are the most common reasons for respiratory failure and are associated with high mortality rates.14 Although use of invasive mechanical ventilation (IMV) in this setting may be lifesaving, for persons with advanced dementia it may be burdensome without a substantial gain in survival.15 Use of IMV may cause both physical and psychological distress for these patients.16,17 A qualitative study of patients who received IMV and were conscious reported that patients experienced feelings of panic, discomfort from the endotracheal tube, and frustration over not being able to make their needs known.18 Similarly, studies of patients being weaned from IMV found that patients experienced anxiety, frustration, and despair.19 For patients with dementia, these feelings of anxiety and psychological distress may be especially distressing as patients may not understand what is happening to them.
Recent studies in the United States,15 Canada,20 and Europe21 have documented an increasing trend in rates of IMV use among hospitalized patients with dementia. Given the potential harms associated with IMV use in patients with advanced dementia and historical data documenting higher rates of life-sustaining therapies among minorities, we sought to compare how rates of IMV use have changed over time in the United States for black compared with white patients with advanced dementia. In this study, we report study findings examining racial differences in IMV using data from a longitudinal cohort study of nursing home residents with advanced dementia hospitalized with pneumonia and/or sepsis between 2001 and 2014. Given the high proportion of nursing home residents who have dementia22 and the frequency with which they experience transitions to the hospital setting,23 these patients represent an important population.
METHODS
Study Population
We conducted a retrospective analysis of fee-for-service Medicare beneficiaries hospitalized between 2001 and 2014 for pneumonia and/or septicemia, and identified patients who had been in a nursing facility during the 120 days immediately preceding the hospital admission. The analysis was restricted to persons identified by the Medicare Beneficiary Enrollment file with a race of either black or white. The Minimum Data Set (MDS), a federally mandated assessment, was linked to Medicare Part A inpatient claims to identify hospitalized Medicare beneficiaries with an MDS assessment that indicated advanced dementia (i.e., equivalent of Cognitive Performance Scale score of 5 or 6) and four or more impairments in the activities of daily living (ADLs).24 The Brown University Institutional Review Board approved the study and waived the requirement for patient consent.
Measures
Sociodemographic data on study participants were abstracted from the Medicare Beneficiary Enrollment file and included race/ethnicity, sex, age, state of residence, and marital status. Race/ethnicity codes in the enrollment file are based on data from the Social Security Administration Master Beneficiary File and are categorized as follows: white, black, Asian, Hispanic, North American Native, other, or unknown. Data on clinical characteristics were based on the MDS assessment and included the presence of a feeding tube, comorbid conditions, impairment in ADLs, and cognitive performance. Receipt of IMV was identified from Medicare claims data using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes 96.70, 96.71, and 96.72. Admission to the hospital, admission to the ICU, length of stay in the hospital, and length of stay in the ICU were based on Medicare claims data.
Statistical Analysis
Descriptive statistics were used to characterize the study sample and examine use of IMV by race over time. To evaluate racial differences in demographic and clinical characteristics, we used chi-square tests for categorical variables and t-tests for continuous variables. Two multivariable logistic models were conducted to examine trends in the association of race (defined as black vs white) and use of IMV. The first model examined the association of race and use of IMV within a hospital (i.e., fixed-effects model), and the second model examined the overall association of race and use of IMV using a random-effects model (i.e., the between-hospital variation in the probability of receiving IMV). Models were adjusted for patients’ demographics (age, sex, and race), cognitive and functional status, comorbid conditions, whether the patient had a hospitalization in the preceding 120 days, and the days from the MDS assessment to hospitalization. Statistical testing was two sided, and P <.05 was considered significant. All analyses were conducted using STATA software, version 15 (StataCorp).
RESULTS
A total of 289,017 black or white nursing home residents with advanced dementia were hospitalized for pneumonia or septicemia between 2000 and 2014. Of the 301,925 hospitalizations experienced by these patients, 63,143 occurred for patients identified as black and 216,874 occurred for patients identified as white, yielding a study sample of 280,017 hospitalizations for analysis. Blacks were more likely than whites to have multiple hospital stays (P < .001). Table 1 displays sample demographic and clinical characteristics. In 2013 to 2014, mean age (standard deviation (SD)) for black patients was 82.7 (8.1) years, and 59.5% were female. For whites, mean age (SD) was 84.0 (7.7) years, and 58.7% were female. Blacks were more likely than whites to have impairment in seven or more ADLs (83.7% vs 75.9%, respectively; P < .001) and a cognitive performance score of 6, signifying severe cognitive impairment (74.3% vs 53.1%, respectively; P < .001). Comorbidities differed between blacks and whites, with blacks being more likely than whites to have diabetes mellitus, type II (46.6% vs 29.4%; P < .001), renal failure (14.9% vs 9.1%; P < .001), stroke (35.6% vs 20.7%; P < .001), and cancer (5.3% vs 3.9%; P = .008). Whites were more likely than blacks to have a hip fracture (3.4% vs 1.2%; P < .001). Throughout the study period (not presented in Table 1), blacks were consistently more likely than whites to have a feeding tube (67.2% vs 34.5%; P < .001) and to be admitted to the ICU during their hospitalization (37.3% vs 27.5%; P < .001).
Table 1.
Characteristics of Black and White Hospitalized Nursing Home Residents with Advanced Dementia
| 2001–2002 | 2005–2006 | 2009–2010a | 2013–2014 | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Blacks | Whites | Blacks | Whites | Blacks | Whites | Blacks | Whites |
| Total No. | 10,712 | 38,450 | 10,466 | 36,374 | 6,722 | 23,696 | 6,394 | 20,277 |
| Received IMV, No. (%) | 893 (8.3) | 1,508 (3.9) | 1,085 (10.4) | 1,710 (4.7) | 1,047 (15.6) | 1910 (8.1) | 1,382 (21.6) | 2,415(11.9) |
| Age, mean (SD) [IQR], y | 83.6 (8.0) [78–89] | 84.3 (7.4) [79–90] | 83.3 (8.0) [78–89] | 84.3 (7.3) [79–89] | 83.1 (8.1) [77–89] | 84.3 (7.5) [79–90] | 82.7 (8.1) [77–89] | 84.0 (7.7) [79–90] |
| Female, % (95% Cl) | 64.7 (63.5–65. 6) | 67.8 (67.4–68.3) | 65.0 (64.1–65.9) | 65.0 (64.5–65.5) | 63.0 (61.9–64.2) | 61.6 (60.9–62.2) | 59.5 (58.3–60.7) | 58.7 (58.0–59.3) |
| 7 ADL dependencies present, % (95% Cl) | 87.8 (87.2–88.4) | 78.4 (77.9–78.8) | 89.2 (88.6–89.8) | 78.4 (78.0–78.9) | 89.8 (89.0–90.5) | 78.0 (77.4–78.5) | 83.7 (82.7–84.6) | 75.9 (75.3–76.5) |
| CPS score of 6, % (95% Cl)b | 88.5 (87.9–89.1) | 74.9 (74.5–75.4) | 86.9 (86.3–87.6) | 68.8 (68.3–69.3) | 82.1 (81.2–83.0) | 61.9 (61.2–62.5) | 74.3 (73.3–75.4) | 53.1 (52.4–53.8) |
| Feeding tube present, % (95% Cl) | 69.5 (68.6–70.3) | 37.8 (37.3–38.2) | 67.6 (66.7–68.5) | 34.5 (34.0–35.0) | 66.3 (65.2–67.4) | 33.5 (32.9–34.1) | 62.8 (61.6–64.0) | 32.1 (31.4–32.7) |
| Comorbidities from preadmission, % (95% Cl) | ||||||||
| Cancer | 7.0 (6.5–7.5) | 6.3 (6.0–6.5) | 6.0 (5.6–6.5) | 6.1 (5.9–6.4) | 6.8 (6.2–7.4) | 5.9 (5.6–6.2) | 5.3 (4.7–5.9) | 3.9 (3.7–4.2) |
| Diabetes mellitus, type II | 35.6 (34.7–36.5) | 22.0 (21.6–22.4) | 43.6 (42.7–44.6) | 26.1 (25.7–26.6) | 48.5 (47.3–49.7) | 30.2 (29.6–30.8) | 46.6 (45.4–47.9) | 29.4 (28.8–30.0) |
| CHF | 22.2 (21.4–23.0) | 25.1 (24.7–25.5) | 23.3 (22.4–24.1) | 24.3 (23.8–24.7) | 20.9 (19.9–21.9) | 22.5 (22.0–23.0) | 19.9 (18.9–20.9) | 19.9(19.3–20.4) |
| Stroke | 44.4 (43.4–45.3) | 28.4 (28.0–28.9) | 44.2 (43.2–45.2) | 27.0 (26.6–27.5) | 43.1 (41.9–44.3) | 25.4 (24.9–26.0) | 35.6 (34.5–36.8) | 20.7 (20.2–21.3) |
| Renal failure | 8.7 (8.2–9.3) | 4.8 (4.6–5.0) | 10.0 (9.4–10.5) | 5.4 (5.1–5.6) | 12.8 (12.0–13.6) | 7.6 (7.3–7.9) | 14.9 (14.0–15.8) | 9.1 (8.7–9.5) |
| Hip fracture | 2.2 (1.9–2.5) | 5.6 (5.3–5.8) | 2.0 (1.7–2.3) | 4.6 (4.4–4.8) | 1.8 (1.5–2.1) | 4.2 (3.9–4.4) | 1.2 (0.9–1.5) | 3.4 (3.2–3.7) |
| Hospital length of stay, mean (SD) [IQR], d | 9.6 (9.2) [4–12] | 7.7 (7.0) [4–9] | 9.1 (8.4) [4–11] | 7.2 (6.4) [4–9] | 8.9 (8.2) [4–11] | 7.0 (6.4) [3–9] | 8.6 (7.4) [4–11] | 6.8 (6.4) [3–8] |
| ICU admission, % (95% Cl) | 24.8 (24.0–25.6) | 15.9 (15.6–16.3) | 32.2 (31.3–33.1) | 22.6 (22.2–23.0) | 43.0 (41.8–44.2) | 34.1 (33.5–34.7) | 56.4 (55.2–57.6) | 45.0 (44.3–45. 7) |
Abbreviations: ADL, activity of daily living; CHF, congestive heart failure; CI, confidence interval; CPS, Cognitive Performance Scale; ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range; SD, standard deviation.
Owing to the change from the Minimum Data Set version 2.0 to version 3.0, 2010 was a partial year and numbers are smaller.
Scores range from 0 to 6, with higher scores indicating greater cognitive impairment.
Temporal changes in the rates of IMV among black and white nursing home residents with advanced dementia between 2001 and 2014 can be seen in Figure 1A. Among white patients who were hospitalized, IMV use increased from 3.7% in 2001 to 12.1% in 2014 (P < .001). Over the same time period, IMV use among black patients who were hospitalized increased from 8.6% to 21.8% (P < .001), with a steeper growth rate beginning in 2005. Figure 1B displays mortality rates by IMV use and race. Mortality rates were high (Figure 1B) and did not differ significantly between black and white patients for either those who received IMV or those who did not. In multivariable analyses, blacks had higher odds of receiving IMV than whites in both the fixed-effects (within-hospital) model (adjusted odds ratio (AOR) = 1.34; 95% confidence interval (CI) = 1.29–1.39) and random-effects (between-hospital) model (AOR = 1.46; 95% CI = 1.40–1.51). Of variance in the probability of receiving IMV for the same individual, 24% was attributed to differences across hospitals (ρ = 0.24; P = .00).
Figure 1.
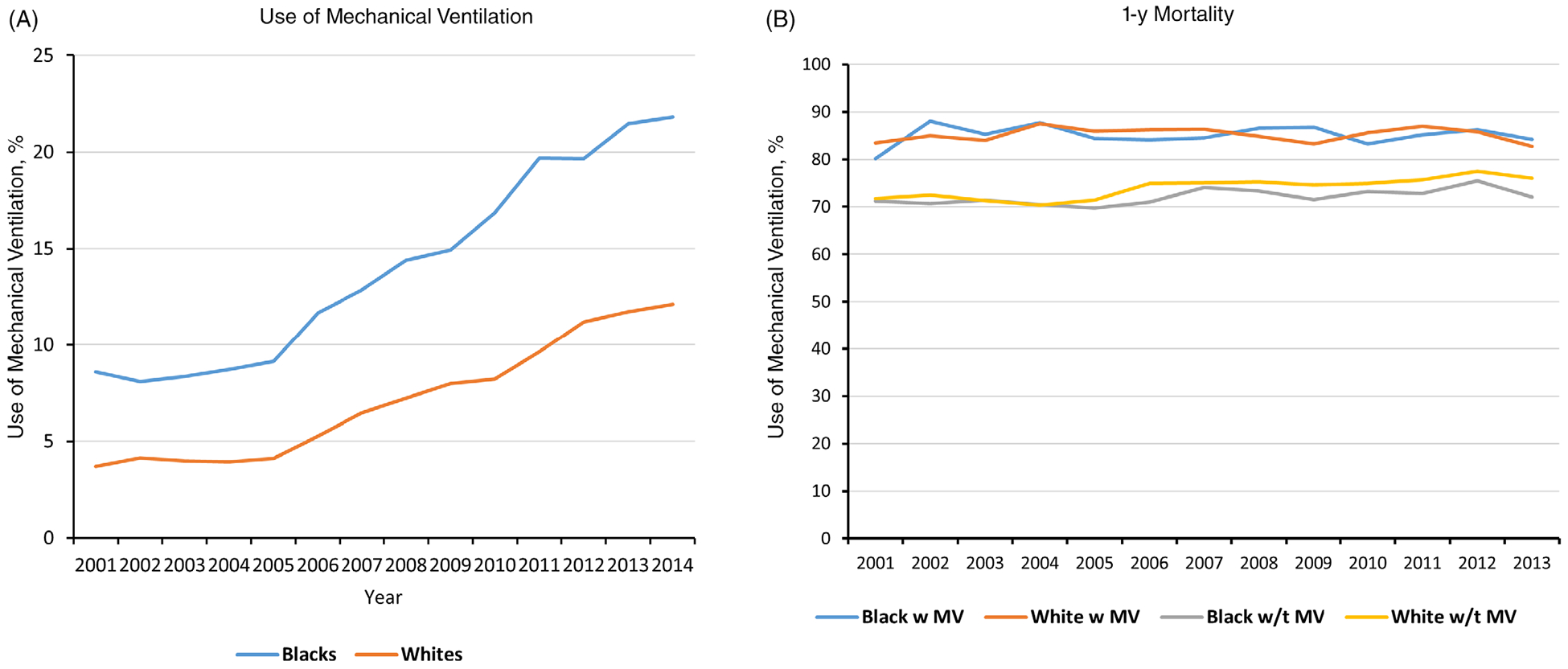
Rates of invasive mechanical ventilation (IMV) and 1-year mortality for black and white patients with advanced dementia hospitalized for pneumonia or septicemia. (A) Percentage of patients who received IMV over time by race. (B) The 1-year mortality rates for those who received mechanical ventilation (“w MV”) compared with those who did not receive mechanical ventilation (“w/t MV”) by race.
DISCUSSION
As the U.S. population ages and becomes more diverse, research into potential inequities in dementia care is an area of increasing importance. Our findings demonstrate racial differences in the intensity of care for patients with advanced dementia, with a higher increase in the use of IMV over time for black patients compared with whites, a difference based, in part, on the hospitals where patients receive care. Our findings are concerning because receipt of intensive interventions in the setting of high mortality rates, where there is no apparent survival benefit associated with higher intensity, may confer unintended distress for patients with advanced dementia.
The association between minority race and greater intensity of care at the end of life has been well documented in other clinical contexts,5 and likely stems from a combination of patient/family (e.g., health literacy and trust), clinician (e.g., training in communication regarding goals of care and implicit bias), and health system–related factors (e.g., structural racism and payment structures).10–12,25,26 Although racial differences in patient preferences have been posited as a key factor,27 there is growing evidence that differences in preferences may reflect disparities in the quality of clinician-patient-family communication about goals of care.28–30 Whether the racial differences we observed reflect disparities in care is unclear in the absence of data on patient preferences regarding end-of-life care, whether goals-of-care discussions occurred, and the quality of those discussions if they did occur. However, our finding that not only do racial differences in IMV use exist within the same hospital, but that these racial differences were also partly explained by hospital variability, suggests that system-level factors may be driving some level of disparities for these patients.
Our findings contrast with those of Mitchell et al.,13 who found decreased rates of feeding tube use in patients with advanced dementia, another invasive intervention with little benefit in this population, among both blacks and whites over a similar time period. It is possible that the widespread dissemination of evidence-based guidelines regarding the lack of clinical benefit associated with feeding tube use in the setting of advanced dementia helps to explain this trend. It is also possible that differences in incentives related to IMV use explain the contrasting trend we observed with use of IMV. Multiple recent studies15,20,21 show an increasing trend in IMV use among patients with advanced dementia in the United States, Canada, and Europe, as well as an association between higher availability of ICU beds and higher use of IMV.15 We previously found15 that persons with advanced dementia admitted to a hospital with an increase of 10 ICU beds during a 2-year period were 6% more likely to receive IMV. Our findings suggest that racial minorities may be at especially high risk for receiving intensive, nonbeneficial treatments of this kind as a result of the hospitals where they receive care.
LIMITATIONS
Several limitations are worth noting. We were unable to evaluate the role of patient preferences, advance directives, or the presence and quality of clinician-patient-family communication about goals of care in driving the observed differences in IMV use. We also lacked data on other patient- and family-level factors that may influence decision-making about IMV, such as trust, health literacy, knowledge about palliative care and hospice, and religiosity. In addition, our findings focused on patients who had resided in a nursing facility before hospitalization, limiting generalizability to other populations.
CONCLUSION
Use of IMV in older adults with advanced dementia increased over the last decade at a higher rate for black patients than for whites, without an associated survival benefit. These findings are in contrast with the decreasing rates of other forms of high-intensity care, such as feeding tube use, noted over a similar period for both black and white patients with advanced dementia. Racial differences in use of IMV were partly explained by the hospitals where patients received care.
ACKNOWLEDGMENTS
Financial Disclosure: This work was funded by program project grants P01 AG027296 and R56 AG063748-02 from the National Institute on Aging. Dr Sharma is supported by an American Cancer Society Mentored Research Scholar Grant (MRSG 14-058-01-PCSM).
Footnotes
Conflict of Interest: The authors have no conflicts.
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the article.
REFERENCES
- 1.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y). 2018;4:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H-Y, Panegyres PK. The role of ethnicity in Alzheimer’s disease: findings from the C-PATH online data repository. J Alzheimers Dis. 2016;51(2): 515–523. [DOI] [PubMed] [Google Scholar]
- 5.Brown CE, Engelberg RA, Sharma R, et al. Race/ethnicity, socioeconomic status, and healthcare intensity at the end of life. J Palliat Med. 2018;21(9):1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chino F, Kamal AH, Leblanc TW, Zafar SY, Suneja G, Chino JP. Place of death for patients with cancer in the United States, 1999 through 2015: racial, age, and geographic disparities. Cancer. 2018;124(22):4408–4419. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Sexton DJ, Drawz P, Ishani A, Reule S. Race, ethnicity, and end-of-life care in dialysis patients in the United States. J Am Soc Nephrol. 2018; 29(9):2387–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng NT, Mukamel DB, Caprio T, Cai S, Temkin-Greener H. Racial disparities in in-hospital death and hospice use among nursing home residents at the end of life. Med Care. 2011;49(11):992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak J, Haley WE, Chiriboga DA. Racial differences in hospice use and in-hospital death among Medicare and Medicaid dual-eligible nursing home residents. Gerontologist. 2008;48(1):32–41. [DOI] [PubMed] [Google Scholar]
- 10.Barnato AE, Berhane Z, Weissfeld LA, Chang C-CH, Linde-Zwirble WT, Angus DC. Racial variation in end-of-life intensive care use: a race or hospital effect? Health Serv Res. 2006;41(6):2219–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamadi H, Moody L, Apatu E, Vossos H, Tafili A, Spaulding A. Impact of hospitals’ referral region racial and ethnic diversity on 30-day readmission rates of older adults. J Community Hosp Intern Med Perspect. 2019;9(3):181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167(12):1233–1239. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube-feeding in US nursing home residents with advanced dementia, 2000–2014. JAMA. 2016; 316(7):769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teno JM, Gozalo P, Khandelwal N, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Intern Med. 2016;176(12):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puntillo KA, Arai S, Cohen NH, et al. Symptoms experienced by intensive care unit patients at high risk of dying. Crit Care Med. 2010;38(11): 2155–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotondi AJ, Chelluri L, Sirio C, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746–752. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson V Being conscious during mechanical ventilator treatment: patients’ and relatives’ experiences [Internet]. 2012. http://hdl.handle.net/2077/27823. Accessed November 11, 2019. [Google Scholar]
- 19.Merchán-Tahvanainen ME, Romero-Belmonte C, Cundín-Laguna M, Basterra-Brun P, San Miguel-Aguirre A, Regaira-Martínez E. Patients’ experience during weaning of invasive mechanical ventilation: a review of the literature. Enferm Intensiva. 2017;28(2):64–79. [DOI] [PubMed] [Google Scholar]
- 20.Borjaille CZ, Hill AD, Pinto R, Fowler RA, Scales DC, Wunsch H. Rates of mechanical ventilation for patients with dementia in Ontario: a population-based cohort study. Anesth Analg. 2019;129(4):e122–e125. [DOI] [PubMed] [Google Scholar]
- 21.Bouza C, Martínez-Alés G, López-Cuadrado T. Effect of dementia on the incidence, short-term outcomes, and resource utilization of invasive mechanical ventilation in the elderly: a nationwide population-based study. Crit Care. 2019;23(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks KL, Black BS, Rabins P. Predictors of mortality in nursing home residents with advanced dementia. Am J Alzheimers Dis Other Demen. 2010; 25(5):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan CM, Tu W, Unroe KT, LaMantia MA, Stump TE, Clark DO. Transitions in care among older adults with dementia in a nationally representative sample of older Americans. J Am Geriatr Soc. 2015;63(8): 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JN, Fries BE, Mehr DR, et al. MDS cognitive performance scale. J Gerontol. 1994;49(4):M174–M182. [DOI] [PubMed] [Google Scholar]
- 25.Kwak J, Haley WE. Current research findings on end-of-life decision making among racially or ethnically diverse groups. Gerontologist. 2005;45(5):634–641. [DOI] [PubMed] [Google Scholar]
- 26.Volandes AE, Paasche-Orlow M, Gillick MR, et al. Health literacy not race predicts end-of-life care preferences. J Palliat Med. 2008;11(5):754–762. [DOI] [PubMed] [Google Scholar]
- 27.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24(6):695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170(17):1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eneanya ND, Wenger JB, Waite K, et al. Racial disparities in end-of-life communication and preferences among chronic kidney disease patients. Am J Nephrol. 2016;44(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich SE, Gruber-Baldini AL, Quinn CC, Zimmerman SI. Discussion as a factor in racial disparity in advance directive completion at nursing home admission. J Am Geriatr Soc. 2009;57(1):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]


