Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus responsible for coronavirus disease 2019 (COVID‐19), is associated with high incidence of multiorgan dysfunction and death. Angiotensin‐converting enzyme 2 (ACE2), which facilitates SARS‐CoV‐2 host cell entry, may be impacted by angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), two commonly used antihypertensive classes. In a multicenter, international randomized controlled trial that began enrollment on March 31, 2020, participants are randomized to continuation vs withdrawal of their long‐term outpatient ACEI or ARB upon hospitalization with COVID‐19. The primary outcome is a hierarchical global rank score incorporating time to death, duration of mechanical ventilation, duration of renal replacement or vasopressor therapy, and multiorgan dysfunction severity. Approval for the study has been obtained from the Institutional Review Board of each participating institution, and all participants will provide informed consent. A data safety monitoring board has been assembled to provide independent oversight of the project.
Keywords: angiotensin receptor blocker, angiotensin‐converting enzyme inhibitor, angiotensin‐converting enzyme inhibitor 2, clinical trial, coronavirus, COVID‐19, hypertension
1. INTRODUCTION
Since its emergence in December 2019, the coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has had a devastating global health impact. 1 COVID‐19 is associated with a high incidence of multiorgan dysfunction and mortality. 2 , 3 Accordingly, there has been an extraordinary response by the international research community to quickly develop trials to evaluate potential disease‐modifying interventions in COVID‐19.
Early in the COVID‐19 pandemic, several reports described hypertension as a risk factor for SARS‐CoV‐2 infection and severity. 4 Angiotensin‐converting enzyme 2 (ACE2), a key counterregulatory component of the renin‐angiotensin system (Figure 1), plays an important role in hypertension pathophysiology and also facilitates SARS‐CoV‐2 host cell entry. 5 ACE2 activity and expression have been implicated as potential contributors to SARS‐CoV‐2 infection and severity. 4 Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), two of the most commonly used antihypertensive classes, 6 may increase ACE2 expression. 4 Consequently, it was speculated that ACEIs and ARBs could worsen the risk of COVID‐19 and COVID‐19‐related adverse outcomes, 7 , 8 , 9 , 10 resulting in widespread media coverage and possibly in empiric discontinuation of these medications. However, there is also evidence suggesting that ACE2 overexpression ameliorates lung injury associated with COVID‐19, 11 , 12 and thus, ACEIs and ARBs could reduce adverse outcomes in patients with this infection. 4 Thus, it remains unclear whether ACEIs and ARBs are beneficial or harmful in patients with COVID‐19.
Figure 1.
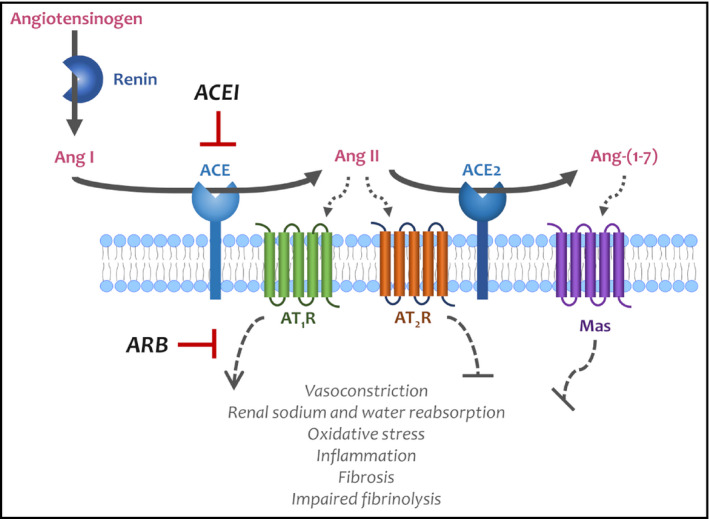
The counterregulatory role of ACE2 in the renin‐angiotensin system. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase ACE2 levels. This effect is speculated to increase the risk of SARS‐CoV‐2 host cell entry but may also have important anti‐inflammatory effects
Given the high prevalence of ACEI and ARB use in the general population and the potential for harm by inappropriate discontinuation of these medications due to their cardio‐ and reno‐protective effects, 6 it is critical to better understand the appropriate acute management of patients with COVID‐19 who use ACEIs and ARBs for the long‐term management of chronic conditions. Thus, we aimed to perform a randomized controlled trial to evaluate the effect of continuation compared with discontinuation of ACEIs and ARBs on hospitalization‐related outcomes in patients admitted with COVID‐19. We developed a hierarchical composite end point of outcomes with public health, clinical, and patient‐centered significance using a global rank score.
2. METHODS AND ANALYSIS
2.1. Overview of the trial design
Figure 2 illustrates the study structure and design. The Randomized Elimination or ProLongation of ACEIs and ARBs in COronaVIrus Disease 2019 trial (REPLACE COVID: NCT04338009) is a prospective randomized open‐label blinded end point (PROBE) trial 13 of continuation vs discontinuation of ACEI/ARB therapy in patients admitted to the hospital with COVID‐19. The primary end point is a novel hierarchical global rank score including time to death, duration of mechanical ventilation, duration of renal replacement therapy or vasopressor therapy, and measures of multiorgan dysfunction.
Figure 2.
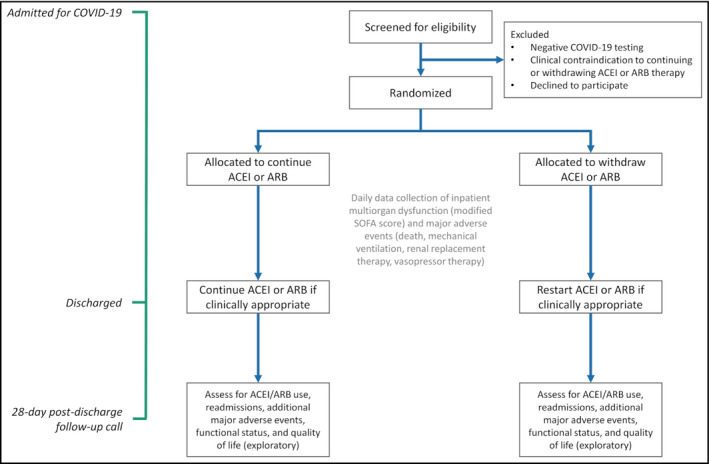
Study overview and design
Participants are being recruited upon admission to the hospital with COVID‐19 at participating centers in the United States, Canada, Mexico, Argentina, Peru, Bolivia, and Sweden (Figure 3). The trial was approved by the Institutional Review Board or the Ethics Committee of each participating center, or via reliance agreements with the Institutional Review Board at the University of Pennsylvania, which is serving as the central Institutional Review Board. All participants provided informed consent either in written form or electronically. Enrollment started on March 31, 2020, and will continue until 152 participants have been enrolled.
Figure 3.
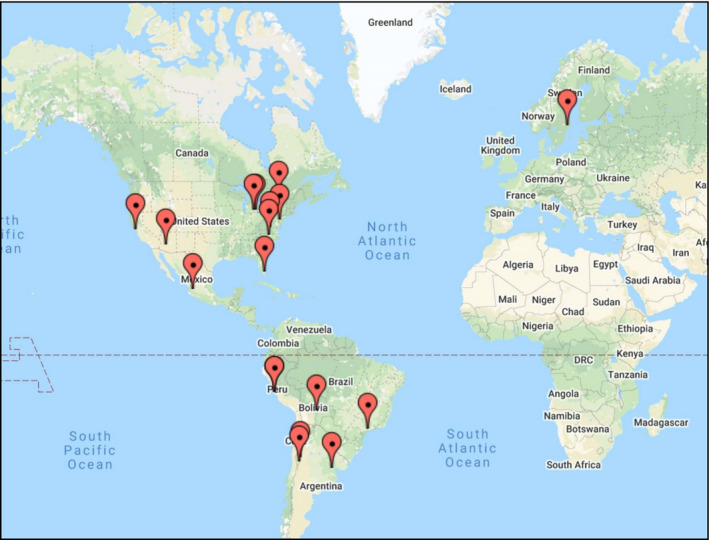
Geographic distribution of study sites enrolling participants in the REPLACE COVID trial. As of August 10, 2020, 136 participants have been enrolled across sites in the United States, Canada, Mexico, Peru, Argentina, Bolivia, and Sweden. Additional sites in the United States and Brazil are initiating enrollment
Participants are randomized to continuation or withdrawal of their outpatient ACEI or ARB for the duration of their hospitalization. Candidates are excluded if they have a major contraindication to either continuing or withdrawing their ACEI or ARB. Participants are followed until the end of their hospitalization and are contacted 28 days following discharge to assess for any changes in their clinical status after discharge.
2.2. Hypothesis
Based on currently available information, there is clinical equipoise regarding the effect of continuation vs discontinuation of ACEIs and ARBs in the setting of COVID‐19. In some experimental models, ACEIs and ARBs increase ACE2 expression in several organs. 14 , 15 , 16 , 17 This could potentially lead to increased SARS‐CoV‐2 virulence, increasing the risk of multiorgan dysfunction and death. However, these findings are not consistent across studies 18 , 19 , 20 and have not been corroborated in humans. 21 , 22 Alternatively, animal models of SARS‐CoV‐1 infection (a virus highly related to SARS‐CoV‐2) suggest that overexpression of ACE2 protects against lung injury associated with these infections. 11 , 12 Thus, ACEIs and ARBs may improve mechanisms of host defense or hyperinflammation, ultimately reducing organ injury and providing direct renal and cardiac protective benefits. 12 Observational studies evaluating the association of ACEI and ARB use with COVID‐19‐related outcomes have demonstrated mixed results. These studies are highly prone to confounding by indication for continuation or withdrawal of these medications based on severity of presentation. 23 Additionally, these studies have not fully addressed important issues with immortal time bias and collider bias. 24 A randomized controlled trial is necessary to adequately address these important limitations.
2.3. Participants
Patients are eligible for inclusion in the REPLACE COVID trial if they are age 18 years or older at the time of their index hospitalization with a clinical presentation consistent with COVID‐19. Individuals are excluded if they have negative SARS‐CoV‐2 testing (among persons under investigation based on clinical suspicion) or clinical contraindications to continuing or withdrawing ACEI or ARB therapy including (1) systolic blood pressure <100 mm Hg; (2) systolic blood pressure >180 mm Hg or >160 if unable to substitute the ACEI or ARB with another antihypertensive class; (3) diastolic blood pressure >110 mm Hg; (4) history of heart failure with unknown left ventricular ejection fraction (LVEF) or known LVEF <40%; (5) serum potassium >5 mEq/L; (6) known pregnancy or breastfeeding; (7) estimated glomerular filtration rate <30 mL/min/1.73 m2; (8) acute kidney injury defined as ≥100% increase in creatinine (and a creatinine >2 mg/dL) compared with the most recent creatinine in the past 6 months, if available; (9) urine protein‐to‐creatinine ratio >3 g/g or >3 g/24‐hours within the past year; or (10) ongoing treatment with aliskiren or sacubitril‐valsartan. Prisoners are also excluded from the trial. Potential participants are typically approached in the first 48 hours of admission unless they develop COVID‐19 during an admission for another cause.
2.4. Intervention
The randomized intervention is the continuation compared with discontinuation of ACEI or ARB therapy (at the dose previously prescribed for patients during their routine care) for the duration of the hospitalization. Among participants randomized to continue these agents, clinicians are encouraged to continue the randomized treatment but are permitted to change the dose of ACEI or ARB or to discontinue these medications if a compelling clinical reason is identified, such as hypotension, hyperkalemia, or significant acute kidney injury. If a participant is prescribed both an ACEI and an ARB prior to admission (anticipated to be rare), that individual will be randomized to continuation of one or both medications, at the clinician's discretion, or discontinuation of both medications. In all participants randomized to discontinuation, treating clinicians are reminded about the medication discontinuation upon discharge and are prompted to consider reinitiating the medication at that time, if clinically appropriate.
2.5. Randomization and blinding
Participants are randomized after providing informed consent either in writing or electronically. We are performing stratified blocked randomization with randomly permuted blocks based on site, sex, and age, given the strong impact of these factors on outcomes in COVID‐19. 3 Each block contains an equal number of allocations to each arm. When the patient is randomized, the study team communicates the randomized treatment strategy to the primary clinician and any relevant consultants.
As in other PROBE trials, 13 treatment allocation is open, with blinding incorporated instead at the level of end point adjudication. A clinician panel at each site is appointed to perform blinded adjudications of the outcome events. Adjudications are performed using a standardized approach to masking patient records so that the clinicians are fully blinded to the randomization arm but are able to assess other key aspects of the participants’ hospitalizations.
2.6. Primary outcome
The primary end point of the trial is a hierarchical global rank score, illustrated in Figure 4. The global rank score is a nonparametric ranked outcome that will be determined at the time of discharge or death. The hierarchical end point approach has been used in several randomized controlled trials to facilitate evaluation of composite outcomes of binary and continuous findings accounting for both the importance of and appropriate censorship for death. 25 , 26 , 27 , 28 , 29 An important benefit of the hierarchical end point is that it provides substantially higher statistical power for any given sample size compared with other commonly used approaches, such as 28‐day ventilator‐free days, time to death, and the World Health Organization COVID‐19 ordinal end point. 30 , 31 , 32 , 33 , 34 The outcome of each patient in the REPLACE COVID trial is ranked against all other participants from worst to best by increasing values of (1) days to death during the hospitalization (ordered lowest to highest); followed by (2) days on invasive mechanical ventilation or extracorporeal membrane oxygenation (ordered highest to lowest); followed by (3) days on renal replacement therapy or inotropic/vasopressor therapy (ordered highest to lowest); followed by (4) area under the curve of a modified Sequential Organ Failure Assessment (SOFA) score.
Figure 4.
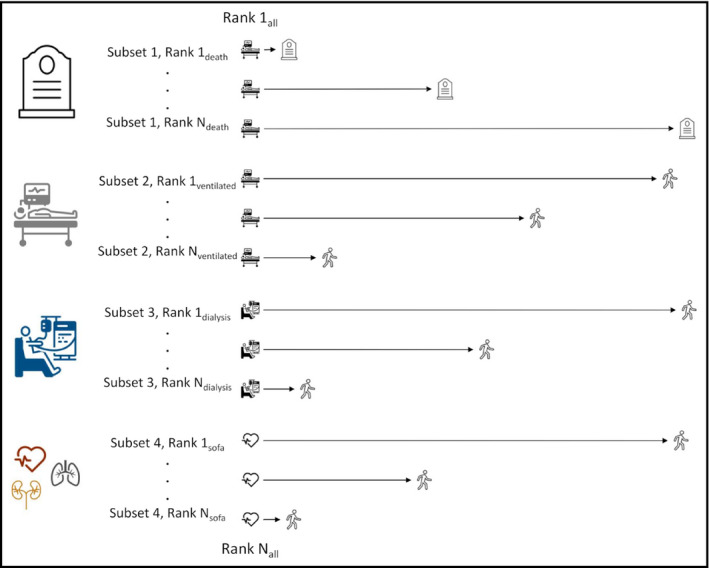
The REPLACE COVID global rank score. Participants are ranked from worst to best outcomes by (1) days in hospital to death; (2) days on invasive mechanical ventilation or extracorporeal membrane oxygenation; (3) days on renal replacement therapy or inotropic/vasopressor therapy; and (4) area under the curve of a modified SOFA score
The modified SOFA score includes the cardiac, respiratory, coagulation, and renal domains of the SOFA score (Table S1). A modified SOFA score is used, rather than the full SOFA score, as the last tier of the global rank score. This decision confers several advantages: (1) The cardiac, renal, respiratory, and coagulation systems are those most likely impacted by our randomized intervention 5 , 14 , 15 , 16 , 17 , 35 ; (2) these SOFA components can be easily and reliably adjudicated using electronic health record review, minimizing workload on the clinical team and maximizing the pragmatic nature of the trial; and (3) the nervous system (Glasgow comma scale) and liver (serum bilirubin) components of the SOFA score are not acquired daily on a routine basis in hospitalized patients in non‐ICU settings. For the respiratory component of the SOFA score, we are applying a modified score used in settings where arterial oxygen saturation is not consistently available, 36 which uses peripheral capillary oxygen saturation instead of arterial oxygen saturation. Use of the modified SOFA score allows all patients in the trial to be compared, even when no major adverse events occur. We will weight the modified SOFA score to account for duration of hospitalization.
In summary, we selected a hierarchical global rank score due to its ability to incorporate biomarkers and important clinical events into a combined, readily interpretable, and reproducible metric. 26 , 27 Our proposed global rank score incorporates patient‐centered factors and health resource utilization, while better resolving the time course of COVID‐19‐related events and with higher statistical power for any given sample size compared with incorporating each individual factor. 30 , 31 , 32 , 33 , 34
2.7. Secondary outcomes
Secondary end points are (1) time to all‐cause death; (2) length of hospital stay; (3) length of intensive care unit stay, invasive mechanical ventilation, or extracorporeal membrane oxygenation; and (4) area under the curve of the modified SOFA score, weighted to account for death and duration of hospitalization. Exploratory end points include (1) intensive care unit admission or respiratory failure requiring mechanical ventilation; (2) hypotension requiring vasopressors, inotropes, or mechanical hemodynamic support such as a ventricular assist device or intra‐aortic balloon pump; (3) number of 28‐day ventilator‐free days; (4) maximal change in NT‐proB‐type natriuretic peptide from baseline (when available); (5) change in serum creatinine between baseline and discharge or time of death; (6) acute kidney injury during hospitalization (defined as Kidney Disease Improving Global Outcomes stage 2 or higher). 37 Additional exploratory outcomes will be ascertained by a follow‐up call 28 days after discharge from the index hospitalization and will include (1) readmissions, (2) major adverse cardiac events, (3) functional status, and (4) quality of life.
2.8. Statistical analysis plan
The global rank score is a nonparametric ranked outcome. 25 , 26 , 27 The primary analyses will be performed on an intent‐to‐treat basis using the total number of participants randomized. Initial descriptive estimates of all measures will be generated for study participants by randomization arm. Primary assessment of the treatment effect will be performed using the nonparametric Wilcoxon rank‐sum test. This will be followed by a more comprehensive linear regression analysis allowing for assessments of the treatment effect while controlling for effects of age, sex, race/ethnicity, history of preexisting heart failure, history of preexisting chronic lung disease, and ACEI vs ARB therapy at baseline. 3 , 38 , 39 , 40 We will utilize nonparametric methods or consider distribution‐stabilizing transformations, as appropriate. The models will include data from participants who drop out. 41 , 42 , 43 Model assumptions will be examined (eg, QQ plots to assess normally distributed residuals for valid Wald tests). Secondary and exploratory time‐to‐event outcomes will be evaluated using Cox proportional hazards models. The proportional hazards assumption will be assessed via weighted versions of Kaplan‐Meier curves using log‐log plots and graphical displays based on the Schoenfeld and scaled Schoenfeld residuals, and violations of the proportional hazards assumption will be addressed with a time‐interaction term. 44 In exploratory analyses, we will assess for effect modification by sex, age, 3 , 38 race, 39 presence of preexisting heart failure or left ventricular dysfunction, baseline ACEI vs ARB therapy, 40 chronic kidney disease, diabetes, and BMI.
We will make every possible effort to minimize missing data and ensure final assessments for participants opting to discontinue study participation. Missing data, however, are an inevitable problem in any study. The mechanism for missingness will be evaluated prior to implementing methodology intended to minimize bias from missing data, such as multiple imputation. 45 We anticipate that <5% of randomized participants will have missing data in the components required to compute the study outcomes.
There is a possibility of systematic bias in the outcomes for those who withdraw from the study or crossover treatment arms. In participants randomized to continuation of these drugs, clinicians will be encouraged to continue the randomized treatment but will be allowed to change the dose of ACEI/ARB or discontinue these medications if compelling clinical reasons are identified. As noted above, in participants randomized to discontinuation, treating clinicians will be reminded about the medication discontinuation upon discharge and will be prompted to consider reinitiating the medication at that time if appropriate, per the clinician's discretion. Sensitivity analyses will be performed on a modified intent‐to‐treat basis, including only data obtained during the period of time in which participants remain on the randomized treatment strategy.
In order to summarize the SOFA score over the course of the hospitalization, we will calculate the area under the curve of the modified SOFA (AUC SOFA) from daily measurements. The AUC SOFA will be ranked from highest to lowest so that lower ranks represent worse outcomes in alignment with the rest of the global rank score.
2.9. Sample size
Assuming feasible distributions of patients across each of the primary end point hierarchies based on the available published evidence at the time of trial inception, 1 , 2 , 3 we performed 10 000 Monte Carlo simulations of rank distributions of 152 patients and determined that there will be >80% power to observe a minimal significant difference of 30% in rank scores between the treatment arms, allowing for interim analyses at 50% of enrollment. 46 , 47 Regarding secondary end points, we will have 90% power to detect a 2‐day difference in length of ICU stay and length of hospital stay assuming a standard deviation of 2 days based on data from initial reports of these outcomes in COVID‐19. 1 , 2 , 3 , 32 Power calculations were performed using python and PASS16. 48
2.10. Data management
The University of Pennsylvania is the Data Coordinating Center (DCC) for the study. The DCC is overseeing randomization, data entry, and data safety monitoring board (DSMB) meetings. The data are being collected using ad hoc electronic case report forms. Data capture and storage are being performed within the framework of the Research Electronic Data Capture (REDCap) project.
2.11. Data Safety Monitoring Board
A DSMB was assembled to provide independent oversight of the project. The DSMB is responsible for assessing (1) baseline comparability between groups; (2) participant accrual rate and retention; (3) data quality with special emphasis on eligibility data; and (4) patient safety. The DSMB will make recommendations regarding study continuation, protocol modification, and review of additional data. The DSMB reviewed planned interim analyses that were performed when the study reached 50% of enrollment. The DSMB also reviews new and emerging data related to the proposed trial that would potentially affect the continuation of the trial.
2.12. Ethics and protection of participants
All partcipants will be adults able to give informed consent or in whom informed consent can be obtained by a legally authorized representative. Persons will be enrolled regardless of sex, race, or ethnicity, aiming to assure adequate representation of women and African Americans. Vulnerable populations such as children, pregnant women, and prisoners will not be enrolled. The risks to study participants are related to the clinical equipoise itself: It is possible that one strategy is better than the other, but at the moment this clinical equipoise is not solvable based on clinical grounds or intuition. Furthermore, after the initial randomized strategy, our trial allows for altering the course of therapy based on clinical grounds that clearly favor one strategy based on clinical assessment as per the treating clinician (such as hypotension, which would prompt discontinuation of ACEIs/ARBs among patients randomized to continued therapy, or pre‐discharge reinitiation of these medications for compelling indications, particularly in the setting of heart failure with a reduced ejection fraction).
The cardiovascular risk of short‐term ACEI or ARB withdrawal is generally minimal. Even in higher risk groups, such as patients with moderately decompensated heart failure (NYHA classes II to III), heart failure decompensation is not observed until 4‐6 weeks following ACEI/ARB withdrawal. 49 This time course far exceeds the typical duration of COVID‐19 hospitalization. However, to ensure an even higher safety threshold, we created an additional exclusion criterion for patients with a LVEF <40%. Patients with heart failure and moderately reduced ejection fraction (LVEF 40%‐50%) should be even less likely to decompensate from ACEI/ARB withdrawal. Furthermore, we are reminding treating clinicians of the potential need to reinitiate therapy at the time of hospital discharge. In patients without heart failure, including those with resistant hypertension, short‐term withdrawal of antihypertensive medications is safe and well‐tolerated. 50 To ensure an additional margin of safety for this group, we created additional exclusion criteria for patients with a baseline systolic blood pressure >180 mm Hg or >160 if unable to substitute another drug class in place of ACEIs or ARBs, and for diastolic blood pressure ≥110 mm Hg.
In order to minimize exposure of the study staff to COVID‐19, many sites are using electronic informed consent forms. When electronic informed consent is performed, potential participants receive the consent form via email and provide consent attestation using a deidentified participant number via an electronic REDCap survey. The informed consent process is performed via phone or video conferencing. The study intervention and potential associated risks are explained to study participants verbally, and they have adequate time to ask questions. No study interventions are initiated until the study team receives either the signed informed consent form or attestation documenting the participants’ agreement to participate. Participants either receive a copy of the signed document via email or a REDCap attestation verification email once they agree to participate.
2.13. Regulatory standards
Because the REPLACE COVID trial is not aimed at obtaining regulatory approval of a novel drug, a labeled approved indication, or repurposing of existing drugs, this trial was formally determined by the Investigational New Drug support unit at the University of Pennsylvania to not require a US Food and Drug Administration Investigational New Drug application.
2.14. Current progress
As of August 10, 2020, the study has enrolled 136 of the planned 152 participants across twelve sites in the United States, Canada, Mexico, Peru, Argentina, Bolivia, and Sweden.
2.15. Trial registration
The trial was registered at ClinicalTrials.gov (NCT04338009).
CONFLICT OF INTEREST
In the last 2 years, JAC has received consulting honoraria from Sanifit, Bristol Myers Squibb, Edwards Lifesciences, Bayer, and JNJ and research grants from the National Institutes of Health, Microsoft, Fukuda‐Denshi, and Bristol Myers Squibb. He has received compensation from the American Heart Association and the American College of Cardiology for editorial roles, and visiting speaker honoraria from Washington University and University of Utah. JS has received speaker honoraria and is on the advisory boards for AstraZeneca, Vifor Pharma, and Novo Nordisk, and has received speaker honoraria from AMGEN. JBB has received research grants from Fast Grants for this study, as well as from the National Institutes of Health. TIC has received funding paid by Janssen Pharmaceuticals to Stanford University; has served as a consultant for Bayer, Janssen Pharmaceuticals, Novo Nordisk, Fresenius Medical Care, Tricida, Gilead, and AstraZeneca; and has received grant support from Satellite Healthcare, the American Heart Association, and the National Institutes of Health.
AUTHOR CONTRIBUTIONS
JAC, JBC, TCH, and VCM conceived the study. JBC, JAC, TCH, MOH, and JC were involved in methodologic design. JBC and JAC drafted the manuscript. JBC, TCH, VCM, PW, NR, NRRS, JERM, JS, TIC, CEA, EBS, JC, CACC, KETV, CRV, and DLE collected the data. JBC, TCH, JC, and MOH statistically analyzed and interpreted the data. All authors critically reviewed and edited the manuscript and acknowledged the final version of the submitted manuscript.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors would like to thank the members of the Data Safety Monitoring Board for their oversight of the trial: John Younger, MD; Raymond R. Townsend, MD; Gustavo Heresi, MD, MS; Todd A. Miano, PharmD, PhD; and Jesse Yenchih Hsu, PhD, MS.
Cohen JB, Hanff TC, Corrales‐Medina V, et al. Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) Trial Protocol. J Clin Hypertens. 2020;22:1780–1788. 10.1111/jch.14011
Clinical Trial Registration Number: clinicaltrials.gov NCT04338009
Funding information
The study was funded by JBC (K23‐HL133843); TCH (T32‐HL007891) JAC (R01‐HL 121510‐01A1, R61‐HL‐146390, R01‐AG058969, 1R01‐HL104106, P01‐HL094307, R03‐HL146874‐01, and R56‐HL136730) CRV (T32‐DK07785); and MOH (R00‐HL141678).
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 3. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sparks MA, Hiremath S, South A, et al. "The Coronavirus Conundrum: ACE2 and Hypertension Edition” NephJC. http://www.nephjc.com/news/covidace2. Accessed April 13, 2020.
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105‐2114. [DOI] [PubMed] [Google Scholar]
- 7. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sommerstein R, Gräni C. Rapid response to: preventing a COVID‐19 pandemic. BMJ. 2020;368:m810. [DOI] [PubMed] [Google Scholar]
- 9. Esler M, Esler D. Can angiotensin receptor‐blocking drugs perhaps be harmful in the COVID‐19 pandemic? J Hypertens. 2020;38:781‐782. [DOI] [PubMed] [Google Scholar]
- 10. Diaz JH. Hypothesis: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID‐19. J Travel Med. 2020;27(3):taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith DH, Neutel JM, Lacourciere Y, Kempthorne‐Rawson J. Prospective, randomized, open‐label, blinded‐endpoint (PROBE) designed trials yield the same results as double‐blind, placebo‐controlled trials with respect to ABPM measurements. J Hypertens. 2003;21:1291‐1298. [DOI] [PubMed] [Google Scholar]
- 14. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605‐2610. [DOI] [PubMed] [Google Scholar]
- 15. Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of Angiotensin‐converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572‐578. [DOI] [PubMed] [Google Scholar]
- 16. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin‐converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970‐976. [DOI] [PubMed] [Google Scholar]
- 17. Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398‐F405. [DOI] [PubMed] [Google Scholar]
- 18. Burrell LM, Risvanis J, Kubota E, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26(4):369‐375; discussion 322–4. [DOI] [PubMed] [Google Scholar]
- 19. Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin‐angiotensin system blockade and angiotensin‐converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond). 2012;123:649‐658. [DOI] [PubMed] [Google Scholar]
- 20. Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID‐19. J Am Soc Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280‐1287. [DOI] [PubMed] [Google Scholar]
- 22. Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13:e0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID‐19 mortality and the renin‐angiotensin system‐a call for epidemiologic investigations. Clin Infect Dis. 2020;71(15):870‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen JB, Hanff TC, South AM. ,Response by Cohen et al. to letter regarding article, "Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized With COVID‐19". Circ Res. 2020;126:e140‐e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation‐targeted adaptive servo‐ventilation therapy in heart failure: the CAT‐HF trial. J Am Coll Cardiol. 2017;69:1577‐1587. [DOI] [PubMed] [Google Scholar]
- 26. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circ Heart Fail. 2010;3:643‐646. [DOI] [PubMed] [Google Scholar]
- 28. Wilson FP, Reese PP, Shashaty MG, et al. A trial of in‐hospital, electronic alerts for acute kidney injury: design and rationale. Clin Trials. 2014;11:521‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury: a single‐blind, parallel‐group, randomised controlled trial. Lancet. 2015;385:1966‐1974.25726515 [Google Scholar]
- 30. O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079‐1087. [PubMed] [Google Scholar]
- 31. Harhay MN, Hill AS, Wang W, et al. Measures of Global health status on dialysis signal early rehospitalization risk after kidney transplantation. PLoS One. 2016;11:e0156532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir‐Ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peterson RL, Vock DM, Babiker A, et al. Comparison of an ordinal endpoint to time‐to‐event, longitudinal, and binary endpoints for use in evaluating treatments for severe influenza requiring hospitalization. Contemp Clin Trials Commun. 2019;15:e100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Senchenkova EY, Russell J, Esmon CT, Granger DN. Roles of Coagulation and fibrinolysis in angiotensin II‐enhanced microvascular thrombosis. Microcirculation. 2014;21:401‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grissom CK, Brown SM, Kuttler KG, et al. A modified sequential organ failure assessment score for critical care triage. Disaster Med Public Health Prep. 2010;4:277‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19–22. [Google Scholar]
- 38. Xie X, Chen J, Wang X, Zhang F, Liu Y. Age‐ and gender‐related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wright JT Jr, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595‐1608. [DOI] [PubMed] [Google Scholar]
- 40. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020;81(5):537‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willan AR, Pater JL. Carryover and the two‐period crossover clinical trial. Biometrics. 1986;42:593‐599. [PubMed] [Google Scholar]
- 42. Brown BW Jr. The crossover experiment for clinical trials. Biometrics. 1980;36:69‐79. [PubMed] [Google Scholar]
- 43. Grizzle JE. The two‐period change‐over design an its use in clinical trials. Biometrics. 1965;21:467‐480. [PubMed] [Google Scholar]
- 44. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model Statistics for Biology and Health. New York, NY: Springer; 2001. [Google Scholar]
- 45. Little RJ. Modeling the drop‐out mechanism in repeated‐measures studies. J Am Stat Assoc. 1995;90:1112‐1121. [Google Scholar]
- 46. Julious SA. Tutorial in Biostatistics. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921‐1986. [DOI] [PubMed] [Google Scholar]
- 47. O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549‐556. [PubMed] [Google Scholar]
- 48. PASS 16 Power Analysis and Sample Size Software. NCSS, LLC. Kaysville, UT. ncss.com/software/pass; 2018. [Google Scholar]
- 49. Pflugfelder PW, Baird MG, Tonkon MJ, DiBianco R, Pitt B. Clinical consequences of angiotensin‐converting enzyme inhibitor withdrawal in chronic heart failure: a double‐blind, placebo‐controlled study of quinapril. The Quinapril Heart Failure Trial Investigators. J Am Coll Cardiol. 1993;22:1557‐1563. [DOI] [PubMed] [Google Scholar]
- 50. Beeftink MM, van der Sande NG, Bots ML, et al. Safety of temporary discontinuation of antihypertensive medication in patients with difficult‐to‐control hypertension. Hypertension. 2017;69:927‐932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


