Abstract
Objective:
To determine the frequency and impact of subjective cognitive complaint (SCC) in Parkinson disease (PD) patients with normal cognition (NC).
Methods:
PD patients with expert consensus-determined NC at baseline were asked a single question regarding presence of SCC. Baseline (N=153) and longitudinal (up to 4 follow-up visits over a 5-year period; N=121) between-group differences in PD patients with (+SCC) and without (-SCC) cognitive complaint were examined, including cognitive test performance and self- and informant-rated functional abilities.
Results:
Eighty-one (53%) participants reported a cognitive complaint. There were no between-group differences in global cognition at baseline. Longitudinally the +SCC group declined more than the -SCC group on global cognition (Mattis Dementia Rating Scale-2 total score (F=(1, 431)=5.71; p=0.02), processing speed (Symbol Digit Modalities Test (F=(1, 425)=7.52; p=0.006) and executive function (Trails B (F=(1, 419)=4.48; p=0.04)), although results were not significant after correction for multiple testing. In addition, the +SCC group was more likely to progress to a diagnosis cognitive impairment over time (hazard ratio=2.61, p=0.02). The +SCC group also demonstrated significantly lower self- and knowledgeable informant-reported cognition-related functional abilities at baseline, and declined more on an assessment of global functional abilities longitudinally.
Conclusions:
PD patients diagnosed with normal cognition, but with SCC, report poorer cognition-specific functional abilities, and long-term are more likely to be diagnosed with cognitive impairment and experience global functional ability decline. These findings suggest that SCC and worse cognition-related functional abilities may be sensitive indicators of initial cognitive decline in PD.
Keywords: Parkinson disease, cognition, cognitive complaint
INTRODUCTION
Parkinson disease (PD) patients frequently exhibit non-motor symptoms such as cognitive impairment, even early in the course of the disease1. Up to 80% of PD patients develop dementia (PDD) in the long-term2, and up to 30% of patients without dementia meet criteria for mild cognitive impairment (PD-MCI)3. Examining patients prior to onset of cognitive decline, one study of established PD patients with normal cognition at baseline found that within 6 years nearly 50% had developed PD-MCI, and all patients with incident PD-MCI subsequently progressed to dementia within 5 years4.
Research in healthy older adults suggests that subjectively-identified cognitive decline may indicate early changes in cognitive functioning not detected on neuropsychological tests5, 6. The value of subjective cognitive complaint (SCC) and its relationship to objective cognitive decline in PD patients without dementia is not well understood. Some studies, with sample sizes ranging from 70 to 250 participants, have shown that PD patients with SCC perform significantly worse on objective cognitive measures than those without SCC7–13 while others have not14, 15. Only four of the studies reported longitudinal follow-up data to examine conversion of non-demented PD patients to PD-MCI or PDD; two of these studies found higher rates of conversion from normal cognition to PD-MCI over a 2-2.5-year-period in PD patients with SCCs8, 11, one study found higher conversion to dementia in PD patients with SCC compared to those without SCC over a 7.5-year-period12, and one study found no change in neuropsychological assessments between non-demented PD patients with and without SCCs at 1 and 2 year follow-up15.
Subjective cognitive complaints, such as problems with attention, processing speed and word finding, are commonly reported among cognitively-normal PD patients, but their significance remains unclear. The goal of the present study was to assess the utility of a single question in cognitively-normal PD patients to identify those with and without cognitive complaint, and compare them cross-sectionally and longitudinally on cognitive and functional measures.
METHODS
Participants
Participants were enrolled through the National Institute of Neurological Disorders and Stroke- funded Morris K. Udall Center for Parkinson’s Disease Research at the University of Pennsylvania. One hundred fifty-three patients with idiopathic PD and normal cognition at baseline were administered cognitive assessments and ratings of functional abilities performed by trained research staff. One hundred twenty-one of the 153 patients were then followed for a minimum of 3 years (corresponding to at least 2 follow-up visits), and up to 5 years, either annually or biennially based on their length of time in the study. The remaining 37 participants did not have at least 2 follow-up visits at the time of analyses and therefore were not included in our longitudinal data. PD diagnosis was made according to UK Brain Bank criteria16. All participants had an expert consensus determination of normal cognition based on Movement Disorders Society (MDS) criteria (see below)3, 17. Patients with a diagnosis of PD-MCI or dementia at baseline were excluded.
Standard protocol, approvals, registrations, and patient consents
Approval from the institutional ethical standards committee on human experimentation was obtained before study initiation, and written informed consent was obtained from all study participants.
Assessments
Clinical assessments
The presence of a cognitive complaint was assessed with a single yes/no question: “Do you feel that your memory and thinking have gotten worse?” If the rater was asked to elaborate on the question, the timeframe of noticeable change in cognition since PD diagnosis was given. Based on response, participants were then divided into two groups: those with cognitive complaint (+SCC) and those without cognitive complaint (-SCC). “Subjective cognitive complaint” is used in the present study as it is a well-known term, however we technically are assessing self-reported cognitive decline. Motor symptom severity was measured with the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III18, and disease severity was measured using the Hoehn & Yahr (H&Y) Scale19. Depression was assessed using the 15-item Geriatric Depression Scale (GDS-15)20. REM sleep behavior disorder (RBD) was assessed with a single item (range 0-4) from the Parkinson’s Disease Sleep Scale (PDSS-2)21. General functional abilities were assessed with the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL)22, and cognition-specific function was assessed with the Penn Parkinson’s Disease Activities Questionnaire-15 (PDAQ-15)23. Knowledgeable Informants (KIs) completed the ADCS-ADL and Knowledgeable Informant version of the PDAQ-15. PD patients completed the PDAQ-15 Patient version.
Neuropsychological assessment
A battery of neuropsychological tests was administered by trained research personnel. The measures were part of the recommended standard battery of cognitive tests for PD patients enrolled in cognitive research studies at Udall Centers24. Global cognition was assessed using the Mattis Dementia Rating Scale-2 (MDRS-2)25 and Montreal Cognitive Assessment (MoCA)26. Measures of attention/processing speed were Trail Making Test Part A27 and the Symbol Digit Modalities Test (SDMT)28. Measures of executive functioning were Letter-Number Sequencing (LNS)29, phonemic verbal fluency (FAS)30, and Trail Making Test Part B27. Memory was assessed using the Hopkins Verbal Learning Test– Revised (HVLT-R)31. Visuospatial measures were the Benton Judgment of Line Orientation (JOLO)32 and the Clock Drawing Test (CDT; command condition)33. Language was assessed using Boston Naming Test (BNT)34 and semantic verbal fluency (animals)30. Due to the timing of assessment introduction into the battery, the number of subjects that completed each assessment varied (see Table 2). All assessments were performed in the PD medication “on” state.
Table 2.
Baseline neuropsychological and functional assessments
| Assessment* | N (-SCC) (+SCC) | -SCC mean (SD) | +SCC mean (SD) | F statistic; p-value** |
|---|---|---|---|---|
| MoCA | 72 81 |
27.21 (2.09) | 27.05 (2.04) | F(1, 150)=0.18, p=0.67 |
| MDRS-2 total score | 72 81 |
141.00 (2.31) | 140.56 (2.51) | F(1, 150)=2.81, p=0.10 |
| HVLT-R immediate recall | 72 79 |
23.88 (5.44) | 22.80 (4.36) | F(1, 148)=2.81, p=0.10 |
| HVLT-R delayed recall | 72 79 |
7.63 (3.20) | 7.51 (3.11) | F(1, 148)=0.03, p=0.87 |
| HVLT-R recognition discrimination | 72 79 |
9.67 (2.44) | 9.38 (2.60) | F(1, 148)=0.27, p=0.61 |
| LNS | 72 80 |
10.5 (2.54) | 10.63 (2.40) | F(1, 149)=0.04, p=0.85 |
| Phonemic verbal fluency (FAS) | 71 80 |
47.63 (12.98) | 46.96 (12.94) | F(1, 148)=0.05, p=0.82 |
| Animal fluency | 71 80 |
21.30 (5.19) | 20.16 (4.40) | F(1, 148)=1.50, p=0.22 |
| Trails A (time) | 72 80 |
38.26 (12.07) | 42.48 (15.08) | F(1, 149)=2.23, p=0.14 |
| Trails B (time) | 72 80 |
87.79 (46.16) | 92.30 (43.49) | F(1, 149)=0.21, p=0.65 |
| SDMT | 71 79 |
41.34 (8.89) | 38.51 (9.19) | F(1, 147)=3.90, p=0.05 |
| JOLO | 72 80 |
24.25 (5.1) | 24.55 (4.15) | F(1, 149)=0.61, p=0.44 |
| Clock Drawing | 41 53 |
6.34 (0.79) | 5.87 (1.11) | F(1, 91)=4.18, p=0.04 |
| BNT | 70 79 |
69.20 (99.32) | 57.57 (3.07) | F(1, 146)=1.32, p=0.25 |
| ADCS-ADL total | 61 69 |
75.48 (3.23) | 73.30 (8.81) | F(1, 127)=1.47, p=0.23 |
| PDAQ-15 KI total | 54 62 |
56.00 (4.86) | 51.63 (9.41) | F(1, 113)=7.00,p=0.009 |
| PDAQ-15 patient total | 64 67 |
54.95 (5.88) | 48.67 (9.03) | F(1, 128)=13.91, p<0.001 |
All scores presented are raw scores.
Bonferroni corrected significance set at p<0.004 for 14 cognitive tests and at p<.02 for 3 functional measures.
Abbreviations: MoCA = Montreal Cognitive Assessment; ADCS-ADL = Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory; BNT = Boston Naming Test; MDRS-2 = Mattis Dementia Rating Scale-2; HVLT-R = Hopkins Verbal Learning Test-Revised; SDMT = Symbol Digit Modalities Test; JOLO = Judgment of Line Orientation; LNS = Letter-Number Sequencing; PDAQ-15 = 15-item Penn Parkinson’s Disease Activities Questionnaire.
Cognitive consensus
Assignment of cognitive status was made for each patient during an annual consensus conference held for each patient by movement disorders specialists and a geriatric psychiatrist affiliated with the Penn Udall Center. The consensus process involved multiple (five on average) pairs of experienced physician raters reviewing demographic and available clinical data, including the clinician or patient impression of cognitive decline compared with premorbid state, the ADCS-ADL, and all raw and standardized cognitive test scores. The physician raters assigned patients a determination of normal cognition, MCI, or dementia based on the available data following the diagnostic criteria proposed by the MDS Task Forces for MCI (level 1 criteria)3 and dementia17. For a given test, a standardized score ≥1.5 SD below the mean was considered impaired, although consensus rater discretion was allowed. The raters within a pair reached agreement on all cases assigned to them. For cases with a between-pair discrepancy in determination, an independent physician rater adjudicated. Inter-rater agreement among pairs was high (kappa=0.80, 95% confidence interval=0.70-0.90)4.
Statistical analyses
To compare demographic and clinical characteristics in the +SCC and -SCC groups, two sample t-test was utilized to examine mean differences for continuous variables and chi-square test for categorical variables. If any significant differences were revealed regarding relevant demographic or clinical characteristics, the significant variable was included as a co-variate in one-way ANCOVA analyses to examine group differences in cognitive and functional performance. To assess group differences in long-term cognitive functioning, linear mixed-effects model analyses were performed. Fixed effects in the mixed-effects model include SCC group status, follow-up time, interaction between SCC group status and follow-up time, along with appropriate covariates. A random intercept was included to account for correlations among repeated measures of cognitive functioning. Kaplan-Meier method was used to estimate the incident impairment probability (rate) from normal cognition to any cognitive impairment between the +SCC and -SCC groups, and Cox regression model was used to examine the association between cognitive complaint status and risk of conversion to MCI or PDD. Kaplan-Meier method was also used to estimate the sensitivity and specificity of SCC by year 4. Although our study was exploratory rather than confirmatory, we present results both without35 and with correction for multiple testing using the Bonferroni method. All statistical tests were two-sided. Statistical analyses were performed using SPSS (version 23).
Data availability statement
Data will be shared at the request of other investigators for purposes of replicating procedures and results.
RESULTS
Participant demographics
Demographic and clinical features are detailed in Table 1. There were a total of 153 PD patients with consensus process-determined normal cognition at baseline, including 81 (52.9%) who reported cognitive complaint. The two groups did not differ significantly in age, sex, disease duration (i.e., time since diagnosis), education, Hoehn & Yahr stage, RBD item score, or UPDRS motor score. The +SCC group had significantly higher GDS-15 scores (t(152)=−2.94; p=.003). Therefore, GDS-15 score was included as a covariate in all subsequent between-group comparisons, including the Cox regression model.
Table 1.
Baseline demographics and clinical characteristics
| Variables | N (-SCC) (+SCC) | Mean -SCC (SD) | Mean +SCC (SD) | t-test; p-value* |
|---|---|---|---|---|
| Age (years) | 72 81 |
68.2 (8.3) | 68.3 (7.9) | 0.92 |
| Sex (% male) | 72 81 |
54% | 59% | 0.62 |
| PD duration (years) | 72 81 |
5.8 (4.0) | 7.0 (5.0) | 0.12 |
| Education (years) | 72 81 |
16.7 (2.1) | 16.4 (2.2) | 0.34 |
| GDS-15 total score | 72 81 |
1.6 (2.0) | 2.7 (2.8) | 0.003 |
| Hoehn & Yahr stage (median (IQR)) |
72 81 |
2.0 (IQR=2-3) | 2.5 (IQR=2-3) | 0.11 |
| UPDRS motor score | 72 81 |
19.7 (11.3) | 23.0 (11.0) | 0.07 |
| REM sleep behavior disorder item score | 58 70 |
0.5 (0.6) | 0.7 (1.1) | 0.18 |
Bonferroni corrected significance set at p<0.006.
Abbreviations: GDS-15 = 15-item Geriatric Depression Scale; UPDRS = Unified Parkinson’s Disease Rating Scale; IQR = interquartile range
Baseline neuropsychological and functional assessments
Results of baseline cognitive and functional assessments are presented in Table 2. The entire sample had MDRS-2 data, and the majority had data for the entire neuropsychological battery. A subset of the sample had data regarding functional measures. At baseline, consistent with having been classified as having normal cognition by expert consensus, the +SCC group did not perform significantly worse than the -SCC group on any of the 14 cognitive tests after correction for multiple comparisons. The +SCC group demonstrated significantly lower scores on the PDAQ-15 Knowledgeable Informant Total (F(1, 113)=7.00, p=0.009) and Patient Total (F(1, 128)=13.91, p<0.001) than the -SCC group. Scores on the ADCS-ADL did not significantly differ between the two groups.
Longitudinal neuropsychological and functional assessments
One hundred twenty-one patients completed at least two follow-up visits, and up to 4 annually-scheduled, post-baseline visits, with the last visit occurring 5 years post-baseline in some participants due to a missed visit. Of the 121 patients, 5 (-SCC=3; +SCC=2) were deemed to have developed cognitive impairment by consensus at some point during follow-up, and then reverted to normal cognition at a subsequent visit. The average duration of follow-up for the entire sample was 2.9 years. For the -SCC group the average duration of follow-up was 2.88 years, and was 2.83 years for the +SCC group. At baseline, 64 (52.9%) of these patients had a cognitive complaint while 57 (47.1%) did not, mirroring the baseline sample. Sixty-eight patients had 2 follow-up visits (+SCC=56%), 30 patients 3 follow-up visits (+SCC=40%), and 23 patients had 4 follow-up visits (+SCC=48%).
On our follow-up analyses, controlling for baseline GDS-15 score and baseline cognitive test score, the +SCC group declined at a significantly faster rate than the -SCC group on the ADCS-ADL (F=(1,404)=12.98; p<0.001). The +SCC group also declined more on the MDRS-2 Total score (F=(1, 431)=5.71; p=0.02), SDMT (F=(1, 425)=7.52; p=0.006) and Trails B (F=(1, 419)=4.48; p=0.04) over time, but this did not withstand correction for multiple testing (Table 3).
Table 3.
Longitudinal neuropsychological and functional assessments
| Assessment* | Annual change (SE) in -SCC group | Annual change (SE) in +SCC group | p-value** (between-group difference in annual change) |
|---|---|---|---|
| MoCA | −0.31 (0.10) | −0.45 (0.09) | 0.28 |
| MDRS-2 total score | −0.45 (0.15) | −0.94 (0.14) | 0.02 |
| HVLT-R immediate recall | 0.10 (0.20) | −0.03 (0.18) | 0.62 |
| HLVT-R delayed recall | 0.04 (0.13) | 0.07 (0.12) | 0.87 |
| HVLT-R recognition discrimination | 0.06 (0.08) | 0.15 (0.08) | 0.48 |
| LNS | −0.22 (0.09) | −0.38 (0.08) | 0.18 |
| Phonemic verbal fluency (FAS) | −1.01 (0.35) | −1.34 (0.33) | 0.48 |
| Animal fluency | −0.77 (0.17) | −0.88 (0.16) | 0.65 |
| Trails A (time) | 2.04 (0.64) | 3.54 (0.61) | 0.09 |
| Trails B (time) | 6.10 (1.79) | 11.31 (1.70) | 0.04 |
| SDMT | −1.32 (0.29) | −2.40 (0.27) | 0.006 |
| JOLO | −0.26 (0.18) | −0.47 (0.17) | 0.41 |
| Clock Drawing | −0.06 (0.05) | −0.01 (0.05) | 0.46 |
| BNT | −0.23 (0.08) | −0.18 (0.08) | 0.64 |
| ADCS-ADL total | −0.51 (0.33) | −2.16 (0.31) | <0.001 |
| PDAQ KI total | −1.11 (0.32) | −1.50 (0.30) | 0.38 |
| PDAQ patient total | −0.62 (0.30) | −0.36 (0.30) | 0.54 |
All scores presented are raw scores.
Bonferroni corrected significance set at p<0.004 for 14 cognitive tests and at p<.02 for 3 functional measures.
Abbreviations: ADCS-ADL = Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory; BNT = Boston Naming Test; MDRS-2 = Mattis Dementia Rating Scale-2; MoCA= Montreal Cognitive Assessment; HVLT-R = Hopkins Verbal Learning Test-Revised; SDMT= Symbol Digit Modalities Test; JOLO = Judgment of Line Orientation; LNS = Letter-Number Sequencing; PDAQ-15 = 15-item Penn Parkinson’s Disease Activities Questionnaire.
Thirty-three patients (28.4%) developed cognitive impairment by consensus determination (either MCI [N=29] or dementia [N=4]) over time. On Kaplan-Meier analysis of these patients, the +SCC group (N=24) had a higher conversion rate to MCI and dementia than did the -SCC group (N=9) (χ2(1)=4.36, p=0.04) (Figure 1). On Cox regression model and controlling for GDS-15 score, +SCC patients were 2.61 times more likely to convert to MCI or dementia than were -SCC patients (hazard ratio=2.61, p=0.02). As CDT was significantly different between groups at baseline before adjustment for multiple comparisons, we ran an additional Cox regression model in the subset of patients with this score available (-SCC N=33; +SCC N=41), and baseline CDT did not predict long-term cognitive decline (p=0.10).
Figure 1.
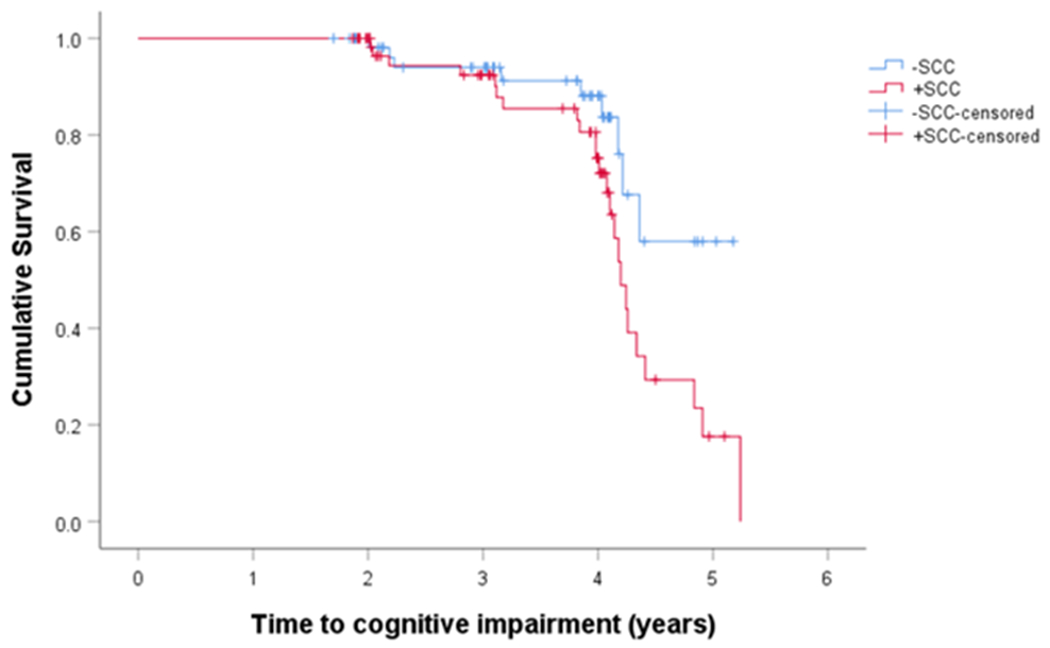
Cognitive Complaint in PD Patients
We also examined the sensitivity and specificity of baseline SCC for predicting future cognitive impairment by year 4 using the Kaplan-Meier method. The sensitivity of SCC was 69% and the specificity of SCC was 51%.
DISCUSSION
To our knowledge, this is the largest study examining the relationship between subjective cognitive complaint and objective decline in a cohort of PD patients comprised exclusively of consensus process-determined cognitively normal patients at baseline. The results of the present study show that about half of established PD patients with normal cognition report cognitive complaint, even when their global and detailed cognitive performance is not clearly distinguishable from patients without such complaint. Additionally, such patients reported or were deemed to have worse function both cross-sectionally and longitudinally. Finally, these patients also performed worse long-term on several cognitive measures, and were more likely to be diagnosed with an incident cognitive disorder over time.
In comparison to some previous longitudinal research examining the impact of SCC on cognitive performance over time in PD patients, we examined global cognitive complaint as opposed to just memory complaint8, included a larger number of patients with SCC8, 11, 12, followed patients over a longer period of time for some8, 15, but not all12, studies, and included important co-variates in our models12, 15.
Presence of cognitive complaints in an overall intact patient might denote a stage of cognitive decline in PD between normal cognition and MCI, as has been reported in the general population5, 6, 36, and SCC in pre-clinical Alzheimer’s disease (AD) is associated with an increased risk for conversion to dementia37, 38. However, in PD it is important to emphasize cognitive complaints broadly, rather than a memory complaint specifically, given the range of cognitive deficits that occurs in non-demented PD patients39, 40. Executive functioning and attention in particular are reliant on fronto-striatal functioning, which is disrupted initially in the course of PD41, 42. The findings of the present study suggest a simple, single clinical question focused on self-perception of general cognitive changes compared with one’s premorbid state may predict future decline in these cognitive domains. A report of subjective cognitive decline is simple to administer and may be meaningful in a clinical setting, alerting clinicians to closely monitor cognition over time and to consider earlier referral for a comprehensive neuropsychological evaluation to establish a clear baseline.
Interestingly, PD patients with cognitive complaint and their KIs both reported functional decline at baseline as assessed by the PDAQ-15. Functional decline has been demonstrated in studies of PD-MCI using performance-based functional assessments43, the PDAQ-1523 and the Parkinson’s Disease-Cognitive Functional Rating Scale44. These results illustrate that even prior to the development of MCI, PD patients with cognitive complaints and their KIs may perceive a subtle decline in everyday cognitive functional abilities. Notably, there was no significant difference at baseline between groups regarding the ADCS-ADL, a functional questionnaire which was developed for use in AD and assesses both basic and instrumental activities of daily living (ADLs). However, on follow-up, the rate of change between groups was significantly different, with the +SCC group declining more quickly on this global functional measure compared with the -SCC group. This highlights the utility of cognition-related functional rating scales developed specifically for PD patients, because while the ADCS-ADL detected functional decline over time, only the PDAQ-15 detected functional differences at baseline. One possible explanation for this is that the PDAQ-15 may be more sensitive to initial functional impairment and therefore show greatest changes early on in the cognitive decline process (+SCC patients on average scored 81-86% of maximum available points on the PDAQ-15 at baseline), while the ADCS-ADL may only start to decline in parallel with more significant changes in cognition (+SCC patients on average scored 94% of maximum available points on the ADCS-ADL at baseline).
The mean baseline GDS-15 scores were 2.7 (SD=2.8) and 1.5 (SD=1.9) in the +SCC and -SCC groups, indicating a higher likelihood of subthreshold depression (SubD) in the +SCC group. A previous study examined the relationship between SubD and subjective cognitive complaint in PD patients and found that SubD patients reported more subjective cognitive complaint than non-depressed patients45. As SCC predicted cognitive decline even when controlling for baseline depression score, the finding suggests that minor depressive symptoms occur secondary to or independent of cognitive complaints.
The sensitivity for baseline SCC to predict future cognitive impairment was acceptable, but the specificity was low. So while approximately 70% of participants who developed cognitive impairment over time had a cognitive complaint while intact, many participants who did not develop cognitive impairment also had subjective complaints at baseline.
Limitations of the current study include a racially and ethnically homogenous sample with a high level of education. Thus, results may not be applicable to the general PD population and should be replicated in a multi-site study with heterogeneous cohorts. Additionally, we used a single, unvalidated question that queried only about “memory” and “thinking” to assess cognitive complaints, and the question was answered only by the patient. Finally, while we examined depression in relation to cognitive complaint, we did not explore other psychiatric symptoms (e.g., apathy or anxiety) or possible confounding variables (e.g., family history of dementia) as potential contributing factors to subjective decline. Research has demonstrated that apathy46, 47 and anxiety48, 49 are associated with cognition in PD, and future research should explore the potential relationship of these factors with subjective cognitive complaint.
This study demonstrates that the presence of subjective cognitive complaint, as determined by a single question, predicts future cognitive decline in PD patients with normal cognition by detailed testing and consensus diagnosis. Additionally, it may serve as a useful indicator for patient and KI perception of mild difficulties performing cognitive activities of daily living. Asking a simple yes/no question to a patient who appears cognitively normal, and who may not spontaneously report cognitive concern, may help clinicians identify those patients at risk for cognitive decline over the next several years. As observational studies and clinical trials in PD shift their focus to preclinical and prodromal patients and testing of possible disease-modifying therapies, identification of cognitively-intact patients with cognitive complaint will allow the study of cognitive decline in PD from its earliest clinical manifestation.
Acknowledgments
Study Funding: This study was funded by a Morris K. Udall Parkinson’s Disease Research Center of Excellence grant from NINDS (NS-053488) and an R01 grant (NS102324).
FINANCIAL DISCLOSURES
Rachael Purri reports no disclosures.
Dr. Laura Brennan consults for Signant Health and Eli Lilly.
Dr. Jacqueline Rick reports no disclosures.
Dr. Sharon Xie has received research funding or support from National Institutes of Health; payments for consulting services related to cancer from F. Hoffman-La Roche Ltd.
Benjamin Deck reports no disclosures.
Dr. Lana M. Chahine receives research support from the Michael J Fox Foundation, has received travel payment from MJFF to MJFF conferences, is a paid consultant to MJFF, receives research support from the UPMC Competitive Medical Research Fund, is study site investigator for a study sponsored by Biogen, is a site sub-investigator for a study sponsored by Voyager, received payment from Elsevier (for book authorship), and receives royalties from Wolters Kluwel (for book authorship).
Dr. Nabila Dahodwala has received support from AbbVie (research grant); Roche, Ely Lilly and Cala Health (clinical trial site investigator); and Acadia (scientific advisory board).
Dr. Alice S. Chen-Plotkin was supported by the NIH (P50 NS053488, and UO1 NS097056), the Alzheimer’s Association/Michael J. Fox Foundation/Weston Brain Institute, and the Parker Family Chair. She reports no conflicts of interest.
Dr. John Duda receives research support from the Department of Veterans Affairs, the Department of Defense, the National Institutes of Health and the Michael J. Fox Foundation.
Dr. James Morley receives research funding from GE Healthcare and Department of Veterans Affairs.
Dr. Rizwan Akhtar reports no disclosures.
Dr. John Q. Trojanowski may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-Inventor and he received revenue from the sale of Avid to Eli Lily as co-inventor on imaging related patents submitted by the University of Pennsylvania.
Dr. Andrew Siderowf has been a consultant to the following companies in the past year: Biogen, Voyager Therapeutics, Merck, Denali, Wave Life Sciences and Prilenia Therapeutics. He has received grant funding from the Michael J. Fox Foundation and NINDS.
Dr. Daniel Weintraub has received research funding or support from Michael J. Fox Foundation for Parkinson’s Research, Alzheimer’s Therapeutic Research Initiative (ATRI), Alzheimer’s Disease Cooperative Study (ADCS), the International Parkinson and Movement Disorder Society (IPMDS); honoraria for consultancy from Acadia, Aptinyx, Biogen, CHDI Foundation, Clintrex LLC, Enterin, F. Hoffmann-La Roche Ltd, Ferring, Promentis, Signant Health, Sunovion, and Takeda; and license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS.
REFERENCES
- 1.Williams-Gray C, Foltynie T, Brayne C, Robbins T, Barker R. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 2007;130:1787–1798. [DOI] [PubMed] [Google Scholar]
- 2.Hely M, Reid W, Adena M, Halliday G, Morris J. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement Disorders 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 3.Litvan I, Goldman J, Troster A, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Movement Disorders 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pigott K, Rick J, Xie S, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology 2015;85:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67:414–422. [DOI] [PubMed] [Google Scholar]
- 6.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 2006;22:471–485. [DOI] [PubMed] [Google Scholar]
- 7.Dujardin K, Duhamel A, Delliaux M, Thomas-Anterion C, Destee A, Defebvre L. Cognitive complaints in Parkinson’s disease: its relationship with objective cognitive decline. J Neurol 2010;257:79–84. [DOI] [PubMed] [Google Scholar]
- 8.Erro R, Santangelo G, Barone P, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol 2014;27:276–281. [DOI] [PubMed] [Google Scholar]
- 9.Lehrner J, Kogler S, Lamm C, et al. Awareness of memory deficits in subjective cognitive decline, mild cognitive impairment, Alzheimer’s disease and Parkinson’s disease. Int Psychogeriatr 2015;27:357–366. [DOI] [PubMed] [Google Scholar]
- 10.Hong JY, Lee Y, Sunwoo MK, Sohn YH, Lee PH. Subjective cognitive complaints and objective cognitive impairment in Parkinson’s disease. J Clin Neurol 2018;14:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong JY, Sunwoo MK, Chung SJ, et al. Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson’s disease. Neurobiol Aging 2014;35:1739–1743. [DOI] [PubMed] [Google Scholar]
- 12.Galtier I, Nieto A, Lorenzo JN, Barroso J. Subjective cognitive decline and progression to dementia in Parkinson’s disease: a long-term follow-up study. J Neurol 2019;266:745–754. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa RP, Mendonca MD, Caetano AP, Lampreia TM, Miguel R, Bugalho PM. Cognitive complaints in Parkinson’s disease patients: from subjective cognitive complaints to dementia and affective disorders. J Neural Transm (Vienna) 2019. [DOI] [PubMed] [Google Scholar]
- 14.Dupouy J, Ory-Magne F, Mekies C, et al. Cognitive complaint in early Parkinson’s disease: A pilot study. Acta Neurol Scand 2018;137:59–66. [DOI] [PubMed] [Google Scholar]
- 15.AlDakheel A, Gasca-Salas C, Armstrong MJ, Duff-Canning S, Marras C. Cognitive complaints in nondemented Parkinson’s disease patients and their close contacts do not predict worse cognitive outcome. Alzheimer Dis Assoc Disord 2019;33:147–153. [DOI] [PubMed] [Google Scholar]
- 16.Hughes A, Daniel S, Kilford L, al. e. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 18.Fahn S, Elton R, members Up. Unified Parkinsons Disease Rating Scale. Florham Park, NJ: Macmillan Healthcare Information, 1987. [Google Scholar]
- 19.Hoehn M, Yahr M. Parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version In: TL B, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press, 1986: 165–173. [Google Scholar]
- 21.Trenkwalder C, Kohnen R, Hogl B, et al. Parkinson’s disease sleep scale--validation of the revised version PDSS-2. Mov Disord 2011;26:644–652. [DOI] [PubMed] [Google Scholar]
- 22.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Disease and Associated Disorders 1997;11(2 Suppl):33S–39S. [PubMed] [Google Scholar]
- 23.Brennan L, Siderowf A, Rubright JD, et al. The Penn Parkinson’s Daily Activities Questionnaire-15: Psychometric properties of a brief assessment of cognitive instrumental activities of daily living in Parkinson’s disease. Parkinsonism Relat Disord 2016;25:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson G, Cholerton B, Gross R, et al. Neuropsychologic assessment in collaborative Parkinson’s disease research: a proposal from the National Institute of Neurological Disorders and Stroke Morris K Udally Centers of Excellence for Parkinson’s Disease Research at the University of Pennsylvania and the University of Washington. Alzheimer’s and Dementia 2013;9:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattis S Dementia Rating Scale-2. Lutz, FL: Psychological Assessment Resources, Inc., 2001. [Google Scholar]
- 26.Nasreddine Z, Phillips N, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 27.Reitan R Validity of the Trail Making Test as an indicator of organic brain disease. Perceptual and Psychomotor Skills 1958;8:271–276. [Google Scholar]
- 28.Smith A Symbol digit modalities test: Manual. Los Angeles: Western Psychological Services, 1982. [Google Scholar]
- 29.Wechsler D WMS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation, 1997. [Google Scholar]
- 30.Gladsjo J, Shuman C, Evans J, Peavy G, Miller S, Heaton R. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 1999;6:147–178. [DOI] [PubMed] [Google Scholar]
- 31.Brandt J, Benedict R. The Hopkins Verbal Learning Test-Revised. Odessa, FL: Psychological Assessment Resources, 2001. [Google Scholar]
- 32.Benton A, Varney N, Hamsher K. Visuospatial judgment: a clinical test. Archives of Neurology 1978;35:364–367. [DOI] [PubMed] [Google Scholar]
- 33.Hubbard E, Santini V, Blankevoort C, et al. Clock drawing performance in cognitively normal elderly. Archives of Clinical Neuropsychology 2008;23:295–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger, 1983. [Google Scholar]
- 35.Bender R, Lange S. Adjusting for multiple testing: when and how? Journal of Clinical Epidemiology 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 36.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr 2008;20:1–16. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand 2014;130:439–451. [DOI] [PubMed] [Google Scholar]
- 38.Burmester B, Leathem J, Merrick P. Subjective cognitive complaints and objective cognitive function in aging: a systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev 2016;26:376–393. [DOI] [PubMed] [Google Scholar]
- 39.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson’s disease: A multicenter pooled analysis. Neurology 2010;75:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan L, Devlin KM, Xie SX, et al. Neuropsychological subgroups in non-demented Parkinson’s disease: a latent class analysis. J Parkinsons Dis 2017;7:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piccirilli M, D’Alessandro P, Finali G, et al. Frontal lobe dysfunction in Parkinson’s disease: prognostic value for dementia? European Neurology 1989;29:71–76. [DOI] [PubMed] [Google Scholar]
- 42.Woods S, Tröster A. Prodromal frontal / executive dysfunction predicts incident dementia in Parkinson’s disease. Journal of International Neuropsychological Society 2003;9:17–24. [DOI] [PubMed] [Google Scholar]
- 43.Pirogovsky E, Schiehser DM, Obtera KM, et al. Instrumental activities of daily living are impaired in Parkinson’s disease patients with mild cognitive impairment. Neuropsychology 2014;28:229–237. [DOI] [PubMed] [Google Scholar]
- 44.Kulisevsky J, Fernandez de Bobadilla R, Pagonabarraga J, et al. Measuring functional impact of cognitive impairment: validation of the Parkinson’s Disease Cognitive Functional Rating Scale. Parkinsonism and Related Disorders 2013;19:812–817. [DOI] [PubMed] [Google Scholar]
- 45.Santangelo G, Vitale C, Trojano L, et al. Subthreshold depression and subjective cognitive complaints in Parkinson’s disease. Eur J Neurol 2014;21:541–544. [DOI] [PubMed] [Google Scholar]
- 46.Dujardin K, Sockeel P, Delliaux M, Destee A, Defebvre L. Apathy may herald cognitive decline and dementia in Parkinson’s disease. Movement Disorders 2009;24:2391–2397. [DOI] [PubMed] [Google Scholar]
- 47.Fitts W, Weintraub D, Massimo L, et al. Caregiver report of apathy predicts dementia in Parkinson’s disease. Parkinsonism Relat Disord 2015;21:992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pontone G, Williams J, Anderson K, et al. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Movement Disorders 2009;24:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bogdanova Y, Cronin-Golomb A. Neurocognitive correlates of apathy and anxiety in Parkinson’s disease. Parkinsons Dis 2012;2012:793076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared at the request of other investigators for purposes of replicating procedures and results.


