Abstract
Background:
Alterations in resting-state functional connectivity (rsFC) have been reported in Posttraumatic Stress Disorder (PTSD). Here, we examined pre- and post-treatment rsFC during a randomized clinical trial to characterize alterations and examine predictors of treatment response.
Methods:
Sixty-four combat veterans with PTSD were randomly assigned to Prolonged Exposure plus placebo, Sertraline plus enhanced medication management, or Prolonged Exposure plus Sertraline. Symptom assessment and resting-state functional MRI scans occurred before and after treatment. Twenty-nine trauma-exposed combat veterans without PTSD served as a control group at intake. Seed-based and ROI-to-ROI connectivities, as well as an exploratory connectome-based approach were used to analyze rsFC patterns. Based on previously reported findings, analyses focused on Salience Network (SN) and Default-Mode Network (DMN).
Results:
At intake, patients with PTSD showed greater DMN-dorsal attention network (DAN) connectivity (between vmPFC and superior parietal lobule; FWE-corrected p= .011), greater SN-DAN connectivity (between insula and middle frontal gyrus; corrected p = .003), and a negative correlation between re-experiencing symptoms and within-DMN connectivity (between PCC and middle temporal gyrus; corrected p < .001). We also found preliminary evidence for associations between rsFC and treatment response. Specifically, high responders (≥ 50% PTSD symptom improvement), compared to low responders, had greater SN-DMN segregation (i.e., less pre-treatment amygdala-PCC connectivity; p= .011) and lower pre-treatment global centrality (p=.042).
Conclusions:
Our findings suggest neural abnormalities in PTSD and may inform future research examining neural biomarkers of PTSD treatment response.
Keywords: PTSD, Prolonged Exposure, Sertraline, FMRI, Functional connectivity, Resting state
1. INTRODUCTION
Posttraumatic Stress Disorder (PTSD) is a debilitating psychiatric disorder affecting approximately 12-23% of combat veterans (Fulton et al., 2015). Evidence from functional MRI (fMRI) studies using resting-state functional connectivity (rsFC) suggests disrupted connectivity in the Default-Mode Network (DMN; associated with internally focused thought and autobiographical memory) and Salience Network (SN; responsible for detecting and orienting to salient stimuli) in individuals with PTSD (Liberzon & Abelson, 2016). Specifically, PTSD has been associated with (1) greater within-SN connectivity (Abdallah, Averill, Ramage, Averill, Goktas, et al., 2019; Brown et al., 2014; Rabinak et al., 2011; Sripada, King, Garfinkel, et al., 2012), (2) lower within-DMN connectivity (Akiki et al., 2018; Bluhm et al., 2009; Lazarov, Zhu, Suarez-Jimenez, Rutherford, & Neria, 2017; Miller et al., 2017; Olson, Kaiser, Pizzagalli, Rauch, & Rosso, 2019; Shang et al., 2014; Viard et al., 2019; Zhang et al., 2017), and (3) greater cross-network SN-DMN connectivity – often called desegregation (Brown et al., 2014; Lanius et al., 2010; Sripada, King, Garfinkel, et al., 2012).
Connectome-based analyses to characterize specific topological properties of whole-brain connectivity (Bullmore & Sporns, 2009) report mostly inconsistent results, with limited evidence of topological alterations in individuals with PTSD (Lei et al., 2015; Long et al., 2013; Niu et al., 2018; Shim, Im, & Lee, 2017; Spielberg, McGlinchey, Milberg, & Salat, 2015; Suo et al., 2015; Xu et al., 2018; H. Zhu et al., 2019). The primary findings thus far suggest that individuals with PTSD have greater “small-worldness” (i.e., more clustering and shorter connections (“paths”) that link regions within the network) and greater centrality (i.e., more central regions (“nodes”) with a high number of paths passing through them) in DMN and SN, further highlighting the roles of these networks in PTSD (Lei et al., 2015; Long et al., 2013). However, it remains unclear how these whole-brain connectivity findings relate to seed-based results. Studies using both seed-based and connectome-based approaches can address this gap.
While effective treatments for PTSD exist, including medication and psychotherapy, response to treatment is often lower than 50% in clinical settings (Kehle-Forbes, Meis, Spoont, & Polusny, 2016; Sripada et al., 2019). Further, individual patients differ in therapeutic response (Berger et al., 2009; Foa, Rothbaum, Riggs, & Murdock, 1991). PTSD response rates to selective serotonin reuptake inhibitors (SSRIs) rarely exceed 60%, and less than 30% of patients achieve full remission (Berger et al., 2009). Foa et al. (1991) showed that among PTSD patients treated with prolonged exposure (PE), 45% continued to meet diagnostic criteria for PTSD and 44% did not have clinically significant improvement three months later. A large meta-analysis of treatment studies of psychotherapy for combat PTSD in VA settings found similar rates of response and remission for PE (Steenkamp, Litz, Hoge, & Marmar, 2015). Importantly, while alterations in rsFC patterns have been identified in PTSD, links between neural connectivity and treatment response remain to be elucidated (for review, see Sheynin & Liberzon, 2017). Recently, King et al. (2016) found that combat PTSD patients had increased PCC-dlPFC and PCC-dACC connectivities following a mindfulness-based exposure therapy compared to an active comparison psychotherapy (present-centered therapy (PCT)). As dlPFC and dACC are associated with executive control of attention, these findings could relate to more effective emotion regulation and control of mental states with treatment (King, Block, Sripada, Rauch, Porter, et al., 2016). Interestingly, Abdallah et al. (2019) found that in combat PTSD patients, PCT decreased connectivity within SN, whereas cognitive processing therapy increased connectivity within the Central Executive Network. In another study, Zhu et al. (2018) reported increased rsFC in patients with PTSD between amygdala, hippocampus and prefrontal regions following exposure therapy – a possible reflection of fear inhibition/extinction and memory processing.
The current study leveraged a multi-center clinical trial (PROGrESS; Rauch, Kim, et al., 2018; Rauch, Simon, et al., 2018), to examine rsFC in patients with PTSD. Clinical and fMRI assessments were conducted before and after treatment, with inclusion of a combat control group assessed at intake (before treatment) only. We aimed to: (1) Confirm and replicate baseline connectivity abnormalities in patients with PTSD compared to combat controls, since existing reports are often limited to specific region(s) with equivocal replication (e.g., Patriat, Birn, Keding, & Herringa, 2016; Sripada, King, Welsh, et al., 2012); (2) Examine whether rsFC pattern abnormalities predict treatment response.
We predicted that patients with PTSD would show lower within-DMN, greater within-SN and greater SN-DMN connectivities, compared to combat controls at intake. In addition, we hypothesized that greater strength of these pre-treatment patterns would predict poorer treatment outcome. We tested each aim using three methodological approaches: seed-based analysis, ROI-ROI network analysis and an exploratory connectome-based analysis. To our knowledge, this is the largest and most comprehensive treatment study to test rsFC in patients with PTSD.
2. METHODS
The PROGrESS study is a four-site randomized-controlled trial, which examined longitudinal outcome data in response to treatment with Prolonged Exposure plus placebo (PE+PLB), Sertraline plus Enhanced Medication Management (SERT+EMM), or combined treatment (PE+SERT). The primary aim was to compare the effects of these treatments, with a secondary aim to identify biomarkers of treatment response. The current paper examined neural biomarkers collected during a resting-state fMRI scan, while those collected during emotion processing and emotion regulation tasks have been reported elsewhere (Duval et al., 2020; Joshi et al., 2020). For a full description of the study design, see Rauch, Simon, et al. (2018).
2.1. Participants
The study was approved by the local Institutional Review Boards at each recruitment site. The pre-treatment dataset included 61 combat veterans with PTSD and 29 combat-exposed healthy controls. Patients with PTSD were Operation Enduring Freedom, Iraqi Freedom, New Dawn veterans and/or active duty with combat-related PTSD with significant impairment (CAPS ≥ 50) for at least three months (DSM IV-TR). Inclusion/exclusion criteria for the “parent study” are previously published (Rauch, Simon, et al., 2018); patients with contraindication for fMRI were excluded from this fMRI study. Patients were randomly assigned to treatments noted above. Treatment period began at week 0 and lasted through week 24, including 13 weeks of the randomly assigned treatment and 12 additional weeks of pill continuation (Sertraline or Placebo; Supplementary Figure 1). PE consisted of 13 standard 90-min sessions, and Sertraline was titrated through week 10 and continued until week 24. Week 24 was chosen as the post-treatment point to allow for maximum therapeutic effect of Sertraline, as well as allowing sufficient time to complete all the PE sessions with flexibility to make up for missed sessions (Rauch, Simon, et al., 2018). Of the 61 participants recruited, 55 patients with a pre-treatment scan had a second CAPS (based on week 24 or the last observed CAPS score up to week 24), which allowed us to test prediction of change in CAPS. Out of these 55 patients, 39 completed a post-treatment scan, which allowed us to also analyze change in rsFC (Table 1). The reduction from 61 to 39 patients was due to: loss to follow-up (n = 12); time constraints (n = 5); decision by PI that treatment was not in best interest (n = 2); excessive motion during MRI scanning (n = 2); and out-of-state move (n = 1). The control group did not perform a second scan. The resting-state scan was the first task participants were asked to perform during the session in 93% of the 129 analyzed scans. High treatment response was defined as 50% or more reduction in CAPS from intake to the last observed CAPS score.
Table 1.
Demographic and clinical characteristics of the different analyzed groups.
| Controls | Patients (total) | Patients (with a 2nd CAPS) | Patients (with a 2nd CAPS and a 2nd scan) | Controls vs (total) Patients | |
|---|---|---|---|---|---|
| n | 29 | 61 | 55 | 39 | - |
| Age (SD) | 35.23 (8.6) | 32.38 (8.27) | 32.71 (8.09) | 32.48 (7.34) | p = .135 |
| Female (%) | 0 | 8.2 | 7.3 | 10.26 | p = .113 |
| CAPS; pre-treatment (SD) | 2 (3.55) | 74.82 (14.62) | 74.76 (15.01) | 72.46 (14.31) | p < .001 |
| CAPS; 2nd timepoint (SD) | - | - | 43.38 (28.24) | 35.85 (24.88) | - |
| PE group (n) | - | - | 10 | 7 | - |
| SERT group (n) | - | - | 23 | 15 | - |
| PE/SERT group (n) | - | - | 22 | 17 | - |
| High responders (%) * | - | - | 43.64 | 56.41 | - |
Defined as ≥ 50% PTSD symptom improvement.
Note: This table does not include two participants who aborted the study before session completion and their data were unusable, and a total of seven scans that were excluded from analyses due to excessive motion.
2.2. fMRI data acquisition and preprocessing
During resting-state scanning, a white fixation cross was displayed at the center of a black screen for eight minutes. Participants were instructed to relax and keep their eyes open with gaze fixed on the cross. Scanning was performed using a Phillips 3-Tesla Achieva X-series MRI scanner with an 8-channel SENSE Head coil. A total of 240 T2*-weighted echo planar gradient-recall volumes were acquired during rest (repetition time/echo time = 2000/25ms, flip angle = 90°, 0mm gap, EPI factor = 43, field of view = 220x220mm, 42 contiguous 2.8mm axial slices per volume). Three volumes before the initiation of the rest period were discarded at the beginning of each run to allow for equilibration of the MRI signal. A high-resolution T1-weighted structural image (3D turbo fast field echo, 1mm isotropic voxel, 0mm gap, 256×200 acquisition matrix, 180 sagittal slices, TR/TE = 9.8/4.6ms, flip angle = 8°) was obtained for anatomical localization.
fMRI data were preprocessed using SPM8 (Wellcome Centre for Human Neuroimaging, London, UK). Functional slices within each volume were sinc-interpolated and realigned, and structural images were coregistered to the mean of the functional images. The structural images were spatially normalized to a standard MNI template using VBM8 and DARTEL high-dimensional warping. Functional images were normalized to MNI space, then smoothed using a 5mm FWHM Gaussian kernel. To control for heartbeat, respiration and motion, a PCA was performed and the top five components of the white matter time series were added to the model as nuisance covariates.
Due to effects of motion on regional correlation, we excluded volumes based on a framewise displacement threshold of 0.5mm. A total of seven scans were excluded from analyses due to excessive motion, based on a criteria of < 3 min of “good” (i.e., without excessive motion) data. Motion parameters (maximum displacement, mean displacement, maximum angle, mean angle) for patients and controls were compared via independent samples t-tests and there were no differences (all p > .050). There were also no motion differences before or after treatment, between patients with high vs. low treatment response (all p > .100).
2.3. fMRI data analyses
Connectivity analyses were performed using the MATLAB toolbox ConnTool (Jelsone-Swain et al., 2010), and followed three complementary approaches. First, we performed seed-based analyses, using seeds within SN (insula, amygdala, dACC) and DMN (PCC, vmPFC, hippocampus) to generate whole-brain connectivity maps between each seed and all other voxels of the brain; see Figure 1. We then performed ROI-ROI network analyses, where we calculated rsFC between all the selected seeds.
Figure 1.
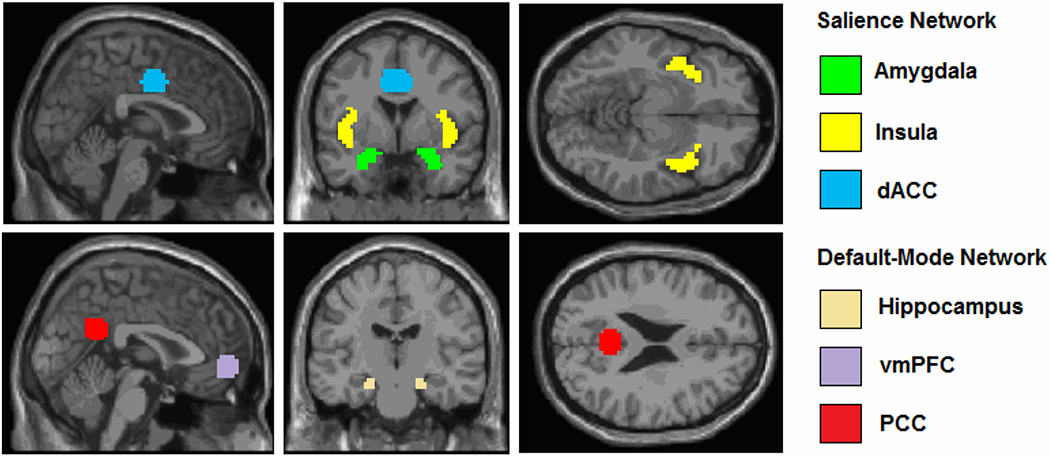
Visualization of the seeds that were used (Sheynin et al., 2020). Coordinates (De Luca et al., 2006): dACC (±4,−6,40) 10mm rad sphere; PCC (±2,−51,27) 10mm rad sphere; vmPFC (±2,54,−4) 10mm rad sphere; Hippocampus (±20,−19,−18) 5mm rad sphere. Anatomical masks were used for insula (Sripada R.K., King A.P., Welsh, R.C., et al., 2012) and amygdala (Sripada R.K., King A.P., Garfinkel S.N., et al., 2012).
In both approaches (seed-based and ROI-ROI) we tested the association between rsFC and PTSD diagnosis before treatment (patients vs. controls and correlation with PTSD severity), prediction of treatment response based on pre-treatment rsFC, and correlation between change in rsFC and change in CAPS across treatment. In all analyses, we controlled for age, sex and motion. When a significant association between rsFC and symptom change was found, we verified that the result was not driven by treatment arm or intake CAPS. To compare each ROI-ROI connectivity between treatment-response groups, we used univariate ANOVA with pre-treatment ROI-ROI connectivity as the dependent variable (DV), and treatment response (high responders, low responders) and treatment arm (PE+PLB, SERT+EMM, PE+SERT) as the independent variables (IVs).
Lastly, we performed exploratory connectome-based analyses, which included graph-theory and connectome-based predictive modeling, and utilized whole-brain connectomes (Power et al., 2011). Graph-theory analyses investigated topological characteristics using measures of network strength (degree of association between all regions (“nodes”) within the network), efficiency (related to the shortest connection (“path”) between various nodes within the network), modularity (ability of the network to form sparsely interconnected modules, each containing densely intraconnected nodes), clustering (amount of interconnections between topologically neighboring nodes), small-worldness (ratio of clustering and path length between the clusters within the network) and centrality (amount of central nodes with a high number of paths passing through them) (Bullmore & Sporns, 2009). In line with previous reports, we calculated area under the curve (AUC) for each network metric, with sparsity thresholds of .10 < S < .34 and an interval of .01 (e.g., Suo et al., 2015). Connectome-based Predictive Modeling (CPM) tested whether pre-treatment connectome features could predict patient/control group membership or response to treatment (Shen et al., 2017).
Second-level maps were initially thresholded at whole-brain p ≤ .001 uncorrected (extent threshold, k = 10), and then cluster thresholded at p ≤ .050, family-wise error (FWE) corrected. Bonferroni correction was used to protect against inflated risk of FWE; using p ≤ .017 for DMN and SN connectivities (three seeds in each network); p ≤ .008 for cross-network SN-DMN connectivities (six seeds), and p ≤ .006 for ROI-ROI SN-DMN connectivities (nine possible connectivities between the six network seeds). Correlations were used to examine whether connectivity patterns were associated with PTSD symptom severity, and were followed with post-hoc analyses to test if the finding was driven by any of the three PTSD symptom clusters (corrected p ≤ .017). A complete list of all the ROI-ROI group analyses is provided in Table 3. Note that the main text includes only findings that reached or approached corrected significance level. We did not apply additional corrections because our aim was to explore whether similar patterns can be identified using three different analytical approaches.
3. RESULTS
In the PROGrESS clinical trial, PTSD symptoms decreased significantly, with no differences between the three treatment groups (Rauch, Kim, et al., 2018). Here, we report the rsFC neuroimaging results.
3.1. Associations with PTSD grouping and symptoms at intake
3.1.1. Primary analyses
Connectivity analysis using Salience-Network (SN) seeds, revealed that patients had greater connectivity between insula and middle frontal gyrus (MFG) (FWE-corrected p = .003; Table 2, Figure 2A). Same analysis using Default-Mode Network (DMN) seeds, revealed that patients had greater connectivity between vmPFC and the superior parietal lobule (corrected p = .011; Table 2, Figure 2B). Additionally, the connectivity between PCC seed and the anterior part of middle temporal gyrus (MTG) was negatively correlated with PTSD severity, and specifically, with re-experiencing symptoms (corrected p < .001; Table 2, Figure 2C). No other seed-based or ROI-ROI results were found when comparing PTSD and control groups or testing correlations with symptoms at intake.
Table 2.
Results from seed-based analyses at intake (before treatment). Ze = peak-level Z-equivalent score.
| Seed and contrast map | Brain region | Connected networks | Figure | MNI [x,y,z] | Ze | Cluster K | Cluster p (FWE-corrected) |
|---|---|---|---|---|---|---|---|
| Insula seed | |||||||
| patients > controls | middle frontal gyrus | SN-DAN | 2A | 27, 14, 43 | 4.86 | 90 | .003 |
| vmPFC seed | |||||||
| patients > controls | superior parietal lobule | DMN-DAN | 2B | 48,−49,61 | 4.77 | 71 | .011 |
| PCC seed | |||||||
| CAPS regressor (patients only; negative correlation) | anterior middle temporal gyrus | within DMN | - | 63,−19,−14 | 4.11 | 51 | .040 |
| Re-experiencing subscale regressor (patients only; negative correlation) | anterior middle temporal gyrus | within DMN | 2C | 57,−19,−17 | 4.22 | 136 | < .001 |
Figure 2.
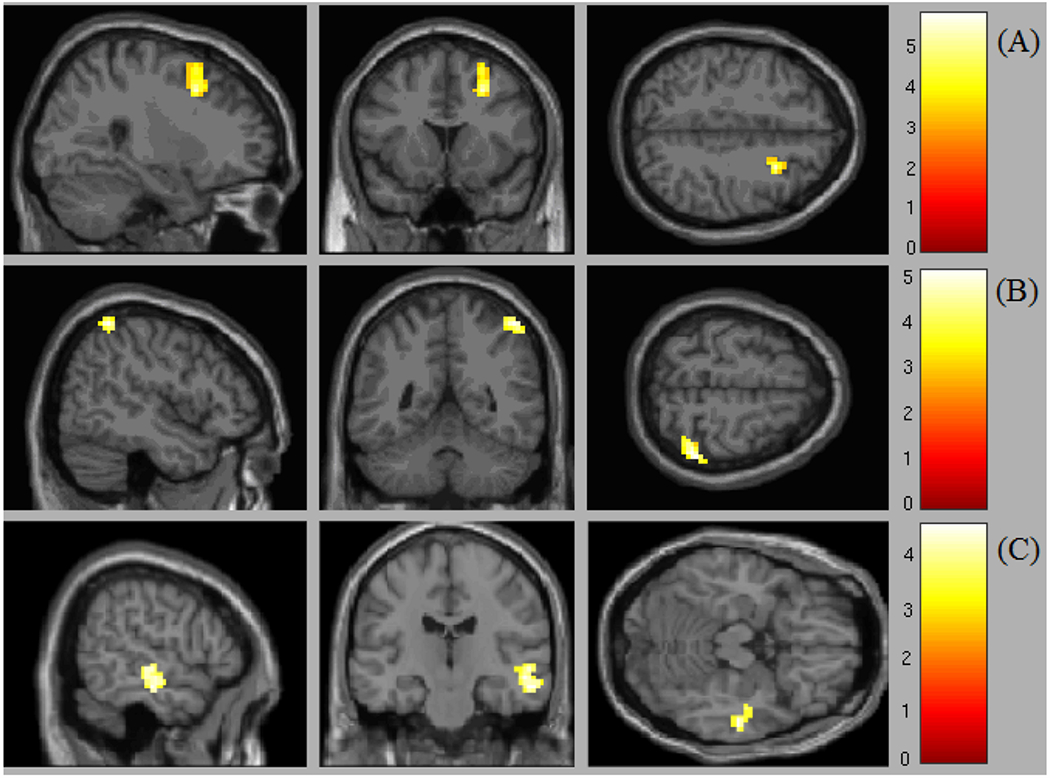
Significant baseline differences in rsFC between PTSD and control groups (seed-based approach). (A) Insula seed. Patients > controls contrast revealed greater connectivity between SN and DAN in patients, and specifically, between insula and MFG (FWE corrected; p = .003). (B) VmPFC seed. Patients > controls contrast revealed greater connectivity between DMN and DAN in patients, and specifically, between vmPFC and superior parietal lobule (corrected p = .011). (C) PCC seed. Within-DMN connectivity, and specifically, between PCC and anterior MTG, was negatively correlated with PTSD severity, and specifically, with re-experiencing symptoms (corrected p < .001).
3.1.2. Exploratory connectome-based analysis
No graph-theory indices differed between PTSD and control groups and no correlations with symptoms were found at intake (all p > .05). Connectome-based Predictive Modeling (CPM) did not predict group membership better than chance.
3.2. Associations with treatment response
3.2.1. Primary analyses
There were no significant associations between seed-based or ROI-ROI connectivities and change in PTSD symptoms from pre- to post-treatment that survived correction for multiple comparisons. There was, however, a trend for a main effect of response status in amygdala-PCC connectivity (Bonferroni-corrected “trend level” defined as .050/9 < p ≤ 1.000/9, based on the nine possible connectivities between the six network seeds for SN-DMN ROI-ROI connectivities), with high treatment responders (≥ 50% symptom improvement) having lower pre-treatment amygdala-PCC connectivity than low responders (F(1,46) = 7.100, p = .011; F(1,45) = 6.133, p = .017 with pre-treatment CAPS as a covariate). Both high responders and control participants had a low levels of amygdala-PCC rsFC at intake, with only low responders having a value greater than zero (p = .010). Following treatment, no difference between high and low responders was found (Figures 3A–B).
Figure 3.
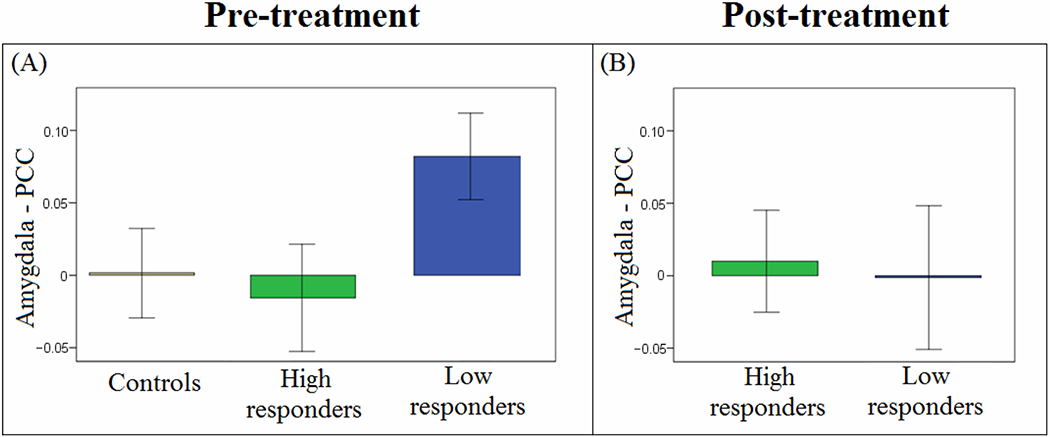
Pre-treatment rsFC is associated with treatment outcome (ROI-ROI analyses). (A) Patients who had high response to treatment ( ≥ 50% change in CAPS) had less amygdala-PCC (SN-DMN) connectivity (i.e. greater segregation) before treatment than patients who had low response to treatment (p = .011). Interestingly, the controls also had a low connectivity value (around zero), and only low responders had a value different (greater) than zero (p = .010). (B) No difference is amygdala-PCC connectivity after treatment.
3.2.2. Exploratory connectome-based analysis
At intake, the functional brain network of high responders had a lower global centrality value than low responders (t(53) = 2.081, p = .042). No other associations between graph-theory measures and change in PTSD symptoms were found (all p > .050). CPM did not predict response to treatment better than chance.
4. DISCUSSION
At intake (before treatment) patients with PTSD showed greater connectivity than healthy controls between vmPFC (a key DMN node) and superior parietal lobule, a node of dorsal attention network (DAN), which is thought to play a key role in orienting attention (Corbetta & Shulman, 2002). Such greater connectivity might underlie hypervigilance to external stimuli, including during rest. PTSD patients relative to controls also showed greater connectivity between insula (a key SN node) and a region in the MFG, also a node of DAN, suggesting greater connectivity between SN and DAN in patients with PTSD. This is consistent with Russman Block et al. (2017) suggesting that SN intrusion upon DAN may contribute to difficulty orienting attention in patients with PTSD. Taken together, these findings of greater connectivity between SN and DMN with DAN are consistent with a growing body of evidence of cross-network desegregation in individuals with PTSD (Lanius et al., 2010; Russman Block et al., 2017; Sripada, King, Welsh, et al., 2012).
In addition, PTSD severity (and specifically re-experiencing symptoms) was negatively correlated with the strength of connectivity between PCC (a key DMN node) and anterior MTG, proposed to be part of a DMN subsystem (Uddin, Kelly, Biswal, Castellanos, & Milham, 2009; Xu et al., 2015). This finding adds to a growing body of evidence suggesting lower within-DMN connectivity in patients with PTSD compared to controls (Akiki et al., 2018; Bluhm et al., 2009; Lazarov et al., 2017; Miller et al., 2017; Olson et al., 2019; Shang et al., 2014; Viard et al., 2019; Zhang et al., 2017). Since DMN is linked to stimulus-independent and internally-focused thought, this finding could represent general disruption in self-referential thought in patients with PTSD. The finding that altered DMN connectivity was driven by re-experiencing symptoms is consistent with prior reports, and may be related to abnormalities in memory formation and retrieval (Spielberg et al., 2015; Yan et al., 2013).
We also identified a preliminary (trend-level) result that suggests that altered rsFC might predict treatment outcome. Specifically, prior to treatment, high treatment responders had less connectivity between amygdala and PCC than low responders, suggesting greater SN-DMN segregation might be associated with better outcome. While there is accumulating evidence for altered connectivity between and within SN and DMN in patients with PTSD, to our knowledge, this is the first study with evidence suggesting that rsFC data might contain predictors of treatment outcome.
Using an exploratory connectome-based approach, we also found preliminary evidence that low treatment responders show greater levels of global centrality before treatment than high responders. This finding is in line with reports of increased neural centrality in PTSD (Lei et al., 2015; Long et al., 2013). Greater global centrality implies the existence of more brain regions that serve as central (e.g., have many paths running through them; Bullmore & Sporns, 2009). It is possible that greater centrality, similarly to the greater SN-DMN connectivity described earlier, reflects forms of neural desegregation previously reported in PTSD, and might also play a role in treatment response. The idea that topological alterations can be used to predict treatment outcome in PTSD is novel and this study provides some initial evidence of potential “bridges” between theory-driven seed-based and data-driven connectome-based approaches.
A number of limitations of this study have to be acknowledged. First, the generalizability of our sample is limited to males who were exposed to one type of trauma (combat) and who were seeking treatment at a VA facility. Second, the control group was not part of the PROGrESS trial and was included to specifically test differences before treatment only. Thus, we cannot isolate effects of treatment from the effects of time. A “post” scan in control subjects in future studies could confirm that the described neural changes are due to treatment effect rather than passage of time alone. Third, while the overall number of subjects and drop-out rate are on par with other large treatment-based studies (e.g., Fonzo et al., 2017), our study was underpowered to detect an effect of treatment type. However, the lack of treatment type effect is consistent with the emotion processing and emotion regulation task-based fMRI findings, as well as with the clinical outcomes, in the parent PROGrESS study (Duval et al., 2020; Joshi et al., 2020; Rauch, Kim, et al., 2018). Another issue related to the small number of participants is the possibility that the results identified in this study are spurious and will fail to replicate. Larger studies to replicate the findings, as well as to test different treatments by collecting and retaining an adequate number of participants in each treatment group, are needed.
In sum, this is one of the first studies to test the relationship between response to treatment and rsFC in PTSD. Our findings suggest greater connectivity between SN, DMN and attention regions (i.e., greater desegregation), and decreased within-DMN connectivity, in patients with PTSD compared to healthy controls. They also offer novel preliminary suggestions that greater segregation between DMN and SN and lower global centrality are neural biomarkers that might be associated with better treatment response. We used several analytical techniques (seed-based, ROI-ROI and connectome-based), suggesting that integrative approaches may be useful and informative (see also Sheynin et al., 2020). While the fact that only few significant results were identified could reflect specificity, it is also possible that larger N studies (including larger treatment arms) are required to identify additional predictors. This study also urges the careful consideration of the number of analyses conducted, and the need to use a stringent statistical correction for multiple analyses, to decrease the risk of reporting spurious results that might fail to replicate. It is our hope that the preliminary findings presented here would facilitate future research in larger treatment samples that ultimately may elucidate neural predictors of treatment response and thereby help personalize and optimize treatment approaches and increase response rates in PTSD.
Supplementary Material
Supplementary Figure 1. Timeline of the ”pre” and “post” scans and CAPS scores in relation to treatment (Rauch et al. Contemp Clin Trials, 2018). Pre-treatment scan and CAPS are based on week 0. Post-treatment scan is based on week 24, and post-treatment CAPS is based week 24 or the last observed score up to week 24. Week 24 was chosen as the post-treatment point to allow for maximum therapeutic effect of Sertraline, as well as allowing sufficient time to complete all the PE sessions with flexibility to make up for missed sessions.
Table 3:
Full list of results from the ROI-ROI analyses. All analyses controlled for age, sex and motion.
| ROI-ROI connectivity | F | p | |
|---|---|---|---|
| Pre-treatment rsFC: patients (n=61) vs. controls (n=29)* | |||
| 1 | Amygdala-Insula | .145 | .705 |
| 2 | Amygdala-dACC | .446 | .506 |
| 3 | Insula-dACC | .214 | .645 |
| 4 | Amygdala-Hippocampus | .316 | .575 |
| 5 | Insula-Hippocampus | .052 | .820 |
| 6 | dACC-Hippocampus | .626 | .431 |
| 7 | Amygdala-PCC | .557 | .458 |
| 8 | Insula-PCC | 3.043 | .085 |
| 9 | dACC-PCC | .113 | .738 |
| 10 | Hippocampus-PCC | .060 | .808 |
| 11 | Amygdala-vmPFC | 1.257 | .265 |
| 12 | Insula-vmPFC | 1.606 | .209 |
| 13 | dACC-vmPFC | .473 | .493 |
| 14 | Hippocampus-vmPFC | 2.270 | .136 |
| 15 | PCC-vmPFC | .012 | .915 |
| Prediction of treatment outcome based on pre-treatment rsFC (low responders (n=31) vs. high responders (n=24))** | |||
| 1 | Amygdala-Insula | <.001 | .995 |
| 2 | Amygdala-dACC | <.001 | .989 |
| 3 | Insula-dACC | .619 | .435 |
| 4 | Amygdala-Hippocampus | .096 | .758 |
| 5 | Insula-Hippocampus | 1.233 | .273 |
| 6 | dACC-Hippocampus | .697 | .408 |
| 7 | Amygdala-PCC | 7.100 | .011 |
| 8 | Insula-PCC | .075 | .785 |
| 9 | dACC-PCC | .218 | .642 |
| 10 | Hippocampus-PCC | 4.133 | .048 |
| 11 | Amygdala-vmPFC | 1.891 | .176 |
| 12 | Insula-vmPFC | .314 | .578 |
| 13 | dACC-vmPFC | .362 | .550 |
| 14 | Hippocampus-vmPFC | .461 | .500 |
| 15 | PCC-vmPFC | 1.003 | .322 |
| Association between changes in rsFC and treatment outcome (low responders (n=17) vs. high responders (n=22))** | |||
| 1 | Amygdala-Insula | 1.213 | .280 |
| 2 | Amygdala-dACC | .143 | .708 |
| 3 | Insula-dACC | 5.142 | .031 |
| 4 | Amygdala-Hippocampus | .334 | .568 |
| 5 | Insula-Hippocampus | .790 | .381 |
| 6 | dACC-Hippocampus | .641 | .430 |
| 7 | Amygdala-PCC | 1.458 | .237 |
| 8 | Insula-PCC | .056 | .815 |
| 9 | dACC-PCC | .055 | .816 |
| 10 | Hippocampus-PCC | 1.597 | .216 |
| 11 | Amygdala-vmPFC | .057 | .813 |
| 12 | Insula-vmPFC | .227 | .637 |
| 13 | dACC-vmPFC | .374 | .545 |
| 14 | Hippocampus-vmPFC | 1.737 | .197 |
| 15 | PCC-vmPFC | .195 | .662 |
IV: group (patients, controls)
IVs: group (low responders, high responders), arm (PE+PLB, SERT+EMM, PE+SERT)
ACKNOWLEDGMENTS
We would like to thank all members of the PROGrESS study team, especially Murray Stein for assistance with project development and execution, Margaret R. Venners for project management, Nita Patel for MRI scanning, Sean Ma for data collection and organization, and Yana Lokshina for assistance with imaging analysis. We thank all of the individuals who participated in this study.
FUNDING AND DISCLOSURE
This work was supported by the U.S. Department of Defense through the U.S. Army Medical Research and Materiel Command (MRMC; Award #W81XWH-11-1-0073; PI: Rauch); the National Center for Advancing Translational Sciences of the National Institutes of Health (Award #UL1TR000433). This material is the result of work supported with resources and the use of facilities at Massachusetts General Hospital, the VA Ann Arbor Healthcare System, Ralph H. Johnson VA Medical Center, and VA San Diego Healthcare System. The views expressed in this article presentation are solely those of the author(s) and do not reflect an endorsement by or the official policy of the Department of Veterans Affairs, Department of Defense, or the U.S. Government, or the official views of the National Institutes of Health. Competing interests: The authors declare no competing interests.
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdallah CG, Averill CL, Ramage AE, Averill LA, Alkin E, Nemati S, … Consortium SS (2019). Reduced Salience and Enhanced Central Executive Connectivity Following PTSD Treatment. Chronic Stress (Thousand Oaks), 3. doi: 10.1177/2470547019838971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill CL, Ramage AE, Averill LA, Goktas S, Nemati S, … Consortium SS (2019). Salience Network Disruption in U.S. Army Soldiers With Posttraumatic Stress Disorder. Chronic Stress (Thousand Oaks), 3. doi: 10.1177/2470547019850467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, Wrocklage KM, Scott JC, Averill LA, Schweinsburg B, … Abdallah CG (2018). Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. Neuroimage, 176, 489–498. doi: 10.1016/j.neuroimage.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, & Figueira I (2009). Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry, 33(2), 169–180. doi: 10.1016/j.pnpbp.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, … Lanius RA (2009). Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci, 34(3), 187–194. [PMC free article] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MW, McCarthy G, & Morey RA (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology, 39(2), 351–359. doi: 10.1038/npp.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, & Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 10(3), 186–198. doi: 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci, 3(3), 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, & Smith SM (2006). fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage, 29(4), 1359–1367. doi: 10.1016/j.neuroimage.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Duval ER, Sheynin J, King AP, Phan KL, Simon NM, Martis B, … Rauch SAM (2020). Neural function during emotion processing and modulation associated with treatment response in a randomized clinical trial for posttraumatic stress disorder. Depress Anxiety. doi: 10.1002/da.23022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Rothbaum BO, Riggs DS, & Murdock TB (1991). Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive-behavioral procedures and counseling. J Consult Clin Psychol, 59(5), 715–723. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, … Etkin A (2017). PTSD Psychotherapy Outcome Predicted by Brain Activation During Emotional Reactivity and Regulation. Am J Psychiatry, 174(12), 1163–1174. doi: 10.1176/appi.ajp.2017.16091072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, … Beckham JC (2015). The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: a meta-analysis. J Anxiety Disord, 31, 98–107. doi: 10.1016/j.janxdis.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Jelsone-Swain LM, Fling BW, Seidler RD, Hovatter R, Gruis K, & Welsh RC (2010). Reduced Interhemispheric Functional Connectivity in the Motor Cortex during Rest in Limb-Onset Amyotrophic Lateral Sclerosis. Front Syst Neurosci, 4, 158. doi: 10.3389/fnsys.2010.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SA, Duval ER, Sheynin J, King AP, Phan KL, Martis B, … Rauch SAM (2020). Neural correlates of emotional reactivity and regulation associated with treatment response in a randomized clinical trial for posttraumatic stress disorder. Psychiatry Res Neuroimaging, 299, 111062. doi: 10.1016/j.pscychresns.2020.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehle-Forbes SM, Meis LA, Spoont MR, & Polusny MA (2016). Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma, 8(1), 107–114. doi: 10.1037/tra0000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, … Liberzon I (2016). Altered Default Mode Network (DMN) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (PTSD) in Combat Veterans of Afghanistan and Iraq. Depress Anxiety, 33(4), 289–299. doi: 10.1002/da.22481 [DOI] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch SA, Porter KE, Favorite TK, … Liberzon I (2016). A Pilot Study of Mindfulness-Based Exposure Therapy in OEF/OIF Combat Veterans with PTSD: Altered Medial Frontal Cortex and Amygdala Responses in Social-Emotional Processing. Front Psychiatry, 7, 154. doi: 10.3389/fpsyt.2016.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, … Brimson M (2010). Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand, 121(1), 33–40. doi: 10.1111/j.1600-0447.2009.01391.x [DOI] [PubMed] [Google Scholar]
- Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, & Neria Y (2017). Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J Psychiatr Res, 94, 15–22. doi: 10.1016/j.jpsychires.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D, Li K, Li L, Chen F, Huang X, Lui S, … Gong Q (2015). Disrupted Functional Brain Connectome in Patients with Posttraumatic Stress Disorder. Radiology, 276(3), 818–827. doi: 10.1148/radiol.15141700 [DOI] [PubMed] [Google Scholar]
- Liberzon I, & Abelson JL (2016). Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron, 92(1), 14–30. doi: 10.1016/j.neuron.2016.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Duan X, Xie B, Du H, Li R, Xu Q, … Chen H (2013). Altered brain structural connectivity in post-traumatic stress disorder: a diffusion tensor imaging tractography study. J Affect Disord, 150(3), 798–806. doi: 10.1016/j.jad.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, & Verfaellie M (2017). Default Mode Network Subsystems are Differentially Disrupted in Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging, 2(4), 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu R, Lei D, Chen F, Chen Y, Suo X, Li L, … Gong Q (2018). Reduced local segregation of single-subject gray matter networks in adult PTSD. Hum Brain Mapp, 39(12), 4884–4892. doi: 10.1002/hbm.24330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Kaiser RH, Pizzagalli DA, Rauch SL, & Rosso IM (2019). Regional Prefrontal Resting-State Functional Connectivity in Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging, 4(4), 390–398. doi: 10.1016/j.bpsc.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriat R, Birn RM, Keding TJ, & Herringa RJ (2016). Default-Mode Network Abnormalities in Pediatric Posttraumatic Stress Disorder. J Am Acad Child Adolesc Psychiatry, 55(4), 319–327. doi: 10.1016/j.jaac.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, … Petersen SE (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi: 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, & Phan KL (2011). Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry, 2, 62. doi: 10.3389/fpsyt.2011.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SAM, Kim HM, Powell C, Tuerk PW, Simon NM, Acierno R, … Hoge CW (2018). Efficacy of Prolonged Exposure Therapy, Sertraline Hydrochloride, and Their Combination Among Combat Veterans With Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2018.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SAM, Simon NM, Kim HM, Acierno R, King AP, Norman SB, … Hoge CW (2018). Integrating biological treatment mechanisms into randomized clinical trials: Design of PROGrESS (PROlonGed ExpoSure and Sertraline Trial). Contemp Clin Trials, 64, 128–138. doi: 10.1016/j.cct.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Russman Block S, King AP, Sripada RK, Weissman DH, Welsh R, & Liberzon I (2017). Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cogn Affect Behav Neurosci, 17(2), 422–436. doi: 10.3758/s13415-016-0488-2 [DOI] [PubMed] [Google Scholar]
- Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, … Zhang W (2014). Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLoS One, 9(5), e96834. doi: 10.1371/journal.pone.0096834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, & Constable RT (2017). Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc, 12(3), 506–518. doi: 10.1038/nprot.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J, Duval ER, Lokshina Y, Scott JC, Angstadt M, Kessler D, … Liberzon I (2020). Altered resting-state functional connectivity in adolescents is associated with PTSD symptoms and trauma exposure. Neuroimage Clin, 26, 102215. doi: 10.1016/j.nicl.2020.102215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J, & Liberzon I (2017). Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett, 649, 133–138. doi: 10.1016/j.neulet.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M, Im CH, & Lee SH (2017). Disrupted cortical brain network in post-traumatic stress disorder patients: a resting-state electroencephalographic study. Transl Psychiatry, 7(9), e1231. doi: 10.1038/tp.2017.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, & Salat DH (2015). Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry, 78(3), 210–216. doi: 10.1016/j.biopsych.2015.02.013 [DOI] [PubMed] [Google Scholar]
- Sripada RK, Blow FC, Rauch SAM, Ganoczy D, Hoff R, Harpaz-Rotem I, & Bohnert KM (2019). Examining the nonresponse phenomenon: Factors associated with treatment response in a national sample of veterans undergoing residential PTSD treatment. J Anxiety Disord, 63, 18–25. doi: 10.1016/j.janxdis.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, & Liberzon I (2012). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci, 37(4), 241–249. doi: 10.1503/jpn.110069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, & Liberzon I (2012). Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med, 74(9), 904–911. doi: 10.1097/PSY.0b013e318273bf33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp MM, Litz BT, Hoge CW, & Marmar CR (2015). Psychotherapy for Military-Related PTSD: A Review of Randomized Clinical Trials. JAMA, 314(5), 489–500. doi: 10.1001/jama.2015.8370 [DOI] [PubMed] [Google Scholar]
- Suo X, Lei D, Li K, Chen F, Li F, Li L, … Gong Q (2015). Disrupted brain network topology in pediatric posttraumatic stress disorder: A resting-state fMRI study. Hum Brain Mapp, 36(9), 3677–3686. doi: 10.1002/hbm.22871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp, 30(2), 625–637. doi: 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Mutlu J, Chanraud S, Guenole F, Egler PJ, Gerardin P, … Guillery-Girard B (2019). Altered default mode network connectivity in adolescents with post-traumatic stress disorder. Neuroimage Clin, 22, 101731. doi: 10.1016/j.nicl.2019.101731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chen F, Lei D, Zhan W, Sun X, Suo X, … Gong Q (2018). Disrupted Functional Network Topology in Children and Adolescents With Post-traumatic Stress Disorder. Front Neurosci, 12, 709. doi: 10.3389/fnins.2018.00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang J, Fan L, Li H, Zhang W, Hu Q, & Jiang T (2015). Tractography-based Parcellation of the Human Middle Temporal Gyrus. Sci Rep, 5, 18883. doi: 10.1038/srep18883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, … Marmar CR (2013). Spontaneous brain activity in combat related PTSD. Neurosci Lett, 547, 1–5. doi: 10.1016/j.neulet.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Zhang XD, Yin Y, Hu XL, Duan L, Qi R, Xu Q, … Li LJ (2017). Altered default mode network configuration in posttraumatic stress disorder after earthquake: A resting-stage functional magnetic resonance imaging study. Medicine (Baltimore), 96(37), e7826. doi: 10.1097/MD.0000000000007826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Li Y, Yuan M, Ren Z, Yuan C, Meng Y, … Zhang W (2019). Increased functional segregation of brain network associated with symptomatology and sustained attention in chronic post-traumatic stress disorder. J Affect Disord, 247, 183–191. doi: 10.1016/j.jad.2019.01.012 [DOI] [PubMed] [Google Scholar]
- Zhu X, Suarez-Jimenez B, Lazarov A, Helpman L, Papini S, Lowell A, … Neria Y (2018). Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depress Anxiety, 35(10), 974–984. doi: 10.1002/da.22816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Timeline of the ”pre” and “post” scans and CAPS scores in relation to treatment (Rauch et al. Contemp Clin Trials, 2018). Pre-treatment scan and CAPS are based on week 0. Post-treatment scan is based on week 24, and post-treatment CAPS is based week 24 or the last observed score up to week 24. Week 24 was chosen as the post-treatment point to allow for maximum therapeutic effect of Sertraline, as well as allowing sufficient time to complete all the PE sessions with flexibility to make up for missed sessions.


