Abstract
Background.
Stem cells present in the vessel wall may be triggered in response to injurious stimuli to undergo differentiation and contribute to vascular disease development. Our aim was to determine the effect of moderate alcohol (EtOH) exposure on the expansion and differentiation of S100 calcium-binding protein B positive (S100β+) resident vascular stem cells and their contribution to pathologic vessel remodeling in a mouse model of arteriosclerosis.
Methods and Results.
Lineage tracing analysis of S100β+ cells was performed in male and female S100β-eGFP/Cre/ERT2–dTomato transgenic mice treated daily with or without EtOH by oral gavage (peak BAC: 15mM or 0.07%) following left common carotid artery ligation for 14 days. Carotid arteries (ligated or sham-operated) were harvested for morphological analysis and confocal assessment of fluorescent-tagged S100β + cells in FFPE carotid cross sections.
Ligation-induced carotid remodeling was more robust in males than in females. EtOH-gavaged mice had less adventitial thickening and markedly reduced neo-intimal formation compared to controls, with a more pronounced inhibitory effect in males compared to females. There was significant expansion of S100β + marked cells in vessels post-ligation, primarily in the neo-intimal compartment. EtOH treatment reduced the fraction of S100β + cells in carotid cross-sections, concomitant with attenuated remodeling. In vitro, EtOH attenuated Sonic Hedgehog-stimulated myogenic differentiation (as evidenced by reduced calponin and myosin heavy chain expression) of isolated murine S100β + vascular stem cells.
Conclusions.
These data highlight resident vascular S100β + stem cells as a novel target population for alcohol, and suggest that regulation of these progenitors in adult arteries, particularly in males, may be an important mechanism contributing to the anti-atherogenic effects of moderate alcohol consumption.
Keywords: Alcohol, Cardioprotective, Atherosclerosis, Stem Cells, Progenitor cells, S100β
INTRODUCTION
Epidemiologic studies indicate that alcohol (ethanol) consumption variously affects the incidence and severity of cardiovascular disease that may result in heart attack and stroke. In general, with respect to atherosclerosis low-moderate consumption (1-2 drinks/day) appears to be protective, whereas episodic binge drinking and chronic abuse is exacerbatory (Rehm and Roerecke, 2017, Piano, 2017, O’Keefe et al., 2014). The precise cell populations and signaling mechanisms targeted by ethanol to mediate these effects continue to be investigated.
Medial vascular smooth muscle cells (vSMC) are a major component of healthy arteries and play a substantial role in atherogenesis (Harman and Jorgensen, 2019). Vascular SMC display phenotypic plasticity; they can reversibly switch between a quiescent ‘contractile’ phenotype and a more active ‘synthetic’ phenotype, and they are a chief source of several heterogenous plaque cell types and extracellular matrix in all phases of atherosclerosis (Basatemur et al., 2019).
In addition to mature medial vSMC, progenitor stem cells present sparsely within the vessel wall may become triggered upon injurious stimuli to differentiate to vSMC and, thus, also importantly contribute to arteriosclerotic pathologies (Majesky et al., 2011, Majesky et al., 2012). These data endorse the concept that cardiovascular disease has a stem cell component (Psaltis and Simari, 2015) and point to a new field of therapeutic options targeting the specific progenitor populations involved.
One such progenitor population is multipotent vascular stem cells discovered by Tang et al., in rodent and human vessels (Tang et al., 2012). This population is smooth muscle myosin heavy chain (Myh11) and calponin (Cnn1) negative, but positive for S100β(i.e., S100 calcium binding protein b,a member of the S100 protein family) SOX10 Sox17 and Nestin. S100 proteins are involved in the regulation of a number of cellular processes such as cell migration, cell cycle progression, and differentiation (Donato et al., 2013). While ethanol is known to affect the growth and migration of mature vSMC (Hendrickson et al., 1998, Cullen et al., 2005, Morrow et al., 2010), any effects on vascular progenitor cells are not as well established. Here, using a murine lineage tracing approach that allows tracking of genomically marked cells and their progeny, we aimed to investigate the contribution of S100β+ vascular stem cells to iatrogenic arteriosclerotic carotid remodeling, and determine any modulatory effect of ‘moderate’ alcohol exposure on this progenitor population in vivo and in vitro.
MATERIALS AND METHODS
Mice for lineage tracing studies.
S100β-EGFP/Cre/ERT2 transgenic mice (JAX Labs, stock #014160, strain name B6;DBA-Tg(S100β-EGFP/cre/ERT2)22Amc/j) express the eGFPCreERT2 (Enhanced Green Fluorescent Protein and tamoxifen inducible cre recombinase/ESR1) fusion gene under the direction of the mouse S100β promoter. Ai9 mice (Jax Labs, stock #007909, strain name B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze /J) express robust tdTomato fluorescence following Cre-mediated LoxP recombination. For lineage tracing experiments S100β-eGFP/Cre/ERT2–dTomato double transgenic mice of both genders were generated by crossing S100β-eGFP/Cre-ERT2 mice with Ai9 reporter mice. The tdTomato transgene expression pattern corresponds to genomically marked S100β, and the eGFP transgene expression pattern corresponds to current expression of S100β.
Mouse carotid artery ligation or sham surgery.
Ligation of the left common carotid artery was performed essentially as described by us previously (Morrow et al., 2010, Liu et al., 2011) (Fig 1a) 1 week after tamoxifen-induced cre-recombination, in male and female S100β-eGFP/Cre/ERT2–dTomato double transgenic mice (Fig 1b). All procedures were approved by the University of Rochester Animal Care Committee and conform to NIH guidelines (Guide for the care and use of laboratory animals). Prior to surgery mice received a single dose of Buprenorphine SR (sustained release) analgesia (0.5-1.0 mg/kg SQ) (and every 72 hrs thereafter as needed). Anesthesia was achieved using inhalational isoflurane with the mouse positioned on a clean operating table, with a warming pad (heated by circulating warm water) to maintain body temperature. The animal was clipped and the surgical site prepped using betadine solution and alcohol. A midline cervical incision was made. With the aid of a dissecting microscope, the left common carotid was isolated and ligated just beneath the bifurcation with 6-0 silk suture. In sham surgery group, the carotid was similarly manipulated but not ligated. The neck incision (2 layers, muscle and skin) was sutured closed and the animal allowed recover under observation.
Figure 1.
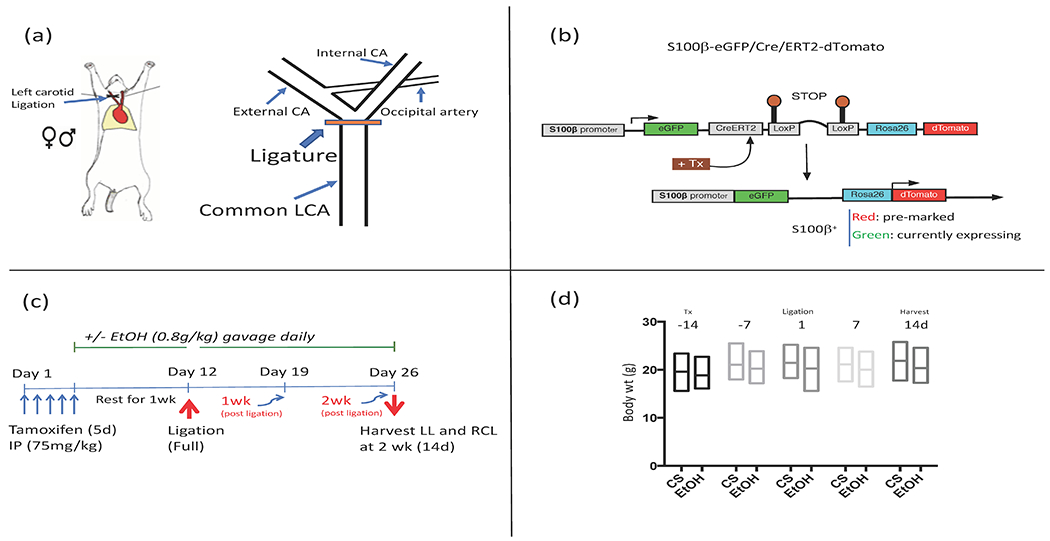
(a) ‘Flow restriction’ model of arteriosclerosis. Placement of ligature on left common carotid, just distal to the bifurcation, is shown. (b) Lineage tracing experiments were performed using S100β-eGFP/Cre/ERT2–dTomato double transgenic mice (S100β-eGFP/tdTm), generated by crossing S100β-eGFP/Cre-ERT2 mice with Ai9 reporter mice. (c) Experimental protocol using S100β-eGFP/tdTm mice showing the time sequence of tamoxifen (Tx) administration (to induce Cre-LoxP recombination and indelibly mark resident S100β+ cells), EtOH or cornstarch (CS) gavage (initiated 1 week prior to ligation, and continued for two weeks post-ligation), and vessel harvest 14d post ligation. LL=left ligated, RCL=Right contralateral. (d) Over the course of the experimental protocol mouse body weights were not significantly different between control (CS) and EtOH groups (Mean +/− SD, n= 13).
Tamoxifen treatment.
To induce Cre-LoxP recombination and indelibly mark resident S100β+ cells, S100β-eGFP/Cre/ERT2–dTomato mice (average weight 20g, 6-8 wks old) were injected with Tamoxifen, 75 mg/kg IP, for 5 consecutive days. They were then rested for 1 wk (during which time they received EtOH by gavage) before ligation surgery or sham operation. (See protocol timeline, Fig 1c).
EtOH treatment.
For 1 week before ligation, mice received the equivalent of 2 drinks daily by oral gavage as described previously by us (Morrow et al., 2010, Liu et al., 2011, Fitzpatrick et al., 2017); i.e., 0.8 g/kg of 200 proof ethanol (ASC/USP grade), maximum total volume 200 μl, giving a peak BAC of 15 mM or 0.07%. For example, a 20 g mouse was gavaged with 18.75 μl EtOH in 200 μl water. This ‘daily moderate’ alcohol feeding regimen was re-continued 1 day post ligation, and continued daily for up to 2 wks when animals were anesthetized and vessels harvested. The control group was gavaged with a calorically matched water-cornstarch mixture. There was no significant effect of EtOH consumption on mouse body weight over the experimental time frame, compared to the cornstarch control group (Fig 1d).
Histomorphometry.
Two weeks post-ligation, mice were anesthetized (ketamine/xylazine) and perfusion fixed with 4% paraformaldehyde in sodium phosphate buffer (pH 7.0). Fixed carotids were embedded in paraffin for sectioning. Starting at the carotid bifurcation landmark (single lumen) a series of cross-sections (10 x 5 μm) were made, every 200 μm through 2 mm length of carotid artery. Cross-sections were de-paraffinized, rehydrated in graded alcohols and stained with Verhoeff-Van Gieson stain for elastic laminae and imaged using a Nikon TE300 microscope equipped with a Spot RT digital camera (Diagnostic Instruments). Digitized images were analyzed using SPOT Advanced imaging software. Assuming a circular structure in vivo, the circumference of the lumen was used to calculate the lumen area, the intimal area was defined by the luminal surface and internal elastic lamina (IEL), the medial area was defined by the IEL and external elastic lamina (EEL), and the adventitial area was the area between the EEL and the outer edge, essentially as described by us previously (Morrow et al., 2010, Liu et al., 2011).
S100β+ Cells in Vivo.
S100β+ cells were identified as either ‘genomically-marked’ red tdTomato expressing cells or ‘currently expressing’ green fluorescent protein (eGFP) cells visualized in deparaffinized S100β-eGFP/Cre/ERT2–dTomato mouse carotid cross sections mounted with Sigma Fluoroshield with DAPI, using an FV1000 Olympus or a Nikon A1R HD laser scanning confocal microscope. Numbers of red or green fluorescent cells and Dapi nuclei (blue) in carotid cross section images from different experimental groups were either analyzed by Fiji ImageJ software (analyze particles function), or those in each vessel compartment were manually counted using a grid system.
Immunohistochemistry.
Carotid cross-sections were stained with mouse monoclonal anti-alpha smooth muscle actin (α-SMA) antibody (Abcam ab7817, 1:200); anti-eNOS antibody (Abcam ab76198, 1:200), anti-CD31 (Abcam ab24590), followed by a goat-anti rabbit IgG secondary Alexa Fluor 647® conjugate (Invitrogen Cat # S32357). Isotype control, and secondary antibody only controls were performed and showed minimal non-specific/background staining. For antigen retrieval, slides were brought to a boil in 10 mM sodium citrate (pH 6.0) then maintained at a sub-boiling temperature for 10 minutes. Slides were cooled on the bench-top for 30 minutes then washed in deionized water three times for 5 min each before being washed in PBS for 5 min. The antigen retrieval protocol diminishes endogenous eGFP and Td tomato transgene signals. Therefore, sections were co-stained with anti-eGFP antibody (Abcam, ab13970, 1:500) and anti-Td tomato antibody (Abcam, ab62341, 1:500).
Isolation and purification of S100β+ vascular stem cells (vSC).
Mouse thoracic aortas (4 at a time) were harvested and placed in cold Hank’s solution for adipose tissue removal. The adventitia was enzymatically removed by incubation of the vessels in Collagenase solution [i.e., MEM β nucleosides GlutaMAX™ (2 ml) containing Collagenase type 1A (0.7 mg/ml), soybean trypsin inhibitor (50 mg/ml), and bovine serum albumin (1 mg/ml)] for 10-20 min at 37°C. Once the adventitia became loose, it was carefully removed as an intact layer using forceps under a dissecting microscope. Remaining aortic tissue was cut into mm-sized pieces with a sterile blade for tissue explant. 5-6 pieces were added to each well of a 6-well plate coated with CELLstart™ (Invitrogen); adventitial side facing upwards. The small pieces were left to air-dry for approx. 30 min before 500 µl neuroectodermal maintenance medium (NE-MM) (i.e., DMEM supplemented with 2% chick embryo extract, 1% FBS, 0.02 βg/ml recombinant mouse FGF basic protein, 1X B-27 Supplement, and 1X CTS N-2 supplement, 1% penicillin-Streptomycin, 50 nM 2-Mercaptoethanol, 100 nM Retinoic acid) was added to each well avoiding tissue detachment. Four hours after incubation at 37°C, 5% CO2, each well was filled with another 500 µl of MM and left to incubate for 7 days in a humidified incubator at 37°C, 5% CO2. Once cells began to explant out of the medial tissue, the tissue pieces were removed. Cells were fed every 2-3 days and passaged every 3-4 days or when ~70% confluent. These cells were positive for S100β and negative for vSMC markers calponin (Cnn1), smooth muscle myosin heavy chain (SM-MHC), and myosin 11 (Myh11). To induce myogenic differentiation S100β+ stem cells were subcultured in SMC differentiation media (DM) consisting of DMEM supplemented with 10% FBS, PDGF-BB (10 ng/ml, Prepro Tech), and TGF-β1 (2 ng/ml, Prepro Tech).
S100β+ vSC immunocytochemistry.
Cells were seeded onto UV sterilized non-coated glass cover slips (20 mm) and grown for 24 hr, then fixed with 3.7% formaldehyde. Samples were permeabilized in 0.025 % Triton X-100 PBS (15 min, RT), blocked using a 5 % BSA, 0.3 M Glycine, 1 % Tween PBS blocking solution (1 hr at room temperature), then incubated with anti-calponin antibody (Cnn1, Sigma Cat No: C2687), or anti-myosin heavy chain antibody, (Myh11, Abcam Cat No: ab683) at the recommended dilutions at room temperature for 1 hr, or 4 °C overnight. Samples were washed twice in PBS and incubated with the recommended concentration of appropriate secondary antibody in blocking buffer for 1 hr or 4 °C overnight. Cell nuclei were stained using DAPI: PBS (dilution 1:1000) at room temperature for 15 min. An Olympus CK30 microscope and FCell software was used to capture images. A threshold of background staining was defined using the secondary antibody control and exposure rates were limited in order to rule out false positives. At least five images from the Olympus CK30 microscopy per experimental group (minimum n=3) were analyzed using ImageJ software and confocal images were analyzed using Zen 2008 software.
Quantitative real-time RT-PCR (qRT-PCR).
Total RNA was extracted from mouse S100β+ stem cells using RNeasy Mini Kit (Cat# 74134, Hilden, Germany) and 1 μg RNA was reverse transcribed to cDNA using iScript cDNA Synthesis kit (Cat# 1708891, Biorad, Hercules, CA, USA). Calponin (Cnn1, a differentiated smooth muscle cell marker), and GIi1 (a sonic hedgehog target gene) mRNA expression was determined by qRT-PCR. The gene specific oligonucleotide sequences were as previously described (Fitzpatrick et al., 2017). GAPDH was used as a housekeeping gene. SYBR Green master mix (Cat# 4309155, ThermoFisher Scientific, Waltham, MA, USA) was used according to manufacturer’s protocol with a QuantStudio 3 Real-Time PCR system (Applied Bosystems, Foster City, CA, USA).
Data analysis.
N=10 mice (consisting of 5 males, and 5 females) were used per experimental group, unless otherwise indicated. All in vitro experiments were performed in triplicate and repeated three times unless otherwise stated. For statistical comparisons, all data was checked for normal gaussian distribution before parametric and non-parametric tests were performed. An unpaired two-sided Student’s t-test was performed to compare differences between two groups. An ANOVA test was performed for multiple comparisons with a Sidak’s multiple comparisons test for parametric data and a Dunn’s Multiple comparison test for non-parametric data using GraphPad Prism software v8™. Results are expressed as Mean +/− SEM. A value of p < 0.05 was considered significant.
RESULTS
Ligation-induced carotid remodeling in S100β-eGFP/Cre/ERT2–dTomato double transgenic mice is attenuated by daily moderate EtOH consumption.
Ligation, or sham operation, of the left common carotid was performed in S100β-eGFP/Cre/ERT2–dTomato mice gavaged with or without ‘daily moderate’ (2 drink equivalent) EtOH. Controls received calorically matched cornstarch solution. Carotids were harvested on day 14 post-ligation and morphologic analysis performed. Ligation injury-induced vessel remodeling manifested itself as increased adventitial and medial compartment volumes, neo-intimal development, and a decreased lumen (Fig. 2a, 2b). Adventitial and neointimal changes were more robust in male than in female mice (Fig. 2a, 2e). EtOH-gavaged mice had significantly less adventitial thickening and markedly reduced neo-intimal formation compared to ligated controls (Fig. 2a, 2e), with a more pronounced inhibitory effect in males compared to females (Fig. 2e). Intima/media ratio was significantly less in the EtOH ligated group compared to ligated controls (Fig 2c). There was no effect of EtOH on sham-operated vessel morphology. Sham-operated vessel compartment volumes were similar in male and female mice (Fig 2d).
Figure 2.

Alcohol attenuates adventitial and neointimal remodeling in S100β-eGFP/tdTm mice. Carotid ligation was performed in S100β-eGFP/Cre/ERT2–dTomato transgenic mice treated with or without “moderate” amounts of EtOH (2 drink equivalent, 0.8 g/kg in 200 μl volume by oral gavage, resulting in a peak BAC of 15 mmol). Control ligated animals received an isocaloric cornstarch solutiuon. Vessels harvested 2 weeks post-ligation were used for morphological analysis. (a) Representative Van Gieson-stained carotid cross-sections. (b) Vessel compartment volumes for sham-operated (S), ligated control (L), and ligated plus EtOH (L+E). Adv = adventitia, Med = media, NI = neointima. Data are mean ± SEM, n=10. *p<0.01 versus sham; #p<0.05 vs L. (c) Intima/media ratio for ligated carotids from control and EtOH mice; *p<0.01. (d) Carotid compartment volumes were similar for sham-operated vessels from either male or female mice. Mean ± SEM, n=3 (male), n=4 (female). (e) Carotid compartment volumes for male and female mice, treated with or without EtOH gavage, showing greater adventitial and neointimal compartment volumes in males following ligation. Adv = adventitia, Med = media, NI = neointima. Data are mean ± SEM, n=5. *p<0.05, #p<0.01.
Expansion of S100β+ cell population following ligation injury.
Perfusion-fixed and paraffin embedded carotid cross sections from sham-operated and ligated experimental groups were evaluated by confocal microscopy for cells with tdTomato (red) or eGFP (green) fluorescence staining, indicative of genomically marked S100β cells and cells currently expressing S100β, respectively. In sham-operated vessels from either control or EtOH treated mice there was a small fraction (1-5%) of adventitial cells that were S100β+ (tdT), with no significant difference between the groups (Fig 3a). There was robust expansion of S100β + cells in carotids from control animals post-ligation (day 14), predominately in the neo-intimal compartment, but also in the media and adventitia (Fig 3b). Note, there were no TdTomato expressing cells (red) found in carotid cross sections from animals that received corn oil rather than tamoxifen (i.e., ‘no tamoxifen’ controls) (Supplemental Figure 1a), and tamoxifen administration had no effect on ligation-induced carotid remodeling (Supplemental Fig 1b).
Figure 3.
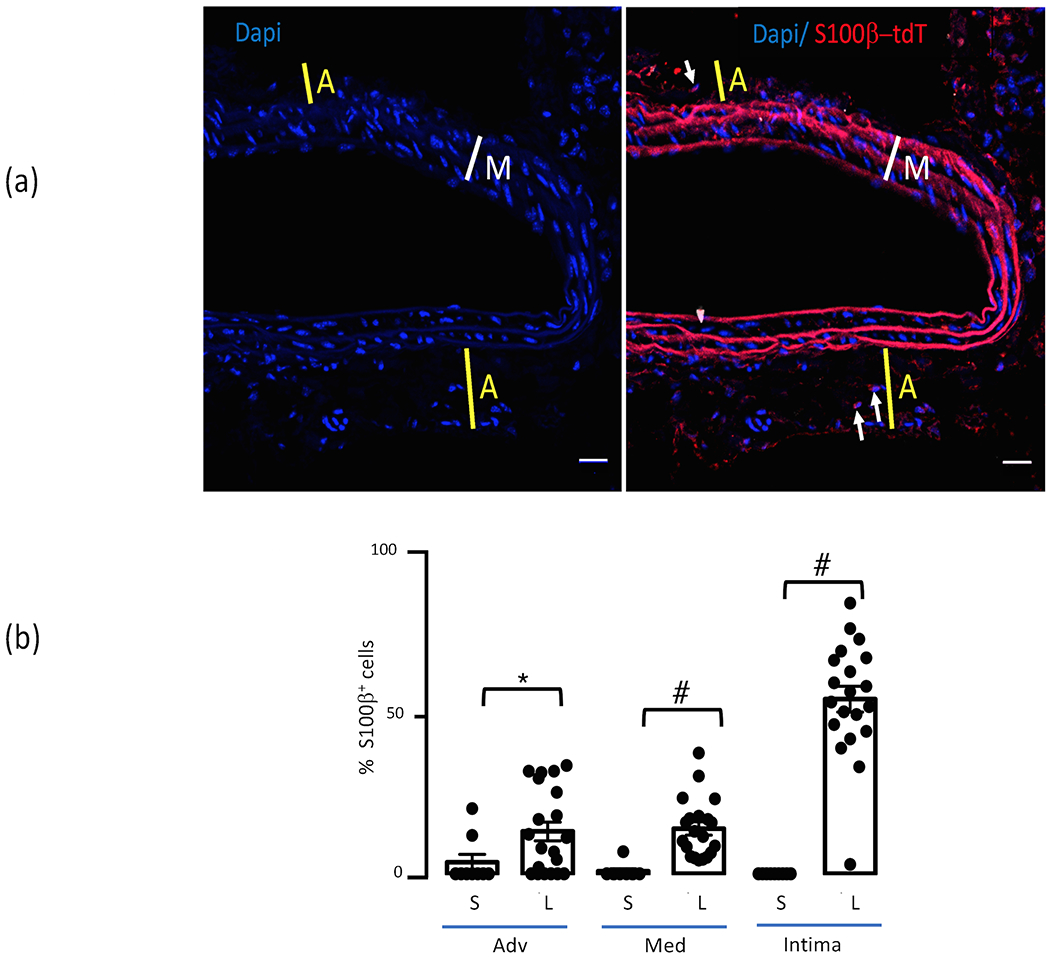
Expansion of S100β+ cells post-ligation. (a) Representative cross section of sham-operated carotid from control mouse showing low number of S100β+ (tdT) cells in the adventitia. Blue= DAPI nuclear stain, red= S100β-Td-Tomato. Thickness of adventitia (A) is indicated by yellow line; media (M) by white line; arrows indicate S100β+ cells. Scale bar=20μM. (b) Bar graph shows cumulative data for S100β+ (tdT) cells as a percentage of total cells (determined by DAPI nuclear staining) in control sham-operated (S) and ligated (L) carotids. Data are mean ± SEM, n= 20 sections from 4 mice. *p<0.05 vs sham, #P< 0.001 vs sham. There was expansion of the S100β+ stem cell population post-ligation injury in the adventitia, the media, and especially in the neo-intima.
EtOH gavage reduces neointimal S100β+ cell expansion following ligation injury.
Daily moderate EtOH gavage attenuated neointima formation (determined by both morphology analysis and Dapi nuclear stain count) concomitant with significantly reduced S100β+ cell expansion. S100β+ populations (both tdTomato- and eGFP-expressing cells) were markedly decreased in the neointima in the EtOH group compared to control; i.e., there were fewer numbers of S100β+ cells, and also a reduced fraction of total cells (Fig 4c). This inhibitory effect was seen in both genders but was greater in males (Fig 5a, b), than in females (Fig 6a, b). There was a slight increase in the number of S100β+ adventitial cells in carotids of EtOH treated males compared to controls (Fig 5b), that was not apparent in females (Fig 6b).
Figure 4.
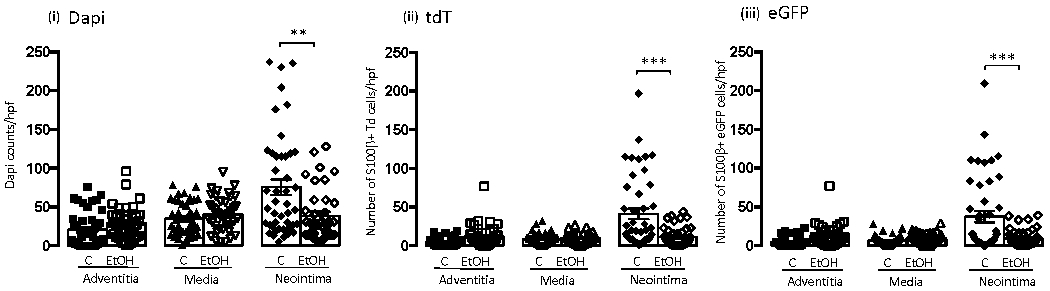
Daily moderate alcohol reduces the expansion of S100β+ stem cells in arteriosclerotic neointima. Bar graph showing cumulative data for (i) total number of cells (determined by Dapi nuclei), (ii) number of S100β+ - TdTomato/red cells, and (iii) number of S100β+ - eGFP/green cells in adventitia, media, and neointima vessel compartments for control (C), and EtOH treated (EtOH) mice. Data are mean ± SEM, n=40 sections from 10 animals. **p<0.001, ***p<0.0001 vs corresponding control.
Figure 5.


Daily moderate alcohol reduces the expansion of S100β+ stem cells in arteriosclerotic neointima: Male cohort. (a) Representative confocal immunofluorescence images (x20 and x60 magnification; scale bars are 50 or 20 μM as indicated) of carotid cross sections from Control ligated (top) or EtOH ligated (bottom) male mice. Blue -> DAPI nuclear stain, red -> S100β-Td-Tomato, green -> S100β-eGFP. Corresponding Van-Gieson (VG) stained cross sections (x20) are also shown. Thickness of adventitia (A) is indicated by yellow line; media (M) by white line; neointima (NI) by red line. (b) Scatter plot with bar graph showing cumulative data for (i) total number of cells (determined by Dapi nuclei), (ii) number of S100β+ - TdTomato/red cells, and (iii) number of S100β+ - eGFP/green cells) in adventitia, media, and neointima vessel compartments for control (C) and EtOH treated males. Data are mean ± SEM, n= 20 sections from 5 animals. *p<0.05, ***p<0.0001 vs corresponding control.
Figure 6.
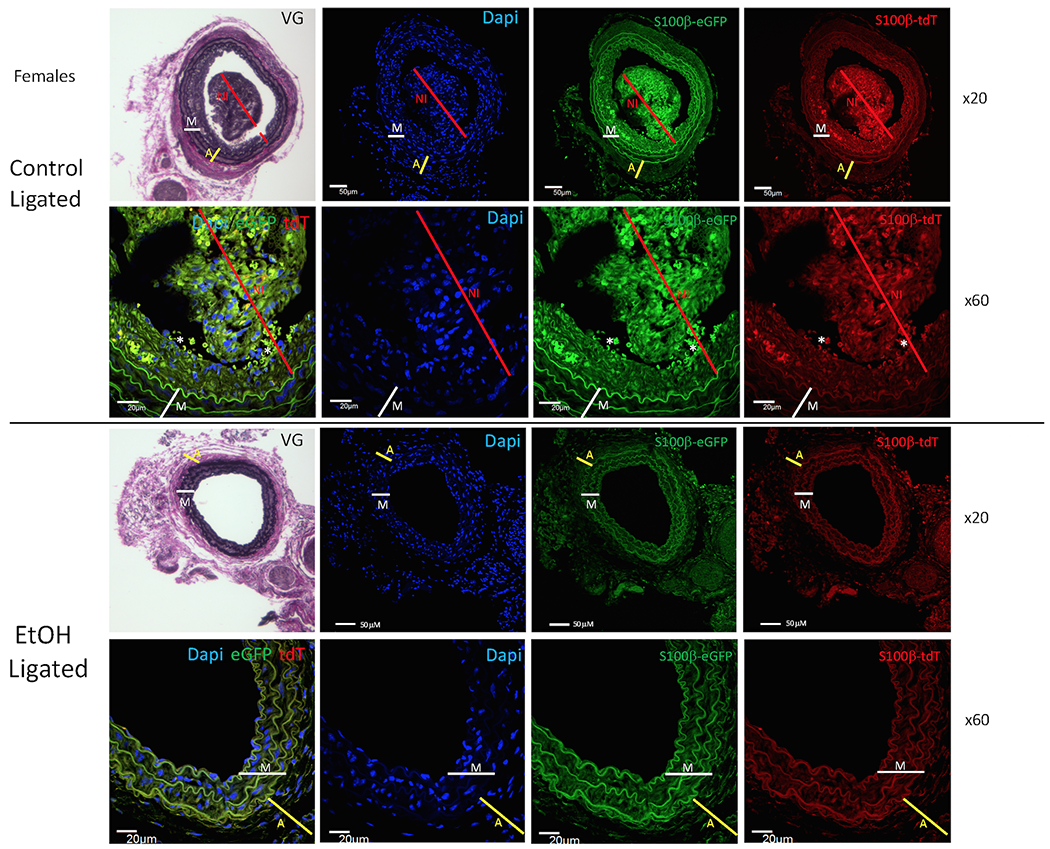

Daily moderate alcohol reduces the expansion of S100β+ stem cells in arteriosclerotic neointima: Female cohort. (a) Representative confocal immunofluorescence images (x20 and x60 magnification, scale bars are 50 or 20 μM as indicated) of carotid cross sections from Control ligated (top) or EtOH ligated (bottom) female mice. Blue -> DAPI nuclear stain, red -> S100β-Td-Tomato, green -> S100β-eGFP. Corresponding Van-Gieson (VG) stained cross sections (x20) are also shown. Thickness of adventitia (A) is indicated by yellow line; media (M) by white line; neointima (NI) by red line. (b) Scatter plot with bar graph showing cumulative data for (i) total number of cells (determined by Dapi (blue) nuclei), (ii) number of S100β+ - TdTomato/red cells, and (iii) number of S100β+ - eGFP/green cells) in adventitia, media, and neointima vessel compartments for control (C) and EtOH treated (EtOH) females. Data are mean ± SEM, n= 20 sections from 5 animals. *p<0.01, **p<0.001 vs corresponding control.
Neointimal cell identification.
To identify the cell types(s) composing the neointima of remodeled vessels (from ligated control and EtOH experimental groups), and to assess their co-expression of S100β,we used markers commonly used for vascular smooth muscle cells (i.e., the contractile protein smooth muscle α-actin; α-SMA), and for endothelial cells (i.e., CD31 [also known as platelet-endothelial cell adhesion molecule, PECAM1], and endothelial nitric oxide synthase; eNOS).
α-actin.
In remodeled carotids, from both ligated control and EtOH groups, cells in the media (i.e., delineated by internal and external elastic laminae) were α-SMA positive as determined by immunohistochemistry (Fig 7a). Many, but not all, neo-intimal cells were α-SMA positive and many of these co-localized with S100β+ cells (tdT, eGFP) (Fig 7a).
Figure 7.
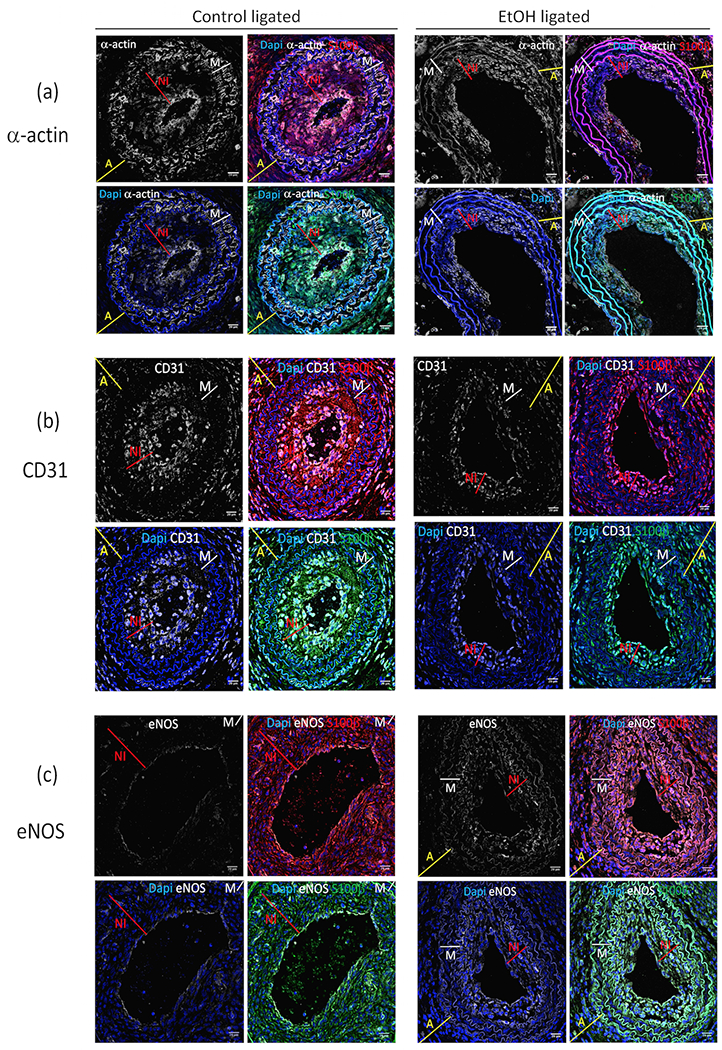
Immunofluorescence images showing (a) α-actin, (b) CD31, and (c) eNOS expression in ligated ‘Control’ and ‘EtOH’ S100β-eGFP/tdTm experimental groups. Immunohistochemistry was performed on sections from carotids harvested 14 days post ligation using anti-alpha-smooth muscle actin (α-SMA), anti-CD31, and anti-eNOS antibodies as described in the Methods section. Representative images shown; Blue=Dapi nuclear stain, white =α–actin (panel ‘a’), CD31 (panel ‘b’) and eNOS (panel ‘c’), red = S100β-Td-Tomato cells, green = S100β-eGFP. x60 magnification (scale bars 20 μM). Thickness of adventitia (A) is indicated by yellow line; media (M) by white line; neointima (NI) by red line.
CD31.
In remodeled carotids (both controls and EtOH) endothelial/intimal cells lining the lumen and some neointimal cells expressed CD31 (Fig 7b), many of which co-localized with S100β+ cells (tdT and eGFP) (Fig 7b). Of note, there was no obvious co-localization of α-actin and CD31 in neointimal cells, indicative of two separate cell populations/phenotypes (Supplemental Figure 1c).
eNOS.
In control ligated vessels eNOS expression determined by immunohistochemistry was seen in the intimal lining cell layer (endothelial cells), and in the occasional neointimal cell. In carotids from ligated EtOH-treated mice, eNOS was apparent in lining endothelial cells and also, to a greater degree than in controls, in some neo-intimal cells (Fig 7c).
EtOH inhibits Sonic hedgehog-induced myogenic differentiation of S100β+ murine vascular stem cells (vSC) in vitro.
We and others have previously reported that Sonic Hedgehog/Gli signaling plays a key role in atherogenesis (Morrow et al., 2007, Redmond et al., 2013, Aravani et al., 2019), and in regulating proliferation and differentiation of a variety of stem cells (Mooney et al., 2015, Milla et al., 2012). To determine whether Sonic Hedgehog drives the differentiation of S100β+ stem cells to smooth muscle-like cells (i.e., myogenic differentiation) and whether EtOH treatment affects this, S100β+/SM-MHC−/CNN1− cells were isolated from mouse thoracic aorta medial explants (Fig 8a). Similar to the effect of control differentiation media (DM), Sonic Hedgehog treatment alone promoted myogenic differentiation of these S100β+ cells as it increased the expression of Calponin1 (CNN1) and Myosin heavy chain 11 (MHC 11) protein levels as determined by immunohistochemistry, an effect blocked by the Hedgehog signaling inhibitor Cyclopamine (Fig 8b). EtOH (5-50 mM) had no toxic effects on S100β+ cells as determined by visual inspection and LDH cytotoxicity assay. As determined by qRT-PCR EtOH treatment decreased DM/Sonic Hedgehog-stimulated Gli target gene mRNA expression (Fig 8c), and attenuated Sonic Hedgehog-stimulated myogenic differentiation of S100β+ cells as evidenced by decreased calponin mRNA expression (Fig 8d).
Figure 8.

EtOH inhibits sonic hedgehog-induced myogenic differentiation of mouse S100β+ vascular stem cells (vSC) in vitro. (a) Isolated mouse vascular stem cells in culture (Phase contrast pic (top), and confocal images showing S100β expression (eGFP) x20, x40). (b) S100β+/Cnn1−/Myh11− vSC were cultured in differentiation media (DM) or sonic hedgehog (SHh, 0.5 μg/ml) +/− its inhibitor Cyclopamine (Cyl, 15 μM) for 7d. Representative immunocytofluoresence images shown, together with cumulative data of the fraction of cells expressing smooth muscle specific markers calponin (Cnn1) and myosin heavy chain 11 (Mhy11) in each experimental group. Data are mean ± SEM, n= 3. # p<0.05 vs MM, * p<0.05 vs SHh. (c) Murine S100β+ vSC were treated (24h) as indicated and mRNA levels of the SHh target gene Gli1 determined by qRT-PCR. MM=Maintenance media, DM=differentiation media, SHh=Sonic hedgehog, Cyl=cyclopamine, EtOH=ethanol (25 mM). Data are mean ± SEM, n= 3. *p<0.05 vs MM, #p<0.05 vs DM+rSHh. (d) Murine S100β+ vSC cultured in maintenance media (MM) were treated with SHh in the absence or presence of cylopamine or various concentrations of EtOH (5, 25, and 50 mM) for 7 days before calponin (Cnn1) mRNA levels were determined by qRT-PCR. Data are mean ± SEM, n= 3. *p<0.05 vs MM; # p<0.05 vs SHh alone.
DISCUSSION
In an attempt to minimize pathological arterial remodeling and its associated compromised blood flow much effort has been focused on understanding and identifying the origin of SMC-like cells that accumulate in the intima. The results reported here indicate that alcohol targets S100β+ resident stem cells in the vasculature that contribute to neointimal hyperplasia and highlight this as a potential mechanism mediating the anti-arteriosclerotic effects of moderate alcohol consumption.
There are plentiful population-based data that (i) light to moderate alcohol consumption has beneficial effects on overall cardiovascular health and mortality, and (ii) that heavy alcohol consumption is associated with poor cardiovascular outcomes and increased mortality (for reviews (Rehm and Roerecke, 2017, Piano, 2017, O’Keefe et al., 2014)). Given the limitations of such observational studies, robust human investigations such as randomized control trials would help establish the direct causality, or not, of these opposing effects of alcohol. In the absence of such human data due primarily to feasibility issues, we previously performed a controlled study in mice comparing the effects of two different patterns of alcohol consumption on atherosclerotic plaque development (Liu et al., 2011). In agreement with many epidemiological studies we found differential effects of ‘daily moderate’ and ‘two day binge’ drinking patterns, beneficial and deleterious, respectively (Liu et al., 2011). Here, we build on that and our other studies in the ligation injury model (Morrow et al., 2010) to probe the cell target populations involved in mediating the protective effects of moderate alcohol on arteriosclerotic remodeling.
Ligation of the left murine carotid artery markedly reduces blood flow that acts locally as a pathological stimulus and results in substantial arterial remodeling in a relatively short time (Korshunov and Berk, 2003). This is a well characterized model for carotid intima-media thickening; two to four weeks post-ligation the adventitia and media are thickened, and there is prominent intimal hyperplasia (i.e., neointimal formation) (Morrow et al., 2010, Liu et al., 2011). The neointima is the basis of vascular occlusive disease (including arteriosclerosis, atherosclerosis, and restenosis after revascularization) that may ultimately result in vessel blockage and cause heart attack or stroke, and intimal medial thickening is measured clinically to diagnose the extent of atherosclerotic vascular disease. Thus, this model is well suited for study of a clinically important phenomenon and its potential modulation.
Morphologically, we found that carotid remodeling was more robust in male than in female mice. Specifically, adventitial and neointimal compartment volumes were increased to a greater extent in males following ligation injury suggesting that sex is a variable in this model, with vessel pathology more progressed in males. This finding is in broad agreement with other studies in mice, albeit using different models (ApoE −/−) (Zhang et al., 2018), and with clinical findings in humans supporting sex differences in the manifestation of cardiovascular disease (Zhang et al., 2020, Man et al., 2020). The precise mechanisms underlying these sex differences are likely complex and not yet fully understood. Sex hormones estrogen and testosterone can certainly affect the development of cardiovascular diseases (Arnold et al., 2017) and may explain, in part, the differences in remodeling we report, but this was not explicitly tested in our study. Regardless of the sex difference seen in the remodeling response in our model, alcohol treatment inhibited neointimal hyperplasia in both males and females to the same extent.
Accumulation of SMC-like cells is central to neointima formation. Neointimal cells were initially thought to arise exclusively via the migration and proliferation of vascular (v) SMC from the media; with contractile, quiescent vSMC switching to a synthetic/secretory phenotype to facilitate this mobilization (Nemenoff et al., 2011). However, recent advances in understanding the biology of intimal hyperplasia point to a more complex picture. In addition to medial vSMC, other cells that may participate include bone marrow-derived progenitor cells, endothelial cells via endothelial-mesenchymal transition, and stem cells (reviewed in (Wang et al., 2015). In particular, much data exists to support the paradigm of resident vascular stem cells (vSCs) being triggered to undergo myogenic differentiation and, thus, also contribute to cell accumulation in the intima during hyperplasia and arteriosclerosis (Tang et al., 2012, Chen et al., 2013). Furthermore, the main contributing cell may vary depending on the experimental vascular disease model studied; e.g., hyperlipidemia vs ligation vs wire injury (Yuan et al., 2017).
Vascular stem cell markers routinely used include CD44, Sox2, Sox 10, Sca-1, S100β, and Nestin (Tang et al., 2012) (Wang et al., 2018). Here, using S100β/EGFP/Cre/ERT2 transgenic mice in lineage tracing experiments we found a small fraction of S100β+ cells in the healthy carotid artery, with a marked expansion of these cells in the remodeled carotid two weeks post-ligation. This expansion, perhaps in response to altered mechanical strain due to reduced blood flow following ligation (Tian et al., 2019), was evident in both males and females across all vessel compartments (i.e., adventitial, medial, neointimal) but occurred chiefly in the neointimal compartment, and to a greater degree in males. We found on average that ~30-60% of neointimal cells in remodeled carotids were S100β-positive and most, but not all, of these were also α-SMA positive. In contrast, in carotids of mice that were ligated but received daily moderate alcohol, there were significantly less S100β+ cells in the neointima (both in number, and as a fraction of total cells), concurrent with attenuated vessel remodeling. These data, together with our in vitro data showing that EtOH inhibits Sonic Hedgehog-driven myogenic differentiation of isolated murine aortic S100β+ vSC, suggest that alcohol (at moderate levels) attenuates neointimal hyperplasia by targeting resident vascular S100β+ progenitor cells. Clinically, less neointima in moderate alcohol consumers would conceivably translate to reduced heart attacks and strokes. Of note, Sonic Hedgehog signaling has previously been implicated in the pathogenesis of vessel disease (Aravani et al., 2019) (Morrow et al., 2007) (Morrow et al., 2009) (Li et al., 2012, Passman et al., 2008, Walshe et al., 2011) as well as in the regulation of stem cell fate especially in different cancers (Clara et al., 2020, Tandon et al., 2019, Sharma et al., 2019). Moreover, several studies have implicated Sonic Hedgehog signaling as an important molecular target for alcohol during development (Ahlgren et al., 2002) and in adult tissues (Latchoumycandane et al., 2015).
The data presented here are in broad agreement with our initial study on alcohol and vascular progenitors that reported alcohol regulation of stem cell antigen-1 positive (Sca1+) stem cells within vascular lesions (Fitzpatrick et al., 2017). However, the origin of these lesional Sca1 progenitors was not determined in that study. Recent studies using single cell RNA sequence analysis (scRNA-seq) suggest that a Sca1+ Myh11-Cre marked SMC subpopulation may give rise to the majority of Sca1+ neointimal cells following vascular injury (Dobnikar et al., 2018). Our current study provides compelling new evidence, using rigorous genetic cell fate mapping of tdT marked perivascular S100β cells before injury, that vascular lesions contain a significant number of S100β+ cells that originate from a non-SMC S100β parent population. Moreover, alcohol attenuates the accumulation of these cells in vivo following injury and inhibits myogenic differentiation of these cells in vitro. Cell fate mapping and scRNA-seq studies have also implicated adventitial Sca1+ cells in generating de novo neointimal SMC-like cells that expand more efficiently than pre-existing SMC (Tang et al., 2020). While several types of adult vascular stem cells have been identified and characterized by niche location, stem cell marker expression, and differentiation potential, in general vascular stem cells remain poorly defined and it may be that different progenitor populations express overlapping ranges of cell markers (Bobryshev et al., 2015). In this context, S100β has been recently shown to maintain an intermediate state of Sca1+ progenitor cells following injury-induced lesion formation (Wu et al., 2019). With this in mind, it is likely that the Sca1+ stem cells previously identified by us in vascular lesions and that are affected by alcohol (Fitzpatrick et al., 2017) originate from a non-SMC parent S100β+ Sca1− stem cell population. However, without definitive Sca1+ cell fate mapping studies, this possibility remains to be investigated.
In carotids from both control and EtOH experimental groups most, but not all, neo-intimal cells were positive for the smooth muscle cell marker α-SMA, and many of these cells co-localized with S100β-expressing cells. There was also a distinct CD31+ cell population apparent in the neointima; these cells were CD31+/α-SMA−/S100β+. These data indicate that heterogeneous cell populations contribute to neointimal hyperplasia following ligation injury. Comparable to smooth muscle cells, endothelial cells may demonstrate plasticity (e.g., in response to altered shear stress) under pathological conditions like vascular remodeling; those within the intima may migrate from their organized layer of cells and transition to mesenchymal or smooth muscle-like phenotype in a process called endothelial-mesenchymal transition (EndMT) (Chen et al., 2015) (Lai et al., 2018). EndMT promotes neointimal hyperplasia and induces atherogenic differentiation of EC (Moonen et al., 2015). Previous lineage tracing studies have implicated endothelial cells that undergo EndMT as contributing to neointimal formation during vein graft remodelling (Cooley et al., 2014, Yuan et al., 2017). The CD31+ cells we observed in the neointima, in both control and EtOH groups, might represent endothelial cells undergoing this transition. We note, however, that although CD31/PECAM1 is typically used as an endothelial cell marker it is expressed in other cells including leucocytes, platelets, and hematopoietic stem cells (Baumann et al., 2004, Liu and Shi, 2012), and multiple functional roles and associations of CD31 to atherosclerosis have been described (review(Woodfin et al., 2007)). It would be of great interest in future studies to determine whether EtOH affects EndMT during arteriosclerosis.
In addition to endothelial cells lining the lumen expressing eNOS, some neointimal cells also appeared to be eNOS+, to a greater degree in the EtOH group compared to controls. Of interest, ethanol has previously been shown to increase eNOS activity and nitric oxide (NO) production in endothelial cells ((Hendrickson et al., 1999, Venkov et al., 1999), (review(Cahill and Redmond, 2012)) and a role for NO in the cardioprotective effect of moderate alcohol is supported in the literature (Abou-Agag et al., 2005, Kleinhenz et al., 2008). Whether this eNOS effect has any functional significance with respect to EtOH’s S100β+ stem cell effect seen here remains to be determined.
In conclusion, these in vivo and in vitro data highlight vascular S100β + stem cells that contribute to intimal hyperplasia as a novel target population for alcohol, and suggest that regulation of these Sonic Hedgehog-responsive progenitor cells in adult arteries, particularly in males, may be an important mechanism contributing to the anti-atherogenic effects of moderate alcohol consumption.
Supplementary Material
Figure 9.
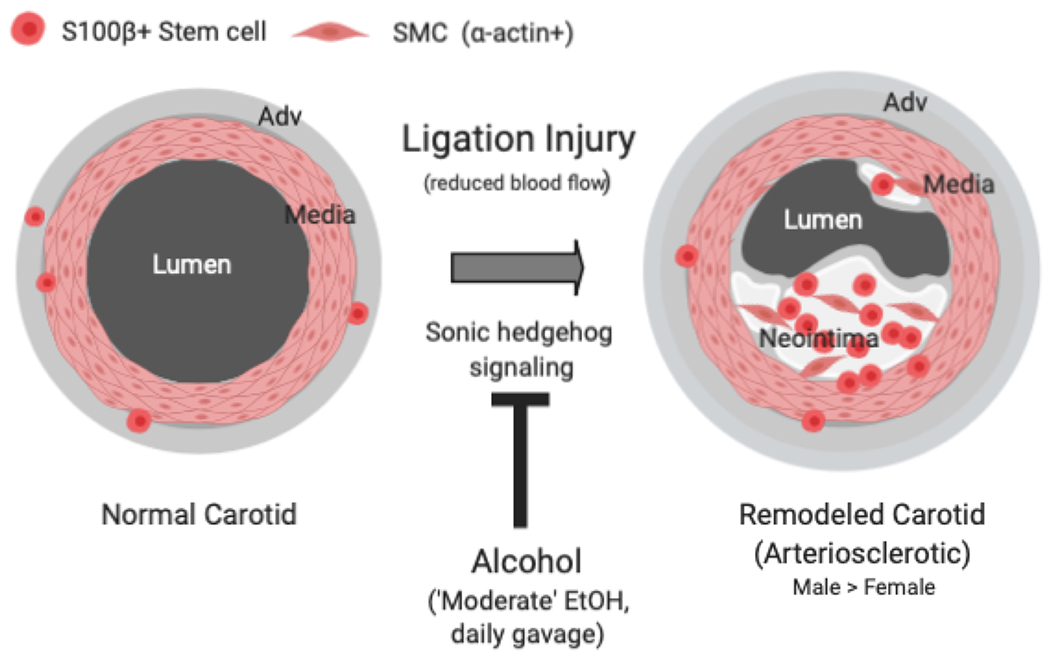
Lineage tracing analysis indicates that S100β+ stem cells, present sparsely in the normal carotid artery wall, expand and undergo differentiation to smooth muscle-like cells (SMC) following ligation-injury and contribute to pathologic neointima formation. Daily moderate alcohol gavage attenuated neointima formation concomitant with reduced S100β+ cell expansion. In vitro, alcohol attenuated Sonic Hedgehog-stimulated myogenic differentiation of isolated murine S100β+ vascular stem cells. Regulation of S100β+ stem cells may be an important mechanism mediating the anti-atherogenic effects of moderate alcohol consumption.
Acknowledgements
We thank Dr Kaye Thomas and Yu (Julie) Zhang for confocal microscope expertise.
Funding
This work was supported in part by grants from the National Institutes of Health R21AA023213 and RO1AA024082 to EMR, and by Science Foundation Ireland grant SFI-11/PI/1128, Health Research Board of Ireland (HRB) grant HRA-POR-2015-1315, and the European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB) to PAC.
Footnotes
Conflict of interest: None declared
REFERENCES
- Abou-Agag LH, Khoo NK, Binsack R, White CR, Darley-Usmar V, Grenett HE, Booyse FM, Digerness SB, Zhou F, Parks DA (2005) Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free radical biology & medicine 39:540–548. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, Thakur V, Bronner-Fraser M (2002) Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proceedings of the National Academy of Sciences of the United States of America 99:10476–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravani D, Morris GE, Jones PD, Tattersall HK, Karamanavi E, Kaiser MA, Kostogrys RB, Ghaderi Najafabadi M, Andrews SL, Nath M, Ye S, Stringer EJ, Samani NJ, Webb TR (2019) HHIPL1, a Gene at the 14q32 Coronary Artery Disease Locus, Positively Regulates Hedgehog Signaling and Promotes Atherosclerosis. Circulation 140:500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K (2017) Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler Thromb Vasc Biol 37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z (2019) Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. [DOI] [PubMed] [Google Scholar]
- Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH (2004) PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 104:1010–1016. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, Orekhov AN, Chistiakov DA (2015) Vascular stem/progenitor cells: current status of the problem. Cell and tissue research 362:1–7. [DOI] [PubMed] [Google Scholar]
- Cahill PA, Redmond EM (2012) Alcohol and cardiovascular disease -modulation of vascular cell function. Nutrients 4:297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M (2015) Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125:4514–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wong MM, Campagnolo P, Simpson R, Winkler B, Margariti A, Hu Y, Xu Q (2013) Adventitial stem cells in vein grafts display multilineage potential that contributes to neointimal formation. Arterioscler Thromb Vasc Biol 33:1844–1851. [DOI] [PubMed] [Google Scholar]
- Clara JA, Monge C, Yang Y, Takebe N (2020) Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol 17:204–232. [DOI] [PubMed] [Google Scholar]
- Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M (2014) TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 6:227ra234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JP, Sayeed S, Kim Y, Theodorakis NG, Sitzmann JV, Cahill PA, Redmond EM (2005) Ethanol inhibits pulse pressure-induced vascular smooth muscle cell migration by differentially modulating plasminogen activator inhibitor type 1, matrix metalloproteinase-2 and -9. Thromb Haemost 94:639–645. [DOI] [PubMed] [Google Scholar]
- Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M, Jorgensen HF (2018) Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun 9:4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL (2013) Functions of S100 proteins. Current molecular medicine 13:24–57. [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick E, Han X, Liu W, Corcoran E, Burtenshaw D, Morrow D, Helt JC, Cahill PA, Redmond EM (2017) Alcohol Reduces Arterial Remodeling by Inhibiting Sonic Hedgehog-Stimulated Stem Cell Antigen-1 Positive Progenitor Stem Cell Expansion. Alcoholism, clinical and experimental research 41:2051–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman JL, Jorgensen HF (2019) The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson RJ, Cahill PA, McKillop IH, Sitzmann JV, Redmond EM (1998) Ethanol inhibits mitogen activated protein kinase activity and growth of vascular smooth muscle cells in vitro. Eur J Pharmacol 362:251–259. [DOI] [PubMed] [Google Scholar]
- Hendrickson RJ, Cahill PA, Sitzmann JV, Redmond EM (1999) Ethanol enhances basal and flow-stimulated nitric oxide synthase activity in vitro by activating an inhibitory guanine nucleotide binding protein. J Pharmacol Exp Ther 289:1293–1300. [PubMed] [Google Scholar]
- Kleinhenz DJ, Sutliff RL, Polikandriotis JA, Walp ER, Dikalov SI, Guidot DM, Hart CM (2008) Chronic ethanol ingestion increases aortic endothelial nitric oxide synthase expression and nitric oxide production in the rat. Alcoholism, clinical and experimental research 32:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA, Berk BC (2003) Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol 23:2185–2191. [DOI] [PubMed] [Google Scholar]
- Lai B, Li Z, He M, Wang Y, Chen L, Zhang J, Yang Y, Shyy JY (2018) Atheroprone flow enhances the endothelial-to-mesenchymal transition. Am J Physiol Heart Circ Physiol 315:H1293–h1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumycandane C, Hanouneh M, Nagy LE, McIntyre TM (2015) Inflammatory PAF Receptor Signaling Initiates Hedgehog Signaling and Kidney Fibrogenesis During Ethanol Consumption. PLoS One 10:e0145691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li J, Li Y, Singh P, Cao L, Xu LJ, Li D, Wang Y, Xie Z, Gui Y, Zheng XL (2012) Sonic hedgehog promotes autophagy of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 303:H1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Shi GP (2012) CD31: beyond a marker for endothelial cells. Cardiovasc Res 94:3–5. [DOI] [PubMed] [Google Scholar]
- Liu W, Redmond EM, Morrow D, Cullen JP (2011) Differential effects of daily-moderate versus weekend binge alcohol consumption on atherosclerotic plaque development in mice. Atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM Jr. (2012) The adventitia: a progenitor cell niche for the vessel wall. Cells, tissues, organs 195:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Regan JN, Hoglund VJ (2011) Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res 108:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man JJ, Beckman JA, Jaffe IZ (2020) Sex as a Biological Variable in Atherosclerosis. Circ Res 126:1297–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla LA, Gonzalez-Ramirez CN, Palma V (2012) Sonic Hedgehog in cancer stem cells: a novel link with autophagy. Biol Res 45:223–230. [DOI] [PubMed] [Google Scholar]
- Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, Harmsen MC (2015) Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 108:377–386. [DOI] [PubMed] [Google Scholar]
- Mooney CJ, Hakimjavadi R, Fitzpatrick E, Kennedy E, Walls D, Morrow D, Redmond EM, Cahill PA (2015) Hedgehog and Resident Vascular Stem Cell Fate. Stem Cells Int 2015:468428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Cullen JP, Liu W, Cahill PA, Redmond EM (2010) Alcohol inhibits smooth muscle cell proliferation via regulation of the Notch signaling pathway. Arterioscler Thromb Vasc Biol 30:2597–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, Collins N, Walls D, Redmond EM, Cahill PA (2009) Sonic Hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol 29:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Sweeney C, Birney YA, Guha S, Collins N, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA (2007) Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. American Journal of Physiology-Cell Physiology 292:C488–C496. [DOI] [PubMed] [Google Scholar]
- Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MC (2011) SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol 31:1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ (2014) Alcohol and cardiovascular health: the dose makes the poison…or the remedy. Mayo Clin Proc 89:382–393. [DOI] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW (2008) A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proceedings of the National Academy of Sciences of the United States of America 105:9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR (2017) Alcohol’s Effects on the Cardiovascular System. Alcohol Res 38:219–241. [PMC free article] [PubMed] [Google Scholar]
- Psaltis PJ, Simari RD (2015) Vascular wall progenitor cells in health and disease. Circ Res 116:1392–1412. [DOI] [PubMed] [Google Scholar]
- Redmond EM, Hamm K, Cullen JP, Hatch E, Cahill PA, Morrow D (2013) Inhibition of patched-1 prevents injury-induced neointimal hyperplasia. Arterioscler Thromb Vasc Biol 33:1960–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Roerecke M (2017) Cardiovascular effects of alcohol consumption. Trends Cardiovasc Med 27:534–538. [DOI] [PubMed] [Google Scholar]
- Sharma A, De R, Javed S, Srinivasan R, Pal A, Bhattacharyya S (2019) Sonic hedgehog pathway activation regulates cervical cancer stem cell characteristics during epithelial to mesenchymal transition. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Tandon I, Waghmode A, Sharma NK (2019) Cancer Stem Cells Equipped with Powerful Hedgehog Signaling and Better Epigenetic Memory: Avenues to Look for Cancer Therapeutics. Curr Cancer Drug Targets 19:877–884. [DOI] [PubMed] [Google Scholar]
- Tang J, Wang H, Huang X, Li F, Zhu H, Li Y, He L, Zhang H, Pu W, Liu K, Zhao H, Bentzon JF, Yu Y, Ji Y, Nie Y, Tian X, Zhang L, Gao D, Zhou B (2020) Arterial Sca1(+) Vascular Stem Cells Generate De Novo Smooth Muscle for Artery Repair and Regeneration. Cell Stem Cell 26:81–96.e84. [DOI] [PubMed] [Google Scholar]
- Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S (2012) Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nature communications 3:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GE, Zhou JT, Liu XJ, Huang YC (2019) Mechanoresponse of stem cells for vascular repair. World J Stem Cells 11:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkov CD, Myers PR, Tanner MA, Su M, Vaughan DE (1999) Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb Haemost 81:638–642. [PubMed] [Google Scholar]
- Walshe TE, Connell P, Cryan L, Ferguson G, Gardiner T, Morrow D, Redmond EM, O’Brien C, Cahill PA (2011) Microvascular retinal endothelial and pericyte cell apoptosis in vitro: role of hedgehog and notch signaling. Investigative ophthalmology & visual science 52:4472–4483. [DOI] [PubMed] [Google Scholar]
- Wang D, Li LK, Dai T, Wang A, Li S (2018) Adult Stem Cells in Vascular Remodeling. Theranostics 8:815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Jacquet L, Karamariti E, Xu Q (2015) Origin and differentiation of vascular smooth muscle cells. J Physiol 593:3013–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Nourshargh S (2007) PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27:2514–2523. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu X, Guo LY, Zhang L, Zheng F, Li S, Li XY, Yuan Y, Liu Y, Yan YW, Chen SY, Wang JN, Zhang JX, Tang JM (2019) S100B is required for maintaining an intermediate state with double-positive Sca-1+ progenitor and vascular smooth muscle cells during neointimal formation. Stem Cell Res Ther 10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Wang D, Xu K, Wang J, Zhang Z, Yang L, Yang GY, Li S (2017) Contribution of Vascular Cells to Neointimal Formation. PLoS One 12:e0168914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li C, Zhu N, Chen Y, Yu Q, Liu E, Wang R (2018) Sex differences in the formation of atherosclerosis lesion in apoE(−/−)mice and the effect of 17beta-estrodiol on protein S-nitrosylation. Biomed Pharmacother 99:1014–1021. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu B, Zhao R, Zhang S, Yu XY, Li Y (2020) The Influence of Sex on Cardiac Physiology and Cardiovascular Diseases. J Cardiovasc Transl Res 13:3–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


