Abstract
Chemotherapy directly or indirectly affects organs in a short-term or continuous manner. Endocrine organs are especially sensitive to cancer treatment, leading to concerns among patients regarding their quality of life afterward. Side-effects to the ovary include damage to the ovarian reserve, resulting in follicle loss, endocrine hormone deficiency, and infertility. It has been previously demonstrated that continuous treatment with 2 mg/kg cisplatin for 15 days can activate primordial follicles, suggesting that the response in the oocytes of primordial follicles was dependent on cisplatin concentration and administration frequency. However, our results demonstrate that continuous treatment with 2 mg/kg cisplatin for 15 days leads to the same consequence as with the continuous treatment of 5 mg/kg cisplatin: the death of oocytes in primordial follicles without indication of activation. Moreover, animals co-injected with melatonin and cisplatin did not display any significant differences from those treated with cisplatin only contrary to the known results. 6-hydroxymelatonin, a metabolite of melatonin, could not prevent follicle destruction, implying that melatonin does not confer the protection of ovarian follicles, either directly or indirectly. Altogether, our data support that fertoprotectants against cisplatin must target molecules that control cell death pathways in the oocytes of primordial follicles.
Keywords: Ovary, Dose, Cisplatin, Melatonin, Apoptosis
Introduction
Cancer patients have been treated primarily with surgery, radiation, and chemotherapy for several dacades. Recently, cancer treatment has been improved and expanded to use immunotherapy such as immune checkpoint inhibitors, cancer treatment vaccines, and monoclonal antibodies. Nonetheless, some groups of cancer patients such as hematologic and solid tumor malignancies still require chemotherapy such as cisplatin. According to the National Cancer Institute, up to 10 to 20% of all cancer patients are prescribed with cisplatin and other platinum-based drugs.
Cisplatin has been used to treat aggressive tumors in children, such as high-risk malignant germ cell tumors, hepatoblastoma, refractory or relapsed medulloblastoma/PNET, etc.
Although advances in cancer therapies have increased the survival rate via damage to cancer cells, these therapies have a wide range of adverse health effects including endocrine and metabolic complications. The most severely and frequently affected organs by radiation and chemotherapy treatment are endocrine organs such as the hypothalamus, pituitary gland, thyroid gland, ovary, and testes (1). These complications often appear in childhood cancer survivors (CCSs) months to years after treatment (2, 3). Monitoring complications in CCSs and studying off-target effects of cancer therapy are critical for improving the quality of life.
One of the most serious side effects is the off-target effect on ovarian germ cells in females (4, 5), causing premature ovarian insufficiency (POI), which clinically presents as endocrine dysfunction and infertility in premenopausal women. It has been proposed that certain intervention agents may have efficacy to protect ovarian reserves against cancer therapies (6). However, there have been no clinical guidelines for the strategic selection of fertoprotective agents due to a lack of thorough knowledge for the exact mechanisms of follicle loss in response to each chemotherapeutic drug. Most of the human data are from ovaries of patients who have been exposed to chemotherapies months or years before collection. Thus, the analysis of human data has not provided an accurate understanding of the mechanism of follicle loss.
Currently, two pathways have been proposed to understand the loss of follicles by chemotherapy: the “burnout theory” and the “apoptotic pathway”. The “burnout theory” states that alkylating agents damage granulosa cells of growing follicles in the ovary. Because granulosa cells from growing follicles produce anti-Mullerian hormone (AMH), which plays a role in suppressing the activation of dormant primordial follicles, damage to granulosa cells decreases the production of AMH (7–9). Indirectly activated primordial follicles via a decrease in AMH secretion undergo folliculogenesis and later atresia, resulting in POI in the ovary. From a signal transduction perspective, chemotherapy may disrupt the pathway that maintains dormancy in primordial follicles, thereby increasing expression of p-AKT, mTOR, and FOXO3A, the main molecules for oocyte activation of primordial follicles, in oocytes. These primordial follicles are irreversibly destined to be activated joining the growing follicle pool (5, 10–18). This observation was supported by the injection of AMH into mice with the combination of chemotherapy (cyclophosphamide, carboplatin, or doxorubicin), proposing that AMH could be a fertoprotective agent against alkylating agent-inducible primordial follicle activation (19–22).
The other pathway for follicle loss predicates that oocytes of primordial follicles undergo atresia via the apoptotic pathway induced by chemotherapy. Our previous works, as well as the work of others, show that oocytes within primordial follicles are highly sensitive to DNA damage (5, 16). Cisplatin binds to DNA or protein and chelates between them in rapidly proliferating cancer cells. The binding produces Pt adducts, leading to activation of DNA repair systems or various signaling cascades including cell death depending on the intensity of the damage. Although oocytes of primordial follicles do not proliferate, these cells are especially vulnerable to chemotherapeutic agents due to specific genomic surveillance molecules. p63, a member of the p53 tumor suppressor family, is present as dimers in oocytes of primordial, primary, and early secondary follicles (15, 16, 23). The knockdown study of p63 showed the critical role of p63 in DNA damage-induced germ cell loss (10, 18). DNA damage initiated by gonadotoxic agents rearranges TAp63 dimers to form TAp63 tetramers through hyperphosphorylation. A recent study demonstrated casein kinase 1 (CK1) primes TAp63a and then CHEK2 induces hyper-phosphorylation of TAp63a in oocytes (24). This cascade with the formation of TAp63 tetramers activates the apoptotic pathway with PUMA-NOXA (10, 11, 13, 25). In addition, our previous study has demonstrated that oocytes of primordial follicles are not activated following X-ray or cisplatin exposure and inhibitors blocking the PI3K/mTORC1 pathway do not protect oocytes of primordial follicles against chemotherapy (5, 10–14, 17).
However, the consequences of continuous cisplatin treatment have remained in question. Previous studies reported that continuous treatment with 2 mg/kg cisplatin leads to the activation of primordial follicles without causing apoptosis (26–30). Thus, molecules suppressing the PI3K-p-AKT-mTORC1 pathway may prevent the loss of primordial follicles. One proposed molecule is melatonin (N-acetyl-5-methoxytryptamine, MT), a hormone produced and secreted by the pineal gland. Due to the antioxidant role of melatonin, it has been proposed that melatonin could protect the ovary from reactive oxygen species (ROS) induced by chemotherapy (26–28).
The purpose of this study was to examine whether the response of primordial follicles to the continuous treatment with 2 mg/kg cisplatin is different from that of continuous treatment with 5 mg/kg cisplatin and thus if fertoprotectants should be considered differently depending on the concentration and frequency of cisplatin. Furthermore, this study investigated whether melatonin can protect the ovary against toxicity from continuous treatment with cisplatin.
Materials and Methods
Animals
All procedures involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska Medical Center. To test the effect of cisplatin in the ovary of six-week-old female CD-1 as the previously reported (26, 27), five-week-old female CD-1 mice were purchased from Charles River Laboratory (Wilmington, MA, USA) and accommodated for a week. Animals were provided with food and water ad libitum and maintained at temperatures of 20–26 °C with a set point of 22 °C, 30–70% humidity with a set point of 40%, and a 10 hours light −14 hours dark photoperiod. They were treated at the University of Nebraska Medical Center in the Comparative Medicine facilities (Omaha, NE, USA). BD 1 ml TB syringes (309623, Becton, Dickson and Company, Franklin Lakes, NJ, USA) were used to inject mice intraperitoneally (i.p.) with cisplatin (P4394, Sigma-Aldrich, St. Louis, MO, USA) (31) or an equivalent volume of DPBS (vehicle) (14190–136, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) at the following doses and times. In this study, we used sixty-four six-week-old female CD-1 mice in total for in vivo cisplatin injection experiments and eight PD5 CD-1 females in total for the in vivo cisplatin TEM experiment. The mice were randomly divided into four groups (n=5 per group). Two groups of mice were injected i.p. once a day at 4 pm with vehicle or 2 mg/kg of cisplatin at 6-weeks of age for 15 days and euthanized 24 hours after the last injection to examine the effect of cisplatin as the previous study performed (26, 27). When mice were euthanized, the weight of liver, kidneys, spleen, and ovaries were measured. The weight of kidneys and ovaries were measured after clearing surrounding adipose tissue. Mice from the other group were treated with 2 mg/kg cisplatin for 15 days and kept for 10 additional days without further injection of 2 mg/kg cisplatin to give time for follicle activation, apoptosis, or recovery. Mice from the last group were treated with 5 mg/kg of cisplatin for 8 days as a positive control, which was originally designed for 15 days of injections to match the 2 mg/kg injection group to compare the effect of cisplatin on the ovary. However, two mice died from 5 mg/kg cisplatin group, and the remaining of the group (n=3) were euthanized at 9 days post initial injection due to toxicity. For cisplatin injections, the volume of injection was calculated with bodyweight × 6.66 (cisplatin stock: 0.75 ug/ul for 5 mg/kg and 0.3 ug/ul for 2 mg/kg). Animals were placed in an uncrowded chamber into which CO2 was introduced from a CO2 tank connected to an appropriate regulator and euthanized. In addition, CD-1 mice were randomly divided by three groups (n=5 per group) and were injected i.p. with 2 mg/kg cisplatin daily for 3, 7, or 10 days and euthanized 24 hours after the last injection to examine the effect of cisplatin on primordial follicles within those timeframes. Control group (n=4) was injected with DPBS (body weight × 6.66). Another group of 6-week-old CD-1 mice was randomly divided into four groups (n=5 per group) and injected i.p. once a day at 2 pm with 30 mg/kg melatonin (M5250, Sigma-Aldrich) with or without 2 mg/kg of cisplatin for 15 days to examine whether melatonin attenuates cisplatin-induced morphological alterations on the ovary as previously demonstrated. 30 mg/kg melatonin was used because that concentration was previously reported to have greater effectiveness than 15 mg/kg melatonin for attenuation of cisplatin-induced morphological alterations (26, 27). Melatonin was prepared in ethanol at a concentration of 50 mg/ml and diluted in saline. For melatonin injections, the volume of injection was calculated with body weight × 10 (melatonin stock: 3 ug/ul). Mice were pretreated with 30 mg/kg melatonin or vehicle two hours before injection of 2 mg/kg cisplatin and were injected for 15 days. These mice were euthanized 24 hours after the last injection of cisplatin.
Whole ovary organ culture
Whole ovary organ culture was performed as previously described (5, 10). Ovaries collected from postnatal day 5 (PD5) CD-1 female mice were placed on Millicell inserts (PICM03050, EMD Millipore Co., Burlington, MA) in 6-well (FB012927, Thermo Fisher Scientific) or 12-well (50-203-138, Thermo Fisher Scientific) plates with α-MEM (32561-037, Gibco, Thermo Fisher Scientific) supplemented with Penicillin-Streptomycin-Glutamine (10-378-016, Thermo Fisher Scientific), bovine serum albumin (BSA) (BP9703-100, Thermo Fisher Scientific) and insulin-transferrin-selenium (ITS) (41400-045, Gibco, Thermo Fisher Scientific). Cisplatin was dissolved in DPBS and added to culture media to final concentration. Ovaries were treated with 1 μM cisplatin for 4, 6, and 8 days to mimic long-term treatment with cisplatin. 4 μM cisplatin treatment for 4 days was used as a positive control based on toxicity data demonstrating induction of over 90% primordial follicle death at 96 hours without damage to oocytes in secondary follicles (5, 10). Additionally, ovaries were treated with 4 μM cisplatin for 4 days with or without 1 μM or 5 μM 6-hydroxymelatonin (21857, Cayman Chemical, Ann Arbor, MI, USA). 6-hydroxymelatonin was used to pretreat the ovaries two hours before the introduction of cisplatin. The ovaries were incubated at 37 °C under 5% CO2. Culture media was changed every 24 hours for both 1 μM and 5 μM 6- hydroxymelatonin and 4 μM cisplatin treatment group and every 48 hours for the 4 μM cisplatin only treatment group. The ovaries were harvested and fixed in Modified Davidson’s fixative for 24 hours at 4 degrees, and then washed for 3 times 20 minutes each with 50 percent ethanol. Then the ovaries were placed in 70 percent ethanol until they were ready to be processed. The ovaries were processed overnight and then embedded into paraffin blocks ready for sectioning, and staining for histological analysis using H&E, 3,3’-Diaminobenzidine (DAB) staining, and immunofluorescence assay. The number of primordial follicles was quantified and compared between treatment groups of different time points and concentrations to identify the toxicity of cisplatin to the ovary.
Follicle counting
Ovaries were harvested and fixed in Modified Davidson’s fixative (64133–10, Electron Microscopy Sciences Inc., Hatfield, PA, USA) at 4 °C for 24 hours after in-vitro culture or in-vivo i.p. injection. The ovarian tissue blocks were sectioned serially at 5 μm thickness for H&E staining, immunofluorescence assay, and DAB staining. H&E staining was performed using standard methods (32). Primordial (PF), primary (PM), secondary (SF), and antral (AF) follicles were counted in every 10th section stained with H&E (5, 10). Follicles with any pre-granulosa cells and healthy oocyte were counted as primordial follicles. Follicles fully covered with one layer of granulosa cells were classified as primary follicles, and follicles with more than one layer of granulosa cells were grouped as secondary follicles. Follicles with any size of antrum were considered as antral follicles. Only healthy secondary or antral follicles with non-atretic and healthy granulosa cells and oocytes were counted for a total number of secondary follicles. Secondary and antral follicles were counted if they had visible nucleoli in the oocyte nucleus to exclude the possibility of duplicate counting. To determine the total number of primordial or primary follicles per ovary, the average number of primordial or primary follicles per section was multiplied by the total number of sections and divided by the average number of sections in which a single primordial or primary follicle appeared (average primordial or primary follicle number per section × total section number × 1/2). The total number of secondary or antral follicles was calculated by multiplying the average number of secondary or antral follicle per section by the total number of sections.
Immunohistochemistry (IHC) and Immunofluorescence (IF)
IHC and IF were performed with ovarian sections as described previously (5, 10, 33). Briefly, tissues collected for immunostaining were fixed with Modified Davidson’s Fixative solution, processed into paraffin, and sectioned at 5 μm. The sections were heated in 10 mM sodium citrate buffer (pH 6.0) containing 0.05% Tween 20 for 30 minutes in a Pressure Cooker. After the antigen retrieval step, the sections were treated with a blocking buffer for 1 hour and then with the primary antibody. The catalog numbers and dilution of primary antibodies were as follows: phospho-AKT (9271S, 1:50), AKT (C67E7, 4691S,1:50), phospho-PTEN (9551T,1:50) from Cell Signaling (Danvers, MA, USA), AMH (MIS) (C-20, sc-6886, 1:100) from Santa Cruz Biotechnology Inc. (Dallas, TX, USA), and anti-phospho-Histone H2A.X (S139, 05–636, 1:100) from MilliporeSigma (Burlington, MA,USA). The secondary antibodies used were horseradish peroxidase-conjugated anti-mouse (BA-9200, 1:400, Vector Laboratories Inc., Burlingame, CA,USA), anti-rabbit (BA-1000, 1:400, Vector), anti-goat (A16003, 1:400, DKXGO BIOAFFINITY, Invitrogen) antibodies, or streptavidin peroxidase (SA-5004, 1:400, Vector Laboratories). After secondary antibody reaction, DAB Peroxidase Substrate Kit (SK-4100, Vector Laboratories, Burlingame, CA) was used for IHC. The ABC kit (NC9206402, Vector Laboratories, Burlingame, CA, USA), and the TSA Plus Fluorescein System Kit (NEL74100KT, PerkinElmer, Inc., Waltham, MA, USA) were used for IF as previously described (5, 10, 34). For reducing bias, the samples were in blind testing. The tissue sections were randomly labeled with numbers and performed the immunofluorescence assay. Then, the images were blindly taken with an EVOS microscope for reducing bias.
Immunoblotting analysis
Ovaries were collected and homogenized in ice-cold lysis buffer (20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1% Triton-X, and 2 mM EDTA) with protease inhibitor cocktail (04693116001, Roche Life Science, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (P0044, MilliporeSigma). Equal amounts (30 μg) of protein measured by the BCA protein assay kit (23225, Thermo Fisher Scientific) were loaded onto an SDS-PAGE gel. Electrophoresis was performed and proteins were transferred to a nitrocellulose membrane (Amersham™Protran™, GE10600015, GE Healthcare Life Science, Chicago, IL,USA) using a transfer apparatus according to the manufacturer’s protocols (Bio-Rad). After incubation with 5% BSA in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.5% Tween 20) for 1 hour at room temperature, primary antibodies were placed at 4 °C for overnight incubation. Antibodies used are the following: phospho-AKT (1:5000, Ser473, 9271S), AKT (1:5000, C67E7, 4691S), phospho-PTEN (1:5000, Ser380, 9551T), PTEN (1:5000, 138G6, 9559S), and beta-actin (1:5000, 4967S) from Cell Signaling. Membranes were washed and incubated with a 1:5000 dilution of secondary antibodies for 1 hour. Proteins were detected by the SuperSignal™ West Pico PLUS Chemiluminescent Substrate (34579, Thermo Fisher Scientific) and exposed using a BioSpectrum 500 Imaging System (P/N 97-0362-01, UVP LLC, Upland, CA, USA).
Transmission Electron Microscopy
Postnatal day 5 CD-1 female mouse (n=8) were injected once with 5 mg/kg cisplatin or DPBS at 4 pm and euthanized at 10 am next day for 18 hours treatment for analysis with transmission electron microscopy. Ovaries were harvested and fixed for 2 hours, then washed 3 times with DPBS for 15 minutes. These samples were post-fixed in osmium tetroxide for 1 to 2 hours and washed 3 times with phosphate buffer for 15 minutes. Ethanol dehydration was performed with a gradient: 50%, 70%, 90%, 95%, and three times with 100% for 20 minutes each. 100% propylene oxide was used for washing 3 times for 15 minutes each step. Then samples were placed in a 1:1 ratio of propylene oxide and Embed 812 in open vials and left overnight in the fume hood. The vials were opened and placed in the fume hood overnight. Tissues were then placed in fresh Embed 812 to soak for 6 hours and put in final molds with fresh Embed 812. The molds were placed in an embedding oven at 65 °C overnight. Molds from the oven were removed and cooled to room temperature before removing polymerized blocks from the molds that were ready for sectioning. Thin sections from the blocks were cut on a Leica UC6 ultramicrotome. Sections were stained with 1% uranyl acetate and Reynold’s lead citrate. Sections were examined on an FEI Tecnai G2 TEM operated at 80 kV.
Statistical analysis
Data were presented by mean +/− SEM. For a comparison of means between more than two independent groups, a one-way analysis of variance was performed. The difference between the two groups was analyzed by Tukey’s range test for statistical comparisons using PRISM version 8.4.2 (464) (GraphPad Software). Values of P<0.05 were considered to be statistically significant. Statistical significance was marked as *, P< 0.05: **, P<0.01; ***, P<0.001; ****, P<0.0001; n.s., not significant.
Results
Cisplatin reduces primordial follicles without increasing the number of growing follicles
To compare the effects of continuous injection with 2 mg/kg cisplatin versus short-term continuous injection with 5 mg/kg cisplatin, 6-week-old CD-1 female mice were tested with two different doses. The mouse strain and age were designed based on previous publications (26, 27). CD-1 mice were injected i.p. once a day at 4 pm with DPBS (vehicle) or 2 mg/kg cisplatin for 15 days. One group of mice that was injected with 2 mg/kg for 15 days (2 mg/kg+10 d) was kept for an extra 10 days to examie whether damaged follicles either survive or die depending on the intensity of damage. The other group of mice were injected i.p. once a day at 4 pm with 5 mg/kg cisplatin for 8 days due to health issues induced by cisplatin toxicity. Mice were harvested 24 hours or 10 days (2 mg/kg+10 d) after continuous daily injection (Figure 1A). Mice in both 5 and 2 mg/kg cisplatin groups were significantly unhealthy in comparison to those in vehicle control. The 5 mg/kg cisplatin injection group was planned for 15 days injections to compare results with the 2 mg/kg cisplatin injection groups. Unfortunately, 2 out of 5 mice died due to toxicity of cisplatin at day 6, resulting in 3 out of 5 mice for analysis. Mice in both 5 and 2 mg/kg cisplatin groups were severely weak, showing reduced activity, low motility, rigid fur, and shivering thermogenesis. Body weight rapidly decreased in the 5 mg/kg cisplatin group throughout the 8-day injection timeframe, while the body weight of the 2 mg/kg cisplatin group slightly decreased during the 15-day injection period. The 2 mg/kg+10 d group showed a gradual recovery of body weight (Figure 1B). A significant decrease in body and ovary weight was found in continuous treatment with a 2 mg/kg cisplatin group (Figure 1C–D). However, the mice that were maintained for another 10 days appeared to recover from the toxicity (Figure 1B). 3’3-diaminobenzidine (DAB) staining with p63 in ovarian tissue sections from mice of each group indicated the presence of healthy oocytes (marked with arrows) (Figure 1E). Accordingly, the number of corpora lutea (CL) dramatically decreased in both groups (Figure 1E). Although many surviving follicles were observed in these groups, significant differences were noted in primordial, primary, secondary, and antral follicles. Abnormal oocytes or granulosa cells remained throughout the tissue sections in the groups with continuous treatment of 2 mg/kg for 15 days cisplatin due to the toxicity of cisplatin (Figure 1Fi). Some primordial follicles lost oocytes, forming empty follicles with abnormal granulosa cells (Figure 1i). Although healthy follicles with clear nuclei in the oocytes and vigorous granulosa cells were identified (Figure 1Fii, arrow), several unhealthy follicles with pyknotic granulosa cells (Figure 1Fiii and 1Fix arrows), unusual oocyte structure (Figure 1Fiv and 1Fviii, arrows), unusual antral follicles with lack of cumulus cells (Figure 1Fv and 1Fix, arrows), suggesting that continuous treatment with 2 mg/kg cisplatin for 15 days once a day is detrimental to ovarian follicles. As shown with follicle counting quantification, the total number of follicles per ovary decreased in all groups. In particular, primordial follicles dramatically decreased. The numbers of primary, secondary, and antral follicles did not increase in 2 or 5 mg/kg cisplatin groups (Figure 1G), suggesting that continuous i.p. injection daily with 2 mg/kg cisplatin for 15 days did not activate primordial follicles. In addition, there was a significant increase in the number of antral follicles of the 2 mg/kg cisplatin + 10 d group compared to that of the 2 mg/kg cisplatin group, suggesting that there was a recovery of surviving secondary follicles.
Figure 1. Cisplatin reduces primordial follicles at any dosage without increasing the number of growing follicles.
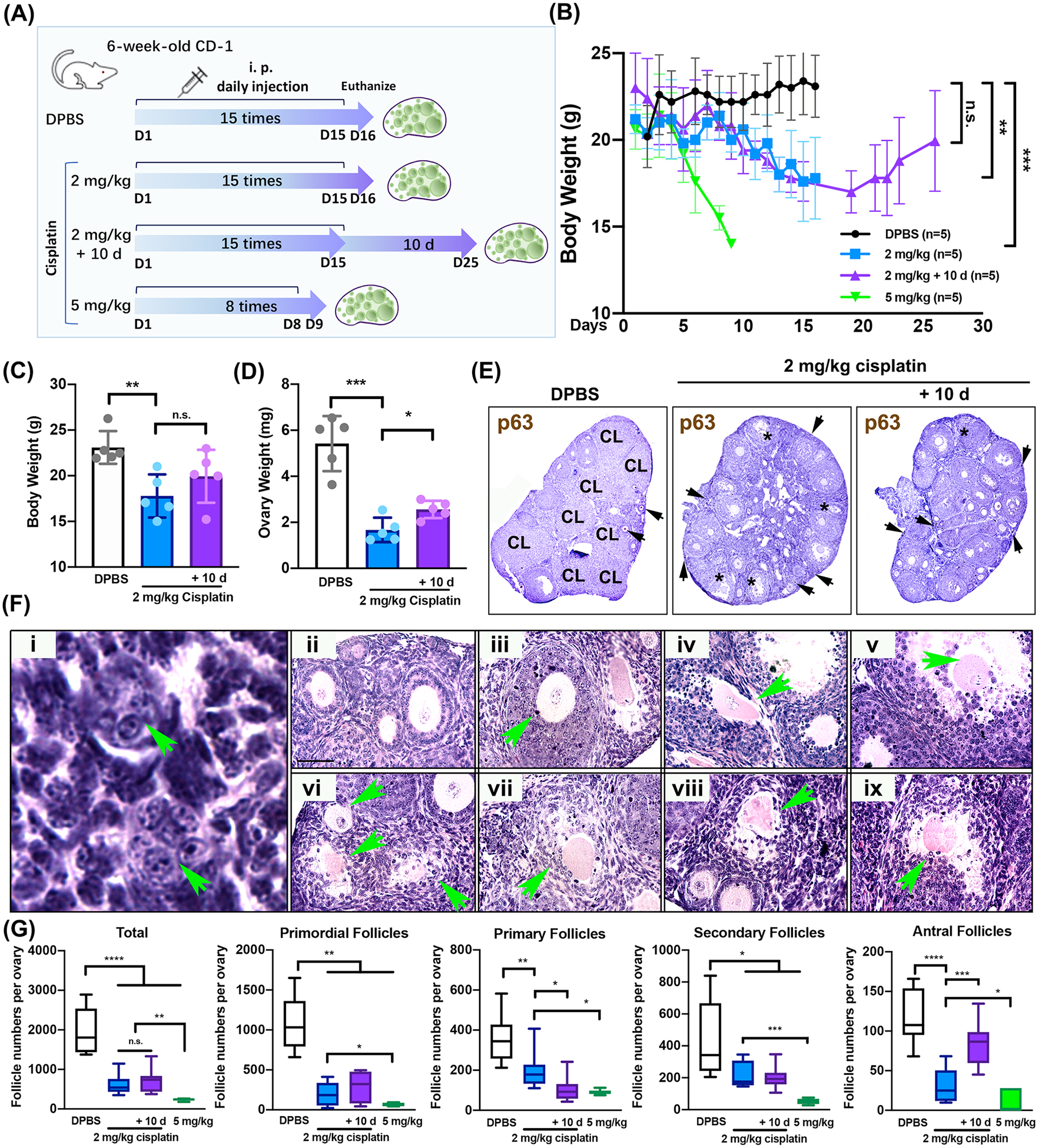
(A) Schematic of daily injection with DPBS and cisplatin (2 mg/kg, 2 mg/kg + 10 d, and 5 mg/kg) to six-week-old CD-1 female mouse (n=5 for each group). Mice of DPBS and cisplatin (2 mg/kg, 2 mg/kg + 10 d) groups were injected once a day for 15 days, while mice of 5 mg/kg cisplatin group were injected for 8 days. (B) Body weight of the mice throughout the injection period was measured every day. (C, D) Body and ovary weight from the mice in DPBS and cisplatin (2 mg/kg and 2 mg/kg+10 d) group at the time of euthanasia. (E) Image of representative DAB staining with p63 biomarker in the ovarian tissue sections from each group. (F) H&E images reflecting normal and abnormal structures (green arrow) in primordial, primary, secondary, and antral follicles from 2 mg/kg cisplatin-treated mice after cisplatin injection for 15 days. Scale bar = 50 μm. (G) Counting of total primordial, primary, secondary, and antral follicles in DPBS, 2 mg/kg, 2 mg/kg+10 d, and 5 mg/kg cisplatin-treated groups. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; n.s., not significant.
The activation signals of p-AKT and p-PTEN were expressed in granulosa cells of growing follicles rather than oocytes of primordial follicles
To investigate whether cisplatin turns on signals of p-AKT and p-PTEN, ovaries from mice exposed with cisplatin were collected and homogenized. The ovary of 2 mg/kg cisplatin group highly expressed both p-AKT and p-PTEN in the ovarian lysate (Figure 2A). Interestingly, the expression of these signals decreased in 2 mg/kg+10 d group, implying that cessation of cisplatin injection decreased the expression of p-AKT and p-PTEN signals in the ovary. To inspect which ovarian cells express these signals in the 2 mg/kg cisplatin group, immunofluorescence (Figure 2B) and immunohistochemistry (Supplemental Figure 1A) analyses were performed. While p-AKT was detected at the basal level in granulosa cells of the DPBS group (Figure 2B), it was highly elevated in granulosa cells of growing follicles in the 2 mg/kg cisplatin group (Figure 2B, arrow and Supplemental Figure 1Av). However, the intensity of p-AKT expression varied depending on the follicles. There was variable expression of p-AKT in granulosa cells within a single growing follicle as shown in Figure 2B. While some small secondary follicles had high expression of p-AKT in all granulosa cells, other small secondary follicles had uneven expression in their granulosa cells. Granulosa cells from antral follicles also had a higher level of p-AKT in comparison to the granulosa cells in control or the 2 mg/kg cisplatin + 10d group. However, p-AKT expression was absent in oocytes of primordial follicles (ooPF in the inset in Figure 2B and green asterisk in the inset of Supplemental Figure 1Av), while it was detectable in a few cuboidal granulosa cells of transitional primordial follicles (inset of Supplemental Figure 1Av). Interestingly, very little p-AKT expression was detected in granulosa cells of growing follicles in the 2 mg/kg+10 d group (Figure 2B, arrow and Supplemental Figure 1Aix). While p-PTEN expression was mainly localized in theca cells and corpora lutea in the DPBS group, it was greatly increased in granulosa cells of small growing follicles of the 2 mg/kg cisplatin group (Figure 2B, arrows and in Supplemental Figure 1Avii). However, the p-PTEN signals became weaker in granulosa cells of small growing follicles in the 2 mg/kg + 10 d group (Supplemental Figure 1Axi) when cisplatin was withdrawn. Patch expression of AMH in the 2 mg/kg cisplatin group reflects unhealthy granulosa cells that lost the ability to secrete AMH (Figure 2B and Supplemental Figure 1Aviii, arrows). To examine whether cisplatin induces DNA damage in oocytes, γH2AX was screened. While the signal of γH2AX was highly detected in oocytes of primordial follicles and granulosa cells of growing follicles, oocytes of growing follicles did not show expression in relation to the concentration of cisplatin. Its expression demonstrates cisplatin-induced prominent DNA breaks in oocytes of primordial follicles (arrows in inset) and granulosa cells of growing follicles with the treatment of 2 mg/kg cisplatin for 15 days, supporting that oocytes of primordial follicles are sensitive to gonadotoxic reagents (Figure 2C). This suggests primordial follicles undergo apoptotic or repair pathways dependent on the intensity of the damage.
Figure 2. The activation signals of p-AKT and p-PTEN were expressed in granulosa cells of growing follicles rather than oocytes of primordial follicles.
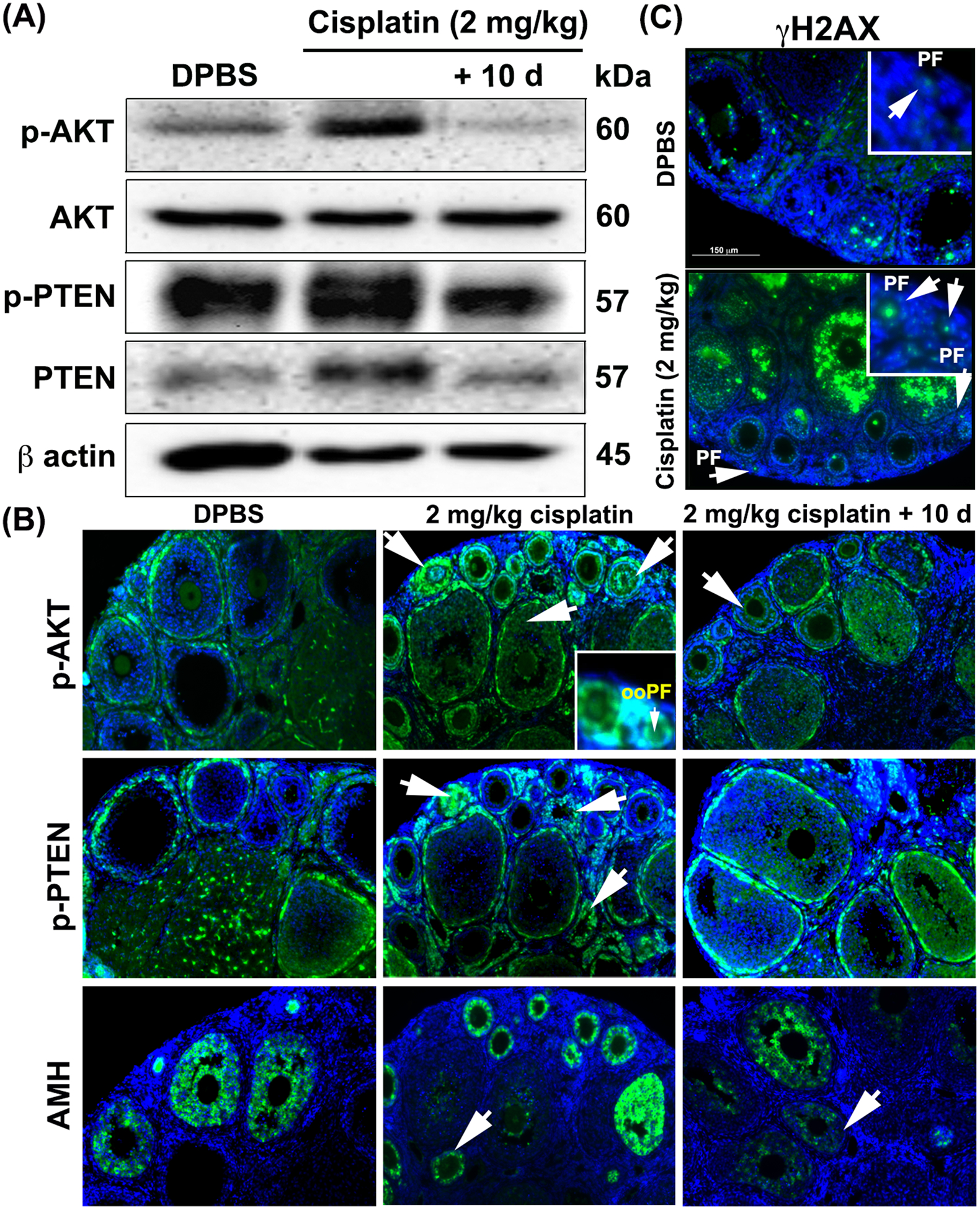
(A) Expression of p-AKT, AKT, p-PTEN, PTEN, and β-actin in whole ovarian extracts from DPBS and cisplatin (2 mg/kg and 2 mg/kg+10 d) group was analyzed by immunoblotting. Molecular weights are indicated next to the blots. (B) Immunofluorescence images of p-AKT, p-PTEN, and AMH expression in the ovaries treated with DPBS and cisplatin (2 mg/kg and 2 mg/kg + 10 d) (n=4). White arrows indicate the localization of expression of p-AKT, p-PTEN, and AMH in all treatment groups. Inset in the 2 mg/kg cisplatin group shows oocyte of primordial follicle (ooPF). p-AKT, p-PTEN, and AMH expression (green) and DAPI (blue). (C) Immunofluorescence images of γH2AX expression in the ovary treated with DPBS and 2 mg/kg cisplatin groups (n=3). Primordial follicles stained with γH2AX (green) are marked with arrows. Inset shows primordial follicles (PF). Scale bar = 150 μm.
Continuous treatment with 2 mg/kg cisplatin for 15 days indirectly and directly causes a detrimental effect on ovarian follicles rather than activation of primordial follicles
To examine the effect of cisplatin on primordial follicles, the injection time frame was modified to 3, 7, or 10 days (Figure 3A). Mice were euthanized after 24 hours of i.p. injection with 2 mg/kg cisplatin for 3, 7, and 10 days. Ovary weight significantly decreased with the increase of cisplatin injection frequency (Figure 3B), showing the toxicity of cisplatin in mouse ovary. Mice injected with 2 mg/kg cisplatin daily at 4 pm for 10 days appeared fragile with smaller body sizes and ragged fur. Fewer CL and smaller growing follicles were present in the ovaries from mice injected with 2 mg/kg cisplatin daily for 10 days in comparison to the 3 or 7 days 2 mg/kg cisplatin groups (Figure 3C). As shown with follicle counting, 2 mg/kg cisplatin daily injection reduced the numbers of all classes of follicles over time with continuous injections (Figure 3D), especially primordial follicles with 7 times daily injection of 2 mg/kg cisplatin. In addition, representative histological images with H&E staining indicated severe damage in follicles followed with 2 mg/kg cisplatin injection. Although healthy follicles were noted in all ovaries (Figure 3E, top row), abnormal follicles were frequently observed in cisplatin-treated ovaries. For instance, follicles appear with oocytes partially or not covered with granulosa cells, pyknotic granulosa cells, and without granulosa cells (Figure 3E). To further investigate the effect of direct and continuous treatment with cisplatin on primordial follicles in the mouse ovary, PD5 mouse ovaries were treated in vitro with 1 μM or 4 μM of cisplatin (Figure 3F). To mimic continuous injection of cisplatin in mice, media containing cisplatin was changed every day for the 1 μM cisplatin group. Consistent with in vivo results, surviving primordial follicles from the mouse ovary with the treatment of 1 μM cisplatin for 96 hours decreased dramatically, with rare primordial follicles after 4 days, and no surviving primordial follicles after 6 or 8 days of in vitro culture. The total numbers of primary and secondary follicles also significantly decreased after 4, 6, and 8 days of in vitro culture (Figure 3G). Representative histological images with H&E staining of the cultured ovaries revealed that direct and continuous treatment with cisplatin damages follicles, in particular primordial follicles, showing pyknotic oocytes (Fig. 3H, arrow). Regardless of dosage or frequency, cisplatin treatment led to a significant decrease in the number of primordial, primary, and secondary follicles, suggesting that cisplatin induces oocyte death instead of activation in primordial follicles.
Figure 3. Continuous treatment with 2 mg/kg cisplatin indirectly and directly causes a detrimental effect on ovarian follicles rather than activation of primordial follicles.
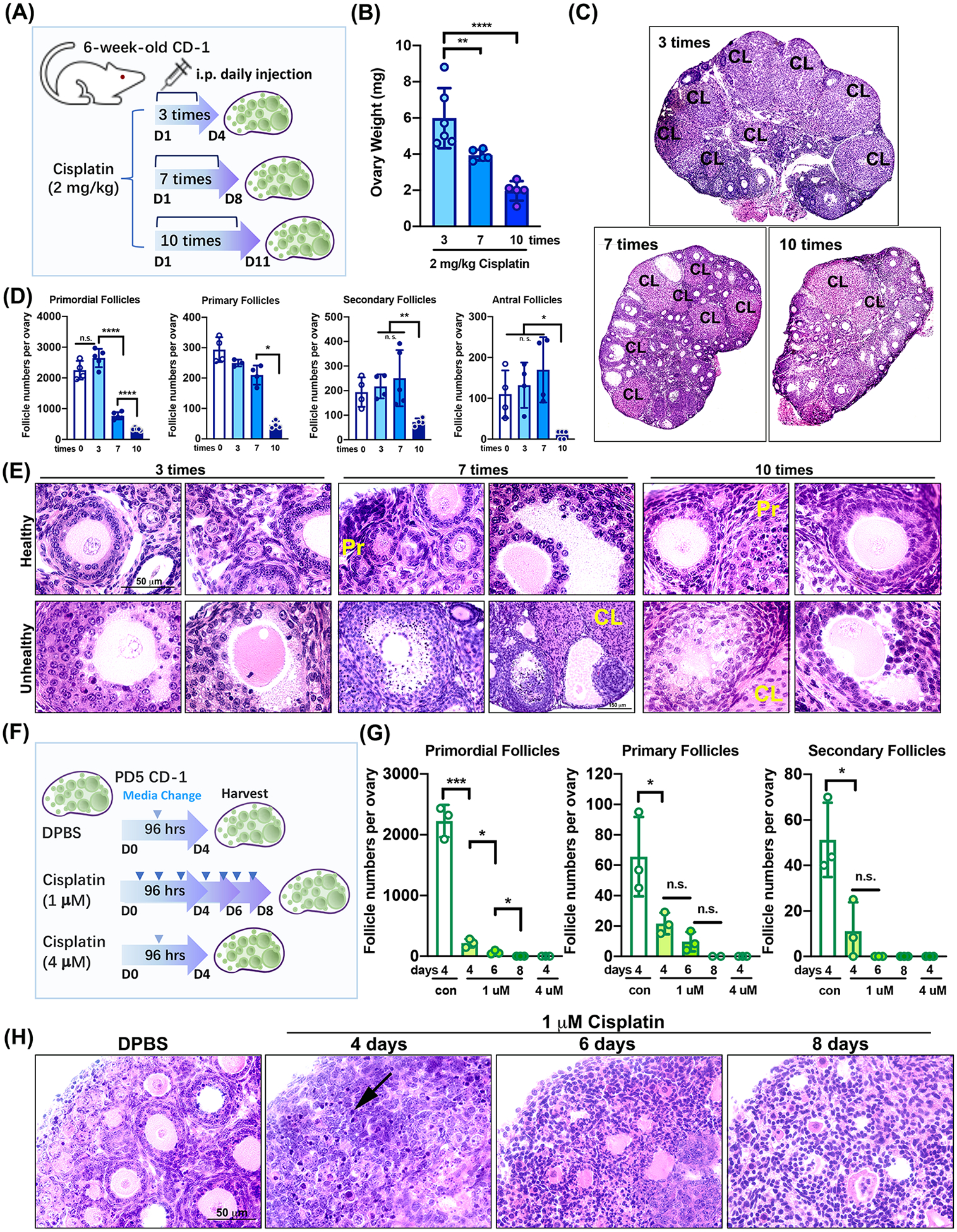
(A) Schematic of i.p. daily injections with 2 mg/kg cisplatin for 3, 7, and 10 days to six-week-old CD-1 female mice (n=5 per group). (B) Ovary weight from 2 mg/kg cisplatin-treated mice at the time of euthanasia. Both ovaries from a mouse were measured. (C) H&E staining of representative ovarian tissue sections from each group. CL = Corpus Luteum. (D) Counting of total primordial, primary, secondary, and antral follicles in control (n=4) and 2 mg/kg cisplatin-treated groups (n=5). (E) Representative histological images with H&E staining showing healthy (top) and unhealthy (bottom) follicles in each group. Primordial follicles (Pr) and corpus luteum (CL). Scale bar = 50 μm. (F) Schematic of treatment of cisplatin in ovarian culture in vitro (n=3). Arrows indicate the time point of media change during culture in vitro. (G) Counting of total primordial, primary, and secondary follicles in each cisplatin-treated group. (H) Representative histological image with H&E staining showing healthy (DPBS) and unhealthy (1 μM cisplatin) follicles in each treatment group. The ovaries were cultured for and harvested at 4, 6, and 8 days. Scale bar = 50 μm. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; n.s., not significant.
Melatonin does not protect ovarian follicles from cisplatin-induced damage
To investigate whether pretreatment with melatonin protects ovarian follicles against the toxicity of cisplatin with or without activation of primordial follicles, 30 mg/kg melatonin was injected i.p. to 6-week-old female mice with or without 2 mg/kg of cisplatin daily for 15 days (Figure 4A). Melatonin was injected i.p. two hours before 2 mg/kg cisplatin i.p. injection for 15 days. Any significant differences between the cisplatin-only group and melatonin + cisplatin group were observed in body and organ (liver, kidney, and spleen) weights, especially ovary (Figure 4B, Supplemental Figure 2). The total number of follicles in melatonin+cisplatin group did not differ significantly from that of the cisplatin-only group (Figure 4C). Although representative histological images of ovaries from melatonin + cisplatin group exhibited some healthy follicles (Figure 4Di, green, primordial; blue, secondary), many follicles revealed damage such as an oocyte surrounded by pyknotic granulosa cells (Figure 4Di, black arrow) and oocytes not covered with granulosa cells (Figure 4Dii–iii). The total number of all classes of follicles implies that the pretreatment of melatonin could not protect ovarian follicles against the toxicity of cisplatin in vivo (Figure 4E). To further investigate whether the direct pretreatment of melatonin protects primordial follicles in the mouse ovary against the toxicity of cisplatin, PD5 mouse ovaries were treated in vitro with 1 μM or 5 μM of 6-hydroxymelatonin (6-OH-Mel), a metabolite of melatonin (Figure 4F). The reason to use postnatal day 5 CD-1 mouse is that the time frame window (PD5) contains the highest primordial follicles after birth and is the best time point to investigate the effects on primordial follicles of the ovary. The 6-hydroxymelatonin (6-OH-Mel) was applied in ovary culture in vitro 2 hours prior to cisplatin treatment. Interestingly, pretreatment with 5 μM of 6-OH-Mel for 96 hours had a detrimental effect on primordial follicles, as evidenced with oocyte death and condensation of nuclei inside oocytes of primordial follicles (Figure 4F). Although follicles appeared healthy following pretreatment with 1 μM of 6-OH-Mel, damage induced by cisplatin was not prevented by priming the ovary with 6-OH-Mel (green arrow, damaged oocyte; blue, damaged granulosa cells) (Figure 4F). The total number of follicles and each class of follicles directly confirmed that ovarian follicles were damaged and pretreatment of 6-OH-Mel did not shield the ovary (Figure 4G).
Figure 4. Melatonin does not protect ovarian follicles from cisplatin-induced damage in the ovary.
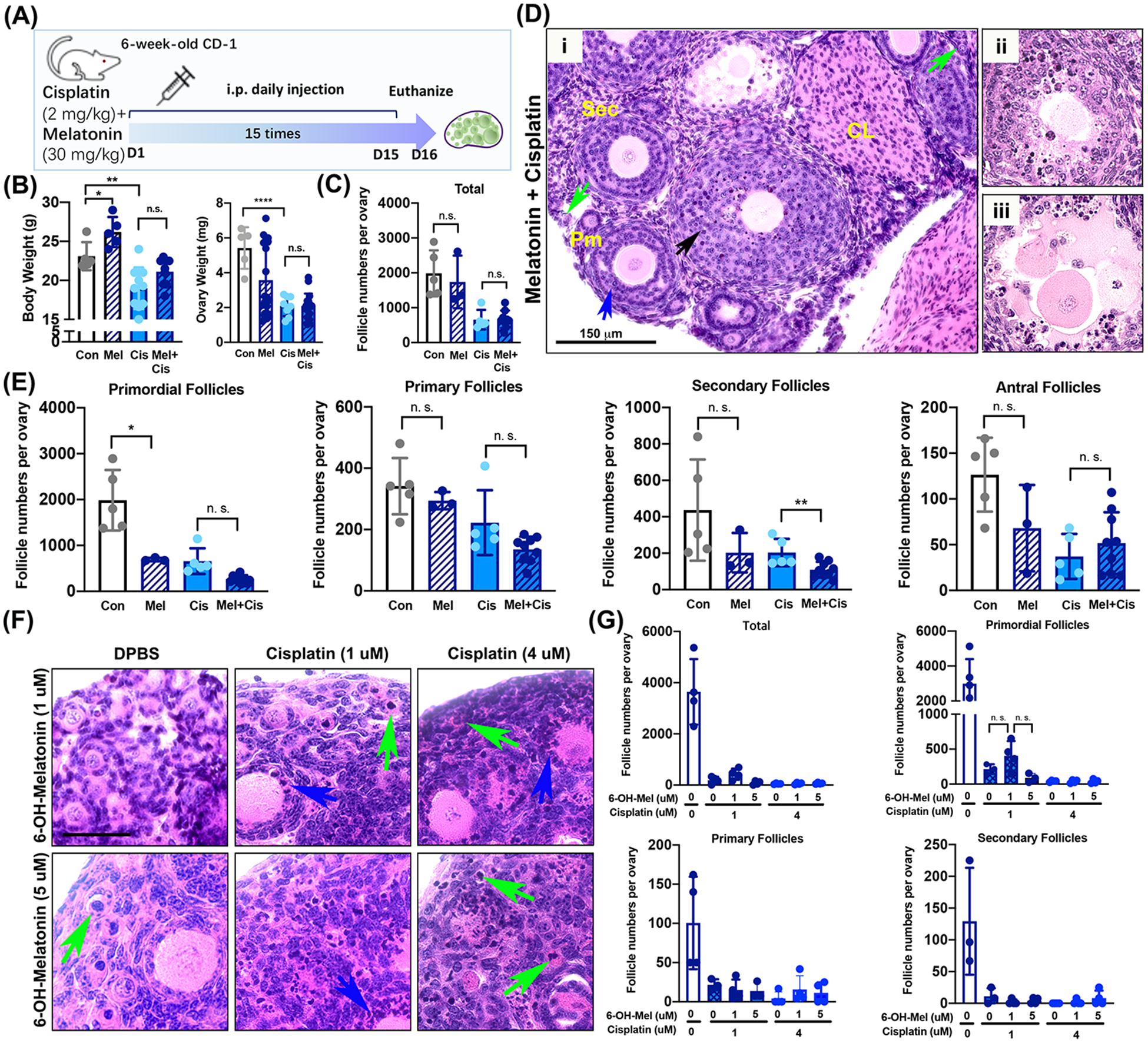
(A) Schematic of i.p. daily injections with 2 mg/kg cisplatin combined with 30 mg/kg melatonin to six-week-old CD-1 female mice (n=5 each for control, melatonin, and cisplatin groups, n=10 for melatonin+cisplatin group). Melatonin was injected two hours before 2 mg/kg cisplatin injection. Mice were euthanized 24 hours after the last injection. (B) Body and ovary weight from female mice of each treatment group at the time of euthanasia. (C) A total number of follicles per treatment group. (D) Representative histological image with H&E staining showing healthy and abnormal follicles (green arrow, primordial follicles; blue arrow, secondary follicles; black arrow, pyknotic granulosa cells). Primary follicles (Pm), secondary follicles (sec), and corpus lutem (CL). Scale bar = 150 μm. (E) Counting of total primordial, primary, and secondary follicles in the ovary from each treatment group. (F) Representative histological images with H&E staining of ovarian tissue treated with 6-hydroxymelatonin (6-OH-Mel) (1 or 5 μM) with or without cisplatin (1 or 4 μM) for 96 hours culture in vitro. Green arrows indicate oocytes with condensed nuclei. Blue arrows indicate pyknotic granulosa cells in growing follicles. Sacle bar = 50 μm (G) Counting of the total, primordial, primary, and secondary follicles from ovaries cultured with 6-OH-Mel with or without cisplatin. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; n.s., not significant.
Cisplatin induces damage of intracellular organelles in the oocyte of primordial follicles as well as granulosa cells of growing follicles
To detail the effects of cisplatin on the organelles of the ovary from the mouse at the organelle level, PD5 CD-1 female mouse were injected once at 4 pm with 5 mg/kg cisplatin or DPBS and euthanized at 10 am the next day after 18 hours treatment of cisplatin or DPBS. Mice did not show any noticeable health problems within 18 hours with the injection of 5 mg/kg cisplatin. Ovarian tissue from mice injected with 5 mg/kg cisplatin or DPBS for 18 hours were fixed for histological analysis (Figure 5A) and transmission electron microscopy (TEM) (Figure 5B). Pyknotic oocytes of primordial follicles (green arrows) and damaged graulosa cells of secondary follicles (pink arrows) were observed within 18 hours of 5 mg/kg cisplatin treatment. However, pregranulosa cells surrounding oocytes of primordial follicles and oocytes of secondary follicles appeared healthy and undamaged (Figure 5A). Accordingly, TEM images displayed that the healthy primordial follicle contains a healthy oocyte surrounded by pre-granulosa cells in the ovary from DPBS-treated mice (Figure 5B, a). The cytoplasm of the oocyte contains healthy mitochondria surrounding the nucleus (Figure 5B, a). The secondary follicle consists of a healthy oocyte that contains heterochromatin with multiple mitochondria and a multilayer of granulosa cells (Figure 5B, b). The mitochondria present spherical or tubular shapes of various sizes and aggregate inside the cytoplasm (Figure 5B, c). In healthy oocytes, other organelles including the Golgi apparatus and lysosomes are observed around the mitochondria (Figure 5B, d). Once the ovary is treated with cisplatin, the nucleus of the oocyte starts to condense (Figure 5B, e), lose its structure and organelles, to be filled with lysosomal vesicles (Figure 5B, f and j), and eventually undergo structural corruption (Figure 5B, g, h, k, and l). In addition, some pre-granulosa cells display accumulation of many lipid droplets (Figure 5B, e and i). However, cisplatin induced death in some granulosa cells of growing follicles (Figure 5B, m and n, dark round cells), but it did not damage the oocytes of growing follicles (Figure 5B, m and n). These granulosa cells contain condensed nuclei, disordered organelle structures, and many lysosomal vesicles, etc (Figure 5B, m and o). The intracellular organelles in oocytes were destroyed, displaying multiple lysosomal vesicles (Figure 5B, o) and loss of the ultrastructure of mitochondrial cristae (Figure 5B, p). This data suggests that cisplatin devastates the oocyte through destruction of intracellular organelles rather than inducing activation of primordial follicles.
Figure 5. Cisplatin induces damage of intracellular organelles in the oocyte of primordial follicles as well as granulosa cells of growing follicles.
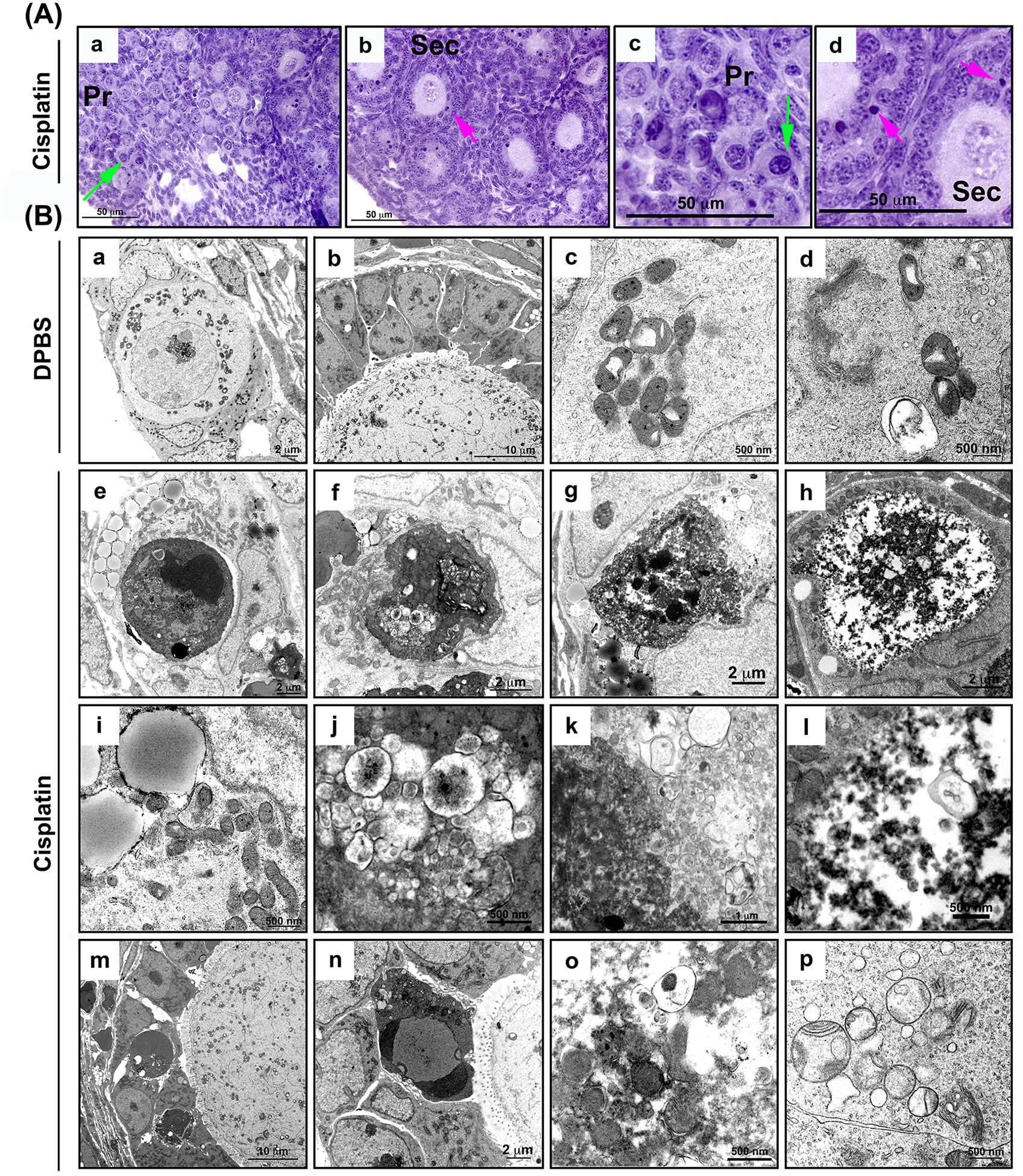
(A) Represetative histological images with H&E staining of ovarian sections from mice treated with 5 mg/kg cisplatin for 18 hours. Pyknotic oocytes of primordial follicles (green arrows) and graulosa cells of secondary follicles (pink arrows) are marked (a-d). (Primordial follicle (Pr) and secondary follicle (sec). Scale bars are labeled. (B) Ovaries from PD5 mice injected with DPBS for 18 hours display primordial follicles with heathy organelles and pregranulosa cells (a), multiple layers of healthy granulosa cells in secondary follicle (b), normal mitochondria, lysosomes, golgi apparatus observed in the oocyte of healthy primordial follicles (c-d). (e-p) TEM images of ovarian cells from mice treated with 5 mg/kg cisplatin for 18 hours. Ovaries display condensed (e) and destructed oocyte of primordial follicles (f, g, h). Lipid droplets observed in primordial follicles in the cisplatin group (e, pregranulosa cells and i, oocyte cytoplasm). High-magnification images of oocyte damage in primordial follicles (j-l). Damaged granulosa cells in secondary follicles (m-n). (o-p) Damaged organelles such as mitochondria and lysosomes in the oocytes of primordial follicles. Scale bars are labeled.
Discussion
Previous studies suggested primordial follicles in the ovary undergo activation when mice are treated daily with 2 mg/kg cisplatin for 15 days, and melatonin prevents activation of primordial follicles following treatment with the dose of cisplatin for 15 days (26, 27). It has been theorized that cotinuouse injection of cisplatin for 15 days activates PI3K pathways in dormant oocytes of primordial follicles. These oocytes express activation markers p-AKT/p-PTEN/FOXO3A following treatment with 2 mg/kg cisplatin for 15 days. In addition, the pretreatment of 30 mg/kg melatonin appeared to suppress cisplatin-induced activation of p-AKT/p-PTEN/FOXO3A in the oocyte of primordial follicles. However, other previous studies including the current study from our group show that one or multiple injections of 5 mg/kg cisplatin induces oocyte death in primordial follicles through the induction of apoptotic pathways (5, 10, 12, 13, 35–37). A major difference between previous studies demonstrating oocyte death and the studies demonstrating follicle activation was the frequency and dosage of cisplatin. Thus, the continuous daily injection with 2 mg/kg cisplatin for 15 days used in previous activation studies with various time points for analysis was used in our study to clarify the effects of cisplatin in oocytes of primordial follicles of the ovaries from CD-1 female mice.
This study demonstrates that cisplatin induces the death of oocytes in primordial follicles following either short-term treatment with 5 mg/kg cisplatin or continuous injections with 2 mg/kg cisplatin once a day for 15 days. The detrimental effects of the condition with continuous injections of 2 mg/kg cisplatin once a day for 15 days were the same as those of short-term treatment of 5 mg/kg cisplatin. Continuous treatment with 2 mg/kg cisplatin decreased the total number of ovarian follicles by destroying dormant and growing follicles with no hallmarks of activation in the oocytes of primordial follicles. These data support our belief that cisplatin is prone to exert toxic effects on the ovarian reserve, as evidenced by the total number of follicles, the number of each class of follicle, and the histological images of mouse ovaries at the point of time-dependent harvest or at the end of in vitro culture. Histological image also showed a decrease in corpora lutea following continuous injections of 2 mg/kg cisplatin once a day for 15 days. The treatment depleted growing follicles by destroying granulosa cells. Furthermore, our results demonstrate that melatonin and its metabolite 6-OH-Mel could not prevent follicle loss in mouse ovaries in vivo and in vitro, respectively. Therefore, the results observed in our study demonstrate that cisplatin is indeed heavily detrimental and induces loss of follicles in the ovarian reserve.
Interestingly, while there was an increase in the total number of antral follicles in the 2 mg/kg cisplatin+10 d group, there was no difference in total numbers of primary and secondary follicles between the 2 mg/kg cisplatin and 2 mg/kg cisplatin+10 d groups. In addition, most of the follicles in 2 mg/kg cisplatin+10 d group had healthy granulosa cells, supporting the notion that healthy granulosa cells in surviving follicles after ceasing cisplatin injection replaced damaged granulosa cell in the 2 mg/kg cisplatin group over the 10 day time period. The increased number of antral follicles supports the growth of surviving secondary follicles. As anticipated, there was no prominent primordial follicle recruitment by a decrease in AMH due to damage to granulosa cells at the end of the 15-day injection period with an additional 10 days. Because the activation of primordial to primary and primordial to secondary in adult cortical primordial follicles of mice is expected to take a minimum of 7–9 days and 23–24 days, respectively (38), the timeframe utilized in this study may not be enough to fully observe follicle activation via decrease of AMH. In addition, it is difficult to estimate the magnitude of change in AMH serum levels needed to recruit a certain number of primordial follicles. Nonetheless, the absence of prominent primary clearly suggests that 2 mg/kg cisplatin does not stimulate primordial follicle activation to primary follicles.
The TEM images support our assertion that cisplatin directly affects the oocytes of primordial follicle and granulosa cells of growing follicles, reinforcing the sensitivity of these cells to the toxicity of cisplatin. In this study, ovaries from PD5 mouse treated once with 5 mg/kg cisplatin were used for TEM analysis to determine the deleterious effects of cisplatin, examining the ultrastructure of damaged organelles. Healthy follicles contain organelles such as mitochondria and the Golgi apparatus. Mitochondria maintain intact cristae structure and aggregate in the cytoplasm. However, the ovary from cisplatin-treated mice showed unhealthy dark-colored follicles, loss of fine ultrastructure, condensation of chromatin aggregates in the nuclei, loss of cellular components, loss of mitochondrial cristae, and formation of gigantic lysosomal vesicles containing mitochondria and other organelles in the oocytes of primordial follicles. The images of primordial follicles reveal that these oocytes are sensitive to harmful cisplatin, resulting in organelle destruction. Meanwhile, the oocytes of secondary follicles do not exhibit any damage from exogenous cisplatin that is administered at the same dosage as the one that damages oocytes of primordial follicles. Therefore, these TEM images directly support the notion of a direct toxic effect of cisplatin on the oocytes of primordial follicles.
Multiple studies have indicated that melatonin grants protective effects on ovarian follicles against the toxicity induced by gonadotoxic agents such as palmitic acid (PA), Di (2-Ethylhexyl) phthalate, and nicotine (39–42). Melatonin’s proposed protective benefits are based on documentation of inhibition of ER stress, reduction of reactive oxygen species (ROS) levels, and inhibition of apoptosis. Previous literature (26, 27) indicated that melatonin significantly decreased the cisplatin-mediated phosphorylation of PTEN, AKT, and translocation of FOXO3a from the nucleus to cytoplasm in primordial oocytes, blocking the activation of primordial follicles in the mouse ovary. However, our current in vivo and in vitro data show that pretreatment for two hours with 30 mg/kg melatonin followed by treatment with 2 mg/kg cisplatin for 15 days did not exhibit any protective effect on follicles in the ovarian reserve and granulosa cells of growing follicles in mice. An interesting difference between previous studies and our current study is the detection of p-PTEN and p-AKT in the ovaries treated with 2 mg/kg cisplatin for 15 days. Although p-PTEN and p-AKT expression appeared to be highly elevated using immunoblotting analysis in both the previous study (26, 27) and our study, the data presented in this study shows that p-AKT and p-PTEN were detected in other cell types such as granulosa cells rather than oocytes of primordial follicles. In line with these observations, the oocytes of primordial follicles from mouse ovaries treated with 2 mg/kg cisplatin for 15 days highly expressed γH2AX, a marker for DNA double-strand break (Fig. 2C), suggesting that oocytes of primordial follicles are highly sensitive to gonadotoxic agents.
Nonetheless, as shown in our study, the fact is that 2 mg/kg cisplatin for 15 consecutive injections significantly affects the health of mouse and is near the lethal dose. The experimental design of this project for the dose and duration was based on previous papers that were published and accepted broadly in the scientific community (26, 27). The papers stated that 5 mg/kg of cisplatin induces apoptosis in follicles, while continuous daily injection of 2mg/kg cisplatin for 15 days induces activation. Our purpose for this study was to investigate whether a high or low dose of cisplatin differentially affects the ovarian reserve. Nonetheless, mice may be at higher risk for cisplatin toxicity because the peak plasma concentration of cisplatin is 2.4–20 times greater in mice than in humans although the half-life of the drug is six-times shorter in mice than human according to previously published toxicity studies (43). Thus, the protocol of cisplatin injection affects the physiology of mice (31).
Therefore, clarifying the mechanism by which cisplatin destroys the oocytes of primordial follicles and granulosa cells of growing follicles are imperative for the discovery and development of adjuvant therapies to protect the ovarian reserve against cisplatin. This study provides a stepping stone for viable adjuvant therapy for future fertility preservation in cancer patients.
Supplementary Material
Acknowledgements
This work was supported by start-up funds from the University of Nebraska Medical Center (S.Y.K), National Institutes of Health (R01HD096042-02), and the University of Nebraska Medical Center Core Facility Grant Program for New Users (S.Y.K). The authors would like to thank Tom Bargar and Nicholas Conoan of the Electron Microscopy Core Facility (EMCF) at the University of Nebraska Medical Center for their technical assistance. The EMCF is supported by state funds from the Nebraska Research Initiative (NRI) and the University of Nebraska Foundation, and institutionally by the Office of the Vice-Chancellor for Research.
Nonstandard Abbreviations
- AMH
Anti-Mullerian hormone
- BSA
Bovine serum albumin
- CK1
Casein kinase 1
- CCSs
Childhood cancers survivors
- CL
Corpora Lutea
- DAB
3’3-Diaminobenzindine
- 6-OH-Mel
6-hydroxymelatonin
- ITS
Insuin-transferrin-selenium
- ooPF
oocyte of primordial follicle
- PA
Palmitic acid
- PD
Post-natal day
- Pm
Primary follicle
- POI
Premature ovarian insufficiency
- Pr
Primordial follicle
- Sec
secondary follicle
- TEM
Transmission electron microscopy
Footnotes
Conflict of Interest
The authors declare no competing interests.
References
- 1.Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr Rev. 2019;40(3):711–67. [DOI] [PubMed] [Google Scholar]
- 2.Chemaitilly W, Sklar CA. Childhood Cancer Treatments and Associated Endocrine Late Effects: A Concise Guide for the Pediatric Endocrinologist. Horm Res Paediatr. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Waguespack SG. Thyroid Sequelae of Pediatric Cancer Therapy. Horm Res Paediatr. 2019;91(2):104–17. [DOI] [PubMed] [Google Scholar]
- 4.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–44. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, et al. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ. 2019;26(3):502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SY, Cho GJ, Davis JS. Consequences of chemotherapeutic agents on primordial follicles and future clinical applications. Obstet Gynecol Sci. 2019;62(6):382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–96. [DOI] [PubMed] [Google Scholar]
- 8.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–84. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–7. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20(8):987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen QN, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, et al. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018;9(6):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen QN, Zerafa N, Liew SH, Findlay JK, Hickey M, Hutt KJ. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod. 2019;25(8):433–44. [DOI] [PubMed] [Google Scholar]
- 13.Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15(10):1179–85. [DOI] [PubMed] [Google Scholar]
- 14.Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343(6170):533–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutandin D, Osterburg C, Srivastav RK, Sumyk M, Kehrloesser S, Gebel J, et al. Quality control in oocytes by p63 is based on a spring-loaded activation mechanism on the molecular and cellular level. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutsch GB, Zielonka EM, Coutandin D, Weber TA, Schafer B, Hannewald J, et al. DNA damage in oocytes induces a switch of the quality control factor TAp63alpha from dimer to tetramer. Cell. 2011;144(4):566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehrloesser S, Tuppi M, Dotsch V. CHK2 sets the stage for CK1 in oocyte quality control. Cell Death Differ. 2018;25(6):1007–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444(7119):624–8. [DOI] [PubMed] [Google Scholar]
- 19.Roness H, Spector I, Leichtmann-Bardoogo Y, Savino AM, Dereh-Haim S, Meirow D. Pharmacological administration of recombinant human AMH rescues ovarian reserve and preserves fertility in a mouse model of chemotherapy, without interfering with anti-tumoural effects. J Assist Reprod Genet. 2019;36(9):1793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepin D, Sabatini ME, Donahoe PK. Mullerian inhibiting substance/anti-Mullerian hormone as a fertility preservation agent. Curr Opin Endocrinol Diabetes Obes. 2018;25(6):399–405. [DOI] [PubMed] [Google Scholar]
- 21.Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, et al. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci U S A. 2017;114(9):E1688–E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonigo C, Beau I, Grynberg M, Binart N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2019;33(1):1278–87. [DOI] [PubMed] [Google Scholar]
- 23.Deutsch GB, Zielonka EM, Coutandin D, Dotsch V. Quality control in oocytes: domain-domain interactions regulate the activity of p63. Cell Cycle. 2011;10(12):1884–5. [DOI] [PubMed] [Google Scholar]
- 24.Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25(3):261–9. [DOI] [PubMed] [Google Scholar]
- 25.Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell. 2012;48(3):343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang H, Na Y, Hong K, Lee S, Moon S, Cho M, et al. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27(Kip1) promoter in primordial follicles. J Pineal Res. 2017;63(3). [DOI] [PubMed] [Google Scholar]
- 27.Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res. 2016;60(3):336–47. [DOI] [PubMed] [Google Scholar]
- 28.Barberino RS, Menezes VG, Ribeiro A, Palheta RC Jr., Jiang X, Smitz JEJ, et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod. 2017;96(6):1244–55. [DOI] [PubMed] [Google Scholar]
- 29.Haghi-Aminjan H, Asghari MH, Farhood B, Rahimifard M, Hashemi Goradel N, Abdollahi M. The role of melatonin on chemotherapy-induced reproductive toxicity. J Pharm Pharmacol. 2018;70(3):291–306. [DOI] [PubMed] [Google Scholar]
- 30.JG DEA, Serra LSM, Lauand L, Kuckelhaus SAS, Sampaio ALL. Protective Effect of Melatonin on Cisplatin-induced Ototoxicity in Rats. Anticancer Res. 2019;39(5):2453–8. [DOI] [PubMed] [Google Scholar]
- 31.Perse M, Veceric-Haler Z. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. Biomed Res Int. 2018;2018:1462802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298(1):149–54. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Ebbert K, Cordeiro MH, Romero M, Zhu J, Serna VA, et al. Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology. 2015;156(4):1464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SY, Ebbert K, Cordeiro MH, Romero MM, Whelan KA, Suarez AA, et al. Constitutive Activation of PI3K in Oocyte Induces Ovarian Granulosa Cell Tumors. Cancer Res. 2016;76(13):3851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan S, Lopes F, Gourley C, Anderson RA, Spears N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS One. 2013;8(7):e70117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart E, Lopes F, Rice S, Nagy B, Anderson RA, Mitchell RT, et al. Chemotherapy drugs cyclophosphamide, cisplatin and doxorubicin induce germ cell loss in an in vitro model of the prepubertal testis. Sci Rep. 2018;8(1):1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr JB, Hutt KJ, Cook M, Speed TP, Strasser A, Findlay JK, et al. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat Med. 2012;18(8):1170–2; author reply 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet. 2014;23(4):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Lei L, Wen D, Yang L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J Ovarian Res. 2019;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Zhang Z, Wang J, Lv D, Zhu T, Wang F, et al. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J Pineal Res. 2019;66(3):e12550. [DOI] [PubMed] [Google Scholar]
- 41.Sun ZY, Zhang P, Wang JJ, Liu JC, Li L, Shen W, et al. Melatonin alleviates meiotic defects in fetal mouse oocytes induced by Di (2-ethylhexyl) phthalate in vitro. Aging (Albany NY). 2018;10(12):4175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang YF, Sun XF, Han ZL, Li L, Ge W, Zhao Y, et al. Protective effects of melatonin against nicotine-induced disorder of mouse early folliculogenesis. Aging (Albany NY). 2018;10(3):463–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Hennik MB, van der Vijgh WJ, Klein I, Elferink F, Vermorken JB, Winograd B, et al. Comparative pharmacokinetics of cisplatin and three analogues in mice and humans. Cancer Res. 1987;47(23):6297–301. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


